Leucine–Histidine Dipeptide Attenuates Microglial Activation and Emotional Disturbances Induced by Brain Inflammation and Repeated Social Defeat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptides
2.2. Animals
2.3. Culture of Primary Mouse Microglia
2.4. LPS Induction of Neuronal Inflammation
2.5. TST
2.6. OFT
2.7. Repeated SDS (R-SDS)
2.8. Social Interaction Test (SIT)
2.9. Elevated Plus Maze (EPM) Test
2.10. Pharmacokinetics of the 14C-LH Peptide
2.11. Statistical Analysis
3. Results
3.1. Effects of the LH Dipeptide on Primary Microglia
3.2. Penetration of the LH Dipeptide through the Blood–Brain Barrier
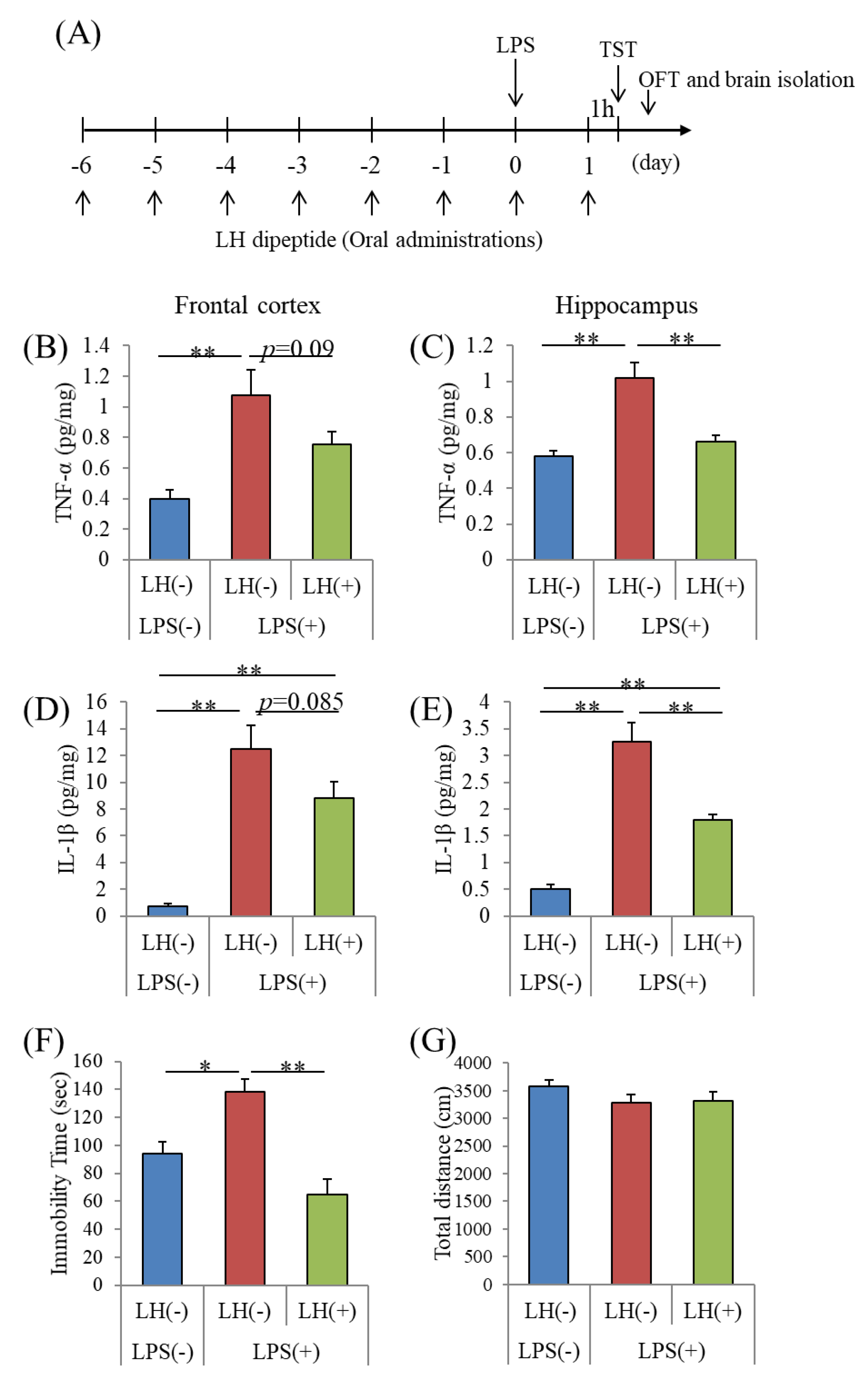
3.3. Effects of the LH Dipeptide on Inflammation in the Brain
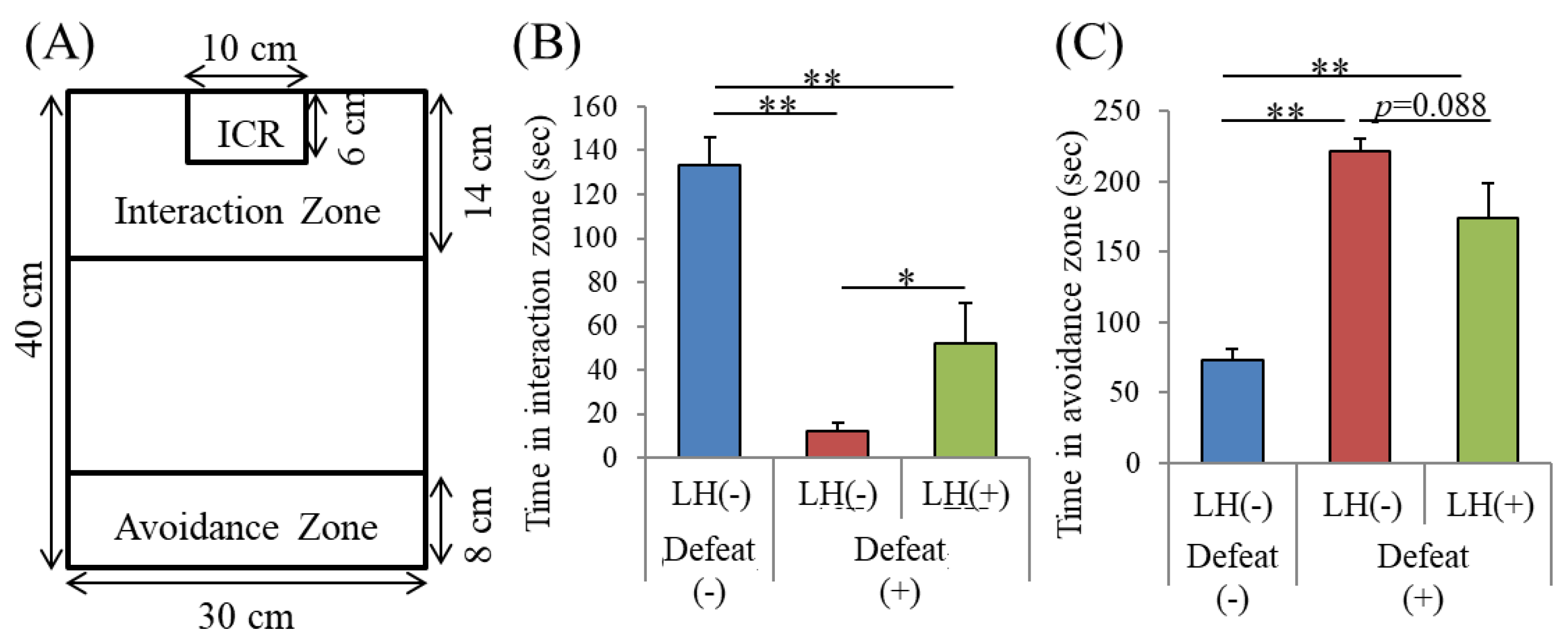
3.4. Effects of the LH Dipeptide on Depression-Associated Emotional Disturbances
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Depression Fact Sheet. Volume 369. 2016. Available online: http://www.who.int/en/news-room/fact-sheets/detail/depression (accessed on 12 June 2019).
- Tanti, A.; Belzung, C. Open questions in current models of antidepressant action. Br. J. Pharmacol. 2010, 159, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Petrik, D. Depression and hippocampal neurogenesis: a road to remission? Science 2012, 338, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I. Managing partial response or nonresponse: switching, augmentation, and combination strategies for major depressive disorder. J. Clin. Psychiatry 2009, 70 (Suppl. 6), 16–25. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E.; Friedman, E.S.; Biggs, M.M.; Wisniewski, S.R.; Trivedi, M.H.; Luther, J.F.; Fava, M.; Nierenberg, A.A.; McGrath, P.J.; Warden, D.; et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am. J. Psychiatry 2007, 164, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Opie, R.S.; Itsiopoulos, C.; Parletta, N.; Sanchez-Villegas, A.; Akbaraly, T.N.; Ruusunen, A.; Jacka, F.N. Dietary recommendations for the prevention of depression. Nutr. Neurosci. 2017, 20, 161–171. [Google Scholar] [CrossRef]
- Roca, M.; Kohls, E.; Gili, M.; Watkins, E.; Owens, M.; Hegerl, U.; van Grootheest, G.; Bot, M.; Cabout, M.; Brouwer, I.A.; et al. Prevention of depression through nutritional strategies in high-risk persons: rationale and design of the MooDFOOD prevention trial. BMC Psychiatry 2016, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Mizushige, T.; Mori, Y.; Shimmura, Y.; Fukutomi, R.; Kanamoto, R.; Ohinata, K. Antidepressant-like effect of food-derived pyroglutamyl peptides in mice. Neuropeptides 2015, 51, 25–29. [Google Scholar] [CrossRef]
- Mizushige, T.; Kanegawa, N.; Yamada, A.; Ota, A.; Kanamoto, R.; Ohinata, K. Aromatic amino acid-leucine dipeptides exhibit anxiolytic-like activity in young mice. Neurosci. Lett. 2013, 543, 126–129. [Google Scholar] [CrossRef]
- Ano, Y.; Ayabe, T.; Kutsukake, T.; Ohya, R.; Takaichi, Y.; Uchida, S.; Yamada, K.; Uchida, K.; Takashima, A.; Nakayama, H. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 2018, 72, 23–31. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, C.; Momma, H.; Ren, Z.; Sugiyama, S.; Guan, L.; Niu, K.; Nagatomi, R. Consumption of low-fat dairy, but not whole-fat dairy, is inversely associated with depressive symptoms in Japanese adults. Soc. Psychiatry Psychiatr. Epidemiol. 2017, 52, 847–853. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Intake of dairy products and calcium and prevalence of depressive symptoms during pregnancy in Japan: a cross-sectional study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.A.; Beurel, E.; Loewenstein, D.A.; Lowell, J.A.; Craighead, W.E.; Dunlop, B.W.; Mayberg, H.S.; Dhabhar, F.; Dietrich, W.D.; Keane, R.W.; et al. Defective Inflammatory Pathways in Never-Treated Depressed Patients Are Associated with Poor Treatment Response. Neuron 2018, 99, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Janelidze, S.; Hagell, P.; Erhardt, S.; Samuelsson, M.; Minthon, L.; Hansson, O.; Bjorkqvist, M.; Traskman-Bendz, L.; Brundin, L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry 2009, 66, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Kitaoka, S.; Tanaka, K.; Segi-Nishida, E.; Imoto, Y.; Ogawa, A.; Nakano, F.; Tomohiro, A.; Nakayama, K.; Taniguchi, M.; et al. The Innate Immune Receptors TLR2/4 Mediate Repeated Social Defeat Stress-Induced Social Avoidance through Prefrontal Microglial Activation. Neuron 2018, 99, 464–479.e467. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.B.; Khandaker, G.M. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 2018, 23, 335–343. [Google Scholar] [CrossRef]
- Kohler, O.; Benros, M.E.; Nordentoft, M.; Farkouh, M.E.; Iyengar, R.L.; Mors, O.; Krogh, J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014, 71, 1381–1391. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Hanke, M.L.; Corona, A.W.; Powell, N.D.; Stiner, L.M.; Bailey, M.T.; Nelson, R.J.; Godbout, J.P.; Sheridan, J.F. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 6277–6288. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Furuyashiki, T.; Kitaoka, S.; Senzai, Y.; Imoto, Y.; Segi-Nishida, E.; Deguchi, Y.; Breyer, R.M.; Breyer, M.D.; Narumiya, S. Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 4319–4329. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ota, K.T.; Li, X.Y.; Sakaue, F.; Li, N.; Dutheil, S.; Banasr, M.; Duric, V.; Yamanashi, T.; Kaneko, K.; et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry 2016, 80, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKim, D.B.; Weber, M.D.; Niraula, A.; Sawicki, C.M.; Liu, X.; Jarrett, B.L.; Ramirez-Chan, K.; Wang, Y.; Roeth, R.M.; Sucaldito, A.D.; et al. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 2018, 23, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Terwilliger, R.; Duman, C.H.; Duman, R.S. Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biol. Psychiatry 2018, 83, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Dohata, A.; Taniguchi, Y.; Hoshi, A.; Uchida, K.; Takashima, A.; Nakayama, H. Iso-alpha-acids, Bitter Components of Beer, Prevent Inflammation and Cognitive Decline Induced in a Mouse Model of Alzheimer’s Disease. J. Biol. Chem. 2017, 292, 3720–3728. [Google Scholar] [CrossRef]
- Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive effects of a fermented dairy product against Alzheimer’s disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS ONE 2015, 10, e0118512. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. JoVE 2012, 39, e3769. [Google Scholar] [CrossRef]
- Shinohara, R.; Taniguchi, M.; Ehrlich, A.T.; Yokogawa, K.; Deguchi, Y.; Cherasse, Y.; Lazarus, M.; Urade, Y.; Ogawa, A.; Kitaoka, S.; et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol. Psychiatry 2018, 23, 1717–1730. [Google Scholar] [CrossRef]
- Higashida, S.; Nagai, H.; Nakayama, K.; Shinohara, R.; Taniguchi, M.; Nagai, M.; Hikida, T.; Yawata, S.; Ago, Y.; Kitaoka, S.; et al. Repeated social defeat stress impairs attentional set shifting irrespective of social avoidance and increases female preference associated with heightened anxiety. Sci. Rep. 2018, 8, 10454. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, J.; Pydi, S.P.; Singh, N.; Aluko, R.E.; Chelikani, P. Bitter taste receptor T2R1 is activated by dipeptides and tripeptides. Biochem. Biophys. Res. Commun. 2010, 398, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Gaida, M.M.; Dapunt, U.; Hansch, G.M. Sensing developing biofilms: the bitter receptor T2R38 on myeloid cells. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Huang, Z.; Sun, X.; Zhu, X.; Zhou, L.; Li, M.; Cheng, B.; Liu, X.; He, C. Microglia Polarization with M1/M2 Phenotype Changes in rd1 Mouse Model of Retinal Degeneration. Front. Neuroanat. 2017, 11, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, A.; Hankey Giblin, P.A. Insights into Macrophage Heterogeneity and Cytokine-Induced Neuroinflammation in Major Depressive Disorder. Pharmaceutics 2018, 11, 64. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Chiba, K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceutics 2014, 7, 1028–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Wu, J.; Fujita, Y.; Yao, W.; Ren, Q.; Yang, C.; Li, S.X.; Shirayama, Y.; Hashimoto, K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2014, 18. [Google Scholar] [CrossRef]
- Lawson, M.A.; Parrott, J.M.; McCusker, R.H.; Dantzer, R.; Kelley, K.W.; O’Connor, J.C. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J. Neuroinflamm. 2013, 10, 87. [Google Scholar] [CrossRef]
- Koenigs, M.; Grafman, J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef]
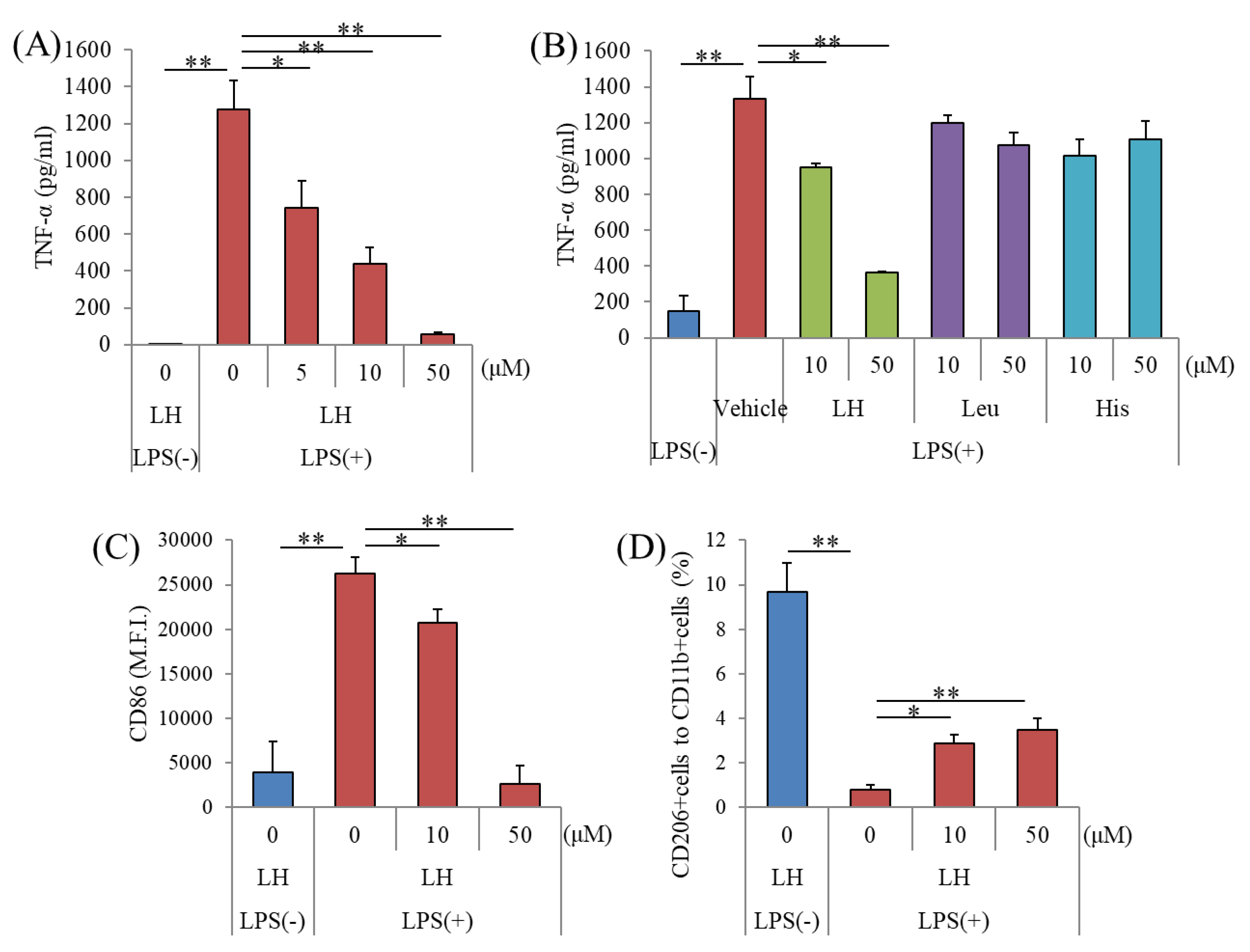

| LPS(−) | LPS(+) | LPS(+) | LPS(+) | |
|---|---|---|---|---|
| LH | 0 μM | 0 μM | 10 μM | 30 μM |
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| IL-1α | 10.85±0.41 | 33.92 ± 4.52 ## | 27.80 ± 0.35* | 21.03 ± 2.76 * |
| IL-1β | N.D. | 461.90 ± 22.31 | 370.28 ± 7.15* | 232.69 ± 65.04 * |
| IL-2 | 0.49±0.16 | 5.11 ± 0.85 ## | 3.83 ± 0.68 | 1.96 ± 1.20 * |
| IL-4 | 11.03±1.87 | 21.10 ± 7.26 | 16.87 ± 2.39 | 10.92 ± 2.53 |
| IL-5 | N.D. | 70.64 ± 6.00 | 59.81 ± 3.63 | 34.33 ± 2.96 * |
| IL-6 | 1.49 ± 0.17 | 1115.34 ± 291.93 ## | 868.39 ± 63.81 | 40.64 ± 4.66 ** |
| IL-9 | N.D. | 0.97 ± 0.51 | 0.95 ± 0.48 | N.D. |
| IL-10 | 4.42 ± 0.88 | 46.67 ± 4.41 ## | 32.60 ± 2.41 | 9.81 ± 6.38 * |
| IL-12p40 | 3.67 ± 0.05 | 2814.93 ± 1672.56 ## | 1285.46 ± 64.50 | 26.13 ± 6.46 ** |
| IL-12p70 | 31.41 ± 7.59 | 274.67 ± 70.58 ## | 255.40 ± 20.96 | 114.16 ± 48.49 |
| IFN-γ | N.D. | 203.20 ± 43.29 | 200.09 ± 13.56 | 205.37 ± 11.01 |
| KC | 249.36 ± 21.45 | 608.00 ± 325.53 | 617.92 ± 54.11 | 164.15 ± 15.42 * |
| G-CSF | 14.82 ± 0.89 | 2553.57 ± 858.49 # | 1526.14 ± 213.37 | 35.49 ± 7.38 ** |
| GM-CSF | 3.24 ± 0.70 | 12.48 ± 3.77 # | 15.78 ± 1.46 | 6.10 ± 1.73 |
| Eotaxin | N.D. | 115.55 ± 114.07 | 5.41 ± 2.50 | N.D. |
| MCP-1 | 341.31 ± 2.31 | 6309.88 ± 874.11 # | 4575.50 ± 224.07 * | 245.64 ± 48.90 ** |
| MIP-1α | 1456.73 ± 57.27 | 7558.73 ± 441.63 ## | 4338.73 ± 1422.37 ** | 1167.67 ± 273.58 ** |
| MIP-1β | 1750.02 ± 82.41 | 34440.79 ± 4285.71 ## | 22361.34 ± 2599.65 * | 1674.58 ± 42.01 ** |
| RANTES | 211.50 ± 3.28 | 13515.62 ± 6828.57 ## | 18915.28 ± 1333.45 | 3393.04 ± 1229.12 |
| TNF-α | N.D. | 4239.37 ± 688.90 | 758.51 ± 115.39 ** | 369.73 ± 84.86 ** |
| Time | Radioactivity (ng/mL) | |
|---|---|---|
| Mean | SE | |
| 2 min | 229.7 | 89.0 |
| 5 min | 568.5 | 126.0 |
| 10 min | 1424.0 | 167.3 |
| 30 min | 2006.7 | 179.4 |
| 1 h | 3839.5 | 389.2 |
| 2 h | 3915.3 | 292.4 |
| 4 h | 3860.1 | 313.9 |
| 6 h | 3360.5 | 362.1 |
| 8 h | 3000.5 | 215.5 |
| 10 h | 2776.0 | 164.2 |
| 24 h | 1704.9 | 147.8 |
| 48 h | 1151.0 | 89.9 |
| 72 h | 867.6 | 37.5 |
| 120 h | 543.2 | 17.5 |
| 168 h | 364.0 | 20.1 |
| Cmax (ng eq./mL) | 3955.6 | 574.3 |
| Tmax(h) | 1.67 | 0.58 |
| T1/2(h) | 76.37 | 1.91 |
| Tissue | Radioactivity Concentration (ng eq. of LH /g or mL) | ||
|---|---|---|---|
| Mean | SE | Ratio | |
| Plasma | 3770.7 | 728.7 | 1.00 |
| Cerebrospinal fluid | 34.4 | 1.9 | 0.01 |
| Cerebrum | 703.7 | 64.3 | 0.19 |
| Cerebral cortex | 788.9 | 32.4 | 0.21 |
| Hippocampus | 722.4 | 38.7 | 0.19 |
| Striatum | 661.7 | 21.1 | 0.18 |
| Thalamus | 702.4 | 63.1 | 0.19 |
| Hypothalamus | 710.0 | 130.1 | 0.19 |
| Midbrain | 812.7 | 42.4 | 0.22 |
| Cerebellum | 704.9 | 293.5 | 0.19 |
| Spinal cord | 553.1 | 29.8 | 0.15 |
| Liver | 8207.5 | 561.5 | 2.18 |
| Kidney | 4023.0 | 168.2 | 1.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ano, Y.; Kita, M.; Kitaoka, S.; Furuyashiki, T. Leucine–Histidine Dipeptide Attenuates Microglial Activation and Emotional Disturbances Induced by Brain Inflammation and Repeated Social Defeat Stress. Nutrients 2019, 11, 2161. https://doi.org/10.3390/nu11092161
Ano Y, Kita M, Kitaoka S, Furuyashiki T. Leucine–Histidine Dipeptide Attenuates Microglial Activation and Emotional Disturbances Induced by Brain Inflammation and Repeated Social Defeat Stress. Nutrients. 2019; 11(9):2161. https://doi.org/10.3390/nu11092161
Chicago/Turabian StyleAno, Yasuhisa, Masahiro Kita, Shiho Kitaoka, and Tomoyuki Furuyashiki. 2019. "Leucine–Histidine Dipeptide Attenuates Microglial Activation and Emotional Disturbances Induced by Brain Inflammation and Repeated Social Defeat Stress" Nutrients 11, no. 9: 2161. https://doi.org/10.3390/nu11092161






