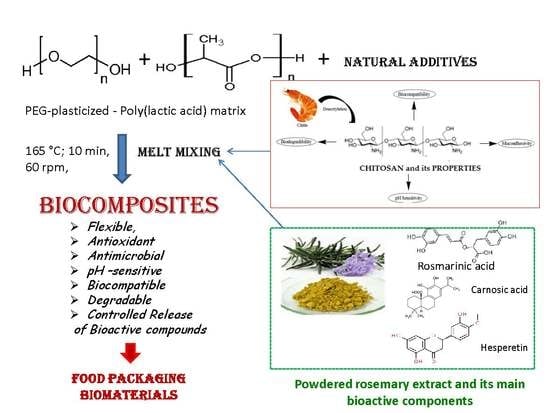
Biocompatible Materials Based on Plasticized Poly(lactic acid), Chitosan and Rosemary Ethanolic Extract I. Effect of Chitosan on the Properties of Plasticized Poly(lactic acid) Materials
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Biocomposites Processing
2.3. Investigation Methods
2.3.1. Processing Behavior
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. ATR–FTIR Spectroscopy
2.3.4. Stress-Strain Measurements
2.3.5. Impact Tests
2.3.6. Dynamic Rheology
2.3.7. Antioxidant Activity Evaluation by ABTS•+ (2, 2’-azino-bis 3-ethylbenzthiazoline-6-sulfonic acid) Radical-cation Scavenging Assay
2.3.8. Antimicrobial Activity
2.3.9. Migration Study of the Active Components within Powdered Rosemary Ethanol Extract into Food Simulant from PLA/PEG/CS-based Films
2.3.10. Biocompatibility Evaluation
3. Results and Discussion
3.1. Processing Behavior
3.2. SEM Results
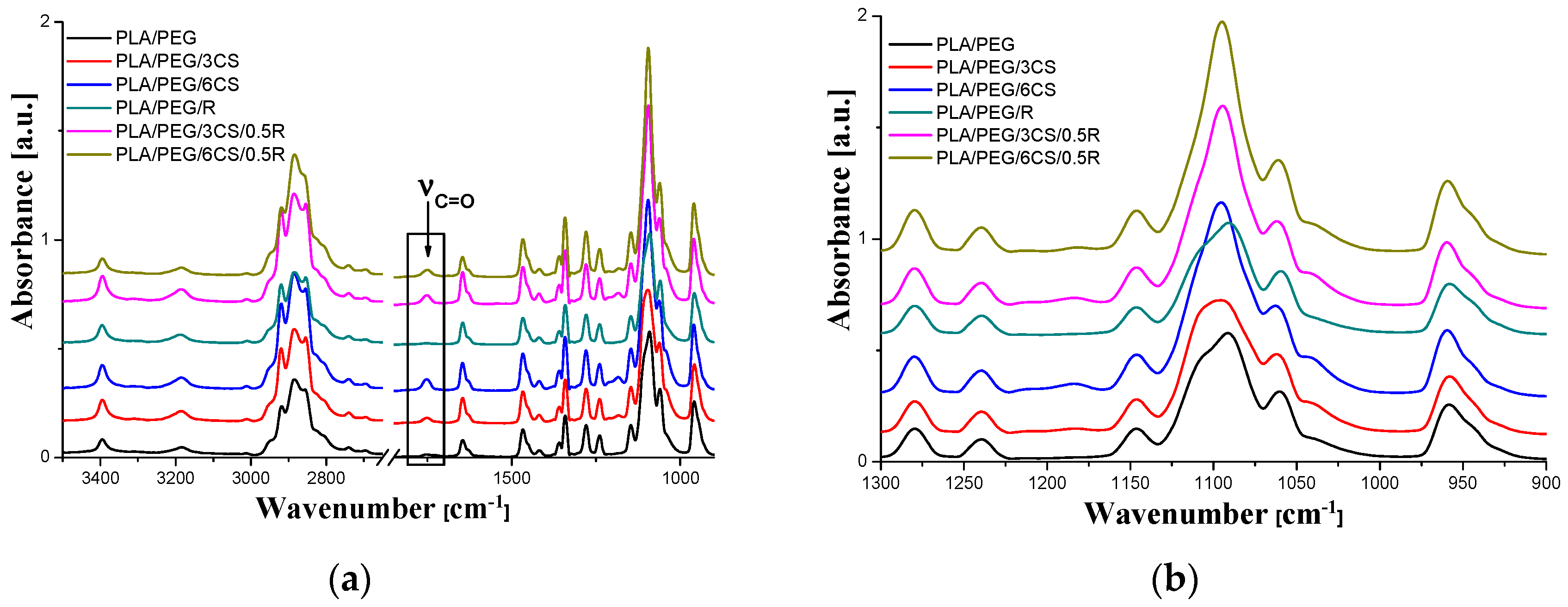
3.3. ATR-FTIR Spectroscopy Results
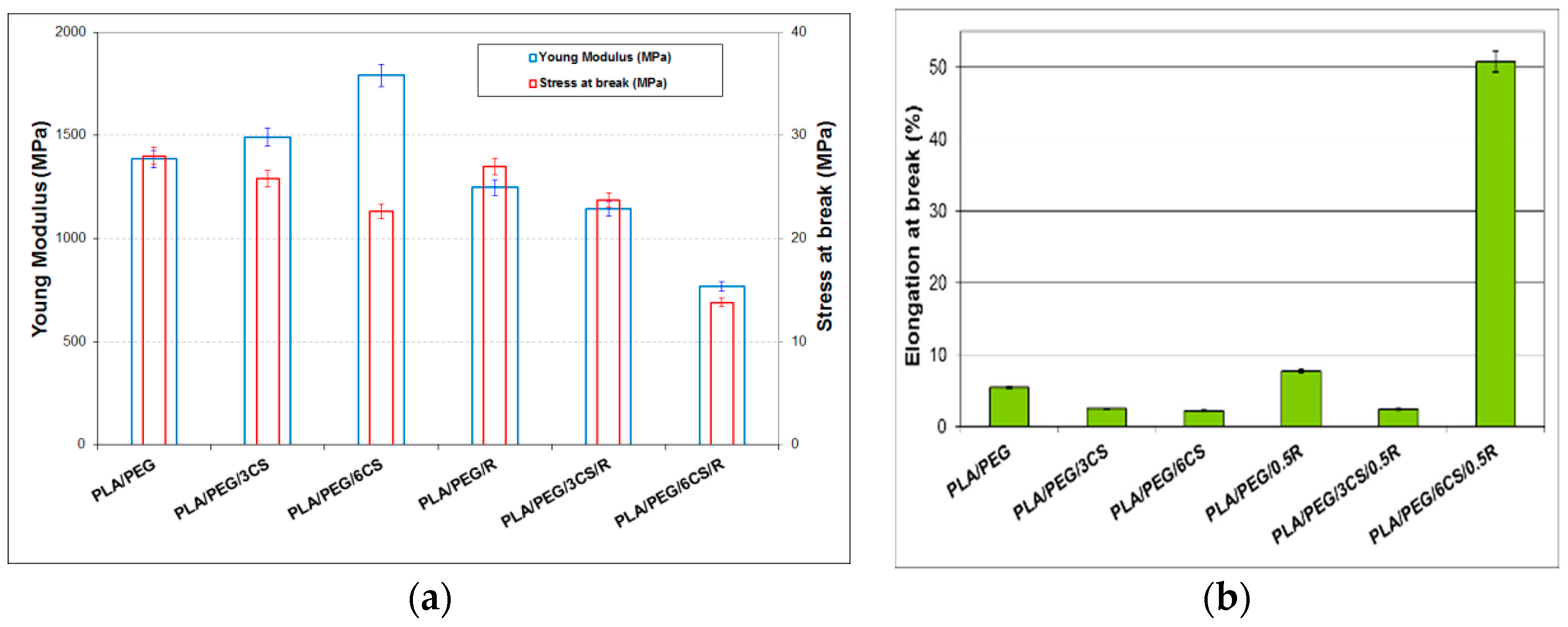
3.4. Mechanical Properties
3.5. Impact Properties
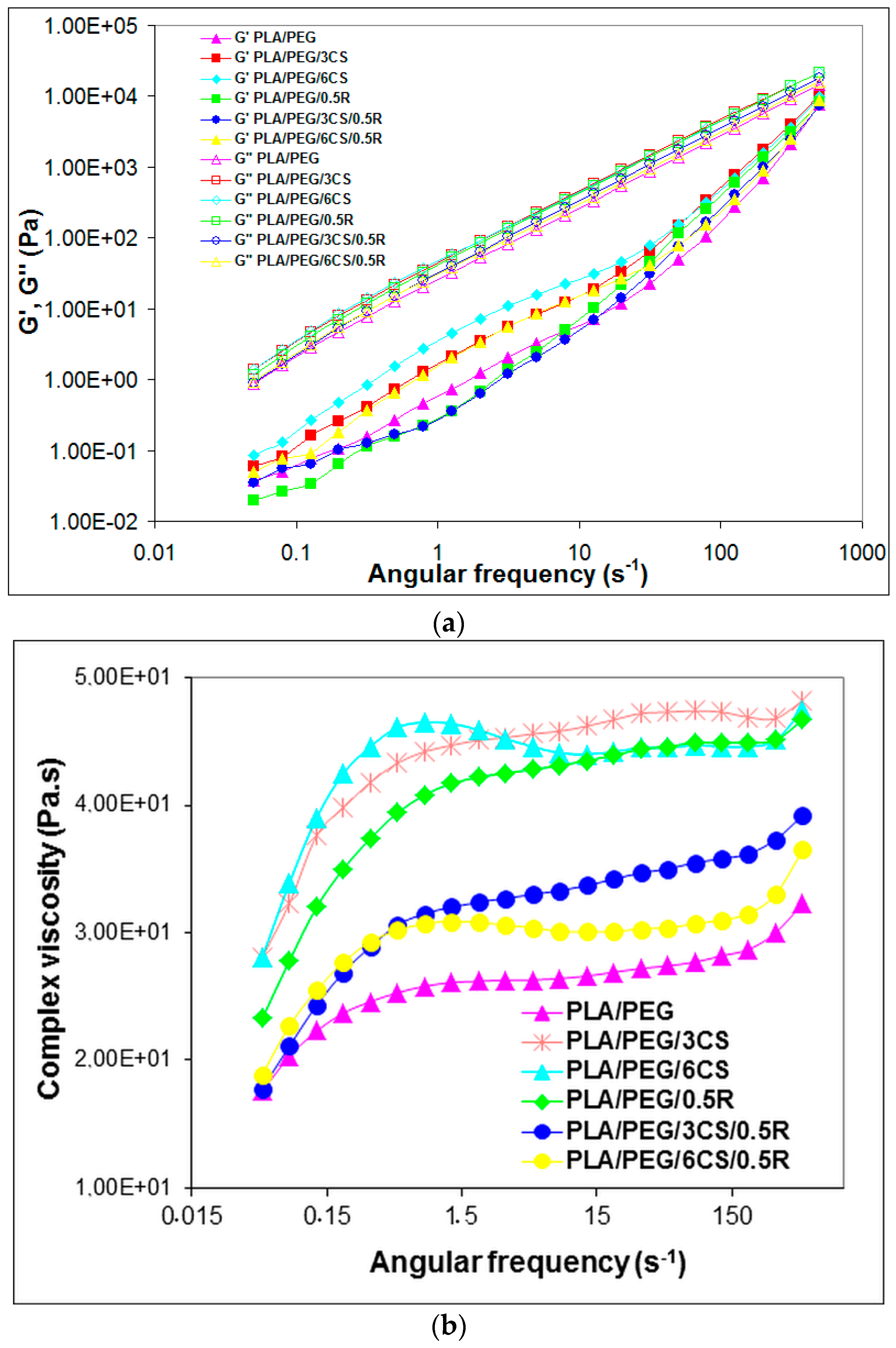
3.6. Rheological Properties
3.7. Antioxidant and Antibacterial Properties
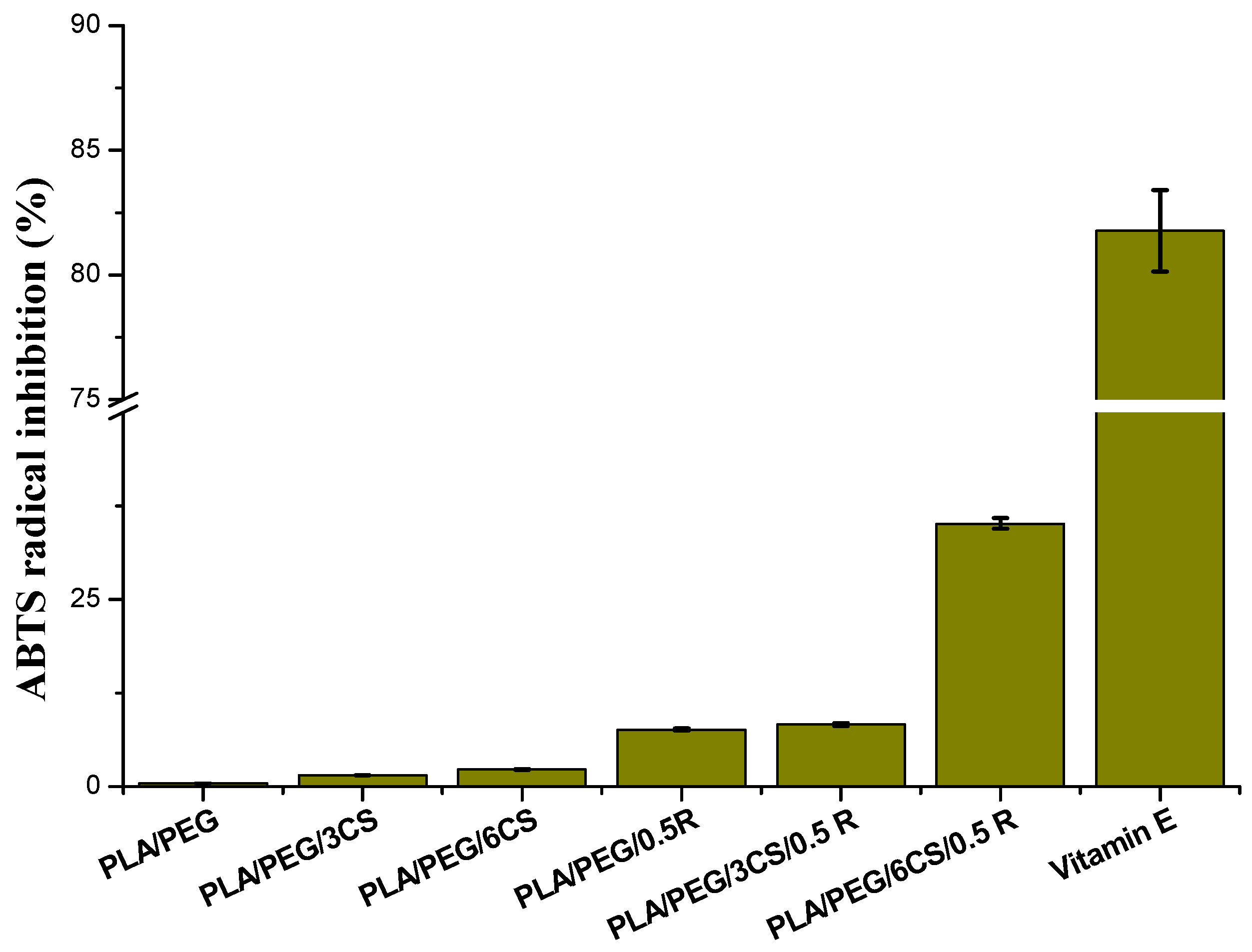
3.7.1. Antioxidant Activity Evaluation
3.7.2. Antibacterial Activity Evaluation
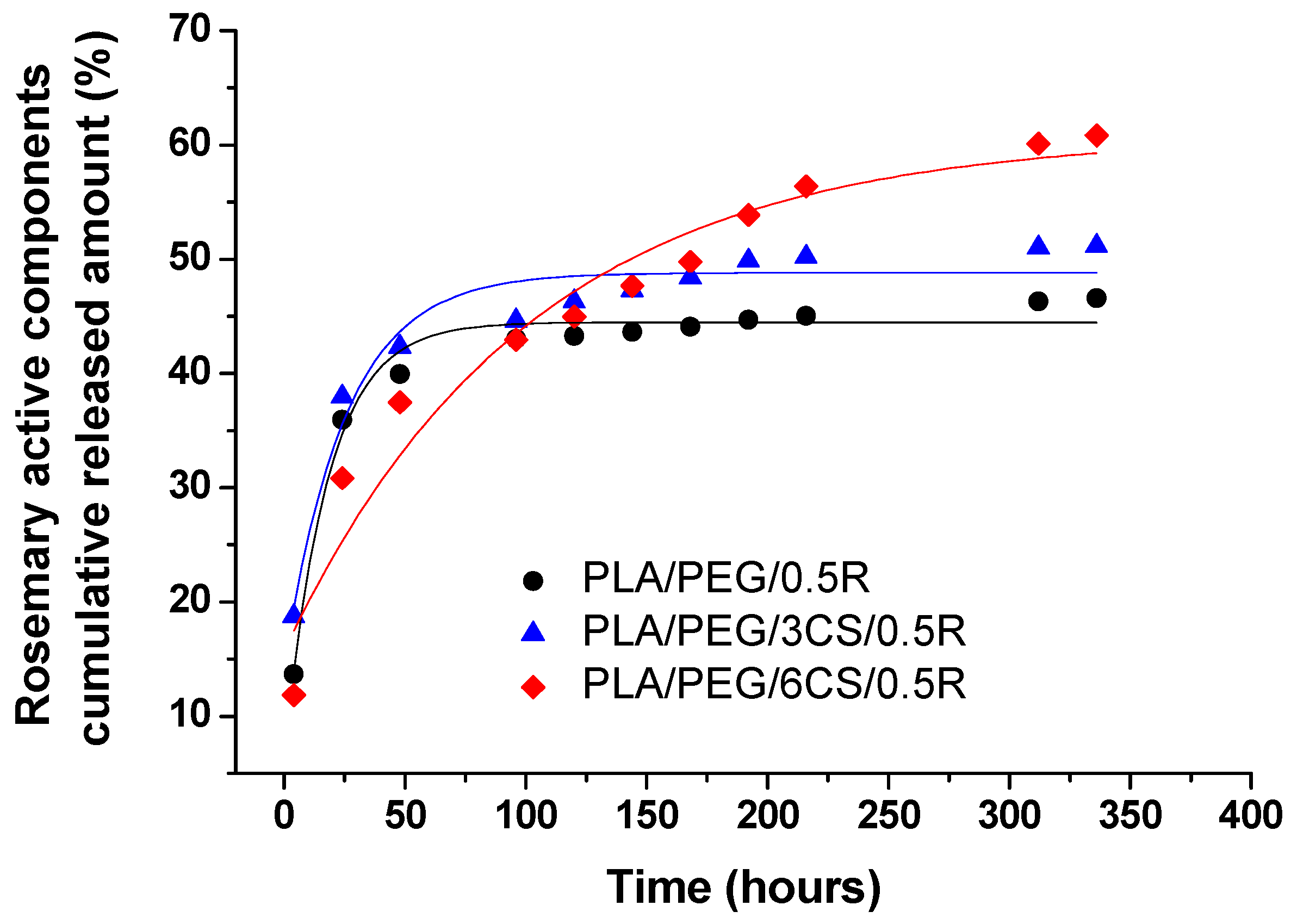
3.8. Migration Study of the Active Components from Powdered Rosemary Alcoholic Extract into Food Simulant from PLA/PEG/CS/R-based Biocomposite Films
3.9. Biocompatibility Study
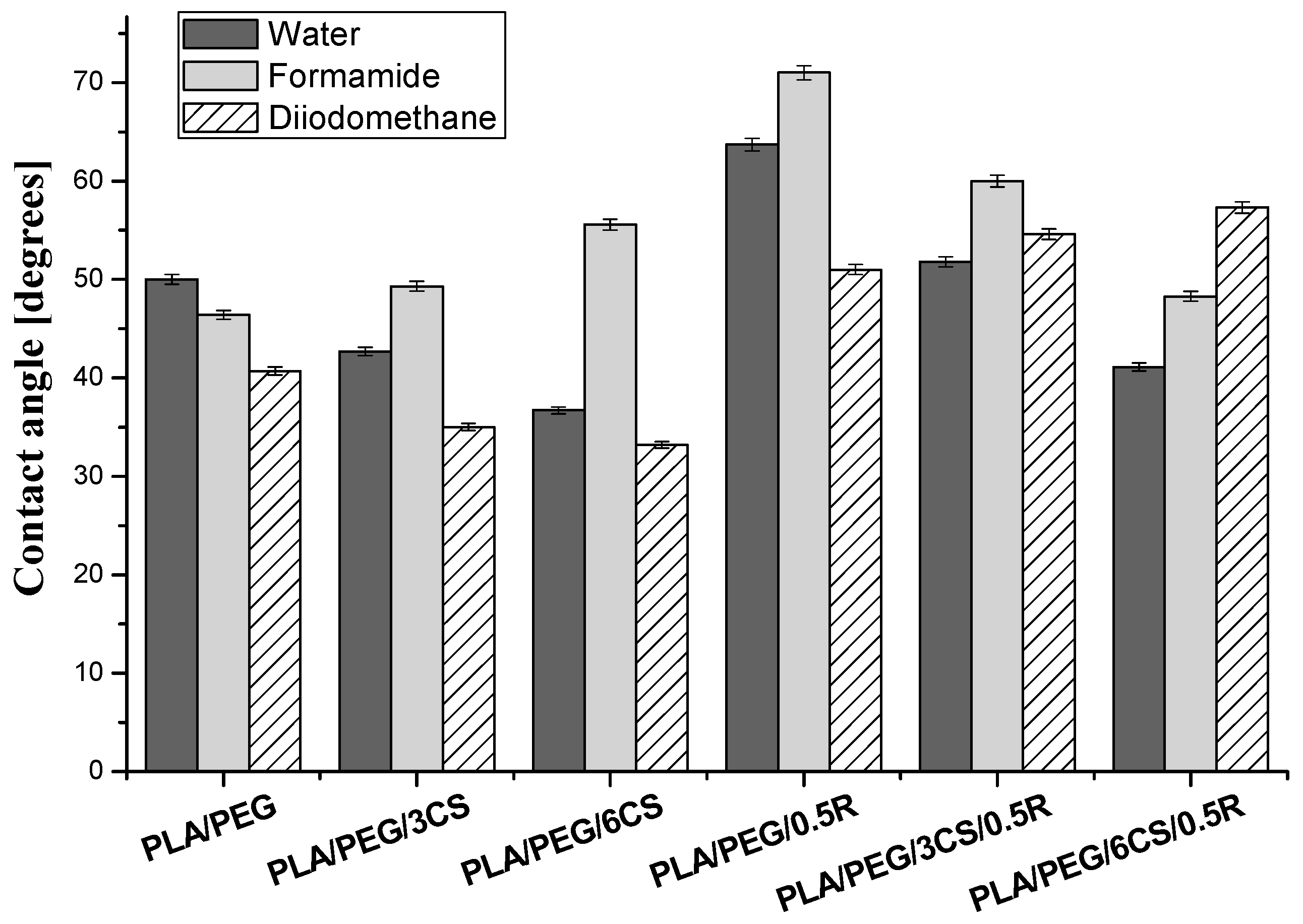
3.9.1. In Vitro Biocompatibility Evaluation by Contact Angle Measurements—Determination of Wettability, Surface Free Energy, and Work of Spreading
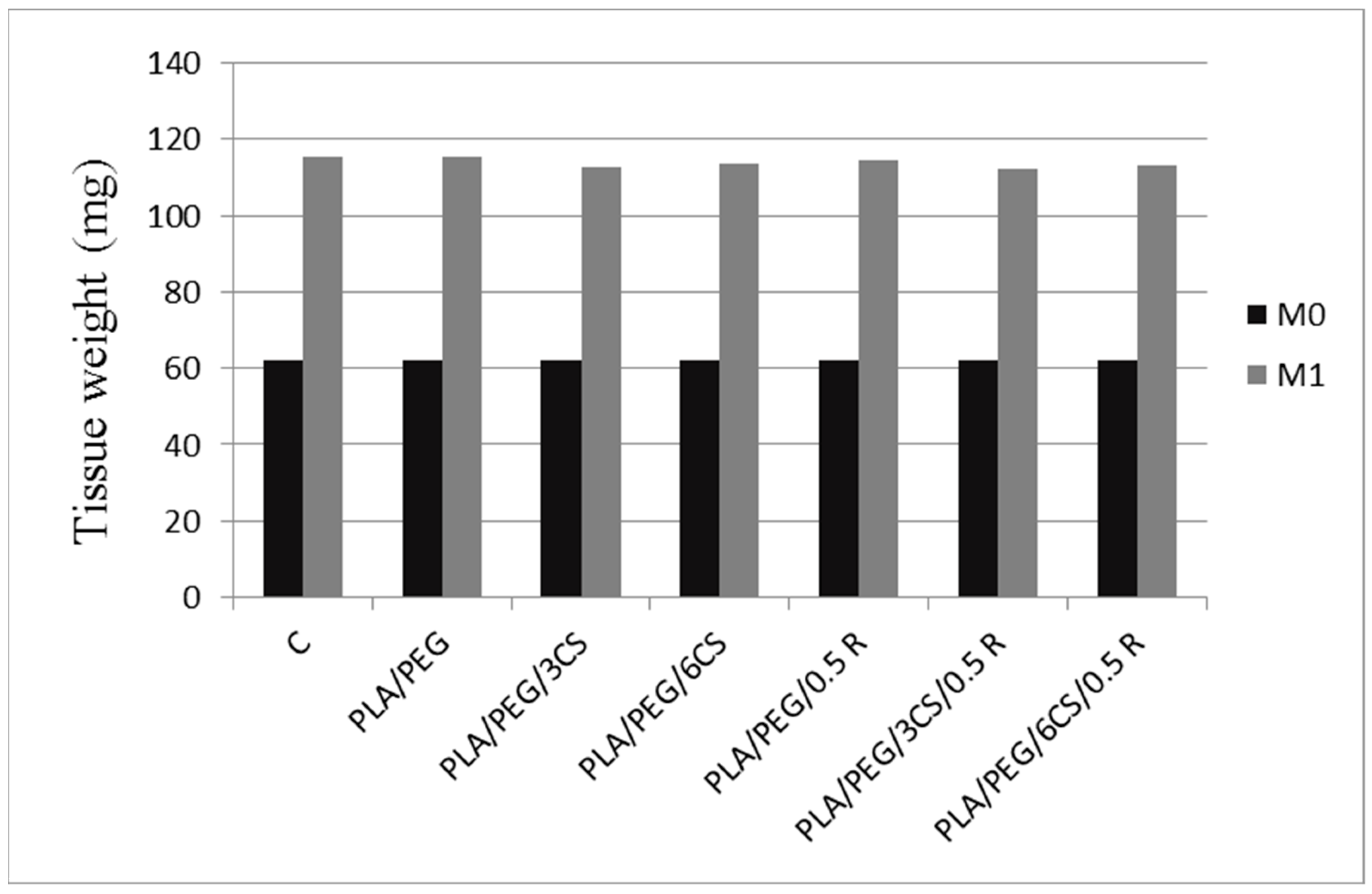
3.9.2. In Vivo Biocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Catala, R.; Lopez-Carballo, G.; Hernandez-Munoz, P.; Gavara, R. PLA and active packaging. In RSC Polymer Chemistry Series No. 12 Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; Jimenez, A., Peltzer, M., Ruseckaiter, R., Eds.; The Royal Society of Chemistry: London, UK, 2015; Chapter 10; pp. 245–268. ISBN 978-1-84973-879-8. [Google Scholar]
- Karkhanis, S.S.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Effect of compounding approaches on fiber dispersion and performance of poly(lactic acid)/cellulose nanocrystal composite blown films. J. Appl. Polym. Sci. 2017, 134, 45212–45222. [Google Scholar] [CrossRef]
- Karkhanis, S.S.; Matuana, L.M.; Stark, N.M.; Sabo, R.C. Effect of Compounding Approaches on Fiber Dispersion and Performance of Poly(Lactic Acid)/Cellulose Nanocrystal Composite Blown Films. ANTEC® 2017 -Anaheim, California, USA May 8–10, 2017; Society of Plastics Engineers: Bethel, CT, USA, 2017; ISBN 978-0-692-88309-9. [Google Scholar]
- Sarazin, P.; Li, G.; Orts, W.J.; Favis, B.D. Binary and ternary blends of polylactide, polycaprolactone and thermoplastic starch. Polymer 2008, 49, 599–609. [Google Scholar] [CrossRef]
- Feng, C.S.; Piao, M.H.; Li, D. Stereocomplex-reinforced pegylated polylactide micelle for optimized drug delivery. Polymers 2016, 8, 165. [Google Scholar] [CrossRef]
- Fiori, S. Industrial uses of PLA. In RSC Polymer Chemistry Series No. 12 Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; Jimenez, A., Peltzer, M., Ruseckaiter, R., Eds.; The Royal Society of Chemistry: London, UK, 2015; Chapter 13; pp. 317–335. ISBN 978-1-84973-879-8. [Google Scholar]
- Martin, O.; Averous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslén, B. The effects of plasticizers on the dynamic mechanical and thermal properties of poly(lactic acid). J. Appl. Polym. Sci. 2002, 86, 1227–1234. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslén, B. Tributylcitrate oligomers as plasticizers for poly (lactic acid): Thermo-mechanical film properties and aging. Polymer 2003, 44, 7679–7688. [Google Scholar] [CrossRef]
- Butnaru, E.; Stoleru, E.; Brebu, M.A.; Darie-Nita, R.N.; Bargan, A.; Vasile, C. Chitosan-based bionanocomposite films prepared by emulsion technique for food preservation. Materials 2019, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Moreno, G.M.; Ramirez, K.; Esquivel, M.; Jimenez, G. Biocomposite films of polylactic acid reinforced with microcrystalline cellulose from pineapple leaf fibers. J. Renew. Mater. 2019, 7, 9–20. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Kenny, J.M. Development and thermal behaviour of ternary PLA matrix composites. Polym. Degrad. Stability 2010, 95, 2200–2206. [Google Scholar] [CrossRef]
- Frone, A.N.; Berlioz, S.; Chailan, J.-F.; Panaitescu, D.M. Morphology and thermal properties of PLA–cellulose nanofibers composites. Carbohydr. Polym. 2013, 91, 377–384. [Google Scholar] [CrossRef]
- Chang, C.-P.; Wang, I.-C.; Perng, Y.-S. Enhanced thermal behavior, mechanical properties and UV shielding of polylactic acid (PLA) composites reinforced with nanocrystalline cellulose and filled with nanosericite. Cellulose Chem. Technol. 2013, 47, 111–123. [Google Scholar]
- Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, crystallization and thermal behaviors of PLA-based composites: Wonderful effects of hybrid GO/PEG via dynamic impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric nanocomposites and nanocoatings for food packaging: A review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Review article. Front. Chem. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Tavassoli, S.; Djomeh, Z.E. Total phenols, antioxidant potential and antimicrobial activity of methanol extract of rosemary (Rosmarinus officinalis L.). Global Veterinaria 2011, 7, 337–341. [Google Scholar]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Rahmat, A.R.; Rahman, W.A.W.A.; Tana, A.-C.; Vikhraman, M. Review. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Han, J.H. Antimicrobial packaging systems plastic films in food packaging. Plast. Des. Libr. 2013, 151–180. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Europ. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Lehr, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.-J.; Chen, S.-Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. Part B 2007, 84B, 131–137. [Google Scholar] [CrossRef]
- Bagheri-Khoulenjani, S.; Taghizadeh, S.M.; Mirzadeh, H. An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohydr. Polym. 2009, 78, 773–778. [Google Scholar] [CrossRef]
- Vaarum, K.M.; Myhr, M.M.; Hjerde, R.J.N.; Smidsroed, O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997, 299, 99–101. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N., Jr. Chitosan in nanostructured thin films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.; Guha, A.K. Clarification of fruit juice with chitosan. Process Biochem. 2004, 39, 2229–2232. [Google Scholar] [CrossRef]
- Baican, M.; Vasile, C. Chitosan containing biomaterials for tissue engineering applications. In Advances in Polymers for Biomedical Applications; Pathania, D., Gupta, B., Eds.; Series: Polymer Science and Technology Nova Science: New York, NY, USA, 2018; Chapter 9; pp. 221–279. ISBN 978-1-53613-612-8. [Google Scholar]
- Cheaburu-Yilmaz, C.N.; Tuncay Tanriverdi, S.; Ozer, O.; Vasile, C. Polysaccharide containing gels for pharmaceutical applications. In Polymer Gels. Science and Fundamentals; Thakur, V.K., Thakur, M.K., Eds.; Springer Nature: Singapore, 2018; Chapter 6; pp. 231–278. ISBN1 978-981-10-6086-1. ISBN2 978-981-10-6085-4. [Google Scholar]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef]
- Shavandia, A.; El-Din, A.; Bekhita, A.; Ali, M.A.; Sun, Z. Bio-mimetic composite scaffold from mussel shells, squid pen and crab chitosan for bone tissue engineering. Intern. J. Biolog. Macromol. 2015, 80, 445–454. [Google Scholar] [CrossRef]
- Shavandia, A.; El-Din, A.; Bekhita, A.; Sun, Z.; Alic, M.A. Bio-scaffolds produced from irradiated squid pen and crab chitosan with hydroxyapatite/β-tricalcium phosphate for bone-tissue engineering. Intern. J. Biolog. Macromol. Part B 2016, 93, 1446–1456. [Google Scholar] [CrossRef]
- Liu, H.; Yin, Y.; Yao, K. Construction of chitosan-gelatin-hyaluronic acid artificial skin in vitro. J. Biomater. Appl. 2007, 21, 413–430. [Google Scholar] [CrossRef]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A special report on the chitosan-based hemostatic dressing: Experience in current combat operations. J. Trauma 2006, 60, 655–658. [Google Scholar] [CrossRef]
- Lin, S.B.; Chen, S.H.; Peng, K.C. Preparation of antibacterial chito-oligosaccharide by altering the degree of deacetylation of β-chitosan in a Trichoderma harzianum chitinase-hydrolysing process. J. Sci. Food Agric. 2009, 89, 238–244. [Google Scholar] [CrossRef]
- Rajalakshmi, A.; Krithiga, N.; Jayachitra, A. Antioxidant activity of the chitosan extracted from shrimp exoskeleton, Middle-East. J. Sci. Res. 2013, 16, 1446–1451. [Google Scholar] [CrossRef]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Chen, C.-Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Chitravadivu, C.; Manian, S.; Kalaichelvi, K. Antimicrobial studies on selected medicinal plants, Erode region, Tamilnadu, India. Middle-East J. Sci. Res. 2009, 4, 147–152. [Google Scholar]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health benefits and use as functional ingredient in meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Angiolella, L.; Sacchetti, G.; Efferth, T. Antimicrobial and antioxidant activities of natural compounds. Evid. Based Complement. Alternat. Med. 2018, 1945179. [Google Scholar] [CrossRef]
- Surendran Nair, M.; Upadhyaya, I.; Amalaradjou, M.A.; Venkitanarayanan, K. Antimicrobial food additives and disinfectants: mode of action and microbial resistance mechanisms. In Food Borne Pathogens and Antibiotic Resistance; Om, V.S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Chapter 12; pp. 275–301. [Google Scholar] [CrossRef]
- Ôzcan, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) extracts on natural olive and sesame oils. Grasas y Aceites 1999, 50, 355–358. [Google Scholar] [CrossRef]
- Wu, J.W.; Lee, M.-H.; Ho, C.-T.; Chang, S.S. Elucidation of the chemical structures of natural antioxidants isolated from rosemary. J. Am. Oil Chem. Soc. 1982, 59, 339–345. [Google Scholar] [CrossRef]
- Ho, C.-T.; Houlihan, C.M.; Chang, S.S. Structural determination of two antioxidants isolated, from rosemary—Abstract of papers. In Proceedings of the 86th American Chemical Society Meeting, Washington, DC, USA, 20–25 March 1983. [Google Scholar]
- Ojeda, A.; Van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Nartop, P.; Gurel, A.; Bedir, E.; Vardar-Sukan, F. Determination of phenolic content and antioxidant activity of extracts obtained from Rosmarinus officinalis’ calli. Plant Physiol. 2007, 164, 1536–1542. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Human Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef]
- Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Suhaj, M. Spice antioxidants isolation and their antiradical activity: A review. J. Food Compos. Anal. 2006, 19, 531–537. [Google Scholar] [CrossRef]
- Maheshwari, N. Overview of Plant-Based Natural Antioxidants and Effect of Thermal Decomposition. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2016. Available online: http://docplayer.net/29265117-Overview -of-plant-based-natural-antioxidants-and-effect-of-thermal-decomposition-neha-maheshwari.html (accessed on 5 April 2019).
- Matejczyk, M.; Swisłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial, rheological, and thermal properties of plasticized polylactide films incorporated with essential oils to inhibit Staphylococcus aureus and Campylobacter jejuni. J. Food Sci. 2016, 81, E419–E429. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, S.H.; Abdullah, L.C.; Aung, M.M.; Ratnam, C.T. A study of mechanical and morphological properties of PLA based biocomposites prepared with EJO vegetable oil based plasticizer and kenaf fibres. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012011. [Google Scholar] [CrossRef]
- Popa, H.C.; Jipa, S.; Zaharescu, T.; Kefalas, P. Kinetic approach of natural antioxidant depletion during thermal oxidation. J. Optoel. Adv. Mat. Rapid Comm. 2010, 4, 223–227. [Google Scholar]
- Darie-Nita, R.N.; Vasile, C.; Stoleru, E.; Pamfil, D.; Zaharescu, T.; Tartau, L.; Tudorachi, N.; Brebu, M.A.; Pricope, G.M.; Dumitriu, R.P.; et al. Evaluation of the rosemary extract effect on the properties of polylactic acid-based materials. Materials 2018, 11, 1825. [Google Scholar] [CrossRef]
- Rapa, M.; Mitelut, A.C.; Tanase, E.E.; Grosu, E.; Popescu, P.; Popa, M.E.; Rosnes, J.T.; Sivertsvik, M.; Darie-Niţă, R.N.; Vasile, C. Influence of chitosan on mechanical, thermal, barrier and antimicrobial properties of PLA-biocomposites for food packaging. Compos. Part B Eng. 2016, 102, 112–121. [Google Scholar] [CrossRef]
- Shen, R.; Xu, W.; Xue, Y.; Chen, L.; Ye, H.; Zhong, E.; Ye, Z.; Gao, J.; Yan, Y. The use of chitosan/PLA nano-fibers by emulsion eletrospinning for periodontal tissue engineering. Artif Cells Nanomed. Biotechnol. 2018, 46, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ding, S.; Zhou, C. Preparation and degradation of PLA/chitosan composite materials. J. Appl. Polym. Sci. 2004, 91, 274–277. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Vargas, M.; Chiralt, A.; Kenny, J.M. Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. J. Food Eng. 2013, 119, 236–243. [Google Scholar] [CrossRef]
- Zaharescu, T.; Jipa, S.; Mariş, D.A.; Mariş, M.; Kappel, W. Effect of rosemary extract on the radiation stability of UHMWPE. Polymers 2009, 149. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood-comparison of different estimation methods. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effect on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lai, W.C.; Liau, W.B.; Lin, T.T. The effect of end groups of PEG on the crystallization behaviors of binary crystalline polymer blends PEG/PLLA. Polymer 2004, 45, 3073–3080. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) no. 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food; Official Journal of the European Union: Brussels, Belgium, 2011; pp. 2–89. [Google Scholar]
- European Commission. Technical Guidelines for Compliance Testing, In the Framework of Regulation (EU) No. 10/2011 on Plastic Food Contact Materials/Draft for Consultation; EU, JRC Science and Policy Reports; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Mulinacci, N.; Innocenti, M.; Bellumori, M.; Giaccherini, C.; Martini, V.; Michelozzi, M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 2011, 85, 167–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar] [PubMed]
- Poças, M.F.; Oliveira, J.C.; Oliveira, F.A.R.; Hogg, T. A critical survey of predictive mathematical models for migration from packaging. Crit. Rev. Food Sci. Nutr. 2008, 48, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, E.; Baican, M.C.; Coroaba, A.; Hitruc, G.E.; Lungu, M.; Vasile, C. Plasma-activated fibrinogen coatings onto poly(vinylidene fluoride) surface for improving biocompatibility with tissues. J. Bioact. Compat. Polym. 2016, 31, 91–108. [Google Scholar] [CrossRef]
- Pascu, M. Contact angle method. In Surface Properties of Polymers; Vasile, C., Pascu, M.C., Eds.; Research Signpost: Trivandrum, India, 2007; pp. 179–201. ISBN 987-81-308-0142-1. [Google Scholar]
- Vasile, C.; Pascu, M.; Bumbu, G.G.; Cojocariu, A. Surface properties of polymers. In Surface Properties of Polymers; Research Signpost: Trivandrum, India, 2007; pp. 1–64. ISBN 987-81-308-0142-1. [Google Scholar]
- Yuan, Y.; Randall Lee, T. Contact angle and wetting properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer Series in Surface Sciences; Springer-Verlag: Berlin, Germany, 2013; pp. 3–34. [Google Scholar]
- Stoleru, E.; Munteanu, B.S.; Darie-Niţă, R.N.; Pricope, G.M.; Lungu, M.; Irimia, A.; Râpă, M.; Lipşa, R.D.; Vasile, C. Complex poly(lactic acid)-based biomaterial for urinary catheters: II. Biocompatibility. Bioinspired Biomim. Nanobiomater. 2016, 5, 152–166. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Pascu, M.-C.; Popescu, M.-C.; Vasile, C. Surface modifications of some nanocomposites containing starch. J. Phys. D Appl. Phys. 2008, 41, 175407. [Google Scholar] [CrossRef]
- Popescu, M.C.; Vasile, C.; Macocinschi, D.; Lungu, M.; Craciunescu, O. Biomaterials based on new polyurethane and hydrolyzed collagen, k-elastin, hyaluronic acid and chondroitin sulfate. Int. J. Biol. Macromol. 2010, 47, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Onofrei, M.D.; Dobos, A.M.; Dunca, S.; Ioanid, E.G.; Ioan, S. Biocidal activity of cellulose materials for medical implants. J. Appl. Polym. Sci. 2015, 132, 41932. [Google Scholar] [CrossRef]
- Macocinschi, D.; Filip, D.; Butnaru, M.; Dimitriu, C.D. Surface characterization of biopolyurethanes based on cellulose derivatives. J. Mater. Sci: Mater. Med. 2009, 20, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, K.; Deepak, K.; Pattanayak, D.K.; Rama Mohan, T.R.; Banerjee, R. Interpenetring blood-biomaterial interactions from surface free energy and work of adhesion. Trends Biomater. Artif. Organs 2005, 18, 73–83. [Google Scholar]
- Albu, R.M.; Avram, E.; Stoica, I.; Ioanid, E.G.; Popovici, D.; Ioan, S. Surface properties and compatibility with blood of new quaternized polysulfones. J. Biomat. Nanobiotech. 2011, 2, 114–124. [Google Scholar] [CrossRef]
- Hodgson, E. A Textbook of Modern Toxicology, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 978-0-470-46206-5. [Google Scholar]
- Wolf, M.F.; Andwraon, J.M. Practical approach to blood compatibility assessments: General considerations and standards. In Biocompatibility and Performance of Medical Devices; Boutrand, J.-P., Ed.; Woodhead Publishing Ltd.: Oxford, UK, 2012; pp. 159–200. [Google Scholar]
- Coleman, K.; Dai, X.; Deng, X.; Lakehal, F.; Tang, X.M. Medical device biocompatibility evaluation: An industry perspective. In Biocompatibility and Performance of Medical Devices; Boutrand, J.-P., Ed.; Woodhead Publishing Series in Biomaterials: Number 50 Ltd.: Oxford, UK, 2012; pp. 201–206. [Google Scholar]
- Lupuşoru, C.E. Imunofarmacologie; ALFA: Iaşi, Romania, 2001; pp. 288–294. [Google Scholar]
- Peacman, M. Clinical & Experimental Immunology; British Society of Immunology, Wiley Library: Hoboken, NJ, USA, 2011. [Google Scholar]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- AVMA Guidelines on Euthanasia, 2007; AVMA Guidelines on Euthanasia (Formerly Report of the AVMA Panel on Euthanasia). June 2007, pp. 6–7. Available online: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (accessed on 27 May 2019).
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized vegetable oils plasticized poly(lactic acid) biocomposites: Mechanical, thermal and morphology properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Del Angel-López, D.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Perales-Castro, M.E.; Flores-Vela, A. Morphological and mechanical properties dependence of PLA amount in PET matrix processed by single screw extrusion. Polym. Plastics Technol. Eng. 2016, 55, 672–683. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Zakaria, Z.; Islam, M.; Hassan, A.; Haafiz, M.K.M.; Arjmandi, R.; Inuwa, I.M.; Hasan, M. Mechanical properties and morphological characterization of PLA/chitosan/epoxidized natural rubber composites. Adv. Mater. Sci. Eng. 2013. [Google Scholar] [CrossRef]
- Bijarimi, M.; Ahmad, S.; Rasid, R.; Khushairi, M.A.; Zakir, M. Poly(lactic acid)/poly(ethylene glycol) blends: Mechanical, thermal and morphological properties. AIP Conf. Proc. 2016, 1727, 020002. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Effect of chitosan and catechin addition on the structural, thermal, mechanical and disintegration properties of plasticized electrospun PLA-PHB biocomposites. Polym. Degrad. Stab. 2016, 132, 145–156. [Google Scholar] [CrossRef]
- Anuar, H.; Noor Azlina, H.; Suzana, A.B.K.; Kaiser, M.R.; Bonnia, N.N.; Surip, S.N.; Abd Razak, S.B. Effect of PEG on impact strength of PLA hybrid biocomposite. In Proceedings of the IEEE Symposium on Business, Engineering and Industrial Applications, Bandung, Indonesia, 23–26 September 2012; pp. 473–476. [Google Scholar] [CrossRef]
- Ghalia, M.A.; Dahman, Y. Investigating the effect of multi-functional chain extenders on PLA/PEG copolymer properties. Intern. J. Biol. Macromol. 2017, 95, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.K.; Bhattacharjee, S.K.; Gaur, S.S.; Pal, A.; Katiyar, V. Chemomechanical, morphological, and rheological studies of chitosan-graft-lactic acid oligomer reinforced poly(lactic acid) bionanocomposite films. J. Appl. Polym. Sci. 2018, 135, 45546. [Google Scholar] [CrossRef]
- Petkova-Parlapanska, K.; Nancheva, V.; Diankov, S.; Hinkov, I.; Karsheva, M. Rheological properties of cosmetic compositions containing rosemary and grapefruit pulp and seeds extracts. J. Chem. Technol. Metall. 2014, 49, 487–493. [Google Scholar]
- Szymańsk, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs. 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Houlihan, C.; Ho, C.; Chang, S. Elucidation of the chemical structure of a novel antioxidant, rosmaridiphenol, isolated from rosemary. J. Am. Oil Chem. Soc. 1984, 61, 1036–1039. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Zhang, H.; Pan, Z. Nanoporous PLA/(Chitosan Nanoparticle) composite fibrous membranes with excellent air filtration and antibacterial performance. Polymers 2018, 10, 1085. [Google Scholar] [CrossRef]
- Bernardes, W.A.; Lucarini, R.; Tazatti, M.G.; Souza, M.; Silva, M.L.; Filho, A.A.; Martins, C.H.; Crotti, A.E.; Pauletti, P.M.; Groppo, M.; et al. Antimicrobial activity of Rosmarinus officinalis against oral pathogens: Relevance of carnosic acid and carnosol. Chem. Biodiv. 2010, 7, 835–1840. [Google Scholar] [CrossRef]
- Harborne, J.B.; Mabry, T.J.; Mabry, H. The Flavonoids: Advances in Research; Harborne, J.B., Mabry, T.J., Eds.; Springer Science Buisness, B.V. Media: London, UK, 1982; ISBN 978-1-4899-2915-0. [Google Scholar]
- Tehrany, E.A.; Desobry, S. Partition coefficients in food/packaging systems: A review. Food Addit. Contam. 2004, 21, 1186–1202. [Google Scholar] [CrossRef] [PubMed]
- Commission Directive. Commission Directive 2002/72/EC Relating to Plastic Materials and Articles Intended to Come into Contact with Foodstuffs; Official Journal of European Communities: Brussels, Belgium, 2002. [Google Scholar]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef]
- Courtney, J.M.; Lamba, N.M.; Sundaram, S.; Forbes, C.D. Biomaterials for blood-contacting applications. Biomaterials 1994, 15, 737–744. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Stoleru, E.; Diaconu, A.; Tudorachi, N. Tailorable polyelectrolyte protein complex based on poly(aspartic acid) and bovine serum albumin. Des. Monomers Polym. 2016, 19, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Liu, M.; Wu, G.; Zhang, X.; Yu, J.; Zhang, Y.; Wang, P.; Yin, X. Enhanced surface hydrophilicity of polylactic acid sutures treated by lipase and chitosan. Textile Res. J. 2018. [Google Scholar] [CrossRef]

| No. | Sample | PLA (wt %) | PEG (wt %) | Chitosan (CS) (wt %) | Rosemary Ethanolic Extract (R) (wt %) |
|---|---|---|---|---|---|
| 1 | PLA/PEG | 80 | 20 | - | - |
| 2 | PLA/PEG/3CS | 77 | 20 | 3 | - |
| 3 | PLA/PEG/6CS | 74 | 20 | 6 | - |
| 4 | PLA/PEG/0.5R | 79.5 | 20 | - | 0.5 |
| 5 | PLA/PEG/3CS/0.5R | 76.5 | 20 | 3 | 0.5 |
| 6 | PLA/PEG/6CS/0.5R | 73.5 | 20 | 6 | 0.5 |
| Liquid | |||||
|---|---|---|---|---|---|
| Water | 72.80 | 21.80 | 51.00 | 25.50 | 25.50 |
| Formamide | 58.00 | 39.00 | 19.00 | 2.28 | 39.6 |
| Methylene iodide | 50.80 | 50.80 | 0.00 | 0.72 | 0.00 |
| Red blood cells (rbc) | 36.56 | 35.2 | 1.36 | 0.01 | 46.2 |
| Platelets (p) | 118.24 | 99.14 | 19.1 | 12.26 | 7.44 |
| Sample | TQmax1 (Nm) | TQ1min (Nm) | TQmax2 (Nm) | TQ5min (Nm) | TQfinal (Nm) |
|---|---|---|---|---|---|
| PLA/PEG | 12.9 | 0.9 | - | 7.3 | 6.5 |
| PLA/PEG/3CS | 8.8 | 1.7 | 11.0 | 6.5 | 6.0 |
| PLA/PEG/6CS | 6.6 | 1.4 | 12.1 | 7.6 | 6.2 |
| PLA/PEG/0.5R | 10.1 | 2.8 | - | 7.3 | 5.7 |
| PLA/PEG/3CS/0.5R | 8.7 | 1.5 | 10.6 | 6.1 | 5.9 |
| PLA/PEG/6CS/0.5R | 9.5 | 2.8 | 12.6 | 7.1 | 5.5 |
| Sample | Impact Strength (kJ/m2) | Impact Energy (J) |
|---|---|---|
| PLA/PEG | 2.21 | 0.11 |
| PLA/PEG/3CS | 3.18 | 0.13 |
| PLA/PEG/6CS | 21.15 | 0.88 |
| PLA/PEG/0.5R | 4.52 | 0.18 |
| PLA/PEG/3CS/0.5R | 15.20 | 0.62 |
| PLA/PEG/6CS/0.5R | 12.39 | 0.51 |
| Sample | Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| Bacillus cereus | Salmonella typhymurium | Escherichia coli | ||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| LDPE | 6 | 18 | 3 | 8 | 7 | 13 |
| PLA/PEG | 45 | 91 | 29 | 77 | 69 | 94 |
| PLA/PEG/3CS | 86 | 100 | 58 | 100 | 73 | 100 |
| PLA/PEG/6CS | 86 | 100 | 58 | 100 | 73 | 100 |
| PLA/PEG/0.5R | 86 | 100 | 48 | 100 | 76 | 100 |
| PLA/PEG/3CS/0.5R | 100 | 100 | 81 | 100 | 76 | 100 |
| PLA/PEG/6CS/0.5R | 100 | 100 | 90 | 100 | 82 | 100 |
| Samples | Peppas/Power Law Model | First Order Kinetic Model | Diffusion Model | KP | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | R2 | k × 103 (h−n) | R2 | k1 × 103 (h−n) | R2 | D × 10−13 (m2/s) | R2 | ||
| PLA/PEG/0.5R | 0.37 | 0.99 | 85.12 | 0.98 | 5.20 | 0.84 | 1.7 | 0.98 | 1.06 |
| PLA/PEG/3CS/0.5R | 0.23 | 0.98 | 147.78 | 0.99 | 5.17 | 0.81 | 2.05 | 0.97 | 0.95 |
| PLA/PEG/6CS/0.5R | 0.38 | 0.99 | 72.92 | 0.99 | 3.7 | 0.92 | 1.05 | 0.99 | 0.64 |
| Sample Code | Ws/rbc | Ws/p | |||||
|---|---|---|---|---|---|---|---|
| PLA/PEG | 39.17 | 1.92 | 0.03 | 34.91 | 41.09 | 4.54 | −69.58 |
| PLA/PEG/3CS | 41.94 | 6.98 | 0.25 | 49.47 | 48.92 | 14.42 | −49.46 |
| PLA/PEG/6CS | 42.76 | 20.34 | 1.54 | 67.30 | 63.10 | 22.22 | −43.83 |
| PLA/PEG/0.5R | 33.64 | 13.95 | 1.27 | 38.25 | 47.59 | 12.27 | −71.51 |
| PLA/PEG/3CS/0.5R | 31.61 | 3.90 | 0.08 | 45.87 | 35.51 | −1.14 | −75.52 |
| PLA/PEG/6CS/0.5R | 30.07 | 7.72 | 0.30 | 49.53 | 37.78 | 0.81 | −75.01 |
| Groups | Variation of the Mean Animal’s Weight (g) |
|---|---|
| C | +8.2 |
| PLA/PEG | +8.1 |
| PLA/PEG/3CS | +7.6 |
| PLA/PEG/6CS | +7.9 |
| PLA/PEG/0.5R | +8.0 |
| PLA/PEG/3CS/0.5R | +7.3 |
| PLA/PEG/6CS/0.5R | +7.7 |
| Groups | Leucocyte Formula (% Values) | |||||
|---|---|---|---|---|---|---|
| PMN | Ly | E | M | B | ||
| Control | 24 h | 29.5 ± 0.83 | 66.3 ± 2.11 | 0.6 ± 0.08 | 3.4 ± 0.10 | 0.2 ± 0.10 |
| 7 days | 29.7 ± 0.47 | 65.9 ± 1.93 | 0.7 ± 0.10 | 3.5 ± 0.10 | 0.2 ± 0.05 | |
| PLA/PEG | 24 h | 29.8 ± 0.89 | 65.7 ± 1.39 | 0.7 ± 0.10 | 3.6 ± 0.05 | 0.2 ± 0.04 |
| 7 days | 29.9 ± 0.55 | 65.5 ± 1.63 | 0.7 ± 0.05 | 3.7 ± 0.12 | 0.2 ± 0.05 | |
| PLA/PEG/3CS | 24 h | 29.1 ± 0.37 | 66.7 ± 2.14 | 0.6 ± 0.08 | 3.4 ± 0.05 | 0.2 ± 0.05 |
| 7 days | 29.4 ± 0.98 | 66.1 ± 1.33 | 0.8 ± 0.1 | 3.5 ± 0.08 | 0.2 ± 0.05 | |
| PLA/PEG/6CS | 24 h | 29.3 ± 0.72 | 66.3 ± 1.55 | 0.7 ± 0.05 | 3.5 ± 0.08 | 0.2 ± 0.04 |
| 7 days | 29.5 ± 0.65 | 66.1 ± 2.04 | 0.7 ± 0.08 | 3.5 ± 0.05 | 0.2 ± 0.05 | |
| PLA/PEG/0.5R | 24 h | 29.8 ± 0.27 | 65.7 ± 1.98 | 0.6 ± 0.10 | 3.7 ± 0.10 | 0.2 ± 0.05 |
| 7 days | 29.9 ± 1.63 | 65.4 ± 1.47 | 0.8 ± 0.13 | 3.7 ± 0.05 | 0.2 ± 0.05 | |
| PLA/PEG/3CS/0.5R | 24 h | 29.5 ± 0.45 | 66.1 ± 1.39 | 0.7 ± 0.08 | 3.5 ± 0.1 | 0.2 ± 0.04 |
| 7 days | 29.2 ± 0.69 | 66.2 ± 1.72 | 0.8 ± 0.05 | 3.6 ± 0.08 | 0.2 ± 0.05 | |
| PLA/PEG/6CS/0.5R | 24 h | 29.6 ± 0.33 | 66.0 ± 1.89 | 0.8 ± 0.1 | 3.4 ± 0.08 | 0.2 ± 0.05 |
| 7 days | 29.7 ± 0.47 | 65.9 ± 1.37 | 0.7 ± 0.08 | 3.5 ± 0.1 | 0.2 ± 0.05 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile, C.; Stoleru, E.; Darie-Niţa, R.N.; Dumitriu, R.P.; Pamfil, D.; Tarţau, L. Biocompatible Materials Based on Plasticized Poly(lactic acid), Chitosan and Rosemary Ethanolic Extract I. Effect of Chitosan on the Properties of Plasticized Poly(lactic acid) Materials. Polymers 2019, 11, 941. https://doi.org/10.3390/polym11060941
Vasile C, Stoleru E, Darie-Niţa RN, Dumitriu RP, Pamfil D, Tarţau L. Biocompatible Materials Based on Plasticized Poly(lactic acid), Chitosan and Rosemary Ethanolic Extract I. Effect of Chitosan on the Properties of Plasticized Poly(lactic acid) Materials. Polymers. 2019; 11(6):941. https://doi.org/10.3390/polym11060941
Chicago/Turabian StyleVasile, Cornelia, Elena Stoleru, Raluca Nicoleta Darie-Niţa, Raluca Petronela Dumitriu, Daniela Pamfil, and Liliana Tarţau. 2019. "Biocompatible Materials Based on Plasticized Poly(lactic acid), Chitosan and Rosemary Ethanolic Extract I. Effect of Chitosan on the Properties of Plasticized Poly(lactic acid) Materials" Polymers 11, no. 6: 941. https://doi.org/10.3390/polym11060941
APA StyleVasile, C., Stoleru, E., Darie-Niţa, R. N., Dumitriu, R. P., Pamfil, D., & Tarţau, L. (2019). Biocompatible Materials Based on Plasticized Poly(lactic acid), Chitosan and Rosemary Ethanolic Extract I. Effect of Chitosan on the Properties of Plasticized Poly(lactic acid) Materials. Polymers, 11(6), 941. https://doi.org/10.3390/polym11060941