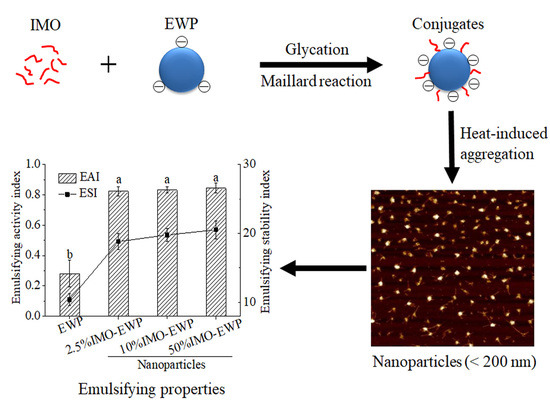
Application of Glycation in Regulating the Heat-Induced Nanoparticles of Egg White Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of IMO-EWP Conjugates
2.3. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Grafting Degree (DG) of the IMO-EWP Conjugates
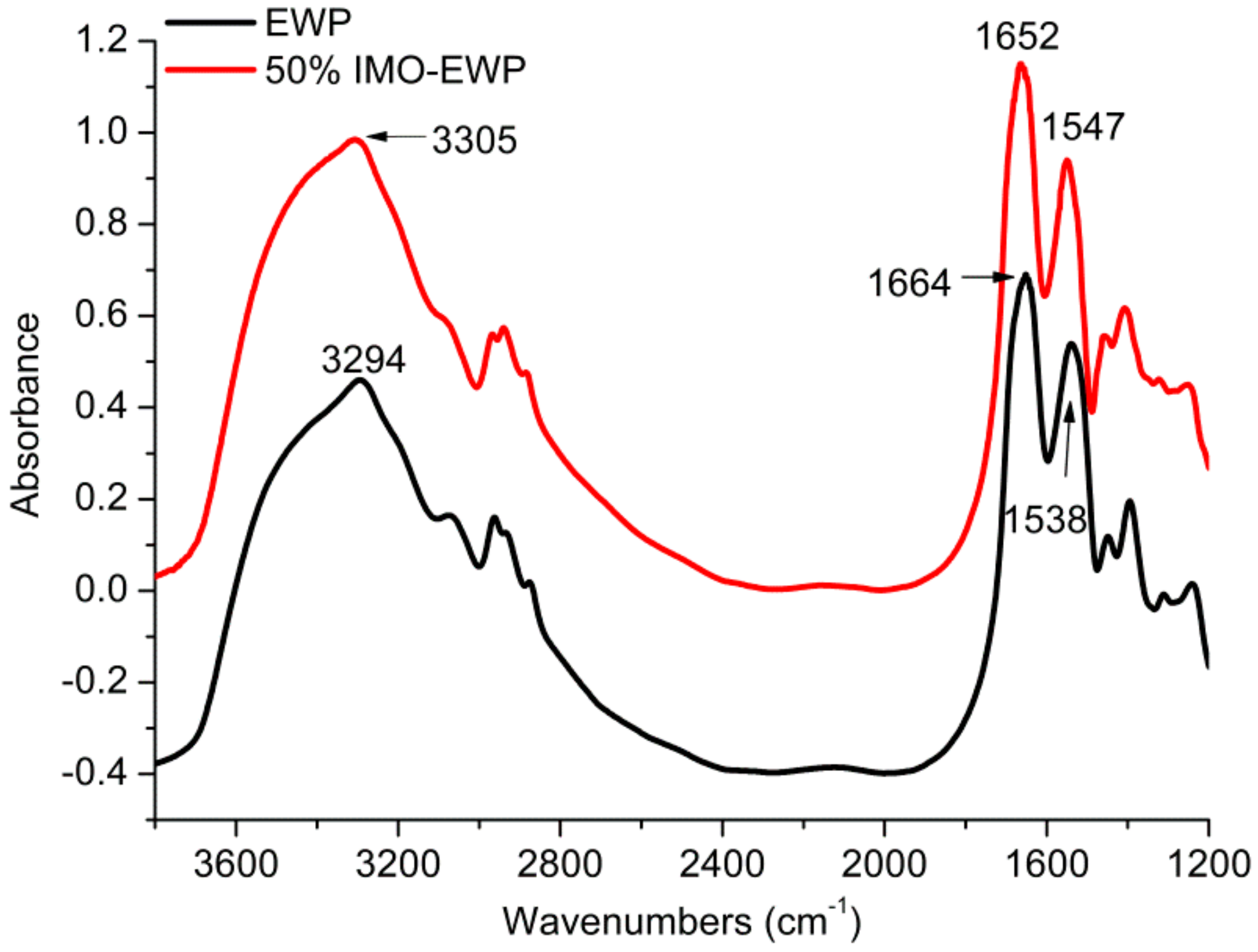
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Differential Scanning Calorimetry (DSC)
2.7. Measurement of Surface Hydrophobicity (Ho)
2.8. Preparation of Heat-Induced Aggregate Particles
2.9. Turbidity Measurement
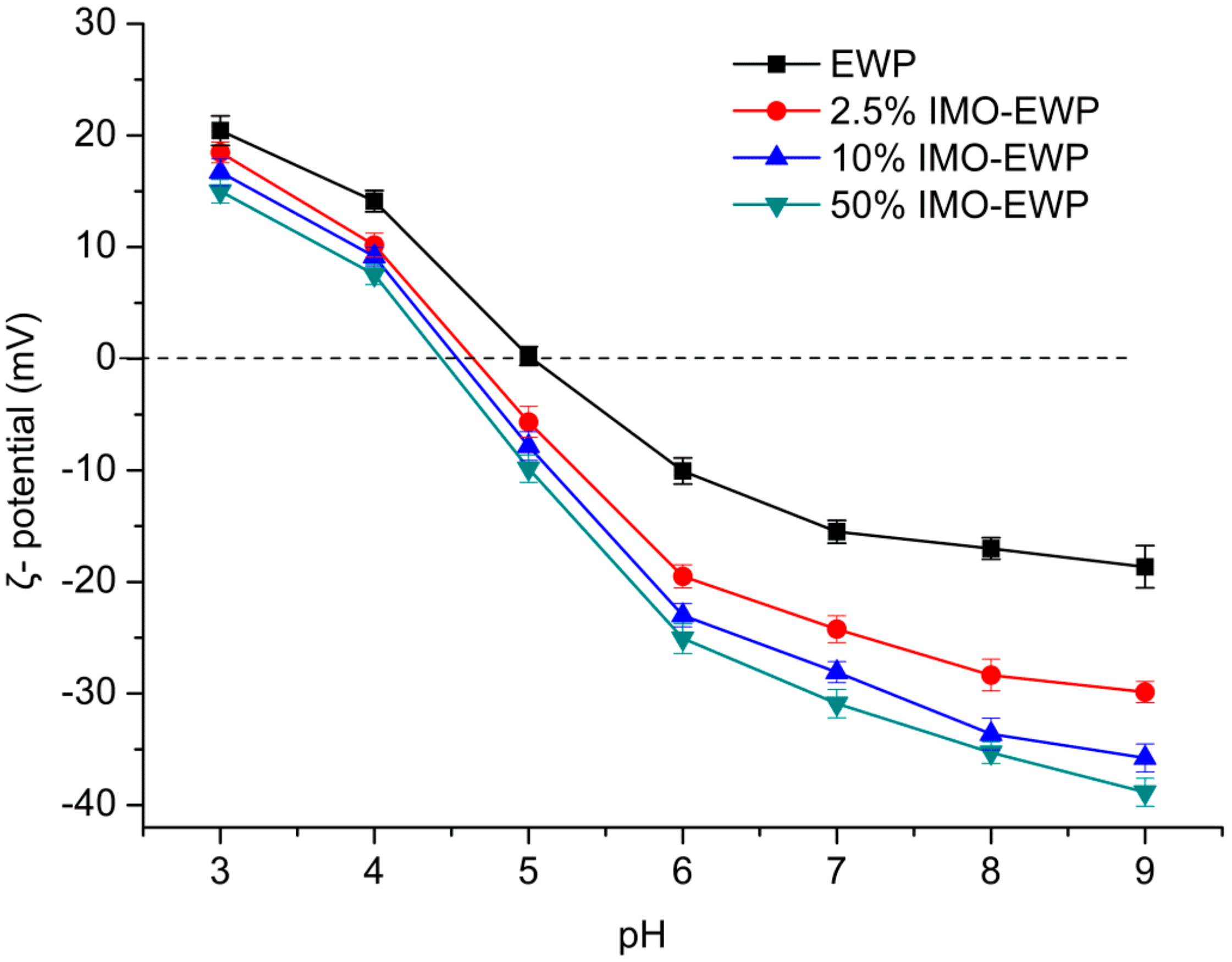
2.10. Determination of Z-Average Hydrodynamic Diameters and ζ-Potentials
2.11. Atomic Force Microscope (AFM)
2.12. Emulsifying Activity Indexe (EAI) and Emulsifying Stability Index (ESI)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Structure Characteristics of IMO-EWP Conjugates
3.2. Thermal Denaturation Properties of IMO-EWP Conjugates
3.3. Surface Hydrophobicity (Ho) and ζ-Potential of IMO-EWP Conjugates
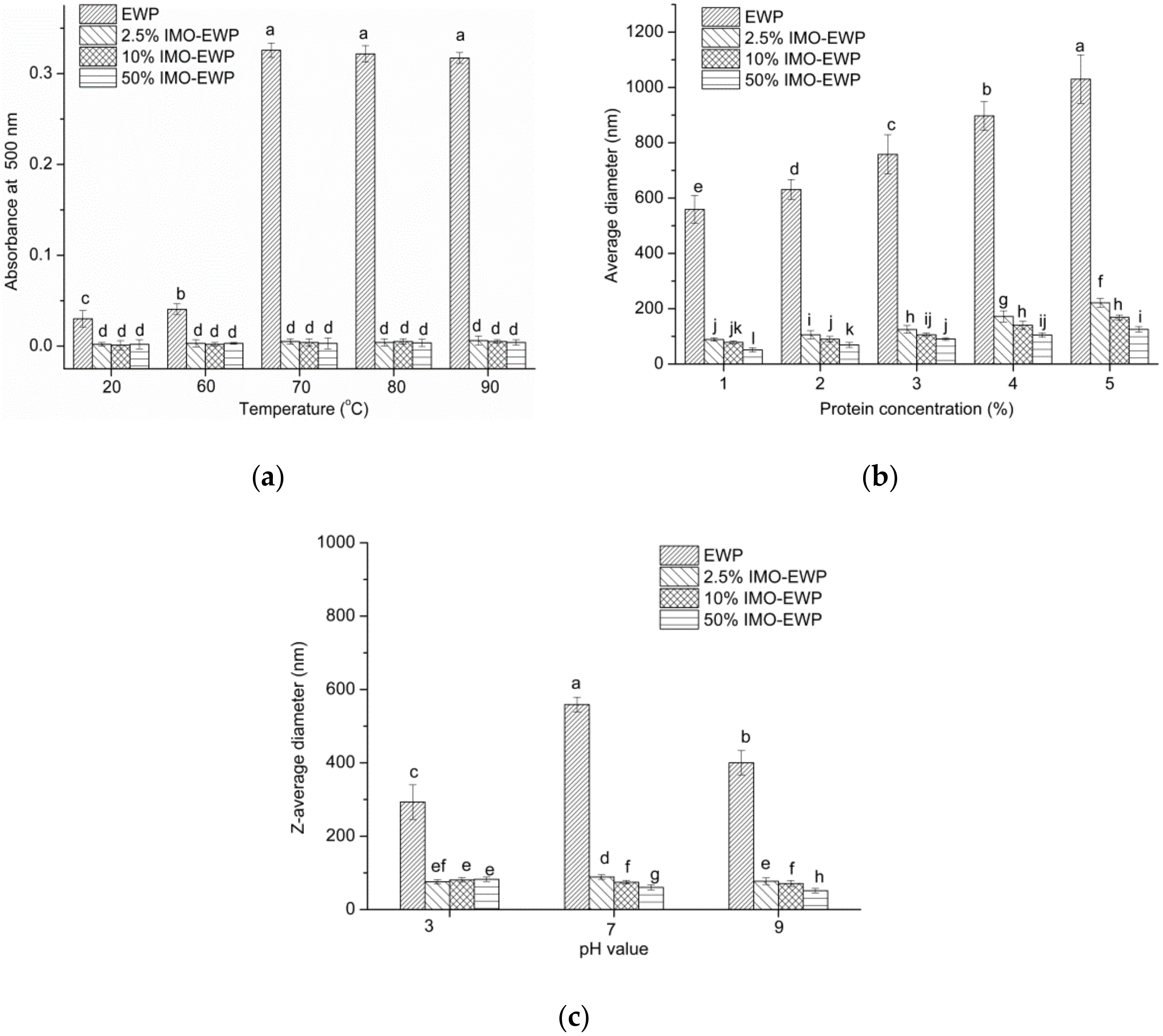
3.4. Effects of Temperature, Protein Concentration, pH and Ionic Strength on the Formation of Nanoparticles
3.5. Emulsifying Activity Index (EAI) and Emulsifying Stability Index (ESI) of Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Effects of pH, temperature and pulsed electric fields on the turbidity and protein aggregation of ovomucin-depleted egg white. Food Res. Int. 2017, 91, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T. Formation and functionality of self-assembled whey protein microgels. Colloids Surf. B Biointerfaces 2016, 137, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Andrade, J.; Engeseth, N.E.; Feng, H. Functionalizing soy protein nano-aggregates with pH-shifting and mano-thermo-sonication. J. Colloid Interface Sci. 2017, 505, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Vries, A.D.; Wesseling, A.; Linden, E.V.D.; Scholten, E. Protein oleogels from heat-set whey protein aggregates. J. Colloid Interface Sci. 2017, 486, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Croguennec, T.; Renault, A.; Beaufils, S.; Dubois, J.-J.; Pezennec, S. Interfacial properties of heat-treated ovalbumin. J. Colloid Interface Sci. 2007, 315, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Raikos, V.; Euston, S.R. Modification of functional properties of egg-white proteins. Nahrung/Food 2003, 47, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Niu, F.; Gu, L.; Li, X.; Yang, H.; Zhou, B.; Wang, J.; Su, Y.; Yang, Y. Formation of fibrous or granular egg white protein microparticles and properties of the integrated emulsions. Food Hydrocoll. 2016, 61, 477–486. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Ramel, J.V.; Santiago, L.G. Protein nanovehicles produced from egg white. Part 1: Effect of pH and heat treatment time on particle size and binding capacity. Food Hydrocoll. 2017, 73, 67–73. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, Q. Thermal aggregation properties of whey protein glycated with various saccharides. Food Hydrocoll. 2013, 32, 87–96. [Google Scholar] [CrossRef]
- Xu, C.H.; Yang, X.Q.; Yu, S.J.; Qi, J.R.; Guo, R.; Sun, W.W.; Yao, Y.J.; Zhao, M. The effect of glycosylation with dextran chains of differing lengths on the thermal aggregation of β-conglycinin and glycinin. Food Res. Int. 2010, 43, 2270–2276. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Yang, B. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2012, 131, 901–906. [Google Scholar] [CrossRef]
- Cai, L.; Lin, C.; Yang, N.; Huang, Z.; Miao, S.; Chen, X.; Pan, J.; Rao, P.; Liu, S. Preparation and characterization of nanoparticles made from co-incubation of SOD and glucose. Nanomaterials 2017, 7, 458. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Cui, B.; Wang, Y.; Jin, W.; Geng, X.; Yan, X.; Li, B. Functional properties of ovalbumin glycosylated with carboxymethyl cellulose of different substitution degree. Food Hydrocoll. 2014, 40, 1–8. [Google Scholar] [CrossRef]
- Rao, Q.; Rocca-Smith, J.R.; Schoenfuss, T.C.; Labuza, T.P. Accelerated shelf-life testing of quality loss for a commercial hydrolysed hen egg white powder. Food Chem. 2012, 135, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Nakai, S. Relationships of hydrophobicity and net charge to the solubility of milk and soy proteins. J. Food Sci. 1985, 50, 486–491. [Google Scholar] [CrossRef]
- Chang, C.; Niu, F.; Su, Y.S.; Qiu, Y.; Gu, L.; Yang, Y. Characteristics and emulsifying properties of acid and acid-heat induced egg white protein. Food Hydrocoll. 2016, 54, 342–350. [Google Scholar] [CrossRef]
- Jing, H.; Yap, M.; Wong, P.Y.Y.; Kitts, D.D. Comparison of physicochemical and antioxidant properties of egg-white proteins and fructose and inulin Maillard reaction products. Food Bioprocess Technol. 2011, 4, 1489–1496. [Google Scholar] [CrossRef]
- Kato, A.; Minaki, K.; Kobayashi, K. Improvement of emulsifying properties of egg white proteins by the attachment of polysaccharide through Maillard reaction in a dry state. J. Agric. Food Chem. 1993, 41, 540–543. [Google Scholar] [CrossRef]
- Sun, W.W.; Yu, S.J.; Zeng, X.A.; Yang, X.Q.; Jia, X. Properties of whey protein isolate-dextran conjugate prepared using pulsed electric field. Food Res. Int. 2011, 44, 1052–1058. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; Weerakkody, R.; Augustin, M.A. Chitosan-glucose conjugates: Influence of extent of Maillard reaction on antioxidant properties. J. Agric. Food Chem. 2010, 58, 12449–12455. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K.; Kawai, S. Preparation and characterization of Maillard reacted chitosan films with hemicellulose model compounds. J. Appl. Polym. Sci. 2008, 108, 2481–2487. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Tabatabaei, R.H.; Pashania, B.; Rajabi, H.Z.; Karim, A.A. Effects of ascorbic acid and sugars on solubility, thermal, and mechanical properties of egg white protein gels. Int. J. Biol. Macromol. 2013, 62, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.D. Study of thermodynamics and kinetics of protein stability by thermal analysis. In Thermal Analysis of Foods; Harwalker, V.R., Ma, C.Y., Eds.; Elsevier Applied Science: New York, USA, 1990; pp. 16–50. [Google Scholar]
- Jiang, J.; Xiong, Y.L.; Chen, J. pH shifting alters solubility characteristics and thermal stability of soy protein isolate and its globulin fractions in different pH, salt concentration, and temperature conditions. J. Agric. Food Chem. 2010, 58, 8035–8042. [Google Scholar] [CrossRef] [PubMed]
- Broersen, K.; Elshof, M.; De Groot, J.; Voragen, A.G.; Hamer, R.J.; De Jongh, H.H. Aggregation of β-lactoglobulin regulated by glucosylation. J. Agric. Food Chem. 2007, 55, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Achouri, A.; Boye, J.I.; Yaylayan, V.A.; Yeboah, F.K. Functional properties of glycated soy 11S glycinin. J. Food Sci. 2005, 70, C269–C274. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Ramel, J.V.; Santiago, L.G. Protein nanovehicles produced from egg white. Part 2: Effect of protein concentration and spray drying on particle size and linoleic acid binding capacity. Food Hydrocoll. 2017, 77, 863–869. [Google Scholar] [CrossRef]
- Datta, D.; Bhattacharjee, S.; Nath, A.; Das, R.; Bhattacharjee, C.; Datta, S. Separation of ovalbumin from chicken egg white using two-stage ultrafiltration technique. Sep. Purif. Technol. 2009, 66, 353–361. [Google Scholar] [CrossRef]
- Hegg, P.O.; Martens, H.; Löfqvist, B. Effects of pH and neutral salts on the formation and quality of thermal aggregates of ovalbumin. A study on thermal aggregation and denaturation. J. Sci. Food Agric. 1979, 30, 981–993. [Google Scholar] [CrossRef]
- Hiller, B.; Lorenzen, P.C. Functional properties of milk proteins as affected by Maillard reaction induced oligomerisation. Food Res. Int. 2010, 43, 1155–1166. [Google Scholar] [CrossRef]
- Medrano, A.; Abirached, C.; Moyna, P.; Panizzolo, L.; Añón, M.C. The effect of glycation on oilewater emulsion properties of β-lactoglobulin. LWT-Food Sci. Technol. 2012, 45, 253–260. [Google Scholar] [CrossRef]

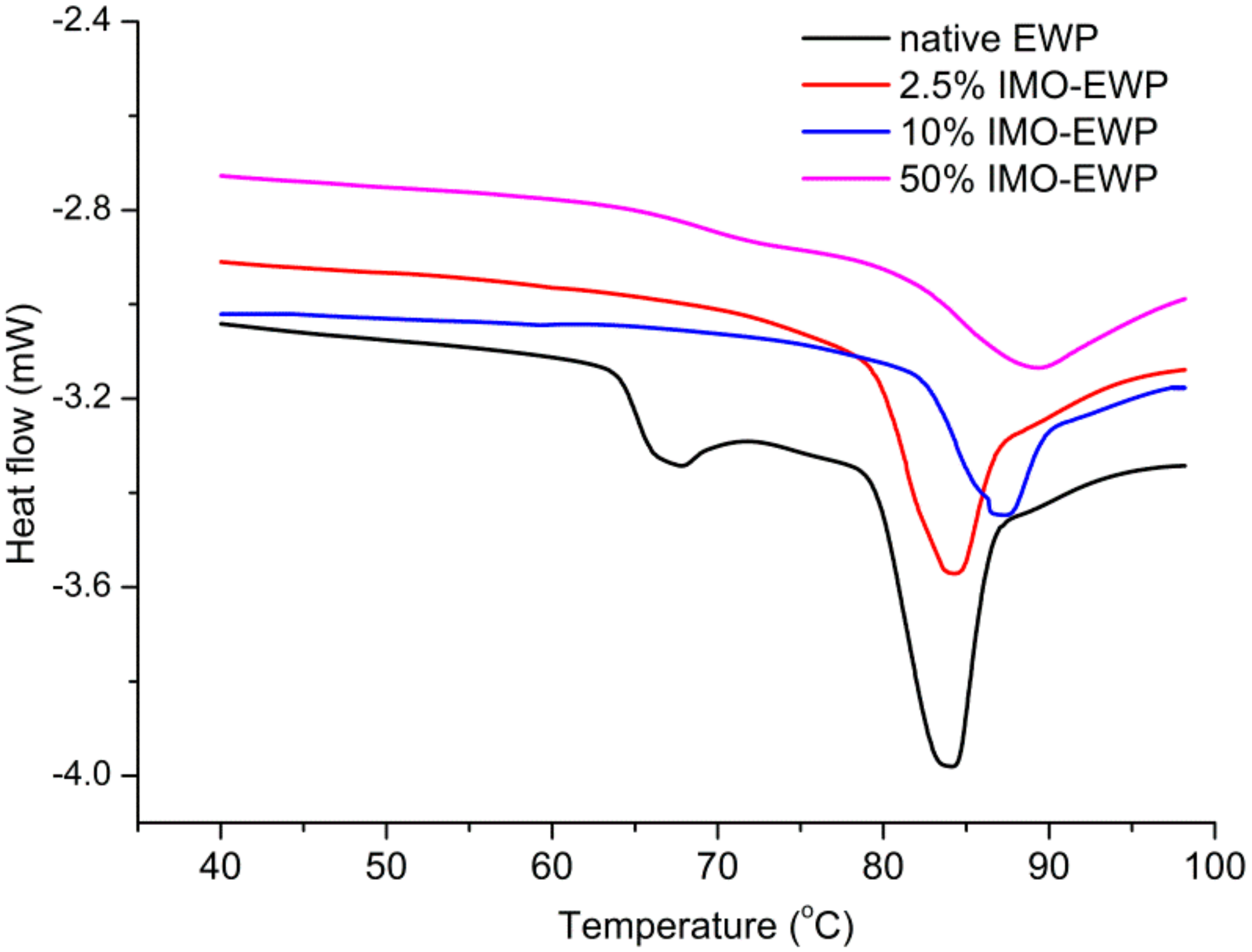



| Samples | Peak 1 | Peak 2 | ||
|---|---|---|---|---|
| Td (°C) | ΔH (J/g) | Td (°C) | ΔH (J/g) | |
| EWP | 66.56 ± 0.04 | 0.67 ± 0.03 | 84.11 ± 0.05 a | 3.94 ± 0.02 a |
| 2.5% IMO-EWP | - | - | 84.06 ± 0.12 a | 3.07 ± 0.04 b |
| 10% IMO-EWP | - | - | 87.19 ± 0.02 b | 2.84 ± 0.05 c |
| 50% IMO-EWP | - | - | 89.17 ± 0.06 c | 2.74 ± 0.02 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Ren, X.; Su, Y.; Yang, Y. Application of Glycation in Regulating the Heat-Induced Nanoparticles of Egg White Protein. Nanomaterials 2018, 8, 943. https://doi.org/10.3390/nano8110943
Wang C, Ren X, Su Y, Yang Y. Application of Glycation in Regulating the Heat-Induced Nanoparticles of Egg White Protein. Nanomaterials. 2018; 8(11):943. https://doi.org/10.3390/nano8110943
Chicago/Turabian StyleWang, Chenying, Xidong Ren, Yujie Su, and Yanjun Yang. 2018. "Application of Glycation in Regulating the Heat-Induced Nanoparticles of Egg White Protein" Nanomaterials 8, no. 11: 943. https://doi.org/10.3390/nano8110943