Hypolipidemic Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program in Overweight and Obese Men: A Double-Blind, Randomized, and Crossover Controlled Trial
Abstract
1. Introduction
2. Results
2.1. Diet
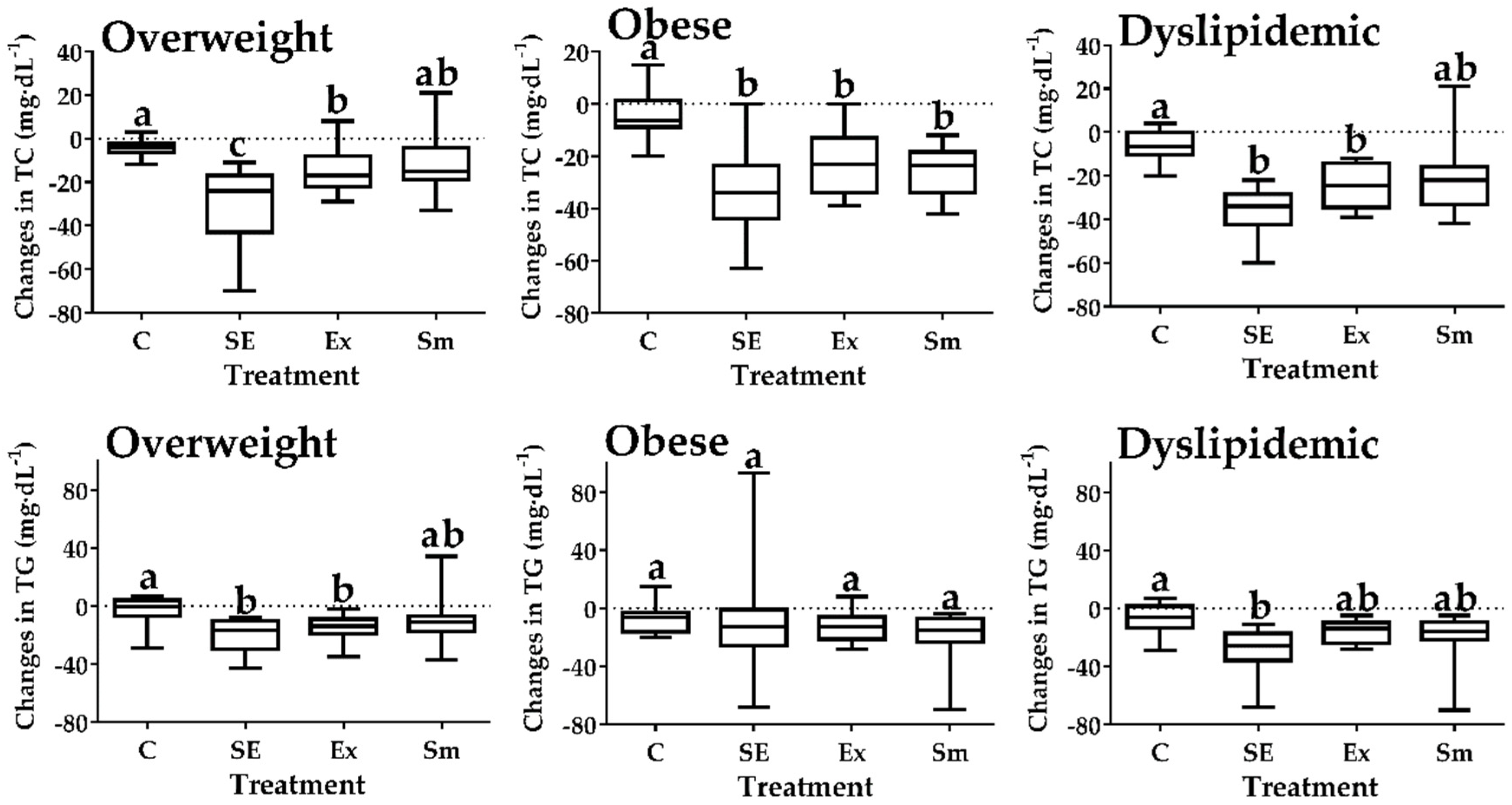
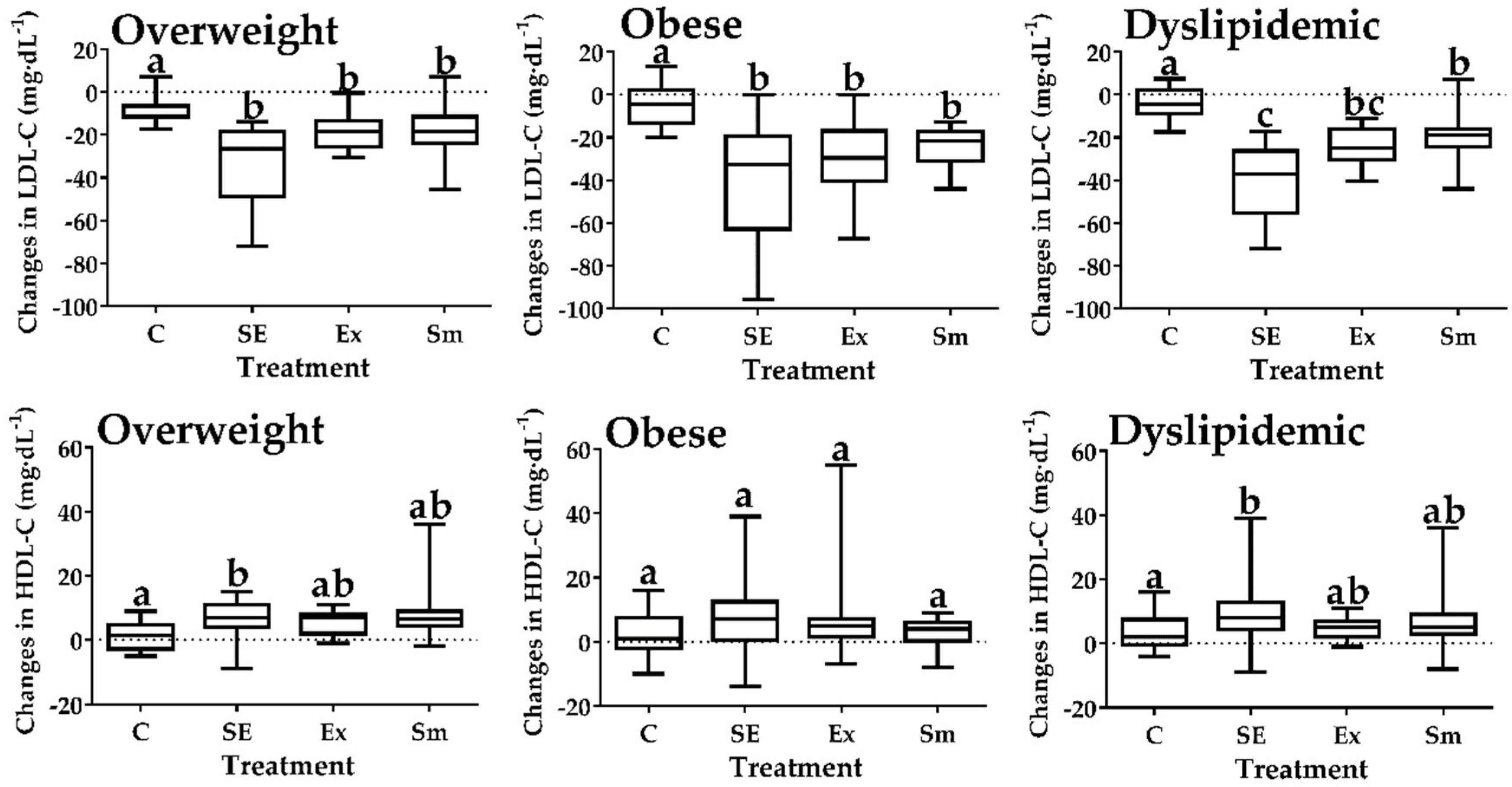
2.2. Intra Group Comparisons (Pre vs. Post) on the Blood Lipid Profile
2.3. Inter-Group Comparisons of the Blood Lipid Profile
2.4. Inter-Group Comparisons on the Body Mass Index
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Participants’ Eligibility
4.3. Baseline Measurements
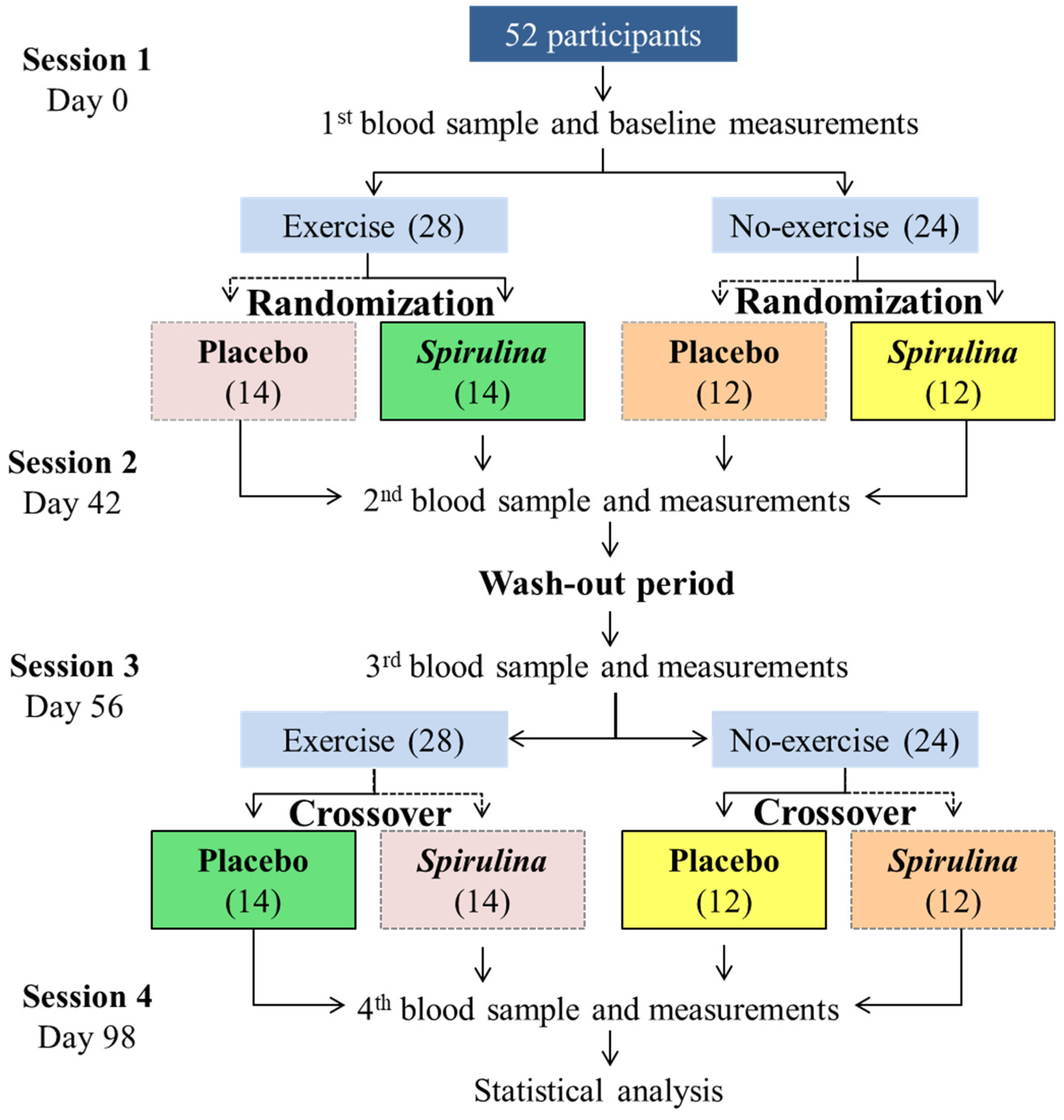
4.4. Study Design
4.5. Blood Sample Collection and Biochemical Analysis
4.6. Dietary Analysis
4.7. Systematic Physical Exercise Protocol
4.8. Main Physiological Outcomes
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, S.C.; Collins, A.; Ferrari, R.; Holmes, D.R.; Logstrup, S.; Vaca, D.; Ralston, J.; Sacco, R.L.; Stam, H.; Taubert, K.; et al. Our time: A call to save preventable death from cardiovascular disease (heart disease and stroke). J. Am. Coll. Cardiol. 2012, 60, 2343–2348. [Google Scholar] [CrossRef]
- D’Adamo, E.; Guardamagna, O.; Chiarelli, F.; Bartuli, A.; Liccardo, D.; Ferrari, F.; Nobili, V. Atherogenic dyslipidemia and cardiovascular risk factors in obese children. Int. J. Endocrinol. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Wall-Medrano, A.; Ramos-Jiménez, A.; Hernández-Torres, R.P.; Villalobos-Molina, R.; Tapia-Pancardo, D.C.; Jiménez-Flores, J.R.; Méndez-Cruz, A.R.; Murguía-Romero, M.; Gallardo-Ortíz, I.A.; Urquídez-Romero, R. Cardiometabolic risk in young adults from northern Mexico: Revisiting body mass index and waist-circumference as predictors. BMC Public Health 2016, 16, 236. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Hudson, R.; Stotz, P.J.; Lam, M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann. Intern. Med. 2015, 162, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.P.; Martin, S.E.; Crouse, S.F. Acute exercise and training alter blood lipid and lipoprotein profiles differently in overweight and obese men and women. Obesity 2012, 20, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A.; Gennat, H.C.; O’Rourke, P.; Del Mar, C. Exercise for overweight or obesity. Cochrane Db. Syst. Rev. 2006, 4, CD003817. [Google Scholar] [CrossRef] [PubMed]
- Shiraev, T.; Barclay, G. Evidence based exercise: Clinical benefits of high intensity interval training. Aust. Fam. Physician 2012, 41, 960. [Google Scholar]
- Chen, G.; Wang, H.; Zhang, X.; Yang, S.-T. Nutraceuticals and functional foods in the management of hyperlipidemia. Crit. Rev. Food Sci. Nutr. 2014, 54, 1180–1201. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Haimeur, A.; Guéno, F.; Meskini, N.; Tremblin, G. Marine microalgae used as food supplements and their implication in preventing cardiovascular diseases. OCL 2015, 22, D409. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef]
- Memije-Lazaro, I.N.; Blas-Valdivia, V.; Franco-Colín, M.; Cano-Europa, E. Arthrospira maxima (Spirulina) and C-phycocyanin prevent the progression of chronic kidney disease and its cardiovascular complications. J. Funct. Food 2018, 43, 37–43. [Google Scholar] [CrossRef]
- Moura, L.P.; Puga, G.M.; Beck, W.R.; Teixeira, I.P.; Ghezzi, A.C.; Silva, G.A.; Mello, M.A.R. Exercise and spirulina control non-alcoholic hepatic steatosis and lipid profile in diabetic Wistar rats. Lipids Health Dis. 2011, 10, 77. [Google Scholar] [CrossRef]
- Mazzola, D.; Fornari, F.; Vigano, G.; Oro, T.; Costa, J.A.V.; Bertolin, T.E. Spirulina platensis Enhances the beneficial effect of exercise on oxidative stress and the lipid profile in rats. Braz. Arch. Biol. Techn. 2015, 58, 961–969. [Google Scholar] [CrossRef]
- Kata, F.S.; Athbi, A.M.; Manwar, E.Q.; Al-Ashoor, A.; Abdel-Daim, M.M.; Aleya, L. Therapeutic effect of the alkaloid extract of the cyanobacterium Spirulina platensis on the lipid profile of hypercholesterolemic male rabbits. Environ. Sci. Pollut. Res. Int. 2018, 25, 19635–19642. [Google Scholar] [CrossRef]
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Wilborn, C.; Beckham, J.; Campbell, B.; Harvey, T.; Galbreat, M.; La Bounty, P.; Nassar, E.; Wismann, J.; Kreider, R. Obesity: prevalence, theories, medical consequences, management, and research directions. J. Int. Soc. Sport. Nutr. 2005, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Mani, U.V.; Desai, S.; Iyer, U. Studies on the long-term effect of spirulina supplementation on serum lipid profile and glycated proteins in NIDDM patients. J. Nutraceuticals Funct. Med. Food 2000, 2, 25–32. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, J.E.; Choi, Y.J.; Huh, K.B.; Kim, W.Y. A randomized study to establish the effects of spirulina in type 2 diabetes mellitus patients. Nutr. Res. Pract. 2008, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kalafati, M.; Jamurtas, A.Z.; Nikolaidis, M.G.; Paschalis, V.; Theodorou, A.A.; Sakellariou, G.K.; Koutedakis, Y.; Kouretas, D. Ergogenic and antioxidant effects of spirulina supplementation in humans. Med. Sci. Sports Exerc. 2010, 42, 142–151. [Google Scholar] [CrossRef]
- Iwata, K.; Inayama, T.; Kato, T. Effects of Spirulina platensis on plasma lipoprotein lipase activity in fructose-induced hyperlipidemic rats. J. Nutr. Sci. Vitaminol. 1990, 36, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Upasani, C.D.; Balaraman, R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytother. Res. 2003, 17, 330–334. [Google Scholar] [CrossRef]
- Sharma, N.K.; Tiwari, S.P.; Tripathi, K.; Rai, A.K. Sustainability and cyanobacteria (blue-green algae): facts and challenges. Appl. Phycol. 2011, 23, 1059–1081. [Google Scholar] [CrossRef]
- Nagaoka, S.; Shimizu, K.; Kaneko, H.; Shibayama, F.; Morikawa, K.; Kanamaru, Y.; Otsuka, A.; Hirahashi, T.; Kato, T. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J. Nutr. 2005, 135, 2425–2430. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Jiao, R.; Ma, K.Y. Cholesterol-lowering nutraceuticals and functional foods. J. Agric. Food Chem. 2008, 56, 8761–8773. [Google Scholar] [CrossRef]
- Thompson, P.D.; Clarkson, P.; Karas, R.H. Statin-associated myopathy. JAMA 2003, 289, 1681–1690. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Z.; Liu, Y.; Wang, X.; Wan, L.; Zhang, L.; Liu, X. Hypolipidemic effect of Fragarianilgerrensis Schlecht. medicine compound on hyperlipidemic rats. Lipids Health Dis. 2018, 17, 222. [Google Scholar] [CrossRef]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef]
- Oscai, L.B.; Essig, D.A.; Palmer, W.K. Lipase regulation of muscle triglyceride hydrolysis. J. Appl. Physiol. 1990, 69, 1571–1577. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Paschalis, V.; Giakas, G.; Fatouros, I.G.; Sakellariou, G.K.; Theodorou, A.A.; Koutedakis, Y.; Jamurtas, A.Z. Favorable and prolonged changes in blood lipid profile after muscle-damaging exercise. Med. Sci. Sports Exerc. 2008, 40, 1483–1489. [Google Scholar] [CrossRef]
- Tan, S.; Wang, J.; Cao, L.; Guo, Z.; Wang, Y. Positive effect of exercise training at maximal fat oxidation intensity on body composition and lipid metabolism in overweight middle-aged women. Clin. Physiol. Funct. Imaging 2016, 36, 225–230. [Google Scholar] [CrossRef]
- Cugusi, L.; Cadeddu, C.; Nocco, S.; Orrù, F.; Bandino, S.; Deidda, M.; Caria, A.; Bassareo, P.P.; Piras, A.; Cabras, S.; et al. Effects of an aquatic-based exercise program to improve cardiometabolic profile, quality of life, and physical activity levels in men with type 2 diabetes mellitus. PM R 2015, 7, 141–148. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Tran, Z.V. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: a meta-analysis of randomized controlled trials. Int. J. Obes. 2005, 29, 881. [Google Scholar] [CrossRef]
- Hernández-Lepe, M.; López-Díaz, J.; Juárez-Oropeza, M.; Hernández-Torres, R.P.; Wall-Medrano, A.; Ramos-Jiménez, A. Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program on the Body Composition and Cardiorespiratory Fitness of Overweight or Obese Subjects: A Double-Blind, Randomized, and Crossover Controlled Trial. Mar. Drugs. 2018, 16, 364. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Magno, C.P.; Lane, K.T.; Hinojosa, M.W.; Lane, J.S. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: Findings from the national health and nutrition examination survey, 1999 to 2004. J. Am. Coll. Surg. 2008, 207, 928–934. [Google Scholar] [CrossRef]
- He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 2001, 50, 817–823. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Wolfe, R.R.; Kelley, D.E. Effects of obesity on substrate utilization during exercise. Obes. Res. 2002, 10, 575–584. [Google Scholar] [CrossRef]
- Das, U.N.; Rao, A.A. Gene expression profile in obesity and type 2 diabetes mellitus. Lipids Health Dis. 2007, 6, 35. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Hernández-Lepe, M.A.; López-Díaz, J.A.; de la Rosa, L.A.; Hernández-Torres, R.P.; Wall-Medrano, A.; Juarez-Oropeza, M.A.; Pedraza-Chaverri, J.; Urquidez-Romero, R.; Ramos-Jiménez, A. Double-blind randomised controlled trial of the independent and synergistic effect of Spirulina maxima with exercise (ISESE) on general fitness, lipid profile and redox status in overweight and obese subjects: Study protocol. BMJ Open 2017, 7, 013744. [Google Scholar] [CrossRef]
- Schulz, K.F.; Grimes, D.A. Generation of allocation sequences in randomised trials: Chance, not choice. Lancet 2002, 359, 515–519. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Hernández-Lepe, M.A.; Wall-Medrano, A.; Juárez-Oropeza, M.A.; Ramos-Jiménez, A.; Hernández-Torres, R.P. Spirulina y su efecto hipolipemiante y antioxidante en humanos: una revisión sistemática. Nutr. Hosp. 2015, 32, 494–500. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Nelson, M. The validation of dietary assessment. In Design Concepts in Nutritional Epidemiology, 2nd ed.; Margetts, B.M., Nelson, M., Eds.; Oxford University Press: Oxford, UK, 1997; pp. 266–295. [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2016; pp. 111–142. [Google Scholar]
- Swain, D.P.; Leutholtz, B.C. Heart rate reserve is equivalent to% VO2 reserve, not to% VO2max. Med. Sci. Sports Exerc. 1997, 29, 410–414. [Google Scholar] [CrossRef]
- Meydani, M.; Hasan, S.T. Dietary polyphenols and obesity. Nutrients 2010, 2, 737–751. [Google Scholar] [CrossRef]

| Total | Overweight | Obese | |
|---|---|---|---|
| n | 52 | 27 | 25 |
| Age (years) | 26 ± 5 | 26 ± 4 | 27 ± 6 |
| Body weight (kg) | 90 ± 13 | 81 ± 8 | 100 ± 12 |
| Height (m) | 1.72 ± 0.1 | 1.72 ± 0.1 | 1.73 ± 0.1 |
| BMI (kg·m−2) | 30.2 ± 4 | 27.4 ± 1.2 | 33.3 ± 3.8 |
| Energy intake (kcal·day−1) | 2054 ± 104 | 1977 ± 139 | 2054 ± 151 |
| Total cholesterol (mg·dL−1) * | 196 ± 36 | 177 ± 28 | 218 ± 30 |
| Triglycerides (mg·dL−1) | 142 ± 41 | 136 ± 35 | 150 ± 46 |
| LDL-C (mg·dL−1) * | 134 ± 36 | 115 ± 27 | 158 ± 31 |
| HDL-C (mg·dL−1) | 34 ± 9.6 | 35.3 ± 8.2 | 32.5 ± 10.9 |
| Blood lipid | 2a (Overweight and obese subjects) | ||||
| C | SE | Ex | Sm | ||
| TC | Basal | 190±31 | 196±35 | 197±38 | 201±39 |
| Final | 186±31 | 163±33 | 177±36 | 183±37 | |
| p value (n) | 0.619 (12) | 0.001 (14) * | 0.053 (14) | 0.103 (12) | |
| TG | Basal | 131±31 | 157±47 | 139±44 | 141±37 |
| Final | 125±32 | 135±37 | 124±42 | 127±34 | |
| p value (n) | 0.517 (12) | 0.070 (14) | 0.212 (14) | 0.176 (12) | |
| LDL-C | Basal | 131±32 | 128±36 | 137±38 | 138±37 |
| Final | 127±30 | 93±34 | 115±36 | 117±38 | |
| p value (n) | 0.680 (12) | 0.001 (14) * | 0.046 (14) * | 0.062 (12) | |
| HDL-C | Basal | 35±12 | 34±9 | 31±9 | 35±10 |
| Final | 37±8 | 42±10 | 36±9 | 40±11 | |
| p value (n) | 0.655 (12) | 0.005 (14) * | 0.059 (14) | 0.071 (12) | |
| Blood lipid | 2b (Dyslipidemic subjects) | ||||
| C | SE | Ex | Sm | ||
| TC | Basal | 219±16 | 226±22 | 232±23 | 233±21 |
| Final | 213±18 | 189±20 | 208±28 | 212±23 | |
| p value (n) | 0.412 (6) | 0.000 (7) * | 0.025 (6) * | 0.029 (6) * | |
| TG | Basal | 160±6 | 184±40 | 180±25 | 167±11 |
| Final | 153±12 | 156±29 | 164±21 | 148±19 | |
| p value (n) | 0.156 (6) | 0.043 (7) * | 0.096 (7) | 0.004 (7) * | |
| LDL-C | Basal | 140±29 | 141±29 | 148±33 | 148±33 |
| Final | 135±27 | 101±34 | 124±33 | 128±32 | |
| p value (n) | 0.650 (9) | 0.001 (10) * | 0.030 (10) * | 0.060 (9) | |
| HDL-C | Basal | 28±8 | 30±6 | 28±6 | 29±6 |
| Final | 31±5 | 40±10 | 33±6 | 35±10 | |
| p value (n) | 0.172 (7) | 0.001 (10) * | 0.019 (10) * | 0.040 (8) * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Lepe, M.A.; Wall-Medrano, A.; López-Díaz, J.A.; Juárez-Oropeza, M.A.; Luqueño-Bocardo, O.I.; Hernández-Torres, R.P.; Ramos-Jiménez, A. Hypolipidemic Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program in Overweight and Obese Men: A Double-Blind, Randomized, and Crossover Controlled Trial. Mar. Drugs 2019, 17, 270. https://doi.org/10.3390/md17050270
Hernández-Lepe MA, Wall-Medrano A, López-Díaz JA, Juárez-Oropeza MA, Luqueño-Bocardo OI, Hernández-Torres RP, Ramos-Jiménez A. Hypolipidemic Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program in Overweight and Obese Men: A Double-Blind, Randomized, and Crossover Controlled Trial. Marine Drugs. 2019; 17(5):270. https://doi.org/10.3390/md17050270
Chicago/Turabian StyleHernández-Lepe, Marco Antonio, Abraham Wall-Medrano, José Alberto López-Díaz, Marco Antonio Juárez-Oropeza, Oscar Iván Luqueño-Bocardo, Rosa Patricia Hernández-Torres, and Arnulfo Ramos-Jiménez. 2019. "Hypolipidemic Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program in Overweight and Obese Men: A Double-Blind, Randomized, and Crossover Controlled Trial" Marine Drugs 17, no. 5: 270. https://doi.org/10.3390/md17050270
APA StyleHernández-Lepe, M. A., Wall-Medrano, A., López-Díaz, J. A., Juárez-Oropeza, M. A., Luqueño-Bocardo, O. I., Hernández-Torres, R. P., & Ramos-Jiménez, A. (2019). Hypolipidemic Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program in Overweight and Obese Men: A Double-Blind, Randomized, and Crossover Controlled Trial. Marine Drugs, 17(5), 270. https://doi.org/10.3390/md17050270







