Corticotropin Releasing Factor Type 1 and 2 Receptor Signaling in the Medial Prefrontal Cortex Modulates Binge-Like Ethanol Consumption in C57BL/6J Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. “Drinking in the Dark” Procedures
2.3. Open Field Procedures
2.4. Surgeries & Drug Administration
2.5. Statistical Analysis
3. Results
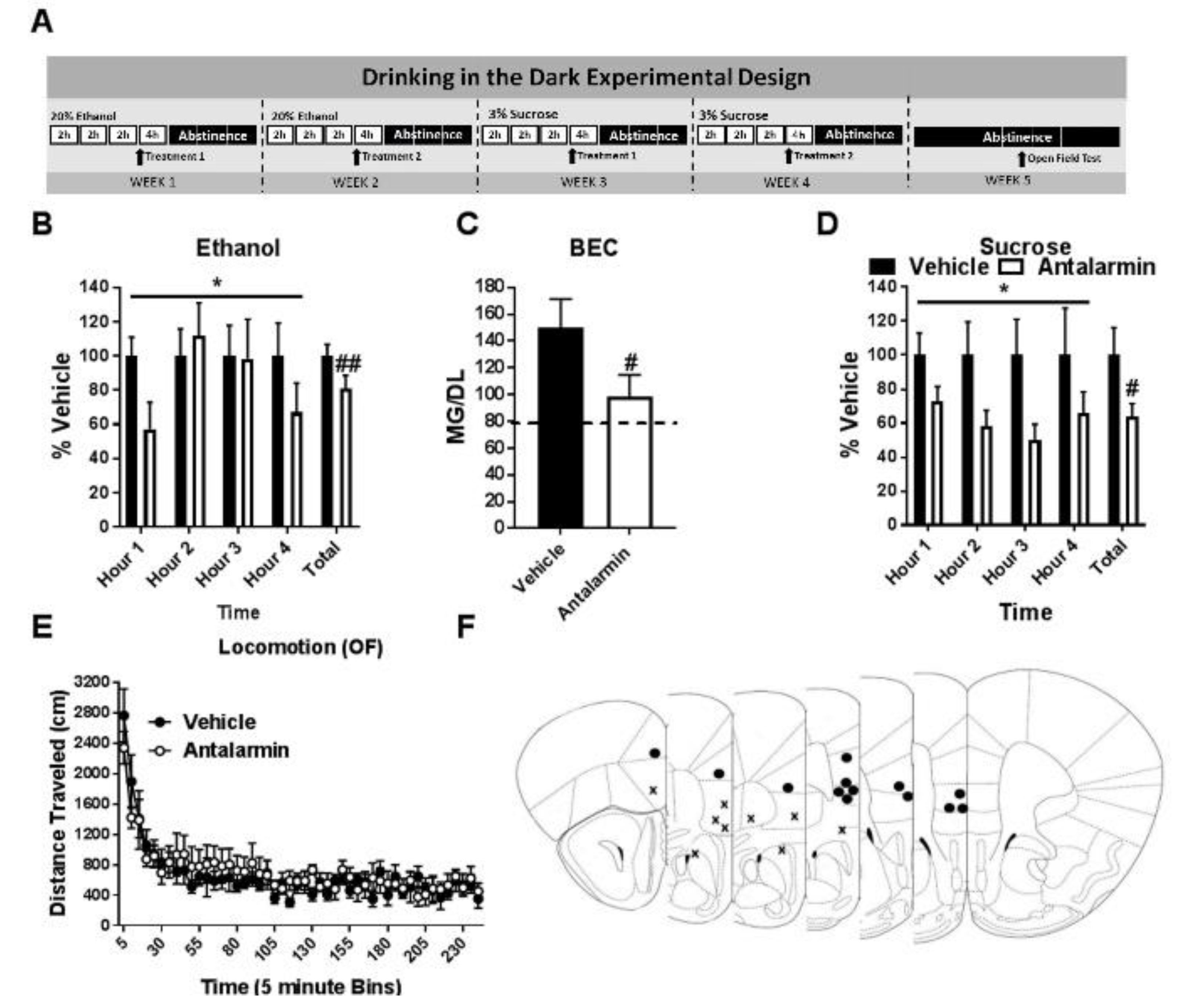
3.1. Pharmacological Inhibition of CRF1R Selectively Reduces Binge-Like Ethanol Consumption
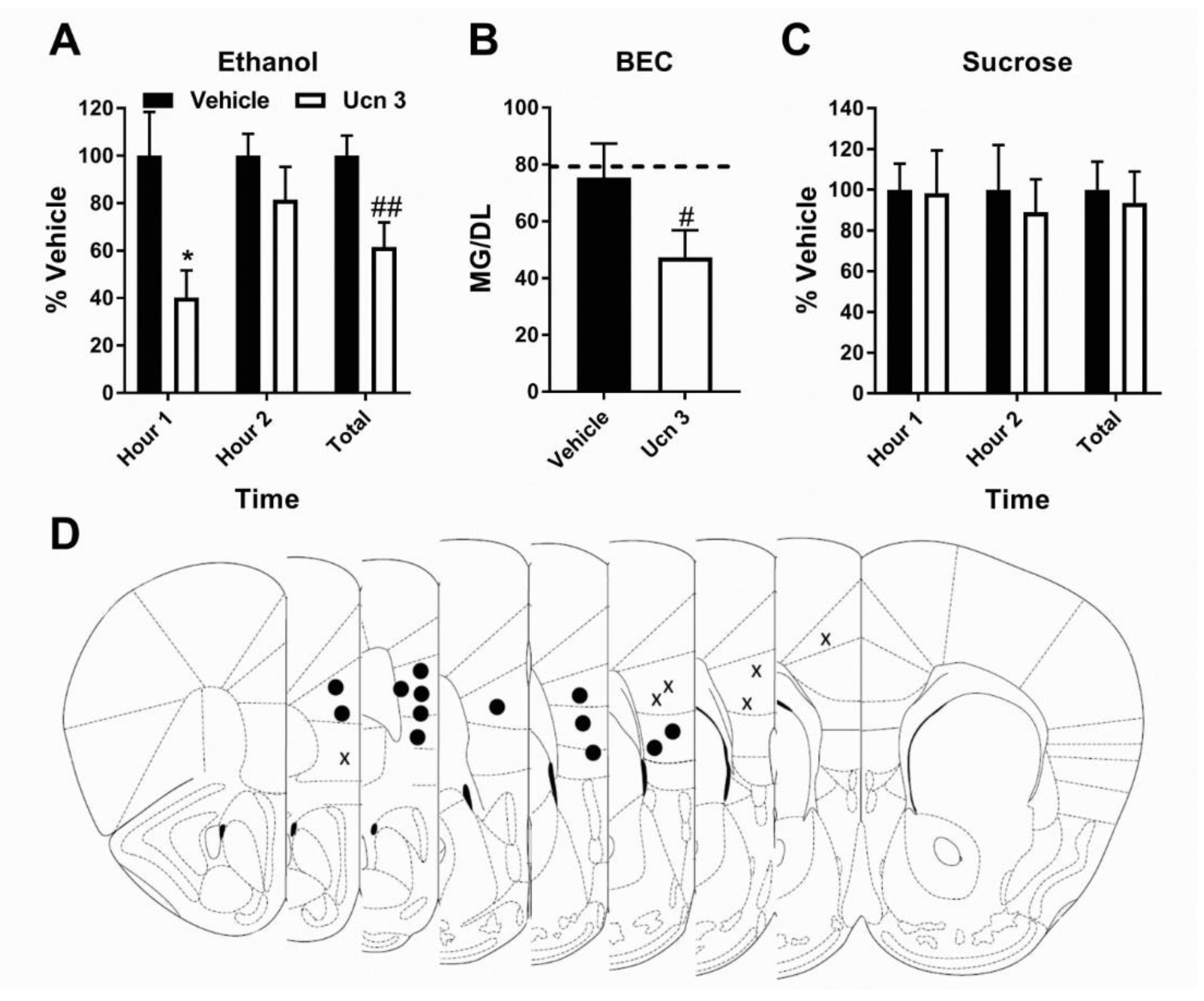
3.2. Pharmacological Activation of CRF2R Selectively Reduces Binge-Like Ethanol Consumption
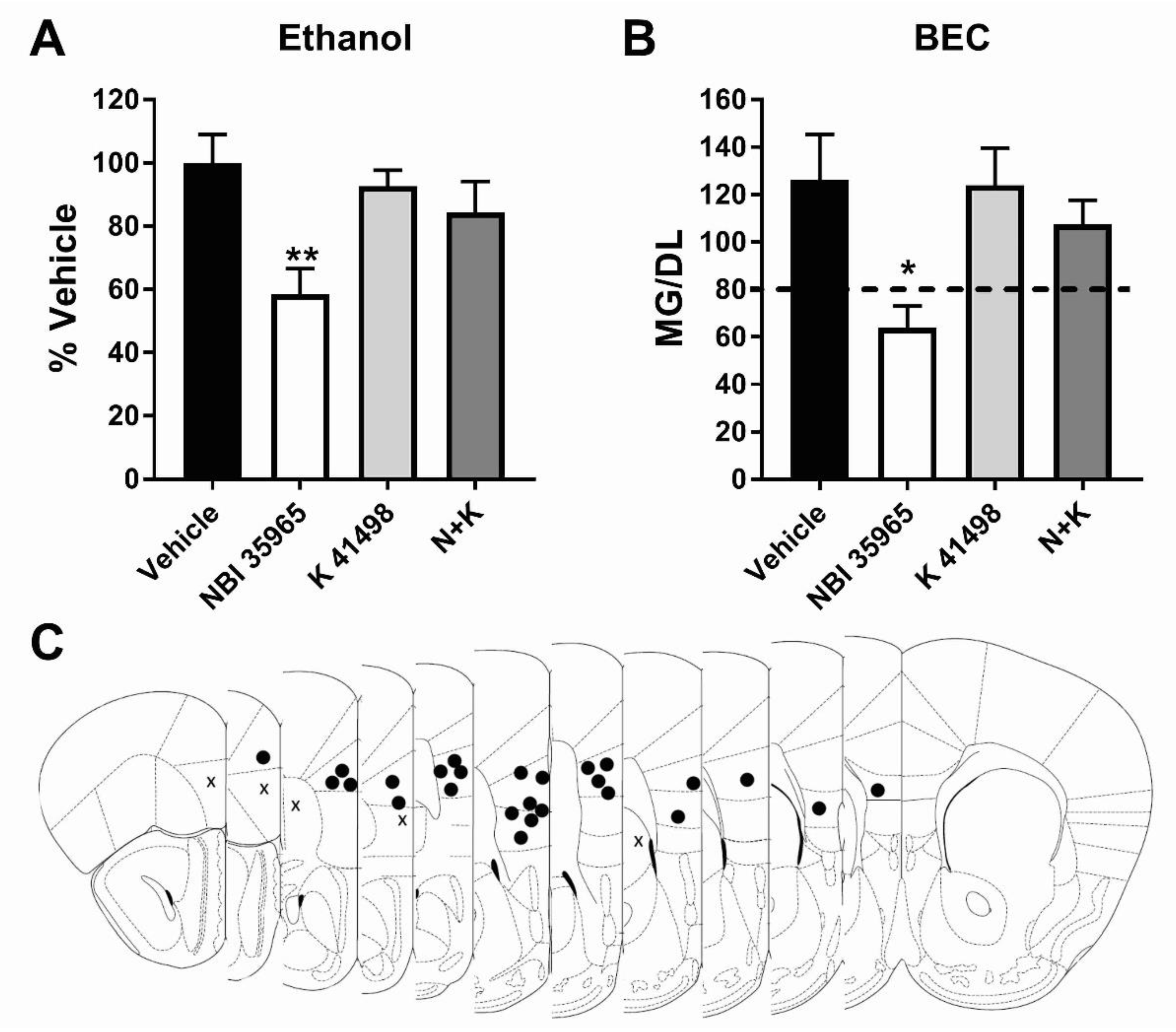
3.3. Co-Antagonism of mPFC CRF2R Attenuates mPFC CRF1R Antagonist Induced Reduction of Binge-Like Ethanol Consumption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waller, M.; McGuire, A.C.; Dobson, A.J. Alcohol use in the military: Associations with health and wellbeing. Subst. Abuse Treat Prev. Policy 2015, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, P.C.; Bestrashniy, J.R.; Nelson, T.F. Problematic Drinking Among Postgraduate Students: Binge Drinking, Prepartying, and Mixing Alcohol with Energy Drinks. Subst. Use Misuse 2016, 51, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Esser, M.B.; Hedden, S.L.; Kanny, D.; Brewer, R.D.; Gfroerer, J.C.; Naimi, T.S. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev. Chronic. Dis. 2014, 11, E206. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.Z.; Russell, M.; Stranges, S.; Dorn, J.; Trevisan, M. Association of lifetime alcohol drinking trajectories with cardiometabolic risk. J. Clin. Endocrinol. Metab. 2008, 93, 154–161. [Google Scholar] [CrossRef]
- Hingson, R.; Heeren, T.; Winter, M.; Wechsler, H. Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24: Changes from 1998 to 2001. Annu. Rev. Public Health 2005, 26, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Naimi, T.S.; Brewer, R.D.; Jones, S.E. Binge drinking and associated health risk behaviors among high school students. Pediatrics 2007, 119, 76–85. [Google Scholar] [CrossRef]
- Thiele, T.E.; Crabbe, J.C.; Boehm, S.L. “Drinking in the Dark” (DID): A simple mouse model of binge-like alcohol intake. Curr. Protoc. Neurosci. 2014, 68, 9–49. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Ford, M.M.; Yu, C.H.; Brown, L.L.; Finn, D.A.; Garland, T., Jr.; Crabbe, J.C. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007, 6, 1–18. [Google Scholar] [CrossRef]
- Zorrilla, E.P.; Logrip, M.L.; Koob, G.F. Corticotropin releasing factor: A key role in the neurobiology of addiction. Front. Neuroendocrinol. 2014, 35, 234–244. [Google Scholar] [CrossRef]
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981, 213, 1394–1397. [Google Scholar] [CrossRef]
- Van Pett, K.; Viau, V.; Bittencourt, J.C.; Chan, R.K.; Li, H.Y.; Arias, C.; Prins, G.S.; Perrin, M.; Vale, W.; Sawchenko, P.E. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000, 428, 191–212. [Google Scholar] [CrossRef]
- Slater, P.G.; Yarur, H.E.; Gysling, K. Corticotropin-Releasing Factor Receptors and Their Interacting Proteins: Functional Consequences. Mol. Pharmacol. 2016, 90, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Lowery, E.G.; Spanos, M.; Navarro, M.; Lyons, A.M.; Hodge, C.W.; Thiele, T.E. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology 2010, 35, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Lowery, E.G.; Thiele, T.E. Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol. Disord. Drug Targets 2010, 9, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lowery-Gionta, E.G.; Navarro, M.; Li, C.; Pleil, K.E.; Rinker, J.A.; Cox, B.R.; Sprow, G.M.; Kash, T.L.; Thiele, T.E. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J. Neurosci. 2012, 32, 3405–3413. [Google Scholar] [CrossRef]
- Rinker, J.A.; Marshall, S.A.; Mazzone, C.M.; Lowery-Gionta, E.G.; Gulati, V.; Pleil, K.E.; Kash, T.L.; Navarro, M.; Thiele, T.E. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol. Psychiatry 2016, 81, 930–940. [Google Scholar] [CrossRef]
- Koob, G.F. Theoretical frameworks and mechanistic aspects of alcohol addiction: Alcohol addiction as a reward deficit disorder. Curr. Top Behav. Neurosci. 2013, 13, 3–30. [Google Scholar]
- Gilpin, N.W.; Herman, M.A.; Roberto, M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol. Psychiatry 2015, 77, 859–869. [Google Scholar] [CrossRef]
- Roberto, M.; Gilpin, N.W.; Siggins, G.R. The central amygdala and alcohol: Role of γ-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb. Perspect. Med. 2012, 2, a012195. [Google Scholar] [CrossRef]
- Ketchesin, K.D.; Stinnett, G.S.; Seasholtz, A.F. Binge Drinking Decreases Corticotropin-Releasing Factor-Binding Protein Expression in the Medial Prefrontal Cortex of Mice. Alcohol Clin. Exp. Res. 2016, 40, 1641–1650. [Google Scholar] [CrossRef]
- Gass, J.T.; Chandler, L.J. The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning. Front. Psychiatry 2013, 4, 46. [Google Scholar] [CrossRef]
- Abernathy, K.; Chandler, L.J.; Woodward, J.J. Alcohol and the prefrontal cortex. Int. Rev. Neurobiol. 2010, 91, 289–320. [Google Scholar]
- Klenowski, P.M. Emerging role for the medial prefrontal cortex in alcohol-seeking behaviors. Addict. Behav. 2018, 77, 102–106. [Google Scholar] [CrossRef]
- Lu, Y.L.; Richardson, H.N. Alcohol, stress hormones, and the prefrontal cortex: A proposed pathway to the dark side of addiction. Neuroscience 2014, 277, 139–151. [Google Scholar] [CrossRef]
- Kubota, Y.; Shigematsu, N.; Karube, F.; Sekigawa, A.; Kato, S.; Yamaguchi, N.; Hirai, Y.; Morishima, M.; Kawaguchi, Y. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb. Cortex 2011, 21, 1803–1817. [Google Scholar] [CrossRef]
- Kubota, Y.; Karube, F.; Nomura, M.; Kawaguchi, Y. The Diversity of Cortical Inhibitory Synapses. Front. Neural Circuits 2016, 10, 27. [Google Scholar] [CrossRef]
- Hupalo, S.; Bryce, C.A.; Bangasser, D.A.; Berridge, C.W.; Valentino, R.J.; Floresco, S.B. Corticotropin-Releasing Factor (CRF) circuit modulation of cognition and motivation. Neurosci. Biobehav. Rev. 2019, 103, 50–59. [Google Scholar] [CrossRef]
- Kirby, L.G.; Freeman-Daniels, E.; Lemos, J.C.; Nunan, J.D.; Lamy, C.; Akanwa, A.; Beck, S.G. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J. Neurosci. 2008, 28, 12927–12937. [Google Scholar] [CrossRef]
- Ohata, H.; Shibasaki, T. Microinjection of different doses of corticotropin-releasing factor into the medial prefrontal cortex produces effects opposing anxiety-related behavior in rats. J. Nippon. Med. Sch. 2011, 78, 286–292. [Google Scholar] [CrossRef]
- Lovenberg, T.W.; Liaw, C.W.; Grigoriadis, D.E.; Clevenger, W.; Chalmers, D.T.; De Souza, E.B.; Oltersdorf, T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. USA 1995, 92, 836–840. [Google Scholar] [CrossRef]
- Carboni, L.; Romoli, B.; Bate, S.T.; Romualdi, P.; Zoli, M. Increased expression of CRF and CRF-receptors in dorsal striatum, hippocampus, and prefrontal cortex after the development of nicotine sensitization in rats. Drug Alcohol Depend. 2018, 189, 12–20. [Google Scholar] [CrossRef]
- Guan, X.; Wan, R.; Zhu, C.; Li, S. Corticotropin-releasing factor receptor type-2 is involved in the cocaine-primed reinstatement of cocaine conditioned place preference in rats. Behav. Brain Res. 2014, 258, 90–96. [Google Scholar] [CrossRef]
- Robinson, S.L.; Marrero, I.M.; Perez-Heydrich, C.A.; Sepulveda-Orengo, M.T.; Reissner, K.J.; Thiele, T.E. Medial prefrontal cortex neuropeptide Y modulates binge-like ethanol consumption in C57BL/6J mice. Neuropsychopharmacology 2019, 44, 1132–1140. [Google Scholar] [CrossRef]
- Marshall, S.A.; McKnight, K.H.; Blose, A.K.; Lysle, D.T.; Thiele, T.E. Modulation of Binge-like Ethanol Consumption by IL-10 Signaling in the Basolateral Amygdala. J. Neuroimmune Pharmacol. 2016. [Google Scholar] [CrossRef]
- Pleil, K.E.; Rinker, J.A.; Lowery-Gionta, E.G.; Mazzone, C.M.; McCall, N.M.; Kendra, A.M.; Olson, D.P.; Lowell, B.B.; Grant, K.A.; Thiele, T.E.; et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat. Neurosci. 2015, 18, 545–552. [Google Scholar] [CrossRef]
- Cox, B.R.; Olney, J.J.; Lowery-Gionta, E.G.; Sprow, G.M.; Rinker, J.A.; Navarro, M.; Kash, T.L.; Thiele, T.E. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin. Exp. Res. 2013, 37, 1688–1695. [Google Scholar] [CrossRef]
- Sparrow, A.M.; Lowery-Gionta, E.G.; Pleil, K.E.; Li, C.; Sprow, G.M.; Cox, B.R.; Rinker, J.A.; Jijon, A.M.; Peňa, J.; Navarro, M.; et al. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacology 2012, 37, 1409–1421. [Google Scholar] [CrossRef]
- Gilpin, N.W. Neuropeptide Y (NPY) in the extended amygdala is recruited during the transition to alcohol dependence. Neuropeptides 2012, 46, 253–259. [Google Scholar] [CrossRef][Green Version]
- Hwa, L.S.; Nathanson, A.J.; Shimamoto, A.; Tayeh, J.K.; Wilens, A.R.; Holly, E.N.; Newman, E.L.; DeBold, J.F.; Miczek, K.A. Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology (Berl) 2015, 232, 2889–2902. [Google Scholar] [CrossRef]
- Moorman, D.E.; James, M.H.; McGlinchey, E.M.; Aston-Jones, G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2015, 1628, 130–146. [Google Scholar] [CrossRef]
- Holmes, A.; Fitzgerald, P.J.; MacPherson, K.P.; DeBrouse, L.; Colacicco, G.; Flynn, S.M.; Masneuf, S.; Pleil, K.E.; Li, C.; Marcinkiewcz, C.A.; et al. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat. Neurosci. 2012, 15, 1359–1361. [Google Scholar] [CrossRef]
- Koob, G.F. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front. Psychiatry 2013, 4, 72. [Google Scholar] [CrossRef]
- Koob, G.F. Negative reinforcement in drug addiction: The darkness within. Curr. Opin. Neurobiol. 2013, 23, 559–563. [Google Scholar] [CrossRef]
- Schreiber, A.L.; Lu, Y.L.; Baynes, B.B.; Richardson, H.N.; Gilpin, N.W. Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology 2017, 113, 323–330. [Google Scholar] [CrossRef]
- Uribe-Mariño, A.; Gassen, N.C.; Wiesbeck, M.F.; Balsevich, G.; Santarelli, S.; Solfrank, B.; Dournes, C.; Fries, G.R.; Masana, M.; Labermeier, C.; et al. Prefrontal Cortex Corticotropin-Releasing Factor Receptor 1 Conveys Acute Stress-Induced Executive Dysfunction. Biol. Psychiatry 2016, 80, 743–753. [Google Scholar]
- Hupalo, S.; Berridge, C.W. Working Memory Impairing Actions of Corticotropin-Releasing Factor (CRF) Neurotransmission in the Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 2733–2740. [Google Scholar] [CrossRef]
- Miguel, T.T.; Gomes, K.S.; Nunes-de-Souza, R.L. Tonic modulation of anxiety-like behavior by corticotropin-releasing factor (CRF) type 1 receptor (CRF1) within the medial prefrontal cortex (mPFC) in male mice: Role of protein kinase A (PKA). Horm. Behav. 2014, 66, 247–256. [Google Scholar] [CrossRef]
- Delli Pizzi, S.; Chiacchiaretta, P.; Mantini, D.; Bubbico, G.; Ferretti, A.; Edden, R.A.; Di Giulio, C.; Onofrj, M.; Bonanni, L. Functional and neurochemical interactions within the amygdala-medial prefrontal cortex circuit and their relevance to emotional processing. Brain Struct. Funct. 2017, 222, 1267–1279. [Google Scholar] [CrossRef]
- Ghosal, S.; Hare, B.; Duman, R.S. Prefrontal Cortex GABAergic Deficits and Circuit Dysfunction in the Pathophysiology and Treatment of Chronic Stress and Depression. Curr. Opin. Behav. Sci. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Jaferi, A.; Bhatnagar, S. Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res. 2007, 1186, 212–223. [Google Scholar] [CrossRef]
- Funk, C.K.; Koob, G.F. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007, 1155, 172–178. [Google Scholar] [CrossRef][Green Version]
- Quadros, I.M.; Macedo, G.C.; Domingues, L.P.; Favoretto, C.A. An Update on CRF Mechanisms Underlying Alcohol Use Disorders and Dependence. Front. Endocrinol. (Lausanne) 2016, 7, 134. [Google Scholar] [CrossRef]
- Crawley, J.N.; Olschowka, J.A.; Diz, D.I.; Jacobowitz, D.M. Behavioral investigation of the coexistence of substance P, corticotropin releasing factor, and acetylcholinesterase in lateral dorsal tegmental neurons projecting to the medial frontal cortex of the rat. Peptides 1985, 6, 891–901. [Google Scholar] [CrossRef]
- Itoga, C.A.; Chen, Y.; Fateri, C.; Echeverry, P.A.; Lai, J.M.; Delgado, J.; Badhon, S.; Short, A.; Baram, T.Z.; Xu, X. New viral-genetic mapping uncovers an enrichment of corticotropin-releasing hormone-expressing neuronal inputs to the nucleus accumbens from stress-related brain regions. J. Comp. Neurol. 2019. [Google Scholar] [CrossRef]
- Lebow, M.A.; Chen, A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 2016, 21, 450–463. [Google Scholar] [CrossRef]
- Valentino, R.J.; Van Bockstaele, E.; Bangasser, D. Sex-specific cell signaling: The corticotropin-releasing factor receptor model. Trends Pharmacol. Sci. 2013, 34, 437–444. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Curtis, A.; Reyes, B.A.; Bethea, T.T.; Parastatidis, I.; Ischiropoulos, H.; Van Bockstaele, E.J.; Valentino, R.J. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: Potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 2010, 15, 896–904. [Google Scholar] [CrossRef]
- Stengel, A.; Taché, Y. CRF and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front. Neurosci. 2014, 8, 52. [Google Scholar] [CrossRef]
- Horst, N.K.; Laubach, M. Reward-related activity in the medial prefrontal cortex is driven by consumption. Front. Neurosci. 2013, 7, 56. [Google Scholar] [CrossRef]
- Reppucci, C.J.; Petrovich, G.D. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: A single and double retrograde tracing study in rats. Brain Struct. Funct. 2016, 221, 2937–2962. [Google Scholar] [CrossRef]
- Delli Pizzi, S.; Chiacchiaretta, P.; Mantini, D.; Bubbico, G.; Edden, R.A.; Onofrj, M.; Ferretti, A.; Bonanni, L. GABA content within medial prefrontal cortex predicts the variability of fronto-limbic effective connectivity. Brain Struct. Funct. 2017, 222, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lv, F.; Yuan, Y.; Fan, C.; Li, J.; Sun, W.; Hu, J. Whole-Brain Mapping of Monosynaptic Afferent Inputs to Cortical CRH Neurons. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, S.L.; Perez-Heydrich, C.A.; Thiele, T.E. Corticotropin Releasing Factor Type 1 and 2 Receptor Signaling in the Medial Prefrontal Cortex Modulates Binge-Like Ethanol Consumption in C57BL/6J Mice. Brain Sci. 2019, 9, 171. https://doi.org/10.3390/brainsci9070171
Robinson SL, Perez-Heydrich CA, Thiele TE. Corticotropin Releasing Factor Type 1 and 2 Receptor Signaling in the Medial Prefrontal Cortex Modulates Binge-Like Ethanol Consumption in C57BL/6J Mice. Brain Sciences. 2019; 9(7):171. https://doi.org/10.3390/brainsci9070171
Chicago/Turabian StyleRobinson, Stacey L., Carlos A. Perez-Heydrich, and Todd E. Thiele. 2019. "Corticotropin Releasing Factor Type 1 and 2 Receptor Signaling in the Medial Prefrontal Cortex Modulates Binge-Like Ethanol Consumption in C57BL/6J Mice" Brain Sciences 9, no. 7: 171. https://doi.org/10.3390/brainsci9070171
APA StyleRobinson, S. L., Perez-Heydrich, C. A., & Thiele, T. E. (2019). Corticotropin Releasing Factor Type 1 and 2 Receptor Signaling in the Medial Prefrontal Cortex Modulates Binge-Like Ethanol Consumption in C57BL/6J Mice. Brain Sciences, 9(7), 171. https://doi.org/10.3390/brainsci9070171




