The Safety and Impact of a Model of Intermittent, Time-Restricted Circadian Fasting (“Ramadan Fasting”) on Hidradenitis Suppurativa: Insights from a Multicenter, Observational, Cross-Over, Pilot, Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Clearance and Study Design
2.2. Patient Selection: Inclusion and Exclusion Criteria
2.3. Socio-Demographic and Clinical Measures
2.4. Statistical Analysis
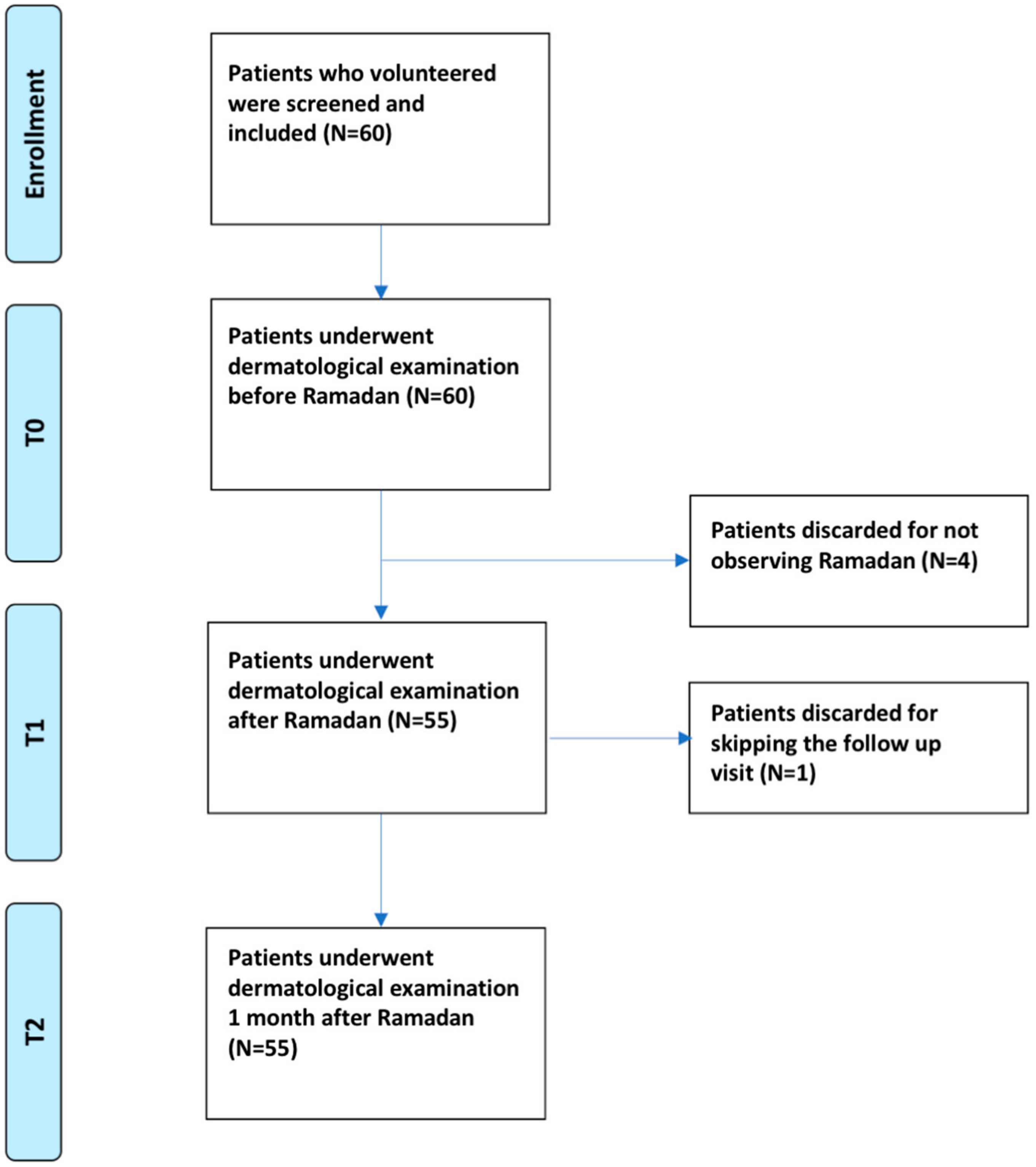
3. Results
3.1. Descriptive Statistics
3.2. Changes in the IHS4 Score: Univariate Analyses
3.3. Changes in the IHS4 score: Multivariate Analyses
3.4. Changes in Nodules: Multivariate Analyses
3.5. Changes in Abscesses: Multivariate Analyses
3.6. Changes in Draining Tunnels: Multivariate Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jemec, G.B. Clinical practice. Hidradenitis suppurativa. N. Engl. J. Med. 2012, 366, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, L.; Ricceri, F.; Prignano, F. Hidradenitis suppurativa and associated diseases. G. Ital. Dermatol. Venereol. 2018, 153, S8–S17. [Google Scholar]
- Danby, F.W.; Jemec, G.B.; Marsch, W.C.; von Laffert, M. Preliminary findings suggest hidradenitis suppurativa may be due to defective follicular support. Br. J. Dermatol. 2013, 168, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Margesson, L.J.; Danby, F.W. Hidradenitis suppurativa. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Damiani, G.; Ceccherini, I.; Berti, E.; Gattorno, M.; Cugno, M. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br. J. Dermatol. 2017, 176, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Ben David, C.; Bragazzi, N.L.; Watad, A.; Sharif, K.; Whitby, A.; Amital, H.; Adawi, M. Hidradenitis suppurativa associated with systemic lupus erythematosus: A case report. Medicine 2018, 97, e0186. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Leone, S.; Fajgenbaum, K.; Bragazzi, N.L.; Pacifico, A.; Conic, R.R.; Pigatto, P.D.; Maiorana, C.; Poli, P.; Berti, E.; et al. Nonalcoholic fatty liver disease prevalence in an Italian cohort of patients with hidradenitis suppurativa: A multi-center retrospective analysis. World J. Hepatol. 2019, 11, 391–401. [Google Scholar] [CrossRef]
- Damiani, G.; di Meo, N.; Marzano, A.V. A unique pneumopathy in a patient with skin nodules and abscesses. Intern. Emerg. Med. 2017, 12, 637–640. [Google Scholar] [CrossRef]
- Shalom, G.; Cohen, A.D. The epidemiology of hidradenitis suppurativa: What do we know? Br. J. Dermatol. 2019, 180, 712–713. [Google Scholar] [CrossRef]
- Tchero, H.; Herlin, C.; Bekara, F.; Fluieraru, S.; Teot, L. Hidradenitis suppurativa: A systematic review and meta-analysis of therapeutic interventions. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 248–257. [Google Scholar]
- Bettoli, V.; Manfredini, M.; Calamo, G.; Forconi, R.; Bencivelli, D.; Mantovani, L.; Pellacani, G.; Corazza, M. Long-term adalimumab treatment of hidradenitis suppurativa: Results and practical insights from a real-life experience. Dermatol. Ther. 2018, 31, e12737. [Google Scholar] [CrossRef]
- Andersen, R.; Jemec, G.B. New treatment strategies for hidradenitis suppurativa. Drugs Today 2016, 52, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, M.; Clark, A.K.; Lee, D.E.; Shi, V.Y. Targeted treatments for hidradenitis suppurativa: A review of the current literature and ongoing clinical trials. J. Dermatolog. Treat. 2018, 29, 441–449. [Google Scholar] [CrossRef]
- Hendricks, A.J.; Hirt, P.A.; Sekhon, S.; Vaughn, A.R.; Lev-Tov, H.A.; Hsiao, J.L.; Shi, V.Y. Non-pharmacologic approaches for hidradenitis suppurativa—A systematic review. J. Dermatolog. Treat. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Danby, F.W. Diet in the prevention of hidradenitis suppurativa (Acne inversa). J. Am. Acad. Dermatol. 2015, 5, S52–S54. [Google Scholar] [CrossRef]
- Boer, J. Resolution of hidradenitis suppurativa after weight loss by dietary measures, especially on frictional locations. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, M.; Platto, J.F.; Shi, V.Y. The role of nutrition in inflammatory pilosebaceous disorders: Implication of the skin-gut axis. Australas. J. Dermatol. 2019, 60, e90–e98. [Google Scholar] [CrossRef] [PubMed]
- Adawi, M.; Watad, A.; Brown, S.; Aazza, K.; Aazza, H.; Zouhir, M.; Sharif, K.; Ghanayem, K.; Farah, R.; Mahagna, H.; et al. Ramadan fasting exerts immunomodulatory effects: Insights from a systematic review. Front. Immunol. 2017, 8, 1144. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Sellami, M.; Salem, I.; Conic, R.; Kimak, M.; Pigatto, P.D.M.; Damiani, G. Fasting and its impact on skin anatomy, physiology, and physiopathology: A Comprehensive review of the literature. Nutrients 2019, 11, 249. [Google Scholar] [CrossRef]
- Adawi, M.; Damiani, G.; Bragazzi, N.L.; Bridgewood, C.; Pacifico, A.; Conic, R.R.Z.; Morrone, A.; Malagoli, P.; Pigatto, P.D.M.; Amital, H.; et al. The impact of intermittent fasting (Ramadan fasting) on psoriatic arthritis disease activity, enthesitis, and dactylitis: A multicentre study. Nutrients 2019, 11, 601. [Google Scholar] [CrossRef]
- Damiani, G.; Watad, A.; Bridgewood, C.; Pigatto, P.D.M.; Pacifico, A.; Malagoli, P.; Bragazzi, N.L.; Adawi, M. The impact of ramadan fasting on the reduction of PASI score, in moderate-to-severe psoriatic patients: A real-life multicenter study. Nutrients 2019, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Watad, A. The impact of fasting on rheumatic diseases. Isr. Med. Assoc. J. 2017, 19, 378–379. [Google Scholar] [PubMed]
- Cazzaniga, S.; Naldi, L.; Damiani, G.; Atzori, L.; Patta, F.; Guidarelli, G.; Bettoli, V. Validation of a visual-aided questionnaire for the self-assessment of hidradenitits suppurativa. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, H.H.; Jemec, G.B. New insights into the diagnosis of hidradenitis suppurativa: Clinical presentations and phenotypes. J. Am. Acad. Dermatol. 2015, 73, S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Della Valle, V.; Iannone, M.; Dini, V.; Marzano, A.V. Autoinflammatory disease damage index (ADDI): A possible newborn also in hidradenitis suppurativa daily practice. Ann. Rheum. Dis. 2017, 76, e25. [Google Scholar] [CrossRef] [PubMed]
- Hurley, H.J. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: Surgical approach. In Roenigk and Roenigk’s Dermatologic Surgery: Principles and Practice, 2nd ed.; Roenigk, R.K., Roenigk, H.H., Jr., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 623–645. [Google Scholar]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. European hidradenitis suppurativa foundation investigator group. Development and validation of the International hidradenitis suppurativa severity score system (IHS4), a novel dynamic scoring system to assess HS severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Stefanadi, E.C.; Dimitrakakis, G.; Antoniou, C.K.; Challoumas, D.; Punjabi, N.; Dimitrakaki, I.A.; Punjabi, S.; Stefanadis, C.I. Metabolic syndrome and the skin: A more than superficial association. Reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol. Metab. Syndr. 2018, 10, 9. [Google Scholar] [CrossRef]
- Napolitano, M.; Megna, M.; Monfrecola, G. Insulin resistance and skin diseases. Sci. World J. 2015, 2015, 479354. [Google Scholar] [CrossRef]
- Monfrecola, G.; Balato, A.; Caiazzo, G.; De Vita, V.; Di Caprio, R.; Donnarumma, M.; Lembo, S.; Fabbrocini, G. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: A possible metabolic loop. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1631–1633. [Google Scholar] [CrossRef]
- Vilanova, I.; Hernández, J.L.; Mata, C.; Durán, C.; García-Unzueta, M.T.; Portilla, V.; Fuentevilla, P.; Corrales, A.; González-Vela, M.C.; González-Gay, M.A.; et al. Insulin resistance in hidradenitis suppurativa: A case-control study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 820–824. [Google Scholar] [CrossRef]
- Akdogan, N.; Alli, N.; Uysal, P.I.; Topcuoglu, C.; Candar, T.; Turhan, T. Visfatin and insulin levels and cigarette smoking are independent risk factors for hidradenitis suppurativa: A case-control study. Arch. Dermatol. Res. 2018, 310, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Charlton, O.; Smith, S.D. Hidradenitis suppurativa and metabolic syndrome—Systematic review and adjusted meta-analysis. Int. J. Dermatol. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Kul, S.; Savaş, E.; Öztürk, Z.A.; Karadağ, G. Does Ramadan fasting alter body weight and blood lipids and fasting blood glucose in a healthy population? A meta-analysis. J. Relig. Health 2014, 53, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.K.; Kumar, V.; Geyfman, M.; Chudova, D.; Ihler, A.T.; Smyth, P.; Paus, R.; Takahashi, J.S.; Andersen, B. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet. 2009, 5, e1000573. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Kondo, N.; Shibasaki, M.; Takano, S.; Tominaga, H.; Katsuura, T. Circadian variation of sweating responses to passive heat stress. Acta Physiol. Scand. 1997, 161, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.; Mariottoni, P.; Corcoran, D.L.; Kirshner, H.F.; Jaleel, T.; Brown, D.A.; Brooks, S.R.; Murray, J.; Morasso, M.I.; MacLeod, A.S. The skin transcriptome in hidradenitis suppurativa uncovers an antimicrobial and sweat gland gene signature which has distinct overlap with wounded skin. PLoS ONE 2019, 14, e0216249. [Google Scholar] [CrossRef]
- Schlapbach, C.; Hänni, T.; Yawalkar, N.; Hunger, R.E. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2011, 65, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Moran, B.; Sweeney, C.M.; Hughes, R.; Malara, A.; Kirthi, S.; Tobin, A.M.; Kirby, B.; Fletcher, J.M. Hidradenitis suppurativa is characterized by dysregulation of the Th17: Treg cell axis, which is corrected by anti-TNF therapy. J. Invest. Dermatol. 2017, 137, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Thomi, R.; Cazzaniga, S.; Seyed Jafari, S.M.; Schlapbach, C.; Hunger, R.E. Association of hidradenitis suppurativa with T helper 1/T helper 17 phenotypes: A semantic map analysis. JAMA Dermatol. 2018, 154, 592–595. [Google Scholar] [CrossRef]
- Vossen, A.R.J.V.; van der Zee, H.H.; Prens, E.P. Hidradenitis suppurativa: A systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front. Immunol. 2018, 9, 2965. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Van Straalen, K.R.; van der Zee, H.H. Lack of photographic documentation undermines assessment of hidradenitis suppurativaphenotypes. Br. J. Dermatol. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Heitmann, B.L.; Andersen, K.W.; Nielsen, O.H.; Sørensen, S.B.; Jawhara, M.; Bygum, A.; Hvid, L.; Grauslund, J.; Wied, J.; et al. Impact of red and processed meat and fibre intake on treatment outcomes among patients with chronic inflammatory diseases: protocol for a prospective cohort study of prognostic factors and personalised medicine. BMJ Open 2018, 8, e018166. [Google Scholar] [CrossRef] [PubMed]
- Andersen, V.; Holmskov, U.; Sørensen, S.B.; Jawhara, M.; Andersen, K.W.; Bygum, A.; Hvid, L.; Grauslund, J.; Wied, J.; Glerup, H.; et al. A proposal for a study on treatment selection and lifestyle recommendations in chronic inflammatory diseases: A Danish multidisciplinary collaboration on prognostic factors and personalised medicine. Nutrients 2017, 9, 499. [Google Scholar] [CrossRef] [PubMed]




| Independent Variables | Coefficient | Standard Error | t | p-Value |
|---|---|---|---|---|
| (Constant) | −3.03 | |||
| ADDI score | 0.16 | 0.09 | 1.79 | 0.0795 |
| Hurley score | 0.66 | 0.11 | 5.93 | <0.0001 |
| Disease duration (years) | 0.02 | 0.01 | 1.65 | 0.1055 |
| Gender | 0.21 | 0.18 | 1.15 | 0.2579 |
| Change in weight | 0.12 | 0.12 | 1.00 | 0.3205 |
| Parameter | B | Standard Deviation | 95% CI Wald | Wald’s Chi-Squared | p-Value | Exp(B) | 95% CI Exp(B) | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | ||||||
| (Intercecpt) | 0.03 | 0.40 | −0.76 | 0.82 | 0.00 | 0.950 | 1.03 | 0.47 | 2.26 |
| No family history of HS | 0.62 | 0.16 | 0.30 | 0.93 | 14.50 | 0.000 | 1.85 | 1.35 | 2.54 |
| HS phenotype (vs. syndromic type) | |||||||||
| Conglobata type | −1.51 | 0.30 | −2.09 | −0.92 | 25.44 | 0.000 | 0.22 | 0.12 | 0.40 |
| Ectopic type | −1.03 | 0.28 | −1.58 | −0.49 | 13.96 | 0.000 | 0.36 | 0.21 | 0.61 |
| Frictional foruncle type | −1.33 | 0.27 | −1.87 | −0.79 | 23.57 | 0.000 | 0.27 | 0.16 | 0.45 |
| Regular type | −0.79 | 0.23 | −1.24 | −0.33 | 11.55 | 0.001 | 0.46 | 0.29 | 0.72 |
| Scarring folliculitis type | −1.11 | 0.25 | −1.60 | −0.62 | 19.72 | 0.000 | 0.33 | 0.20 | 0.54 |
| Disease duration | 0.02 | 0.01 | −0.01 | 0.04 | 2.15 | 0.143 | 1.02 | 1.00 | 1.04 |
| Hurley score | 0.44 | 0.09 | 0.25 | 0.62 | 21.35 | 0.000 | 1.55 | 1.29 | 1.86 |
| Change in weight | −0.13 | 0.11 | −0.34 | 0.08 | 1.42 | 0.234 | 0.88 | 0.71 | 1.09 |
| Parameter | B | Standard Deviation | Wald 95% CI | Wald’s Chi-Squared | p-Value | Exp(B) | 95% CI Exp(B) | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | ||||||
| (Intercept) | −0.86 | 0.38 | −1.59 | −0.12 | 5.22 | 0.022 | 0.42 | 0.20 | 0.89 |
| No family history of HS | 0.14 | 0.13 | −0.11 | 0.40 | 1.18 | 0.278 | 1.15 | 0.89 | 1.49 |
| HS phenotype (vs. syndromic type) | |||||||||
| Conglobata type | 0.33 | 0.22 | −0.11 | 0.76 | 2.15 | 0.143 | 1.38 | 0.90 | 2.14 |
| Ectopic type | −0.17 | 0.51 | −1.17 | 0.83 | 0.11 | 0.741 | 0.84 | 0.31 | 2.30 |
| Frictional foruncle type | 0.95 | 0.20 | 0.56 | 1.34 | 22.59 | 0.000 | 2.59 | 1.75 | 3.83 |
| Regular type | −0.09 | 0.22 | −0.53 | 0.34 | 0.18 | 0.668 | 0.91 | 0.59 | 1.40 |
| Scarring folliculitis type | 0.71 | 0.18 | 0.36 | 1.05 | 15.99 | 0.000 | 2.03 | 1.43 | 2.87 |
| Non biologics | |||||||||
| Topical antibiotics | −0.47 | 0.28 | −1.02 | 0.07 | 2.96 | 0.085 | 0.62 | 0.36 | 1.07 |
| Systemic antibiotics | −0.08 | 0.13 | −0.34 | 0.18 | 0.38 | 0.539 | 0.92 | 0.71 | 1.20 |
| Hurley score | 0.59 | 0.12 | 0.36 | 0.82 | 25.67 | 0.000 | 1.80 | 1.44 | 2.27 |
| Change in weight | −0.04 | 0.06 | −0.17 | 0.08 | 0.47 | 0.493 | 0.96 | 0.85 | 1.09 |
| Parameter | B | Standard Deviation | Wald’s 95% CI | Wald’s Chi-Squared | p-Value | Exp(B) | 95% CI Exp(B) | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | ||||||
| (Intercept) | 0.71 | 0.30 | 0.11 | 1.30 | 5.44 | 0.020 | 2.02 | 1.12 | 3.66 |
| No family history of HS | −0.01 | 0.13 | −0.26 | 0.24 | 0.01 | 0.928 | 0.99 | 0.77 | 1.27 |
| HS phenotype | |||||||||
| Conglobata type | −0.45 | 0.24 | −0.91 | 0.02 | 3.55 | 0.060 | 0.64 | 0.40 | 1.02 |
| Ectopic type | −0.73 | 0.20 | −1.12 | −0.33 | 12.87 | 0.000 | 0.48 | 0.33 | 0.72 |
| Frictional foruncle type | −1.19 | 0.16 | −1.51 | −0.87 | 53.30 | 0.000 | 0.30 | 0.22 | 0.42 |
| Regular type | −0.77 | 0.17 | −1.11 | −0.43 | 20.17 | 0.000 | 0.46 | 0.33 | 0.65 |
| Scarring folliculitis type | −1.29 | 0.16 | −1.60 | −0.97 | 64.93 | 0.000 | 0.28 | 0.20 | 0.38 |
| Non biologics | |||||||||
| Topical antibiotics | −0.05 | 0.19 | −0.43 | 0.33 | 0.06 | 0.804 | 0.95 | 0.65 | 1.39 |
| Systemic antibiotics | 0.07 | 0.12 | −0.16 | 0.30 | 0.37 | 0.543 | 1.07 | 0.85 | 1.35 |
| Hurley score | 0.23 | 0.09 | 0.05 | 0.41 | 6.35 | 0.012 | 1.26 | 1.05 | 1.51 |
| Change in weight | 0.09 | 0.09 | −0.08 | 0.25 | 0.98 | 0.322 | 1.09 | 0.92 | 1.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiani, G.; Mahroum, N.; Pigatto, P.D.M.; Pacifico, A.; Malagoli, P.; Tiodorovic, D.; Conic, R.R.; Amital, H.; Bragazzi, N.L.; Watad, A.; et al. The Safety and Impact of a Model of Intermittent, Time-Restricted Circadian Fasting (“Ramadan Fasting”) on Hidradenitis Suppurativa: Insights from a Multicenter, Observational, Cross-Over, Pilot, Exploratory Study. Nutrients 2019, 11, 1781. https://doi.org/10.3390/nu11081781
Damiani G, Mahroum N, Pigatto PDM, Pacifico A, Malagoli P, Tiodorovic D, Conic RR, Amital H, Bragazzi NL, Watad A, et al. The Safety and Impact of a Model of Intermittent, Time-Restricted Circadian Fasting (“Ramadan Fasting”) on Hidradenitis Suppurativa: Insights from a Multicenter, Observational, Cross-Over, Pilot, Exploratory Study. Nutrients. 2019; 11(8):1781. https://doi.org/10.3390/nu11081781
Chicago/Turabian StyleDamiani, Giovanni, Naim Mahroum, Paolo Daniele Maria Pigatto, Alessia Pacifico, Piergiorgio Malagoli, Danica Tiodorovic, Rosalynn RZ Conic, Howard Amital, Nicola Luigi Bragazzi, Abdulla Watad, and et al. 2019. "The Safety and Impact of a Model of Intermittent, Time-Restricted Circadian Fasting (“Ramadan Fasting”) on Hidradenitis Suppurativa: Insights from a Multicenter, Observational, Cross-Over, Pilot, Exploratory Study" Nutrients 11, no. 8: 1781. https://doi.org/10.3390/nu11081781
APA StyleDamiani, G., Mahroum, N., Pigatto, P. D. M., Pacifico, A., Malagoli, P., Tiodorovic, D., Conic, R. R., Amital, H., Bragazzi, N. L., Watad, A., & Adawi, M. (2019). The Safety and Impact of a Model of Intermittent, Time-Restricted Circadian Fasting (“Ramadan Fasting”) on Hidradenitis Suppurativa: Insights from a Multicenter, Observational, Cross-Over, Pilot, Exploratory Study. Nutrients, 11(8), 1781. https://doi.org/10.3390/nu11081781








