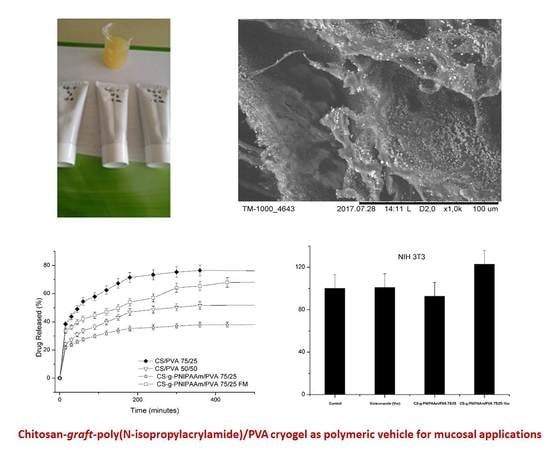
Chitosan-Graft-Poly(N-Isopropylacrylamide)/PVA Cryogels as Carriers for Mucosal Delivery of Voriconazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Freeze–Thaw Hydrogels CS-g-PNIPAAm/PVA
2.3. Preparation and Optimization of Pharmaceutical Formulation
2.4. Characterization Methods
2.4.1. FTIR Spectroscopy
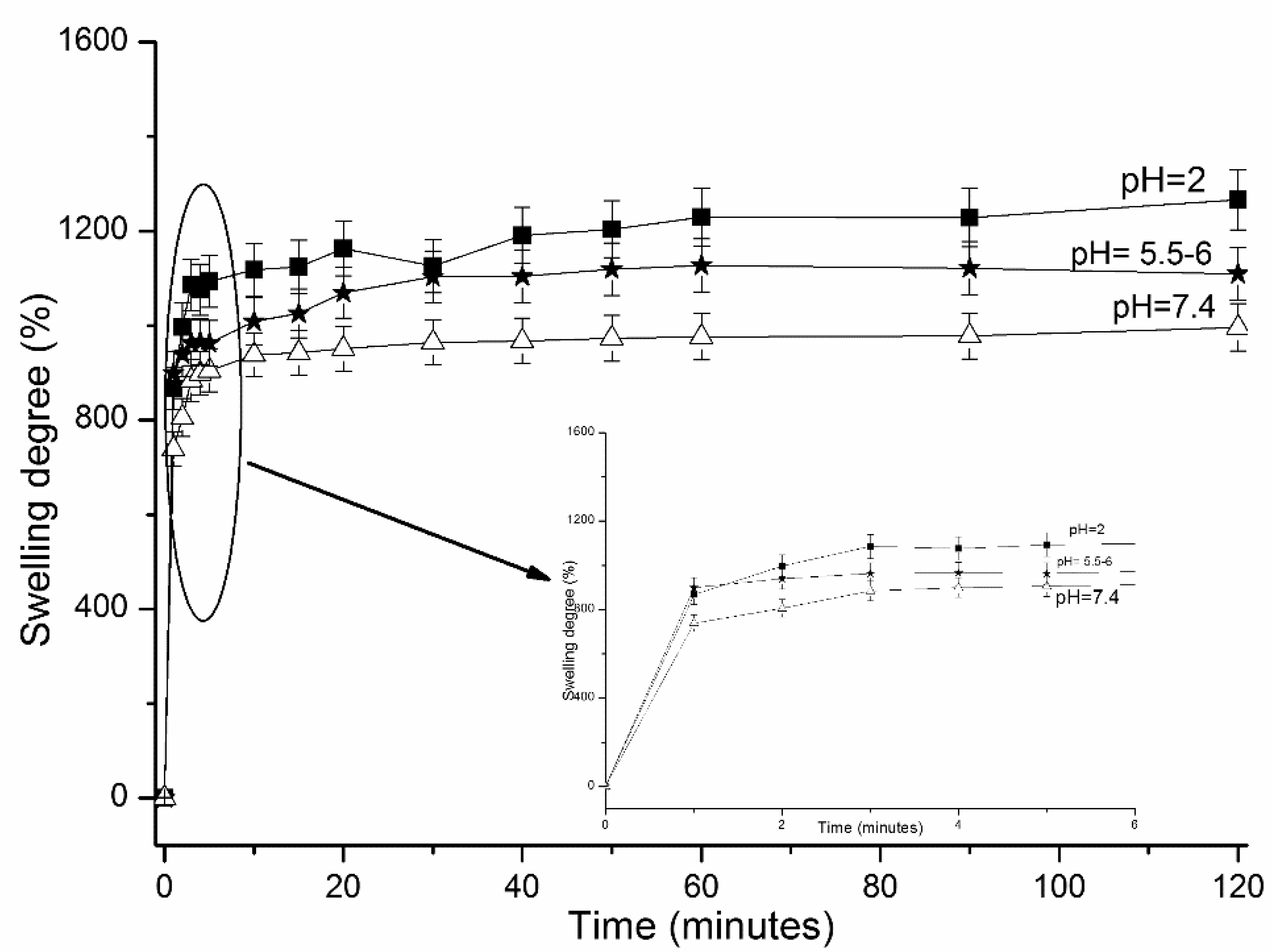
2.4.2. Swelling Behavior of the Polymeric Hydrogels
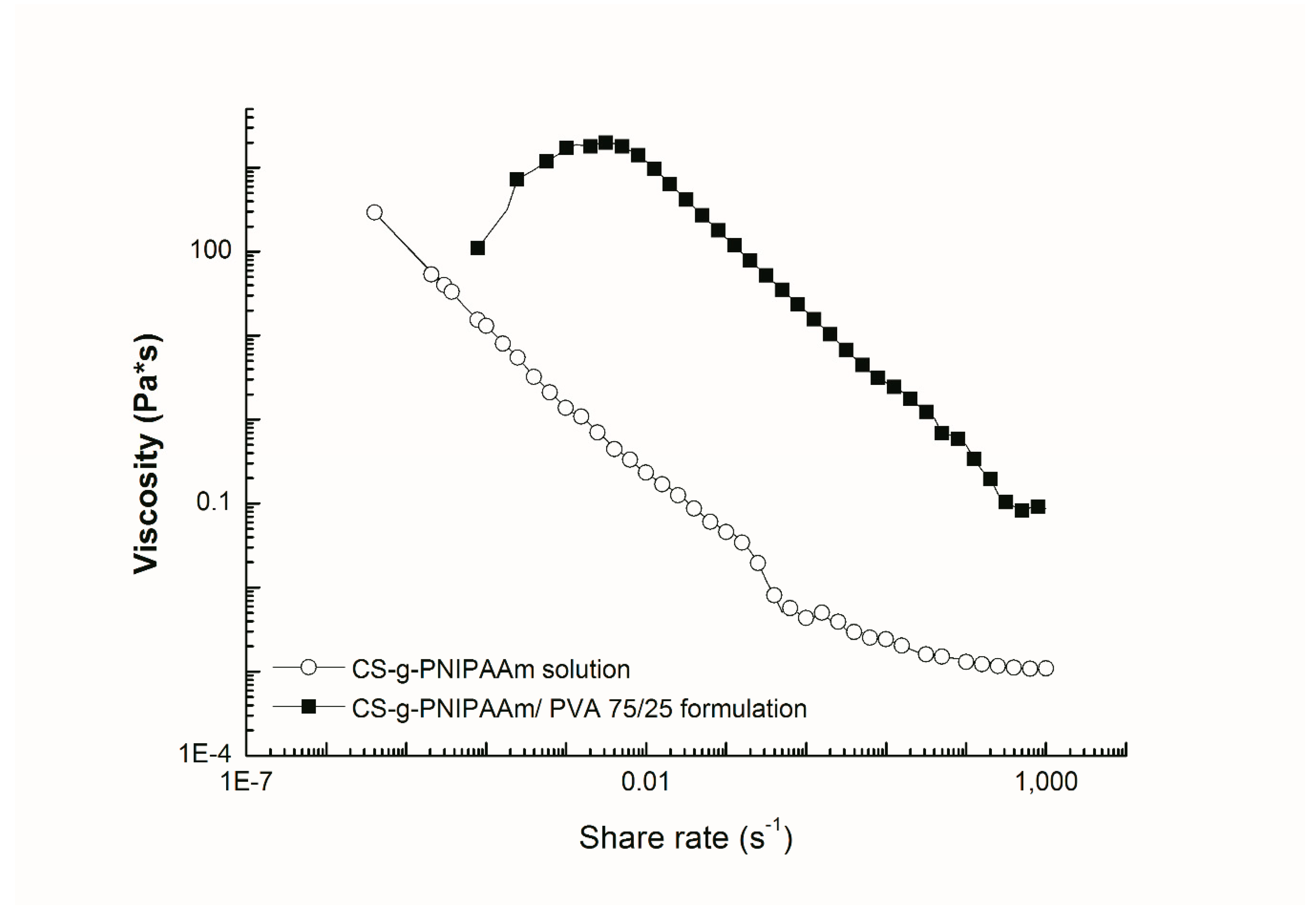
2.4.3. Rheological Measurements
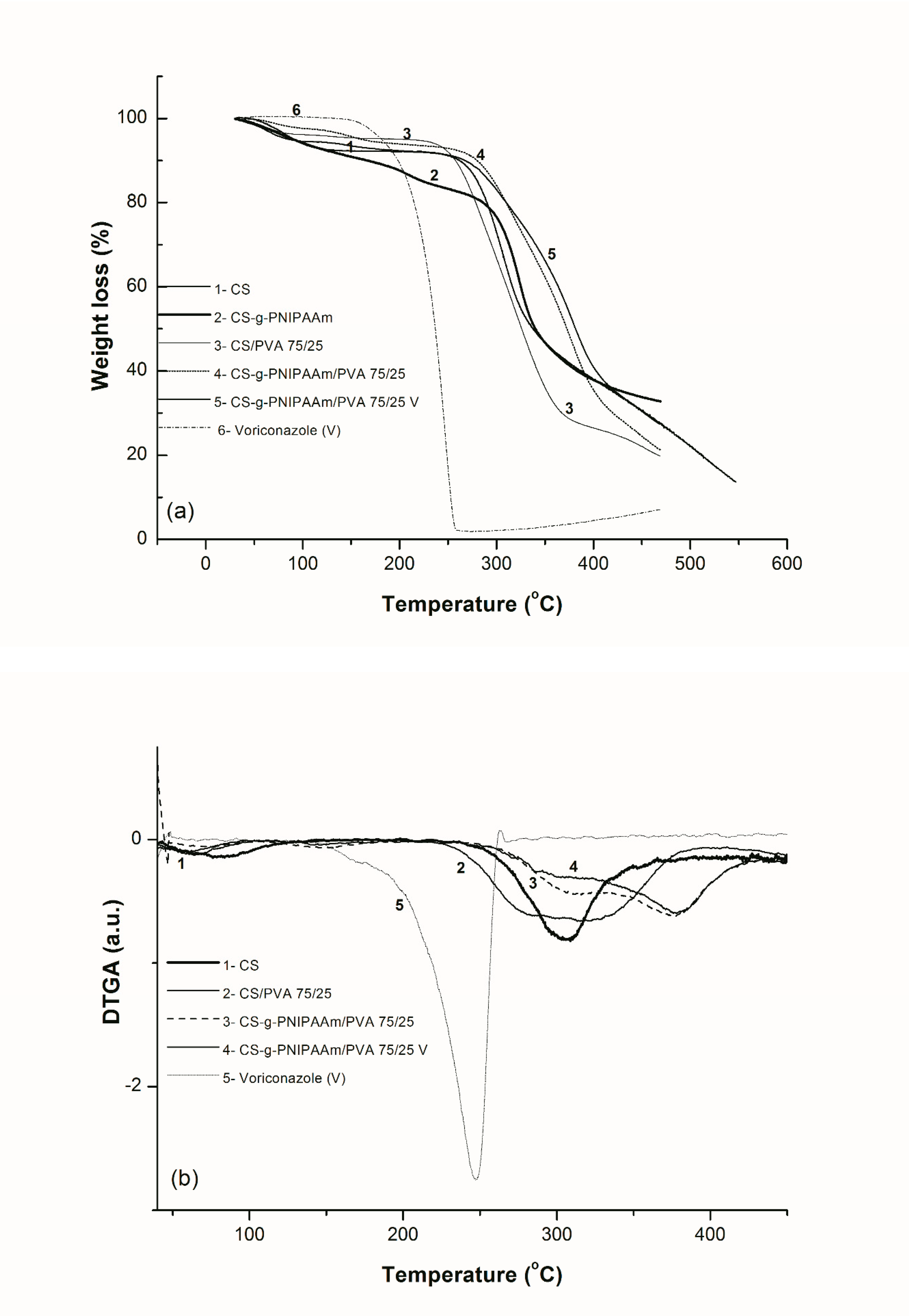
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Microscopic Observations of Formulations
2.4.7. Texture Profile Analysis (TPA)
2.4.8. Analytical Method for Drug Determination
2.4.9. In Vitro Release Study of Formulations
2.4.10. Stability Studies
2.4.11. Cell Culture and Cytotoxicity Tests
3. Results and Discussions
3.1. Optimization Studies
3.2. Structure Identification of Hydrogels
3.2.1. FTIR Spectra
3.2.2. Thermal Behavior
3.2.3. Swelling Ability of CS-g-PNIPAAm/PVA
3.2.4. In Vitro Release of Voriconazole
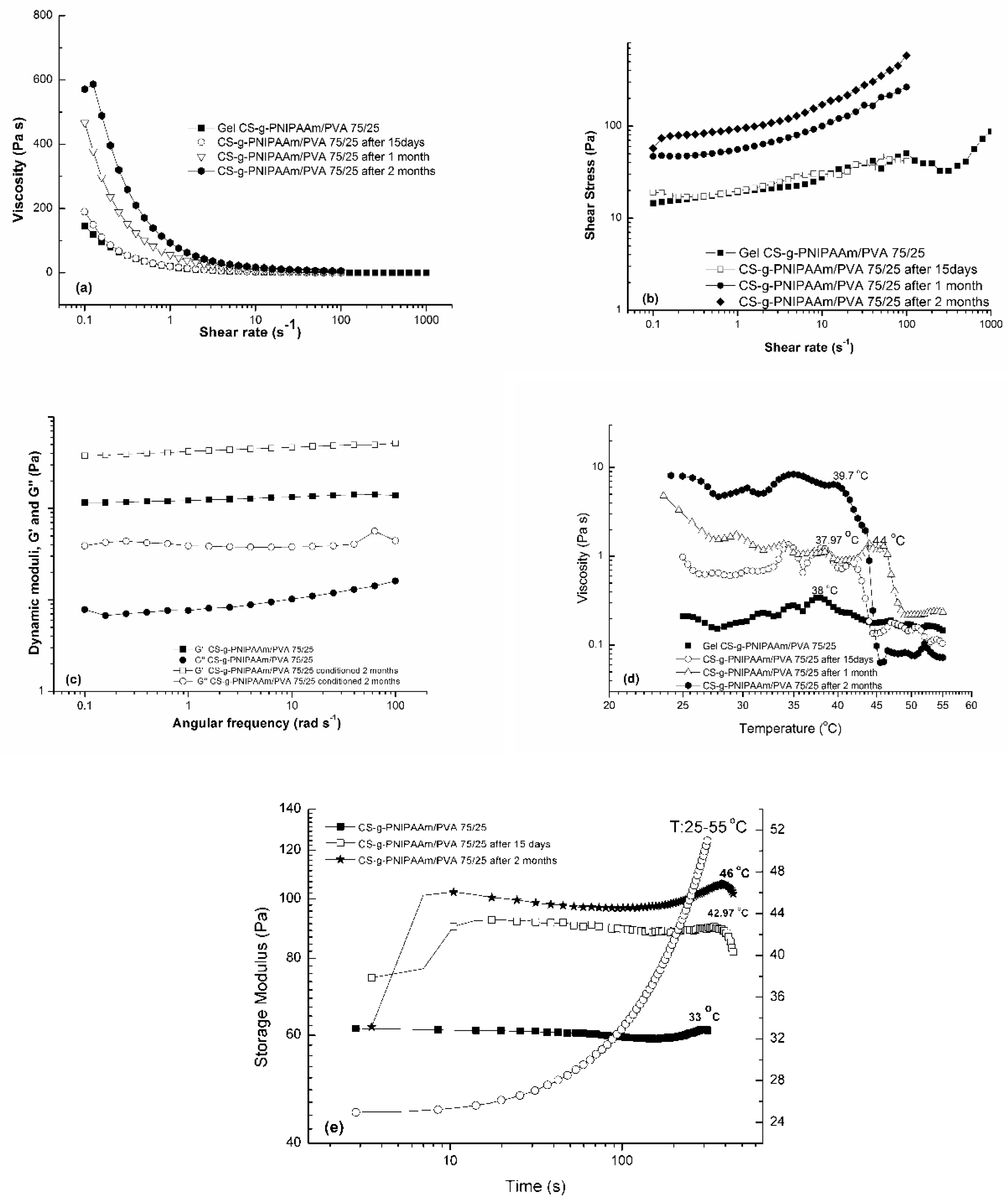
3.2.5. Stability of CS-g-PNIPAAm/PVA Cryogels
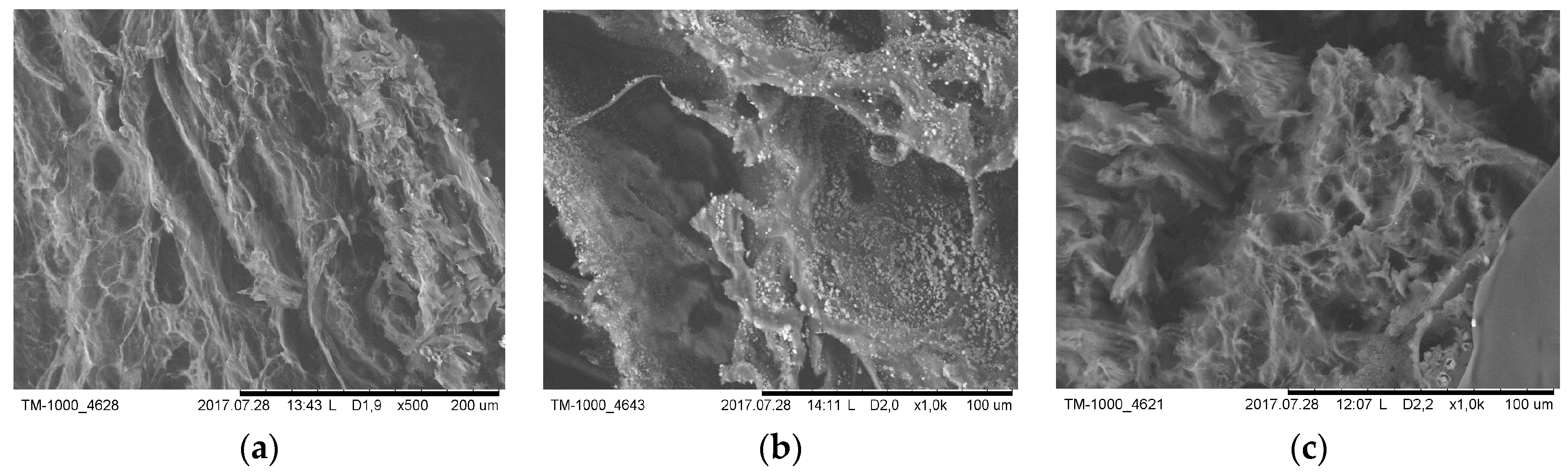
3.2.6. Microscopic Observations
3.2.7. Cytotoxicity and Cell Culture Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan graft copolymers. In Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC: Boca Raton, FL, USA, 1996; Volume 2, pp. 1208–1217. [Google Scholar]
- Sonia, T.A.; Sharma Chandra, P. Chitosan and its derivatives for drug delivery perspective. Adv. Polym. Sci. 2011, 243, 23–54. [Google Scholar]
- Kurita, K. Schiff’s base formation and alkylation. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A new horizon in modifications of chitosan: syntheses and applications. Crit. Rev. Ther. Drug 2013, 30, 91–181. [Google Scholar] [CrossRef]
- Luckanagul, J.A.; Pitakchatwong, C.; Bhuket, P.R.N.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Conzatti, G.; Ayadi, F.; Cavalie, S.; Carrère, N.; Tourrette, A. Thermosensitive PNIPAM grafted alginate/chitosan PEC. Appl. Surf. Sci. 2019, 467–468, 940–948. [Google Scholar] [CrossRef]
- Eskandari, P.; Abousalman-Rezvani, Z.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Mardani, H. Polymer grafting on graphene layers by controlled radical polymerization. Adv. Colloid Interf.Sci. 2019, 273, 102021. [Google Scholar] [CrossRef] [PubMed]
- Lepoittevin, B.; Elzein, T.; Dragoe, D.; Bejjani, A.; Lemée, F.; Levillain, J.; Bazin, P.; Roger, P.; Dez, I. Hydrophobization of chitosan films by surface grafting with fluorinated polymer brushes. Carbohydr. Polym. 2019, 205, 437–446. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Karavana, S.Y.; Yilmaz, O. Functionalized chitosan for pharmaceutical applications. Curr. Org. Synth. 2017, 14, 1–13. [Google Scholar]
- Cheaburu-Yilmaz, C.N.; Karavana, S.Y.; Yilmaz, O. Functionalized chitosan based matrices for in vitro delivery of Voriconazole. In Proceedings of the 21th International Conference of Inventics, Iasi, Romania, 25–26 May 2017; pp. 37–46. [Google Scholar]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Nugent, M.J.D.; Hanley, A.; Tomkins, P.T.; Higginbotham, C.L. Investigation of a novel freeze-thaw process for the production of drug delivery hydrogels. J. Mater. Sci. Mater. Med. 2005, 16, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.K.; Campbell, G.; Zhang, Z.F.; Hui, A.J.; Boughner, D.R. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J. Biomed. Mater. Res. B 2002, 63, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; Van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Parparita, E.; Cheaburu, C.N.; Patachia, S.F.; Vasile, C. Polyvinyl alcohol/chitosan/montmorillonite nanocomposites preparation by freeze/thaw cycles and characterization. Acta Chem. Iasi 2014, 22, 75–96. [Google Scholar] [CrossRef][Green Version]
- Tuncay Tanrıverdi, S.; Cheaburu-Yilmaz, C.N.; Carbone, S.; Ozer, O. Preparation and in vitro evaluation of melatonin-loaded HA/PVA gel formulations. Pharm. Dev. Technol. 2018, 23, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Koutsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: a review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Cheaburu-Yilmaz, C.N.; Pamfil, D.; Vasile, C.; Bibire, N.; Lupuşoru, R.V.; Zamfir, C.L.; Lupusoru, C.E. Toxicity, biocompatibility, pH-responsiveness and methotrexate release from PVA/Hyaluronic Acid cryogels for psoriasis therapy. Polymers 2017, 9, 123. [Google Scholar] [CrossRef]
- Veloso, D.F.M.C.; Benedetti, N.I.G.M.; Avila, R.I.; Bastos, T.S.A.; Silva, T.C.; Silva, M.R.R.; Batista, A.C.; Valadares, M.C.; Lim, E.M. Intravenous delivery of a liposomal formulation of voriconazole improves drug pharmacokinetics, tissue distribut ion, and enhances antifungal activity. Drug Deliv. 2018, 25, 1585–1594. [Google Scholar] [CrossRef]
- Okur, N.Ü.; Yoltaş, A.; Yozgatlı, V. Development and characterization of voriconazole loaded in situ gel formulations for ophthalmic application. Turk. J. Pharm. Sci. 2016, 13, 311–317. [Google Scholar]
- Song, S.H.; Lee, K.M.; Kang, J.B.; Lee, S.G.; Kang, M.J.; Choi, Y.W. Improved skin delivery of voriconazole with a nanostructured lipid carrier-based hydrogel formulation. Chem. Pharm. Bull. 2014, 62, 793–798. [Google Scholar] [CrossRef]
- Rençber, S.; Karavana, S.Y.; Ay Şenyiğit, Z.; Eraç, B.; Hoşgör Limoncu, M.; Baloğlu, E. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: formulation, preparation, and in vitro/in vivo evaluation. Pharm. Dev. Technol. 2016, 22, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Rençber, S.; Cheaburu-Yilmaz, C.N.; Aydin Kose, F.; Karavana, S.Y.; Yilmaz, O. Preparation and characterization of alginate and chitosan IPC based gel formulation for mucosal application. Cell. Chem. Technol. 2019, in press. [Google Scholar]
- Korsmeyer, R.W.; Lustig, S.R; Peppas, N.A. Solute and penetrant diffusion in swellable polymers. I. mathematical modeling. J. Polym. Sci. Part B: Polymer Physics 1986, 24, 395–408. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Smith, A.L. The Coblentz Society Desk Book of Infrared Spectra, 2nd ed.; Carver, C.D., Ed.; The Coblentz Society: Kirkwood, MO, USA, 1982; pp. 1–24. [Google Scholar]
- Cheaburu-Yilmaz, C.N.; Dumitriu, R.P.; Nistor, M.T.; Lupusoru, C.; Popa, M.I.; Profire, L.; Silvestre, C.; Vasile, C. Biocompatible and biodegradable chitosan / clay nanocomposites as new carriers for theophylline controlled release. British J. Pharm. Res. 2015, 6, 228–254. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook, 2nd ed.; Vincentz Network GmbH & Co. KG: Hannover, Germany, 2006. [Google Scholar]
- Bakonyi, M.; Berkó, S.; Kovács, A.; Budai-Szűcs, M.; Kis, N.; Erős, G.; Csóka, I.; Csányi, E. Application of quality by design principles in the development and evaluation of semisolid drug carrier systems for the transdermal delivery of lidocaine. J. Drug. Deliv. Sci. Technol. 2018, 44, 136–145. [Google Scholar] [CrossRef]
- Geurtsen, W.; Leinenbach, F.; Krage, T.; Leyhausen, G. Cytotoxicity of four root canal sealers in permanent 3T3 cells and primary human periodontal ligament fibroblast cultures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 592–597. [Google Scholar] [CrossRef]
- Flores, I.L.; Gamba, T.O.; De Carvalho, R.V.; Lund, R.G.; Etges, A. In vitro cytotoxicity of plantago australis ethanol extract used as an anti-ınflammatory for the treatment of oral pathologies. Jentashapir J. Health Res. 2016, 7, e28515. [Google Scholar] [CrossRef]
- Topcuoglu, N.; Lacin, C.C.; Erguven, M.; Bilir, A.; Sutlupinar, N.; Kulekci, G. Antibacterial effect of kenger gum on mutans streptococci and its cytotoxic effect on the 3T3 fibroblast cell line. Oral Health Prev. Dent. 2015, 13, 157–162. [Google Scholar]
- Severino, P.; Chaud, M.V.; Shimojo, A.; Antonini, D.; Lancelloti, M.; Santana, M.H.; Souto, E.B. Sodium alginate-cross-linked polymyxin B sulphate-loaded solid lipid nanoparticles: Antibiotic resistance tests and HaCat and NIH/3T3 cell viability studies. Colloids Surf. B: Biointerf. 2015, 129, 191–197. [Google Scholar] [CrossRef]
- Kang, S.; Lee, M.; Kang, M.; Noh, M.; Jeon, J.; Lee, Y.; Seo, J.H. Development of anti-biofouling interface on hydroxyapatite surface by coating zwitterionic MPC polymer containing calcium-binding moieties to prevent oral bacterial adhesion. Acta Biomater. 2016, 40, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ferrua, C.P.; Leal, F.B.; de Oliveira Gazal, M.; Ghisleni, G.C.; de Carvalho, R.V.; Demarco, F.F.; Ogliari, F.A.; Nedel, F. Iodonium salt incorporation in dental adhesives and its relation with degree of conversion, ultimate tensile strength, cell viability, and oxidative stress. Clin. Oral Investig. 2018, 23, 1143–1151. [Google Scholar] [CrossRef] [PubMed]





| Code | Formulation |
|---|---|
| CS-g-PNIPAAm/PVA 75/25 FM with Vor | Hydrogel CS-g-PNIPAAm physically crosslinked with PVA with a composition 75/25 v/v % by three cycles of −20/25 °C freeze–thaw; loading of Voriconazole was done by solution mixing after the hydrogel preparation |
| CS-g-PNIPAAm/PVA 75/25 with Vor | Hydrogel CS-g-PNIPAAm physically crosslinked with PVA with a composition 75/25 v/v % loading of Voriconazole was done during the preparation of hydrogel |
| CS/PVA 75/25 | Physically crosslinked hydrogel between simple chitosan and PVA with a composition 75/25 v/v % by freeze–thaw −20/25 °C (three cycles) |
| CS/PVA 75/25 with Vor | Physically crosslinked hydrogel between simple chitosan and PVA with a composition 75/25 v/v % by freeze–thaw −20/25 °C (three cycles); loading of Voriconazole was done during the hydrogel preparation |
| CS/PVA 50/50 | Physically crosslinked hydrogel between simple chitosan and PVA with a composition 50/50 v/v % by freeze–thaw −20/25 °C (three cycles) |
| CS/PVA 50/50 with Vor | Physically crosslinked hydrogel between simple chitosan and PVA with a composition 50/50 v/v % by freeze–thaw −20/25 °C (three cycles); loading of Voriconazole was done during the hydrogel preparation |
| System | Hardness | Compressibility | Adhesiveness | Cohesivity | Elasticity |
|---|---|---|---|---|---|
| CS-g-PNIPAAm/PVA 75/25 FM with Vor | 0.948 ± 0.07 | 1.357 ± 0.13 | −0.643 ± 0.01 | 0.89 | 0.97 |
| CS-g-PNIPAAm/PVA with Vor | 0.62 ± 0.02 | 1.20 ± 0.01 | −0.57 ± 0.00 | 1.01 | 0.97 |
| CS/PVA 75/25 | 7.56 ± 1.04 | 15.80 ± 4.57 | −5.62 ± 0.38 | 0.77 | 0.90 |
| CS/PVA 75/25 with Vor | 2.48 ± 0.12 | 3.63 ± 0.33 | −4.14 ± 0.22 | 0.92 | 1.11 |
| CS/PVA 50/50 | 8.42 ± 0.81 | 16.67 ± 2.65 | −6.78 ± 1.09 | 0.73 | 0.78 |
| CS/PVA 50/50 with Vor | 15 ± 0.12 | 26.56 ± 20.32 | −1.82 ± 0.67 | 0.67 | 0.88 |
| Formulation | Percent Released from Total Amount in 6 h (%) | K (min)−n | n | R2 |
|---|---|---|---|---|
| CS/PVA 75/25 | 76 | 1.50 | 0.18 | 0.98 |
| CS/PVA 50/50 | 52 | 0.80 | 0.20 | 0.99 |
| CS-g-PNIPAAm/PVA 75/25 FM | 68 | 1.12 | 0.17 | 0.98 |
| CS-g-PNIPAAm/PVA 75/25 | 38 | 0.20 | 0.17 | 0.97 |
| System | pH | Organoleptic Properties | Drug Content (μg/mL) | Mechanical Properties from TPA Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| H (N) | C (Ns) | E | Ch | A | ||||
| CS-g-PNIPAAm/PVA 75/25 | 5.0 | Gel, pale yellow | 6.55 | 10.02 | 36.93 | 0.97 | 0.96 | 4.48 |
| CS-g-PNIPAAm/PVA 75/25, after 15 days | 4.85 | Gel, pale yellow turned to light pink, no change in odor, no phase separation | 6.85 | 14.44 | 16.80 | 0.98 | 0.84 | 4.47 |
| CS-g-PNIPAAm/PVA 75/25, after 1 month | 4.8 | Gel, light pink-yellowish, no change in odor, no phase separation | 6.78 | 10.30 | 18.92 | 0.94 | 0.86 | 4.80 |
| CS-g-PNIPAAm/PVA 75/25, after 2 months | 4.75 | Gel, light pink-yellowish, no change in odor, no phase separation | 6.58 | 12.09 | 18.52 | 0.95 | 0.91 | 4.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheaburu-Yilmaz, C.N.; Yilmaz, O.; Aydin Kose, F.; Bibire, N. Chitosan-Graft-Poly(N-Isopropylacrylamide)/PVA Cryogels as Carriers for Mucosal Delivery of Voriconazole. Polymers 2019, 11, 1432. https://doi.org/10.3390/polym11091432
Cheaburu-Yilmaz CN, Yilmaz O, Aydin Kose F, Bibire N. Chitosan-Graft-Poly(N-Isopropylacrylamide)/PVA Cryogels as Carriers for Mucosal Delivery of Voriconazole. Polymers. 2019; 11(9):1432. https://doi.org/10.3390/polym11091432
Chicago/Turabian StyleCheaburu-Yilmaz, Catalina Natalia, Onur Yilmaz, Fadime Aydin Kose, and Nela Bibire. 2019. "Chitosan-Graft-Poly(N-Isopropylacrylamide)/PVA Cryogels as Carriers for Mucosal Delivery of Voriconazole" Polymers 11, no. 9: 1432. https://doi.org/10.3390/polym11091432
APA StyleCheaburu-Yilmaz, C. N., Yilmaz, O., Aydin Kose, F., & Bibire, N. (2019). Chitosan-Graft-Poly(N-Isopropylacrylamide)/PVA Cryogels as Carriers for Mucosal Delivery of Voriconazole. Polymers, 11(9), 1432. https://doi.org/10.3390/polym11091432