Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Diets
2.3. Survival Study
2.4. Prospective Detailed Assessment Study
2.5. Tissue Sampling
2.6. Blood Lipoprotein Separation
2.7. Blood Cholesterol Determinations
2.8. Determination of Plasma Levels TNF-α and IL-10
2.9. Liver Function Tests
2.10. Analysis of High-Density Lipoprotein (HDL)-Containing Plasma Antioxidant Activity
2.11. Determination of Plasma Malondialdehyde Levels
2.12. Histological Characterization of Aortic Root Atherosclerosis
2.13. Cardiovascular Magnetic Resonance Imaging
2.14. MRI Data Analysis
2.15. Statistical Analyses
3. Results
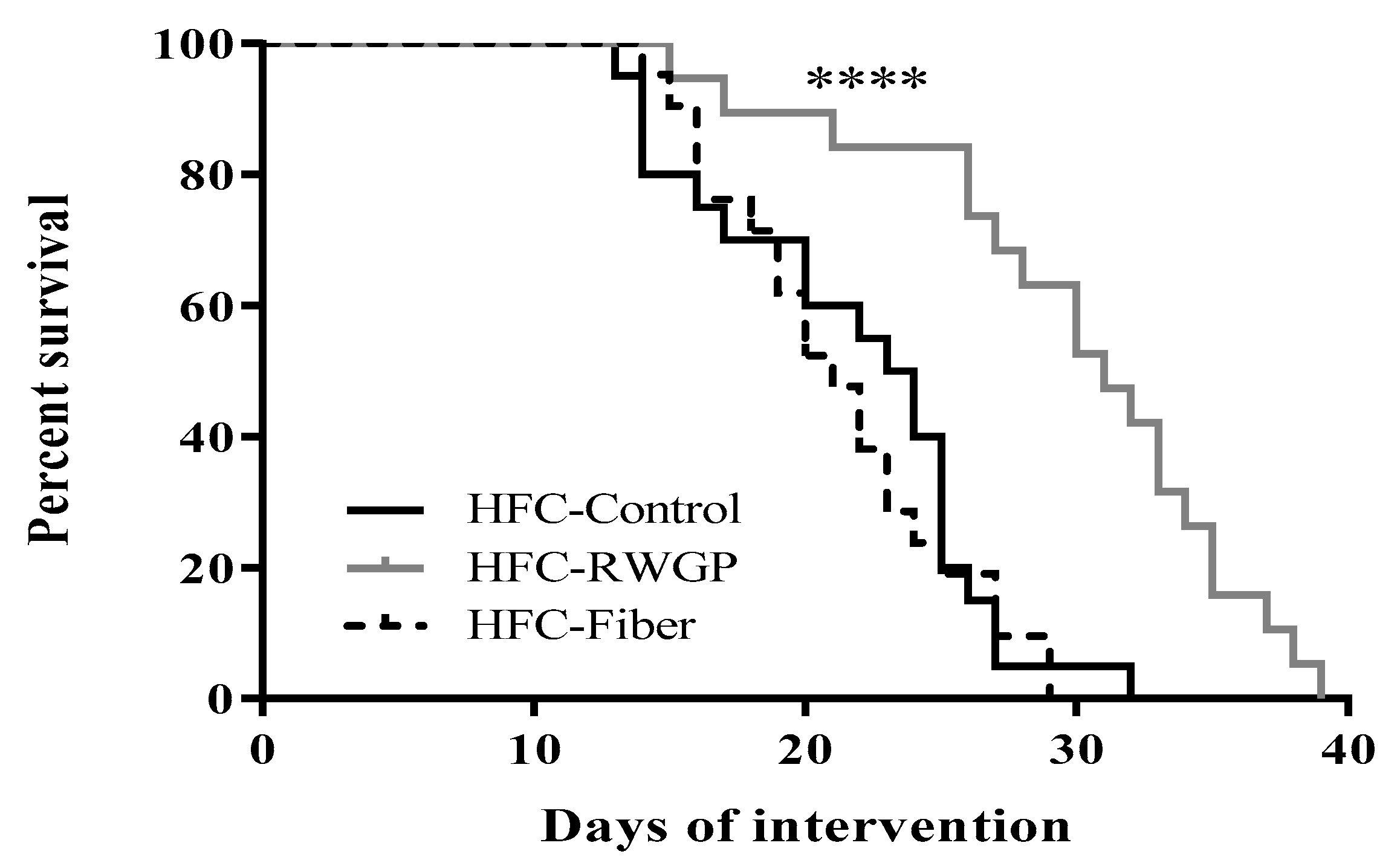
3.1. RWGP Supplementation Attenuated Premature Death in Atherogenic Diet-Fed SR-B1 KO/ApoER61h/h Mice
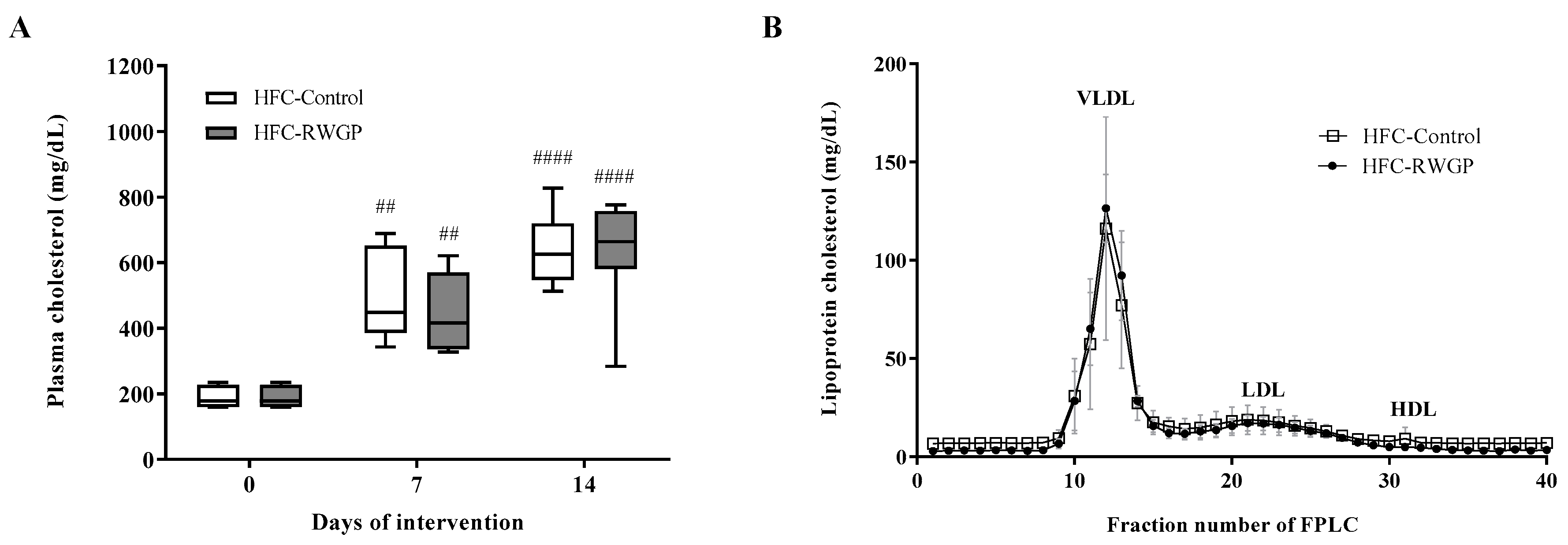
3.2. Total and Lipoprotein Cholesterol Levels were Unaffected by RWGP in Atherogenic Diet-Fed SR-B1 KO/ApoER61h/h Mice
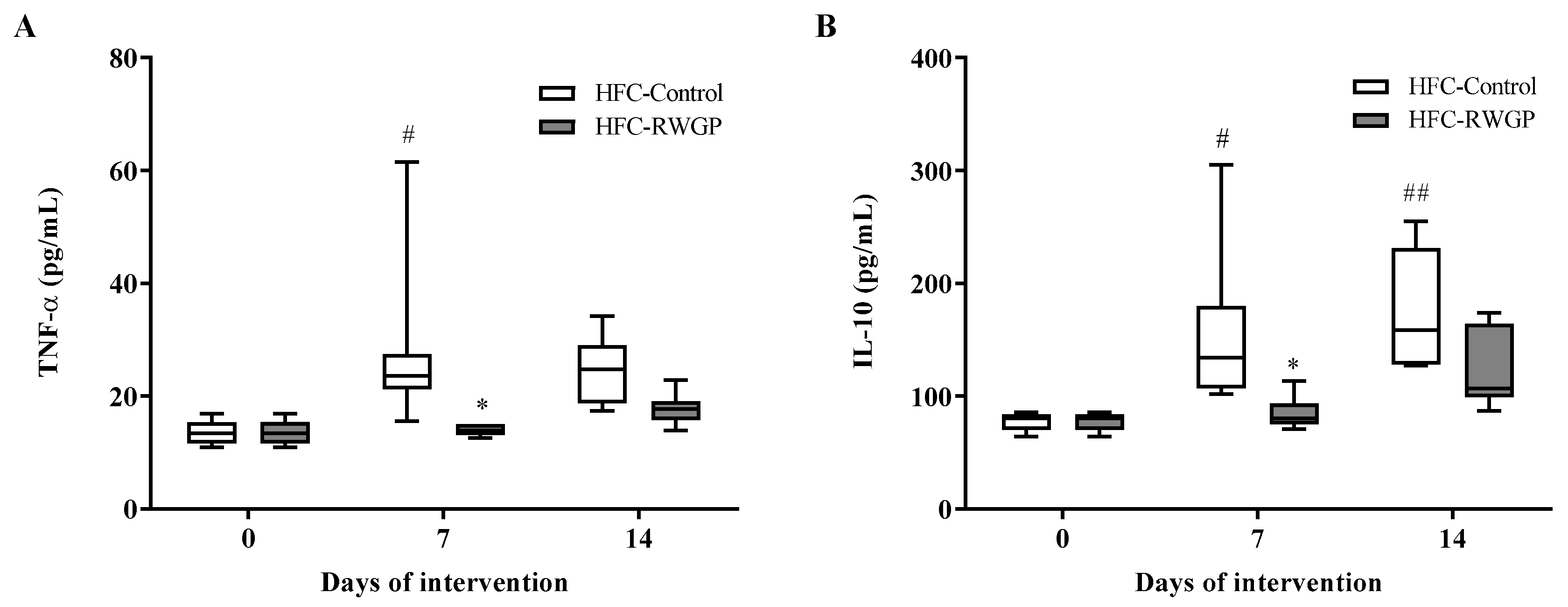
3.3. RWGP Supplementation Modulated Inflammatory Cytokines in Atherogenic Diet-Fed SR-B1 KO/ApoER61h/h Mice
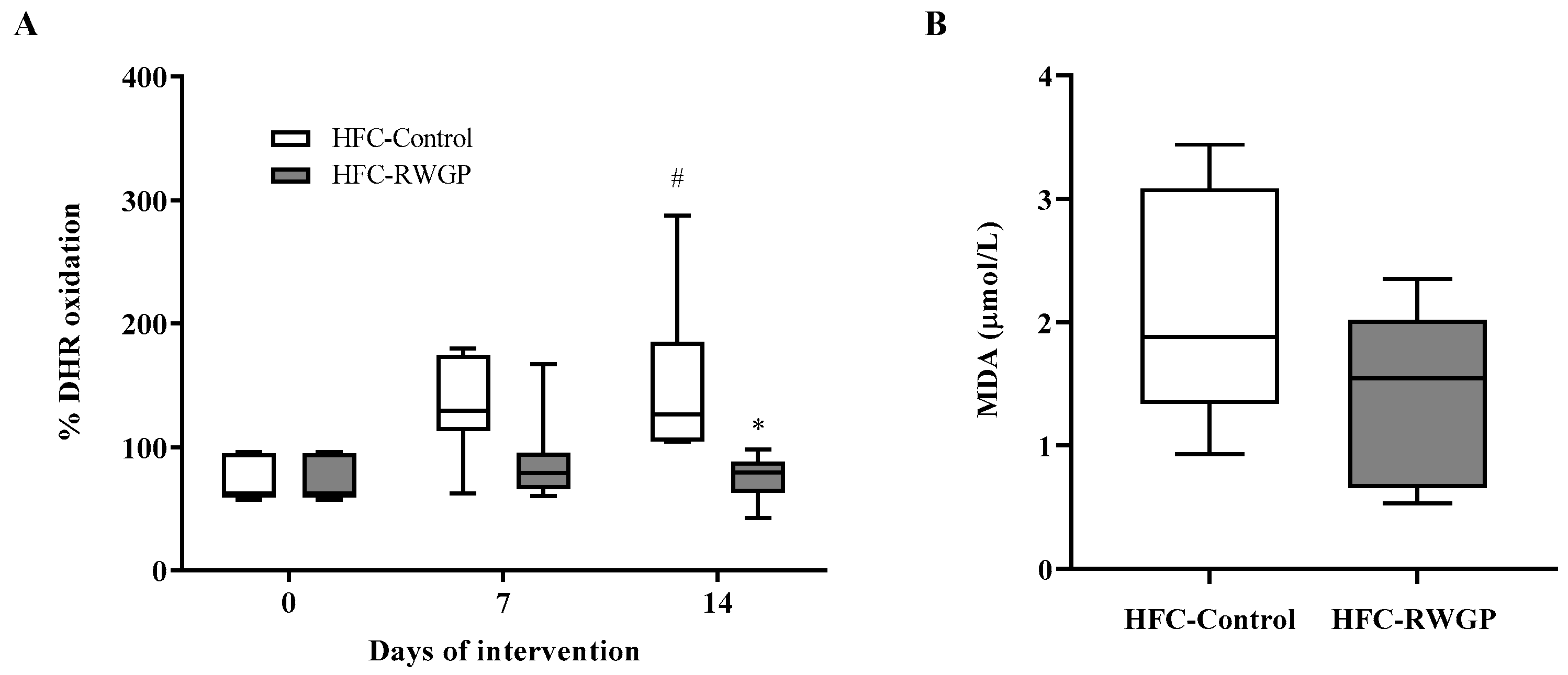
3.4. RWGP Supplementation Increases HDL-Containing Plasma Antioxidant Activity in Atherogenic Diet-Fed SR-B1 KO/ApoER61h/h Mice
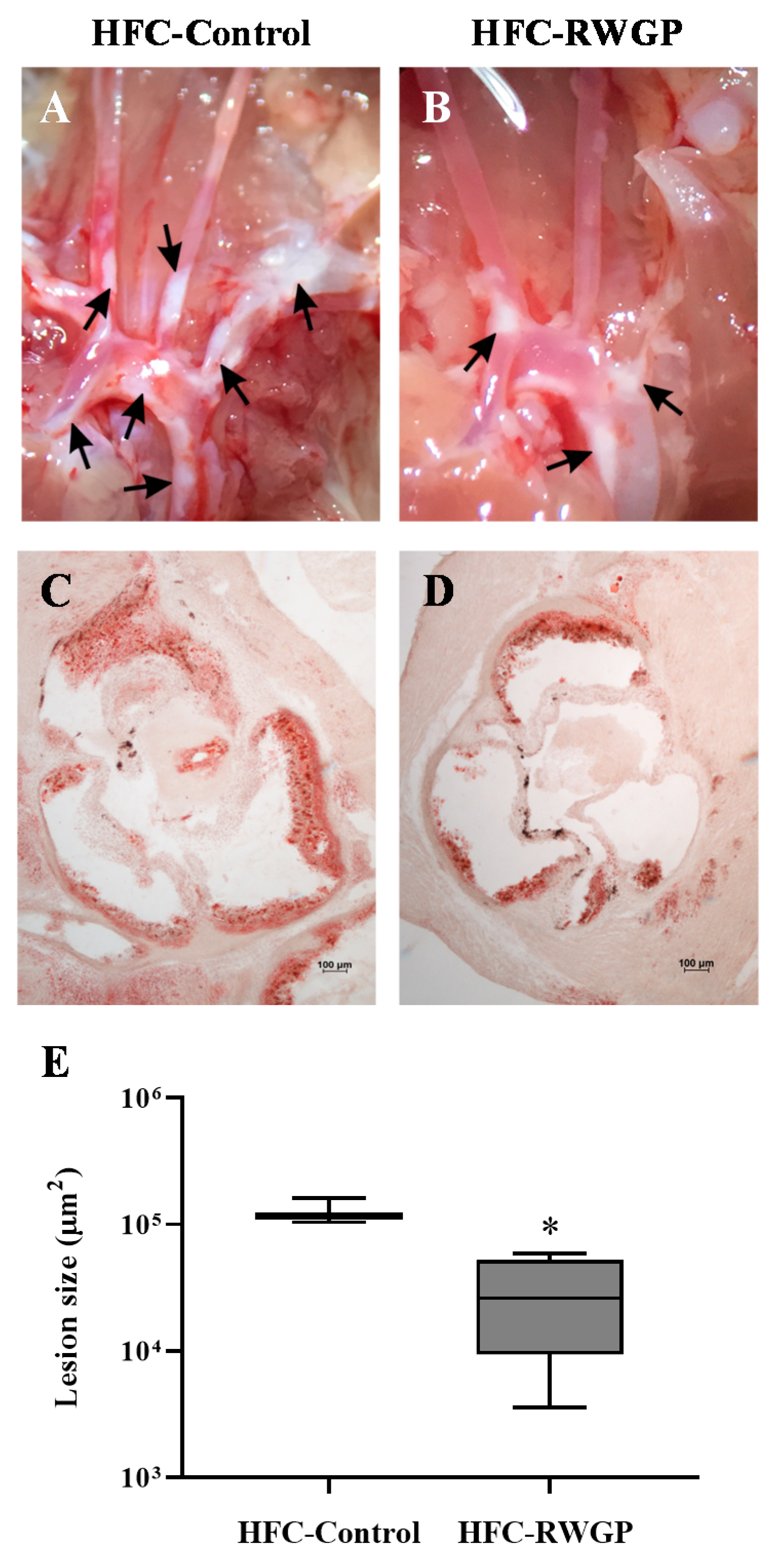
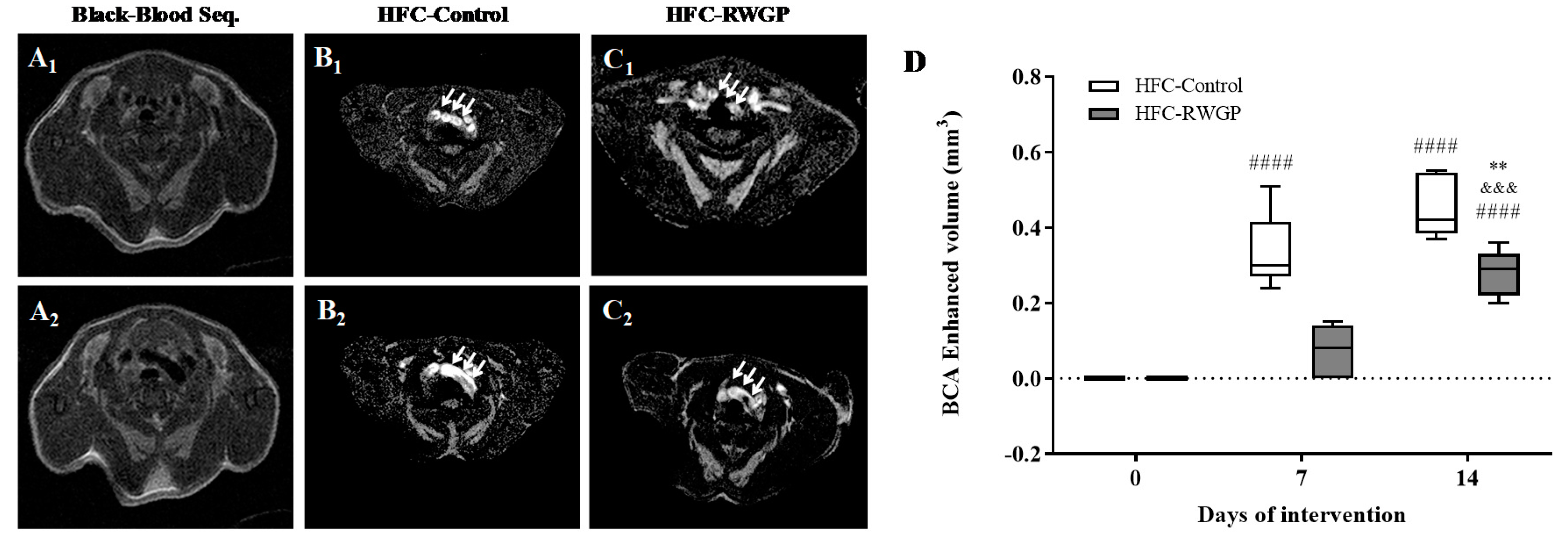
3.5. Decreased Atherosclerotic Lesions and Improved MRI-Assessed Endothelial Function Induced by RWGP Supplementation in Atherogenic Diet-Fed SR-B1 KO/ApoER61h/h Mice
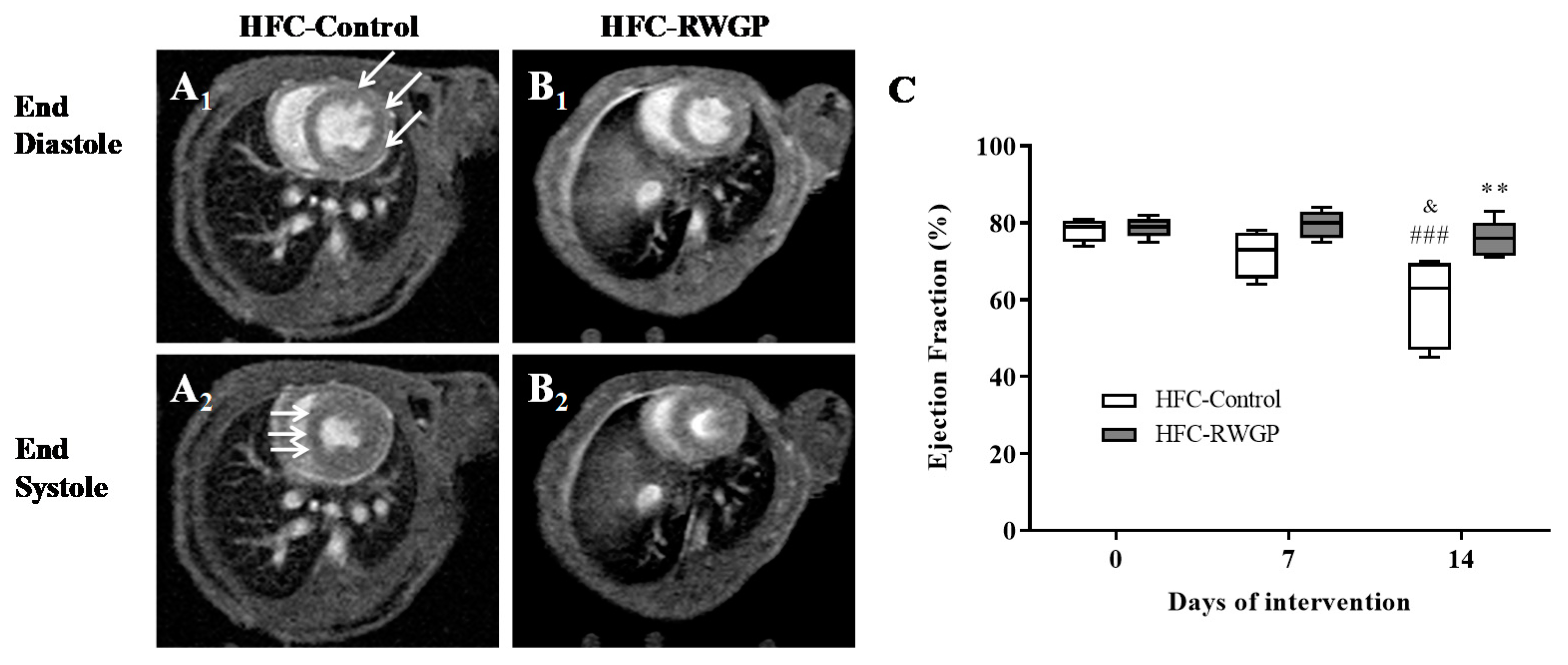
3.6. RWGP Supplementation Attenuated Myocardial Dysfunction and Reduced Myocardial Infarction in Atherogenic Diet-Fed SR-B1 KO/ApoER61h/h Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics-2013 update: A Report from the American Heart Association. Circulation 2013, 127, 143–152. [Google Scholar] [CrossRef]
- Trigatti, B.L.; Krieger, M.; Rigotti, A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1732–1738. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Fruchart, J.C.; Davignon, J.; Hermans, M.P.; Al-Rubeaan, K.; Amarenco, P.; Assmann, G.; Brown, W.V.; Ceska, R.; Chapman, M.J.; Dodson, P.M.; et al. Residual macrovascular risk in 2013: What have we learned? Cardiovasc. Diabetol. 2014, 13, 26. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Jiménez, J.P.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Saura-Calixto, F. Effects of grape antioxidant dietary fiber in cardiovascular disease risk factors. Nutrition 2008, 24, 646–653. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A Systematic Review of the Evidence Supporting a Causal Link Between Dietary Factors and Coronary Heart Disease. Arch. Intern Med. 2009, 169, 659–669. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary Fiber as a Carrier of Dietary Antioxidants: An Essential Physiological Function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Rosenblat, M.; Hayek, T.; Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 2006, 187, 363–371. [Google Scholar] [CrossRef]
- Al-Jarallah, A.; Igdoura, F.; Zhang, Y.; Tenedero, C.B.; White, E.J.; MacDonald, M.E.; Igdoura, S.A.; Trigatti, B.L. The effect of pomegranate extract on coronary artery atherosclerosis in SR-BI/APOE double knockout mice. Atherosclerosis 2013, 228, 80–89. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health—New Evidence. Eur. J. Clin. Nutr. 2019, 72, 55–59. [Google Scholar] [CrossRef]
- Hartz, S.M.; Oehlert, M.; Horton, A.; Grucza, R.A.; Fisher, S.L.; Culverhouse, R.C.; Nelson, K.G.; Sumeral, S.W.; Neal, P.C.; Regnier, P.; et al. Daily Drinking Is Associated with Increased Mortality. Alcohol. Clin. Exp. Res. 2018, 42, 2246–2255. [Google Scholar] [CrossRef]
- Martínez, N.; Casós, K.; Simonetti, P.; Sáiz, M.P.; Moreno, J.J.; Mitjavila, M.T. De-alcoholised white and red wines decrease inflammatory markers and NF-κB in atheroma plaques in apoE-deficient mice. Eur. J. Nutr. 2013, 52, 737–747. [Google Scholar] [CrossRef]
- Rodriguez Lanzi, C.; Perdicaro, D.J.; Antoniolli, A.; Fontana, A.R.; Miatello, R.M.; Bottini, R.; Vazquez Prieto, M.A. Grape pomace and grape pomace extract improve insulin signaling in high-fat-fructose fed rat-induced metabolic syndrome. Food Funct. 2016, 7, 1544–1553. [Google Scholar] [CrossRef]
- Urquiaga, I.; D’Acuña, S.; Pérez, D.; Dicenta, S.; Echeverría, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial. Biol. Res. 2015, 48, 49. [Google Scholar] [CrossRef]
- Hernández-Salinas, R.; Decap, V.; Leguina, A.; Cáceres, P.; Perez, D.; Urquiaga, I.; Iturriaga, R.; Velarde, V. Antioxidant and anti hyperglycemic role of wine grape powder in rats fed with a high fructose diet. Biol. Res. 2015, 48, 53. [Google Scholar] [CrossRef]
- Zhang, S.; Picard, M.H.; Vasile, E.; Zhu, Y.; Raffai, R.L.; Weisgraber, K.H.; Krieger, M. Diet-induced occlusive coronary atherosclerosis, myocardial infarction, cardiac dysfunction, and premature death in scavenger receptor class B type I-deficient, hypomorphic apolipoprotein ER61 mice. Circulation 2005, 111, 3457–3464. [Google Scholar] [CrossRef]
- Braun, A.; Trigatti, B.L.; Post, M.J.; Sato, K.; Simons, M.; Edelberg, J.M.; Rosenberg, R.D.; Schrenzel, M.; Krieger, M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ. Res. 2002, 90, 270–276. [Google Scholar] [CrossRef]
- Rigotti, A.; Trigatti, B.L.; Penman, M.; Rayburn, H.; Herz, J.; Krieger, M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl. Acad. Sci. USA 1997, 94, 12610–12615. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar]
- Warnick, G.R.; Benderson, J.; Albers, J.J. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 1982, 28, 1379–1388. [Google Scholar]
- Kelesidis, T.; Currier, J.S.; Huynh, D.; Meriwether, D.; Charles-Schoeman, C.; Reddy, S.T.; Fogelman, A.M.; Navab, M.; Yang, O.O. A biochemical fluorometric method for assessing the oxidative properties of HDL. J. Lipid Res. 2011, 52, 2341–2351. [Google Scholar] [CrossRef]
- Templar, J.; Kon, S.P.; Milligan, T.P.; Newman, D.J.; Raftery, M.J. Increased plasma malondialdehyde levels in glomerular disease as determined by a fully validated HPLC method. Nephrol. Dial. Transpl. 1999, 14, 946–951. [Google Scholar] [CrossRef]
- Nakagawa-Toyama, Y.; Zhang, S.; Krieger, M. Dietary Manipulation and Social Isolation Alter Disease Progression in a Murine Model of Coronary Heart Disease. PLoS ONE 2012, 7, e47965. [Google Scholar] [CrossRef]
- Leiva, A.; Contreras-Duarte, S.; Amigo, L.; Sepúlveda, E.; Boric, M.; Quiñones, V.; Busso, D.; Rigotti, A. Gugulipid causes hypercholesterolemia leading to endothelial dysfunction, increased atherosclerosis, and premature death by ischemic heart disease in male mice. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef]
- Contreras-Duarte, S.; Chen, P.; Andía, M.; Uribe, S.; Irarrázaval, P.; Kopp, S.; Kern, S.; Marsche, G.; Busso, D.; Wadsack, C.; et al. Attenuation of atherogenic apo B-48-dependent hyperlipidemia and high density lipoprotein remodeling induced by vitamin C and E combination and their beneficial effect on lethal ischemic heart disease in mice. Biol. Res. 2018, 51, 34. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; van Erk, M.J.; Nikolsky, Y.; Cnubben, N.H.P.; Verheij, E.R.; Smilde, A.K.; Hendriks, H.F.J.; Zadelaar, S.; Smith, G.J.; et al. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: A combined transcriptomics and metabolomics analysis. Genome Biol. 2007, 8, R200. [Google Scholar] [CrossRef]
- Papac-Milicevic, N.; Busch, C.-L.; Binder, C.J. Malondialdehyde Epitopes as Targets of Immunity and the Implications for Atherosclerosis. Adv. Immunol. 2016, 131, 1–59. [Google Scholar]
- Lobbes, M.B.I.; Heeneman, S.; Passos, V.L.; Welten, R.; Kwee, R.M.; van der Geest, R.J.; Wiethoff, A.J.; Caravan, P.; Misselwitz, B.; Daemen, M.J.; et al. Gadofosveset-enhanced magnetic resonance imaging of human carotid atherosclerotic plaques: A proof-of-concept study. Investig. Radiol. 2010, 45, 275–281. [Google Scholar] [CrossRef]
- Reith, C.; Armitage, J. Management of residual risk after statin therapy. Atherosclerosis 2016, 245, 161–170. [Google Scholar] [CrossRef]
- Van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of Wine, Alcohol and Polyphenols on Cardiovascular Disease Risk Factors: Evidences from Human Studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef]
- Myung, S.K.; Ju, W.; Cho, B.; Oh, S.W.; Park, S.M.; Koo, B.K.; Park, B.J.; Korean Meta-Analysis Study Group. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 346, f10. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.-L.; Zhang, L.; Zou, L.-X.; Chen, C.; Liu, Y.; Xia, Y.-L.; Li, H.-H. Gallic Acid Suppresses Cardiac Hypertrophic Remodeling and Heart Failure. Mol. Nutr. Food Res. 2019, 63, 1800807. [Google Scholar] [CrossRef]
- Hayek, T.; Fuhrman, B.; Vaya, J.; Rosenblat, M.; Belinky, P.; Coleman, R.; Elis, A.; Aviram, M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2744–2752. [Google Scholar] [CrossRef]
- Nomura, S.; Monobe, M.; Ema, K.; Matsunaga, A.; Maeda-Yamamoto, M.; Horie, H. Effects of flavonol-rich green tea cultivar (Camellia sinensis L.) on plasma oxidized LDL levels in hypercholesterolemic mice. Biosci. Biotechnol. Biochem. 2016, 80, 360–362. [Google Scholar] [CrossRef]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F., Jr. Antioxidant and Anti-Inflammatory Effects in RAW264.7 Macrophages of Malvidin, a Major Red Wine Polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar] [CrossRef]
- Martín-Carrón, N.; Goñi, I.; Larrauri, J.A.; García-Alonso, A.; Saura-Calixto, F. Reduction in serum total and LDL cholesterol concentrations by a dietary fiber and polyphenol-rich grape product in hypercholesterolemic rats. Nutr. Res. 1999, 19, 1371–1381. [Google Scholar] [CrossRef]
- Zheng, X.X.; Xu, Y.L.; Li, S.H.; Liu, X.X.; Hui, R.; Huang, X.H. Green tea intake lowers fasting serum total and LDL cholesterol in adults: A meta-analysis of 14 randomized controlled trials. Am. J. Clin. Nutr. 2011, 94, 601–610. [Google Scholar] [CrossRef]
- Bertrand, M.-J.; Tardif, J.-C. Inflammation and beyond: New directions and emerging drugs for treating atherosclerosis. Expert Opin. Emerg. Drugs 2017, 22, 1–26. [Google Scholar] [CrossRef]
- Klinghammer, L.; Urschel, K.; Cicha, I.; Lewczuk, P.; Raaz-Schrauder, D.; Achenbach, S.; Garlichs, C.D. Impact of telmisartan on the inflammatory state in patients with coronary atherosclerosis—Influence on IP-10, TNF-α and MCP-1. Cytokine 2013, 62, 290–296. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Wang, J.; Liu, Y.-M.; Zheng, Q.-S.; Li, C.-Y. Inhibitory effect of Malvidin on TNF-α-induced inflammatory response in endothelial cells. Eur. J. Pharmacol. 2014, 723, 67–72. [Google Scholar] [CrossRef]
- Tu, S.; Xiao, F.; Min, X.; Chen, H.; Fan, X.; Cao, K. Catechin Attenuates Coronary Heart Disease in a Rat Model by Inhibiting Inflammation. Cardiovasc. Toxicol. 2018, 18, 393–399. [Google Scholar] [CrossRef]
- Rodríguez-Morgado, B.; Candiracci, M.; Santa-María, C.; Revilla, E.; Gordillo, B.; Parrado, J.; Castaño, A. Obtaining from Grape Pomace an Enzymatic Extract with Anti-inflammatory Properties. Plant Foods Hum. Nutr. 2015, 70, 42–49. [Google Scholar] [CrossRef]
- Shmarina, G.V.; Pukhalsky, A.L.; Kokarovtseva, S.N.; Pukhalskaya, D.A.; Shabalova, L.A.; Kapranov, N.I.; Kashirskaja, N.J. Tumor necrosis factor-alpha/interleukin-10 balance in normal and cystic fibrosis children. Mediat. Inflamm. 2001, 10, 191–197. [Google Scholar] [CrossRef]
- Wang, G.; Kim, R.Y.; Imhof, I.; Honbo, N.; Luk, F.S.; Li, K.; Kumar, N.; Zhu, B.Q.; Eberlé, D.; Ching, D.; et al. The Immunosuppressant FTY720 Prolongs Survival in a Mouse Model of Diet-induced Coronary Atherosclerosis and Myocardial Infarction. J. Cardiovasc. Pharmacol. 2014, 63, 132–143. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Assini, J.M.; Sutherland, B.G.; DiMattia, A.S.; Khami, M.; Koppes, J.B.; Sawyez, C.G.; Withman, S.C.; Huff, M.W. Naringenin Decreases Progression of Atherosclerosis by Improving Dyslipidemia in High-Fat–Fed Low-Density Lipoprotein Receptor–Null Mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 742–748. [Google Scholar] [CrossRef]
- Phinikaridou, A.; Andia, M.E.; Protti, A.; Indermuehle, A.; Shah, A.; Smith, A.; Warley, A.; Botnar, R.M. Noninvasive Magnetic Resonance Imaging Evaluation of Endothelial Permeability in Murine Atherosclerosis Using an Albumin-Binding Contrast Agent. Circulation 2012, 126, 707–719. [Google Scholar] [CrossRef]
- Luk, F.S.; Kim, R.Y.; Li, K.; Ching, D.; Wong, D.K.; Joshi, S.K.; Imhof, I.; Honbo, N.; Hoover, H.; Zhu, B.-Q.; et al. Immunosuppression With FTY720 Reverses Cardiac Dysfunction in Hypomorphic ApoE Mice Deficient in SR-BI Expression That Survive Myocardial Infarction Caused by Coronary Atherosclerosis. J. Cardiovasc. Pharmacol. 2016, 67, 47–56. [Google Scholar] [CrossRef][Green Version]
- Loffredo, L.; Perri, L.; Di Castelnuovo, A.; Iacoviello, L.; De Gaetano, G.; Violi, F. Supplementation with vitamin E alone is associated with reduced myocardial infarction: A meta-analysis. Nutr Metab. Cardiovasc. Dis. 2015, 25, 354–363. [Google Scholar] [CrossRef]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef]
- Jiao, Y.; Shang, J.; Ohta, Y.; Yan, H.; Liu, X.; Li, X.; Morihara, R.; Nakano, Y.; Fukui, Y.; Shi, X.; et al. Neuroprotective Effects of Tocovid Pretreatment in a Mouse Stroke Model. J. Stroke Cerebrovasc. Dis. 2018, 27, 2166–2174. [Google Scholar] [CrossRef]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.; Urzúa, C.; Pérez, D.; Dicenta, S.; De la Cerda, P.; Amigo, L.; Carreño, J.C.; Echeverría, G.; et al. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients 2018, 10, 1388. [Google Scholar] [CrossRef]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef]
- Costabile, G.; Vitale, M.; Luongo, D.; Naviglio, D.; Vetrani, C.; Ciciola, P.; Tura, A.; Castello, F.; Mena, P.; Del Rio, D.; et al. Grape pomace polyphenols improve insulin response to a standard meal in healthy individuals: A pilot study. Clin. Nutr. 2018, S0261-5614, 32561–32565. [Google Scholar] [CrossRef]
| HFC-Control | HFC-RWGP | HFC-Fiber | |
|---|---|---|---|
| Proximate analysis and fiber (g/100 g of supplemented diet) | |||
| Fat | 14.5 | 15.1 | 13.4 |
| Protein | 20.1 | 17.2 | 17.2 |
| Carbohydrates a | 37.4 | 30.6 | 33.2 |
| Dietary fiber | 15.0 | 22.1 | 22.8 |
| Soluble | 2.6 | 3.6 | 2.5 |
| Insoluble | 12.4 | 18.5 | 20.3 |
| Ash | 5.1 | 5.1 | 4.4 |
| Moisture | 8.0 | 9.9 | 9.1 |
| Antioxidants and antioxidant capacity | |||
| Polyphenols (mg GE/g) | 1.9 ± 0.08 | 6.3 ± 0.42 | 1.6 ± 0.10 |
| α-tocopherol (μg/g) | 2.1 ± 0.13 | 8.2 ± 0.88 | 1.7 ± 0.08 |
| γ-tocopherol (μg/g) | 15.2 ± 1.20 | 18.3 ± 1.16 | 9.2 ± 0.41 |
| δ-tocopherol (μg/g) | 7.9 ± 0.62 | 5.2 ± 0.74 | 3.9 ± 0.21 |
| Total tocopherols (μg/g) | 25.2 ± 1.95 | 31.7 ± 2.78 | 14.8 ± 0.70 |
| Vitamin C (μg/g) | 200.8 ± 8.58 | 248.6 ± 8.12 | 260.1 ± 3.27 |
| ORAC (μmoles TE/g) | 35.8 ± 2.61 | 75.4 ± 3.61 | 36.3 ± 2.05 |
| Day 7; Number of Hearts (%) | Day 14; Number of Hearts (%) | |||||
|---|---|---|---|---|---|---|
| Treatment | Infarcted | Non-Infarcted | Total | Infarcted | Non-Infarcted | Total |
| HFC-Control | 1 (14.3%) | 6 (85.7%) | 7 | 7 (100%) | 0 (0%) | 7 |
| HFC-RWGP | 0 (0%) | 7 (100%) | 7 | 1 (14.3%) | 6 (85.7%) a | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, K.; Salas-Pérez, F.; Echeverría, G.; Urquiaga, I.; Dicenta, S.; Pérez, D.; de la Cerda, P.; González, L.; Andia, M.E.; Uribe, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients 2019, 11, 2135. https://doi.org/10.3390/nu11092135
Rivera K, Salas-Pérez F, Echeverría G, Urquiaga I, Dicenta S, Pérez D, de la Cerda P, González L, Andia ME, Uribe S, et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients. 2019; 11(9):2135. https://doi.org/10.3390/nu11092135
Chicago/Turabian StyleRivera, Katherine, Francisca Salas-Pérez, Guadalupe Echeverría, Inés Urquiaga, Sara Dicenta, Druso Pérez, Paula de la Cerda, Leticia González, Marcelo E. Andia, Sergio Uribe, and et al. 2019. "Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease" Nutrients 11, no. 9: 2135. https://doi.org/10.3390/nu11092135
APA StyleRivera, K., Salas-Pérez, F., Echeverría, G., Urquiaga, I., Dicenta, S., Pérez, D., de la Cerda, P., González, L., Andia, M. E., Uribe, S., Tejos, C., Martínez, G., Busso, D., Irarrázaval, P., & Rigotti, A. (2019). Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients, 11(9), 2135. https://doi.org/10.3390/nu11092135






