Protective Effects of Hydrolyzed Chicken Extract (Probeptigen®/Cmi-168) on Memory Retention and Brain Oxidative Stress in Senescence-Accelerated Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Food Intake
2.2. Animal Treatment and Tissue Collection
2.3. ProBeptigen/CMI-168 Preparation
2.4. The Degree of Senescence in Samp8 Mice
2.5. Memory Behavioural Tests
2.5.1. Open Field Test (Locomotion)
2.5.2. Passive Avoidance Test
2.5.3. Active (Shuttle) Avoidance Test
2.6. Assessment of Antioxidant Enzymes
2.6.1. Superoxide Dismutase Assay (SOD)
2.6.2. Catalase Assay (CAT)
2.6.3. Glutathione Peroxidase Assay (GPx), Glutathione Reductase (GSH Rd) and Glucose-6-Phosphate Dehydrogenase (G6pdh) Assays
2.7. Oxidative Stress Measures
2.8. Determination of 8-Hydroxy-2′-Deoxyguanosine in Mitochondrial DNA
2.9. Determination of Dopamine Content
2.10. RNA Extraction and Quality
2.11. DNA Microarray Chip-Based Whole Genome Brain Transcriptome Profiling
2.12. Statistical Analysis
3. Results
3.1. Effect of ProBeptigen on Changes in Body Weight, Food Intake and Locomotor Activity
3.2. Effect of ProBeptigen on Total Grading Score of Senescence
3.3. Effect of ProBeptigen on Cognition
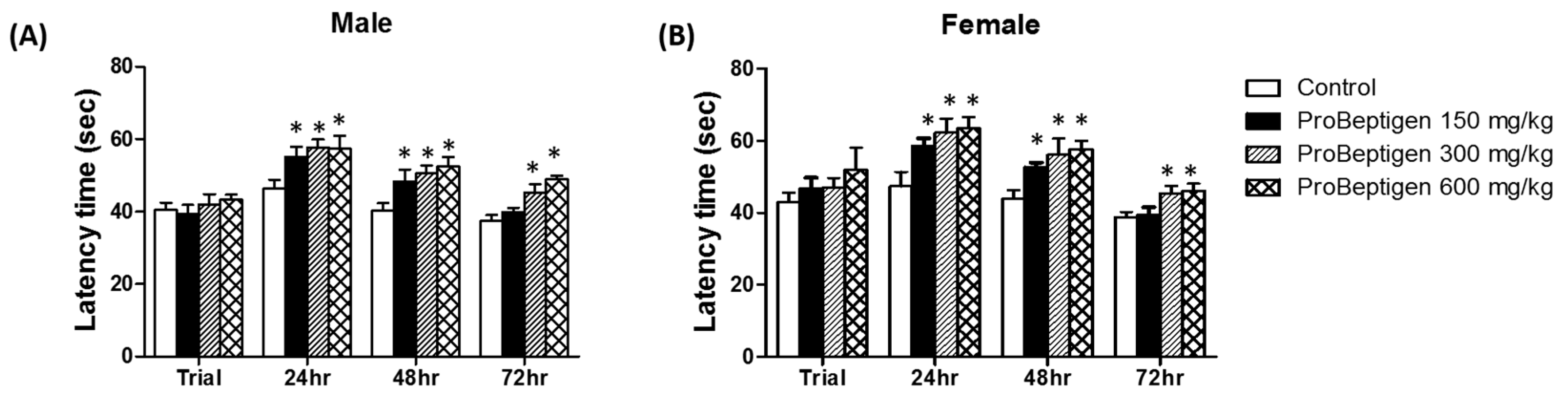
3.3.1. Effect of ProBeptigen on the Latency of Samp8 Mice in the Passive Avoidance Test
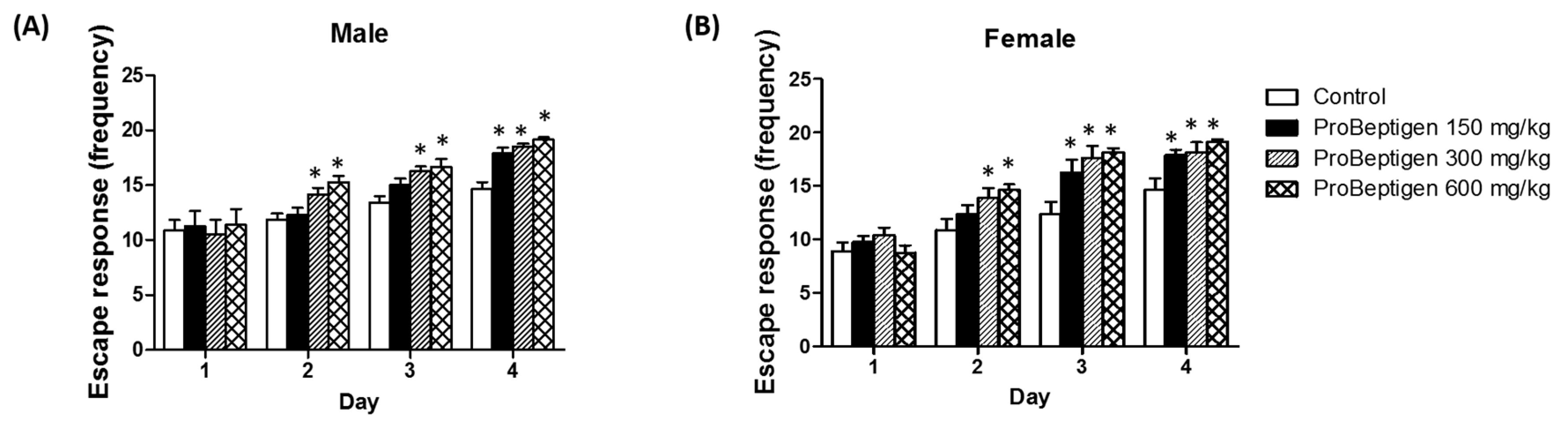
3.3.2. Effect of ProBeptigen on the SAMP8 Mice in the Active Avoidance Test
3.4. Effect of ProBeptigen on Oxidation of Brain
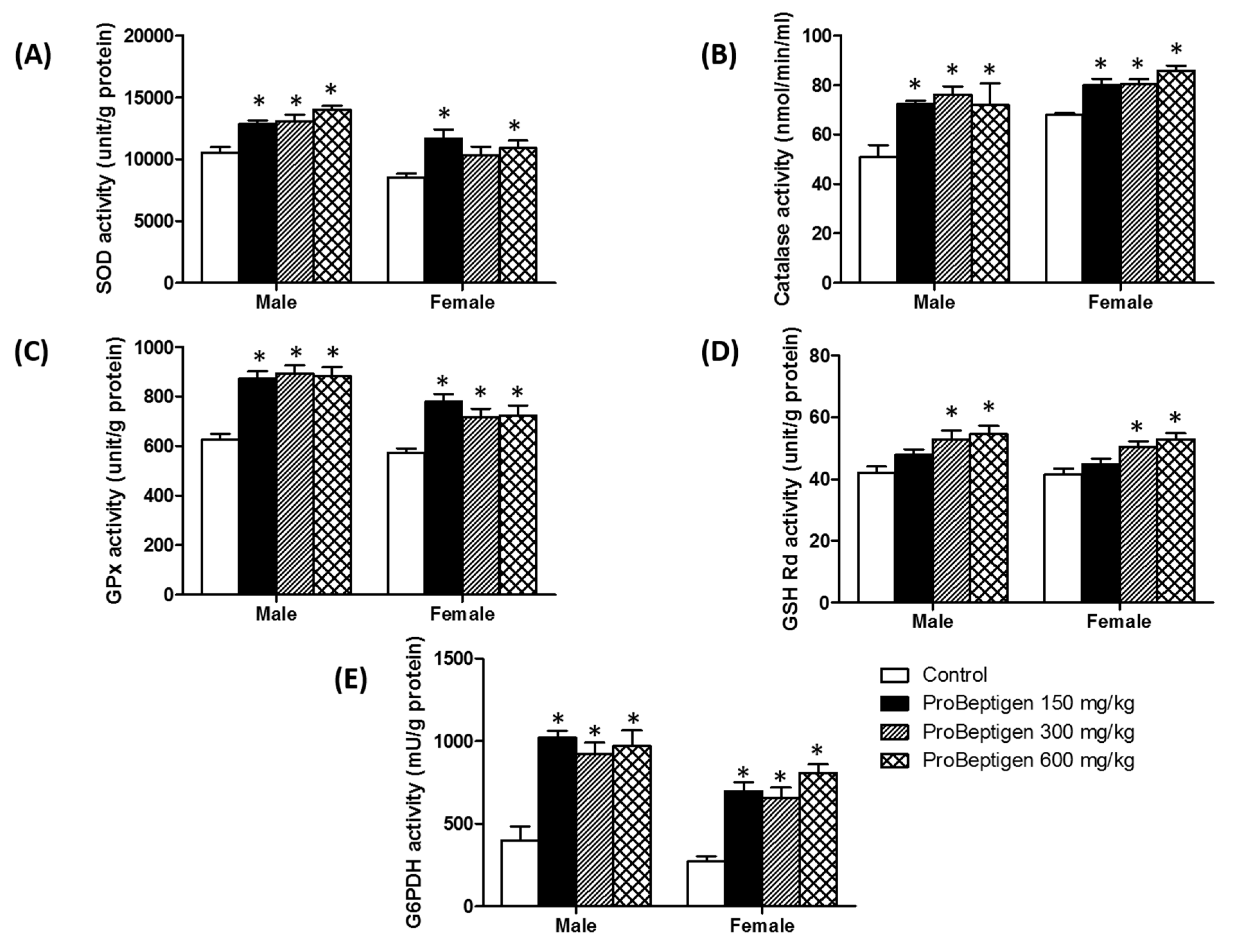
3.4.1. Effect of ProBeptigen on the Anti-Oxidative Enzymes in the Brain of Samp8 Mice
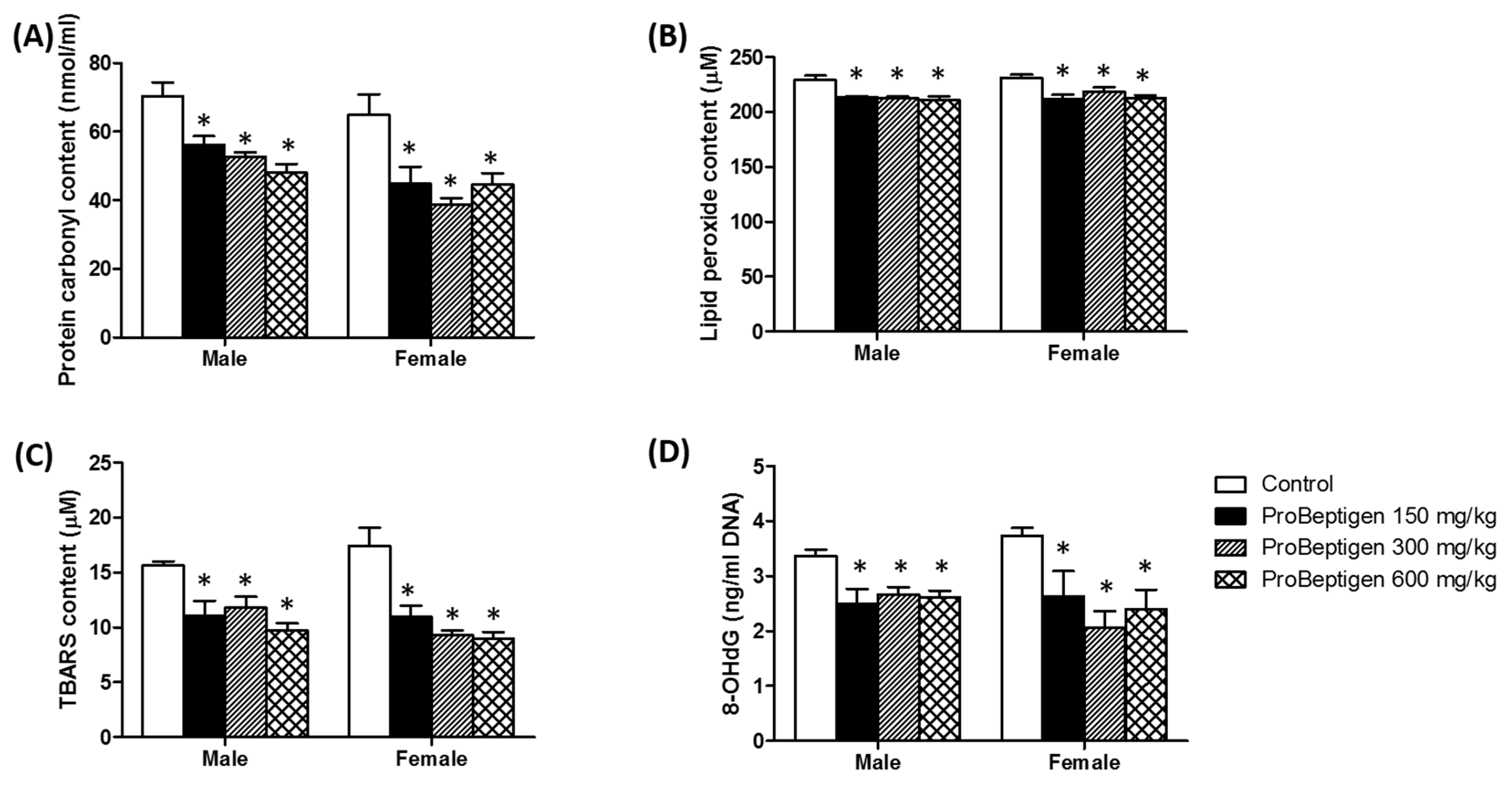
3.4.2. Effect of ProBeptigen on the Protein and Lipid Peroxidation in the Brain of Samp8 Mice
3.4.3. Effect of ProBeptigen on Brain Dopamine Concentration
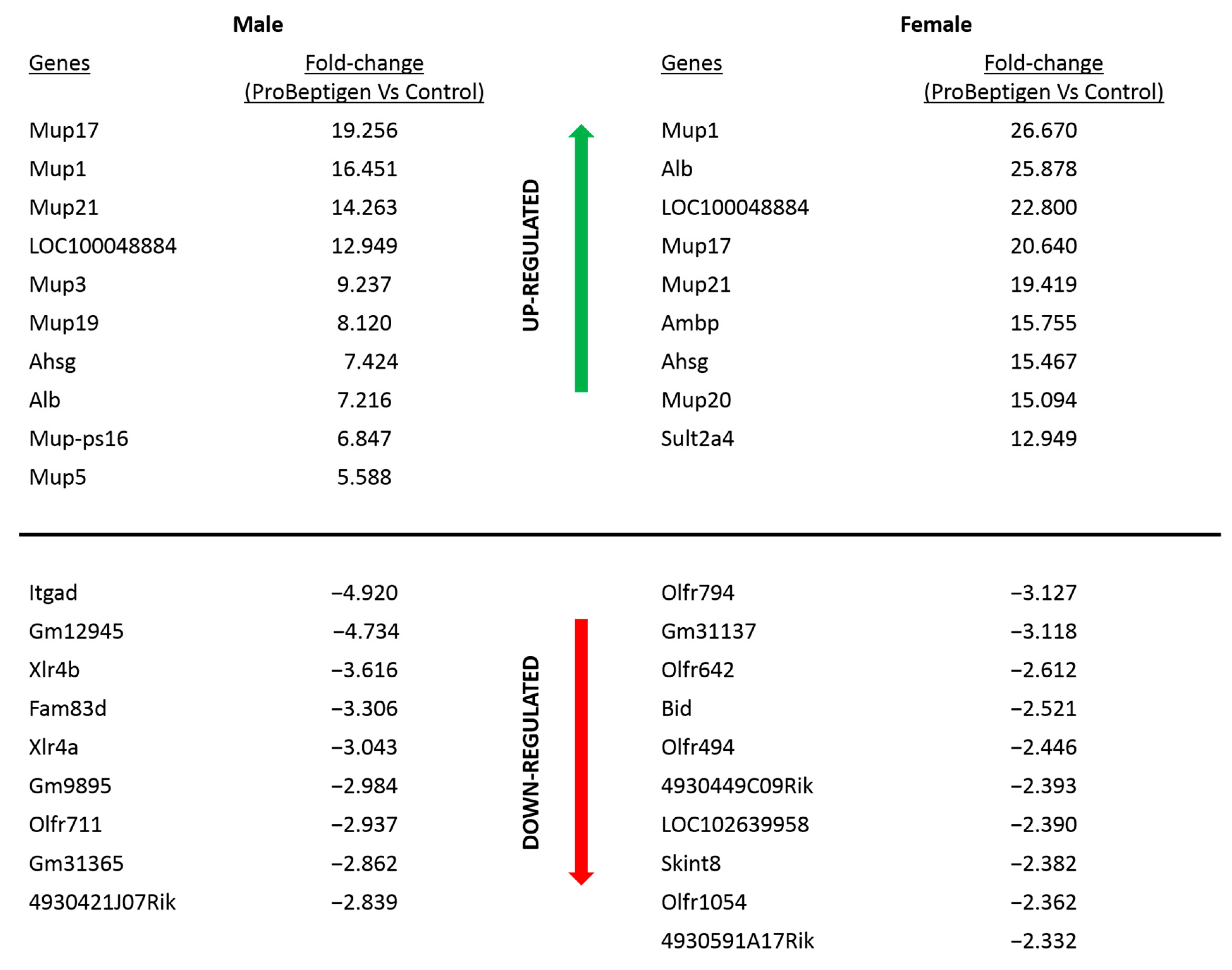
3.5. Transcript Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Mohanakumar, K.P. Aging and Neurodegeneration: A Tangle of Models and Mechanisms. Aging Dis. 2016, 7, 111–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, T.R. Environmental Enrichment: Aging and Memory. Yale J. Biol. Med. 2012, 85, 491–500. [Google Scholar] [PubMed]
- Brayne, C. The elephant in the room - healthy brains in later life, epidemiology and public health. Nat. Rev. Neurosci. 2007, 8, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. When does age-related cognitive decline begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzilai, N.; Atzmon, G.; Derby, C.A.; Bauman, J.M.; Lipton, R.B. A genotype of exceptional longevity is associated with preservation of cognitive function. Neurology 2006, 67, 2170–2175. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 526–533. [Google Scholar] [CrossRef]
- Reiter, R.J.; Acuña-Castroviejo, D.; Tan, D.X.; Burkhardt, S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann. N. Y. Acad. Sci. 2001, 939, 200–215. [Google Scholar] [CrossRef]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: from a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Liu, J.; Head, E.; Gharib, A.M.; Yuan, W.; Ingersoll, R.T.; Hagen, T.M.; Cotman, C.W.; Ames, B.N. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, A.M.; Moraes, C.T. What role does mitochondrial stress play in neurodegenerative diseases? Methods Mol. Biol. Clifton NJ 2010, 648, 63–78. [Google Scholar]
- Rodolfo, C.; Ciccosanti, F.; Giacomo, G.D.; Piacentini, M.; Fimia, G.M. Proteomic analysis of mitochondrial dysfunction in neurodegenerative diseases. Expert Rev. Proteomics 2010, 7, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-H.; Chan, Y.-C.; Liao, J.-W.; Wang, M.-F.; Yen, G.-C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J. Nutr. Biochem. 2010, 21, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging: Alzheimer’s disease pathogenesis. AGE 1995, 18, 97–119. [Google Scholar] [CrossRef]
- Takeda, T.; Hosokawa, M.; Takeshita, S.; Irino, M.; Higuchi, K.; Matsushita, T.; Tomita, Y.; Yasuhira, K.; Hamamoto, H.; Shimizu, K.; et al. A new murine model of accelerated senescence. Mech. Ageing Dev. 1981, 17, 183–194. [Google Scholar] [CrossRef]
- Takeda, T.; Hosokawa, M.; Higuchi, K.; Hosono, M.; Akiguchi, I.; Katoh, H. A novel murine model of aging, Senescence-Accelerated Mouse (SAM). Arch. Gerontol. Geriatr. 1994, 19, 185–192. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Koppal, T.; Howard, B.; Subramaniam, R.; Hall, N.; Hensley, K.; Yatin, S.; Allen, K.; Aksenov, M.; Aksenova, M.; et al. Structural and Functional Changes in Proteins Induced by Free Radical-mediated Oxidative Stress and Protective Action of the Antioxidants N-tert-Butyl-α-phenylnitrone and Vitamin Ea. Ann. N. Y. Acad. Sci. 1998, 854, 448–462. [Google Scholar] [CrossRef]
- Farr, S.A.; Poon, H.F.; Dogrukol-Ak, D.; Drake, J.; Banks, W.A.; Eyerman, E.; Butterfield, D.A.; Morley, J.E. The antioxidants α-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J. Neurochem. 2003, 84, 1173–1183. [Google Scholar] [CrossRef]
- Flood, J.F.; Morley, J.E. Learning and memory in the SAMP8 mouse. Neurosci. Biobehav. Rev. 1998, 22, 1–20. [Google Scholar] [CrossRef]
- Chen, G.-H.; Wang, C.; Yangcheng, H.-Y.; Liu, R.-Y.; Zhou, J.-N. Age-related changes in anxiety are task-specific in the senescence-accelerated prone mouse 8. Physiol. Behav. 2007, 91, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Flood, J.F.; Morley, J.E. Age-related changes in footshock avoidance acquisition and retention in senescence accelerated mouse (SAM). Neurobiol. Aging 1993, 14, 153–157. [Google Scholar] [CrossRef]
- Markowska, A.L.; Spangler, E.L.; Ingram, D.K. Behavioral assessment of the senescence-accelerated mouse (SAM P8 and R1). Physiol. Behav. 1998, 64, 15–26. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kiyota, Y.; Nishiyama, M.; Nagaoka, A. Senescence-accelerated mouse (SAM): age-related reduced anxiety-like behavior in the SAM-P/8 strain. Physiol. Behav. 1992, 51, 979–985. [Google Scholar] [CrossRef]
- Yagi, H.; Katoh, S.; Akiguchi, I.; Takeda, T. Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Res. 1988, 474, 86–93. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Stadtman, E.R. Protein Oxidation Processes in Aging Brain. In Advances in Cell Aging and Gerontology; The Aging Brain; Timiras, P.S., Bittar, E.E., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Chapter 7; Volume 2, pp. 161–191. [Google Scholar]
- Morley, J.E. The SAMP8 mouse: a model of Alzheimer disease? Biogerontology 2002, 3, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Pallas, M.; Camins, A.; Smith, M.A.; Perry, G.; Lee, H.; Casadesus, G. From aging to Alzheimer’s disease: unveiling “the switch” with the senescence-accelerated mouse model (SAMP8). J. Alzheimers Dis. JAD 2008, 15, 615–624. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Poon, H.F. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Kurokawa, T.; Oda, N.; Ishibashi, S. Early appearance of abnormality of microperoxisomal enzymes in the cerebral cortex of senescence-accelerated mouse. Mech. Ageing Dev. 1996, 92, 175–184. [Google Scholar] [CrossRef]
- Fujibayashi, Y.; Yamamoto, S.; Waki, A.; Konishi, J.; Yonekura, Y. Increased mitochondrial DNA deletion in the brain of SAMP8, a mouse model for spontaneous oxidative stress brain. Neurosci. Lett. 1998, 254, 109–112. [Google Scholar] [CrossRef]
- Nishiyama, N.; Zhou, Y.; Saito, H. Ameliorative effects of chronic treatment using DX-9386, a traditional Chinese prescription, on learning performance and lipid peroxide content in senescence accelerated mouse. Biol. Pharm. Bull. 1994, 17, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Higuchi, O.; Tateshita, K.; Tomobe, K.; Okuma, Y.; Nomura, Y. Antioxidative activity and ameliorative effects of memory impairment of sulfur-containing compounds in Allium species. BioFactors Oxf. Engl. 2006, 26, 135–146. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Hosoda, K.; Tsai, C.-J.; Yamamoto, S.; Wang, M.-F. Favorable effects of tea on reducing the cognitive deficits and brain morphological changes in senescence-accelerated mice. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, L.; Xie, B.; Liao, Y.; Yang, E.; Sun, Z. Ameliorative effects of lotus seedpod proanthocyanidins on cognitive deficits and oxidative damage in senescence-accelerated mice. Behav. Brain Res. 2008, 194, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Price, T.O.; Dominguez, L.J.; Motisi, A.; Saiano, F.; Niehoff, M.L.; Morley, J.E.; Banks, W.A.; Ercal, N.; Barbagallo, M. Extra Virgin Olive Oil Improves Learning and Memory in SAMP8 Mice. J. Alzheimers Dis. 2012, 28, 81–92. [Google Scholar] [CrossRef] [PubMed]
- He, X.-L.; Zhou, W.-Q.; Bi, M.-G.; Du, G.-H. Neuroprotective effects of icariin on memory impairment and neurochemical deficits in senescence-accelerated mouse prone 8 (SAMP8) mice. Brain Res. 2010, 1334, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Harada, M.; Nakagawa, M.; Tanaka, T.; Gunadi, B.; Setiabudi, M.L.; Uktolseja, J.L.; Miyata, Y. Effects of chicken extract on the recovery from fatigue caused by mental workload. Appl. Hum. Sci. J. Physiol. Anthropol. 1996, 15, 281–286. [Google Scholar] [CrossRef]
- Zain, A.M.; Syedsahiljamalulail, S. Effect of taking chicken essence on stress and cognition of human volunteers. Malays. J. Nutr. 2003, 9, 19–29. [Google Scholar] [PubMed]
- Young, H.; Benton, D.; Carter, N. The effect of chicken extract on mood, cognition and heart rate variability. Nutrients 2015, 7, 887–904. [Google Scholar] [CrossRef]
- Huang, S.-W.; Hsu, Y.-J.; Lee, M.-C.; Li, H.-S.; Yeo, P.C.W.; Lim, A.L.; Huang, C.-C. In Vitro and In Vivo Functional Characterization of Essence of Chicken as an Ergogenic Aid. Nutrients 2018, 10, 1943. [Google Scholar] [CrossRef]
- Azhar, Z.M.; Zubaidah, J.O.; Norjan, K.O.; Zhuang, C.Y.-J.; Tsang, F. A pilot placebo-controlled, double-blind, and randomized study on the cognition-enhancing benefits of a proprietary chicken meat ingredient in healthy subjects. Nutr. J. 2013, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-F.; Chang, C.-Y.; Yong, S.-M.; Lim, A.-L.; Nakao, Y.; Chen, S.-J.; Kuo, Y.-M. A Hydrolyzed Chicken Extract CMI-168 Enhances Learning and Memory in Middle-Aged Mice. Nutrients 2019, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Bickford, P.C.; Gould, T.; Briederick, L.; Chadman, K.; Pollock, A.; Young, D.; Shukitt-Hale, B.; Joseph, J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000, 866, 211–217. [Google Scholar] [CrossRef]
- Emilien, G.; Beyreuther, K.; Masters, C.L.; Maloteaux, J.M. Prospects for pharmacological intervention in Alzheimer disease. Arch. Neurol. 2000, 57, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Introini, I.B.; McGaugh, J.L.; Baratti, C.M. Pharmacological evidence of a central effect of naltrexone, morphine, and β-endorphin and a peripheral effect of met- and leu-enkephalin on retention of an inhibitory response in mice. Behav. Neural Biol. 1985, 44, 434–446. [Google Scholar] [CrossRef]
- Janković, B.D.; Marić, D.; Veljić, J. Cerebrally mediated modulation of anaphylactic shock by methionine-enkephalin. Int. J. Neurosci. 1990, 51, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A.J.; Olson, R.D.; Schally, A.V.; Coy, D.H. CNS effects of peripherally administered brain peptides. Life Sci. 1979, 25, 401–414. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Dorado-Martínez, C.; Borgonio-Pérez, G.; Hiriart-Urdanivia, M.; Verdugo-Diaz, L.; Durán-Vázquez, A.; Colin-Baranque, L.; Avila-Costa, M.R. Effects of taurine on ozone-induced memory deficits and lipid peroxidation levels in brains of young, mature, and old rats. Environ. Res. 2000, 82, 7–17. [Google Scholar] [CrossRef]
- Di Bernardini, R.; Harnedy, P.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A.M.; Hayes, M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011, 124, 1296–1307. [Google Scholar] [CrossRef]
- Peña-Ramos, E.A.; Xiong, Y.L.; Arteaga, G.E. Fractionation and characterisation for antioxidant activity of hydrolysed whey protein. J. Sci. Food Agric. 2004, 84, 1908–1918. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant Activity of Peptides Obtained from Porcine Myofibrillar Proteins by Protease Treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: a review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Demejia, E. A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Compr. Rev. Food Sci. Food Saf. 2005, 4, 63–78. [Google Scholar] [CrossRef]
- Smith, Q.R. Transport of Glutamate and Other Amino Acids at the Blood-Brain Barrier. J. Nutr. 2000, 130, 1016S–1022S. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, R.L.; Viña, J.R.; Simpson, I.; Zaragozá, R.; Mokashi, A.; Hawkins, R.A. Cationic amino acid transport across the blood-brain barrier is mediated exclusively by system y+. Am. J. Physiol.-Endocrinol. Metab. 2006, 291, E412–E419. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, G.M.; Pettigrew, K.D.; Patlak, C.S.; Hertz, M.M.; Paulson, O.B. Asymmetrical transport of amino acids across the blood-brain barrier in humans. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1990, 10, 698–706. [Google Scholar] [CrossRef]
- Hawkins, R.A.; O’Kane, R.L.; Simpson, I.A.; Viña, J.R. Structure of the Blood–Brain Barrier and Its Role in the Transport of Amino Acids. J. Nutr. 2006, 136, 218S–226S. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, D.; Guo, Y.; Li, J. Purification of chicken breast protein hydrolysate and analysis of its antioxidant activity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 3397–3404. [Google Scholar] [CrossRef]
- Armbrecht, H.J.; Siddiqui, A.M.; Green, M.; Farr, S.A.; Kumar, V.B.; Banks, W.A.; Patrick, P.; Shah, G.; Morley, J.E. SAMP8 mice have altered hippocampal gene expression in LTP, phosphatidylinositol signaling, and endocytosis pathways. Neurobiol. Aging 2014, 35, 159–168. [Google Scholar] [CrossRef]
- Verbitsky, M.; Yonan, A.L.; Malleret, G.; Kandel, E.R.; Gilliam, T.C.; Pavlidis, P. Altered hippocampal transcript profile accompanies an age-related spatial memory deficit in mice. Learn. Mem. Cold Spring Harb. N 2004, 11, 253–260. [Google Scholar] [CrossRef]
- Kaisho, Y.; Miyamoto, M.; Shiho, O.; Onoue, H.; Kitamura, Y.; Nomura, S. Expression of neurotrophin genes in the brain of senescence-accelerated mouse (SAM) during postnatal development. Brain Res. 1994, 647, 139–144. [Google Scholar] [CrossRef]
- Kumar, V.B.; Franko, M.W.; Farr, S.A.; Armbrecht, H.J.; Morley, J.E. Identification of age-dependent changes in expression of senescence-accelerated mouse (SAMP8) hippocampal proteins by expression array analysis. Biochem. Biophys. Res. Commun. 2000, 272, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Okuma, Y.; Nomura, J.; Nagashima, K.; Nomura, Y. Age-related alterations in the expression of glial cell line-derived neurotrophic factor in the senescence-accelerated mouse brain. J. Pharmacol. Sci. 2003, 92, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, Y.; Zhou, J. Alzheimer’s disease-related gene expression in the brain of senescence accelerated mouse. Neurosci. Lett. 1999, 268, 139–142. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Drake, J.; Kanski, J.; Varadarajan, S.; Tsoras, M.; Butterfield, D.A. Elevation of brain glutathione by gamma-glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. J. Neurosci. Res. 2002, 68, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, T.; Asada, S.; Nishitani, S.; Hazeki, O. Age-related changes in manganese superoxide dismutase activity in the cerebral cortex of senescence-accelerated prone and resistant mouse. Neurosci. Lett. 2001, 298, 135–138. [Google Scholar] [CrossRef]
- Morley, J.E.; Armbrecht, H.J.; Farr, S.A.; Kumar, V.B. The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1822, 650–656. [Google Scholar] [CrossRef]
- Shi, C.; Xiao, S.; Liu, J.; Guo, K.; Wu, F.; Yew, D.T.; Xu, J. Ginkgo biloba extract EGb761 protects against aging-associated mitochondrial dysfunction in platelets and hippocampi of SAMP8 mice. Platelets 2010, 21, 373–379. [Google Scholar] [CrossRef]
- Sandi, C. Stress and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Robinson, O.J.; Vytal, K.; Cornwell, B.R.; Grillon, C. The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front. Hum. Neurosci. 2013, 7, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Fels, I.M.J.; te Wierike, S.C.M.; Hartman, E.; Elferink-Gemser, M.T.; Smith, J.; Visscher, C. The relationship between motor skills and cognitive skills in 4–16 year old typically developing children: A systematic review. J. Sci. Med. Sport 2015, 18, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Matsugo, S.; Kitagawa, T.; Minami, S.; Esashi, Y.; Oomura, Y.; Tokumaru, S.; Kojo, S.; Matsushima, K.; Sasaki, K. Age-dependent changes in lipid peroxide levels in peripheral organs, but not in brain, in senescence-accelerated mice. Neurosci. Lett. 2000, 278, 105–108. [Google Scholar] [CrossRef]
- Nomura, Y.; Wang, B.X.; Qi, S.B.; Namba, T.; Kaneko, S. Biochemical changes related to aging in the senescence-accelerated mouse. Exp. Gerontol. 1989, 24, 49–55. [Google Scholar] [CrossRef]
- Wu, H.C.; Pan, B.S.; Chang, C.L.; Shiau, C.Y. Low-molecular-weight peptides as related to antioxidant properties of chicken essence. J. Food Drug Anal. 2005, 13, 176–183. [Google Scholar]
- Dringenberg, H.C. Alzheimer’s disease: more than a “cholinergic disorder” - evidence that cholinergic-monoaminergic interactions contribute to EEG slowing and dementia. Behav. Brain Res. 2000, 115, 235–249. [Google Scholar] [CrossRef]
- Gottfries, C.G. Neurochemical aspects on aging and diseases with cognitive impairment. J. Neurosci. Res. 1990, 27, 541–547. [Google Scholar] [CrossRef]
- Karasawa, N.; Nagatsu, I.; Sakai, K.; Nagatsu, T.; Watanabe, K.; Onozuka, M. Immunocytochemical study of catecholaminergic neurons in the senescence-accelerated mouse (SAM-P8) brain. J. Neural Transm. Vienna Austria 1996 1997, 104, 1267–1275. [Google Scholar] [CrossRef]
- Flood, J.F.; Morley, J.E.; La Reginna, M. Age-related changes in the pharmacological improvement of retention in senescence accelerated mouse (SAM). Neurobiol. Aging 1993, 14, 159–166. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Boyko, S.S.; Akparov, V.K.; Ostrovskaya, R.U.; Skoldinov, S.P.; Rozantsev, G.G.; Voronina, T.A.; Zherdev, V.P.; Seredenin, S.B. Identification of a novel endogenous memory facilitating cyclic dipeptide cyclo-prolylglycine in rat brain. FEBS Lett. 1996, 391, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Prasad, C. Bioactive cyclic dipeptides. Peptides 1995, 16, 151–164. [Google Scholar] [CrossRef]
- Cooke, J.P. The pivotal role of nitric oxide for vascular health. Can. J. Cardiol. 2004, 20 (Suppl. B), 7B–15B. [Google Scholar] [PubMed]
- Cooke, J.P.; Dzau, V.J. Nitric oxide synthase: role in the genesis of vascular disease. Annu. Rev. Med. 1997, 48, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; de Nigris, F.; Williams-Ignarro, S.; Pignalosa, O.; Sica, V.; Ignarro, L.J. Nitric oxide and atherosclerosis: an update. Nitric Oxide Biol. Chem. 2006, 15, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Faden, A.I.; Knoblach, S.M.; Movsesyan, V.A.; Cernak, I. Novel small peptides with neuroprotective and nootropic properties. J. Alzheimers Dis. JAD 2004, 6, S93–S97. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.R.C.; Tang, Y.; Kozikowski, A.P.; Flippen-Anderson, J.L.; Knoblach, S.M.; Faden, A.I. Synthesis and biological activity of novel neuroprotective diketopiperazines. Bioorg. Med. Chem. 2002, 10, 3043–3048. [Google Scholar] [CrossRef]
- Tsuruoka, N.; Beppu, Y.; Koda, H.; Doe, N.; Watanabe, H.; Abe, K. A DKP cyclo(L-Phe-L-Phe) found in chicken essence is a dual inhibitor of the serotonin transporter and acetylcholinesterase. PloS One 2012, 7, e50824. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Mathai, S.; Harris, P.; Wen, J.-Y.; Zhang, R.; Brimble, M.; Gluckman, P. Peripheral administration of a novel diketopiperazine, NNZ 2591, prevents brain injury and improves somatosensory-motor function following hypoxia-ischemia in adult rats. Neuropharmacology 2007, 53, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Alamri, Y.; Liu, K.; MacAskill, M.; Harris, P.; Brimble, M.; Dalrymple-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.; et al. Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, M.; Zurita, E.; Malakoutikhah, M.; Tarragó, T.; Giralt, E. Diketopiperazines as a Tool for the Study of Transport across the Blood−Brain Barrier (BBB) and Their Potential Use as BBB-Shuttles. J. Am. Chem. Soc. 2007, 129, 11802–11813. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Zhu, W.; Wang, Y.; Lam, K.S.L.; Zhang, J.; Wu, D.; Kraegen, E.W.; Li, Y.; Xu, A. Major urinary protein-1 increases energy expenditure and improves glucose intolerance through enhancing mitochondrial function in skeletal muscle of diabetic mice. J. Biol. Chem. 2009, 284, 14050–14057. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, L.; Rui, L. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J. Biol. Chem. 2009, 284, 11152–11159. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, I.K.; Lamprecht, R. Fear conditioning leads to alteration in specific genes expression in cortical and thalamic neurons that project to the lateral amygdala. J. Neurochem. 2015, 132, 313–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, G.P.; Rubin, M.A.; Mello, C.F. Modulation of learning and memory by natural polyamines. Pharmacol. Res. 2016, 112, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Berlese, D.B.; Sauzem, P.D.; Carati, M.C.; Guerra, G.P.; Stiegemeier, J.A.; Mello, C.F.; Rubin, M.A. Time-dependent modulation of inhibitory avoidance memory by spermidine in rats. Neurobiol. Learn. Mem. 2005, 83, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.P.; Mello, C.F.; Bochi, G.V.; Pazini, A.M.; Fachinetto, R.; Dutra, R.C.; Calixto, J.B.; Ferreira, J.; Rubin, M.A. Hippocampal PKA/CREB pathway is involved in the improvement of memory induced by spermidine in rats. Neurobiol. Learn. Mem. 2011, 96, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Rubin, M.A.; Boemo, R.L.; Jurach, A.; Rojas, D.B.; Zanolla, G.R.; Obregon, A.D.; Souza, D.O.; Mello, C.F. Intrahippocampal spermidine administration improves inhibitory avoidance performance in rats. Behav. Pharmacol. 2000, 11, 57–61. [Google Scholar] [CrossRef]


| Ingredients | Amount (%) |
|---|---|
| Proteins and peptides | 91.38 |
| Free amino acids | 4.23 |
| Diketopiperazines | 7.598 |
| Carbohydrate | 1.01 |
| Lipid | 1.64 |
| Minerals | |
| Sodium | 0.31 |
| Potassium | 0.34 |
| Calcium | 0.08 |
| Magnesium | 0.1 |
| Chloride | 0.53 |
| Sex | Group | Body Weight (g) | Food Intake (g/day) | Water Consumption (ml/day) | ||
|---|---|---|---|---|---|---|
| Initial | Final | Gain | ||||
| Male | Control | 29.19 ± 0.49 | 29.71 ± 0.34 | 0.52 ± 0.43 | 4.61 ± 0.20 | 6.43 ± 0.18 |
| ProBeptigen (150 mg/kg) | 29.91 ± 0.93 | 32.36 ± 1.39 | 2.45 ± 0.63 | 4.76 ± 0.16 | 6.57 ± 0.13 | |
| ProBeptigen (300 mg/kg) | 28.89 ± 0.89 | 30.63 ± 0.85 | 1.74 ± 0.79 | 4.98 ± 0.18 | 6.60 ± 0.22 | |
| ProBeptigen (600mg/kg) | 29.14 ± 0.67 | 30.24 ± 0.68 | 1.09 ± 0.50 | 4.51 ± 0.22 | 6.36 ± 0.33 | |
| Female | Control | 25.86 ± 0.55 | 26.47 ± 0.57 | 0.61 ± 0.31 | 4.25 ± 0.16 | 5.32 ± 0.18 |
| ProBeptigen (150 mg/kg) | 26.16 ± 0.96 | 27.00 ± 0.95 | 0.85 ± 0.74 | 4.21 ± 0.16 | 5.48 ± 0.16 | |
| ProBeptigen (300 mg/kg) | 26.25 ± 0.60 | 28.16 ± 1.11 | 1.91 ± 0.81 | 3.21 ± 0.10 | 5.40 ± 0.23 | |
| ProBeptigen (600 mg/kg) | 24.88 ± 0.83 | 26.44 ± 1.02 | 1.56 ± 0.57 | 4.28 ± 0.24 | 5.42 ± 0.12 | |
| Group | Control | ProBeptigen (150 mg/kg) | ProBeptigen (300 mg/kg) | ProBeptigen (600 mg/kg) |
|---|---|---|---|---|
| Behavior | ||||
| Reactivity | 0.50 ± 0.19 | 0.38 ± 0.18 | 0.13 ± 0.13 | 0.00 ± 0.00 |
| Passivity | 0.63 ± 0.18 | 0.38 ± 0.18 | 0.25 ± 0.16 | 0.13 ± 0.13 |
| Skin and hair | ||||
| Glossiness | 0.88 ± 0.23 | 0.75 ± 0.16 | 0.50 ± 0.19 | 0.63 ± 0.18 |
| Coarseness | 1.00 ± 0.19 | 0.88 ± 0.13 | 0.75 ± 0.16 | 0.75 ± 0.25 |
| Hair loss | 0.63 ± 0.26 | 0.50 ± 0.19 | 0.25 ± 0.16 | 0.13 ± 0.13 |
| Ulcer | 0.38 ± 0.18 | 0.25 ± 0.16 | 0.13 ± 0.13 | 0.13 ± 0.13 |
| Eyes | ||||
| Periophthalmic lesion | 0.38 ± 0.18 | 0.25 ± 0.16 | 0.13 ± 0.13 | 0.38 ± 0.18 |
| Spine | ||||
| Lordokyphosis | 0.88 ± 0.23 | 0.63 ± 0.26 | 0.50 ± 0.27 | 0.63 ± 0.18 |
| Total | 5.25 ± 0.59 | 4.00 ± 0.85 | 2.63 ± 0.68 * | 2.75 ± 0.59 * |
| Group | Control | ProBeptigen (150 mg/kg) | ProBeptigen (300 mg/kg) | ProBeptigen (600 mg/kg) |
|---|---|---|---|---|
| Behavior | ||||
| Reactivity | 0.38 ± 0.18 | 0.38 ± 0.18 | 0.25 ± 0.16 | 0.25 ± 0.16 |
| Passivity | 0.50 ± 0.19 | 0.25 ± 0.16 | 0.38 ± 0.18 | 0.25 ± 0.16 |
| Skin and hair | ||||
| Glossiness | 0.63 ± 0.18 | 0.38 ± 0.18 | 0.25 ± 0.16 | 0.38 ± 0.18 |
| Coarseness | 0.63 ± 0.18 | 0.50 ± 0.19 | 0.38 ± 0.18 | 0.50 ± 0.19 |
| Hair loss | 0.88 ± 0.30 | 0.38 ± 0.18 | 0.25 ± 0.16 | 0.25 ± 0.16 |
| Ulcer | 0.75 ± 0.25 | 0.50 ± 0.19 | 0.25 ± 0.16 | 0.25 ± 0.16 |
| Eyes | ||||
| Periophthalmic lesion | 1.00 ± 0.27 | 0.38 ± 0.18 | 0.63 ± 0.18 | 0.50 ± 0.19 |
| Spine | ||||
| Lordokyphosis | 0.50 ± 0.19 | 0.75 ± 0.16 | 0.50 ± 0.19 | 0.63 ± 0.18 |
| Total | 5.25 ± 0.56 | 3.50 ± 0.42 * | 2.88 ± 0.55 * | 3.00 ± 0.46 * |
| GO Term/Pathway | Class Members on Array | p-Value |
|---|---|---|
| Heme binding | 19 | 1.71 × 10−14 |
| Regulation of insulin-like growth factor (IGF) transport and uptake by insulin-like growth factor | 14 | 1.42 × 10−8 |
| Carboxylic acid metabolic process | 27 | 3.68 × 10−8 |
| Oxidation-reduction process | 28 | 7.37 × 10−8 |
| Negative regulation of endopeptidase activity | 12 | 4.68 × 10−5 |
| Transition metal ion binding | 24 | 8.14 × 10−5 |
| Acute phase response | 6 | 1.68 × 10−3 |
| Innate immune system | 22 | 4.13 × 10−3 |
| Positive regulation of lipid catabolic process | 5 | 1.39 × 10−2 |
| Acylglycerol metabolic process | 7 | 3.16 × 10−2 |
| Regulation of lipoprotein lipase activity | 4 | 4.83 × 10−2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, M.-Y.; Chen, Y.-J.; Lin, L.-H.; Nakao, Y.; Lim, A.L.; Wang, M.-F.; Yong, S.M. Protective Effects of Hydrolyzed Chicken Extract (Probeptigen®/Cmi-168) on Memory Retention and Brain Oxidative Stress in Senescence-Accelerated Mice. Nutrients 2019, 11, 1870. https://doi.org/10.3390/nu11081870
Chou M-Y, Chen Y-J, Lin L-H, Nakao Y, Lim AL, Wang M-F, Yong SM. Protective Effects of Hydrolyzed Chicken Extract (Probeptigen®/Cmi-168) on Memory Retention and Brain Oxidative Stress in Senescence-Accelerated Mice. Nutrients. 2019; 11(8):1870. https://doi.org/10.3390/nu11081870
Chicago/Turabian StyleChou, Ming-Yu, Ying-Ju Chen, Liang-Hung Lin, Yoshihiro Nakao, Ai Lin Lim, Ming-Fu Wang, and Shan May Yong. 2019. "Protective Effects of Hydrolyzed Chicken Extract (Probeptigen®/Cmi-168) on Memory Retention and Brain Oxidative Stress in Senescence-Accelerated Mice" Nutrients 11, no. 8: 1870. https://doi.org/10.3390/nu11081870




