Preconditioning in an Inflammatory Milieu Augments the Immunotherapeutic Function of Mesenchymal Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of BM-MSCs and AD-MSCs
2.2. Culture Conditions
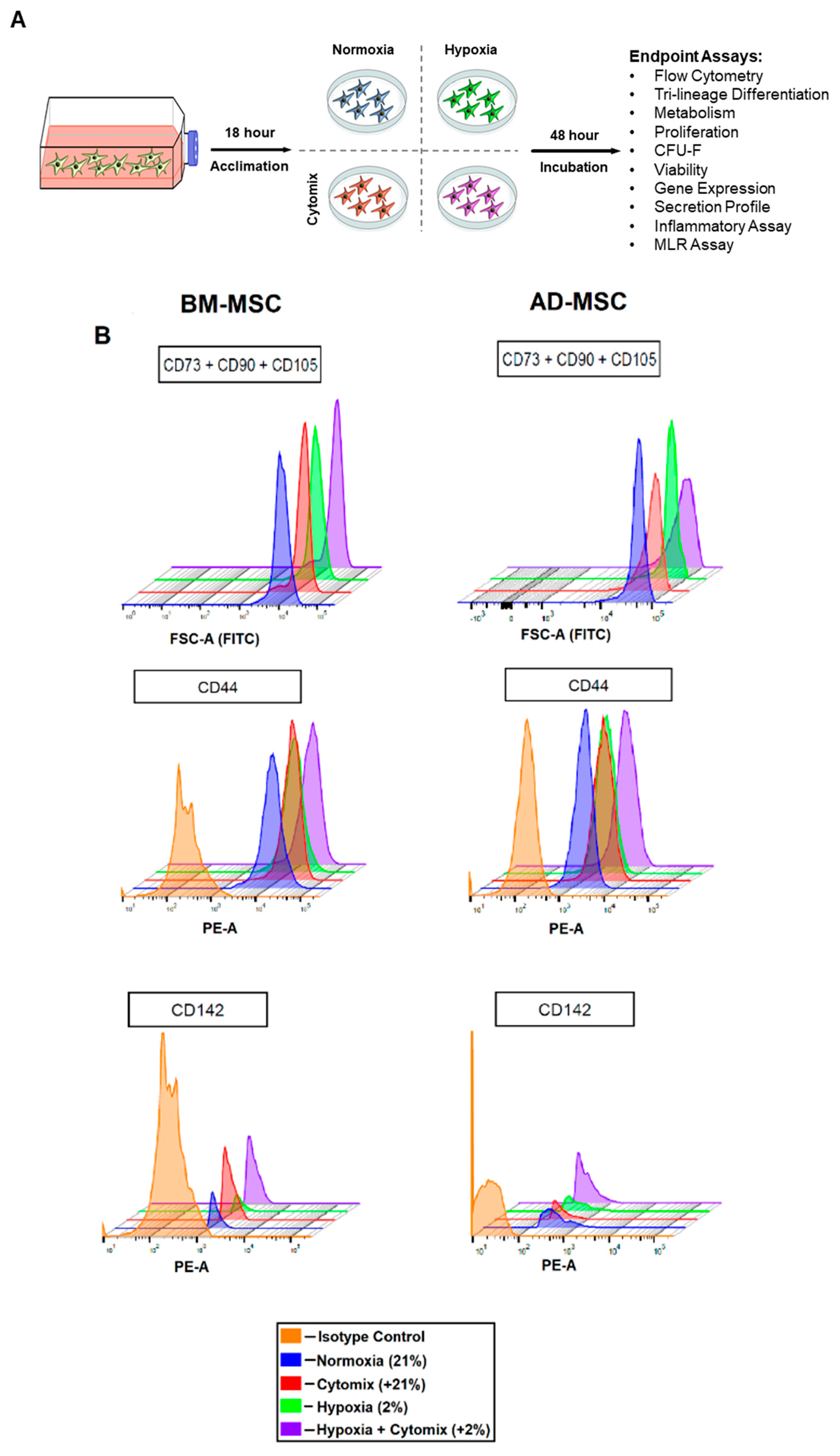
2.3. BM-MSC and AD-MSC Surface Markers
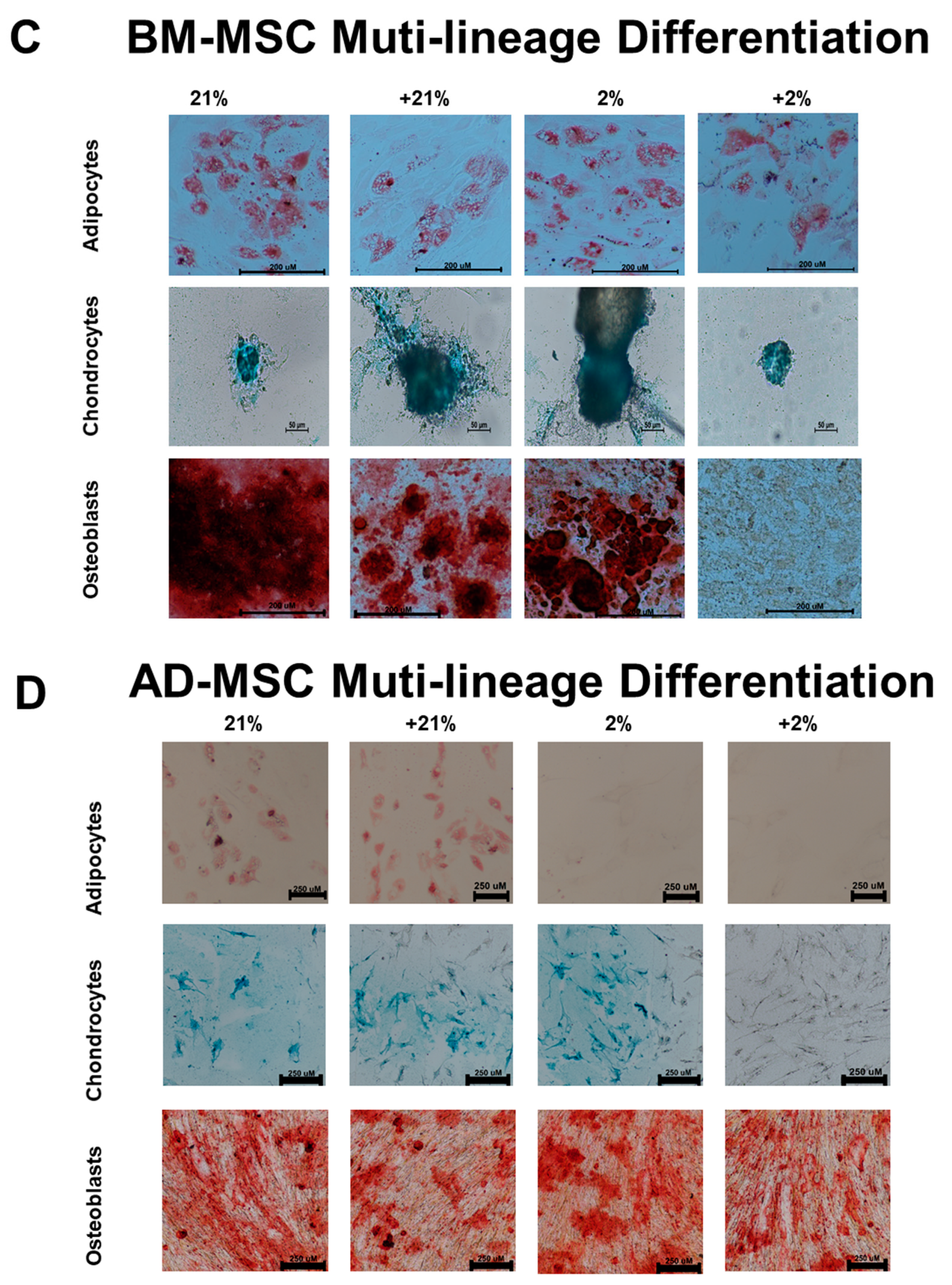
2.4. Multi-Lineage Differentiation
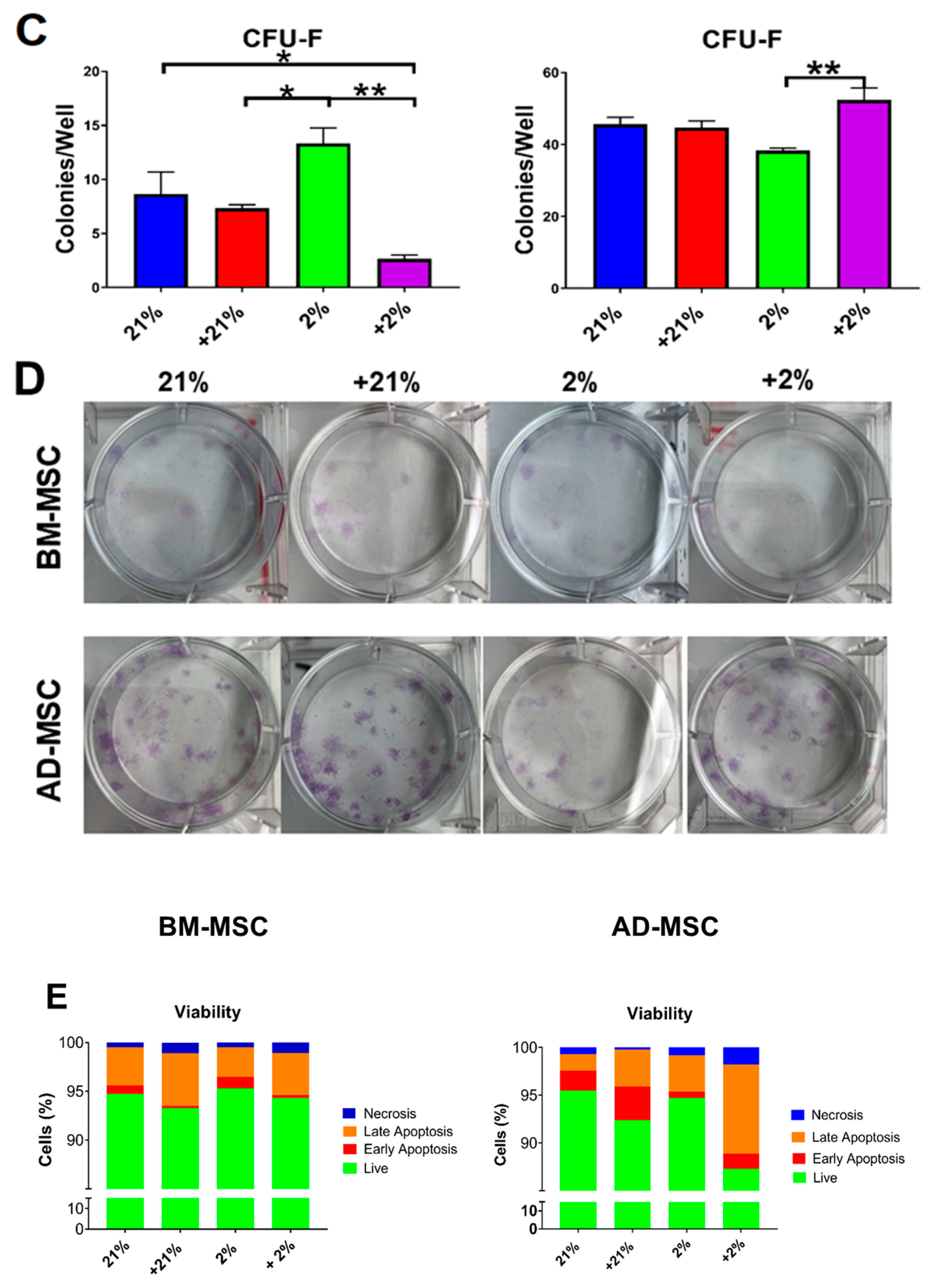
2.5. Colony Forming Unit-Fibroblast (CFU-F) Assay
2.6. Cell Apoptosis/Viability and Morphology
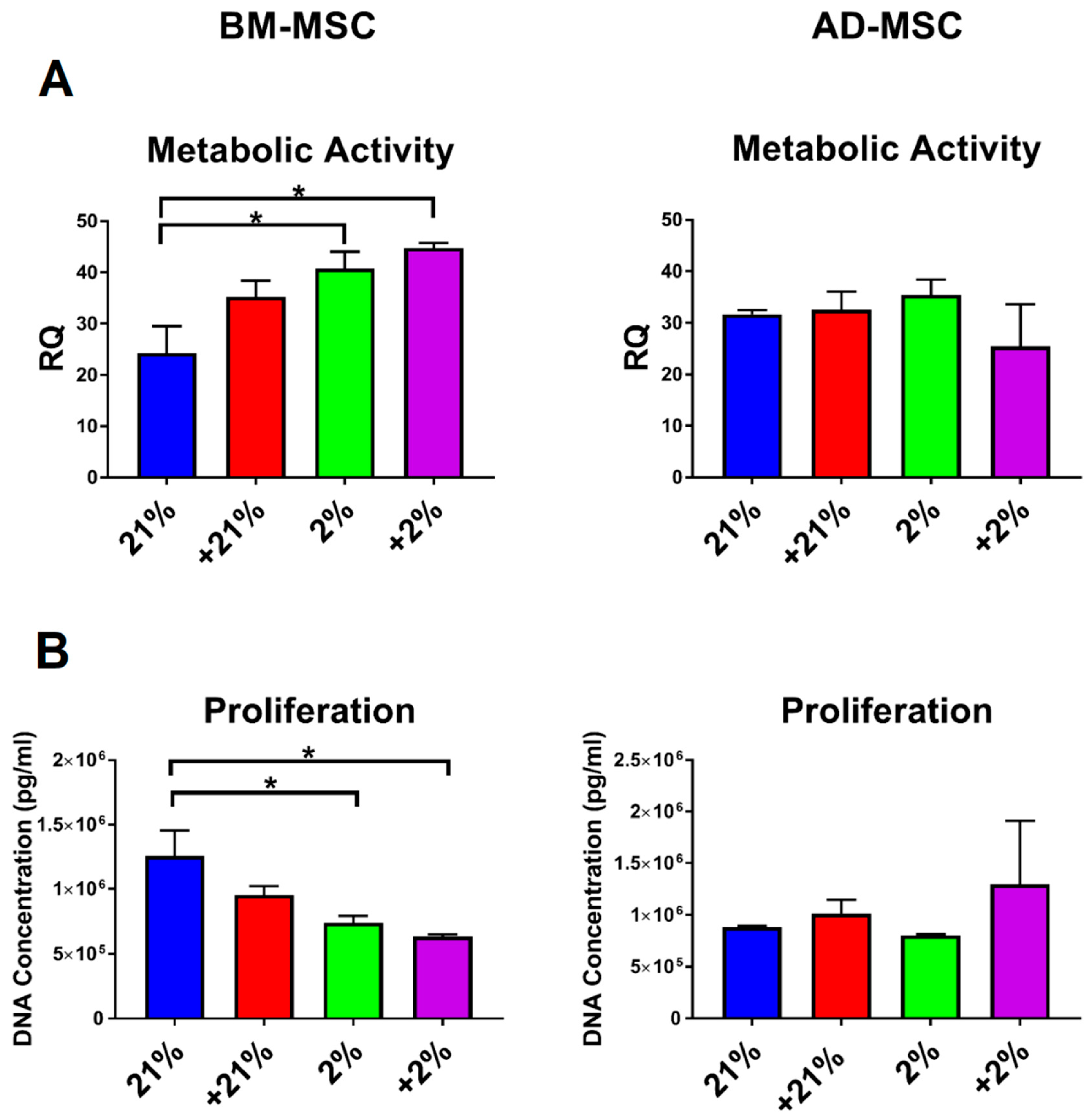
2.7. Metabolic and Proliferation Assays
2.8. Gene Expression
2.9. Secretion Profile
2.10. Inflammatory Assay
2.11. Mixed Lymphocyte Reaction
2.12. Statistical Analysis
3. Results
3.1. Surface Marker Expression and Differentiation
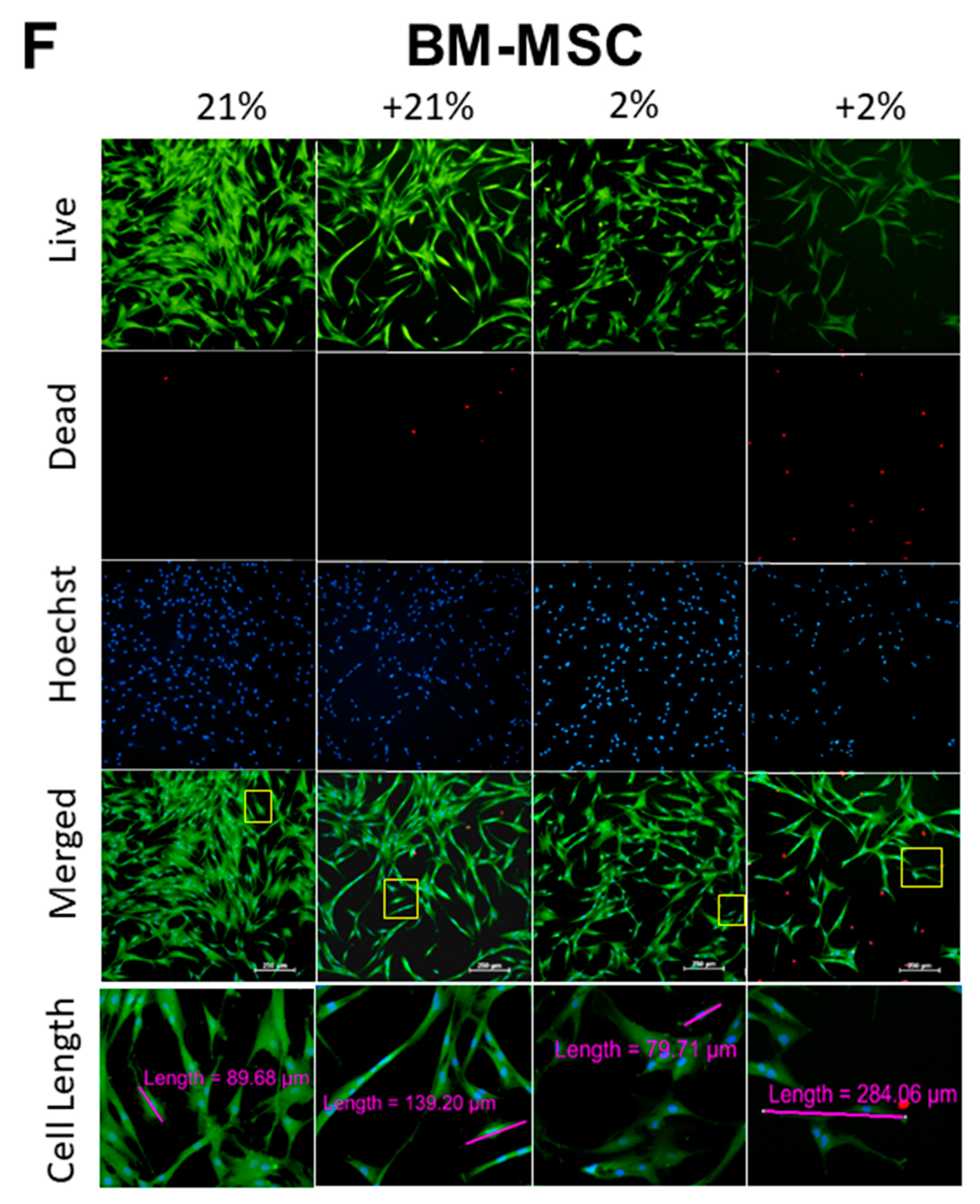
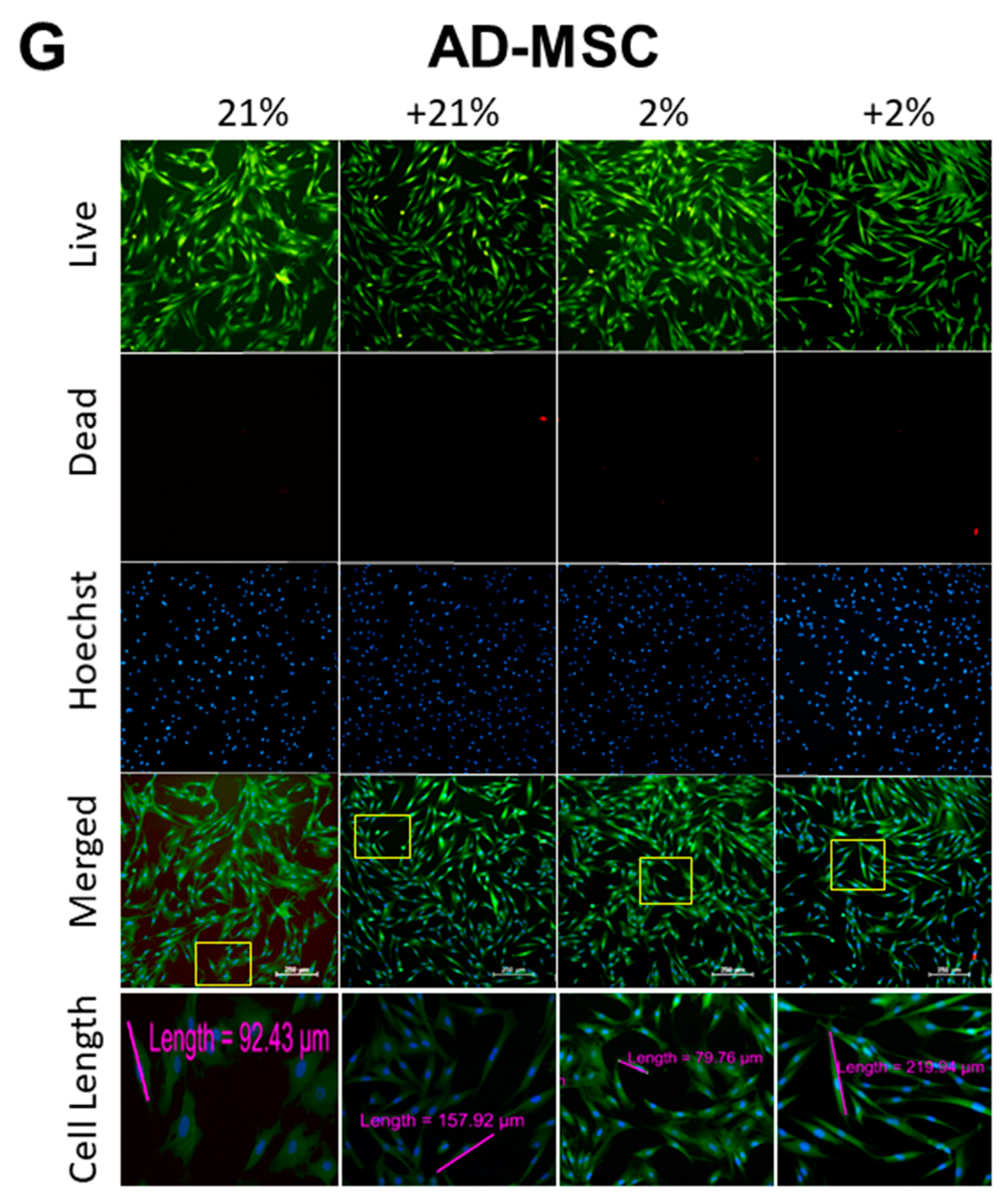
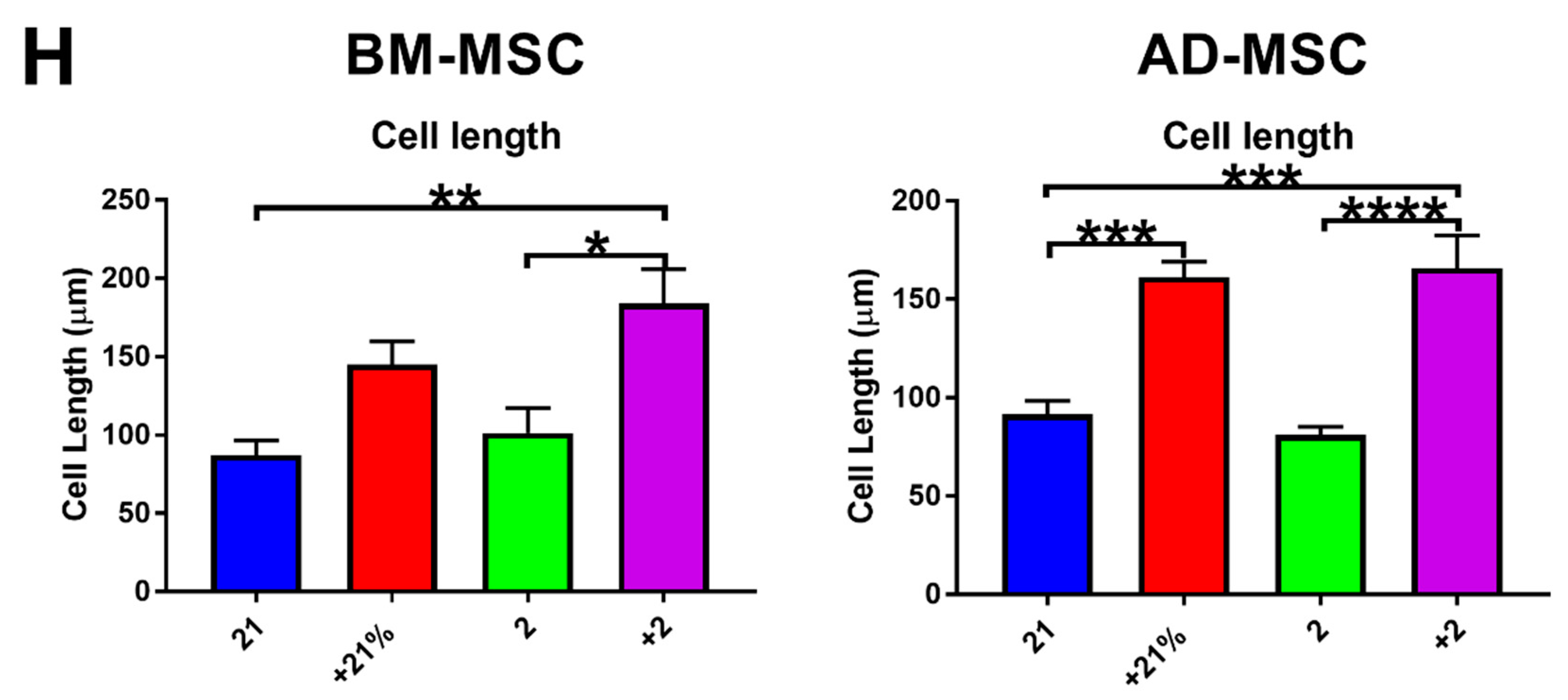
3.2. Cell Metabolism, Proliferation, Clonogenicity, Viability, and Morphology
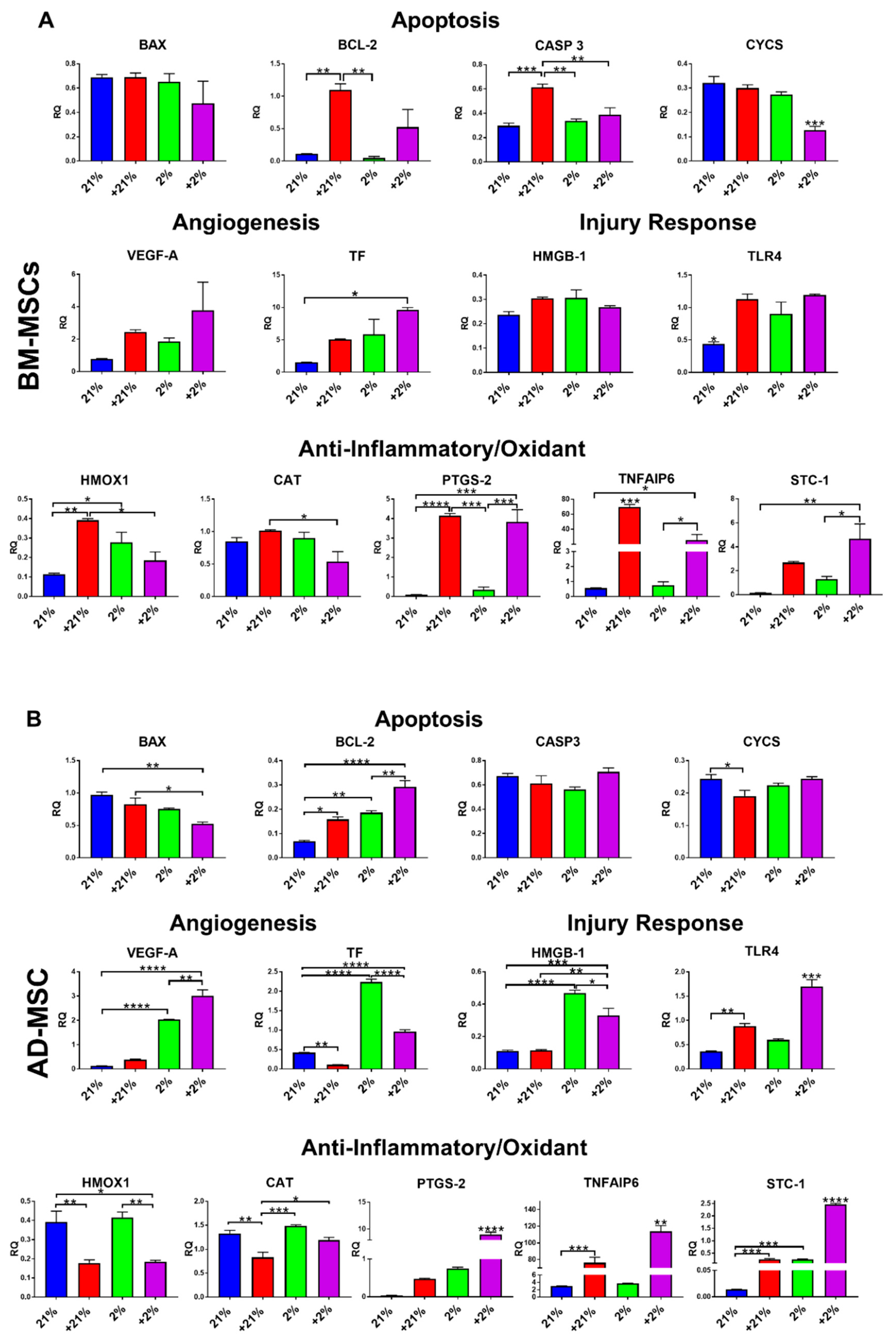
3.3. Gene Expression
3.4. Secretion Profile
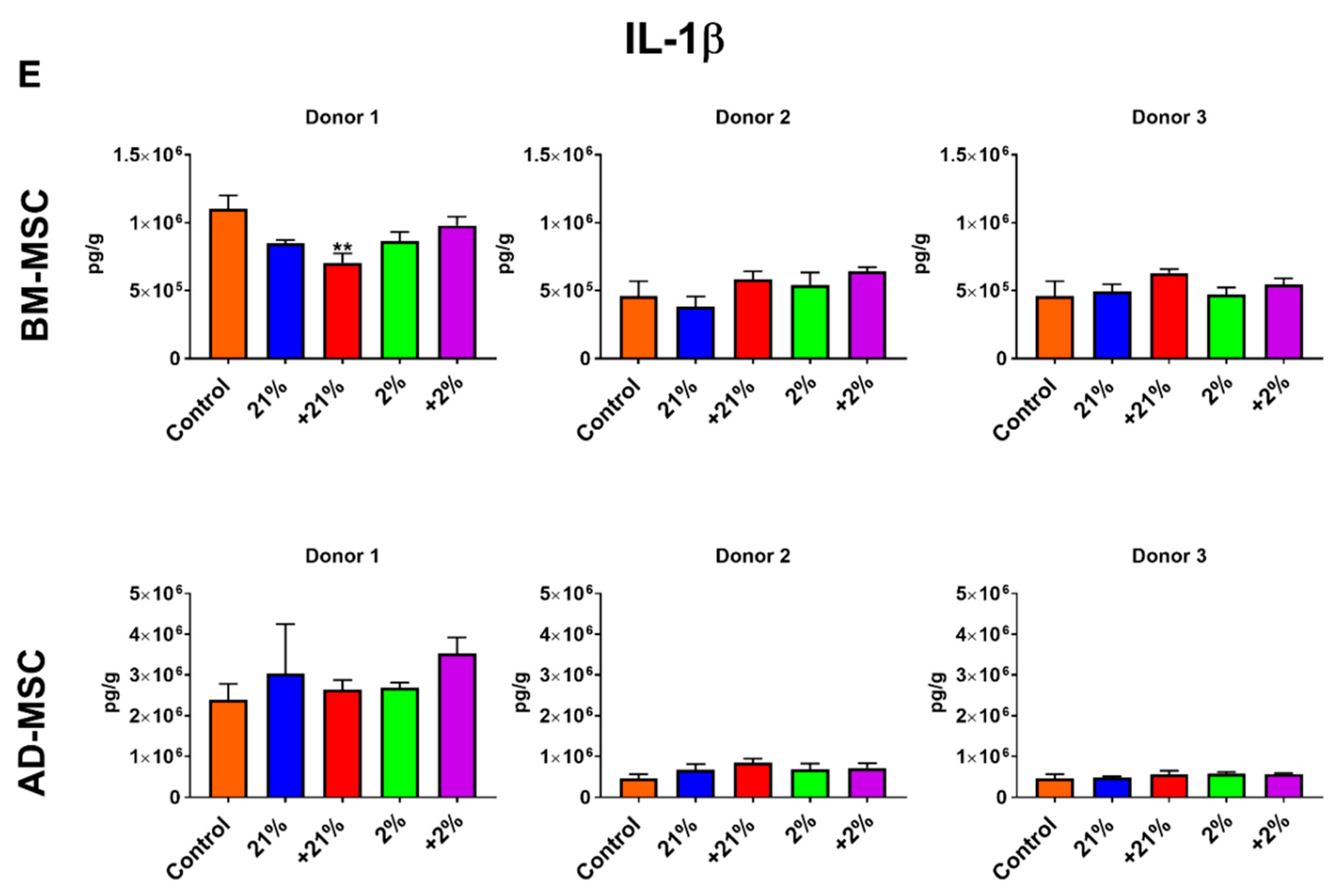
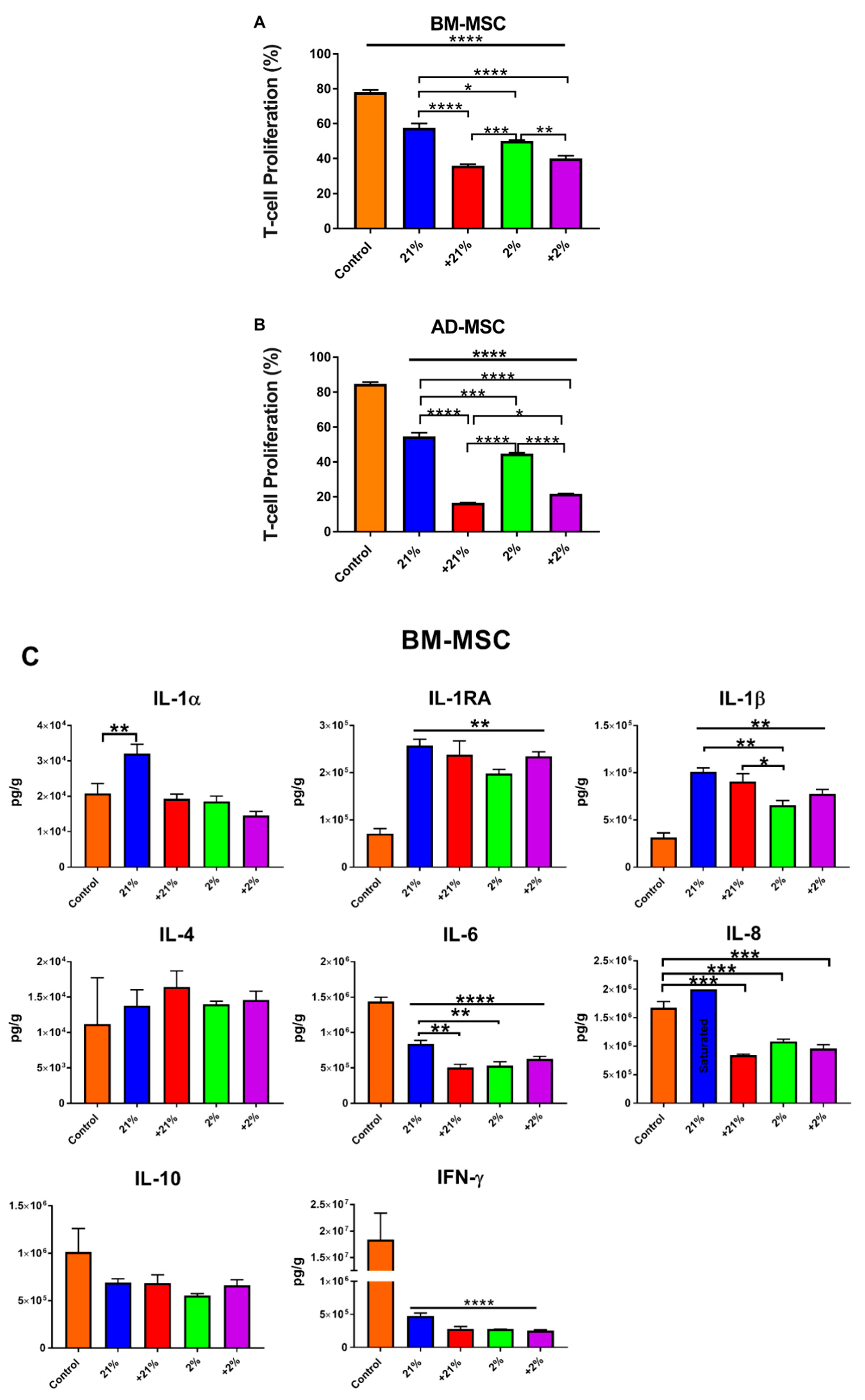
3.5. Inflammatory Assay
3.6. Mixed Lymphocyte Reaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Antebi, B.; Mohammadipoor, A.; Batchinsky, A.I.; Cancio, L.C. The promise of mesenchymal stem cell therapy for acute respiratory distress syndrome. J. Trauma Acute Care Surg. 2018, 84, 183–191. [Google Scholar] [CrossRef]
- Wang, L.T.; Ting, C.H.; Yen, M.L.; Liu, K.J.; Sytwu, H.K.; Wu, K.K.; Yen, B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J. Biomed. Sci. 2016, 23, 76. [Google Scholar] [CrossRef]
- Baghaei, K.; Hashemi, S.M.; Tokhanbigli, S.; Asadi Rad, A.; Assadzadeh-Aghdaei, H.; Sharifian, A.; Zali, M.R. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol. Hepatol. Bed Bench 2017, 10, 208–213. [Google Scholar] [PubMed]
- Leto Barone, A.A.; Khalifian, S.; Lee, W.P.; Brandacher, G. Immunomodulatory effects of adipose-derived stem cells: Fact or fiction? BioMed Res. Int. 2013, 2013, 383685. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Gordon, P.L.; Koo, W.K.; Marx, J.C.; Neel, M.D.; McNall, R.Y.; Muul, L.; Hofmann, T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA 2002, 99, 8932–8937. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Gotherstrom, C.; Hassan, M.; Uzunel, M.; Ringden, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Duijvestein, M.; Vos, A.C.; Roelofs, H.; Wildenberg, M.E.; Wendrich, B.B.; Verspaget, H.W.; Kooy-Winkelaar, E.M.; Koning, F.; Zwaginga, J.J.; Fidder, H.H.; et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: Results of a phase I study. Gut 2010, 59, 1662–1669. [Google Scholar] [CrossRef]
- Boregowda, S.V.; Krishnappa, V.; Chambers, J.W.; Lograsso, P.V.; Lai, W.T.; Ortiz, L.A.; Phinney, D.G. Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a p53-dependent mechanism: Implications for long-term culture expansion. Stem Cells 2012, 30, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L. Adipose tissue oxygenation: Effects on metabolic function. Adipocyte 2014, 3, 75–80. [Google Scholar] [CrossRef]
- Berniakovich, I.; Giorgio, M. Low oxygen tension maintains multipotency, whereas normoxia increases differentiation of mouse bone marrow stromal cells. Int. J. Mol. Sci. 2013, 14, 2119–2134. [Google Scholar] [CrossRef] [PubMed]
- Fotia, C.; Massa, A.; Boriani, F.; Baldini, N.; Granchi, D. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. Cytotechnology 2015, 67, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, N.; Morimoto, N.; Ogawa, T.; Taketani, S.; Kusumoto, K. Hypoxia Enhances Proliferation of Human Adipose-Derived Stem Cells via HIF-1a Activation. PLoS ONE 2015, 10, e0139890. [Google Scholar] [CrossRef]
- Dos Santos, F.; Andrade, P.Z.; Boura, J.S.; Abecasis, M.M.; da Silva, C.L.; Cabral, J.M. Ex vivo expansion of human mesenchymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell. Physiol. 2010, 223, 27–35. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of TNFalpha in pulmonary pathophysiology. Respir. Res. 2006, 7, 125. [Google Scholar] [CrossRef]
- Potier, E.; Ferreira, E.; Andriamanalijaona, R.; Pujol, J.P.; Oudina, K.; Logeart-Avramoglou, D.; Petite, H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 2007, 40, 1078–1087. [Google Scholar] [CrossRef]
- Hung, S.C.; Pochampally, R.R.; Chen, S.C.; Hsu, S.C.; Prockop, D.J. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 2007, 25, 2363–2370. [Google Scholar] [CrossRef]
- Przybyt, E.; Krenning, G.; Brinker, M.G.; Harmsen, M.C. Adipose stromal cells primed with hypoxia and inflammation enhance cardiomyocyte proliferation rate in vitro through STAT3 and Erk1/2. J. Transl. Med. 2013, 11, 39. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef]
- Antebi, B.; Zhang, Z.; Wang, Y.; Lu, Z.; Chen, X.D.; Ling, J. Stromal-cell-derived extracellular matrix promotes the proliferation and retains the osteogenic differentiation capacity of mesenchymal stem cells on three-dimensional scaffolds. Tissue Eng. Part C Methods 2015, 21, 171–181. [Google Scholar] [CrossRef]
- Broekman, W.; Amatngalim, G.D.; de Mooij-Eijk, Y.; Oostendorp, J.; Roelofs, H.; Taube, C.; Stolk, J.; Hiemstra, P.S. TNF-alpha and IL-1beta-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir. Res. 2016, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Ohashi, K.; Matsubara, Y.; Kohori, A.; Ohno, T.; Kakidachi, H.; Horii, A.; Kanegae, K.; Utoh, R.; Iwata, T.; et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem. Biophys. Res. Commun. 2013, 431, 203–209. [Google Scholar] [CrossRef]
- Lim, R.; Malhotra, A.; Tan, J.; Chan, S.T.; Lau, S.; Zhu, D.; Mockler, J.C.; Wallace, E.M. First-In-Human Administration of Allogeneic Amnion Cells in Premature Infants With Bronchopulmonary Dysplasia: A Safety Study. Stem Cells Transl. Med. 2018, 7, 628–635. [Google Scholar] [CrossRef]
- Ozdemir, E.; Kansu, E. Deep Vein Thrombosis Following Non-myeloablative Allogeneic Stem Cell Transplantation: Presentation of Three Cases and Literature Review. Turkish J. Haematol. Off. J. Turkish Soc. Haematol. 2013, 30, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Christy, B.A.; Herzig, M.C.; Montgomery, R.K.; Delavan, C.; Bynum, J.A.; Reddoch, K.M.; Cap, A.P. Procoagulant activity of human mesenchymal stem cells. J. Trauma Acute Care Surg. 2017, 83, S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Stephenne, X.; Vosters, O.; Najimi, M.; Beuneu, C.; Dung, K.N.; Wijns, W.; Goldman, M.; Sokal, E.M. Tissue factor-dependent procoagulant activity of isolated human hepatocytes: Relevance to liver cell transplantation. Liver Transplant. Off. Publ. Am. Assoc. Study of Liver Dis. Int. Liver Transplant. Soc. 2007, 13, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Rodriguez, L.A., 2nd; Walker, K.P., 3rd; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef]
- Antebi, B.; Walker, K.P., 3rd; Mohammadipoor, A.; Rodriguez, L.A.; Montgomery, R.K.; Batchinsky, A.I.; Cancio, L.C. The effect of acute respiratory distress syndrome on bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Cheng, X.; Harris, J.N.; Gower, L.B.; Chen, X.D.; Ling, J. Biomimetic collagen-hydroxyapatite composite fabricated via a novel perfusion-flow mineralization technique. Tissue Eng. Part C Methods 2013, 19, 487–496. [Google Scholar] [CrossRef]
- Sharma, R.R.; Pollock, K.; Hubel, A.; McKenna, D. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion 2014, 54, 1418–1437. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Ivanova-Todorova, E.; Bochev, I.; Mourdjeva, M.; Dimitrov, R.; Bukarev, D.; Kyurkchiev, S.; Tivchev, P.; Altunkova, I.; Kyurkchiev, D.S. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol. Lett. 2009, 126, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef]
- English, K.; Barry, F.P.; Field-Corbett, C.P.; Mahon, B.P. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 2007, 110, 91–100. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Majore, I.; Kasper, C.; Hass, R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun. Signal. 2010, 8, 18. [Google Scholar] [CrossRef]
- Ejtehadifar, M.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Dehdilani, N.; Abbasi, P.; Molaeipour, Z.; Saleh, M. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv. Pharm. Bull. 2015, 5, 141–149. [Google Scholar] [CrossRef]
- Michiels, C. Physiological and pathological responses to hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef]
- Killer, M.C.; Nold, P.; Henkenius, K.; Fritz, L.; Riedlinger, T.; Barckhausen, C.; Frech, M.; Hackstein, H.; Neubauer, A.; Brendel, C. Immunosuppressive capacity of mesenchymal stem cells correlates with metabolic activity and can be enhanced by valproic acid. Stem Cell Res. Ther. 2017, 8, 100. [Google Scholar] [CrossRef]
- Klinker, M.W.; Marklein, R.A.; Lo Surdo, J.L.; Wei, C.H.; Bauer, S.R. Morphological features of IFN-gamma-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E2598–E2607. [Google Scholar] [CrossRef]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Liu, Y.; Akiyama, K.; Chen, C.; Qu, C.; Jin, Y.; Shi, S. IFN-gamma and TNF-alpha synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFkappaB signaling. Stem Cells 2013, 31, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.B.; Porter, R.M.; Evans, C.H.; Ritter, T.; Shaw, G.; Barry, F.; Murphy, J.M. TNFalpha and IL-1beta influence the differentiation and migration of murine MSCs independently of the NF-kappaB pathway. Stem Cell Res. Ther. 2014, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Perlman, H.; Zhang, X.; Chen, M.W.; Walsh, K.; Buttyan, R. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ. 1999, 6, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Salakou, S.; Kardamakis, D.; Tsamandas, A.C.; Zolota, V.; Apostolakis, E.; Tzelepi, V.; Papathanasopoulos, P.; Bonikos, D.S.; Papapetropoulos, T.; Petsas, T.; et al. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo 2007, 21, 123–132. [Google Scholar]
- Matsumoto, K.; Ema, M. Roles of VEGF-A signalling in development, regeneration, and tumours. J. Biochem. 2014, 156, 1–10. [Google Scholar] [CrossRef]
- Han, K.H.; Kim, A.K.; Kim, M.H.; Kim, D.H.; Go, H.N.; Kang, D.; Chang, J.W.; Choi, S.W.; Kang, K.S.; Kim, D.I. Protein profiling and angiogenic effect of hypoxia-cultured human umbilical cord blood-derived mesenchymal stem cells in hindlimb ischemia. Tissue Cell 2017, 49, 680–690. [Google Scholar] [CrossRef]
- Liu, J.; Hao, H.; Xia, L.; Ti, D.; Huang, H.; Dong, L.; Tong, C.; Hou, Q.; Zhao, Y.; Liu, H.; et al. Hypoxia pretreatment of bone marrow mesenchymal stem cells facilitates angiogenesis by improving the function of endothelial cells in diabetic rats with lower ischemia. PLoS ONE 2015, 10, e0126715. [Google Scholar] [CrossRef]
- Gleeson, B.M.; Martin, K.; Ali, M.T.; Kumar, A.H.; Pillai, M.G.; Kumar, S.P.; O’Sullivan, J.F.; Whelan, D.; Stocca, A.; Khider, W.; et al. Bone Marrow-Derived Mesenchymal Stem Cells Have Innate Procoagulant Activity and Cause Microvascular Obstruction Following Intracoronary Delivery: Amelioration by Antithrombin Therapy. Stem Cells 2015, 33, 2726–2737. [Google Scholar] [CrossRef]
- Abe, K.; Shoji, M.; Chen, J.; Bierhaus, A.; Danave, I.; Micko, C.; Casper, K.; Dillehay, D.L.; Nawroth, P.P.; Rickles, F.R. Regulation of vascular endothelial growth factor production and angiogenesis by the cytoplasmic tail of tissue factor. Proc. Natl. Acad. Sci. USA 1999, 96, 8663–8668. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, T.; Wada, H.; Shigemori, C.; Miki, C.; Gabazza, E.C.; Nobori, T.; Nakamura, S.; Shiku, H. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am. J. Hematol. 2002, 69, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Diaz de la Guardia, R.; Lopez-Millan, B.; Lavoie, J.R.; Bueno, C.; Castano, J.; Gomez-Casares, M.; Vives, S.; Palomo, L.; Juan, M.; Delgado, J.; et al. Detailed Characterization of Mesenchymal Stem/Stromal Cells from a Large Cohort of AML Patients Demonstrates a Definitive Link to Treatment Outcomes. Stem Cell Rep. 2017, 8, 1573–1586. [Google Scholar] [CrossRef]
- Rizzardini, M.; Zappone, M.; Villa, P.; Gnocchi, P.; Sironi, M.; Diomede, L.; Meazza, C.; Monshouwer, M.; Cantoni, L. Kupffer cell depletion partially prevents hepatic heme oxygenase 1 messenger RNA accumulation in systemic inflammation in mice: Role of interleukin 1beta. Hepatology 1998, 27, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Terry, C.M.; Clikeman, J.A.; Hoidal, J.R.; Callahan, K.S. Effect of tumor necrosis factor-alpha and interleukin-1 alpha on heme oxygenase-1 expression in human endothelial cells. Am. J. Physiol. 1998, 274, H883–H891. [Google Scholar]
- Carraway, M.S.; Ghio, A.J.; Carter, J.D.; Piantadosi, C.A. Expression of heme oxygenase-1 in the lung in chronic hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L806–L812. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Jiang, B.H.; Chin, B.Y.; Iyer, N.V.; Alam, J.; Semenza, G.L.; Choi, A.M. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 1997, 272, 5375–5381. [Google Scholar] [CrossRef]
- Panchenko, M.V.; Farber, H.W.; Korn, J.H. Induction of heme oxygenase-1 by hypoxia and free radicals in human dermal fibroblasts. Am. J. Physiol. Cell Physiol. 2000, 278, C92–C101. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R.; Bassi, R.; Calabrese, V.; Clark, J.E.; Green, C.J. Endothelial heme oxygenase-1 induction by hypoxia. Modulation by inducible nitric-oxide synthase and S-nitrosothiols. J. Biol. Chem. 2000, 275, 13613–13620. [Google Scholar] [CrossRef]
- Chabannes, D.; Hill, M.; Merieau, E.; Rossignol, J.; Brion, R.; Soulillou, J.P.; Anegon, I.; Cuturi, M.C. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007, 110, 3691–3694. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Withers, C.M.; Bartz, R.R.; MacGarvey, N.C.; Fu, P.; Sweeney, T.E.; Welty-Wolf, K.E.; Suliman, H.B. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J. Biol. Chem. 2011, 286, 16374–16385. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. CMLS 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, W.; Ren, H.Z.; Zhao, X.; Wang, S.; Ma, H.C.; Shi, X.L. Mesenchymal stem cells increase expression of heme oxygenase-1 leading to anti-inflammatory activity in treatment of acute liver failure. Stem Cell Res. Ther. 2017, 8, 70. [Google Scholar] [CrossRef]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. J. Int. Soc. Matrix Biol. 2018. [Google Scholar] [CrossRef]
- Foskett, A.M.; Bazhanov, N.; Ti, X.; Tiblow, A.; Bartosh, T.J.; Prockop, D.J. Phase-directed therapy: TSG-6 targeted to early inflammation improves bleomycin-injured lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L120–L131. [Google Scholar] [CrossRef]
- Mittal, M.; Tiruppathi, C.; Nepal, S.; Zhao, Y.-Y.; Grzych, D.; Soni, D.; Prockop, D.J.; Malik, A.B. TNF a -stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc. Natl. Acad. Sci. USA 2016, 113, E8151–E8158. [Google Scholar] [CrossRef]
- Lee, R.H.; Yu, J.M.; Foskett, A.M.; Peltier, G.; Reneau, J.C.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 16766–16771. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Lee, R.H.; Prockop, D.J.; Bartosh, T.J. Stanniocalcin-1 attenuates ischemic cardiac injury and response of differentiating monocytes/macrophages to inflammatory stimuli. Transl. Res. J. Lab. Clin. Med. 2016, 177, 127–142. [Google Scholar] [CrossRef]
- Ono, M.; Ohkouchi, S.; Kanehira, M.; Tode, N.; Kobayashi, M.; Ebina, M.; Nukiwa, T.; Irokawa, T.; Ogawa, H.; Akaike, T.; et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 549–560. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Huang, L.; Tong, H.; Chen, P.; Lai, D.; Hu, Y.; Cheng, B.; Shu, Q.; et al. Adipose-derived mesenchymal stem cells modulate CD14(++)CD16(+) expression on monocytes from sepsis patients in vitro via prostaglandin E2. Stem Cell Res. Ther. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Xu, H.; Xu, C.; Cai, W.; Li, Q.; Cheng, Y.; Jin, M.; Wang, R.X.; Peng, Y.; Zhang, Y.; et al. Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy 2014, 10, 1301–1315. [Google Scholar] [CrossRef]
- Wang, C.; Lv, D.; Zhang, X.; Ni, Z.A.; Sun, X.; Zhu, C. Interleukin-10-Overexpressing Mesenchymal Stromal Cells Induce a Series of Regulatory Effects in the Inflammatory System and Promote the Survival of Endotoxin-Induced Acute Lung Injury in Mice Model. DNA Cell Biol. 2018, 37, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bustos, M.L.; Huleihel, L.; Meyer, E.M.; Donnenberg, A.D.; Donnenberg, V.S.; Sciurba, J.D.; Mroz, L.; McVerry, B.J.; Ellis, B.M.; Kaminski, N.; et al. Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem Cells Transl. Med. 2013, 2, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Leeper-Woodford, S.K.; Carey, P.D.; Byrne, K.; Jenkins, J.K.; Fisher, B.J.; Blocher, C.; Sugerman, H.J.; Fowler, A.A., 3rd. Tumor necrosis factor. Alpha and beta subtypes appear in circulation during onset of sepsis-induced lung injury. Am. Rev. Respir. Dis. 1991, 143, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, B.C.; McIntyre, R.C.; Meldrum, D.R.; Fullerton, D.A. Pentoxifylline treatment attenuates pulmonary vasomotor dysfunction in acute lung injury. J. Surg. Res. 1997, 71, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yin, S.; Liu, W.; Li, N.; Zhang, W.; Cao, Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007, 13, 1185–1195. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Buscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 241–248. [Google Scholar] [CrossRef]
- Tse, W.T.; Pendleton, J.D.; Beyer, W.M.; Egalka, M.C.; Guinan, E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 2003, 75, 389–397. [Google Scholar] [CrossRef]
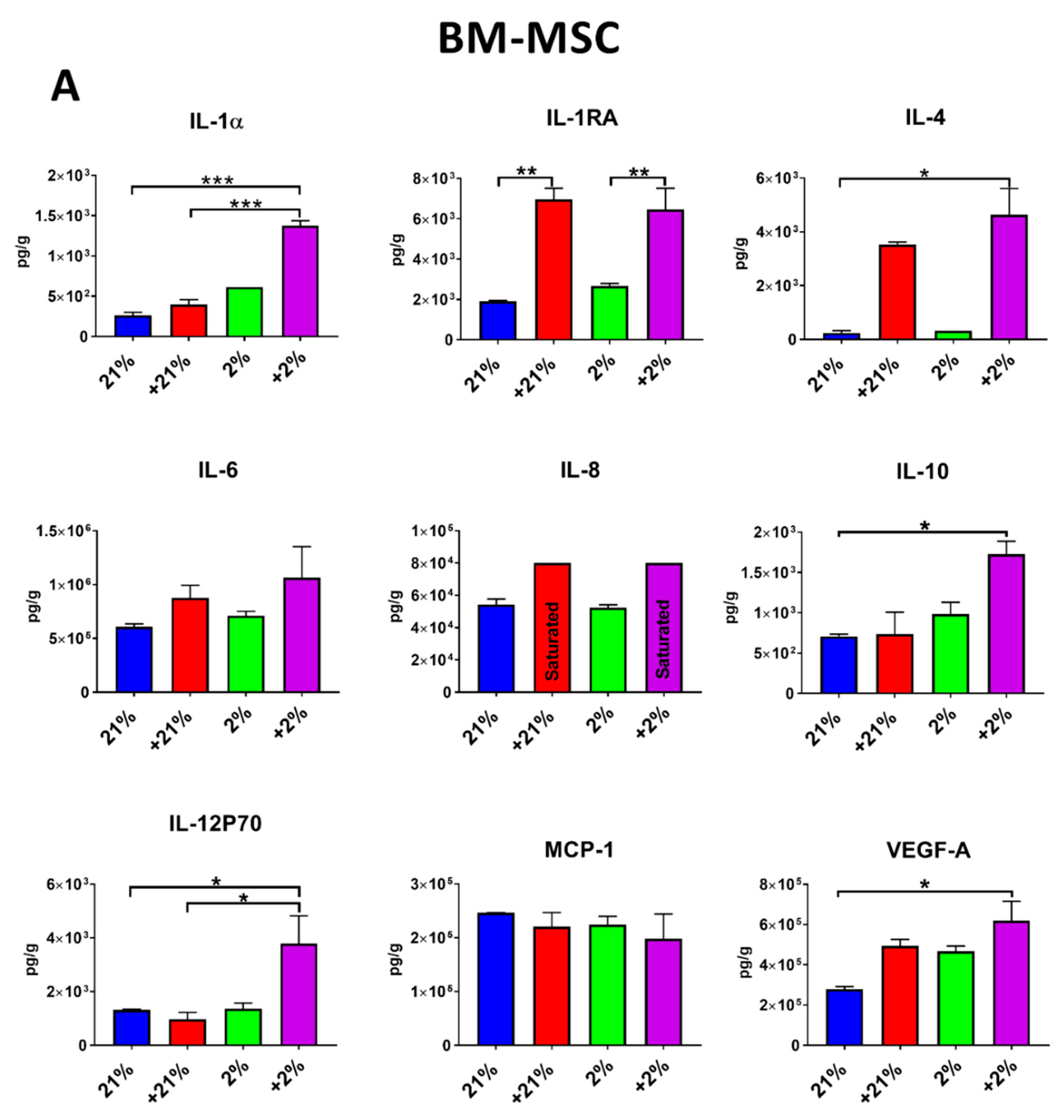
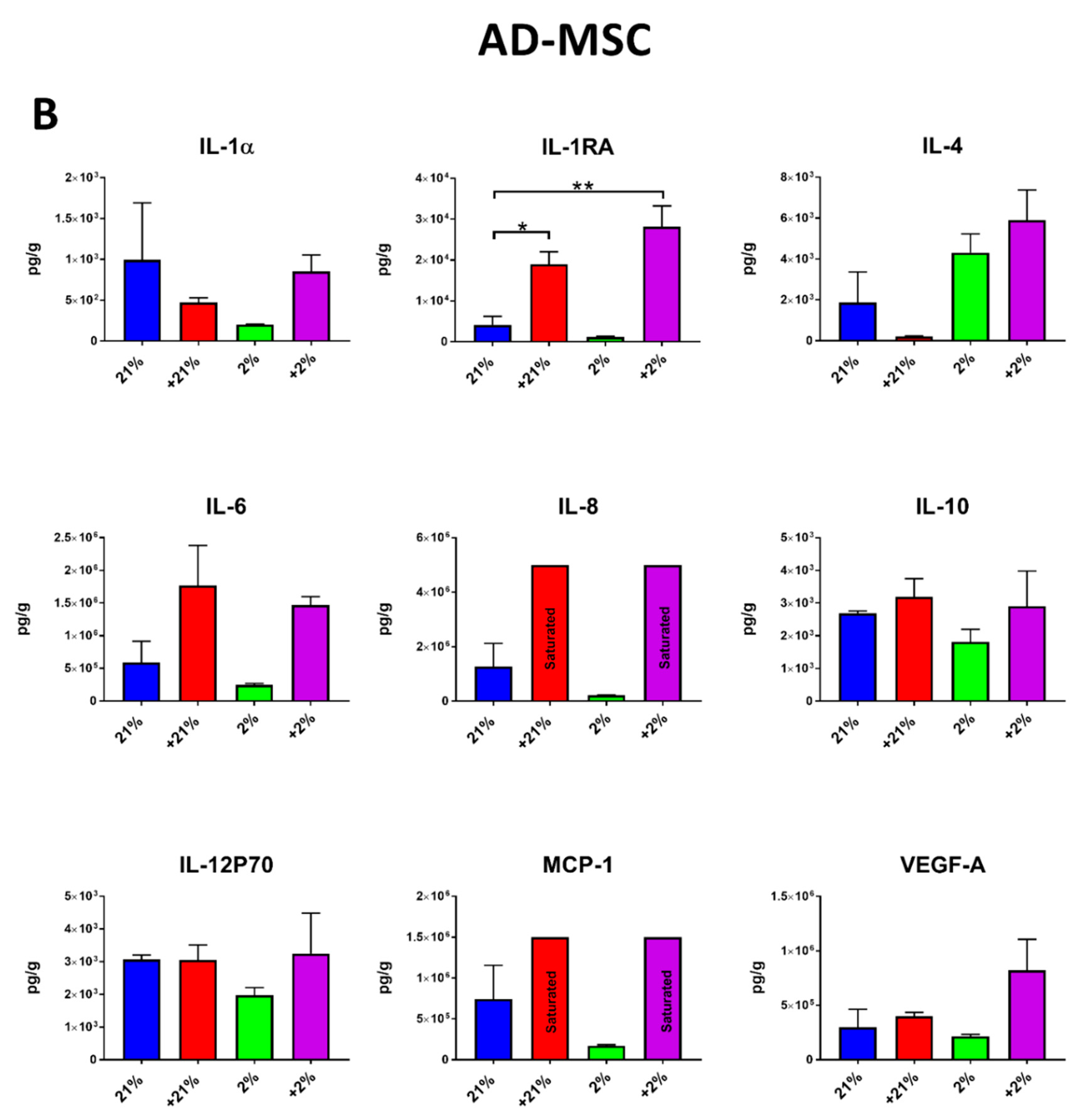
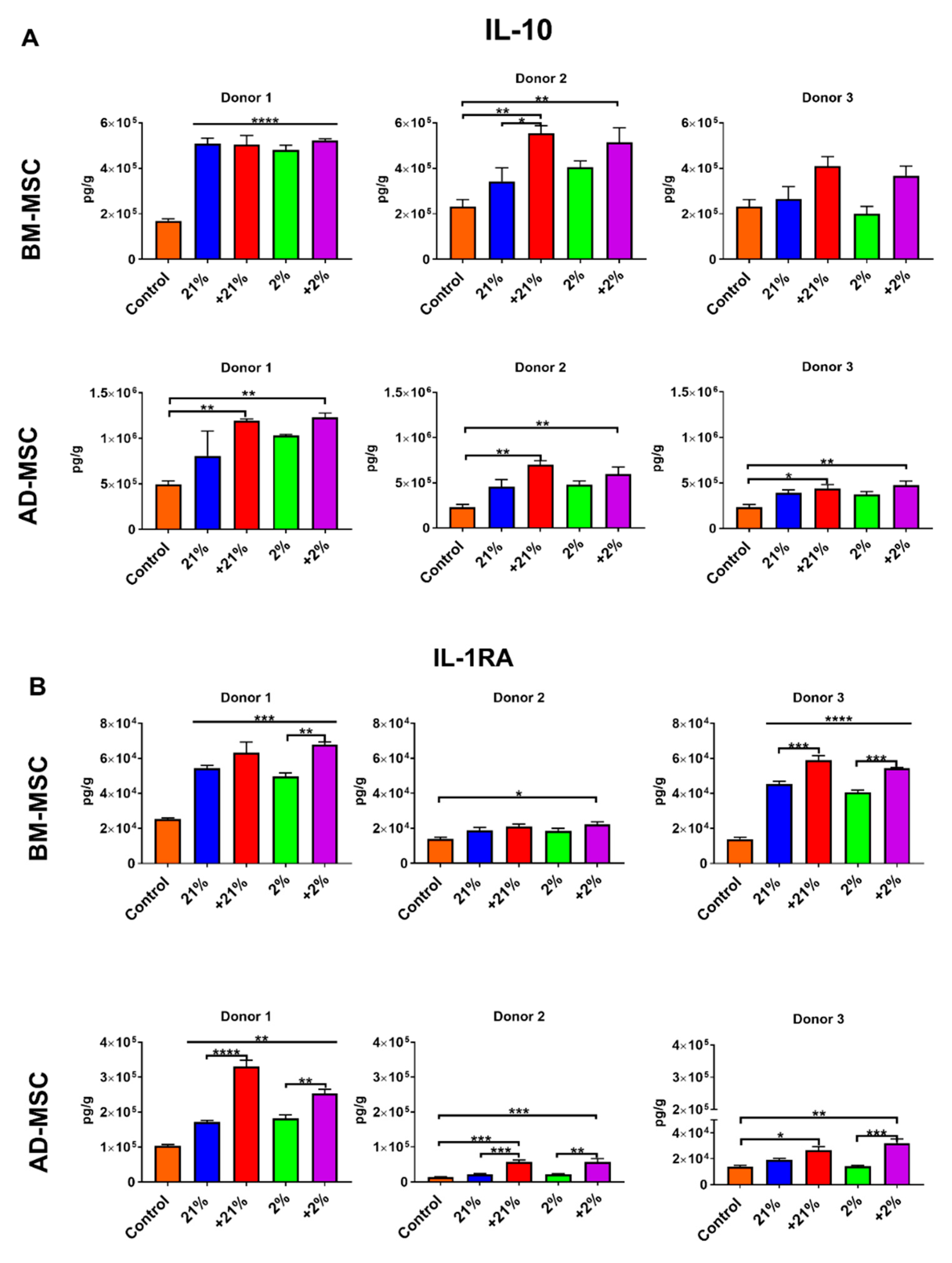
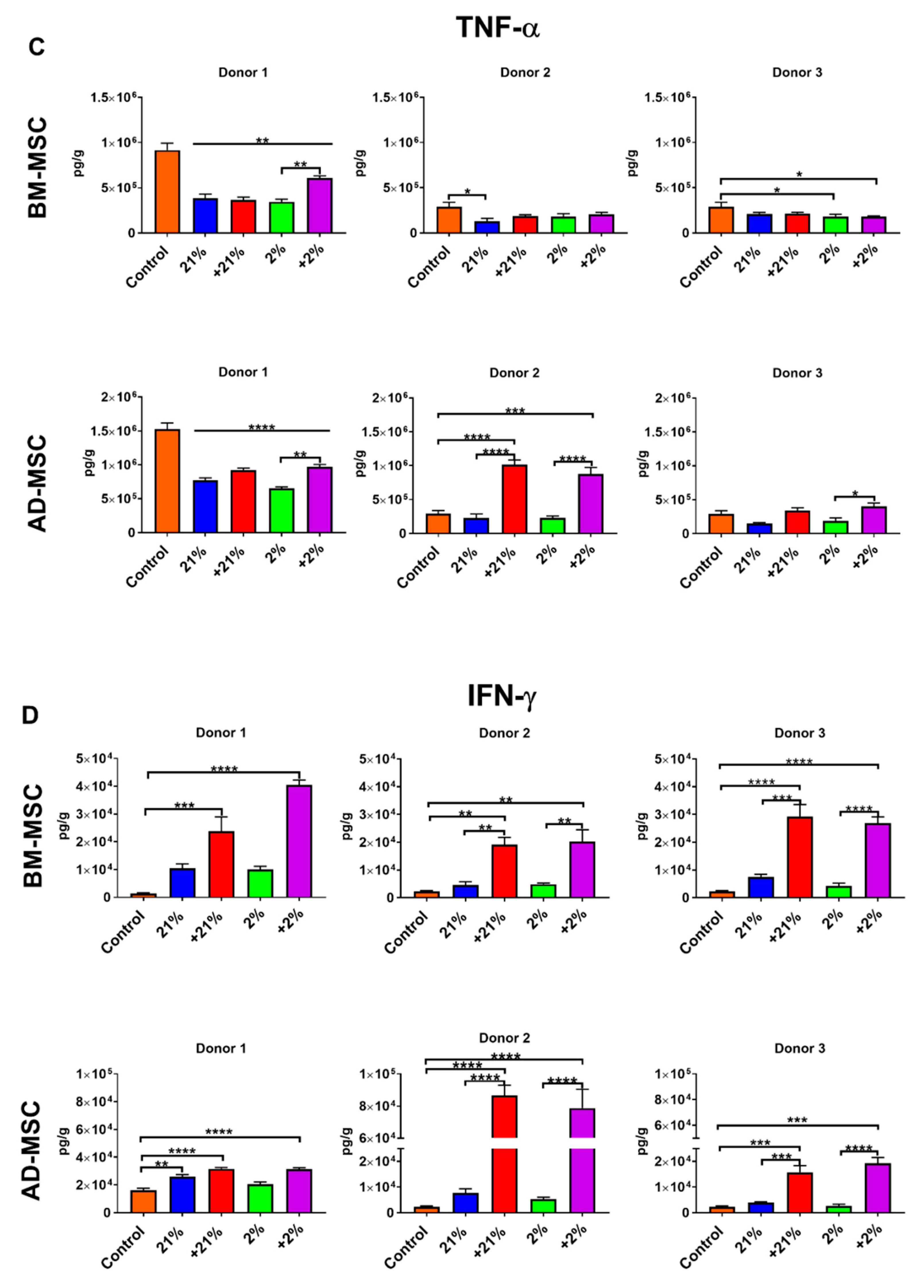

| 21% | +21% | 2% | +2% | |||||
|---|---|---|---|---|---|---|---|---|
| BM | AD | BM | AD | BM | AD | BM | AD | |
| Gene Expression | ||||||||
| TF (F3) | - | - | - | ↓↓ | - | ↑↑↑↑ | ↑↑ | ↑↑↑ |
| VEGF-A | - | - | - | - | - | ↑↑↑↑ | - | ↑↑↑↑ |
| BCL-2 | - | - | ↑↑ | ↑ | - | ↑↑ | - | ↑↑↑↑ |
| HMOX1 | - | - | ↑↑ | ↓↓ | ↑ | - | - | ↓ |
| TNFAIP6 | - | - | ↑↑↑ | ↑↑↑ | - | ↑↑ | ↑ | - |
| MSC Secretion | ||||||||
| IL-1RA | - | - | ↑↑ | ↑ | - | - | ↑↑ | ↑↑ |
| IL-8 | - | - | ↑↑↑↑ | ↑↑↑↑ | - | - | ↑↑↑↑ | ↑↑↑↑ |
| Immunosuppression (MLR Assay) | ↑ | ↑ | ↑↑ | ↑↑↑↑ | ↑ | ↑ | ↑↑ | ↑↑↑ |
| Immunomodulation (MLR Assay) | ||||||||
| IFN-γ | ↓↓↓↓ | ↓↓↓↓ | ↓↓↓↓ | ↓↓↓↓ | ↓↓↓↓ | ↓↓↓↓ | ↓↓↓↓ | ↓↓↓↓ |
| IL-1β | ↑↑ | - | ↑↑ | - | ↑ | - | ↑↑ | - |
| IL-6 | ↓ | - | ↓↓ | - | ↓↓ | - | ↓ | - |
| IL-8 | ↑↑↑↑ | - | ↓↓↓ | - | ↓↓↓ | - | ↓↓↓ | - |
| IL-1RA | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, L.A., II; Mohammadipoor, A.; Alvarado, L.; Kamucheka, R.M.; Asher, A.M.; Cancio, L.C.; Antebi, B. Preconditioning in an Inflammatory Milieu Augments the Immunotherapeutic Function of Mesenchymal Stromal Cells. Cells 2019, 8, 462. https://doi.org/10.3390/cells8050462
Rodriguez LA II, Mohammadipoor A, Alvarado L, Kamucheka RM, Asher AM, Cancio LC, Antebi B. Preconditioning in an Inflammatory Milieu Augments the Immunotherapeutic Function of Mesenchymal Stromal Cells. Cells. 2019; 8(5):462. https://doi.org/10.3390/cells8050462
Chicago/Turabian StyleRodriguez, Luis A., II, Arezoo Mohammadipoor, Lucero Alvarado, Robin M. Kamucheka, Amber M. Asher, Leopoldo C. Cancio, and Ben Antebi. 2019. "Preconditioning in an Inflammatory Milieu Augments the Immunotherapeutic Function of Mesenchymal Stromal Cells" Cells 8, no. 5: 462. https://doi.org/10.3390/cells8050462
APA StyleRodriguez, L. A., II, Mohammadipoor, A., Alvarado, L., Kamucheka, R. M., Asher, A. M., Cancio, L. C., & Antebi, B. (2019). Preconditioning in an Inflammatory Milieu Augments the Immunotherapeutic Function of Mesenchymal Stromal Cells. Cells, 8(5), 462. https://doi.org/10.3390/cells8050462







