Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter
Abstract
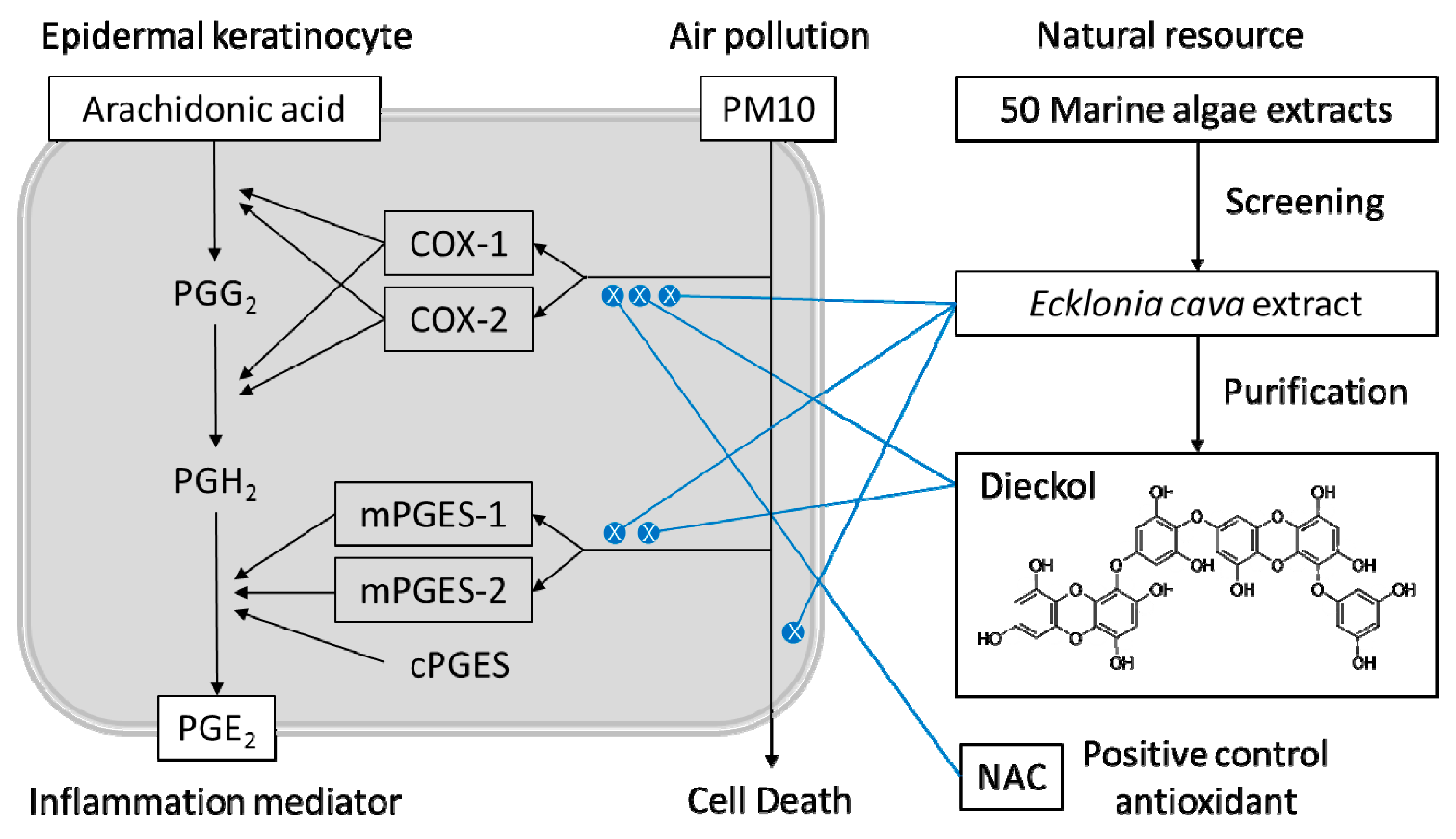
1. Introduction
2. Materials and Methods
2.1. Marine Alga Extracts
2.2. Purification of Dieckol from E. cava
2.3. Instrumental Analysis
2.4. High Performance Liquid Chromatography (HPLC)
2.5. Cell Culture
2.6. Treatment of Cells with PM10
2.7. Cell Viability Assay
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Quantitative Reverse-Transcriptase Polymerase Chain Reaction (qRT-PCR) Analysis
2.10. Assay for Cellular ROS Production
2.11. The 3D-reconstructed Human Skin Models
2.12. Statistical Analysis
3. Results
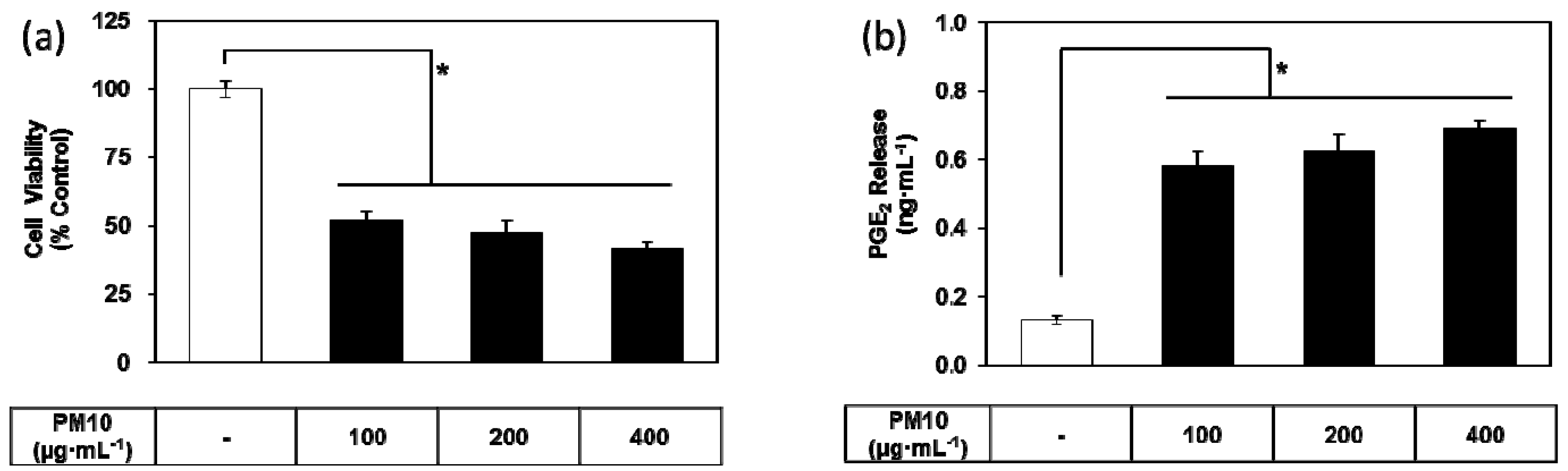
3.1. PM10 Induces Cytotoxicity and PGE2 Release of Keratinocytes
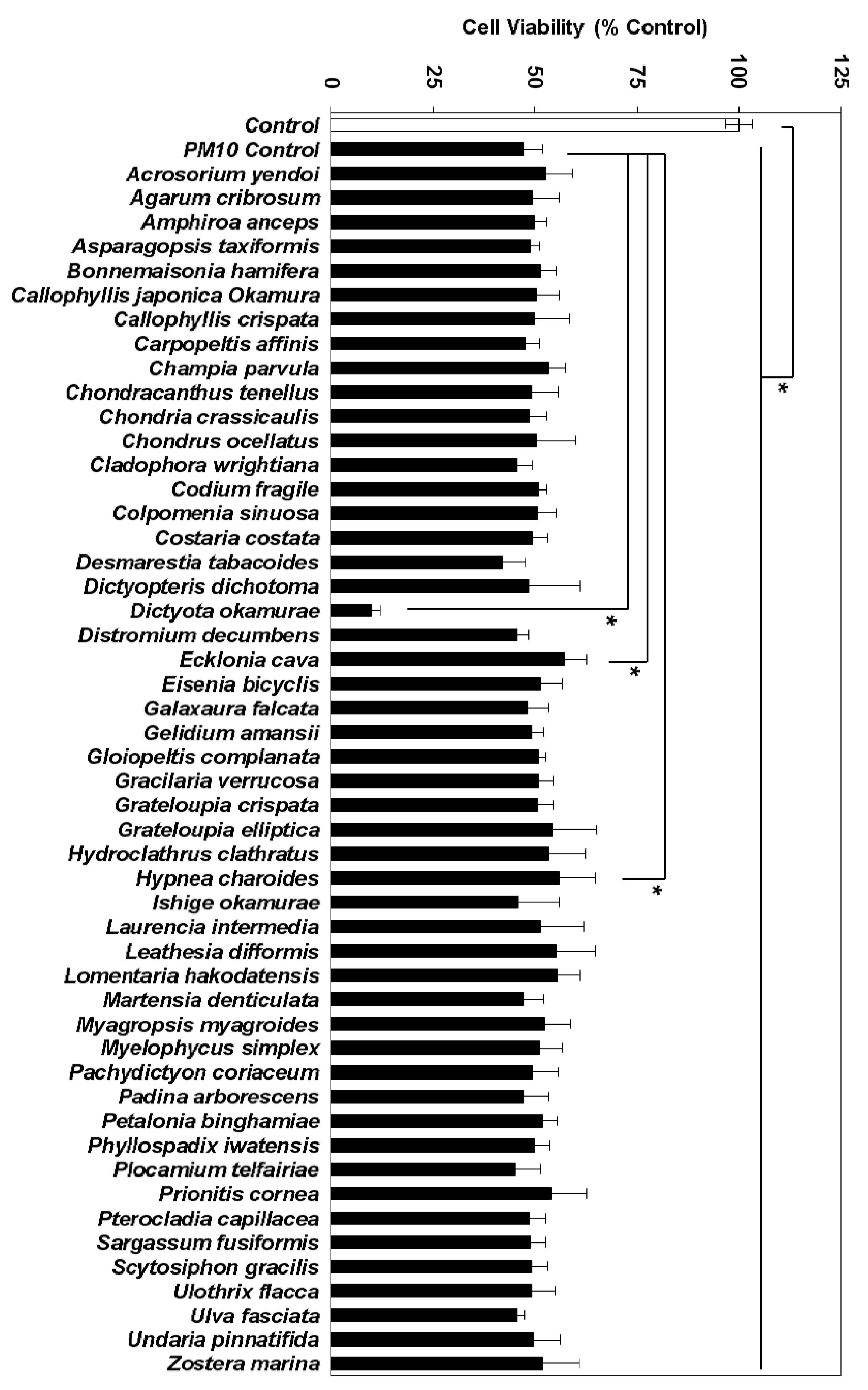
3.2. Effects of Marine Alga Extracts on PM10-induced Cytotoxicity
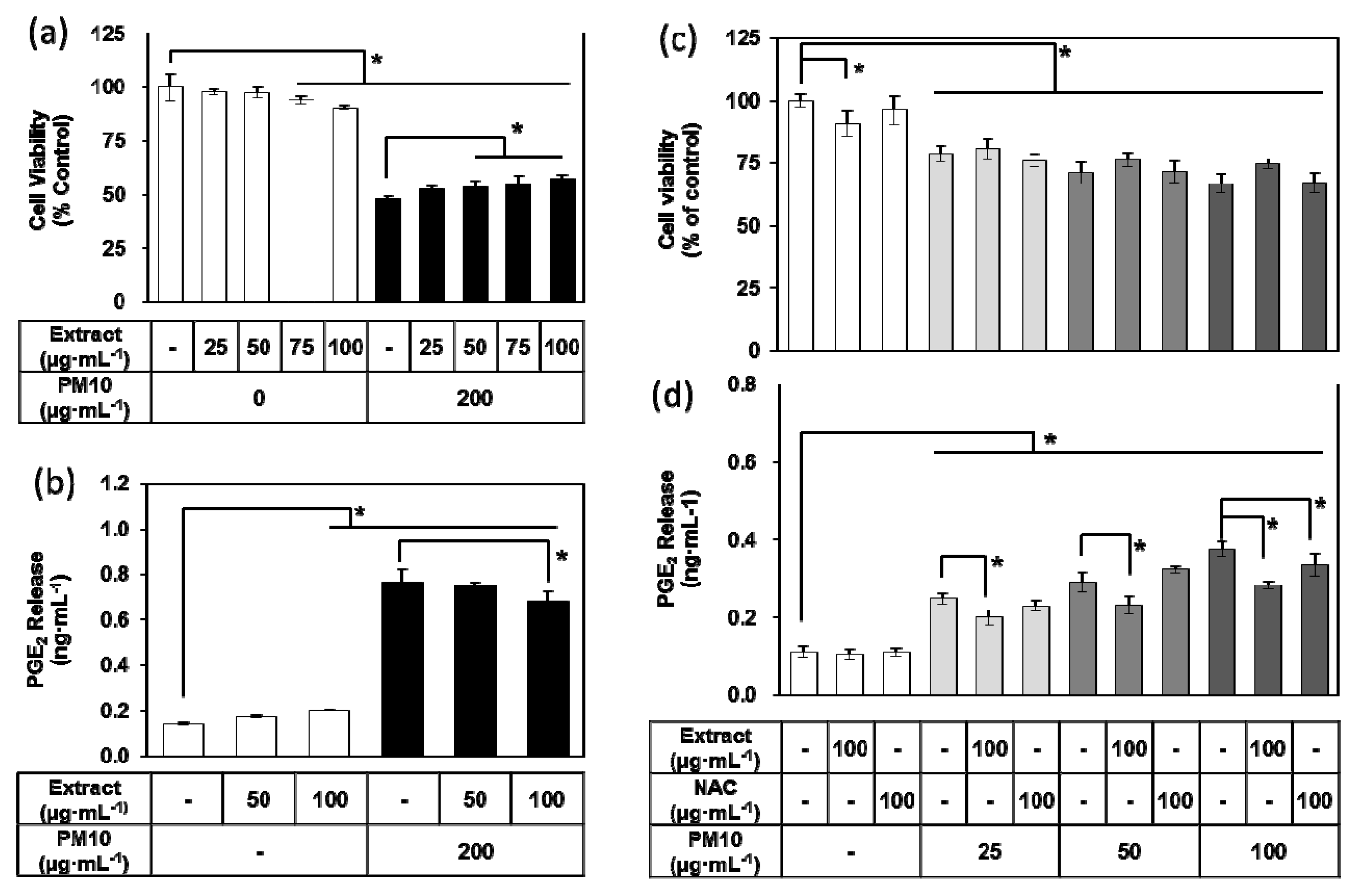
3.3. Effects of E. cava Extract on PM10-induced Cytotoxicity and PGE2 Release
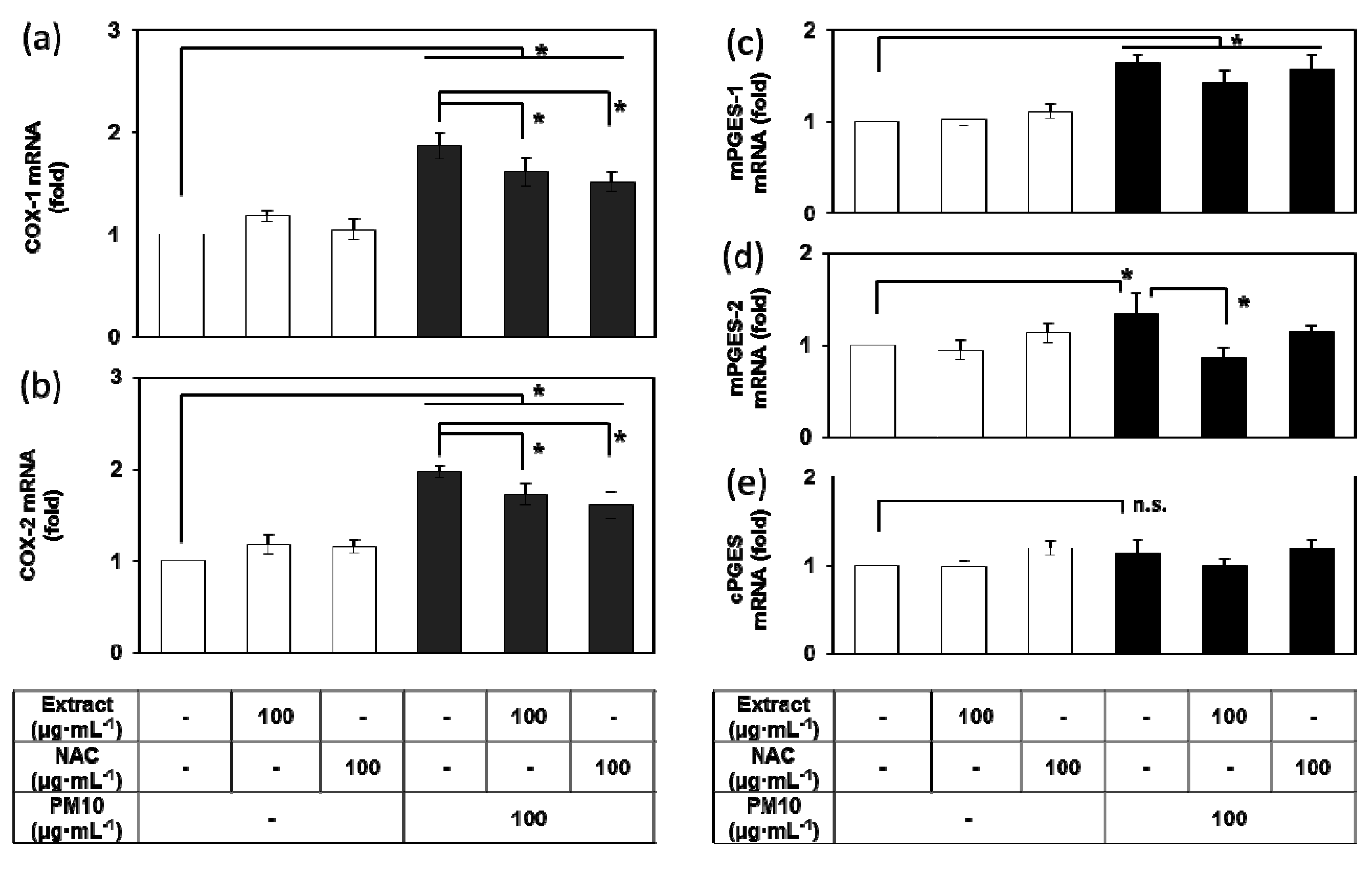
3.4. Effects of E. cava Extract on the PM10-induced Gene Expression of the Enzymes Involved in the PGE2 Synthesis
3.5. Purification of Dieckol from E. cava
3.6. Analysis of Chemical Structure of Dieckol
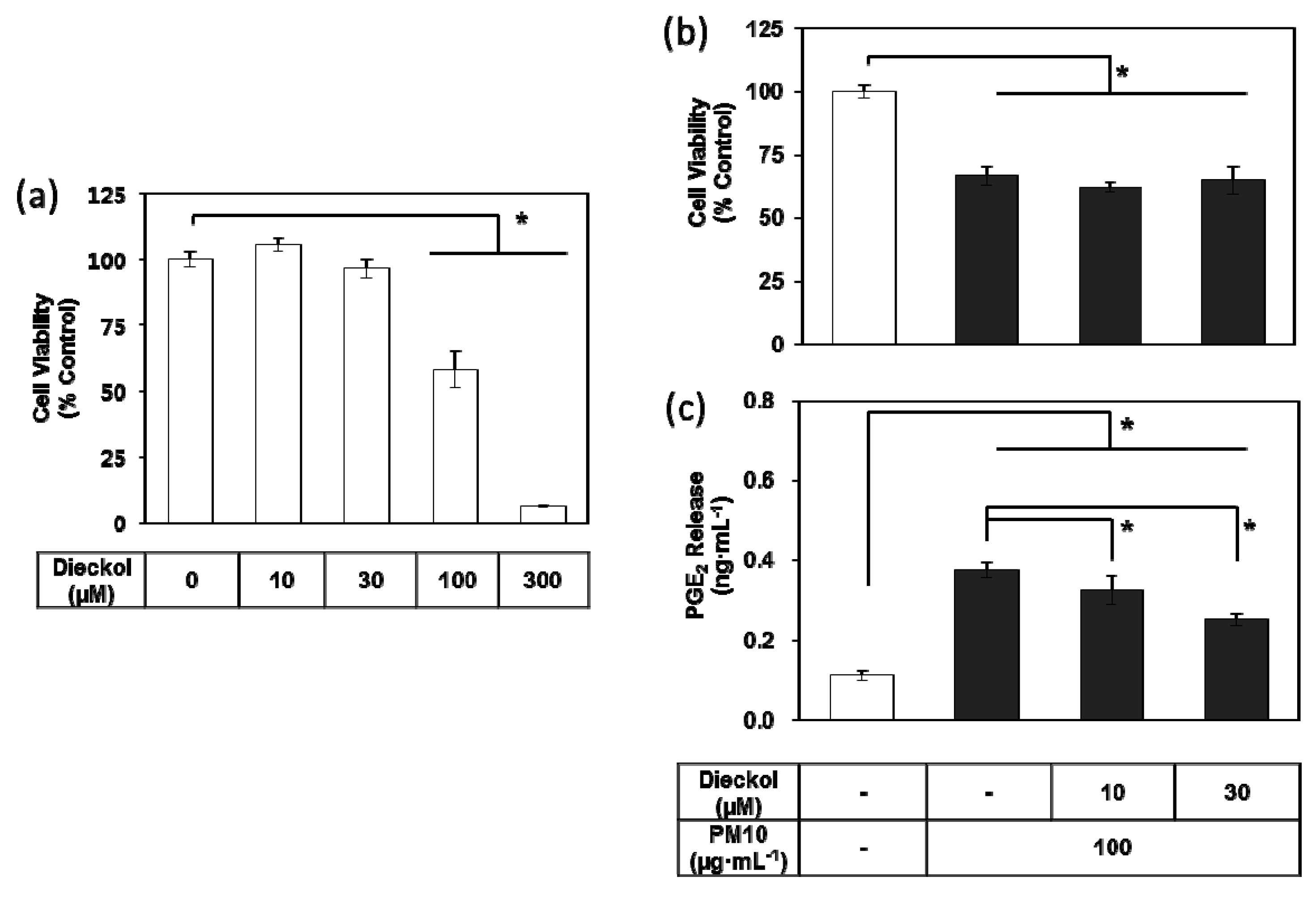
3.7. Effects of Dieckol on PM10-induced Cytotoxicity and PGE2 Release of Keratibnocytes
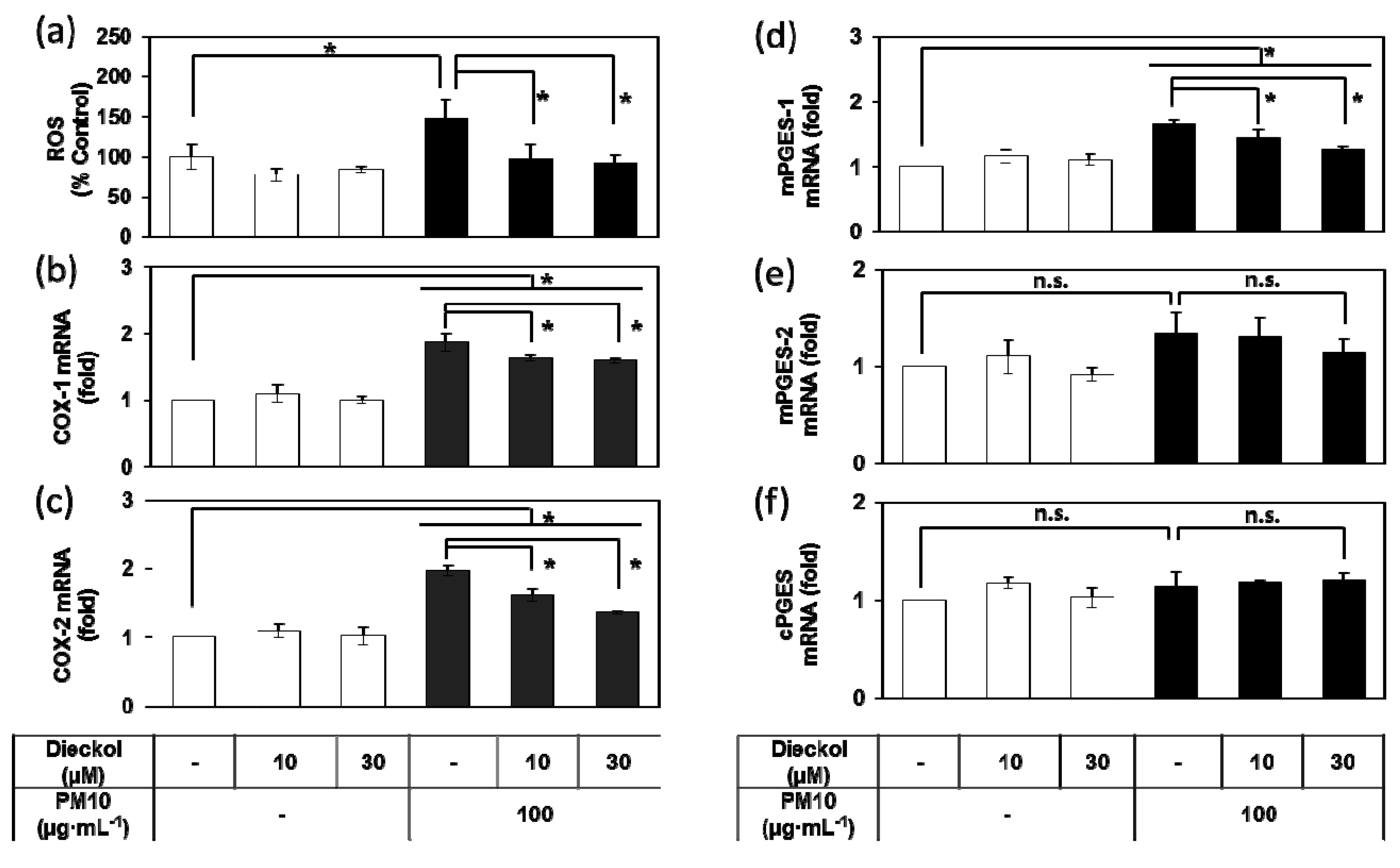
3.8. Effects of Dieckol on the PM10-Induced ROS Production and the PM10-Induced Gene Expression of the Enzymes Involved in the PGE2 Synthesis.
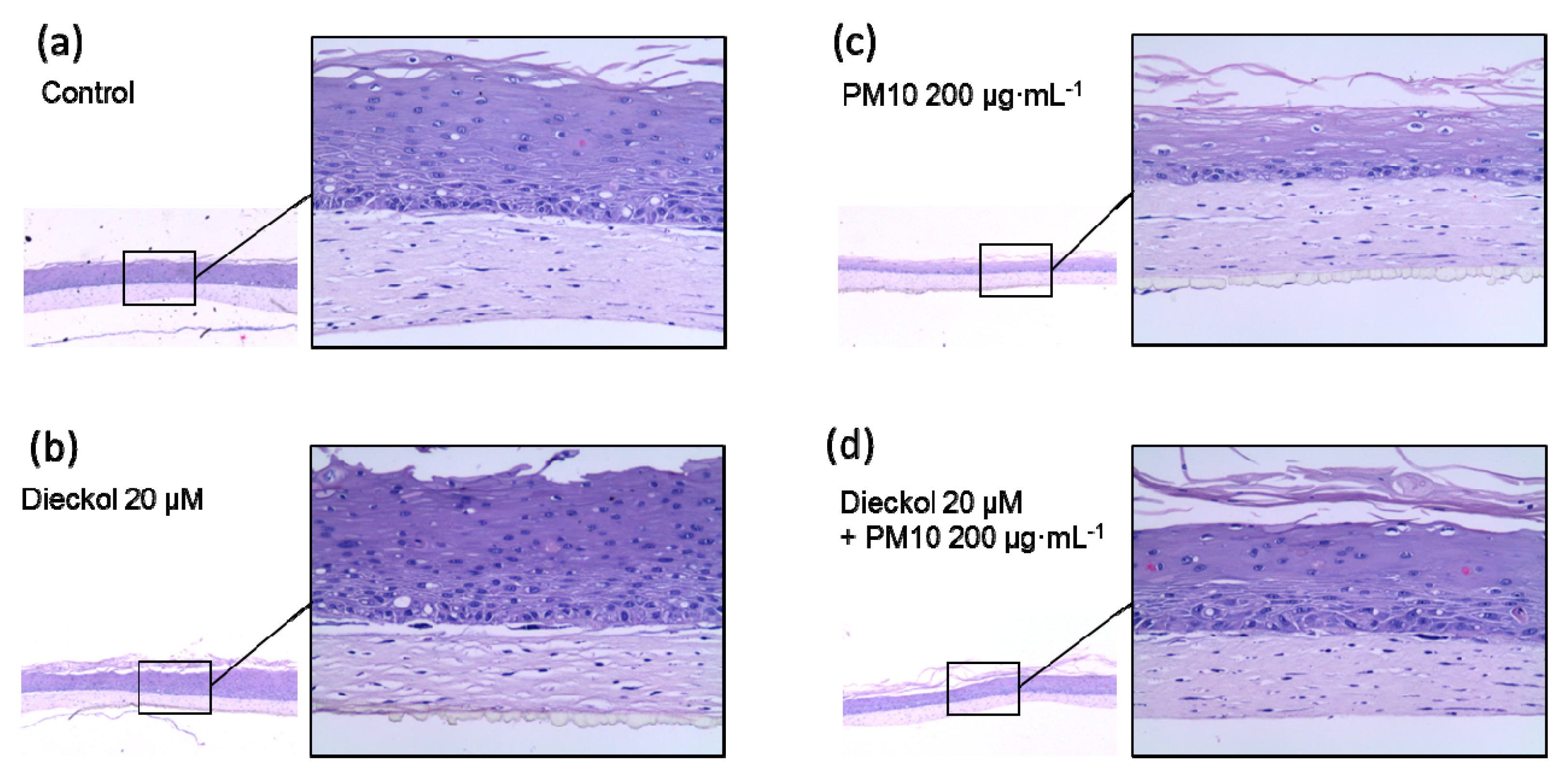
3.9. Protective Effects of Dieckol against PM10 in a 3D-reconstructed Skin Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krzyzanowski, M. WHO air quality guidelines for Europe. J. Toxicol. Environ. Health A 2008, 71, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Y.; Wang, Q.; Liu, T. Toxicity research of PM2.5 compositions in vitro. Int. J. Environ. Res. Public Health 2017, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Vargas, M.P.; Teran, L.M. Air pollution: impact and prevention. Respirology 2012, 17, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [PubMed]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd. Mortality effects of longer term exposures to fine particulate air pollution: Review of recent epidemiological evidence. Inhal. Toxicol. 2007, 19, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, S.; Lefranc, A.; Gault, G.; Chatignoux, E.; Couvy, F.; Jouves, B.; Filleul, L. Are the short-term effects of air pollution restricted to cardiorespiratory diseases? Am. J. Epidemiol. 2009, 169, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Bakke, J.V.; Wieslander, G.; Norback, D.; Moen, B.E. Eczema increases susceptibility to PM10 in office indoor environments. Arch. Environ. Occup. Health 2012, 67, 15–21. [Google Scholar] [CrossRef]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef]
- Pan, T.L.; Wang, P.W.; Aljuffali, I.A.; Huang, C.T.; Lee, C.W.; Fang, J.Y. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015, 78, 51–60. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Vierkotter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Kramer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.E. Pollution as a risk factor for the development of melasma and other skin disorders of facial hyperpigmentation is there a case to be made? J. Drugs Dermatol. 2015, 14, 337–341. [Google Scholar] [PubMed]
- Soeur, J.; Belaidi, J.P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Datzmann, T.; Markevych, I.; Trautmann, F.; Heinrich, J.; Schmitt, J.; Tesch, F. Outdoor air pollution, green space, and cancer incidence in Saxony: A semi-individual cohort study. BMC Public Health 2018, 18, 715. [Google Scholar] [CrossRef]
- Park, S.Y.; Byun, E.J.; Lee, J.D.; Kim, S.; Kim, H.S. Air pollution, autophagy, and skin aging: Impact of particulate matter (PM10) on human dermal fibroblasts. Int. J. Mol. Sci. 2018, 19, 2727. [Google Scholar] [CrossRef]
- Romani, A.; Cervellati, C.; Muresan, X.M.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Benedusi, M.; Evelson, P.; Valacchi, G. Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mech. Ageing Dev. 2018, 172, 86–95. [Google Scholar] [CrossRef]
- Magnani, N.D.; Muresan, X.M.; Belmonte, G.; Cervellati, F.; Sticozzi, C.; Pecorelli, A.; Miracco, C.; Marchini, T.; Evelson, P.; Valacchi, G. Skin damage mechanisms related to airborne particulate matter exposure. Toxicol. Sci. 2016, 149, 227–236. [Google Scholar] [CrossRef]
- Morales-Barcenas, R.; Chirino, Y.I.; Sanchez-Perez, Y.; Osornio-Vargas, A.R.; Melendez-Zajgla, J.; Rosas, I.; Garcia-Cuellar, C.M. Particulate matter (PM10) induces metalloprotease activity and invasion in airway epithelial cells. Toxicol. Lett. 2015, 237, 167–173. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hu, S.C.; Chiang, Y.C.; Hsu, L.F.; Lin, Y.C.; Lee, I.T.; Tsai, M.H.; Fang, J.Y. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016, 6, 27995. [Google Scholar] [CrossRef]
- Alfaro-Moreno, E.; Martinez, L.; Garcia-Cuellar, C.; Bonner, J.C.; Murray, J.C.; Rosas, I.; Rosales, S.P.; Osornio-Vargas, A.R. Biologic effects induced in vitro by PM10 from three different zones of Mexico City. Environ. Health Perspect. 2002, 110, 715–720. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hsu, L.F.; Fang, J.Y.; Chiang, Y.C.; Tsai, M.H.; Lee, M.H.; Li, S.Y.; Hu, S.C.; Lee, I.T.; et al. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants–exposed human keratinocytes through ROS/MAPKs pathways. J. Ethnopharmacol. 2016, 189, 300–309. [Google Scholar] [CrossRef]
- Tsai, M.H.; Hsu, L.F.; Lee, C.W.; Chiang, Y.C.; Lee, M.H.; How, J.M.; Wu, C.M.; Huang, C.L.; Lee, I.T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-kappaB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef]
- Burke, K.E. Mechanisms of aging and development—A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech. Ageing Dev. 2018, 172, 123–130. [Google Scholar] [CrossRef]
- Kim, M.M.; Ta, Q.V.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: an edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Lee, H.; Kang, C.; Jung, E.S.; Kim, J.S.; Kim, E. Antimetastatic activity of polyphenol-rich extract of Ecklonia cava through the inhibition of the Akt pathway in A549 human lung cancer cells. Food Chem. 2011, 127, 1229–1236. [Google Scholar] [CrossRef]
- Choi, J.G.; Kang, O.H.; Brice, O.O.; Lee, Y.S.; Chae, H.S.; Oh, Y.C.; Sohn, D.H.; Park, H.; Choi, H.G.; Kim, S.G.; et al. Antibacterial activity of Ecklonia cava against methicillin-resistant Staphylococcus aureus and Salmonella spp. Foodborne Pathog. Dis. 2010, 7, 435–441. [Google Scholar] [CrossRef]
- Kang, C.; Jin, Y.B.; Lee, H.; Cha, M.; Sohn, E.T.; Moon, J.; Park, C.; Chun, S.; Jung, E.S.; Hong, J.S.; et al. Brown alga Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt signaling pathways. Food Chem. Toxicol. 2010, 48, 509–516. [Google Scholar] [CrossRef]
- Shin, H.C.; Hwang, H.J.; Kang, K.J.; Lee, B.H. An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch. Pharm. Res. 2006, 29, 165–171. [Google Scholar] [CrossRef]
- Kang, S.M.; Cha, S.H.; Ko, J.Y.; Kang, M.C.; Kim, D.; Heo, S.J.; Kim, J.S.; Heu, M.S.; Kim, Y.T.; Jung, W.K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Lee, J.W.; Seok, J.K.; Boo, Y.C. Ecklonia cava extract and dieckol attenuate cellular lipid peroxidation in keratinocytes exposed to PM10. Evid. Based Complementary Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Seok, J.K.; Suh, H.J.; Choi, Y.H.; Hong, S.S.; Kim, D.S.; Boo, Y.C. Antimelanogenic effects of luteolin 7-sulfate isolated from Phyllospadix iwatensis Makino. Br. J. Dermatol. 2016, 175, 501–511. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, S.H.; Kim, J.E.; Choi, J.Y.; Park, R.W.; Yong Park, J.; Park, H.S.; Sohn, Y.S.; Lee, D.S.; Bae Lee, E.; et al. Betaig-h3 supports keratinocyte adhesion, migration, and proliferation through alpha3beta1 integrin. Biochem. Biophys. Res. Commun. 2002, 294, 940–948. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lin, W.; Li, Z. Blueberries inhibit cyclooxygenase-1 and cyclooxygenase-2 activity in human epithelial ovarian cancer. Oncol. Lett. 2017, 13, 4897–4904. [Google Scholar] [CrossRef]
- Xu, J.; Wu, W.; Zhang, H.; Yang, L. Berberine alleviates amyloid beta25-35-induced inflammatory response in human neuroblastoma cells by inhibiting proinflammatory factors. Exp. Ther. Med. 2018, 16, 4865–4872. [Google Scholar]
- Molloy, E.S.; Morgan, M.P.; Doherty, G.A.; McDonnell, B.; O’Byrne, J.; Fitzgerald, D.J.; McCarthy, G.M. Microsomal prostaglandin E2 synthase 1 expression in basic calcium phosphate crystal-stimulated fibroblasts: role of prostaglandin E2 and the EP4 receptor. Osteoarthr. Cartil. 2009, 17, 686–692. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar]
- Ferrer, M.D.; Busquests-Cortes, C.; Capo, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem. 2019, 26, 1–15. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Yoon, J.S.; Kasin Yadunandam, A.; Kim, S.J.; Woo, H.C.; Kim, H.R.; Kim, G.D. Dieckol, isolated from Ecklonia stolonifera, induces apoptosis in human hepatocellular carcinoma Hep3B cells. J. Nat. Med. 2013, 67, 519–527. [Google Scholar] [CrossRef]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.; Jeon, Y.J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues—Is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. 2009, 13, 3753–3763. [Google Scholar] [CrossRef]
- Hara, S. Prostaglandin terminal synthases as novel therapeutic targets. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 703–723. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Perspective of microsomal prostaglandin E2 synthase-1 as drug target in inflammation-related disorders. Biochem. Pharmacol. 2015, 98, 1–15. [Google Scholar] [CrossRef]
- Jung, W.K.; Heo, S.J.; Jeon, Y.J.; Lee, C.M.; Park, Y.M.; Byun, H.G.; Choi, Y.H.; Park, S.G.; Choi, I.W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446. [Google Scholar] [CrossRef]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.C.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann. Ist. Super. Sanita 2003, 39, 405–410. [Google Scholar]
- Ishii, H.; Fujii, T.; Hogg, J.C.; Hayashi, S.; Mukae, H.; Vincent, R.; Van Eeden, S.F. Contribution of IL-1 beta and TNF-alpha to the initiation of the peripheral lung response to atmospheric particulates (PM10). Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L176–L183. [Google Scholar] [CrossRef]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (-)-epigallocatechin-3-gallate rescue cell viability and attenuate inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. Skin Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Park, S.; Seok, J.K.; Kwak, J.Y.; Suh, H.J.; Kim, Y.M.; Boo, Y.C. Anti-inflammatory effects of pomegranate peel extract in THP-1 cells exposed to particulate matter PM10. Evid. Based Complement. Alternat. Med. 2016, 2016, 6836080. [Google Scholar] [CrossRef]
- Cho, D.Y.; Le, W.; Bravo, D.T.; Hwang, P.H.; Illek, B.; Fischer, H.; Nayak, J.V. Air pollutants cause release of hydrogen peroxide and interleukin-8 in a human primary nasal tissue culture model. Int. Forum Allergy Rhinol. 2014, 4, 966–971. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Lassegue, B.; Griendling, K.K. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 653–661. [Google Scholar] [CrossRef]
- Seok, J.K.; Cho, M.A.; Ha, J.W.; Boo, Y.C. Role of dual oxidase 2 in reactive oxygen species production induced by airborne particulate matter PM10 in human epidermal keratinocytes. J. Soc. Cosmet. Sci. Korea 2018, 45, 57–67. [Google Scholar]
- Choi, M.A.; Seok, J.K.; Lee, J.W.; Lee, S.Y.; Kim, Y.M.; Boo, Y.C. Effects of resveratrol and resveratryl triacetate on the inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. J. Soc. Cosmet. Sci. Korea 2018, 44, 249–258. [Google Scholar]
- Donaldson, K.; Stone, V.; Borm, P.J.A.; Jimenez, L.A.; Gilmour, P.S.; Schins, R.P.F.; Knaapen, A.M.; Rahman, I.; Faux, S.P.; Brown, D.M.; et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic. Biol. Med. 2003, 34, 1369–1382. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, R.; Cao, L.; Shen, Z.X.; Cao, Y.X. The role of MAPK pathways in airborne fine particulate matter-induced upregulation of endothelin receptors in rat basilar arteries. Toxicol. Sci. 2016, 149, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Tsatsanis, C.; Androulidaki, A.; Venihaki, M.; Margioris, A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006, 38, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, M.K.; Li, K.; Hu, C.; Lu, M.H.; Jie, S.T. Eupafolin nanoparticle improves acute renal injury induced by LPS through inhibiting ROS and inflammation. Biomed. Pharmacother. 2017, 85, 704–711. [Google Scholar] [CrossRef] [PubMed]

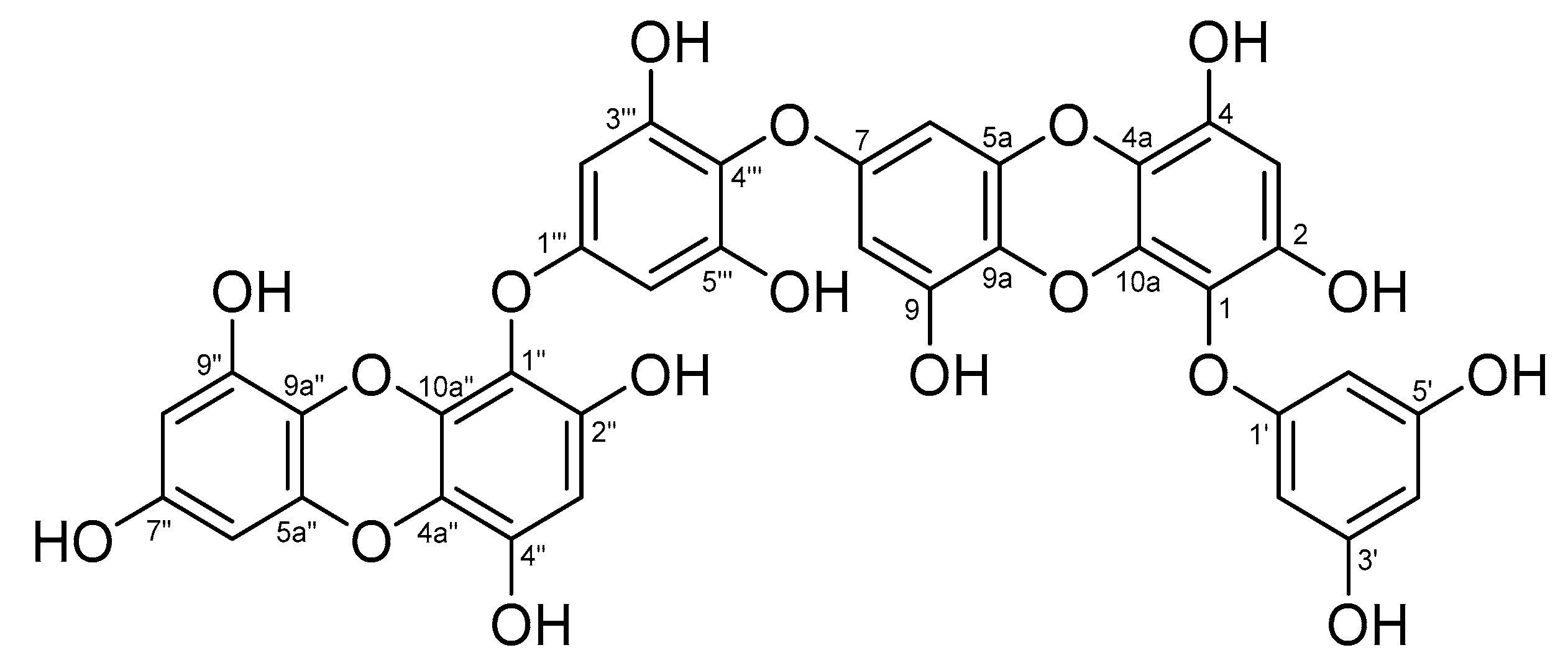
| Position | δH Multiplicity, (J Hz) | δC Multiplicity | Position | δH Multiplicity, (J Hz) | δC Multiplicity |
|---|---|---|---|---|---|
| 1 | 124.7 c s | 1″ | 124.8 c s | ||
| 2 | 147.5 s | 2″ | 147.4 s | ||
| 3 | 6.14 s | 99.5 d | 3″ | 6.16 s | 99.6 d |
| 4 | 143.4 s | 4″ | 143.5 s | ||
| 4a | 125.8 d s | 4a″ | 125.7 d s | ||
| 5a | 143.3 s | 5a″ | 144.4 s | ||
| 6 | 6.07 d (2.8) | 96.0 d | 6″ | 5.96 d (2.8) | 95.9 d |
| 7 | 156.1 s | 7″ | 154.7 s | ||
| 8 | 6.06 d (2.8) | 99.9 d | 8″ | 5.99 d (2.8) | 100.0 d |
| 9 | 147.1 s | 9″ | 147.3 s | ||
| 9a | 126.3 s | 9a″ | 125.0 s | ||
| 10a | 138.6 s | 10a″ | 138.8 s | ||
| 1′ | 162.0 s | 1‴ | 157.9 s | ||
| 2′, 6′ | 5.93 b | 95.5 d | 2‴, 6‴ | 6.09 s | 96.3 d |
| 3′, 5′ | 160.3 s | 3‴, 5‴ | 152.5 s | ||
| 4′ | 5.93 b | 97.8 d | 4‴ | 126.6 s |
| Gene Name | GenBank Accession Number | Primer Sequences | Ref. |
|---|---|---|---|
| Cyclooxygenase 1 (COX-1); Prostaglandin-endoperoxide synthase 1 (PTGS1) | NM_000962.4 | Forward: 5′-CAGAGCCAGATGGCTGTGGG-3′ Reverse: 5′-AAGCTGCTCATCGCCCCAGG-3′ | [38] |
| Cycloxygenase 2 (COX-2); Prostaglandin-endoperoxide synthase 2 (PTGS2) | NM_000963.3 | Forward: 5′-CTGCGCCTTTTCAAGGATGG-3′ Reverse: 5′-CCCCACAGCAAACCGTAGAT-3′ | [39] |
| Microsomal prostaglandin E synthase 1 (mPGES-1); Prostaglandin E synthase 1 (PTGES 1) | NM_004878.5 | Forward: 5′-AACCCTTTTGTCGCCTG-3′ Reverse: 5′-GTAGGCCACGGTGTGT-3′ | [40] |
| Microsomal prostaglandin E synthase 1 (mPGES-2); Prostaglandin E synthase 2 (PTGES 2) | NM_025072.7 | Forward: 5′-GAAAGCTCGCAACAACTAAAT-3′ Reverse: 5′-CTTCATGGCTGGGTAGTAG-3′ | [40] |
| Cytosolic prostaglandin E synthase (cPGES); Prostaglandin E synthase 3 (PTGES3), | NM_006601.6 | Forward:5′-ATAAAAGAACGGACAGATCAA-3′ Reverse:5′-CACTAAGCCAATTAAGCTTTG-3′ | [40] |
| GAPDH (glyceraldehyde 3-phosphate dehydrogenase) | NM_002046.3 | Forward: 5′-ATGGGGAAGGTGAAGGTCG-3′ Reverse: 5′-GGGGTCATTGATGGCAACAA-3′ | [33] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants 2019, 8, 190. https://doi.org/10.3390/antiox8060190
Ha JW, Song H, Hong SS, Boo YC. Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants. 2019; 8(6):190. https://doi.org/10.3390/antiox8060190
Chicago/Turabian StyleHa, Jae Won, Hyerim Song, Seong Su Hong, and Yong Chool Boo. 2019. "Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter" Antioxidants 8, no. 6: 190. https://doi.org/10.3390/antiox8060190
APA StyleHa, J. W., Song, H., Hong, S. S., & Boo, Y. C. (2019). Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants, 8(6), 190. https://doi.org/10.3390/antiox8060190






