CD44 Promotes Migration and Invasion of Docetaxel-Resistant Prostate Cancer Cells Likely via Induction of Hippo-Yap Signaling
Abstract
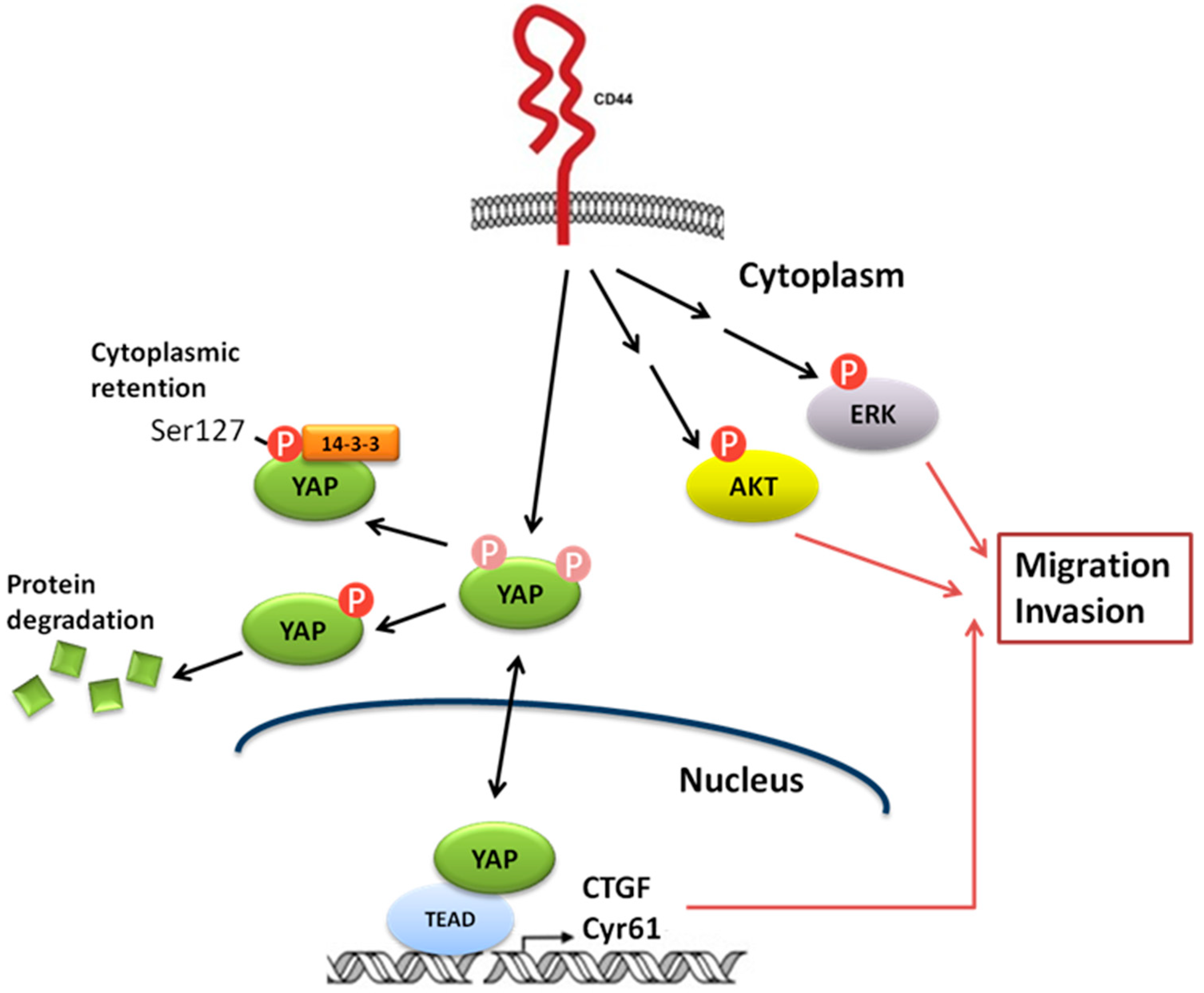
:1. Introduction
2. Results
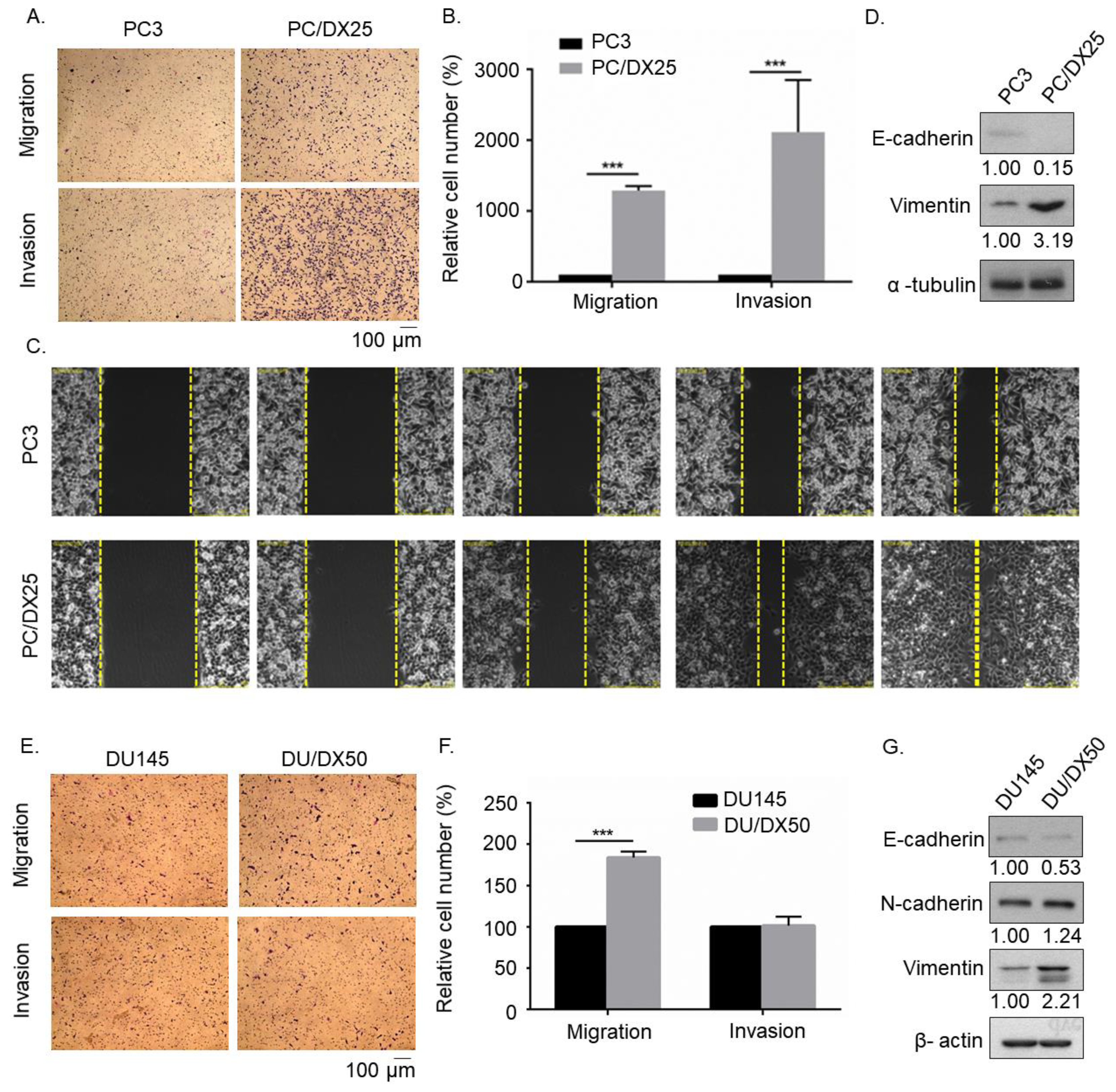
2.1. Docetaxel-Resistance Prostate Cancer (PCa) Cells Acquired Higher Migration and Invasion Ability than Parental PCa cells
2.2. Docetaxel-Resistant PCa Cells Contain Higher CD44+ Population
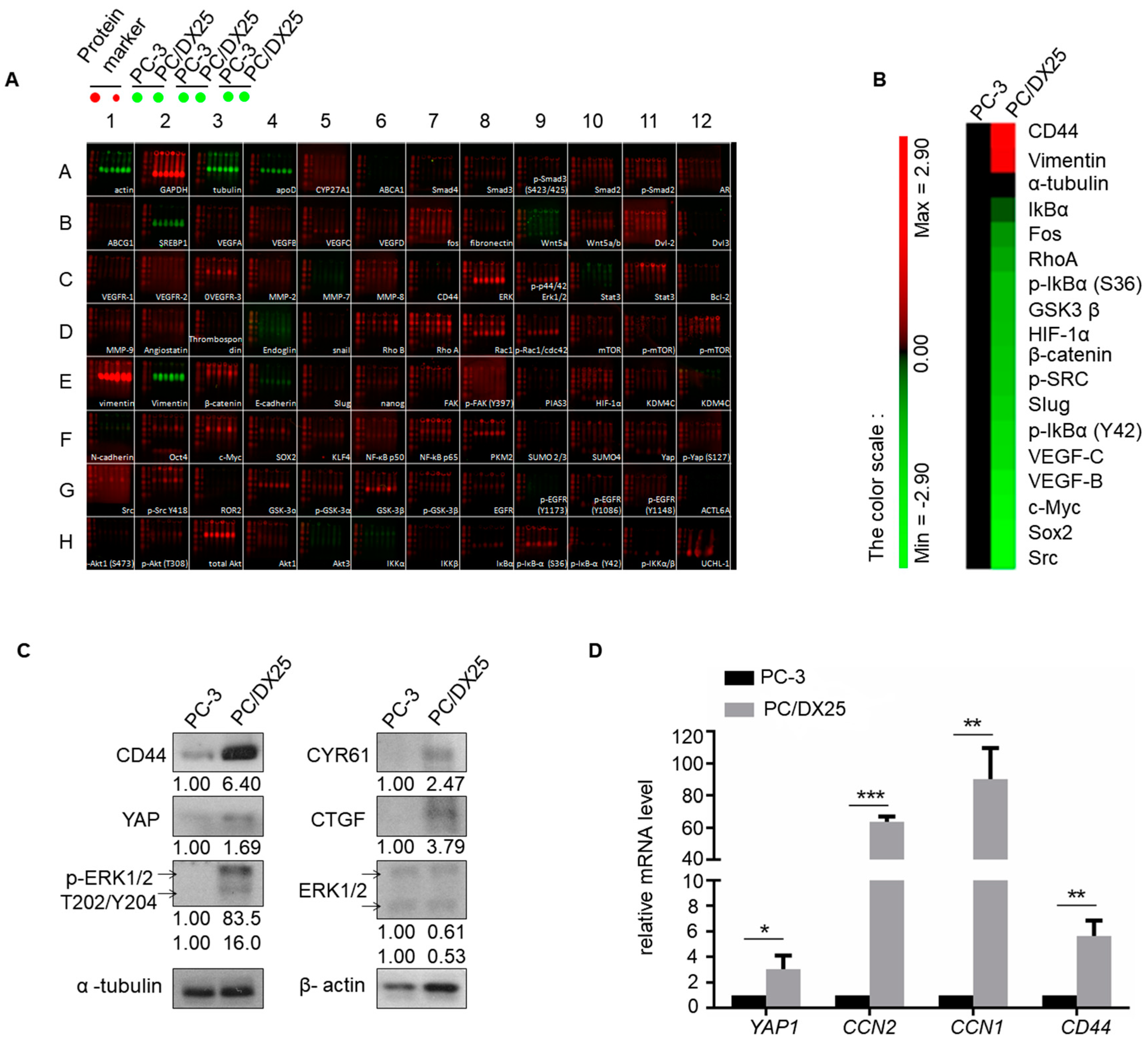
2.3. Docetaxel Resistant PCa Cells Express Higher Level of Proteins Involved in Hippo-YAP Pathway
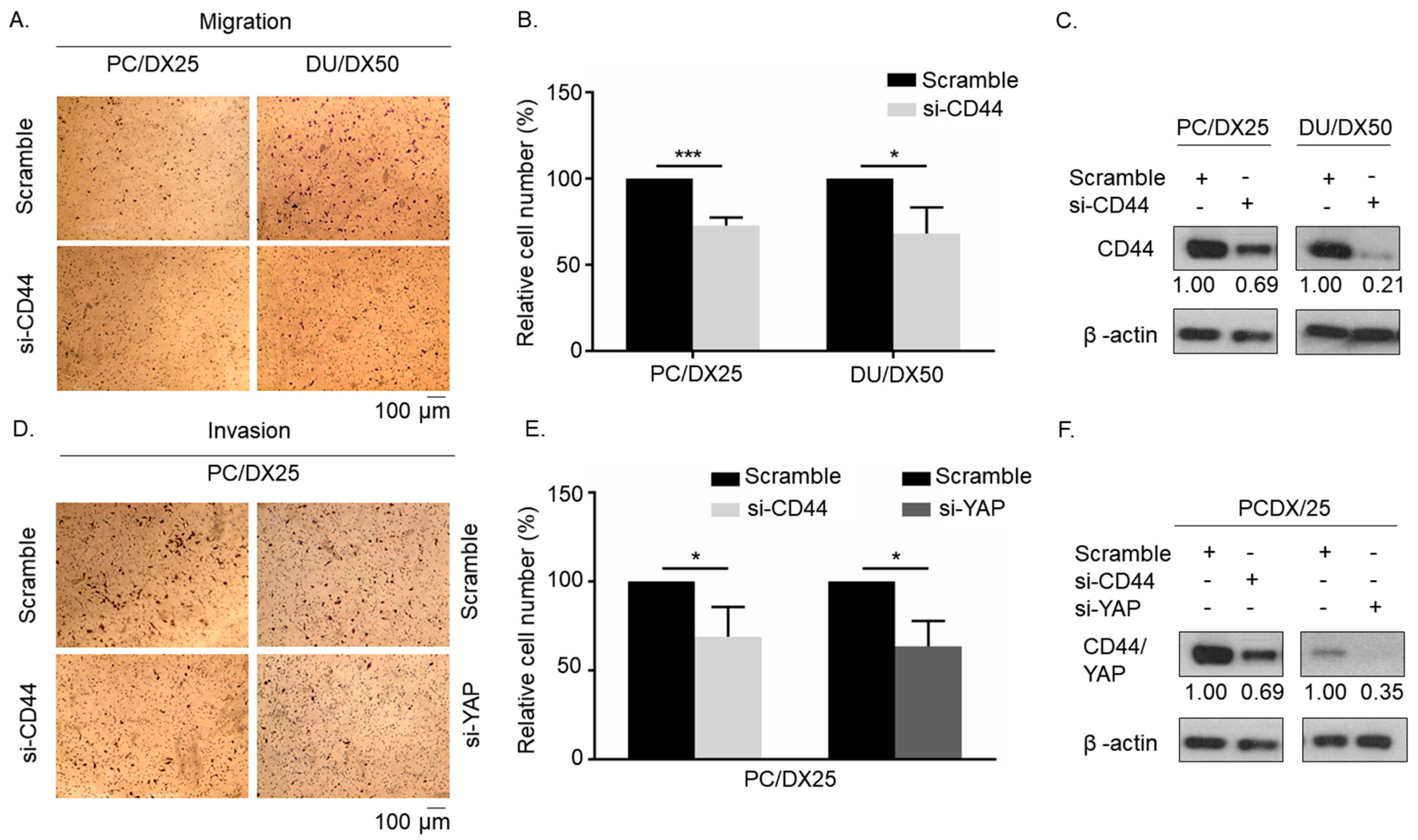
2.4. Knockdown of CD44 or YAP Suppresses Migration and Invasion of Docetaxel-Resistant PCa Cells
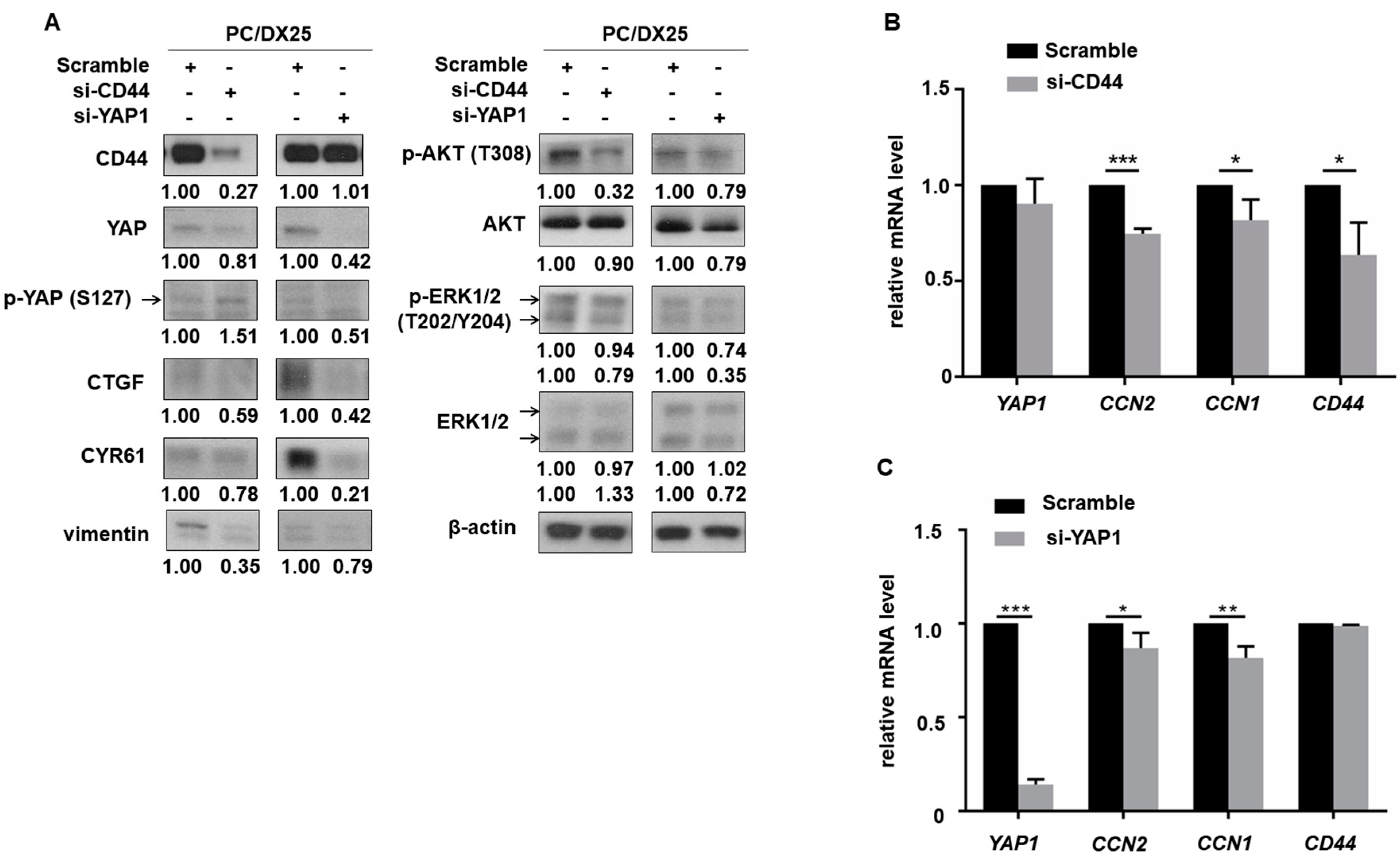
2.5. CD44 Regulated the Expression of YAP in Docetaxel-Resistant PCa Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Transwell Migration Assay
4.4. Transwell Invasion Assay
4.5. Wound Healing Assay
4.6. Flow Cytometry
4.7. Micro-Western Arrays (MWA)
4.8. Real-Time Quantitative PCR
4.9. Knockdown of CD44 or YAP with Small Interfering RNA
4.10. Western Blot Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hellerstedt, B.A.; Pienta, K.J. The current state of hormonal therapy for prostate cancer. CA Cancer J. Clin. 2002, 52, 154–179. [Google Scholar] [CrossRef]
- Chuu, C.P.; Kokontis, J.M.; Hiipakka, R.A.; Fukuchi, J.; Lin, H.P.; Lin, C.Y.; Huo, C.; Su, L.C. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. J. Biomed. Sci. 2011, 18, 63. [Google Scholar] [CrossRef]
- Gilligan, T.; Kantoff, P.W. Chemotherapy for prostate cancer. Urology 2002, 60, 94–100. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N., Jr.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef]
- Ploussard, G.; Terry, S.; Maille, P.; Allory, Y.; Sirab, N.; Kheuang, L.; Soyeux, P.; Nicolaiew, N.; Coppolani, E.; Paule, B.; et al. Class iii beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Res. 2010, 70, 9253–9264. [Google Scholar] [CrossRef] [PubMed]
- Hour, T.C.; Chung, S.D.; Kang, W.Y.; Lin, Y.C.; Chuang, S.J.; Huang, A.M.; Wu, W.J.; Huang, S.P.; Huang, C.Y.; Pu, Y.S. Egfr mediates docetaxel resistance in human castration-resistant prostate cancer through the akt-dependent expression of abcb1 (mdr1). Arch. Toxicol. 2015, 89, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Keizman, D.; Zhang, Z.; Gurel, B.; Lotan, T.L.; Hicks, J.L.; Fedor, H.L.; Carducci, M.A.; De Marzo, A.M.; Eisenberger, M.A. An immunohistochemical signature comprising pten, myc, and ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer 2012, 118, 6063–6071. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, M.F.; Wagner, J.P.; Chuu, C.P.; Lauffenburger, D.A.; Jones, R.B. Systems analysis of egf receptor signaling dynamics with microwestern arrays. Nat. Methods 2010, 7, 148–155. [Google Scholar] [CrossRef]
- Maitland, N.J.; Collins, A.T. Prostate cancer stem cells: A new target for therapy. J. Clin. Oncol. 2008, 26, 2862–2870. [Google Scholar] [CrossRef]
- Hao, J.; Madigan, M.C.; Khatri, A.; Power, C.A.; Hung, T.T.; Beretov, J.; Chang, L.; Xiao, W.; Cozzi, P.J.; Graham, P.H.; et al. In vitro and in vivo prostate cancer metastasis and chemoresistance can be modulated by expression of either cd44 or cd147. PLoS ONE 2012, 7, e40716. [Google Scholar] [CrossRef] [PubMed]
- Naor, D.; Wallach-Dayan, S.B.; Zahalka, M.A.; Sionov, R.V. Involvement of cd44, a molecule with a thousand faces, in cancer dissemination. Semin. Cancer Biol. 2008, 18, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Ponta, H.; Sherman, L.; Herrlich, P.A. Cd44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.; Peyrollier, K.; Xia, W.; Gilad, E. Hyaluronan-cd44 interaction activates stem cell marker nanog, stat-3-mediated mdr1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 2008, 283, 17635–17651. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Liu, G.; Chakrabarty, S. Isolation and characterization of calcium sensing receptor null cells: A highly malignant and drug resistant phenotype of colon cancer. Int. J. Cancer 2013, 132, 1996–2005. [Google Scholar] [CrossRef]
- Lu, S.; Labhasetwar, V. Drug resistant breast cancer cell line displays cancer stem cell phenotype and responds sensitively to epigenetic drug saha. Drug Deliv. Transl. Res. 2013, 3, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Reinke, L.M.; Damerow, M.S.; Perez, D.; Chodosh, L.A.; Yang, J.; Cheng, C. Cd44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J. Clin. Invest. 2011, 121, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Patrawala, L.; Calhoun-Davis, T.; Schneider-Broussard, R.; Tang, D.G. Hierarchical organization of prostate cancer cells in xenograft tumors: The cd44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007, 67, 6796–6805. [Google Scholar] [CrossRef] [PubMed]
- Hurt, E.M.; Kawasaki, B.T.; Klarmann, G.J.; Thomas, S.B.; Farrar, W.L. Cd44+ cd24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br. J. Cancer 2008, 98, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Dubrovska, A.; Kim, S.; Salamone, R.J.; Walker, J.R.; Maira, S.M.; Garcia-Echeverria, C.; Schultz, P.G.; Reddy, V.A. The role of pten/akt/pi3k signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. USA 2009, 106, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.; Tapon, N. The salvador-warts-hippo pathway—An emerging tumour-suppressor network. Nat. Rev. Cancer 2007, 7, 182–191. [Google Scholar] [CrossRef]
- Pan, D. Hippo signaling in organ size control. Genes Dev. 2007, 21, 886–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; Hong, W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 2008, 13, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of yap oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef]
- Hao, Y.; Chun, A.; Cheung, K.; Rashidi, B.; Yang, X. Tumor suppressor lats1 is a negative regulator of oncogene yap. J. Biol. Chem. 2008, 283, 5496–5509. [Google Scholar] [CrossRef]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.Y.; Chinnaiyan, A.M.; et al. Tead mediates yap-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef]
- Zhang, H.; Pasolli, H.A.; Fuchs, E. Yes-associated protein (yap) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl. Acad. Sci. USA 2011, 108, 2270–2275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, S.; Chen, X.; Stauffer, S.; Yu, F.; Lele, S.M.; Fu, K.; Datta, K.; Palermo, N.; Chen, Y.; et al. The hippo pathway effector yap regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol. Cell Biol. 2015, 35, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cui, J.; Ji, Z.; Cheng, C.; Zhang, K.; Zhang, C.; Chu, M.; Zhao, Q.; Yu, Z.; Zhang, Y.; et al. Mir-302/367/lats2/yap pathway is essential for prostate tumor-propagating cells and promotes the development of castration resistance. Oncogene 2017, 36, 6336–6347. [Google Scholar] [CrossRef]
- Yu, S.; Cai, X.; Wu, C.; Wu, L.; Wang, Y.; Liu, Y.; Yu, Z.; Qin, S.; Ma, F.; Thiery, J.P.; et al. Adhesion glycoprotein cd44 functions as an upstream regulator of a network connecting erk, akt and hippo-yap pathways in cancer progression. Oncotarget 2015, 6, 2951–2965. [Google Scholar] [CrossRef]
- Xu, Y.; Stamenkovic, I.; Yu, Q. Cd44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010, 70, 2455–2464. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, H.; Ge, X.; Chen, Q.; Yuan, D.; Chen, Q.; Leng, W.; Chen, L.; Tang, Q.; Bi, F. Cd44 acts through rhoa to regulate yap signaling. Cell Signal 2014, 26, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xia, H.; Xu, H.; Ma, J.; Zhou, S.; Hou, W.; Tang, Q.; Gong, Q.; Nie, Y.; Bi, F. Standard cd44 modulates yap1 through a positive feedback loop in hepatocellular carcinoma. Biomed. Pharmacother. 2018, 103, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Osada, H.; Murakami-Tonami, Y.; Horio, Y.; Hida, T.; Sekido, Y. Statin suppresses hippo pathway-inactivated malignant mesothelioma cells and blocks the yap/cd44 growth stimulatory axis. Cancer Lett. 2017, 385, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.C.; Lin, C.Y.; Su, L.C.; Fu, H.H.; Yang, S.D.; Chuu, C.P. Cape suppresses migration and invasion of prostate cancer cells via activation of non-canonical wnt signaling. Oncotarget 2016, 7, 38010–38024. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-J.; Lin, C.-Y.; Liao, W.-Y.; Hour, T.-C.; Wang, H.-D.; Chuu, C.-P. CD44 Promotes Migration and Invasion of Docetaxel-Resistant Prostate Cancer Cells Likely via Induction of Hippo-Yap Signaling. Cells 2019, 8, 295. https://doi.org/10.3390/cells8040295
Lai C-J, Lin C-Y, Liao W-Y, Hour T-C, Wang H-D, Chuu C-P. CD44 Promotes Migration and Invasion of Docetaxel-Resistant Prostate Cancer Cells Likely via Induction of Hippo-Yap Signaling. Cells. 2019; 8(4):295. https://doi.org/10.3390/cells8040295
Chicago/Turabian StyleLai, Chih-Jen, Ching-Yu Lin, Wen-Ying Liao, Tzyh-Chyuan Hour, Horng-Dar Wang, and Chih-Pin Chuu. 2019. "CD44 Promotes Migration and Invasion of Docetaxel-Resistant Prostate Cancer Cells Likely via Induction of Hippo-Yap Signaling" Cells 8, no. 4: 295. https://doi.org/10.3390/cells8040295
APA StyleLai, C.-J., Lin, C.-Y., Liao, W.-Y., Hour, T.-C., Wang, H.-D., & Chuu, C.-P. (2019). CD44 Promotes Migration and Invasion of Docetaxel-Resistant Prostate Cancer Cells Likely via Induction of Hippo-Yap Signaling. Cells, 8(4), 295. https://doi.org/10.3390/cells8040295





