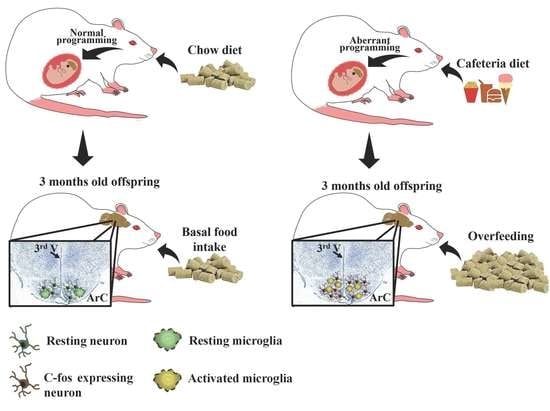
Priming of Hypothalamic Ghrelin Signaling and Microglia Activation Exacerbate Feeding in Rats’ Offspring Following Maternal Overnutrition
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animals and Housing
2.3. Diets
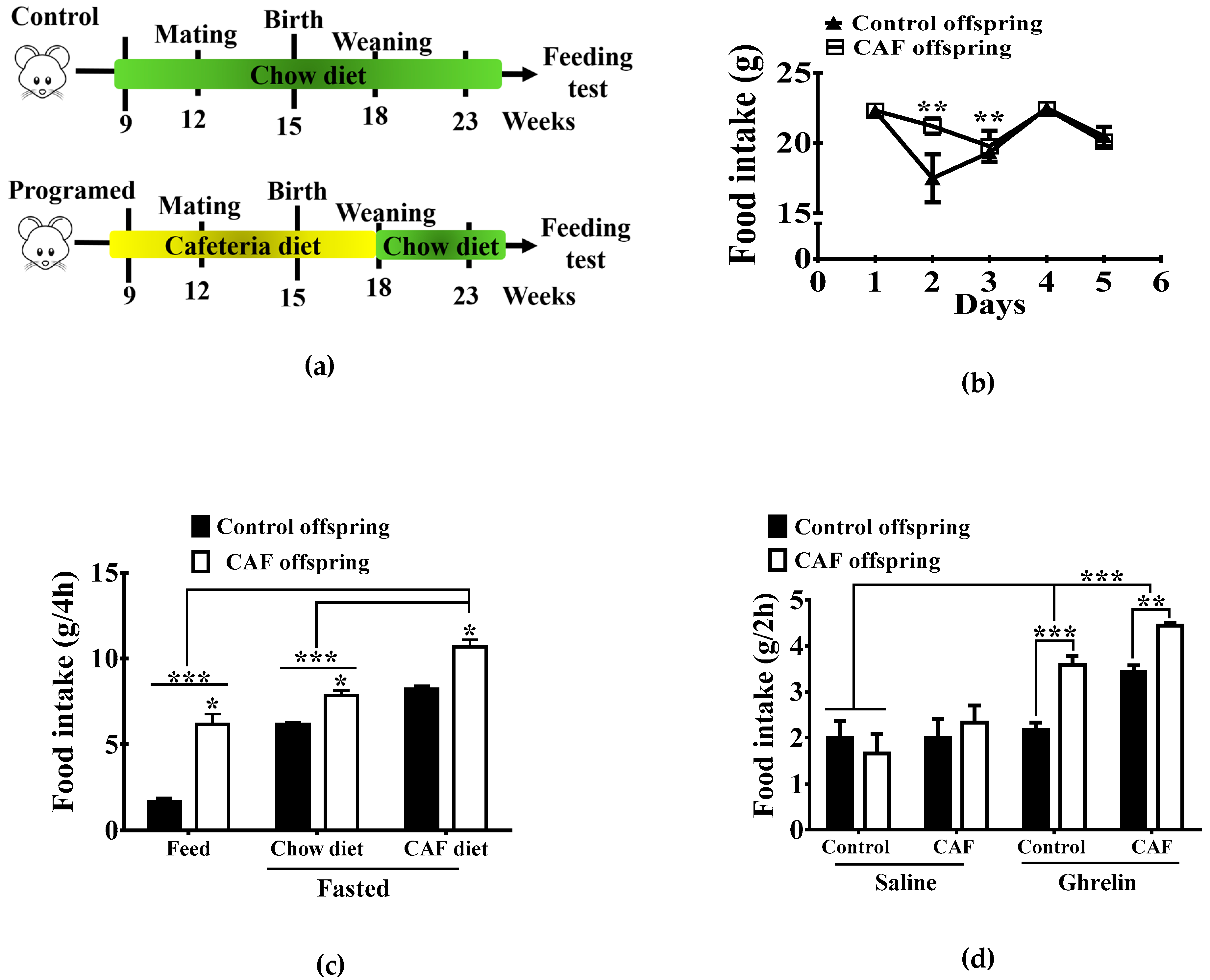
2.4. Maternal Nutritional Programming Model
2.5. Analysis of Ghrelin Signaling for Chow and CAF Exposure in Offspring
2.6. Intracardiac Perfusion
2.7. Tissue Sample Collection
2.8. Stereotaxic Surgery and I.C.V. Ghrelin Administration
2.9. Glucose and Insulin Tolerance Test (GTT, ITT) and Ghrelin Sensitivity Assessments
2.10. Microglia Primary Culture and Treatments
2.11. Cytokine Measurements
2.12. Immunohistochemistry Analysis by Confocal Imaging
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results
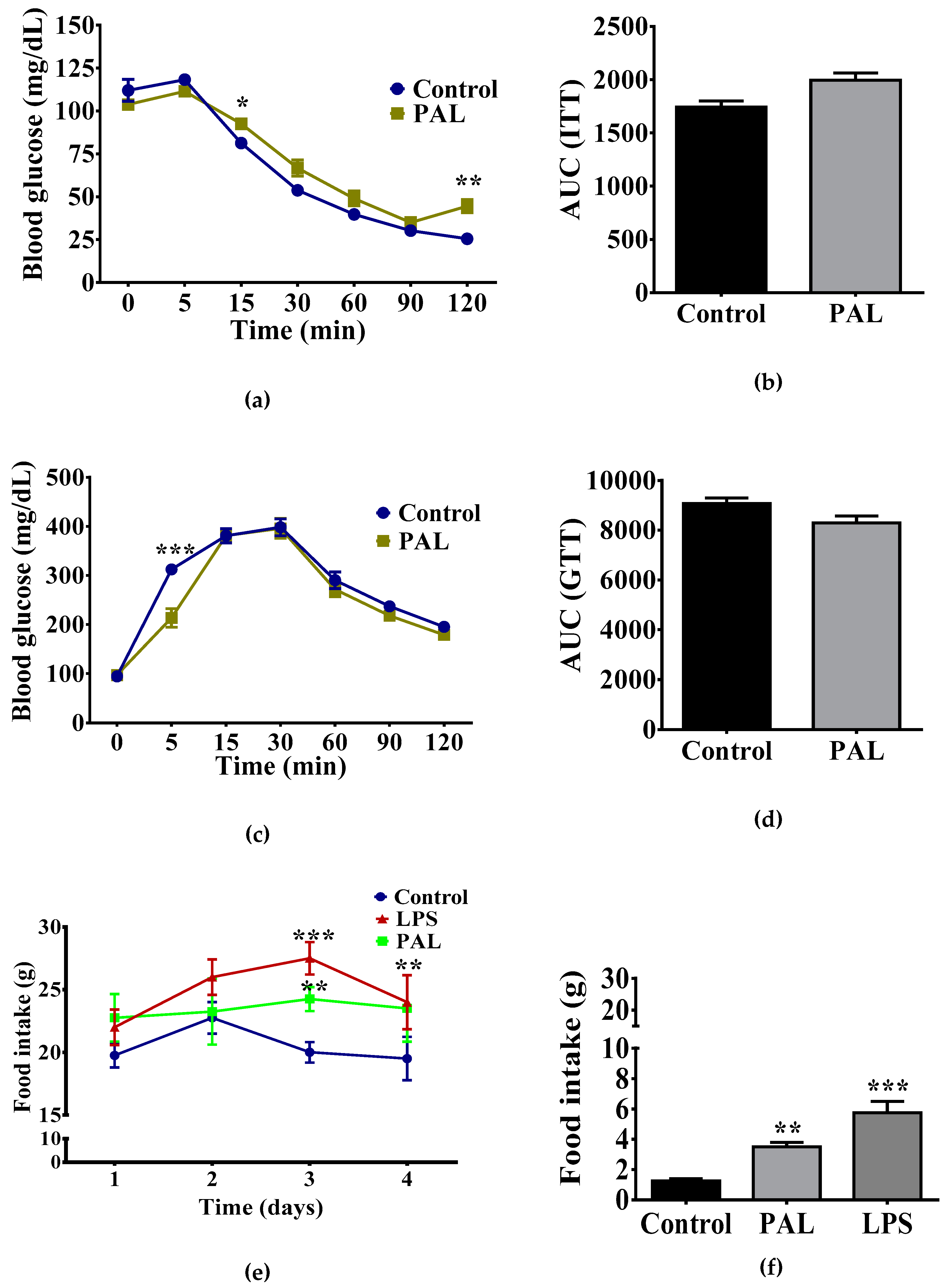
3.1. Maternal Nutritional Programming Exacerbates Ghrelin Sensitivity in Offspring, Leading to an Increase in Food Intake
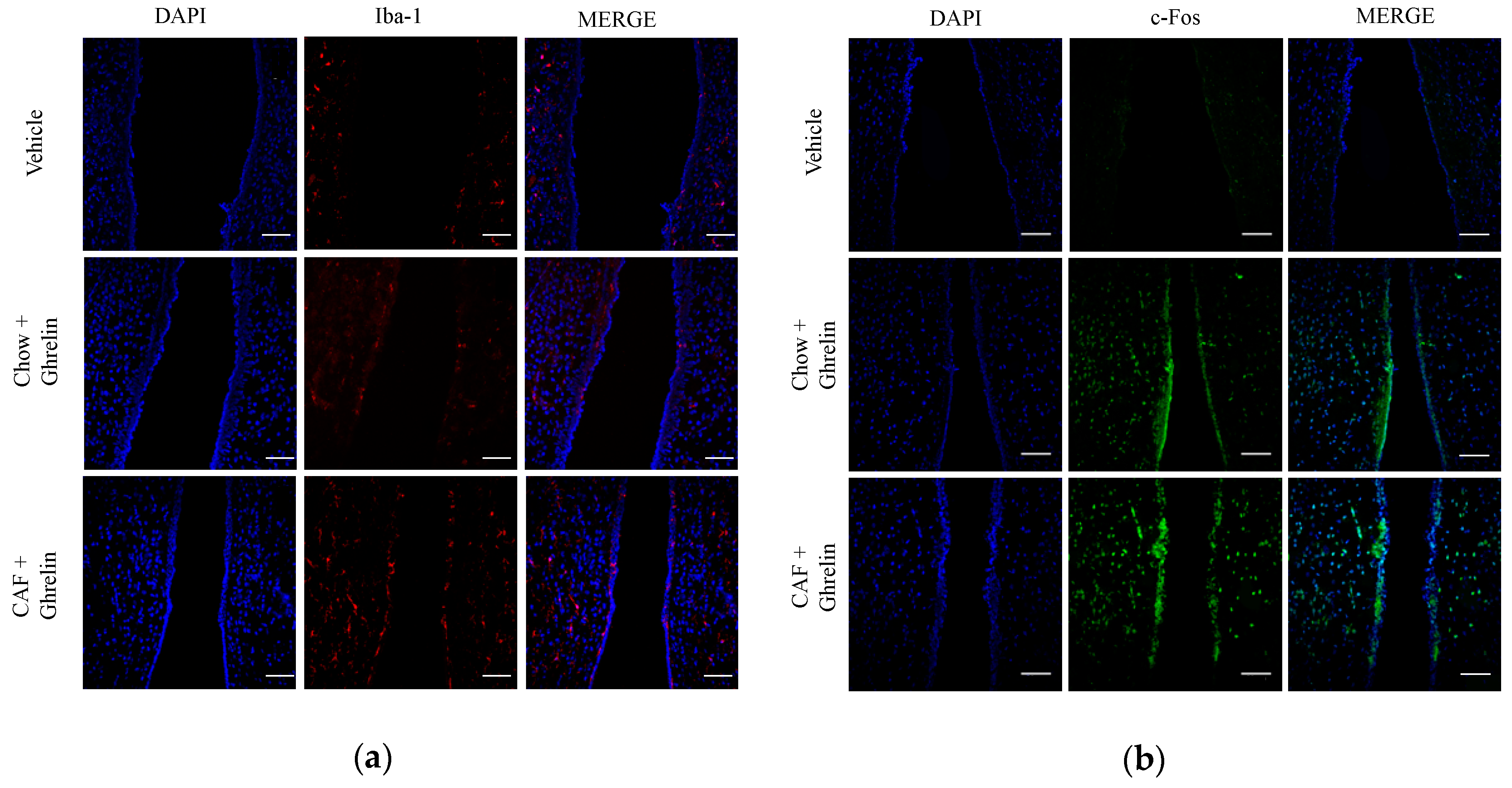
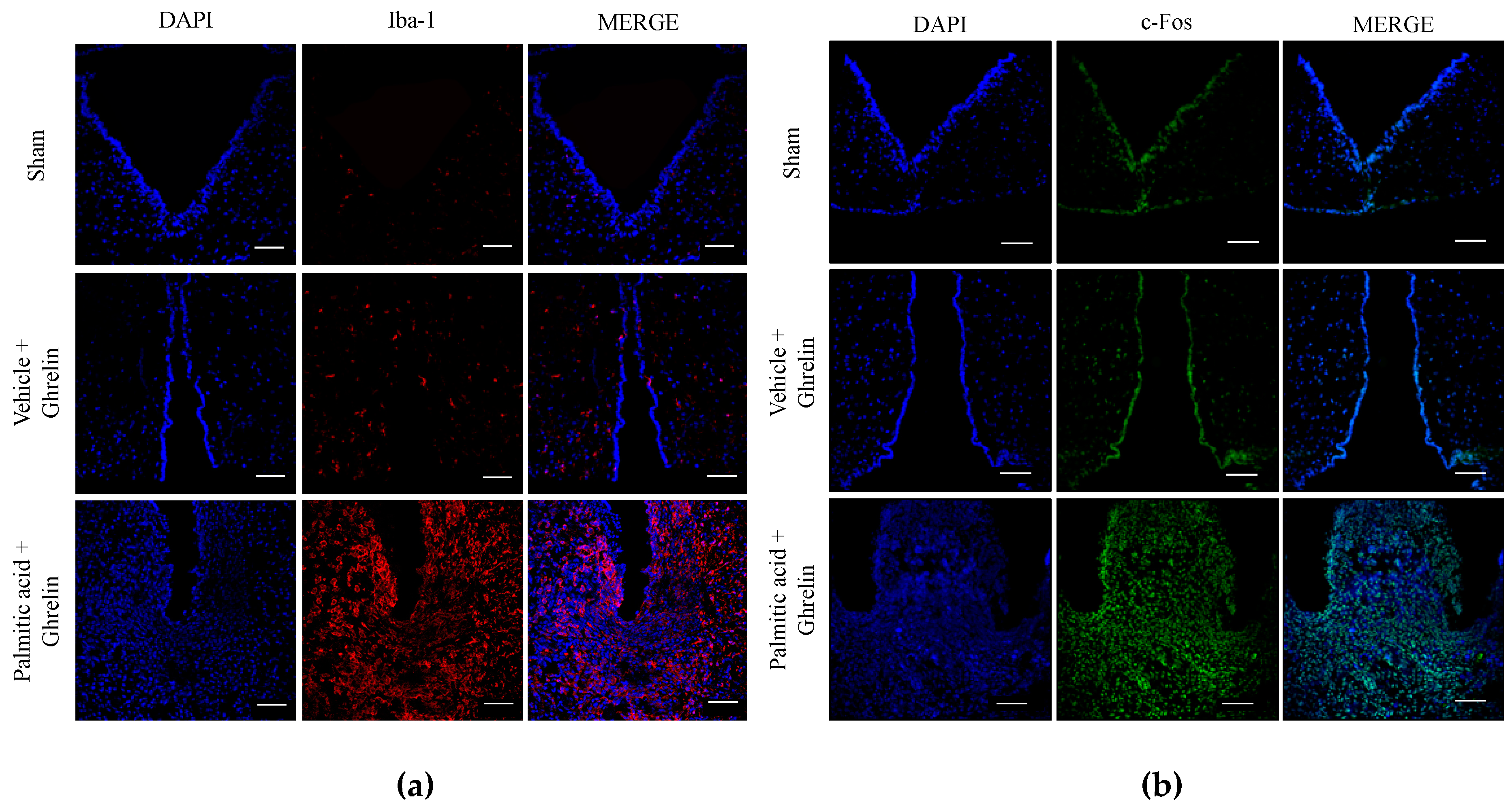
3.2. Maternal Programming by CAF Exposure Sets Activation of Hypothalamic Ghrelin-Sensitive C-Fos Neurons in Offspring
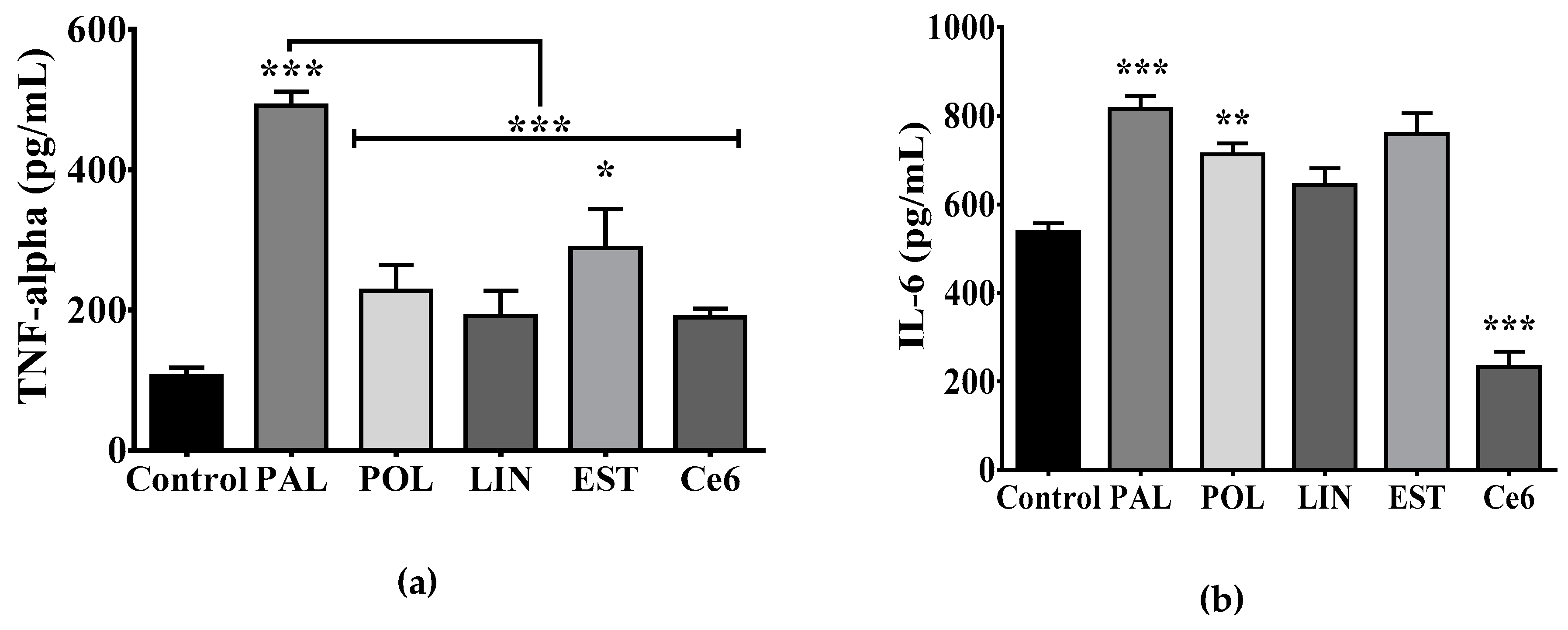
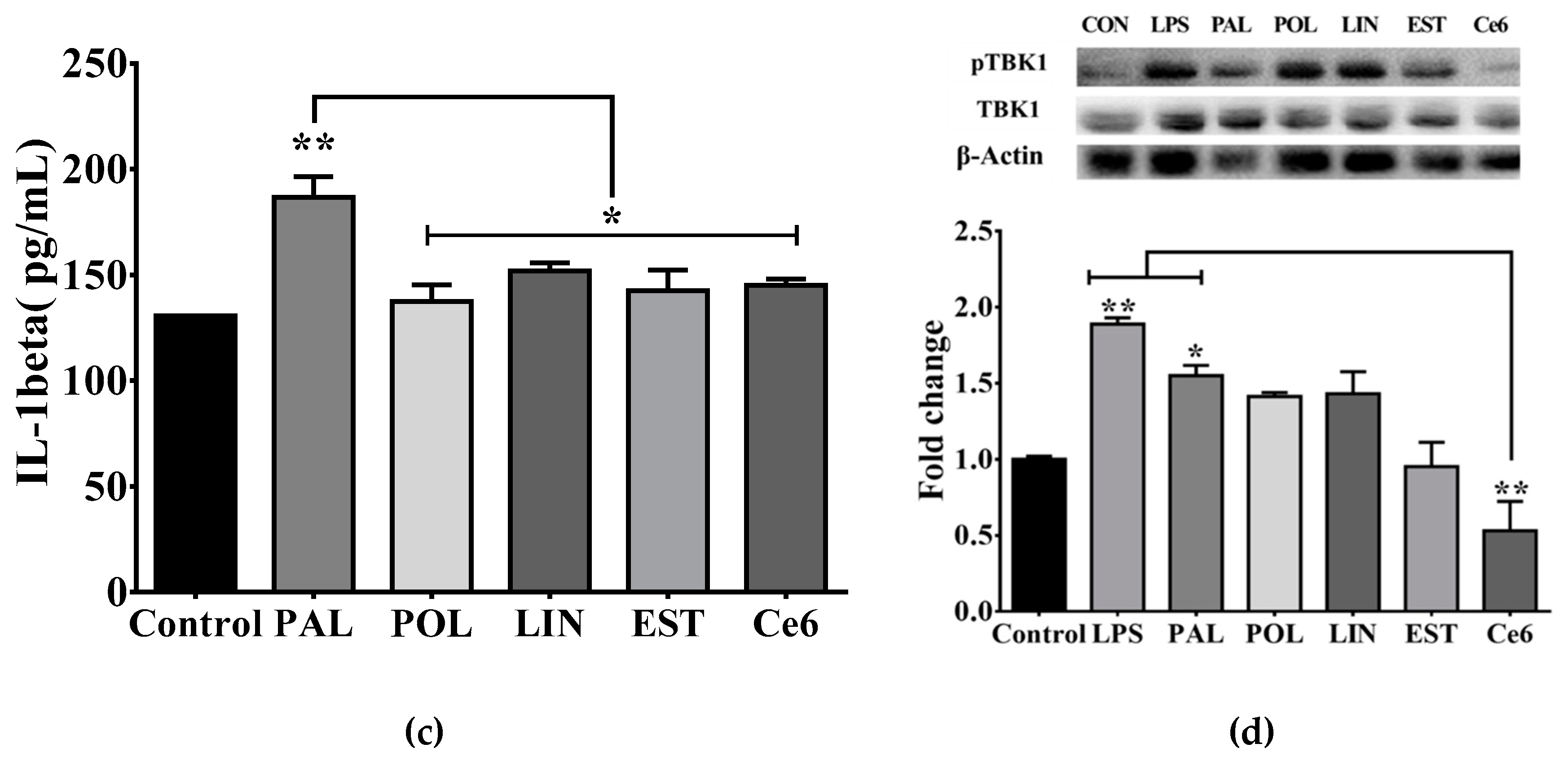
3.3. Saturated Lipids Activate a Pro-Inflammatory Stage in Microglia
3.4. Hypothalamic Palmitic Acid Inoculation Promotes Increase in Total Food Intake
3.5. Hypothalamic Saturated Lipids Stimulation Activates Microglia and Ghrelin-Sensitive Neurons
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alfaradhi, M.Z.; Kusinski, L.C.; Fernandez-Twinn, D.S.; Pantaleão, L.C.; Carr, S.K.; Ferland-McCollough, D.; Yeo, G.S.H.; Bushell, M.; Ozanne, E.S. Maternal Obesity in Pregnancy Developmentally Programs Adipose Tissue Inflammation in Young, Lean Male Mice Offspring. Endocrinology 2016, 157, 4246–4256. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.M.; Segovia, S.A.; Vickers, M.H. Experimental models of maternal obesity and neuroendocrine programming of metabolic disorders in offspring. Front. Endocrinol. (Lausanne) 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Frihauf, J.B.; Fekete, É.M.; Nagy, T.R.; Levin, B.E.; Zorrilla, E.P. Maternal Western diet increases adiposity even in male offspring of obesity-resistant rat dams: Early endocrine risk markers. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R1045–R1059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, P.D.; Samuelsson, A.-M.; Poston, L. Maternal obesity and the developmental programming of hypertension: A role for leptin. Acta Physiol. 2014, 210, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Perez, R.E.; Fuentes-Mera, L.; de la Garza, A.L.; Torre-Villalvazo, I.; Reyes-Castro, L.A.; Rodriguez-Rocha, H.; Garcia-Garcia, A.; Corona-Castillo, J.C.; Tovar, A.R.; Zambrano, E.; et al. Maternal overnutrition by hypercaloric diets programs hypothalamic mitochondrial fusion and metabolic dysfunction in rat male offspring. Nutr. Metab. (London) 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Montalvo-Martinez, L.; Cardenas-Perez, R.E.; Fuentes-Mera, L.; Garza-Ocañas, L. Obesogenic diet intake during pregnancy programs aberrant synaptic plasticity and addiction-like behavior to a palatable food in offspring. Behav. Brain Res. 2017, 330, 46–55. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Perelló, M.; Zigman, J.M. The Role of Ghrelin in Reward-Based Eating. Biol. Psychiatry 2012, 72, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Słupecka, M.; Romanowicz, K.; Woliński, J. Maternal high-fat diet during pregnancy and lactation influences obestatin and ghrelin concentrations in milk and plasma of Wistar rat dams and their offspring. Int. J. Endocrinol. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sominsky, L.; Ziko, I.; Spencer, S.J. Neonatal overfeeding disrupts pituitary ghrelin signalling in female rats long-term; Implications for the stress response. PLoS ONE 2017, 12, e0173498. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017, 26, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2139. [Google Scholar] [CrossRef]
- Chang, G.-Q.; Gaysinskaya, V.; Karatayev, O.; Leibowitz, S.F. Maternal high-fat diet and fetal programming: Increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J. Neurosci. 2008, 28, 12107–12119. [Google Scholar] [CrossRef] [PubMed]
- Dieberger, A.; de Rooij, S.; Korosi, A.; Vrijkotte, T. Maternal lipid concentrations during early pregnancy and eating behaviour and energy intake in the offspring. Nutrients 2018, 10, 1026. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.; Calder, P. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Maldonado-Ruiz, R.; Fuentes-Mera, L.; Camacho, A. Central modulation of neuroinflammation by neuropeptides and energy-sensing hormones during obesity. Biomed Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, M.; Haghiac, M.; Catalano, P.M.; O’Tierney-Ginn, P.; Mouzon, S.H. Causal relationship between obesity-related traits and TLR4-driven responses at the maternal–fetal interface. Diabetologia 2016, 59, 2459–2466. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, G.; Li, Y.; Wang, Y.; Liu, Z. Knockdown of Tlr4 in the arcuate nucleus improves obesity related metabolic disorders. Sci. Rep. 2017, 7, 7441. [Google Scholar] [CrossRef]
- Delint-Ramirez, I.; Ruiz, R.M.; Torre-Villalvazo, I.; Fuentes-Mera, L.; Ocañas, L.G.; Tovar, A.; Camacho, A. Genetic obesity alters recruitment of TANK-binding kinase 1 and AKT into hypothalamic lipid rafts domains. Neurochem. Int. 2015, 80, 23–32. [Google Scholar] [CrossRef]
- Naznin, F.; Toshinai, K.; Waise, T.M.Z.; NamKoong, C.; Moin, A.S.M.; Sakoda, H.; Nakazato, M. Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J. Endocrinol. 2015, 226, 81–92. [Google Scholar] [CrossRef]
- De Luca, S.N.; Sominsky, L.; Soch, A.; Wang, H.; Ziko, I.; Rank, M.M.; Spencer, S.J. Conditional microglial depletion in rats leads to reversible anorexia and weight loss by disrupting gustatory circuitry. Brain Behav. Immun. 2019, 77, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Gottero, C.; Benso, A.; Prodam, F.; Destefanis, S.; Gauna, C.; Maccario, M.; Deghenghi, R.; van der Lely, A.J.; Ghigo, E. Effects of ghrelin on the insulin and glycemic responses to glucose, arginine, or free fatty acids load in humans. J. Clin. Endocrinol. Metab. 2003, 88, 4268–4272. [Google Scholar] [CrossRef] [PubMed]
- Delhanty, P.J.D.; van Kerkwijk, A.; Huisman, M.; van de Zande, B.; Verhoef-Post, M.; Gauna, C.; Hofland, L.; Themmen, A.P.N.; van der Lely, A.J. Unsaturated fatty acids prevent desensitization of the human growth hormone secretagogue receptor by blocking its internalization. AJP Endocrinol. Metab. 2010, 299, E497–E505. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007; 456p. [Google Scholar]
- Cabral, A.; Valdivia, S.; Fernandez, G.; Reynaldo, M.; Perello, M. divergent neuronal circuitries underlying acute orexigenic effects of peripheral or central ghrelin: Critical role of brain accessibility. J. Neuroendocrinol. 2014, 26, 542–554. [Google Scholar] [CrossRef]
- De la Garza, A.; Garza-Cuellar, M.; Silva-Hernandez, I.; Cardenas-Perez, R.; Reyes-Castro, L.; Zambrano, E.; Gonzalez-Hernandez, B.; Garza-Ocañas, L.; Fuentes-Mera, L.; Camacho, A. Maternal flavonoids intake reverts depression-like behaviour in rat female offspring. Nutrients 2019, 11, 572. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Smith, M.S.; Grove, K.L. perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology 2011, 93, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, D.; Zhao, H.; Chen, Y.; Liu, Y.; Cao, C.; Han, L.; Liu, G. Ghrelin inhibits cell apoptosis induced by lipotoxicity in pancreatic β-cell line. Regul. Pept. 2010, 161, 43–50. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, Y.; Fan, X. Inhibition of ghrelin o-acyltransferase attenuated lipotoxicity by inducing autophagy via AMPK–mTOR pathway. Drug Des. Devel. Ther. 2018, 12, 873–885. [Google Scholar] [CrossRef]
- Mosa, R.M.H.; Zhang, Z.; Shao, R.; Deng, C.; Chen, J.; Chen, C. Implications of ghrelin and hexarelin in diabetes and diabetes-associated heart diseases. Endocrine 2015, 49, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.E.; Howard, V.G.; Lemus, M.B.; Jois, T.; Andrews, Z.B.; Sleeman, M.W. The Ghrelin/GOAT system regulates obesity-induced inflammation in male mice. Endocrinology 2017, 158, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Chen, X.; Hu, J.; Fu, Y.; Peng, J.; Li, Y.; Chen, J.; Li, P.; Liu, L.; Cao, J.; et al. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-α/NF-κB signaling pathways. Sci. Rep. 2019, 9, 1348. [Google Scholar] [CrossRef]
- Maldonado-Ruiz, R.; Montalvo-Martínez, L.; Fuentes-Mera, L.; Camacho, A. Microglia activation due to obesity programs metabolic failure leading to type two diabetes. Nutr. Diabetes 2017, 7, e254. [Google Scholar] [CrossRef]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.; Kim, M. Hesperetin, a Citrus flavonoid, attenuates lps-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, Y.; Mendlovich, S.; Riwkes, S.; Braw, Y.; Levkovitch-Verbin, H.; Gal, G.; Fennig, S.; Treves, I.; Kron, S. A Double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry 2010, 71, 138–149. [Google Scholar] [CrossRef]
- Ziko, I.; de Luca, S.; Dinan, T.; Barwood, J.M.; Sominsky, L.; Cai, G.; Kenny, R.; Stokes, L.; Jenkins, T.A.; Spencer, S.J. Neonatal overfeeding alters hypothalamic microglial profiles and central responses to immune challenge long-term. Brain. Behav. Immun. 2014, 41, 32–43. [Google Scholar] [CrossRef]
- Van Niekerk, G.; Isaacs, A.W.; Nell, T.; Engelbrecht, A.-M. Sickness-Associated Anorexia: Mother Nature’s Idea of Immunonutrition? Mediators Inflamm. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wisse, B.E.; Ogimoto, K.; Tang, J.; Harris, M.K.; Raines, E.W.; Schwartz, M.W. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology 2007, 148, 5230–5237. [Google Scholar] [CrossRef]
- Djogo, T.; Robins, S.C.; Schneider, S.; Kryzskaya, D.; Liu, X.; Mingay, A.; Gillon, C.J.; Kim, J.H.; Storch, K.-F.; Boehm, U.; et al. Adult NG2-glia are required for median eminence-mediated leptin sensing and body weight control. Cell Metab. 2016, 23, 797–810. [Google Scholar] [CrossRef]
- Thaler, J.; Yi, C.; Schur, E.; Guyenet, S.; Hwang, B.; Dietrich, M.; Zhao, X.; Sarruf, D.; Izgur, V.; Maravilla, K.; et al. Obesity is associated with hipothalamic injury in rodents and humans. J. Clin. Investig. 2011, 122, 153. [Google Scholar] [CrossRef]
- Morton, G.J.; Meek, T.H.; Schwartz, M.W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 2014, 15, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navas, C.; Morselli, E.; Clegg, D.J. Sexually dimorphic brain fatty acid composition in low and high fat diet-fed mice. Mol. Metab. 2016, 5, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Mainardi, M.; Scala, F.; Natale, F.; Lapenta, R.; Mattera, A.; Rinaudo, M.; Puma, D.D.L.; Ripoli, C.; et al. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Kang, M.; Lee, A.; Yoo, H.J.; Kim, M.; Kim, M.; Shin, D.Y.; Lee, J.H. Association between increased visceral fat area and alterations in plasma fatty acid profile in overweight subjects: a cross-sectional study. Lipids Health Dis. 2017, 16, 248. [Google Scholar] [CrossRef]
- Dumas, J.A.; Bunn, J.Y.; Nickerson, J.; Crain, K.I.; Ebenstein, D.B.; Tarleton, E.K.; Makarewicz, J.; Poynter, M.E.; Kien, C.L. Dietary saturated fat and monounsaturated fat have reversible effects on brain function and the secretion of pro-inflammatory cytokines in young women. Metabolism 2016, 65, 1582–1588. [Google Scholar] [CrossRef]
- Hsu, T.M.; Kanoski, S.E. Blood-brain barrier disruption: Mechanistic links between Western diet consumption and dementia. Front. Aging Neurosci. 2014, 6, 6. [Google Scholar] [CrossRef]

| Reagent | Catalog | Application (conc.) | Manufacturer |
|---|---|---|---|
| Ghrelin Dulbecco´s Modified Eagle’s Medium high glucose | G8903 D5648 | i.c.v PC | Sigma-Aldrich, St. Louis, MO, USA Sigma-Aldrich, St. Louis, MO, USA |
| L-15 Medium (Leibovitz) | L4386 | PC | Sigma-Aldrich, St. Louis, MO, USA |
| Palmitic acid | P0500 | i.c.v. and PC | Sigma-Aldrich, St. Louis, MO, USA |
| Palmitoleic acid | P9417 | PC | Sigma-Aldrich, St. Louis, MO, USA |
| Linoleic acid | L1376 | PC | Sigma-Aldrich, St. Louis, MO, USA |
| Stearic acid | S47S1 | PC | Sigma-Aldrich, St. Louis, MO, USA |
| N-hexanoyl-D-esfingosin | H6524 | PC | Sigma-Aldrich, St. Louis, MO, USA |
| lipopolysaccharide (LPS) Escherichia coli 0111: B4 | L2630 | i.c.v. and PC | Sigma-Aldrich, St. Louis, MO, USA |
| Rat TNF-α ELISA Ready-SET-Go! | 88-7340 | ELISA | eBioscience, San Diego, CA, USA |
| Rat IL-6 ELISA kit | RAB0311 | ELISA | Sigma-Aldrich, St. Louis, MO, USA |
| Rat IL-1β ELISA kit | RAB0277 | ELISA | Sigma-Aldrich, St. Louis, MO, USA |
| Antibody | Catalog | Application (conc.) | Host | Manufacturer |
|---|---|---|---|---|
| Anti-NAK | ab40676 | WB (1:1000) | Rabbit | abcam, Cambridge, MA, USA |
| Anti-p-NAK S172 | ab109272 | WB (1:1000) | Rabbit | abcam, Cambridge, MA, USA |
| Anti-p-NF-κB p65 S536 | 3033S | WB (1:1000) | Rabbit | Cell Signaling, Beverly, MA, USA |
| Anti-p- NF-κB p65 | 8242S | WB (1:1000) | Rabbit | Cell Signaling, Beverly, MA, USA |
| Anti- β-Actin | 8457P | WB (1:5000) | Rabbit | Cell Signaling, Beverly, MA, USA |
| Anti-rabbit IgG-HRP | sc-2370 | WB (1:1000) | Cow | Santa Cruz Biotech., Dallas, TX, USA |
| Alexa fluor 488 anti-rabbit | A-11034 | IF (1:1000) | Goat | Thermo Fisher Scientific, Waltham, MA, USA |
| Alexa fluor 546 anti-rabbit | A-11035 | IF (1:1000) | Goat | Thermo Fisher Scientific, Waltham, MA, USA |
| Anti-c-fos | ab190289 | IF (1:1000) | Rabbit | abcam, Cambridge, MA, USA |
| Anti-Iba-1 | ab178847 | IF (1:200) | Rabbit | abcam, Cambridge, MA, USA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Ruiz, R.; Cárdenas-Tueme, M.; Montalvo-Martínez, L.; Vidaltamayo, R.; Garza-Ocañas, L.; Reséndez-Perez, D.; Camacho, A. Priming of Hypothalamic Ghrelin Signaling and Microglia Activation Exacerbate Feeding in Rats’ Offspring Following Maternal Overnutrition. Nutrients 2019, 11, 1241. https://doi.org/10.3390/nu11061241
Maldonado-Ruiz R, Cárdenas-Tueme M, Montalvo-Martínez L, Vidaltamayo R, Garza-Ocañas L, Reséndez-Perez D, Camacho A. Priming of Hypothalamic Ghrelin Signaling and Microglia Activation Exacerbate Feeding in Rats’ Offspring Following Maternal Overnutrition. Nutrients. 2019; 11(6):1241. https://doi.org/10.3390/nu11061241
Chicago/Turabian StyleMaldonado-Ruiz, Roger, Marcela Cárdenas-Tueme, Larisa Montalvo-Martínez, Roman Vidaltamayo, Lourdes Garza-Ocañas, Diana Reséndez-Perez, and Alberto Camacho. 2019. "Priming of Hypothalamic Ghrelin Signaling and Microglia Activation Exacerbate Feeding in Rats’ Offspring Following Maternal Overnutrition" Nutrients 11, no. 6: 1241. https://doi.org/10.3390/nu11061241
APA StyleMaldonado-Ruiz, R., Cárdenas-Tueme, M., Montalvo-Martínez, L., Vidaltamayo, R., Garza-Ocañas, L., Reséndez-Perez, D., & Camacho, A. (2019). Priming of Hypothalamic Ghrelin Signaling and Microglia Activation Exacerbate Feeding in Rats’ Offspring Following Maternal Overnutrition. Nutrients, 11(6), 1241. https://doi.org/10.3390/nu11061241