Metaflammation’s Role in Systemic Dysfunction in Obesity: A Comprehensive Review
Abstract
1. Introduction: Redefining Obesity as a Systemic Dysfunction
1.1. Various Types and Key Contributing Axes
1.2. The Need for Integrated Approaches
2. Adipose Tissue: A Dynamic Immunometabolic Organ
2.1. From Energy Reservoir to Active Organ
2.2. Adipocyte Hypertrophy and Dysfunction
2.3. Adipokines and Systemic Impact
2.4. Immune Cell Infiltration and Remodelling
3. Metaflammation: The Core Inflammatory Driver of Obesity
3.1. Definition and Characteristics
3.2. Cellular and Molecular Triggers
3.3. Systemic Spillover and Organ-Specific Impact
4. Gut Microbiota Dysbiosis: A Key Modulator of Metabolic Dysfunction in Obesity
4.1. Dysbiosis and Metabolic Endotoxemia
4.2. Microbiota-Bile Acid Axis
4.3. Microbiota and Gut Hormones Modulation
5. Neuroendocrine Dysregulation and Obesity
5.1. Hypothalamic Inflammation
5.2. Leptin and Insulin Resistance
5.3. Hormonal Imbalance
5.4. Gut–Brain Axis
6. Epigenetic Imprinting: Transgenerational Influences
6.1. Epigenetic Mechanisms in Obesity
6.2. Early Metabolic Programming
6.3. Reversibility and Microbiota Influence
7. Therapeutic Strategies: Towards Integrated and Personalised Interventions
7.1. Targeting Metaflammation
7.2. Microbiota Modulation
7.3. Precision Nutrition and Multi-Omics
7.4. Targeting Macrophage Dynamics
7.5. Extreme Obesity and Bariatric Surgery
7.6. Shifting Paradigms
8. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Valk, E.S.; Savas, M.; van Rossum, E.F.C. Stress and Obesity: Are There More Susceptible Individuals? Curr. Obes. Rep. 2018, 7, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Pecht, T.; Shaco-Levy, R.; Harman-Boehm, I.; Kirshtein, B.; Kuperman, Y.; Chen, A.; Blüher, M.; Shai, I.; Rudich, A. Adipose Tissue Foam Cells Are Present in Human Obesity. J. Clin. Endocrinol. Metab. 2013, 98, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B. The Complex Role of Adipokines in Obesity, Inflammation, and Autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Seong, J.; Kang, J.Y.; Sun, J.S.; Kim, K.W. Hypothalamic Inflammation and Obesity: A Mechanistic Review. Arch. Pharmacal Res. 2019, 42, 383–392. [Google Scholar] [CrossRef]
- Ye, J. Emerging Role of Adipose Tissue Hypoxia in Obesity and Insulin Resistance. Int. J. Obes. 2008, 33, 54–66. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, H.; Zhao, X.; Hou, D.; Yan, Y.; Cianflone, K.; Li, M.; Mi, J. Leptin and Leptin-to-Adiponectin Ratio Predict Adiposity Gain in Nonobese Children over a Six-Year Period. Child. Obes. 2017, 13, 213–221. [Google Scholar] [CrossRef]
- Lafortuna, C.L. Clinical Functional Behavioural and Epigenomic Biomarkers of Obesity. Front. Biosci. 2017, 22, 1655–1681. [Google Scholar] [CrossRef]
- Campión, J.; Milagro, F.I.; Martínéz, J.A. Individuality and Epigenetics in Obesity. Obes. Rev. 2009, 10, 383–392. [Google Scholar] [CrossRef]
- Lima, R.P.A.; Hayashi, D.N.; Lima, K.Q.d.F.; Gomes, N.I.G.; Ribeiro, M.R.; Prada, P.O.; Costa, M.J.d.C. The Role of Epigenetics in the Etiology of Obesity: A Review. J. Clin. Epigenetics 2017, 3, 41. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Sarma, D.K.; Kumawat, M.; Tiwari, R.; Verma, V.; Nagpal, R.; Kumar, M. Implication of Obesity and Gut Microbiome Dysbiosis in the Etiology of Colorectal Cancer. Cancers 2023, 15, 1913. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.; Yan, M.R. Evolution Not Revolution: Nutrition and Obesity. Nutrients 2017, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Lu, H.-C.; Chou, Y.; Liu, P.; Chen, H.; Huang, M.; Lin, C.-H.; Tsai, C. Gut Microbial Signatures for Glycemic Responses of GLP-1 Receptor Agonists in Type 2 Diabetic Patients: A Pilot Study. Front. Endocrinol. 2022, 12, 814770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, C.; Chen, Y.; Chen, C.; Cheng, J.; Xia, F.; Wang, N.; Lu, Y. LH/FSH Ratio Is Associated With Visceral Adipose Dysfunction in Chinese Women Older Than 55. Front. Endocrinol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much More than a Hunger Hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Tuero, C.; Becerril, S.; Ramírez, B.; Catalán, V.; Cienfuegos, J.A.; Burrell, M.A.; Valentí, V.; Moncada, R.; Gómez-Ambrosi, J.; Rodríguez, A.; et al. Changes in Ghrelin Isoforms after Sleeve Gastrectomy or Gastric Plication and Their Association with Adiposity and Metabolic Profile. Eur. J. Clin. Investig. 2025, 55, e70031. [Google Scholar] [CrossRef]
- Obradović, M.; Sudar-Milovanović, E.; Šoškić, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenović, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Napoli, N.; Pozzilli, P. Obesity and Glucose Metabolism; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Dey, S.; Murmu, N.; Bose, M.; Ghosh, S.; Giri, B. Obesity and Chronic Leptin Resistance Foster Insulin Resistance: An Analytical Overview. BLDE Univ. J. Health Sci. 2021, 6, 7–21. [Google Scholar] [CrossRef]
- Jungheim, E.S.; Travieso, J.; Carson, K.R.; Moley, K.H. Obesity and Reproductive Function. Obstet. Gynecol. Clin. N. Am. 2012, 39, 479–493. [Google Scholar] [CrossRef]
- Iavarone, I.; Mele, D.; Caprio, F.; Andreoli, G.; Vastarella, M.G.; Franciscis, P.D.; Ronsini, C. Obesity May Impair Response to Ovarian Stimulation.A Retrospective Observational Study on Oocyte Quality. Front. Cell Dev. Biol. 2024, 12, 1461132. [Google Scholar] [CrossRef]
- Lainez, N.M.; Coss, D. Obesity, Neuroinflammation, and Reproductive Function. Endocrinology 2019, 160, 2719–2736. [Google Scholar] [CrossRef] [PubMed]
- Eng, P.C.; Phylactou, M.; Qayum, A.; Woods, C.; Lee, H.; Aziz, S.; Moore, B.; Miras, A.D.; Comninos, A.; Tan, T.; et al. Obesity-Related Hypogonadism in Women. Endocr. Rev. 2023, 45, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Tsamis, K.I.; Kanaka-Gantenbein, C.; Johnson, E.O.; Chrousos, G.P. Neurobehavioral and Neuroendocrine Regulation of Energy Homeostasis. Postgrad. Med. 2019, 131, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D. Appetite Regulation: Hormones, Peptides, and Neurotransmitters and Their Role in Obesity. Am. J. Lifestyle Med. 2017, 13, 586–601. [Google Scholar] [CrossRef]
- Suzuki, K.; Jayasena, C.; Bloom, S.R. Obesity and Appetite Control. Exp. Diabetes Res. 2012, 2012, 824305. [Google Scholar] [CrossRef]
- Howe, S.; Hand, T.; Manore, M.M. Exercise-Trained Men and Women: Role of Exercise and Diet on Appetite and Energy Intake. Nutrients 2014, 6, 4935–4960. [Google Scholar] [CrossRef]
- Dionysopoulou, S.; Charmandari, E.; Bargiota, A.; Vlahos, N.; Mastorakos, G.; Valsamakis, G. The Role of Hypothalamic Inflammation in Diet-Induced Obesity and Its Association with Cognitive and Mood Disorders. Nutrients 2021, 13, 498. [Google Scholar] [CrossRef]
- Ekberg, N.R.; Falhammar, H.; Näslund, E.; Brismar, K. Predictors of Normalized HbA1c after Gastric Bypass Surgery in Subjects with Abnormal Glucose Levels, a 2-Year Follow-up Study. Sci. Rep. 2020, 10, 15127. [Google Scholar] [CrossRef]
- Wu, D.; Wang, H.; Xie, L.; Hu, F. Cross-Talk Between Gut Microbiota and Adipose Tissues in Obesity and Related Metabolic Diseases. Front. Endocrinol. 2022, 13, 908868. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to Obesity with Adipose Tissue Inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Castoldi, Â.; de Souza, C.N.; Câmara, N.O.S.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.; AlHarthi, H.; Alghamdi, A.; Sabico, S.; Al-Daghri, N.M. Role of NLRP3 Inflammasome Activation in Obesity-Mediated Metabolic Disorders. Int. J. Environ. Res. Public Health 2021, 18, 511. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.-L.; Cheung, S.W.M.; Cheng, K.K.Y. NLRP3 Inflammasome Activation in Adipose Tissues and Its Implications on Metabolic Diseases. Int. J. Mol. Sci. 2020, 21, 4184. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Xu, Z.; Han, B.; Su, D.; Liu, C. NLRP3 Inflammasome and Its Inhibitors: A Review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xiao, H.; Wu, Z.; Yang, Z.; Ding, B.; Jin, Z.; Yang, Y. NLRP3 Sparks the Greek Fire in the War against Lipid-related Diseases. Obes. Rev. 2020, 21, e13045. [Google Scholar] [CrossRef]
- Neuwirt, E.; Gorka, O.; Saller, B.S.; Groß, C.J.; Madl, T.; Groß, O. NLRP3 as a Sensor of Metabolism Gone Awry. Curr. Opin. Biotechnol. 2021, 68, 300–309. [Google Scholar] [CrossRef]
- Meiliana, A.; Wijaya, A. Metaflammation, NLRP3 Inflammasome Obesity and Metabolic Disease. Indones. Biomed. J. 2011, 3, 168. [Google Scholar] [CrossRef]
- Jorquera, G.; Russell, J.; Monsalves-Álvarez, M.; Cruz, G.; Valladares, D.; Basualto-Alarcón, C.; Barrientos, G.; Estrada, M.; Llanos, P. NLRP3 Inflammasome: Potential Role in Obesity Related Low-Grade Inflammation and Insulin Resistance in Skeletal Muscle. Int. J. Mol. Sci. 2021, 22, 3254. [Google Scholar] [CrossRef]
- Frühbeck, G.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Valentí, V.; Moncada, R.; Becerril, S.; Unamuno, X.; Silva, C.; Salvador, J.; et al. Novel Protective Role of Kallistatin in Obesity by Limiting Adipose Tissue Low Grade Inflammation and Oxidative Stress. Metabolism 2018, 87, 123–135. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Béguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.; Paquot, N. Inflammation as a Link between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Faloia, E.; Grazia, M.; Marco, D.R.; Paola, L.M.; Furlani, G.; Marco, B. Inflammation as a Link between Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012, 2012, 476380. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Debnath, M.; Agrawal, S.; Agrawal, A.; Dubey, G.P. Metaflammatory Responses during Obesity: Pathomechanism and Treatment. Obes. Res. Clin. Pract. 2015, 10, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Koźniewski, K. Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes. Molecules 2020, 25, 2224. [Google Scholar] [CrossRef]
- Sharma, P. Inflammation and the Metabolic Syndrome. Indian J. Clin. Biochem. 2011, 26, 317–318. [Google Scholar] [CrossRef]
- Hu, R.; Xie, Y.; Lü, B.; Li, Q.; Chen, F.; Li, L.; Hu, J.; Huang, Y.; Li, Q.; Ye, W.; et al. Metabolic Inflammatory Syndrome: A Novel Concept of Holistic Integrative Medicine for Management of Metabolic Diseases. AME Med. J. 2018, 3, 51. [Google Scholar] [CrossRef]
- Patel, H.S.; Patel, V. Inflammation and Metabolic Syndrome: An Overview. Curr. Res. Nutr. Food Sci. J. 2015, 3, 263–268. [Google Scholar] [CrossRef]
- Reddy, P.B.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.J.; Jialal, I. Metabolic Syndrome Is an Inflammatory Disorder: A Conspiracy between Adipose Tissue and Phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Deng, L.; Wang, R.; Li, H.; Zhang, C.; Zhao, L.; Zhang, M. miRNA-Gene Regulatory Network in Gnotobiotic Mice Stimulated by Dysbiotic Gut Microbiota Transplanted from a Genetically Obese Child. Front. Microbiol. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Fang, H.; E-Lacerda, R.R.; Schertzer, J.D. Obesity Promotes a Leaky Gut, Inflammation and Pre-Diabetes by Lowering Gut Microbiota That Metabolise Ethanolamine. Gut 2023, 72, 1809–1811. [Google Scholar] [CrossRef]
- Ragheb, R.; Medhat, A.M. Mechanisms of Fatty Acid-Induced Insulin Resistance in Muscle and Liver. J. Diabetes Metab. 2011, 2, 1000127. [Google Scholar] [CrossRef]
- Yang, J.; Gibson, B.; Snider, J.; Jenkins, C.M.; Han, X.; Gross, R.W. Submicromolar Concentrations of Palmitoyl-CoA Specifically Thioesterify Cysteine 244 in Glyceraldehyde-3-Phosphate Dehydrogenase Inhibiting Enzyme Activity: A Novel Mechanism Potentially Underlying Fatty Acid Induced Insulin Resistance. Biochemistry 2005, 44, 11903–11912. [Google Scholar] [CrossRef]
- Kraegen, E.W.; Cooney, G.J. Free Fatty Acids and Skeletal Muscle Insulin Resistance. Curr. Opin. Lipidol. 2008, 19, 235–241. [Google Scholar] [CrossRef]
- Holland, W.L.; Knotts, T.A.; Chavez, J.A.; Wang, L.; Hoehn, K.L.; Summers, S.A. Lipid Mediators of Insulin Resistance. Nutr. Rev. 2008, 65, S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, D.; Demir, S.Ç.; Sezer, H. Insulin Resistance, Obesity, and Lipotoxicity. Adv. Exp. Med. Biol. 2024, 1460, 391–430. [Google Scholar] [CrossRef] [PubMed]
- Lair, B.; Laurens, C.; Bosch, B.V.D.; Moro, C. Novel Insights and Mechanisms of Lipotoxicity-Driven Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6358. [Google Scholar] [CrossRef] [PubMed]
- Lipke, K.; Kubis-Kubiak, A.; Piwowar, A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States-Current View of Knowledge. Cells 2022, 11, 844. [Google Scholar] [CrossRef]
- Shen, X.; Yang, L.; Yan, S.; Zheng, H.; Liang, L.; Cai, X.; Liao, M. Fetuin A Promotes Lipotoxicity in β Cells through the TLR4 Signaling Pathway and the Role of Pioglitazone in Anti-Lipotoxicity. Mol. Cell. Endocrinol. 2015, 412, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Han, T.-T.; Liu, Y.; Zheng, S.; Zhang, Y.; Liu, W.; Hu, Y. Insulin Resistance Caused by Lipotoxicity Is Related to Oxidative Stress and Endoplasmic Reticulum Stress in LPL Gene Knockout Heterozygous Mice. Atherosclerosis 2015, 239, 276–282. [Google Scholar] [CrossRef]
- Burak, M.F.; Stanley, T.L.; Lawson, E.A.; Campbell, S.L.; Lynch, L.; Hasty, A.H.; Domingos, A.I.; Dixit, V.D.; Hotamıs ̧lıgil, G.S.; Sheedy, F.J.; et al. Adiposity, Immunity, and Inflammation: Interrelationships in Health and Disease: A Report from 24th Annual Harvard Nutrition Obesity Symposium 2023. Am. J. Clin. Nutr. 2024, 120, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Hotamışlıgil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.; Sahlin, K.; Fernström, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Højlund, K. Mitochondrial Respiration Is Decreased in Skeletal Muscle of Patients With Type 2 Diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, S.; Deng, Q.; Tang, Y.; Yao, P.; Tang, H.; Dong, X.-Y. Linseed Oil Improves Hepatic Insulin Resistance in Obese Mice through Modulating Mitochondrial Quality Control. J. Funct. Foods 2018, 53, 166–175. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Hung, W.; Hung, W.; Lee, Y.-J.; Chen, Y.-C.; Lee, C.; Tsai, Y.; Dai, C. Circulating Short-Chain Fatty Acids and Non-Alcoholic Fatty Liver Disease Severity in Patients with Type 2 Diabetes Mellitus. Nutrients 2023, 15, 1712. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Westerbacka, J. The Fatty Liver and Insulin Resistance. Curr. Mol. Med. 2005, 5, 287–295. [Google Scholar] [CrossRef]
- Münte, E.; Hartmann, P. The Role of Short-Chain Fatty Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease and Other Metabolic Diseases. Biomolecules 2025, 15, 469. [Google Scholar] [CrossRef]
- Li, X.; He, M.; Yi, X.; Lu, X.; Zhu, M.; Xue, M.; Tang, Y.; Zhu, Y. Short-Chain Fatty Acids in Nonalcoholic Fatty Liver Disease: New Prospects for Short-Chain Fatty Acids as Therapeutic Targets. Heliyon 2024, 10, e26991. [Google Scholar] [CrossRef]
- Kang, M.; Lee, H.-G.; Kim, H.; Song, K.; Chun, Y.G.; Lee, M.H.; Kim, B.; Jeon, Y. Anti-Obesity Effects of Sargassum Thunbergii via Downregulation of Adipogenesis Gene and Upregulation of Thermogenic Genes in High-Fat Diet-Induced Obese Mice. Nutrients 2020, 12, 3325. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.; Gho, Y.S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Gao, W.; Liu, J.; Lü, X.; Yang, Q. Epigenetic Regulation of Energy Metabolism in Obesity. J. Mol. Cell Biol. 2021, 13, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R.A.; Choi, M. microRNAs in the Regulation of Adipogenesis and Obesity. Curr. Mol. Med. 2011, 11, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M.; Amri, H.E.; Bakillah, A. The Clinical Potential of Adipogenesis and Obesity-Related microRNAs. Nutr. Metab. Cardiovasc. Dis. 2017, 28, 91–111. [Google Scholar] [CrossRef]
- Abente, E.J.; Subramanian, M.; Ramachandran, V.; Najafi-Shoushtari, S.H. MicroRNAs in Obesity-Associated Disorders. Arch. Biochem. Biophys. 2015, 589, 108–119. [Google Scholar] [CrossRef]
- Gharanei, S.; Shabir, K.; Brown, J.E.; Weickert, M.O.; Barber, T.M.; Kyrou, I.; Randeva, H. Regulatory microRNAs in Brown, Brite and White Adipose Tissue. Cells 2020, 9, 2489. [Google Scholar] [CrossRef]
- Mercado, C. MicroRNAs: A New Class of Master Regulators of Adipogenesis. Hum. Genet. Embryol. 2013, 3, 108. [Google Scholar] [CrossRef]
- Son, Y.H.; Ka, S.; Kim, A.; Kim, J.B. Regulation of Adipocyte Differentiation via MicroRNAs. Endocrinol. Metab. 2014, 29, 122–135. [Google Scholar] [CrossRef]
- Quintanilha, B.J.; Reis, B.Z.; Corrêa, T.A.F.; Duarte, G.B.d.S.; Rogero, M.M. MicroRNAs and Inflammation Biomarkers in Obesity. In Elsevier eBooks; Elsevier BV: Amsterdam, The Netherlands, 2019; p. 179. [Google Scholar] [CrossRef]
- Alexander, R.; Lodish, H.F.; Sun, L. MicroRNAs in Adipogenesis and as Therapeutic Targets for Obesity. Expert Opin. Ther. Targets 2011, 15, 623–636. [Google Scholar] [CrossRef]
- Kaushik, P.; Anderson, J.T. Obesity: Epigenetic Aspects. Biomol. Concepts 2016, 7, 145–155. [Google Scholar] [CrossRef]
- Samblas, M.; Milagro, F.I.; Martínéz, J.A. DNA Methylation Markers in Obesity, Metabolic Syndrome, and Weight Loss. Epigenetics 2019, 14, 421–444. [Google Scholar] [CrossRef]
- Majnik, A.; Gunn, V.L.; Fu, Q.; Lane, R.H. Epigenetics: An Accessible Mechanism through Which to Track and Respond to an Obesogenic Environment. Expert Rev. Endocrinol. Metab. 2014, 9, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Youngson, N.A.; Morris, M.J. What Obesity Research Tells Us about Epigenetic Mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2012, 368, 20110337. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yin, R. Recent Progress in Epigenetics of Obesity. Diabetol. Metab. Syndr. 2022, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Lama, D.; Rabhi, N. Childhood Obesity from the Genes to the Epigenome. Front. Endocrinol. 2024, 15, 1393250. [Google Scholar] [CrossRef]
- Rajamoorthi, A.; LeDuc, C.A.; Thaker, V. The Metabolic Conditioning of Obesity: A Review of the Pathogenesis of Obesity and the Epigenetic Pathways That “Program” Obesity from Conception. Front. Endocrinol. 2022, 13, 1032491. [Google Scholar] [CrossRef]
- Arneth, B. Interactions among Nutrition, Metabolism and the Immune System in the Context of Starvation and Nutrition-Stimulated Obesity. Nutr. Diabetes 2025, 15, 26. [Google Scholar] [CrossRef]
- San-Cristóbal, R.; Navas-Carretero, S.; Martínez-González, M.Á.; Ordovás, J.M.; Martínéz, J.A. Contribution of Macronutrients to Obesity: Implications for Precision Nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320. [Google Scholar] [CrossRef]
- Mukherjee, S.; Skrede, S.; Haugstøyl, M.E.; López, M.; Fernø, J. Peripheral and Central Macrophages in Obesity. Front. Endocrinol. 2023, 14, 1232171. [Google Scholar] [CrossRef]
- Li, W.; Chen, W. Weight Cycling Based on Altered Immune Microenvironment as a Result of Metaflammation. Nutr. Metab. 2023, 20, 13. [Google Scholar] [CrossRef]
- Fei, Q.; Huang, J.; He, Y.; Zhang, Y.; Zhang, X.; Wang, J.; Fu, Q. Immunometabolic Interactions in Obesity: Implications for Therapeutic Strategies. Biomedicines 2025, 13, 1429. [Google Scholar] [CrossRef]
- Stenman, L.K.; Lehtinen, M.J.; Meland, N.; Christensen, J.E.; Yeung, N.; Saarinen, M.; Courtney, M.J.; Burcelin, R.; Lähdeaho, M.; Linros, J.; et al. Probiotic With or Without Fiber Controls Body Fat Mass, Associated With Serum Zonulin, in Overweight and Obese Adults—Randomized Controlled Trial. EBioMedicine 2016, 13, 190–200. [Google Scholar] [CrossRef]
- Belančić, A. Gut Microbiome Dysbiosis and Endotoxemia—Additional Pathophysiological Explanation for Increased COVID-19 Severity in Obesity. Obes. Med. 2020, 20, 100302. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.; Saad, M.J.A. Influence of Gut Microbiota on Subclinical Inflammation and Insulin Resistance. Mediat. Inflamm. 2013, 2013, 986734. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Castagliuolo, I.; Leo, V.D.; Buda, A.; Pinzani, M.; Palù, G.; Martines, D. Increased Intestinal Permeability in Obese Mice: New Evidence in the Pathogenesis of Nonalcoholic Steatohepatitis. AJP Gastrointest. Liver Physiol. 2006, 292, G518–G525. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.S.; Collado, M.C.; Ferreira, C.L.L.F.; Bressan, J.; Pelúzio, M.d.C.G. Potential Mechanisms for the Emerging Link between Obesity and Increased Intestinal Permeability. Nutr. Res. 2012, 32, 637–647. [Google Scholar] [CrossRef]
- Wang, K.; Lai, W.; Min, T.; Wei, J.; Bai, Y.; Cao, H.; Guo, J.; Su, Z. The Effect of Enteric-Derived Lipopolysaccharides on Obesity. Int. J. Mol. Sci. 2024, 25, 4305. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Li, R.; Andreu-Sánchez, S.; Kuipers, F.; Fu, J. Gut Microbiome and Bile Acids in Obesity-Related Diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101493. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, F.; Zhao, A.; Lei, S.; Zhang, Y.; Xie, G.; Chen, T.; Qu, C.; Rajani, C.; Dong, B.; et al. Bile Acid Is a Significant Host Factor Shaping the Gut Microbiome of Diet-Induced Obese Mice. BMC Biol. 2017, 15, 120. [Google Scholar] [CrossRef]
- Wei, M.; Huang, F.; Zhao, L.; Zhang, Y.; Yang, W.; Wang, S.; Li, M.; Han, X.; Ge, K.; Qu, C.; et al. A Dysregulated Bile Acid-Gut Microbiota Axis Contributes to Obesity Susceptibility. eBioMedicine 2020, 55, 102766. [Google Scholar] [CrossRef] [PubMed]
- González-Regueiro, J.A.; Moreno-Castañeda, L.; Uribe, M.; Chávez-Tapia, N.C. The Role of Bile Acids in Glucose Metabolism and Their Relation with Diabetes. Ann. Hepatol. 2017, 16, S15–S20. [Google Scholar] [CrossRef] [PubMed]
- Sah, D.K.; Arjunan, A.; Park, S.Y.; Jung, Y.D. Bile Acids and Microbes in Metabolic Disease. World J. Gastroenterol. 2022, 28, 6846–6866. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Wang, Y.; Jin, L.; Huang, W. Bile Acids, Gut Microbiota and Metabolic Surgery. Front. Endocrinol. 2022, 13, 929530. [Google Scholar] [CrossRef]
- Rohm, T.V.; Keller, L.; Bosch, A.J.T.; AlAsfoor, S.; Baumann, Z.; Thomas, A.; Wiedemann, S.J.; Steiger, L.; Dalmas, É.; Wehner, J.; et al. Targeting Colonic Macrophages Improves Glycemic Control in High-Fat Diet-Induced Obesity. Commun. Biol. 2022, 5, 370. [Google Scholar] [CrossRef]
- Das, P.; Marcišauskas, S.; Ji, B.; Nielsen, J. Metagenomic Analysis of Bile Salt Biotransformation in the Human Gut Microbiome. BMC Genom. 2019, 20, 517. [Google Scholar] [CrossRef]
- Zheng, Z.; Yuan, F.; Li, J.; Zhu, X.; Jia, R.; Wei, J.; Jia, F.; Hou, X.; Zhang, Z.; Wang, Y.; et al. Gut Microbiota-Mediated Bile Acid Metabolism Regulates Colorectal Cancer Liver Metastasis by Altering Neutrophil Recruitment. Cancer Res. 2025, OF1–OF18. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling Pathways in Obesity: Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef]
- López-Ortega, O.; Moreno-Corona, N.C.; Cruz-Holguín, V.J.; Garcia-Gonzalez, L.D.; Helguera-Repetto, A.C.; Romero-Valdovinos, M.; Arevalo-Romero, H.; Cedillo-Barrón, L.; León-Juárez, M. The Immune Response in Adipocytes and Their Susceptibility to Infection: A Possible Relationship with Infectobesity. Int. J. Mol. Sci. 2022, 23, 6154. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.M.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Tzeravini, E.; Koliaki, C.; Dalamaga, Μ.; Kokkinos, A. The Role of Mitochondrial Adaptation and Metabolic Flexibility in the Pathophysiology of Obesity and Insulin Resistance: An Updated Overview. Curr. Obes. Rep. 2021, 10, 191–213. [Google Scholar] [CrossRef]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic Effects of Obesity: A Review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Yanovski, J.A. Progress in Pharmacotherapy for Obesity. JAMA 2021, 326, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Aktar, N.; Qureshi, N.K.; Ferdous, H.S. Obesity: A Review of Pathogenesis and Management Strategies in Adult. Delta Med. Coll. J. 2017, 5, 35–48. [Google Scholar] [CrossRef]
- Dâmaso, A.R.; Masquio, D.C.L.; Campos, R.M.d.S.; Corgosinho, F.C.; Cercato, C. Effects of Multidisciplinary Therapy on Energy Balance, Inflammation, and Metabolic Diseases in Adolescents with Obesity: A Narrative Review. Ann. N. Y. Acad. Sci. 2024, 1542, 25–50. [Google Scholar] [CrossRef] [PubMed]
- Bomberg, E.M.; Ryder, J.R.; Brundage, R.C.; Straka, R.J.; Fox, C.K.; Gross, A.C.; Oberle, M.M.; Bramante, C.T.; Sibley, S.D.; Kelly, A.S. Precision Medicine in Adult and Pediatric Obesity: A Clinical Perspective. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819863022. [Google Scholar] [CrossRef]
- Hurtado, M.D.; Acosta, A. Precision Medicine and Obesity. Gastroenterol. Clin. N. Am. 2021, 50, 127–139. [Google Scholar] [CrossRef]
- Siegel, R.M.; Haemer, M.; Kharofa, R.Y.; Christison, A.L.; Hampl, S.; Tinajero-Deck, L.; Lockhart, M.K.; Reich, S.; Pont, S.J.; Stratbucker, W.; et al. Community Healthcare and Technology to Enhance Communication in Pediatric Obesity Care. Child. Obes. 2018, 14, 453–460. [Google Scholar] [CrossRef]
- Henriques, F.; Bedard, A.H.; Batista, M.L. Adipose Tissue Inflammation and Metabolic Disorders; IntechOpen Ebooks: London, UK, 2019. [Google Scholar] [CrossRef]
- Guerreiro, V.; Carvalho, D.; Freitas, P. Obesity, Adipose Tissue, and Inflammation Answered in Questions. J. Obes. 2022, 2022, 2252516. [Google Scholar] [CrossRef]
- Blüher, M.; Müller-Wieland, D. Editorial: Adipose Tissue Dysfunction. Front. Endocrinol. 2022, 13, 999188. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.K.; Foster, M.T. Adipose Tissue: An Endocrine Organ Playing a Role in Metabolic Regulation. Horm. Mol. Biol. Clin. Investig. 2016, 26, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Oliveira, V.; Câmara, N.O.S.; Moraes-Vieira, P.M. Adipokines as Drug Targets in Diabetes and Underlying Disturbances. J. Diabetes Res. 2015, 2015, 681612. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Artis, D. Immune Regulation of Metabolic Homeostasis in Health and Disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Kita, S.; Maeda, N.; Shimomura, I. Interorgan Communication by Exosomes, Adipose Tissue, and Adiponectin in Metabolic Syndrome. J. Clin. Investig. 2019, 129, 4041–4049. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Li, N.; Zhao, S.; Zhang, Z.; Zhu, Y.; Gliniak, C.; Vishvanath, L.; An, Y.; Wang, M.-Y.; Deng, Y.; Zhu, Q.; et al. Adiponectin Preserves Metabolic Fitness during Aging. eLife 2021, 10, e65108. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Jha, A.; Agarwal, A.; Misra, A. The Impact of Obesity on Inflammatory Markers Used in the Assessment of Disease Activity in Rheumatoid Arthritis—A Cross-Sectional Study. Reumatol./Rheumatol. 2020, 58, 9–14. [Google Scholar] [CrossRef]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Bensussen, A.; Torres-Magallanes, J.A.; de Álvarez-Buylla, E.R. Molecular Tracking of Insulin Resistance and Inflammation Development on Visceral Adipose Tissue. Front. Immunol. 2023, 14, 1014778. [Google Scholar] [CrossRef]
- Yan, K. Recent Advances in the Effect of Adipose Tissue Inflammation on Insulin Resistance. Cell. Signal. 2024, 120, 111229. [Google Scholar] [CrossRef]
- Ramón-Krauel, M.; Leal-Witt, M.J.; Osorio-Conles, Ó.; Amat-Bou, M.; Lerín, C.; Selva, D.M. Relationship between Adiponectin, TNFα, and SHBG in Prepubertal Children with Obesity. Mol. Cell. Pediatr. 2021, 8, 3. [Google Scholar] [CrossRef]
- Li, X.; Ren, Y.; Chang, K.-W.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose Tissue Macrophages as Potential Targets for Obesity and Metabolic Diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Qi, Y.; Yi, H.; Mao, C.; Meng, Q.; Wang, H.; Zheng, C. The Roles of Adipose Tissue Macrophages in Human Disease. Front. Immunol. 2022, 13, 908749. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and Glucose Metabolism in White Adipocytes: Pathways, Dysfunction and Therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Trinh, M.N.; Brown, M.S.; Goldstein, J.L.; Han, J.; Vale, G.; McDonald, J.G.; Seemann, J.; Mendell, J.T.; Lu, F. Last Step in the Path of LDL Cholesterol from Lysosome to Plasma Membrane to ER Is Governed by Phosphatidylserine. Proc. Natl. Acad. Sci. USA 2020, 117, 18521–18529. [Google Scholar] [CrossRef]
- Toubal, A.; Kiaf, B.; Beaudoin, L.; Cagninacci, L.; Rhimi, M.; Fruchet, B.; Silva, J.D.; Corbett, A.J.; Simoni, Y.; Lantz, O.; et al. Mucosal-Associated Invariant T Cells Promote Inflammation and Intestinal Dysbiosis Leading to Metabolic Dysfunction during Obesity. Nat. Commun. 2020, 11, 3755. [Google Scholar] [CrossRef]
- Yao, J.; Wu, D.; Qiu, Y. Adipose Tissue Macrophage in Obesity-Associated Metabolic Diseases. Front. Immunol. 2022, 13, 977485. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Z.; Qiu, Y.; Gao, Y.; Fan, Y.; Wang, Q.; Zhou, Q. NLRP3 Inflammasome Activation in Response to Metals. Front. Immunol. 2023, 14, 1055788. [Google Scholar] [CrossRef]
- Lempesis, I.; van Meijel, R.L.J.; Manolopoulos, K.; Goossens, G.H. Oxygenation of Adipose Tissue: A Human Perspective. Acta Physiol. 2019, 228, e13298. [Google Scholar] [CrossRef]
- Pezhman, L.; Tahrani, A.A.; Chimen, M. Dysregulation of Leukocyte Trafficking in Type 2 Diabetes: Mechanisms and Potential Therapeutic Avenues. Front. Cell Dev. Biol. 2021, 9, 624184. [Google Scholar] [CrossRef]
- Remmerie, A.; Scott, C.L. Macrophages and Lipid Metabolism. Cell. Immunol. 2018, 330, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Ieronymaki, E.; Daskalaki, M.; Lyroni, K.; Tsatsanis, C. Insulin Signaling and Insulin Resistance Facilitate Trained Immunity in Macrophages Through Metabolic and Epigenetic Changes. Front. Immunol. 2019, 10, 1330. [Google Scholar] [CrossRef] [PubMed]
- Püschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, 11, 4358. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D. Macrophages Switch: The Fate of Adipose Tissue in Obesity. MOJ Immunol. 2016, 3, 11–12. [Google Scholar] [CrossRef]
- Herrada, A.A.; Olate-Briones, A.; Rojas, A.; Liu, C.; Escobedo, N.; Piesche, M. Adipose Tissue Macrophages as a Therapeutic Target in Obesity-associated Diseases. Obes. Rev. 2021, 22, e13200. [Google Scholar] [CrossRef]
- Radushev, V.; Karkossa, I.; van den Berg, J.M.; von Bergen, M.; Engelmann, B.; Rolle-Kampczyk, U.; Blüher, M.; Wagner, U.; Schubert, K.; Rossol, M. Dysregulated Cytokine and Oxidative Response in Hyper-Glycolytic Monocytes in Obesity. Front. Immunol. 2024, 15, 1416543. [Google Scholar] [CrossRef]
- Qin, Y.; Jia, L.; Liu, H.; Ma, W.; Ren, X.; Li, H.; Liu, Y.; Li, H.; Ma, S.; Liu, M.; et al. Macrophage Deletion of Noc4l Triggers Endosomal TLR4/TRIF Signal and Leads to Insulin Resistance. Nat. Commun. 2021, 12, 6121. [Google Scholar] [CrossRef]
- Sharma, M.; Boytard, L.; Hadi, T.; Koelwyn, G.J.; Simon, R.; Ouimet, M.; Seifert, L.; Spiro, W.; Yan, B.; Hutchison, S.; et al. Enhanced Glycolysis and HIF-1α Activation in Adipose Tissue Macrophages Sustains Local and Systemic Interleukin-1β Production in Obesity. Sci. Rep. 2020, 10, 5555. [Google Scholar] [CrossRef]
- Orliaguet, L.; Ejlalmanesh, T.; Alzaïd, F. Metabolic and Molecular Mechanisms of Macrophage Polarisation and Adipose Tissue Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 5731. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, F.; Vilahur, G.; Badimón, L.; Palomo, I. Mechanisms of Chronic State of Inflammation as Mediators That Link Obese Adipose Tissue and Metabolic Syndrome. Mediat. Inflamm. 2013, 2013, 136584. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Gorgani-Firuzjaee, S.; Panahi, G.; Meshkani, R. Molecular and Cellular Mechanisms Linking Inflammation to Insulin Resistance and β-Cell Dysfunction. Transl. Res. 2015, 167, 228–256. [Google Scholar] [CrossRef]
- Jager, J.; Grémeaux, T.; Cormont, M.; Marchand-Brustel, Y.L.; Tanti, J. Interleukin-1β-Induced Insulin Resistance in Adipocytes through Down-Regulation of Insulin Receptor Substrate-1 Expression. Endocrinology 2006, 148, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D. Molecular Mechanisms for the Vicious Cycle between Insulin Resistance and the Inflammatory Response in Obesity. Int. J. Mol. Sci. 2023, 24, 9818. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Sheikh, N. Role of Immune Cells in Obesity Induced Low Grade Inflammation and Insulin Resistance. Cell. Immunol. 2017, 315, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tanti, J.; Ceppo, F.; Jager, J.; Berthou, F. Implication of Inflammatory Signaling Pathways in Obesity-Induced Insulin Resistance. Front. Endocrinol. 2013, 3, 181. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.; Matteo, G.D.; Ingallina, C.; Ambroselli, D.; Carradori, S.; Gallorini, M.; Giusti, A.M.; Salvo, A.; Grosso, M.; Mannina, L. Modulatory Properties of Food and Nutraceutical Components Targeting NLRP3 Inflammasome Activation. Nutrients 2022, 14, 490. [Google Scholar] [CrossRef]
- Schleh, M.W.; Caslin, H.L.; Garcia, J.N.; Mashayekhi, M.; Srivastava, G.; Bradley, A.B.; Hasty, A.H. Metaflammation in Obesity and Its Therapeutic Targeting. Sci. Transl. Med. 2023, 15, eadf9382. [Google Scholar] [CrossRef]
- Orliaguet, L.; Dalmas, É.; Drareni, K.; Venteclef, N.; Alzaïd, F. Mechanisms of Macrophage Polarization in Insulin Signaling and Sensitivity. Front. Endocrinol. 2020, 11, 62. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Daryabor, G.; Kabelitz, D.; Kalantar, K. An Update on Immune Dysregulation in Obesity-related Insulin Resistance. Scand. J. Immunol. 2018, 89, e12747. [Google Scholar] [CrossRef]
- Takatsu, K. The Inflammasomes and Immunometabolism: A Small Molecule Inhibitor of the NLRP3 Inflammasome. Biochem. Biophys. Res. Commun. 2022, 633, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, S.; Rocca, C.; Gianquinto, E.; Granieri, M.C.; Boscaro, V.; Blua, F.; Rolando, B.; Marini, E.; Gallicchio, M.; De Bartolo, A.; et al. Discovery of a Novel 1,3,4-Oxadiazol-2-One-Based NLRP3 Inhibitor as a Pharmacological Agent to Mitigate Cardiac and Metabolic Complications in an Experimental Model of Diet-Induced Metaflammation (Version 1). arXiv 2023, arXiv:2309.07939. [Google Scholar] [CrossRef]
- Wen, H.; Gris, D.; Lei, Y.L.; Jha, S.; Zhang, L.; Huang, M.T.; Brickey, W.J.; Ting, J.P.-Y. Fatty Acid–Induced NLRP3-ASC Inflammasome Activation Interferes with Insulin Signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Verdú, E.; Homs, J.; Boadas-Vaello, P. Physiological Changes and Pathological Pain Associated with Sedentary Lifestyle-Induced Body Systems Fat Accumulation and Their Modulation by Physical Exercise. Int. J. Environ. Res. Public Health 2021, 18, 13333. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Liu, A.; Chen, X.; Cheng, W.; Dirsch, O.; Dahmen, U. The Severity of LPS Induced Inflammatory Injury Is Negatively Associated with the Functional Liver Mass after LPS Injection in Rat Model. J. Inflamm. 2018, 15, 21. [Google Scholar] [CrossRef]
- Jakkawanpitak, C.; Hutadilok-Towatana, N.; Sermwittayawong, D. Fungal-like Particles and Macrophage-Conditioned Medium Are Inflammatory Elicitors for 3T3-L1 Adipocytes. Sci. Rep. 2020, 10, 9437. [Google Scholar] [CrossRef]
- Lü, B.; Lü, Y.; Moser, A.H.; Shigenaga, J.K.; Grünfeld, C.; Feingold, K.R. LPS and Proinflammatory Cytokines Decrease Lipin-1 in Mouse Adipose Tissue and 3T3-L1 Adipocytes. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1502–E1509. [Google Scholar] [CrossRef]
- Salazar-León, J.; Valdez-Hernández, A.L.; García-Jiménez, S.; Román-Domínguez, L.; Huanosta-Murillo, E.; Bonifaz, L.C.; Pérez-Martínez, L.; Pedraza-Alva, G. Nlrp1b1 Negatively Modulates Obesity-Induced Inflammation by Promoting IL-18 Production. Sci. Rep. 2019, 9, 13815. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Z.; Hong, L.; Liu, J.; Ma, X.; Wang, W.; Pan, R.; Lu, W.; Luo, Q.; Gao, S.; et al. Apolipoprotein E (ApoE) Orchestrates Adipose Tissue Inflammation and Metabolic Disorders through NLRP3 Inflammasome. Mol. Biomed. 2023, 4, 47. [Google Scholar] [CrossRef]
- Pang, H.; Ling, D.; Cheng, Y.; Akbar, R.; Jin, L.; Ren, J.; Wu, H.; Chen, B.; Zhou, Y.; Zhu, H.; et al. Gestational High-fat Diet Impaired Demethylation of Pparα and Induced Obesity of Offspring. J. Cell. Mol. Med. 2021, 25, 5404–5416. [Google Scholar] [CrossRef]
- Chen, P.; Chiu, W.; Hsu, P.; Lin, S.; Peng, I.; Wang, C.; Tsai, S. Pathophysiological Implications of Hypoxia in Human Diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef]
- Kang, G.-S.; Jo, H.-J.; Lee, Y.-R.; Oh, T.; Park, H.-J.; Ahn, G. Sensing the Oxygen and Temperature in the Adipose Tissues—Who’s Sensing What? Exp. Mol. Med. 2023, 55, 2300–2307. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Xie, C.; Jiang, C. The Role of Hypoxia-Inducible Factors in Metabolic Diseases. Nat. Rev. Endocrinol. 2018, 15, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Okahara, F.; Osaki, N.; Shimotoyodome, A. Increased GIP Signaling Induces Adipose Inflammation via a HIF-1α-Dependent Pathway and Impairs Insulin Sensitivity in Mice. Am. J. Physiol. Endocrinol. Metab. 2014, 308, E414–E425. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, Z. Fundamental Roles for Hypoxia Signalling in Adipose Tissue Metabolism and Inflammation in Obesity. Curr. Opin. Physiol. 2019, 12, 39–43. [Google Scholar] [CrossRef]
- Lin, Q.; Yun, Z. The Hypoxia-Inducible Factor Pathway in Adipocytes: The Role of HIF-2 in Adipose Inflammation and Hypertrophic Cardiomyopathy. Front. Endocrinol. 2015, 6, 39. [Google Scholar] [CrossRef]
- Takikawa, A.; Mahmood, A.; Nawaz, A.; Kado, T.; Okabe, K.; Yamamoto, S.; Aminuddin, A.; Senda, S.; Tsuneyama, K.; Ikutani, M.; et al. HIF-1α in Myeloid Cells Promotes Adipose Tissue Remodeling Toward Insulin Resistance. Diabetes 2016, 65, 3649–3659. [Google Scholar] [CrossRef]
- Andrei, A.M.; Berbecaru-Iovan, A.; Din-Anghel, F.R.I.; Stănciulescu, C.E.; Berbecaru-Iovan, S.; Baniţă, I.M.; Pisoschi, C.G. Interplay between Hypoxia, Inflammation and Adipocyte Remodeling in the Metabolic Syndrome. In Hypoxia and Human Disease, Chapter 16; InTech Ebooks: London, UK, 2017. [Google Scholar] [CrossRef]
- Li, J.; Yu, R.; Zhang, P.; Wen, S.; Wang, S.; Zhang, X.; Xu, Q.; Kong, L. Dietary Fructose-Induced Gut Dysbiosis Promotes Mouse Hippocampal Neuroinflammation: A Benefit of Short-Chain Fatty Acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef]
- Winer, D.A.; Winer, S.; Dranse, H.J.; Lam, T.K.T. Immunologic Impact of the Intestine in Metabolic Disease. J. Clin. Investig. 2017, 127, 33–42. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, J.; Zhu, S.; Xin, L.; Yu, C.; Shen, Z. The Role of Short Chain Fatty Acids in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2022, 28, 540–548. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.; Zhang, Y.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.; Zhou, H.; Wang, Y.; Xu, Z. Short-Chain Fatty Acids in Diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Wijdeveld, M.; Schrantee, A.; Hagemeijer, A.; Nederveen, A.J.; Scheithauer, T.P.M.; Levels, J.H.M.; Prodan, A.; de Vos, W.M.; Nieuwdorp, M.; IJzerman, R.G. Intestinal Acetate and Butyrate Availability Is Associated with Glucose Metabolism in Healthy Individuals. iScience 2023, 26, 108478. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Konop, M.; Bielińska, K.; Hutsch, T.; Dziekiewicz, M.; Banaszkiewicz, A.; Ufnal, M. Inflammatory Bowel Disease Is Associated with Increased Gut-to-blood Penetration of Short-chain Fatty Acids: A New, Non-invasive Marker of a Functional Intestinal Lesion. Exp. Physiol. 2019, 104, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Sebastian-Valverde, M.; Pasinetti, G.M. The NLRP3 Inflammasome as a Critical Actor in the Inflammaging Process. Cells 2020, 9, 1552. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Duewell, P. NLRP3 Inflammasome Activation in Inflammaging. Semin. Immunol. 2018, 40, 61–73. [Google Scholar] [CrossRef]
- Ramachandran, R.; Manan, A.; Kim, J.; Choi, S. NLRP3 Inflammasome: A Key Player in the Pathogenesis of Life-Style Disorders. Exp. Mol. Med. 2024, 56, 1488–1500. [Google Scholar] [CrossRef]
- Wang, L.; Hauenstein, A.V. The NLRP3 Inflammasome: Mechanism of Action, Role in Disease and Therapies. Mol. Asp. Med. 2020, 76, 100889. [Google Scholar] [CrossRef]
- Villalva, M.; Martínez-García, J.J.; Jaime, L.; Santoyo, S.; Pelegrín, P.; Pérez-Jiménez, J. Polyphenols as NLRP3 Inflammasome Modulators in Cardiometabolic Diseases: A Review of in vivo studies. Food Funct. 2023, 14, 9534–9553. [Google Scholar] [CrossRef]
- Nani, A.; Tehami, W. Targeting Inflammasome Pathway by Polyphenols as a Strategy for Pancreatitis, Gastrointestinal and Liver Diseases Management: An Updated Review. Front. Nutr. 2023, 10, 1157572. [Google Scholar] [CrossRef]
- Gritsenko, A.; Green, J.; Brough, D.; López-Castejón, G. Mechanisms of NLRP3 Priming in Inflammaging and Age Related Diseases. Cytokine Growth Factor Rev. 2020, 55, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Witztum, J.L.; Steinberg, D. Role of Oxidized Low Density Lipoprotein in Atherogenesis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Ramprasad, M.P.; Terpstra, V.; Kondratenko, N.; Quehenberger, O.; Steinberg, D. Cell Surface Expression of Mouse Macrosialin and Human CD68 and Their Role as Macrophage Receptors for Oxidized Low Density Lipoprotein. Proc. Natl. Acad. Sci. USA 1996, 93, 14833–14838. [Google Scholar] [CrossRef] [PubMed]
- Rios, F.J.; Koga, M.M.; Pecenin, M.F.; Ferracini, M.; Gidlund, M.; Jancar, S. Oxidized LDL Induces Alternative Macrophage Phenotype through Activation of CD36 and PAFR. Mediat. Inflamm. 2013, 2013, 198193. [Google Scholar] [CrossRef]
- Houben, T.; Oligschlaeger, Y.; Bitorina, A.V.; Hendrikx, T.; Walenbergh, S.M.A.; Lenders, M.-H.; Gijbels, M.J.; Verheyen, F.; Lütjohann, D.; Hofker, M.H.; et al. Blood-Derived Macrophages Prone to Accumulate Lysosomal Lipids Trigger oxLDL-Dependent Murine Hepatic Inflammation. Sci. Rep. 2017, 7, 12550. [Google Scholar] [CrossRef]
- van Tits, L.J.H.; Stienstra, R.; van Lent, P.L.; Netea, M.G.; Joosten, L.A.B.; Stalenhoef, A.F.H. Oxidized LDL Enhances Pro-Inflammatory Responses of Alternatively Activated M2 Macrophages: A Crucial Role for Krüppel-like Factor 2. Atherosclerosis 2010, 214, 345–349. [Google Scholar] [CrossRef]
- Rosenfeld, M.E.; Khoo, J.C.; Miller, E.R.; Parthasarathy, S.; Palinski, W.; Witztum, J.L. Macrophage-Derived Foam Cells Freshly Isolated from Rabbit Atherosclerotic Lesions Degrade Modified Lipoproteins, Promote Oxidation of Low-Density Lipoproteins, and Contain Oxidation-Specific Lipid-Protein Adducts. J. Clin. Investig. 1991, 87, 90–99. [Google Scholar] [CrossRef]
- Schilke, R.M.; Blackburn, C.M.R.; Bamgbose, T.T.; Woolard, M.D. Interface of Phospholipase Activity, Immune Cell Function, and Atherosclerosis. Biomolecules 2020, 10, 1449. [Google Scholar] [CrossRef]
- Quinn, M.T.; Parthasarathy, S.; Fong, L.G.; Steinberg, D. Oxidatively Modified Low Density Lipoproteins: A Potential Role in Recruitment and Retention of Monocyte/Macrophages during Atherogenesis. Proc. Natl. Acad. Sci. USA 1987, 84, 2995–2998. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Bezsonov, E.E.; Orekhova, V.A.; Popkova, T.V.; Starodubova, A.V.; Orekhov, A.N. Recognition of Oxidized Lipids by Macrophages and Its Role in Atherosclerosis Development. Biomedicines 2021, 9, 915. [Google Scholar] [CrossRef]
- Obri, A.; Serra, D.; Herrero, L.; Mera, P. The Role of Epigenetics in the Development of Obesity. Biochem. Pharmacol. 2020, 177, 113973. [Google Scholar] [CrossRef]
- Deng, L.; Kersten, S.; Stienstra, R. Triacylglycerol Uptake and Handling by Macrophages: From Fatty Acids to Lipoproteins. Prog. Lipid Res. 2023, 92, 101250. [Google Scholar] [CrossRef]
- Brunner-Weinzierl, M.C.; Gruber, M.; Schmid, D.; Baran, H.; Moeslinger, T. Proliferation of Macrophages Due to the Inhibition of Inducible Nitric Oxide Synthesis by Oxidized Low-Density Lipoproteins. EXCLI J. 2015, 14, 439–451. [Google Scholar] [CrossRef]
- Dang, E.V.; Cyster, J.G. Loss of Sterol Metabolic Homeostasis Triggers Inflammasomes—How and Why. Curr. Opin. Immunol. 2018, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rajamäki, K.; Lappalainen, J.; Öörni, K.; Välimäki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol Crystals Activate the NLRP3 Inflammasome in Human Macrophages: A Novel Link between Cholesterol Metabolism and Inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Takahashi, M. The Crystal-Induced Activation of NLRP3 Inflammasomes in Atherosclerosis. Inflamm. Regen. 2017, 37, 18. [Google Scholar] [CrossRef]
- Sheedy, F.J.; Grebe, A.; Rayner, K.J.; Kalantari, P.; Ramkhelawon, B.; Carpenter, S.; Becker, C.; Ediriweera, H.; Mullick, A.E.; Golenbock, D.T.; et al. CD36 Coordinates NLRP3 Inflammasome Activation by Facilitating Intracellular Nucleation of Soluble Ligands into Particulate Ligands in Sterile Inflammation. Nat. Immunol. 2013, 14, 812–820. [Google Scholar] [CrossRef]
- Baumer, Y.; Ng, Q.; Sanda, G.E.; Dey, A.K.; Teague, H.; Sorokin, A.V.; Dagur, P.K.; Silverman, J.I.; Harrington, C.; Rodante, J.; et al. Chronic Skin Inflammation Accelerates Macrophage Cholesterol Crystal Formation and Atherosclerosis. JCI Insight 2018, 3, e97179. [Google Scholar] [CrossRef]
- Ayala, T.S.; Tessaro, F.H.G.; Jannuzzi, G.P.; Bella, L.M.; Ferreira, K.S.; Martins, J.O. High Glucose Environments Interfere with Bone Marrow-Derived Macrophage Inflammatory Mediator Release, the TLR4 Pathway and Glucose Metabolism. Sci. Rep. 2019, 9, 11447. [Google Scholar] [CrossRef]
- Pavlou, S.; Lindsay, J.; Ingram, R.J.; Xu, H.; Chen, M. Sustained High Glucose Exposure Sensitizes Macrophage Responses to Cytokine Stimuli but Reduces Their Phagocytic Activity. BMC Immunol. 2018, 19, 24. [Google Scholar] [CrossRef]
- Moganti, K.; Li, F.; Schmuttermaier, C.; Riemann, S.; Klüter, H.; Gratchev, A.; Harmsen, M.C.; Kzhyshkowska, J. Hyperglycemia Induces Mixed M1/M2 Cytokine Profile in Primary Human Monocyte-Derived Macrophages. Immunobiology 2016, 222, 952–959. [Google Scholar] [CrossRef]
- Ding, S.; Xu, S.; Ma, Y.; Liu, G.; Jang, H.; Fang, J. Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules 2019, 9, 850. [Google Scholar] [CrossRef]
- Stienstra, R.; van Diepen, J.A.; Tack, C.J.; Zaki, H.; van de Veerdonk, F.L.; Perera, D.; Neale, G.; Hooiveld, G.; Hijmans, A.; Vroegrijk, I.O.C.M.; et al. Inflammasome Is a Central Player in the Induction of Obesity and Insulin Resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 15324–15329. [Google Scholar] [CrossRef]
- Matuschik, L.; Riabov, V.; Schmuttermaier, C.; Sevastyanova, T.; Weiß, C.; Klüter, H.; Kzhyshkowska, J. Hyperglycemia Induces Inflammatory Response of Human Macrophages to CD163-Mediated Scavenging of Hemoglobin-Haptoglobin Complexes. Int. J. Mol. Sci. 2022, 23, 1385. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C. Role of Free Fatty Acids in Endothelial Dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef]
- Chaurasia, B.; Talbot, C.L.; Summers, S.A. Adipocyte Ceramides-The Nexus of Inflammation and Metabolic Disease. Front. Immunol. 2020, 11, 576347. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Bikman, B.T.; Wang, L.; Guan, Y.; Sargent, K.M.; Bulchand, S.; Knotts, T.A.; Shui, G.; Clegg, D.J.; Wenk, M.R.; et al. Lipid-Induced Insulin Resistance Mediated by the Proinflammatory Receptor TLR4 Requires Saturated Fatty Acid–Induced Ceramide Biosynthesis in Mice. J. Clin. Investig. 2011, 121, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, X.; Zheng, Z.; Yu, S.; Zhou, B.; Liu, Y.; Liu, D.; Chen, Y.; Qian, X. Beneficial Effect of ER Stress Preconditioning in Protection against FFA-Induced Adipocyte Inflammation via XBP1 in 3T3-L1 Adipocytes. Mol. Cell. Biochem. 2019, 463, 45–55. [Google Scholar] [CrossRef]
- Ormseth, M.J.; Ll, S.; Fazio, S.; Linton, M.; Raggi, P.; Solus, J.; Oeser, A.; Bian, A.; Gebretsadik, T.; Shintani, A.; et al. Free Fatty Acids Are Associated with Metabolic Syndrome and Insulin Resistance but Not Inflammation in Systemic Lupus Erythematosus. Lupus 2012, 22, 26–33. [Google Scholar] [CrossRef]
- Sokołowska, E.; Błachnio-Zabielska, A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Badawi, A.; Klip, A.; Haddad, P.; Cole, D.E.; Bailo, B.G.; El-Sohemy, A.; Karmali, M. Type 2 Diabetes Mellitus and Inflammation: Prospects for Biomarkers of Risk and Nutritional Intervention. Diabetes Metab. Syndr. Obes. 2010, 3, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M.L. The Role of Fatty Acids in Insulin Resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Chen, X.-Y.; Hu, Q.; Wang, M.; Jin, R.; Zhang, Q.; Wang, W.; Wang, R.; Kang, L.-L.; et al. Reactive Oxygen Species-Induced TXNIP Drives Fructose-Mediated Hepatic Inflammation and Lipid Accumulation Through NLRP3 Inflammasome Activation. Antioxid. Redox Signal. 2015, 22, 848–870. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tate, M.; Mathew, G.; Vince, J.E.; Ritchie, R.H.; de Haan, J.B. Oxidative Stress and NLRP3-Inflammasome Activity as Significant Drivers of Diabetic Cardiovascular Complications: Therapeutic Implications. Front. Physiol. 2018, 9, 114. [Google Scholar] [CrossRef]
- Maurya, C.K.; Arha, D.; Kumar, A.; Kumar, S.; Pandey, J.; Avisetti, D.R.; Kalivendi, S.V.; Klip, A.; Tamrakar, A.K. NOD2 Activation Induces Oxidative Stress Contributing to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle Cells. Free Radic. Biol. Med. 2015, 89, 158–169. [Google Scholar] [CrossRef]
- Li, X.; Xiao, G.-Y.; Guo, T.; Song, Y.; Li, Q. Potential Therapeutic Role of Pyroptosis Mediated by the NLRP3 Inflammasome in Type 2 Diabetes and Its Complications. Front. Endocrinol. 2022, 13, 986565. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Javaid, H.M.A.; Ko, E.J.; Joo, E.J.; Kwon, S.H.; Park, J.; Shin, S.; Cho, K.W.; Huh, J.Y. TNFα-Induced NLRP3 Inflammasome Mediates Adipocyte Dysfunction and Activates Macrophages through Adipocyte-Derived Lipocalin 2. Metabolism 2023, 142, 155527. [Google Scholar] [CrossRef]
- Lindhorst, A.; Raulien, N.; Wieghofer, P.; Eilers, J.; Rossi, F.; Bechmann, I.; Gericke, M. Adipocyte Death Triggers a Pro-Inflammatory Response and Induces Metabolic Activation of Resident Macrophages. Cell Death Dis. 2021, 12, 579. [Google Scholar] [CrossRef]
- Sun, S.; Ji, Y.; Kersten, S.; Qi, L. Mechanisms of Inflammatory Responses in Obese Adipose Tissue. Annu. Rev. Nutr. 2012, 32, 261–286. [Google Scholar] [CrossRef]
- Kuroda, M.; Sakaue, H. Adipocyte Death and Chronic Inflammation in Obesity. J. Med. Investig. 2017, 64, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, X.; Ibrahim, M.A.; Peltzer, N. Cell Death and Inflammation during Obesity: “Know My Methods, WAT(Son)”. Cell Death Differ. 2022, 30, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nagai, Y.; Honda, H.; Okamoto, N.; Yanagibashi, T.; Ogasawara, M.; Yamamoto, S.; Imamura, R.; Takasaki, I.; Hara, H.; et al. Bidirectional Crosstalk between Neutrophils and Adipocytes Promotes Adipose Tissue Inflammation. FASEB J. 2019, 33, 11821–11835. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, Y.; Takatsu, K. Activation and Regulation of the Pattern Recognition Receptors in Obesity-Induced Adipose Tissue Inflammation and Insulin Resistance. Nutrients 2013, 5, 3757–3778. [Google Scholar] [CrossRef] [PubMed]
- RIVERS, S.L.; Klip, A.; Giacca, A. NOD1: An Interface Between Innate Immunity and Insulin Resistance. Endocrinology 2019, 160, 1021–1030. [Google Scholar] [CrossRef]
- ZHOU, Y.; Zhou, H.; Li, Y.; Song, Y. NOD1 Activation Induces Innate Immune Responses and Insulin Resistance in Human Adipocytes. Diabetes Metab. 2012, 38, 538–543. [Google Scholar] [CrossRef]
- Jaïs, A.; Brüning, J.C. Hypothalamic Inflammation in Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef]
- Meiliana, A.; Dewi, N.M.; Wijaya, A. Hypothalamic Microinflammation: New Paradigm in Obesity and Metabolic Disease. Indones. Biomed. J. 2020, 12, 201–213. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, M. Homeostatic Regulation of Glucose Metabolism by the Central Nervous System. Endocrinol. Metab. 2022, 37, 9–25. [Google Scholar] [CrossRef]
- Saitoh, S.; van Wijk, K.; Nakajima, O. Crosstalk between Metabolic Disorders and Immune Cells. Int. J. Mol. Sci. 2021, 22, 10017. [Google Scholar] [CrossRef]
- Pan, D.; Li, G.; Jiang, C.; Hu, J.; Hu, X. Regulatory Mechanisms of Macrophage Polarization in Adipose Tissue. Front. Immunol. 2023, 14, 1149366. [Google Scholar] [CrossRef]
- Romeo, G.; Lee, J.; Shoelson, S.E. Metabolic Syndrome, Insulin Resistance, and Roles of Inflammation—Mechanisms and Therapeutic Targets. Arter. Thromb. Vasc. Biol. 2012, 32, 1771–1776. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut Microbiota and Metabolic Syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Hemarajata, P.; Versalovic, J. The Human Gut Microbiome and Body Metabolism: Implications for Obesity and Diabetes. Clin. Chem. 2013, 59, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Long, S.L.; Gahan, C.G.M.; Joyce, S.A. Interactions between Gut Bacteria and Bile in Health and Disease. Mol. Asp. Med. 2017, 56, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.A.; Theriot, C.M. Diversification of Host Bile Acids by Members of the Gut Microbiota. Gut Microbes 2019, 11, 158–171. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of Gut Microbiota with Bile Acid Metabolism and Its Influence on Disease States. Appl. Microbiol. Biotechnol. 2016, 101, 47–64. [Google Scholar] [CrossRef]
- Bourgin, M.; Kriaa, A.; Mkaouar, H.; Mariaule, V.; Jablaoui, A.; Maguin, E.; Rhimi, M. Bile Salt Hydrolases: At the Crossroads of Microbiota and Human Health. Microorganisms 2021, 9, 1122. [Google Scholar] [CrossRef]
- Yao, L.; Seaton, S.C.; Ndousse-Fetter, S.; Adhikari, A.A.; DiBenedetto, N.; Mina, A.I.; Banks, A.S.; Bry, L.; Devlin, A.S. A Selective Gut Bacterial Bile Salt Hydrolase Alters Host Metabolism. eLife 2018, 7, e37182. [Google Scholar] [CrossRef]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G.M. Regulation of Host Weight Gain and Lipid Metabolism by Bacterial Bile Acid Modification in the Gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Talavera, O.; Tailleux, A.; Lefèbvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Receptors FXR and TGR5 Signaling in Fatty Liver Diseases and Therapy. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Lu, M.; Xu, Y.; Wang, Q.; Gu, X.; Li, Y.; Zhuang, T.; Xia, C.; Zhang, T.; Gou, X.; et al. The Role of Gut Microbiota-Bile Acids Axis in the Progression of Non-Alcoholic Fatty Liver Disease. Front. Microbiol. 2022, 13, 908011. [Google Scholar] [CrossRef]
- Larabi, A.; Masson, H.L.P.; Bäumler, A.J. Bile Acids as Modulators of Gut Microbiota Composition and Function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Giannini, C.; Mastromauro, C.; Scapaticci, S.; Gentile, C.; Chiarelli, F. Role of Bile Acids in Overweight and Obese Children and Adolescents. Front. Endocrinol. 2022, 13, 1011994. [Google Scholar] [CrossRef]
- Ma, H.; Patti, M. Bile Acids, Obesity, and the Metabolic Syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 573–583. [Google Scholar] [CrossRef]
- Fleishman, J.S.; Kumar, S. Bile Acid Metabolism and Signaling in Health and Disease: Molecular Mechanisms and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 97. [Google Scholar] [CrossRef]
- Schmid, A.; Schlegel, J.; Thomalla, M.; Karrasch, T.; Schäffler, A. Evidence of Functional Bile Acid Signaling Pathways in Adipocytes. Mol. Cell. Endocrinol. 2018, 483, 1–10. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, J.; Liu, C.; Le, T.N.; Lu, Y.; Feng, F.; Zhao, M. High-Fat Diet-Induced Decreased Circulating Bile Acids Contribute to Obesity Associated with Gut Microbiota in Mice. Foods 2024, 13, 699. [Google Scholar] [CrossRef]
- Duranti, S.; Ferrario, C.; van Sinderen, D.; Ventura, M.; Turroni, F. Obesity and Microbiota: An Example of an Intricate Relationship. Genes Nutr. 2017, 12, 18. [Google Scholar] [CrossRef]
- Xu, M.; Lan, R.; Qiao, L.; Lin, X.; Hu, D.; Zhang, S.; Yang, H.J.; Zhou, J.; Ren, Z.; Li, X.; et al. Bacteroides Vulgatus Ameliorates Lipid Metabolic Disorders and Modulates Gut Microbial Composition in Hyperlipidemic Rats. Microbiol. Spectr. 2023, 11, e02517-22. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Gu, Y.; Wang, X.; Xie, R.; Sun, Y.; Wang, B.; Cao, H. Regulation of Gut Microbiota-Bile Acids Axis by Probiotics in Inflammatory Bowel Disease. Front. Immunol. 2022, 13, 974305. [Google Scholar] [CrossRef]
- Heinken, A.; Ravcheev, D.A.; Baldini, F.; Heirendt, L.; Fleming, R.M.T.; Thiele, I. Systematic Assessment of Secondary Bile Acid Metabolism in Gut Microbes Reveals Distinct Metabolic Capabilities in Inflammatory Bowel Disease. Microbiome 2019, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.Y.; de Valdez, G.F.; Fadda, S.; Taranto, M.P. New Insights into Bacterial Bile Resistance Mechanisms: The Role of Bile Salt Hydrolase and Its Impact on Human Health. Food Res. Int. 2018, 112, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, H.; Li, M.; He, T.; Guo, S.; Zhu, L.; Tan, J.; Wang, B. Novel Approaches in IBD Therapy: Targeting the Gut Microbiota-Bile Acid Axis. Gut Microbes 2024, 16, 2356284. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cowley, E.S.; Wolf, P.G.; Doden, H.L.; Murai, T.; Caicedo, K.Y.O.; Ly, L.K.; Sun, F.-R.; Takei, H.; Nittono, H.; et al. Formation of Secondary Allo-Bile Acids by Novel Enzymes from Gut Firmicutes. Gut Microbes 2022, 14, 2132903. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Alves, J.M.P.; Hylemon, P.B.; Bajaj, J.S. Cirrhosis, Bile Acids and Gut Microbiota. Gut Microbes 2013, 4, 382–387. [Google Scholar] [CrossRef]
- Ndou, S.P.; Tun, H.M.; Kiarie, E.G.; Walsh, M.C.; Khafipour, E.; Nyachoti, C.M. Dietary Supplementation with Flaxseed Meal and Oat Hulls Modulates Intestinal Histomorphometric Characteristics, Digesta- and Mucosa-Associated Microbiota in Pigs. Sci. Rep. 2018, 8, 5880. [Google Scholar] [CrossRef]
- Beckers, K.F.; Flanagan, J.P.; Sones, J.L. Microbiome and Pregnancy: Focus on Microbial Dysbiosis Coupled with Maternal Obesity. Int. J. Obes. 2023, 48, 439, Correction in Int. J. Obes. 2025, 49, 1421. [Google Scholar] [CrossRef]
- Sutoyo, D.A.R.; Atmaka, D.R.; Sidabutar, L.M.G.B. Dietary factors affecting firmicutes and bacteroidetes ratio in solving obesity problem: A literature review. Media Gizi Indones. 2020, 15, 94–109. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The Critical Role of Gut Microbiota in Obesity. Front. Endocrinol. 2022, 13, 1025706. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhu, L.; Li, Y. Gut and Obesity/Metabolic Disease: Focus on Microbiota Metabolites. MedComm 2022, 3, e171. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Sarmiento-Andrade, Y.; Suárez, R.; Quintero, B.; Garrochamba, K.; Chapela, S. Gut Microbiota and Obesity: New Insights. Front. Nutr. 2022, 9, 1018212. [Google Scholar] [CrossRef]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A Taxonomic Signature of Obesity in a Large Study of American Adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef]
- Min, Y.W.; Rezaie, A.; Pimentel, M. Bile Acid and Gut Microbiota in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2022, 28, 549–561. [Google Scholar] [CrossRef]
- Li, M.; Liu, S.; Wang, M.; Hu, H.; Yin, J.; Liu, C.; Huang, Y. Gut Microbiota Dysbiosis Associated with Bile Acid Metabolism in Neonatal Cholestasis Disease. Sci. Rep. 2020, 10, 7686. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Tian, T.; Xie, J.; Wang, X.; Deng, W.; Hao, N.-B.; Li, C. Relationship between Gut Microbiota Dysbiosis and Bile Acid in Patients with Hepatitis B-Induced Cirrhosis. BMC Gastroenterol. 2025, 25, 552. [Google Scholar] [CrossRef]
- Baars, A.M.; Oosting, A.; Lohuis, M.A.M.; Koehorst, M.; Aidy, S.E.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex Differences in Lipid Metabolism Are Affected by Presence of the Gut Microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef]
- Beau, A.; Benoit, B.; Barz, M.L.; Meugnier, E.; Penhoat, A.; Calzada, C.; Pinteur, C.; Loizon, E.; Chanon, S.; Vieille-Marchiset, A.; et al. Inhibition of Intestinal FXR Activity as a Possible Mechanism for the Beneficial Effects of a Probiotic Mix Supplementation on Lipid Metabolism Alterations and Weight Gain in Mice Fed a High Fat Diet. Gut Microbes 2023, 15, 2281015. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velázquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 Cannabinoid Receptor Alters Gut Microbiota and Attenuates Inflammation and Diet-Induced Obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef]
- Iwaza, R.; Wasfy, R.M.; Dubourg, G.; Raoult, D.; Lagier, J. Akkermansia muciniphila: The State of the Art, 18 Years after Its First Discovery. Front. Gastroenterol. 2022, 1, 1024393. [Google Scholar] [CrossRef]
- Luo, Y.; Lan, C.; Li, H.; Ouyang, Q.; Kong, F.; Wu, A.; Ren, Z.; Tian, G.; Cai, J.; Yu, B.; et al. Rational Consideration of Akkermansia muciniphila Targeting Intestinal Health: Advantages and Challenges. npj Biofilm. Microbiomes 2022, 8, 81. [Google Scholar] [CrossRef]
- Hagi, T.; Belzer, C. The Interaction of Akkermansia muciniphila with Host-Derived Substances, Bacteria and Diets. Appl. Microbiol. Biotechnol. 2021, 105, 4833–4841. [Google Scholar] [CrossRef]
- Depommier, C.; Vitale, R.M.; Iannotti, F.A.; Silvestri, C.; Flamand, N.; Druart, C.; Everard, A.; Pelicaen, R.; Maiter, D.; Thissen, J.; et al. Beneficial Effects of Akkermansia Muciniphila Are Not Associated with Major Changes in the Circulating Endocannabinoidome but Linked to Higher Mono-Palmitoyl-Glycerol Levels as New PPARα Agonists. Cells 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia Muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia muciniphila: Key Player in Metabolic and Gastrointestinal Disorders. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8075–8083. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, F.; Sotoodehnejadnematalahi, F.; Hajebrahimi, Z.; Fateh, A.; Siadat, S.D. Effects of Active, Inactive, and Derivatives of Akkermansia muciniphila on the Expression of the Endocannabinoid System and PPARs Genes. Sci. Rep. 2022, 12, 10031. [Google Scholar] [CrossRef]
- Goldsammler, M.; Merhi, Z.; Büyük, E. Role of Hormonal and Inflammatory Alterations in Obesity-Related Reproductive Dysfunction at the Level of the Hypothalamic-Pituitary-Ovarian Axis. Reprod. Biol. Endocrinol. 2018, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.; Cryan, J.F.; Schellekens, H. Gut Peptides and the Microbiome: Focus on Ghrelin. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L. The Microbiome and Risk for Obesity and Diabetes. JAMA 2016, 317, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2011, 61, 364–371. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int. J. Obes. 2014, 39, 424. [Google Scholar] [CrossRef]
- Christiansen, C.; Gabe, M.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The Impact of Short-Chain Fatty Acids on GLP-1 and PYY Secretion from the Isolated Perfused Rat Colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef]
- Rusch, J.; Layden, B.T.; Dugas, L.R. Signalling Cognition: The Gut Microbiota and Hypothalamic-Pituitary-Adrenal Axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Akiba, Y.; Inoue, T.; Kaji, I.; Higashiyama, M.; Narimatsu, K.; Iwamoto, K.; Watanabe, M.; Guth, P.H.; Engel, E.; Kuwahara, A.; et al. Short-chain Fatty Acid Sensing in Rat Duodenum. J. Physiol. 2014, 593, 585–599. [Google Scholar] [CrossRef]
- Abdalqadir, N.; Adeli, K. GLP-1 and GLP-2 Orchestrate Intestine Integrity, Gut Microbiota, and Immune System Crosstalk. Microorganisms 2022, 10, 2061. [Google Scholar] [CrossRef]
- Farzi, A.; Ip, C.K.; Reed, F.; Enriquez, R.F.; Zenz, G.; Durdević, M.; Zhang, L.; Holzer, P.; Herzog, H. Lack of Peptide YY Signaling in Mice Disturbs Gut Microbiome Composition in Response to High-fat Diet. FASEB J. 2021, 35, e21435. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Byndloss, M.X.; Devkota, S.; Duca, F.A.; Niess, J.H.; Nieuwdorp, M.; Orho-Melander, M.; Sanz, Y.; Tremaroli, V.; Zhao, L. The Gut Microbiota and Diabetes: Research, Translation, and Clinical Applications—2023 Diabetes, Diabetes Care, and Diabetologia Expert Forum. Diabetologia 2024, 67, 1760–1782. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Z.; Han, J.; Qian, L.; Wang, W.; Lei, J.; Wang, H. Endocrine, Genetic, and Microbiome Nexus of Obesity and Potential Role of Postbiotics: A Narrative Review. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2023, 28, 84. [Google Scholar] [CrossRef] [PubMed]
- Landgraaf, R.G.; Bloem, M.N.; Fumagalli, M.; Benninga, M.A.; de Lorijn, F.; Nieuwdorp, M. Acupuncture as Multi-Targeted Therapy for the Multifactorial Disease Obesity: A Complex Neuro-Endocrine-Immune Interplay. Front. Endocrinol. 2023, 14, 1236370. [Google Scholar] [CrossRef] [PubMed]
- Gómez, W.A.; Humeres, G.; Orozco, C.A.; Cannataro, R.; Muñoz-Contreras, A.M.; Gómez-Miranda, L.M.; Petro, J.L.; Bonilla, D.A. Leptin Signaling and Its Relationship with Obesity-Induced Insulin Resistance: A Bioinformatics-Assisted Review. Gene Expr. 2025, 24, 56–63. [Google Scholar] [CrossRef]
- Hummel, J.; Benkendorff, C.; Fritsche, L.; Prystupa, K.; Vosseler, A.; Gancheva, S.; Trenkamp, S.; Birkenfeld, A.L.; Preißl, H.; Roden, M.; et al. Brain Insulin Action on Peripheral Insulin Sensitivity in Women Depends on Menstrual Cycle Phase. Nat. Metab. 2023, 5, 1475–1482. [Google Scholar] [CrossRef]
- Badr, M.A.A.; El-Rabaa, G.; Freiha, M.; Kędzia, A.; Niechciał, E. Endocrine Consequences of Childhood Obesity: A Narrative Review. Front. Endocrinol. 2025, 16, 1584861. [Google Scholar] [CrossRef]
- Nonogaki, K. The Regulatory Role of the Central and Peripheral Serotonin Network on Feeding Signals in Metabolic Diseases. Int. J. Mol. Sci. 2022, 23, 1600. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Zhou, Q.; Chen, Y.; Lei, X.; Chen, Y.; Chen, Q. Circulating Acyl and Des-Acyl Ghrelin Levels in Obese Adults: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 2679. [Google Scholar] [CrossRef]
- Nokoff, N.; Thurston, J.; Hilkin, A.; Pyle, L.; Zeitler, P.; Nadeau, K.J.; Santoro, N.; Kelsey, M.M. Sex Differences in Effects of Obesity on Reproductive Hormones and Glucose Metabolism in Early Puberty. J. Clin. Endocrinol. Metab. 2019, 104, 4390–4397. [Google Scholar] [CrossRef]
- Stamatiades, G.A.; Kaiser, U.B. Gonadotropin Regulation by Pulsatile GnRH: Signaling and Gene Expression. Mol. Cell. Endocrinol. 2017, 463, 131–141. [Google Scholar] [CrossRef]
- Stárka, L.; Hill, M.; Pospíšilová, H.; Dušková, M. Estradiol, Obesity and Hypogonadism. Physiol. Res. 2020, 69, S273–S278. [Google Scholar] [CrossRef] [PubMed]
- Mlyczyńska, E.; Kieżun, M.; Kurowska, P.; Dawid, M.; Pich, K.; Respekta, N.; Daudon, M.; Rytelewska, E.; Dobrzyń, K.; Kamińska, B.; et al. New Aspects of Corpus Luteum Regulation in Physiological and Pathological Conditions: Involvement of Adipokines and Neuropeptides. Cells 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Nteeba, J.; Ganesan, S.; Keating, A.F. Progressive Obesity Alters Ovarian Folliculogenesis with Impacts on Pro-Inflammatory and Steroidogenic Signaling in Female Mice1. Biol. Reprod. 2014, 91, 86. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.A.; Ranganathan, P. Emerging Role of Gut Microbiota Dysbiosis in Neuroinflammation and Neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef]
- Upreti, K.; George, J.P.; Upreti, S.; Mahajan, S. Polycystic Ovary Syndrome Diagnosis: The Promise of Artificial Intelligence for Improved Clinical Accuracy. Biomed. Pharmacol. J. 2025, 18, 353–372. [Google Scholar] [CrossRef]
- Thornton, T.; Mills, D.E.; Bliss, E. The Impact of Lipopolysaccharide on Cerebrovascular Function and Cognition Resulting from Obesity-Induced Gut Dysbiosis. Life Sci. 2023, 336, 122337. [Google Scholar] [CrossRef]
- Abildinova, G.; Benberin, V.; Vochshenkova, T.; Afshar, A.; Mussin, N.M.; Kaliyev, A.A.; Zhussupova, Z.; Tamadon, A. The Gut-Brain-Metabolic Axis: Exploring the Role of Microbiota in Insulin Resistance and Cognitive Function. Front. Microbiol. 2024, 15, 1463958. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, H.; Zheng, X. Amino Acids at the Intersection of Nutrition and Insulin Sensitivity. Drug Discov. Today 2019, 24, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pollock, C.A.; Saad, S. Aberrant DNA Methylation Mediates the Transgenerational Risk of Metabolic and Chronic Disease Due to Maternal Obesity and Overnutrition. Genes 2021, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. A Maternal High-Fat Diet Induces DNA Methylation Changes That Contribute to Glucose Intolerance in Offspring. Front. Endocrinol. 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Harmancıoğlu, B.; Kabaran, S. Maternal High Fat Diets: Impacts on Offspring Obesity and Epigenetic Hypothalamic Programming. Front. Genet. 2023, 14, 1158089. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. A Maternal High-Fat Diet Disturbs DNA Methylation Changes That Contribute to Glucose Intolerance in Offspring. Front. Endocrinol. Front. Endocrinol. 2021, 12, 705827. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L.; Liu, J.; Li, Y.; Zhang, J. Long-Term Effects of Maternal Low-Protein Diet and Post-Weaning High-Fat Feeding on Glucose Metabolism and Hypothalamic POMC Promoter Methylation in Offspring Mice. Front. Nutr. 2021, 8, 657848. [Google Scholar] [CrossRef]
- Parrillo, L.; Spinelli, R.; Nicolò, A.; Longo, M.; Mirra, P.; Raciti, G.A.; Miele, C.; Bèguinot, F. Nutritional Factors, DNA Methylation, and Risk of Type 2 Diabetes and Obesity: Perspectives and Challenges. Int. J. Mol. Sci. 2019, 20, 2983. [Google Scholar] [CrossRef]
- Tellechea, M.L.; Mensegue, M.F.; Pirola, C.J. The Association between High Fat Diet around Gestation and Metabolic Syndrome-Related Phenotypes in Rats: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 5086. [Google Scholar] [CrossRef]
- Miyoshi, M.; Saito, K.; Jia, H.; Kato, H. Maternal Protein Restriction and Post-Weaning High-Fat Feeding Alter Plasma Amino Acid Profiles and Hepatic Gene Expression in Mice Offspring. Foods 2022, 11, 753. [Google Scholar] [CrossRef]
- Moody, L.; Shao, J.; Chen, H.; Pan, Y. Maternal Low-Fat Diet Programs the Hepatic Epigenome despite Exposure to an Obesogenic Postnatal Diet. Nutrients 2019, 11, 2075. [Google Scholar] [CrossRef]
- Ren, J.; Cheng, Y.; Ming, Z.; Dong, X.; Zhou, Y.-Z.; Ding, G.; Pang, H.; Rahman, T.U.; Akbar, R.; Huang, H.; et al. Intrauterine Hyperglycemia Exposure Results in Intergenerational Inheritance via DNA Methylation Reprogramming on F1 PGCs. Epigenetics Chromatin 2018, 11, 20. [Google Scholar] [CrossRef]
- Kvaløy, K.; Page, C.M.; Holmen, T.L. Epigenome-Wide Methylation Differences in a Group of Lean and Obese Women—A HUNT Study. Sci. Rep. 2018, 8, 16330. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Dhana, K.; Braun, K.V.E.; Nano, J.; Voortman, T.; Demerath, E.W.; Guan, W.; Fornage, M.; van Meurs, J.B.J.; Uitterlinden, A.G.; Hofman, A.; et al. An Epigenome-Wide Association Study of Obesity-Related Traits. Am. J. Epidemiol. 2018, 187, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, S.J.; Tellam, R.L.; Morrison, J.L.; Mühlhäusler, B.S.; Molloy, P.L. Recent Developments on the Role of Epigenetics in Obesity and Metabolic Disease. Clin. Epigenetics 2015, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Keleher, M.R.; Zaidi, S.N.F.; Hicks, L.E.; Shah, S.; Xing, X.; Li, D.; Wang, T.; Cheverud, J.M. A High-Fat Diet Alters Genome-Wide DNA Methylation and Gene Expression in SM/J Mice. BMC Genom. 2018, 19, 888. [Google Scholar] [CrossRef]
- Wahl, S.; Drong, A.; Lehne, B.; Loh, M.; Scott, W.R.; Kunze, S.; Tsai, P.-C.; Ried, J.S.; Zhang, W.; Yang, Y.; et al. Epigenome-Wide Association Study of Body Mass Index, and the Adverse Outcomes of Adiposity. Nature 2016, 541, 81–86. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, D.; Cline, M.A.; Gilbert, E.R. Chronic Stress, Epigenetics, and Adipose Tissue Metabolism in the Obese State. Nutr. Metab. 2020, 17, 88. [Google Scholar] [CrossRef]
- Ortega, F.; Moreno, M.; Mercader, J.M.; Moreno-Navarrete, J.M.; Fuentes-Batllevell, N.; Sabater, M.; Ricart, W.; Fernández-Real, J.M. Inflammation Triggers Specific microRNA Profiles in Human Adipocytes and Macrophages and in Their Supernatants. Clin. Epigenetics 2015, 7, 49. [Google Scholar] [CrossRef]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ba-Ssalamah, A.; de Gier, C.; Valent, I.; Item, C.B.; Greber-Platzer, S.; Zeyda, M. Circulating microRNAs 34a, 122, and 192 Are Linked to Obesity-Associated Inflammation and Metabolic Disease in Pediatric Patients. Int. J. Obes. 2021, 45, 1763–1772. [Google Scholar] [CrossRef]
- Liu, L.; Li, X. Downregulation of miR-320 Alleviates Endoplasmic Reticulum Stress and Inflammatory Response in 3T3-L1 Adipocytes. Exp. Clin. Endocrinol. Diabetes 2019, 129, 131–137. [Google Scholar] [CrossRef]
- Landrier, J.; Derghal, A.; Mounien, L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells 2019, 8, 859. [Google Scholar] [CrossRef]
- Jiang, X.; Xue, M.; Fu, Z.; Ji, C.; Guo, X.; Zhu, L.; Xu, L.; Pang, L.; Xu, M.; Qu, H. Insight into the Effects of Adipose Tissue Inflammation Factors on miR-378 Expression and the Underlying Mechanism. Cell. Physiol. Biochem. 2014, 33, 1778–1788. [Google Scholar] [CrossRef]
- Miranda, K.; Yang, X.; Bam, M.; Murphy, E.A.; Nagarkatti, P.; Nagarkatti, P. MicroRNA-30 Modulates Metabolic Inflammation by Regulating Notch Signaling in Adipose Tissue Macrophages. Int. J. Obes. 2018, 42, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Leff, T.; Granneman, J.G. Adipose Tissue in Health and Disease; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Youssef, E.M.; Elfiky, A.M.; Soliman, B.; Abu-Shahba, N.; ElHefnawi, M. Expression Profiling and Analysis of Some miRNAs in Subcutaneous White Adipose Tissue during Development of Obesity. Genes Nutr. 2020, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Belmonte, T.; González-Domínguez, R. Childhood Obesity, Metabolic Syndrome, and Oxidative Stress: MicroRNAs Go on Stage. Rev. Endocr. Metab. Disord. 2023, 24, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Siani, A. Role of microRNAs in Obesity and Obesity-Related Diseases. Genes Nutr. 2017, 12, 23. [Google Scholar] [CrossRef]
- Sanada, T.; Sano, T.; Sotomaru, Y.; Alshargabi, R.; Yamawaki, Y.; Yamashita, A.; Matsunaga, H.; Iwashita, M.; Shinjo, T.; Kanematsu, T.; et al. Anti-Inflammatory Effects of miRNA-146a Induced in Adipose and Periodontal Tissues. Biochem. Biophys. Rep. 2020, 22, 100757. [Google Scholar] [CrossRef]
- Zhong, H.; Ma, M.; Liang, T.; Guo, L. Role of MicroRNAs in Obesity-Induced Metabolic Disorder and Immune Response. J. Immunol. Res. 2018, 2018, 2835761. [Google Scholar] [CrossRef]
- Assmann, T.S.; Cuevas-Sierra, A.; Riezu-Boj, J.I.; Milagro, F.I.; Martínéz, J.A. Novel Interactions Between Circulating microRNAs and Gut Microbiota Composition in Human Obesity. Res. Sq. 2020, 21, 9509. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Wang, F.; Tsai, M.; Chen, C.; Lian, W. MicroRNA-29a Compromises Hepatic Adiposis and Gut Dysbiosis in High Fat Diet-Fed Mice via Downregulating Inflammation. Mol. Nutr. Food Res. 2023, 67, 2200348. [Google Scholar] [CrossRef]
- de Proença, L.G.B.; Macedo, A.C.G. Relationship among nutrients, gut microbiota, and microRNAs for healthy weight loss: A systematic review. Int. J. Nutrology 2025, 18, 9509. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, X.; Zeng, S.; He, M.; Xing, X.; Wang, C. miRNA-10a-5p Alleviates Insulin Resistance and Maintains Diurnal Patterns of Triglycerides and Gut Microbiota in High-Fat Diet-Fed Mice. Mediat. Inflamm. 2020, 2020, 8192187. [Google Scholar] [CrossRef]
- Zhang, M. A Review of the Role of Gut Microbiome in Obesity. E3S Web Conf. 2020, 218, 3010. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated with Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2020, 160, 573–599. [Google Scholar] [CrossRef]
- Turpin, T.; Thouvenot, K.; Gonthier, M. Adipokines and Bacterial Metabolites: A Pivotal Molecular Bridge Linking Obesity and Gut Microbiota Dysbiosis to Target. Biomolecules 2023, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Banerjee, D.; Ramprasad, P.; Roy, S.; Pal, D.; Dasgupta, S. Recent Insights of Obesity-Induced Gut and Adipose Tissue Dysbiosis in Type 2 Diabetes. Front. Mol. Biosci. 2023, 10, 1224982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, M.; Wang, M.C.; Gao, Y.; Zhang, C.; Liu, S.; Qu, S.; Liu, Z.; Zhang, C. Integrated Multiomic Analysis Reveals the High-Fat Diet Induced Activation of the MAPK Signaling and Inflammation Associated Metabolic Cascades via Histone Modification in Adipose Tissues. Front. Genet. 2021, 12, 650863. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleky, H.M.; Zhou, J. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Whitt, J.; Woo, V.; Lee, P.; Moncivaiz, J.; Haberman, Y.; Denson, L.A.; Tso, P.; Alenghat, T. Disruption of Epithelial HDAC3 in Intestine Prevents Diet-Induced Obesity in Mice. Gastroenterology 2018, 155, 501–513. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-Chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Qin, Y.; Roberts, J.D.; Grimm, S.A.; Lih, F.B.; Deterding, L.J.; Li, R.; Chrysovergis, K.; Wade, P.A. An Obesity-Associated Gut Microbiome Reprograms the Intestinal Epigenome and Leads to Altered Colonic Gene Expression. Genome Biol. 2018, 19, 7. [Google Scholar] [CrossRef]
- Chiodi, V.; Rappa, F.; Re, O.L.; Chaldakov, G.N.; Lelouvier, B.; Micale, V.; Domenici, M.R.; Vinciguerra, M. Deficiency of Histone Variant macroH2A1.1 Is Associated with Sexually Dimorphic Obesity in Mice. Sci. Rep. 2023, 13, 19123. [Google Scholar] [CrossRef]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef]
- Hauck, A.K.; Zhou, T.; Upadhyay, A.; Sun, Y.; O’Connor, M.B.; Chen, Y.; Bernlohr, D. Histone Carbonylation Is a Redox-Regulated Epigenomic Mark That Accumulates with Obesity and Aging. Antioxidants 2020, 9, 1210. [Google Scholar] [CrossRef]
- Gallardo-Escribano, C.; Buonaiuto, V.A.; Ruiz-Moreno, M.I.; Vargas-Candela, A.; Vilches-Perez, A.; Benítez-Porres, J.; Romance-García, Á.R.; Ruiz-Moreno, A.; Gómez-Huelgas, R.; Bernal-López, M.R. Epigenetic Approach in Obesity: DNA Methylation in a Prepubertal Population Which Underwent a Lifestyle Modification. Clin. Epigenetics 2020, 12, 144. [Google Scholar] [CrossRef]
- Schones, D.E.; Leung, A.; Natarajan, R. Chromatin Modifications Associated With Diabetes and Obesity. Arter. Thromb. Vasc. Biol. 2015, 35, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.N.L.; Chandanathil, M.; Millis, R.M. Epigenetic Mechanisms in Obesity: Broadening Our Understanding of the Disease. Cureus 2023, 15, e47875. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. A Reversible Epigenetic Link between Obesity and Cancer Risk. Trends Endocrinol. Metab. 2018, 29, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cirera, S.; Taşöz, E.; Liu, Y.; Olsen, L.H.; Christoffersen, B.Ø.; Pedersen, H.D.; Ludvigsen, T.P.; Kirk, R.K.; Schumacher-Petersen, C.; et al. Diet-Dependent Changes of the DNA Methylome Using a Göttingen Minipig Model for Obesity. Front. Genet. 2021, 12, 632859. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Maccani, J.Z.J.; Hawley, N.L.; Wing, R.; Kelsey, K.T.; McCaffery, J.M. Epigenetic Patterns in Successful Weight Loss Maintainers: A Pilot Study. Int. J. Obes. 2014, 39, 865–868. [Google Scholar] [CrossRef] [PubMed]
- ElGendy, K.; Malcomson, F.C.; Bradburn, D.M.; Mathers, J.C. Effects of Bariatric Surgery on DNA Methylation in Adults: A Systematic Review and Meta-Analysis. Surg. Obes. Relat. Dis. 2019, 16, 128–136. [Google Scholar] [CrossRef]
- Gancheva, S.; Ouni, M.; Jeleník, T.; Koliaki, C.; Szendroedi, J.; Toledo, F.G.S.; Markgraf, D.F.; Pesta, D.; Mastrototaro, L.; Filippo, E.D.; et al. Dynamic Changes of Muscle Insulin Sensitivity after Metabolic Surgery. Nat. Commun. 2019, 10, 4179. [Google Scholar] [CrossRef]
- Toro-Martin, J.; Guenard, F.; Tchernof, A.; Deshaies, Y.; Marceau, P.; Vohl, M.C. Bariatric Surgery Induces Hypomethylation of Genes Related to Type 2 Diabetes and Insulin Resistance. Int. J. Mol. Biol. 2017, 2, 00020. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Díaz-Lagares, Á. DNA Methylation in Obesity and Associated Diseases. In Elsevier eBooks; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 313–329. [Google Scholar] [CrossRef]
- Campbell, I.C.; Mill, J.; Uher, R.; Schmidt, U. Eatig Disorders, Gene–Environment Interactions and Epigenetics. Neurosci. Biobehav. Rev. 2010, 35, 784–793. [Google Scholar] [CrossRef]
- Aurich, S.; Müller, L.; Kovács, P.; Keller, M. Implication of DNA Methylation during Lifestyle Mediated Weight Loss. Front. Endocrinol. 2023, 14, 1181002. [Google Scholar] [CrossRef]
- Keller, M.; Meir, A.Y.; Wolf, S.; Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Tsaban, G.; Zelicha, H.; Hopp, L.; Müller, L.; et al. DNA Methylation Signature in Blood Mirrors Successful Weight-Loss during Lifestyle Interventions: The CENTRAL Trial. Genome Med. 2020, 12, 97. [Google Scholar] [CrossRef]
- Šket, R.; Slapnik, B.; Kotnik, P.; Črepinšek, K.; Kern, B.Č.; Tesovnik, T.; Bizjan, B.J.; Vrhovšek, B.; Remec, Ž.I.; Debeljak, M.; et al. Integrating Genetic Insights, Technological Advancements, Screening, and Personalized Pharmacological Interventions in Childhood Obesity. Adv. Ther. 2025, 2, 72–93. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Chen, F.; Zhou, B. Epigenetics in Obesity: Mechanisms and Advances in Therapies Based on Natural Products. Pharmacol. Res. Perspect. 2024, 12, e1171. [Google Scholar] [CrossRef]
- Müller, M.; Geisler, C. EDITORIAL Defining Obesity as a Disease. Eur. J. Clin. Nutr. 2017, 71, 1256–1258. [Google Scholar] [CrossRef]
- Faeza, M.; Benincasa, G.; Docimo, L.; Nicoletti, G.F.; Napolli, C. Clinical epigenetics and restoring of metabolic health in severely obese patients undergoing batriatric and metabolic surgery. Updates Surg. 2021, 74, 431–438. [Google Scholar] [CrossRef]
- Gawlik, A.; Salonen, A.; Jian, C.; Yanover, C.; Antosz, A.; Shmoish, M.; Wasniewska, M.; Bereket, A.; Wudy, S.A.; Hartmann, M.F.; et al. Personalized Approach to Childhood Obesity: Lessons from Gut Microbiota and Omics Studies. Narrative Review and Insights from the 29th European Childhood Obesity Congress. Pediatr. Obes. 2021, 16, e12835. [Google Scholar] [CrossRef]
- Strzelec, M.; Detka, J.; Mieszczak, P.; Sobocińska, M.; Majka, M. Immunomodulation—A General Review of the Current State-of-the-Art and New Therapeutic Strategies for Targeting the Immune System. Front. Immunol. 2023, 14, 1127704. [Google Scholar] [CrossRef]
- Nascimento, S.S.d.C.; de Queiroz, J.L.C.; de Medeiros, A.F.; de Nunes, A.C.F.; Piuvezam, G.; Maciel, B.L.L.; Passos, T.S.; de Morais, A.H.A. Anti-Inflammatory Agents as Modulators of the Inflammation in Adipose Tissue: A Systematic Review. PLoS ONE 2022, 17, e0273942. [Google Scholar] [CrossRef]
- Claycombe-Larson, K.; Alvine, T.; Wu, D.; Kalupahana, N.S.; Moustaïd-Moussa, N.; Roemmich, J.N. Nutrients and Immunometabolism: Role of Macrophage NLRP3. J. Nutr. 2020, 150, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.; Starodovna, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D. Personalized Medicine and Molecular Diagnostics for Obesity: Metabolic Systems Reconstruction and Gut Microbiome Biomarkers. Curr. Pharmacogenomics Pers. Med. 2011, 9, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M. Impact of the Gut Microbiota on Inflammation, Obesity, and Metabolic Disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2022, 38, 42–50. [Google Scholar] [CrossRef]
- Mehta, N.H.; Huey, S.L.; Kuriyan, R.; Peña-Rosas, J.P.; Finkelstein, J.L.; Kashyap, S.R.; Mehta, S. Potential Mechanisms of Precision Nutrition-Based Interventions for Managing Obesity. Adv. Nutr. 2024, 15, 100186. [Google Scholar] [CrossRef]
- León-Mimila, P.; Wang, J.; Huertas-Vázquez, A. Relevance of Multi-Omics Studies in Cardiovascular Diseases. Front. Cardiovasc. Med. 2019, 6, 91. [Google Scholar] [CrossRef]
- Lin, Z.; Feng, W.; Liu, Y.; Ma, C.; Arefan, D.; Zhou, D.; Cheng, X.; Yu, J.; Gao, L.; Du, L.; et al. Machine Learning to Identify Metabolic Subtypes of Obesity: A Multi-Center Study. Front. Endocrinol. 2021, 12, 713592. [Google Scholar] [CrossRef]
- Pérez-Campos, E.; Mayoral, L.P.; Andrade, G.; Mayoral, E.-C.; Hernández-Huerta, M.T.; Canseco, S.; Canales, F.R.; Cabrera-Fuentes, H.; Cruz, M.; Pérez- Santiago, A.D.; et al. Obesity Subtypes, Related Biomarkers & Heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef]
- Yang, C.-H.; Fagnocchi, L.; Apostle, S.; Wegert, V.; Casaní-Galdón, S.; Landgraf, K.; Panzeri, I.; Dror, E.; Heyne, S.; Wörpel, T.; et al. Independent Phenotypic Plasticity Axes Define Distinct Obesity Sub-Types. Nat. Metab. 2022, 4, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Qi, L. Gene-Diet Interaction and Precision Nutrition in Obesity. Int. J. Mol. Sci. 2017, 18, 787. [Google Scholar] [CrossRef] [PubMed]
- Pardali, E.C.; Cholevas, C.; Androutsos, O.; Tsigalou, C.; Poulimeneas, D.; Bogdanos, D.P.; Dalamaga, Μ.; Goulis, D.G.; Grammatikopoulou, M.G. Interventional Approaches to Combat Obesity: Exploring the Metabolomic Signature of Weight Loss Trials. Metab. Open 2025, 27, 100373. [Google Scholar] [CrossRef]
- Watanabe, K.; Wilmanski, T.; Diener, C.; Earls, J.C.; Zimmer, A.; Lincoln, B.; Hadlock, J.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T.; et al. Multiomic Signatures of Body Mass Index Identify Heterogeneous Health Phenotypes and Responses to a Lifestyle Intervention. Nat. Med. 2023, 29, 996–1008. [Google Scholar] [CrossRef]
- Gazan, R.; Barré, T.; Pérignon, M.; Maillot, M.; Soler, L.G.; Darmon, N.; Vieux, F. Validation of an Aggregated Food Dataset Based on Nutritional Quality-Prices Relationships. Ann. Nutr. Metab. 2015, 67 (Suppl. S1), 1–601. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Rakıcıoğlu, N. The Future of Obesity Management through Precision Nutrition: Putting the Individual at the Center. Curr. Nutr. Rep. 2024, 13, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.M. Nutrigenomics—New Approaches for Human Nutrition Research. J. Sci. Food Agric. 2006, 86, 1156–1163. [Google Scholar] [CrossRef]
- Keijer, J.; Escoté, X.; Galmés, S.; Palou-March, A.; Serra, F.; Aldubayan, M.A.; Pigsborg, K.; Magkos, F.; Baker, E.J.; Calder, P.C.; et al. Omics Biomarkers and an Approach for Their Practical Implementation to Delineate Health Status for Personalized Nutrition Strategies. Crit. Rev. Food Sci. Nutr. 2023, 64, 8279–8307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Zou, X.; Chai, Y.; Yao, Y. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Henninger, A.M.J.; Eliasson, B.; Jenndahl, L.; Hammarstedt, A. Adipocyte Hypertrophy, Inflammation and Fibrosis Characterize Subcutaneous Adipose Tissue of Healthy, Non-Obese Subjects Predisposed to Type 2 Diabetes. PLoS ONE 2014, 9, e105262. [Google Scholar] [CrossRef]
- Roberts, J.; Fallon, P.G.; Hams, E. The Pivotal Role of Macrophages in Metabolic Distress. In IntechOpen eBooks; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Cho, Y.K.; Lee, S.; Lee, J.R.; Doh, J.; Park, J.; Jung, Y.; Lee, Y. Lipid Remodeling of Adipose Tissue in Metabolic Health and Disease. Exp. Mol. Med. 2023, 55, 1955–1973. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Prebiotic Fiber Modulation of the Gut Microbiota Improves Risk Factors for Obesity and the Metabolic Syndrome. Gut Microbes 2012, 3, 29–34. [Google Scholar] [CrossRef]
- Ferrarese, R.; Ceresola, E.R.; Preti, A.; Canducci, F. Probiotics, Prebiotics and Synbiotics for Weight Loss and Metabolic Syndrome in the Microbiome Era. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7588–7605. [Google Scholar] [CrossRef]
- Mocanu, V.; Zhang, Z.; Deehan, E.C.; Kao, D.; Hotte, N.; Karmali, S.; Birch, D.W.; Samarasinghe, K.K.; Walter, J.; Madsen, K. Fecal Microbial Transplantation and Fiber Supplementation in Patients with Severe Obesity and Metabolic Syndrome: A Randomized Double-Blind, Placebo-Controlled Phase 2 Trial. Nat. Med. 2021, 27, 1272–1279. [Google Scholar] [CrossRef]
- Zhang, F.; Zuo, T.; Wan, Y.; Xu, Z.; Cheung, C.; Li, A.Y.; Zhu, W.; Tang, W.; Chan, P.K.S.; Chan, F.K.L.; et al. Mul-ti-Omic Analyses Identify Mucosa Bacteria and Fecal Metabolites Associated with Weight Loss after Fecal Micro-biota Transplantation. Innov. 2022, 3, 100304. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral Administration of Blautia Wexlerae Ameliorates Obesity and Type 2 Diabetes via Metabolic Remodeling of the Gut Microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef] [PubMed]
- Woldemariam, S.; Dorner, T.E.; Wiesinger, T.; Stein, K.V. Multi-Omics Approaches for Precision Obesity Management. Wien. Klin. Wochenschr. 2023, 135, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Siebert, J.; Stanislawski, M.A.; Zaman, A.; Ostendorf, D.M.; Konigsberg, I.R.; Jambal, P.; Ir, D.; Bing, K.; Wayland, L.; Scorsone, J.J.; et al. Multiomic Predictors of Short-Term Weight Loss and Clinical Outcomes During a Behavioral-Based Weight Loss Intervention. Obesity 2021, 29, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Kanameni, S.; Chang, R.; Nair, V.; Safe, S.; Bazer, F.W.; Zhou, B. Interferon Tau Alleviates Obesity-Induced Adipose Tissue Inflammation and Insulin Resistance by Regulating Macrophage Polarization. PLoS ONE 2014, 9, e98835. [Google Scholar] [CrossRef]
- Chylíková, J.; Dvořáčková, J.; Tauber, Z.; Kamarád, V. M1/M2 Macrophage Polarization in Human Obese Adipose Tissue. Biomed. Pap. 2018, 162, 79–82. [Google Scholar] [CrossRef]
- Ma, C.; Jian, C.; Guo, L.; Li, W.; Zhang, C.; Wang, L.; Yuan, M.; Zhang, P.; Dong, J.; He, P.; et al. Adipose Tissue Targeting Ultra-Small Hybrid Nanoparticles for Synergistic Photodynamic Therapy and Browning Induction in Obesity Treatment. Small 2023, 20, 2308962. [Google Scholar] [CrossRef]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Styne, D.M.; Arslanian, S.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.; Yanovski, J.A. Pediatric Obesity—Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Javed, A.; Jumean, M.; Murad, M.H.; Okorodudu, D.; Kumar, S.; Somers, V.K.; Sochor, O.; López-Jimenez, F. Diagnostic Performance of Body Mass Index to Identify Obesity as Defined by Body Adiposity in Children and Adolescents: A Systematic Review and Meta-analysis. Pediatr. Obes. 2014, 10, 234–244. [Google Scholar] [CrossRef]
- Hampl, S.E.; Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Avila Edwards, K.C.; Eneli, I.; Hamre, R.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef]
- Müller, M.J. From BMI to Functional Body Composition. Eur. J. Clin. Nutr. 2013, 67, 1119–1121. [Google Scholar] [CrossRef]
- Steinbeck, K.; Lister, N.B.; Gow, M.L.; Baur, L.A. Treatment of Adolescent Obesity. Nat. Rev. Endocrinol. 2018, 14, 331–344. [Google Scholar] [CrossRef]
- Tarabra, E.; Nouws, J.; Vash-Margita, A.; Nadzam, G.; Goldberg, R.; Name, M.V.; Pierpont, B.; Knight, J.; Shulman, G.I.; Caprio, S. The Omentum of Obese Girls Harbors Small Adipocytes and Browning Transcripts. JCI Insight 2020, 5, e135448. [Google Scholar] [CrossRef]
- Thornton, P.; Reader, V.; Digby, Z.; Smolak, P.; Lindsay, N.; Harrison, D.; Clarke, N.; Watt, A. Reversal of High Fat Diet-Induced Obesity, Systemic Inflammation, and Astrogliosis by the NLRP3 Inflammasome Inhibitors NT-0249 and NT-0796S. J. Pharmacol. Exp. Ther. 2024, 388, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Tee, C.C.L.; Cooke, M.B.; Chong, M.C.; Yeo, W.K.; Camera, D.M. Mechanisms for Combined Hypoxic Conditioning and Divergent Exercise Modes to Regulate Inflammation, Body Composition, Appetite, and Blood Glucose Homeostasis in Overweight and Obese Adults: A Narrative Review. Sports Med. 2022, 53, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qian, H.; Xin, X.; Liu, Q. Effects of Different Exercise Modalities on Inflammatory Markers in the Obese and Overweight Populations: Unraveling the Mystery of Exercise and Inflammation. Front. Physiol. 2024, 15, 1405094. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, H.; Liu, T.; Wang, R. Exercise Alleviates Aging of Adipose Tissue through Adipokine Regulation. Metabolites 2024, 14, 135. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, X. Anti-Inflammatory Effect of Exercise Training through Reducing Inflammasome Activation-Related Inflammatory Cytokine Levels in Overweight/Obese Populations: A Systematic Review and Meta-Analysis. Complement. Ther. Clin. Pract. 2022, 49, 101656. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, A.S.; Cardona, A.E. The IL-1β Phenomena in Neuroinflammatory Diseases. J. Neural Transm. 2017, 125, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The Role of Interleukin-1 in General Pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Sarkar, C.; Rawat, V.S.; Kalita, D.; Deka, S.; Agnihotri, A. Promise of the NLRP3 Inflammasome Inhibitors in In Vivo Disease Models. Molecules 2021, 26, 4996. [Google Scholar] [CrossRef]
- Sharma, A.; Choi, J.S.Y.; Stefanovic, N.; Al-Sharea, A.; Simpson, D.S.; Mukhamedova, N.; Jandeleit-Dahm, K.; Murphy, A.; Sviridov, D.; Vince, J.E.; et al. Specific NLRP3 Inhibition Protects Against Diabetes-Associated Atherosclerosis. Diabetes 2020, 70, 772–787. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Shoelson, S.E. Therapeutic Approaches Targeting Inflammation for Diabetes and Associated Cardiovascular Risk. J. Clin. Investig. 2017, 127, 83–93. [Google Scholar] [CrossRef]
- Li, D.; Zhong, J.; Zhang, Q.; Zhang, J. Effects of Anti-Inflammatory Therapies on Glycemic Control in Type 2 Diabetes Mellitus. Front. Immunol. 2023, 14, 1125116. [Google Scholar] [CrossRef]
- Alfadul, H.; Sabico, S.; Al-Daghri, N.M. The Role of Interleukin-1β in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 901616. [Google Scholar] [CrossRef]
- Reilly, S.M.; Chiang, S.-H.; Decker, S.J.; Chang, L.; Uhm, M.; Larsen, M.J.; Rubin, J.R.; Mowers, J.; White, N.M.; Hochberg, I.; et al. An Inhibitor of the Protein Kinases TBK1 and IKK-ɛ Improves Obesity-Related Metabolic Dysfunctions in Mice. Nat. Med. 2013, 19, 313–321. [Google Scholar] [CrossRef]
- Rafey, M.; Fang, C.E.H.; Ioana, I.; Griffin, H.; Hynes, M.L.; O’Brien, T.; McAnena, O.J.; O’Shea, P.; Collins, C.; Davenport, C.; et al. The Leptin to Adiponectin Ratio (LAR) Is Reduced by Sleeve Gastrectomy in Adults with Severe Obesity: A Prospective Cohort Study. Sci. Rep. 2020, 10, 16270. [Google Scholar] [CrossRef]
- Ferraz-Bannitz, R.; Welendorf, C.R.; Coelho, P.O.; Salgado, W.; Nonino, C.B.; Beraldo, R.A.; Foss-Freitas, M.C. Bariatric Surgery Can Acutely Modulate ER-Stress and Inflammation on Subcutaneous Adipose Tissue in Non-Diabetic Patients with Obesity. Diabetol. Metab. Syndr. 2021, 13, 19. [Google Scholar] [CrossRef]
- Camastra, S.; Vitali, A.; Anselmino, M.; Gastaldelli, A.; Bellini, R.; Berta, R.; Severi, I.; Baldi, S.; Astiarraga, B.; Barbatelli, G.; et al. Muscle and Adipose Tissue Morphology, Insulin Sensitivity and Beta-Cell Function in Diabetic and Nondiabetic Obese Patients: Effects of Bariatric Surgery. Sci. Rep. 2017, 7, 9007. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Ramanjaneya, M.; Moin, A.S.M.; Hunt, S.C.; Atkin, S.L. Clinical Improvement May Not Reflect Metabolic Homeostasis Normalization in Subjects with and without Roux-En-Y Bariatric Surgery after 12 Years: Comparison of Surgical Subjects to a Lean Cohort. Front. Endocrinol. 2023, 14, 1228853. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, H.; Han, B.; Zhang, X. Mechanisms through Which Laparoscopic Sleeve Gastrectomy Mitigates Atherosclerosis Risk: A Focus on Visceral Adipose Tissue. Eur. J. Med. Res. 2025, 30, 370. [Google Scholar] [CrossRef] [PubMed]
- Agareva, M.; Michurina, S.; Tomilova, A.; Shestakova, E.; Voznesenskaya, A.A.; Sineokaya, M.; Zubkova, E.; Ratner, E.I.; Stafeev, I.; Parfyonova, Y.; et al. Incretin-Based Approaches for Type 2 Diabetes Therapy: Effects on Circulating Cytokines and Adipocyte’s Secretome. BMC Endocr. Disord. 2025, 25, 182. [Google Scholar] [CrossRef]
- Dadson, P.; Honka, M.; Suomi, T.; Haridas, P.A.N.; Rokka, A.; Palani, S.; Goltseva, E.; Wang, N.; Roivainen, A.; Salminen, P.; et al. Proteomic Profiling Reveals Alterations in Metabolic and Cellular Pathways in Severe Obesity and Following Metabolic Bariatric Surgery. Am. J. Physiol.-Endocrinol. Metab. 2025, 328, E311–E324. [Google Scholar] [CrossRef]
- Lobato, C.B.; Pereira, S.S.; Guimarães, M.; Soares, B.; Hartmann, B.; Nora, M.; Holst, J.J.; Monteiro, M.P. Glycemic Variability and Entero-Pancreatic Hormones Signatures after Different Bariatric Surgery Procedures: A Cross-Sectional Study. Int. J. Obes. 2025, 49, 2042–2050. [Google Scholar] [CrossRef]
- Maltais-Payette, I.; Lajeunesse-Trempe, F.; Nadeau, M.; Bouvet-Bouchard, L.; Hould, F.; Biertho, L.; Tchernof, A. Circulating Amino Acid Changes Three Years After Bariatric Surgery. Metabolites 2025, 15, 297. [Google Scholar] [CrossRef]
- Salehi, M.; D’Alessio, D.A. Effects of Glucagon like Peptide-1 to Mediate Glycemic Effects of Weight Loss Surgery. Rev. Endocr. Metab. Disord. 2014, 15, 171–179. [Google Scholar] [CrossRef]
- Brzozowska, M.M.; Isaacs, M.; Bliuc, D.; Baldock, P.A.; Eisman, J.A.; White, C.; Greenfield, J.R.; Center, J.R. Effects of Bariatric Surgery and Dietary Intervention on Insulin Resistance and Appetite Hormones over a 3 Year Period. Sci. Rep. 2023, 13, 6032. [Google Scholar] [CrossRef]
- Patti, M.; Goldfine, A.B. Hypoglycemia After Gastric Bypass: The Dark Side of GLP-1. Gastroenterology 2014, 146, 605–608. [Google Scholar] [CrossRef]
- Valenzano, A.; Tartaglia, N.; Ambrosi, A.; Tafuri, D.; Monda, M.; Messina, A.; Sessa, F.; Campanozzi, A.; Monda, V.; Cibelli, G.; et al. The Metabolic Rearrangements of Bariatric Surgery: Focus on Orexin-A and the Adiponectin System. J. Clin. Med. 2020, 9, 3327. [Google Scholar] [CrossRef]
- Arterburn, D.; Courcoulas, A.P. Bariatric Surgery for Obesity and Metabolic Conditions in Adults. BMJ 2014, 349, g3961. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of Obesity: An Update on the Available Medications and Drugs under Investigation. eClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Bain, S.C.; Kanamarlapudi, V. The Regulation of Metabolic Homeostasis by Incretins and the Metabolic Hormones Produced by Pancreatic Islets. Diabetes Metab. Syndr. Obes. 2024, 17, 2419–2456. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, N.; Xu, L.; Ping, F.; Li, W.; Li, Y.; Zhang, H. Subpopulation Differences in the Cardiovascular Efficacy of Long-Acting Glucagon-Like Peptide 1 Receptor Agonists in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Ther. 2020, 11, 2121–2143. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.; Nesti, L.; Tricò, D.; Ferrannini, E. Effects of GLP-1 Receptor Agonists and SGLT-2 Inhibitors on Cardiac Structure and Function: A Narrative Review of Clinical Evidence. Cardiovasc. Diabetol. 2021, 20, 196. [Google Scholar] [CrossRef]
- Spósito, A.C.; Berwanger, O.; de Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1RAs in Type 2 Diabetes: Mechanisms That Underlie Cardiovascular Effects and Overview of Cardiovascular Outcome Data. Cardiovasc. Diabetol. 2018, 17, 157, Correction in Cardiovasc. Diabetol. 2019, 18, 23.. [Google Scholar] [CrossRef]
- Varnum, A.A.; Pozzi, E.; Deebel, N.A.; Evans, A.; Eid, N.; Sadeghi-Nejad, H.; Ramasamy, R. Impact of GLP-1 Agonists on Male Reproductive Health—A Narrative Review. Medicina 2023, 60, 50. [Google Scholar] [CrossRef]
- Lisco, G.; Bartolomeo, N.; Tullio, A.D.; Pergola, G.D.; Guastamacchia, E.; Jirillo, E.; Piazzolla, G.; Triggiani, V.; Giagulli, V.A. Long-acting Glucagon-like Peptide 1 Receptor Agonists Boost Erectile Function in Men with Type 2 Diabetes Mellitus Complaining of Erectile Dysfunction: A Retrospective Cohort Study. Andrology 2023, 12, 633–642. [Google Scholar] [CrossRef]
- Izzi-Engbeaya, C.; Jones, S.; Crustna, Y.; Machenahalli, P.; Papadopoulou, D.; Modi, M.; Panayi, C.; Starikova, J.; Eng, P.C.; Phylactou, M.; et al. Effects of Glucagon-like Peptide-1 on the Reproductive Axis in Healthy Men. J. Clin. Endocrinol. Metab. 2020, 105, 1119–1125. [Google Scholar] [CrossRef]
- Gargiulo, P.; Savarese, G.; D’Amore, C.; Martino, F.D.; Lund, L.H.; Marsico, F.; Dellegrottaglie, S.; Marciano, C.; Trimarco, B.; Filardi, P.P. Efficacy and Safety of Glucagon-like Peptide-1 Agonists on Macrovascular and Microvascular Events in Type 2 Diabetes Mellitus: A Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1081–1088. [Google Scholar] [CrossRef]
- Monami, M.; Zannoni, S.; Pala, L.; Silverii, G.A.; Andreozzi, F.; Sesti, G.; Mannucci, E. Effects of Glucagon-like Peptide-1 Receptor Agonists on Mortality and Cardiovascular Events: A Comprehensive Meta-Analysis of Randomized Controlled Trials. Int. J. Cardiol. 2017, 240, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E.; Banerji, M.A. Non-Insulin Injectable Treatments (Glucagon-Like Peptide-1 and Its Analogs) and Cardiovascular Disease. Diabetes Technol. Ther. 2012, 14, S43. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 Receptor Agonists (GLP-1RAs): Cardiovascular Actions and Therapeutic Potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.; Prato, S.D.; Khurmi, N.S.; Lam, C.S.P.; Lópes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Koliaki, C.; Doupis, J. Incretin-Based Therapy: A Powerful and Promising Weapon in the Treatment of Type 2 Diabetes Mellitus. Diabetes Ther. 2011, 2, 101–121. [Google Scholar] [CrossRef]
- Guglielmi, V.; Bettini, S.; Sbraccia, P.; Busetto, L.; Pellegrini, M.; Yumuk, V.; Colao, A.; Ghoch, M.E.; Muscogiuri, G. Beyond Weight Loss: Added Benefits Could Guide the Choice of Anti-Obesity Medications. Curr. Obes. Rep. 2023, 12, 127–146. [Google Scholar] [CrossRef]
- AlZaim, I.; Hammoud, S.H.; Al-Koussa, H.; Ghazi, A.; Eid, A.H.; El-Yazbi, A.F. Adipose Tissue Immunomodulation: A Novel Therapeutic Approach in Cardiovascular and Metabolic Diseases. Front. Cardiovasc. Med. 2020, 7, 602088. [Google Scholar] [CrossRef]
- Blumberg, B.; Chang, R.; Egusquiza, R.J.; Amato, A.A.; Li, Z.; Joloya, E.M.; Wheeler, H.B.; Nguyen, A.; Shioda, K.; Odajima, J.; et al. Heritable Changes in Chromatin Contacts Linked to Transgenerational Obesity. Res. Sq. 2023, rs.3, rs-3570919. [Google Scholar] [CrossRef]
- Pinel, A.; Rigaudière, J.; Morio, B.; Capel, F. Adipose Tissue Dysfunctions in Response to an Obesogenic Diet Are Reduced in Mice after Transgenerational Supplementation with Omega 3 Fatty Acids. Metabolites 2021, 11, 838. [Google Scholar] [CrossRef]
- Ruden, D.M.; Singh, A.; Rappolee, D.A. Pathological Epigenetic Events and Reversibility Review: The Intersection between Hallmarks of Aging and Developmental Origin of Health and Disease. Epigenomics 2023, 15, 741–754. [Google Scholar] [CrossRef]
- Abraham, M.J.; Sherbini, A.E.; El-Diasty, M.; Askari, S.; Szewczuk, M.R. Restoring Epigenetic Reprogramming with Diet and Exercise to Improve Health-Related Metabolic Diseases. Biomolecules 2023, 13, 318. [Google Scholar] [CrossRef]
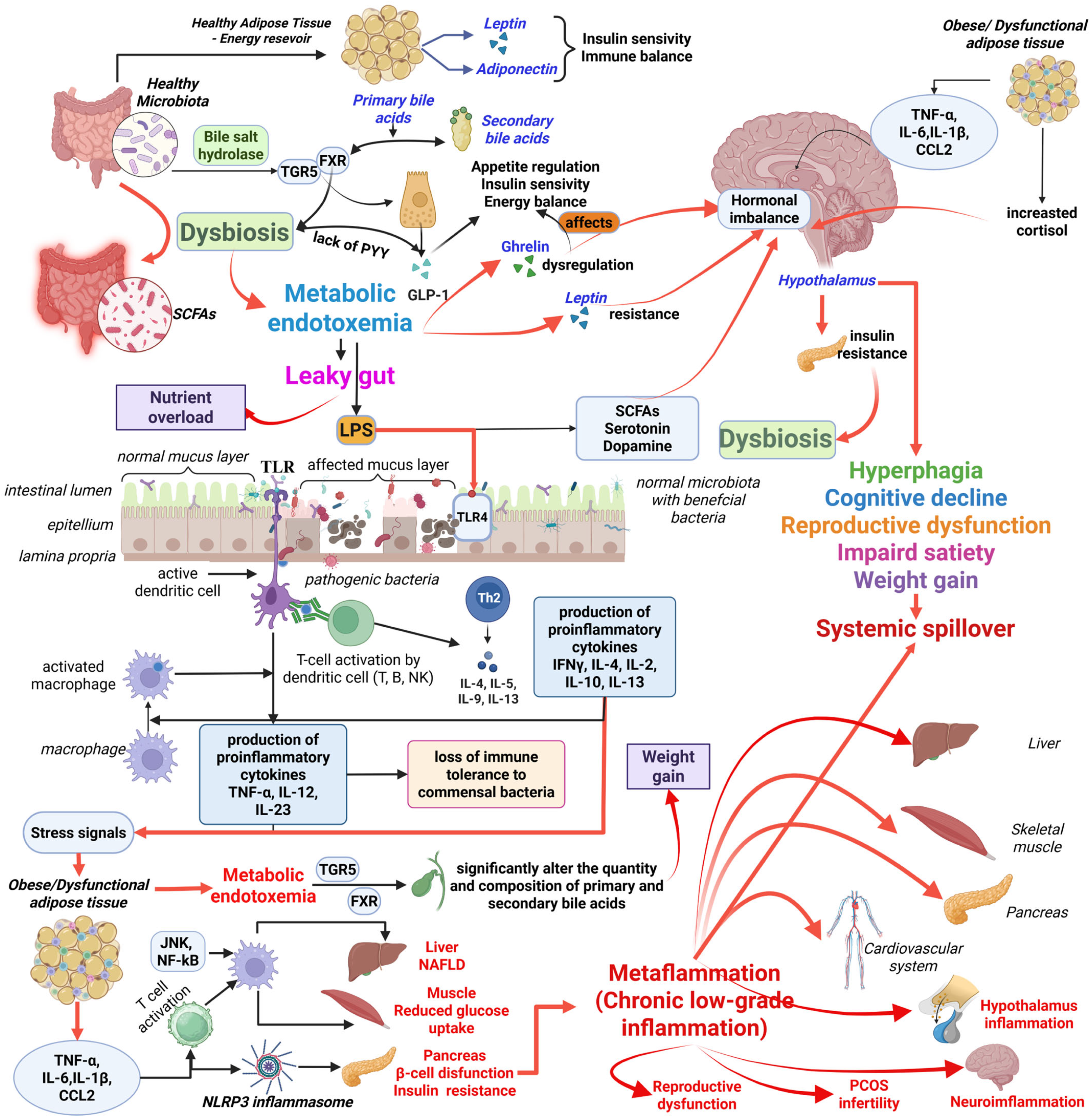
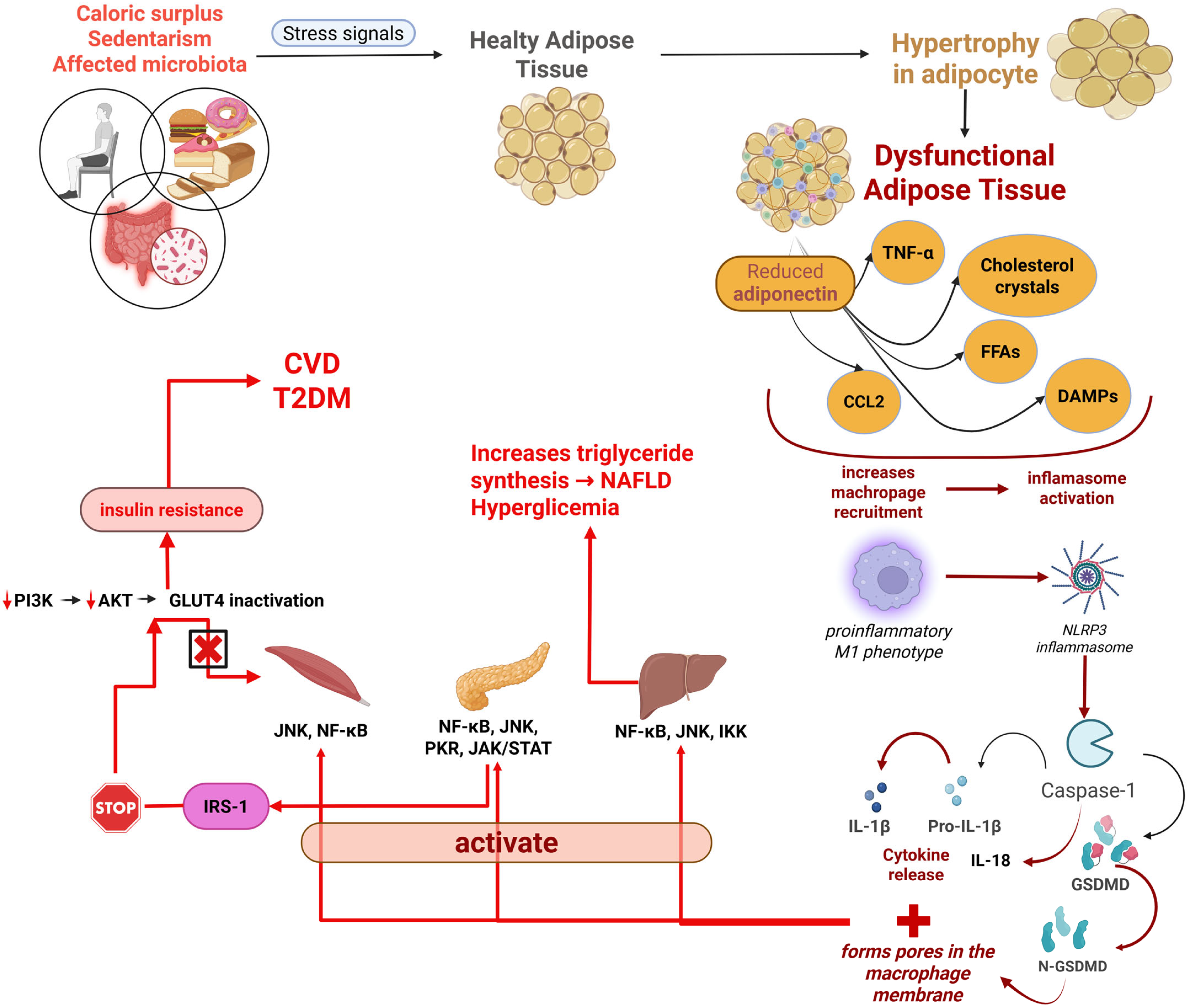

| Affected System | Main Mechanism | Associated Effects | Experimental Model | Principal Findings |
|---|---|---|---|---|
| Neuroendocrine | Leptin resistance (impaired transport across blood–brain barrier (BBB), defective receptor signalling, suppressor of cytokine signalling 3 (SOCS3) activation, hypothalamic inflammation (microglial activation, astrogliosis, cytokine secretion), ghrelin dysregulation (decreased active acyl-ghrelin), hormonal imbalances (e.g., cortisol elevation, altered adipokines), impaired glucose-sensing in hypothalamus, altered neuroendocrine axes [17,19] | Appetite dysregulation (hyperphagia), impaired satiety signalling, reduced fertility (anovulation, impaired spermatogenesis) [20,21,22], hypogonadism [23], disrupted pubertal timing, increased central fat accumulation, PCOS, impaired glucose homeostasis [24,25], altered energy expenditure [26,27], cognitive and mood disorders [28] | Cohort study of 99 obese subjects undergoing Roux-en-Y gastric bypass surgery [29]. | The study by Ekberg et al. [29] aimed to identify and compare clinical biomarkers for insulin sensitivity and other parameters in predicting the normalisation of HbA1c after RYGB surgery in subjects with abnormal glucose levels. While specific predictors were not detailed in the available excerpts, the research focused on these factors post-surgery [29]. |
| Immune | Chronic low-grade inflammation (metaflammation) due to nutritionally overloaded metabolic cells, M1 macrophage infiltration and polarization (driven by altered lipid metabolism, increased glycolysis, hypoxia-inducible factor-1α (HIF-1α) activation), Nucleotide-binding oligomerization domain, Leucine-rich repeat and Pyrin domain containing pyrin domain-containing protein—3 (NLRP)-3) inflammasome activation (by oxidized LDL, cholesterol crystals, hyperglycaemia, free fatty acids, excess ATP, reactive oxygen species), dysfunctional adipocyte signalling, immune cell infiltration into adipose tissue (T lymphocytes, NK cells, mast cells, dendritic cells), persistent epigenetic reprogramming (trained immunity) [30,31,32,33,34,35,36,37,38,39,40] | Insulin resistance (impaired insulin signalling via serine phosphorylation of IRS-1, activation of JNK, IKK, PKR) [41,42,43,44,45,46], increased pro-inflammatory cytokine secretion (TNF-α, IL-1β, IL-6), enhanced inflammatory gene expression, exacerbation of T2DM, endothelial dysfunction, systemic chronic low-grade inflammation [47,48,49,50], impaired adipose tissue function. | Gnotobiotic mice stimulated by dysbiotic gut microbiota transplant from a genetically obese child [51]. Obese mice and humans (mechanism studied by Fang et al. [52]). | Deng et al. [51] observed upregulated pro-inflammatory genes in the colon and liver, and altered glucagon-like peptide 1/insulin receptor signalling. Fang et al. [52] identified that obesity promotes a leaky gut, inflammation, and pre-diabetes by lowering gut microbiota that metabolise ethanolamine. |
| Metabolic | Insulin resistance, lipotoxicity (excess free fatty acid release, accumulation of diacylglycerol and ceramides), reduced short-chain fatty acids from dysbiosis, mitochondrial dysfunction, endoplasmic reticulum stress, glucose-fatty acid cycle imbalance [18,19,53,54,55,56,57,58,59,60,61,62,63,64,65] | T2DM, hepatic steatosis [66,67,68,69], dyslipidemia, metabolic syndrome, impaired glucose uptake in muscle [18], altered energy metabolism, increased oxidative stress, impaired β-cell function, cardiovascular disease [70] | Gnotobiotic mice stimulated by dysbiotic gut microbiota transplant from a genetically obese child [51]. Obese mice (diet-induced obesity) treated with Akkermansia muciniphila [71]. Cohort study of 99 obese subjects undergoing Roux-en-Y gastric bypass surgery [29]. Obese mice and humans (mechanism studied by Fang et al. [52]). | Deng et al. [51] found lipid and cholesterol accumulation in the liver and decreased insulin receptor signalling. Depommier et al. [71] demonstrate that Akkermansia muciniphila improves metabolic parameters, enhances fatty acid oxidation, and improves glucose homeostasis by increasing mono-palmitoyl-glycerol (1-PG), a PPARα agonist. Ekberg et al. [29] aimed to predict HbA1c normalisation post-surgery. Fang et al. [52] proposed that reduced ethanolamine-metabolising microbiota contribute to leaky gut and metabolic dysfunction. |
| Epigenetic | DNA methylation (e.g., at the level of LEP, adiponectin (ADIPOQ), Peroxisome Proliferator-Activated Receptor γ(PPAR-γ), PGC-1a genes), microRNA (miRNA) deregulation (e.g., miR-27a, miR-143, miR-146a), histone modifications, early metabolic programming due to maternal hypernutrition/gestational inflammation, environmental chemical exposure [4,9,10,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] | Transgenerational risk transmission of obesity and metabolic disorders, altered gene activity and expression patterns in adipogenesis, lipid metabolism, insulin signalling, and inflammatory responses [9,10,85,86], potential for biomarkers and therapeutic targets [75,80,81], influence on susceptibility to diseases [10], reversible with lifestyle changes [10,82] | Gnotobiotic mice stimulated by dysbiotic gut microbiota transplant from a genetically obese child [51]. | Deng et al. [51] investigated miRNA-gene regulatory networks in response to dysbiotic gut microbiota, revealing altered gene expression patterns relevant to host phenotype changes and demonstrating molecular changes before body fat changes. |
| Microbiota | Gut microbiota dysbiosis (reduced diversity, altered Firmicutes/Bacteroidetes ratios), increased intestinal permeability (“leaky gut”) allowing bacterial lipopolysaccharides translocation, metabolic endotoxemia, altered bile acid metabolism, modulation of gut hormones (ghrelin, glucagon-like peptide-1 (GLP-1), Peptide tyrosine (PYY) [11,52,71,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106] | Systemic inflammation, persistent obesity, reduced short-chain fatty acid production, impaired glucose homeostasis, insulin resistance, increased energy harvest from diet, activation of pro-inflammatory pathways, hepatic steatosis [107], altered central control of appetite and metabolic processes, influence on neuroinflammation via gut–brain axis, and increased oxidative stress. | Metagenomic analysis of human gut microbiome [108]. Gnotobiotic mice stimulated by dysbiotic gut microbiota transplant from a genetically obese child [51]. Obese mice (diet-induced obesity) treated with Akkermansia muciniphila [71]. Obese mice and humans (mechanism studied by Fang et al. [52]). Microbial manipulation in mouse CRC models (mechanisms studied by Zheng et al. [109]) | Das et al. [108] identified specific bacterial enzymes and pathways involved in bile salt biotransformation, which in turn influence host lipid and cholesterol metabolism. Deng et al. [51] found that dysbiotic gut microbiota transplantation led to molecular changes, which were observed as early as the second week, two weeks before changes in body fat occurred. Depommier et al. [71] showed that A. muciniphila influences lipid mediators (like 1-PG) to improve metabolic health. Fang et al. [52] suggest that obesity leads to leaky gut and metabolic dysfunction by reducing ethanolamine-metabolising gut microbiota. |
| Trigger Factor | Target Tissue | Metabolic Effect | Experimental Model | Principal Findings | References |
|---|---|---|---|---|---|
| LPS | Adipocytes, Macrophages | IL-1β, IL-18 activation; systemic inflammation | Murine models (C57BL/6J mice) [167], rat models [168], THP-1 macrophages [169]. In vitro studies on bone marrow derived macrophages and 3T3-L1 adipocytes [170,171]. | LPS induces an inflammatory cascade in macrophages and adipocytes via TLR4, leading to the production of inflammatory cytokines like TNF-α, IL-1β, and iNOS [169]. In mice, LPS causes a marked decrease in lipin-1 mRNA in adipose tissue [170]. LPS stimulation of peritoneal and bone marrow-derived macrophages decreases ApoE mRNA levels [172]. Intravascular infusion of free fatty acids activates NF-kB in adipose tissue, involving TLR4 in macrophages and adipocytes [167]. | [13,34,111,167,169,170,171,172,173] |
| Adipocyte hypoxia | Adipose tissue | HIF-1α and inflammasome activation | Mouse models of obesity (ob/ob and dietary obese mice) [174], adipocyte-specific Hif-1α KO mice [175]. | Hypoxia in expanding white adipose tissue leads to increased HIF-1α expression [174]. HIF-1α in adipocytes regulates lysophosphatidylcholine metabolism and is necessary for homocysteine-induced insulin resistance [176]. Adipocyte-specific HIF-1α deletion prevents adipose tissue inflammation and insulin resistance induced by a high-fat diet in mice [177]. Hypoxia triggers ROS production, ER stress, and inflammatory responses [174]. | [33,151,174,175,176,177,178,179,180,181] |
| Reduced SCFA | Intestine, Adipose tissue | Leaky gut, dysbiosis; impaired glucose metabolism | Fructose-fed C57BL/6N mice [182]. Studies linking gut microbiota metabolites to intestinal and systemic health [183]. | Reduced SCFAs lead to intestinal epithelial barrier impairment and gut dysbiosis in fructose-fed mice [182]. SCFA butyrate is effective in alleviating diet-induced obesity [11]. Microbiota-derived SCFAs, such as propionate, activate ileal free fatty acid receptor 2 to lower hepatic glucose production [184]. | [11,182,183,184,185,186,187,188,189] |
| Molecular byproducts of ageing, physical inactivity, Western diet | Systemic | Systemic chronic low-grade inflammation | Human cohorts and animal models studying inflammaging and metabolic stress [190,191]. | Western societies’ lifestyle and excess calorie consumption can aggravate age-related inflammatory responses, known as metaflammation [191]. The NLRP3 inflammasome is centrally involved in recognizing triggers during physiological aging and metabolic stress [191]. A sedentary lifestyle and high-fat diet can lead to obesity and chronic low-grade inflammation [192]. | [35,39,190,191,192,193,194,195,196] |
| Oxidised low-density lipoprotein | Macrophages | Increased inflammation | Animal models of atherosclerosis (e.g., cholesterol-fed rabbits, LDL-receptor-deficient mice) [197,198], human THP-1 macrophages [199]. | OxLDL is taken up by macrophages, leading to cholesterol accumulation and the formation of foam cells, which contributes to atherogenesis [198,200]. OxLDL can induce alternative macrophage phenotypes, affecting cytokine production [199]. OxLDL enhances pro-inflammatory responses of M2 macrophages, shifting them towards a pro-inflammatory profile [201]. | [35,197,198,199,200,201,202,203,204,205,206,207,208] |
| Cholesterol crystals | Macrophages | Increased inflammation | ApoE-knockout mice fed a high-cholesterol diet [209], human macrophages [210]. | Cholesterol crystals in atherosclerotic lesions drive IL-1β production in macrophages, and this effect is lost in NLRP3- and ASC-deficient macrophages [209]. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages, linking cholesterol metabolism and inflammation [210]. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals [211]. | [36,192,209,210,211,212,213] |
| Hyperglycaemia | THP-1-derived macrophages, 3T3-L1 mature adipocytes, human adipose tissue, pancreatic islets | Impaired glucose metabolism; insulin resistance; increased IL-1β and IL-18 production | THP-1-derived macrophages, 3T3-L1 adipocytes, human adipose tissue [34]. Bone marrow-derived macrophages from diabetic mice [214]. | Hyperglycaemia stimulates NLRP3 inflammasome activation in various cell types [34]. High glucose treatment increases IL-1β expression in macrophages [215]. Hyperglycaemia itself can induce a mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages, supporting diabetes-associated inflammation [216]. | [34,35,159,169,171,214,215,216,217,218,219] |
| Free fatty acids, Ceramides, Excess ATP | Adipose tissue, Systemic | Insulin resistance; pro-inflammatory response; contributes to metabolic syndrome | Mouse liver cell lines [220], various cell types (macrophages, adipocytes) [221], rodent models [222]. | Palmitate activates NLRP3, enhances ROS generation, and can suppress insulin-induced Akt phosphorylation, suggesting FFA-mediated insulin resistance [220]. Saturated fatty acids induce ceramide formation, which are potent antagonists of insulin action [222]. In both macrophages and adipocytes, ceramides activate the NLRP3 inflammasome [221]. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signalling [166]. | [33,34,35,161,166,218,220,221,222,223,224,225,226,227] |
| Reactive oxygen species | Systemic | Activates NLRP3 inflammasome; leads to insulin resistance and metabolic syndrome | Rat hepatocytes [228], human studies on diabetic complications [229]. | ROS drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation [228]. Elevated mitochondrial ROS in myeloid cells of T2DM patients are associated with increased production of inflammasome-dependent cytokines IL-1β and IL-18 [159]. NOD2 activation enhances ROS production in skeletal muscle cells, contributing to mitochondrial dysfunction and insulin resistance [230]. | [33,39,159,192,228,229,230,231,232] |
| Metabolic insults | Adipose tissue | Exacerbates insulin resistance; promotes adipose tissue dysfunction | Human and animal studies [34,232]. | Metabolic insults, including SFAs, pro-inflammatory adipokines, hyperglycaemia, and endotoxemia, are major stimuli for NLRP3 inflammasome activation in adipose tissue [34]. NLRP3 inflammasome activity in adipose tissues positively correlates with obesity and its metabolic complications [34]. | [34,38,39,63,206,211,232,233] |
| Danger signals from stressed/dying adipocytes | Adipocytes | Activation of NLRP3-ASC inflammasome; interferes with insulin signalling | In vitro studies on adipocytes [234]. | Endogenous danger signals activate NLRP3 inflammasome, leading to processing and secretion of IL-1β and IL-18 [235]. Released fatty acids induce NLRP3-ASC inflammasome activation, interfering with insulin signalling and reducing insulin sensitivity [166]. Adipocyte death triggers a pro-inflammatory response and metabolic activation of resident macrophages [234]. | [31,33,63,137,166,234,235,236,237] |
| Microorganism | Bile Acid Metabolism | Metabolic Effect | Experimental Model | Principal Findings | References |
|---|---|---|---|---|---|
| Bacteroidetes (phylum) | Possesses bile salt hydrolase activity, deconjugating primary bile acids | Affects host lipid and cholesterol metabolism, influences weight gain | Gnotobiotic mice monocolonized with wild-type or BSH-deleted Bacteroides thetaiotaomicron strains [254]. Germ-free and conventionally raised murine models with bacterial BSH expression [265]. High-fat diet-induced hyperlipidemic rats treated with Bacteroides vulgatus [266]. | Bacteroides thetaiotaomicron with BSH activity selectively modulated levels of tauro-β-muricholic acid [254]. High-level BSH expression reduced host weight gain, plasma cholesterol, and liver triglycerides in mice [265]. Bacteroides vulgatus ameliorated serum lipid profiles and systemic inflammation in hyperlipidemic rats by changing bile acid metabolism [266]. BSH mediates a microbe-host dialogue regulating host lipid and cholesterol metabolism and weight gain [255]. | [250,254,255,265,266,267,268,269,270] |
| Firmicutes (phylum) | Some species involved in the formation of secondary allo-bile acids can be associated with increased energy harvesting | Altered bile acid profile; influences energy balance and contributes to obesity | Metagenomic and phylogenetic analyses of human stool samples [271]. Studies on primary bile acid (cholic acid) influence on gut microbiota composition [272]. | Firmicutes generate secondary allo-bile acids (allo-DCA and allo-LCA) through novel enzymes (BaiA1, BaiP, BaiJ) [271]. Increased primary bile acid levels can shift the gut microbiome towards Firmicutes, particularly Clostridium cluster XIVa, leading to increased deoxycholic acid production [272]. | [89,90,108,265,271,272,273,274,275,276,277,278] |
| Clostridium, Enterococcus, Lactobacillus, Bifidobacterium, Ruminococcus, Xanthomonas, Eubacterium | Contribute to the transformation of primary into secondary bile acids | Altered secondary bile acid profile, influencing host metabolic pathways | Rodent models fed high-fat diets [279]. Human metagenome analysis [280]. Studies identifying bile acid-converting bacteria [281]. | Clostridium scindens and Clostridium hylemonae were abundant in obesity-prone rodents, modifying BA metabolism and promoting obesity [279]. Species within Clostridium and Eubacterium generate secondary bile acids that can modulate adiposity via FXR or TGR5 signalling [280]. Gut bacteria that convert primary BAs to secondary BAs belong to a limited number of species, mainly Clostridiales [281]. | [270,273,279,280,281,282,283,284] |
| Akkermansia muciniphila | Indirectly influences bile acid-related metabolic health through modulation of the endocannabinoid system, body weight, and food intake regulation | Associated with improved metabolic parameters; indirectly linked to bile acid-influenced metabolic health | High-fat diet-fed mice administered A. muciniphila [285,286,287]. Obese and type 2 diabetic mice [286]. | A. muciniphila administration reversed high-fat diet-induced metabolic disorders, including fat-mass gain, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance, by increasing intestinal endocannabinoids [286]. Oral transfer of live A. muciniphila ameliorated obese and diabetic phenotypes, reduced metabolic endotoxemia, host adiposity, and improved glucose metabolism [287]. It influences endocannabinoid concentrations in the ileum, such as 2-AG, associated with improved metabolic dysfunctions [285]. | [90,285,286,287,288,289,290,291,292,293,294] |
| General dysbiotic state | Decreased beneficial microbiota and increased opportunistic pathogens (e.g., Bacilli, Enterobacteriales, Streptococcaceae, Veillonella, Lactobacillales increase; Clostridia, Clostridiales decrease) | Correlates with higher bile acid levels; reflects or contributes to altered bile acid metabolism in disease states, including those related to obesity | Human cohort studies of obese and metabolically unhealthy individuals [279]. High-fat diet-fed mice [102,264]. | Unhealthy obese subjects had a significantly lower proportion of secondary bile acids compared to primary ones, suggesting altered BA composition is involved in different metabolic states of obesity [279]. A high-fat diet caused rapid increases in the intestinal BA pool and altered microbial composition, driving modifications of BA and microbiota compositions that trigger metabolic disorders [102]. Long-term HFD feeding decreased hepatic and serum BA levels and impaired gut microbiota, with higher bile salt hydrolase activity in ileal microbes [264]. | [101,102,103,264,272,279,283] |
| Type of Modification | Trigger Factor | Consequences | Experimental Model | Principal Findings | References |
|---|---|---|---|---|---|
| DNA methylation | High-fat maternal diet | Increased T2DM risk in offspring; altered gene activity and changes in DNA methylation patterns predisposing to obesity and related comorbidities; may be transgenerationally inherited | Pregnant mice or rats fed a high-fat diet [173,326,327,328]. Studies on intrauterine exposure to obesogenic environments. | Maternal high-fat diet induces DNA methylation changes in offspring’s brown adipose tissue, impacting obesity progression [329] and contributes to glucose intolerance [327]. Leads to lasting epigenetic alterations, programming metabolic pathways and adipogenesis. Gestational high-fat diet impaired demethylation of PPARα in offspring’s liver, inducing obesity [173]. Maternal overnutrition alters DNA methylation in early life, predisposing offspring to metabolic diseases [326]. | [173,326,327,328,329,330,331,332,333,334,335] |
| DNA methylation | Environmental factors | Altered gene activity, changes in DNA methylation patterns predisposing to obesity and related comorbidities; risk of dyslipidemia; potential for biomarkers and therapeutic targets | Human cohorts (e.g., obese vs. lean individuals) [336,337,338], animal studies on obesogenic environments [84,339]. | Obese individuals exhibit distinct epigenetic signatures. Environmental factors link to altered gene activity and obesity phenotypes [337]. Differential methylation of genes like PPARγ, LEP, and FTO correlates with adipocyte differentiation and insulin resistance. Global hypomethylation in subcutaneous adipose tissue and leukocytes of obese individuals has been observed [336]. | [10,84,336,337,338,339,340,341,342] |
| miRNA deregulation | Chronic inflammation | Pro-inflammatory gene expression, dysregulation of central lipid and glucose metabolism, impaired target gene expression in obese adipose tissue, insulin resistance, altered lipid metabolism; potential for biomarkers and therapeutic targets | Human primary mature adipocytes and macrophages [343], pediatric cohorts with severe obesity [344], 3T3-L1 adipocytes [345]. | Altered expression of miRNAs (e.g., miR-27a, miR-143, miR-146a) contributes to insulin resistance, adipose tissue inflammation, and impaired lipid metabolism. Circulating miRNAs (e.g., miR-34a, miR-122, miR-192) are associated with obesity-related inflammation and metabolic disease in pediatric patients [344]. Downregulation of miR-320 alleviates endoplasmic reticulum stress and inflammatory response in 3T3-L1 adipocytes [345]. | [74,80,343,344,345,346,347,348,349,350,351,352,353,354] |
| miRNA deregulation | Gut microbiota dysbiosis | Regulation of white adipose tissue inflammation and obesity | Human cohorts comparing obese and eutrophic individuals [355], mouse models of high-fat diet-induced obesity [356], gnotobiotic mice stimulated by dysbiotic gut microbiota transplant [51]. | Host-secreted miRNAs regulate the gut microbiota, and the gut microbiota affects the host via inducing special miRNA expression [357]. Circulating miRNAs and gut microbiota composition show novel interactions in human obesity [355]. Dysbiotic gut microbiota transplantation in mice led to miRNA deregulation and altered gene expression impacting host phenotype [51]. | [51,292,352,355,356,357,358,359,360,361,362] |
| Histone modifications | Dysbiosis and oxidative stress | Altered transcription of metabolic genes, impaired energy metabolism, changes in adipogenesis | Mouse models of high-fat diet [363], studies on gut dysbiosis [364], intestinal epithelial cells [365]. | Gut dysbiosis can induce excessive production of reactive oxygen species, leading to inflammation and epigenetic alterations [364]. Microbiota-derived short-chain fatty acids can cause epigenetic imprinting in utero [366]. Diet-microbiota interactions mediate global histone acetylation and methylation in multiple host tissues [367]. HFD induces histone modification in adipose tissues, activating genes related to lipogenesis, energy metabolism, and inflammation [363]. | [206,342,363,364,366,367,368,369,370,371] |
| Histone modifications | Environmental factors | Influence on gene expression without altering the DNA sequence | Human and animal studies on environmental exposures [84,339]. | Histone modifications mediate the interaction between genetic predisposition and environmental exposures. Environmental factors, including nutrition, inflammation, hypoxia, and physical activity, can induce epigenetic changes [372]. Chromatin modifications are associated with the progression of diabetes and obesity [373]. | [10,82,84,206,339,372,373,374] |
| General epigenetic changes | Multifactorial | Influence susceptibility to diseases like obesity, contribute to disordered energy metabolism; can be reversed with lifestyle changes; provide insights into pathophysiological processes for prevention and treatment; potential for new therapeutic approaches | Weight loss interventions (lifestyle, pharmacotherapy, bariatric surgery) [88,375,376,377,378,379,380]. | Epigenetic phenomena are dynamic and reversible with intensive lifestyle changes [82]. Weight loss interventions (dietary changes, exercise, surgical interventions) can reverse epigenetic modifications, including methylation patterns of leptin and adiponectin genes [88]. Bariatric surgery can lead to significant improvements in methylation of genes regulating insulin sensitivity and inflammatory responses [379,381]. | [10,82,88,206,372,375,376,377,378,379,380,381,382,383,384,385,386,387] |
| Intervention | Main Target | Systemic Effects | Examples/References |
|---|---|---|---|
| targeting metaflammation | Chronic low-grade inflammation, oxidative stress, NLRP3 inflammasome | Ameliorates metaflammation and its metabolic consequences, restores adipose homeostasis, mitigates obesity-associated cardiometabolic complications | Novel anti-inflammatory compounds, gut microbiota modulators, NLRP3 inhibitors, inhibiting inflammatory responses by neutralising cytokines/chemokines, deleting Toll-like receptors, blocking neutrophil recruitment [5] |
| microbiota modulation | Gut microbiota composition and function, intestinal barrier integrity, microbial metabolites | Mitigates metaflammation, improves metabolic health, reduces intestinal low-grade inflammation, improves gut barrier integrity, ameliorates metabolic balance, promotes weight loss, improved glucose homeostasis and satiety | Dietary interventions (fermentable fibres, prebiotics) [415], Probiotics (e.g., Lactobacillus gasseri) [93,416], Faecal Microbiota Transplantation [93,417,418], manipulating microbial metabolites [73], Akkermansia muciniphila [93,292], Blautia wexlerae [419] |
| precision nutrition and multi-omics | Individual metabolic and microbial profiles, specific biomarkers and pathways implicated in metaflammation, individual’s genetic, environmental, lifestyle characteristics, obesity subtypes/phenotypes | Optimises anti-inflammatory responses, fosters sustained metabolic health, enables individualised prevention and treatment strategies, enhances treatment effectiveness and tolerability | Integration of omics data (genomic, epigenomic, proteomic, metabolomic, microbiomic profiling) [90,119,390,403,404,405,420], DNA methylation, metabolomics, gut microbiome data to predict weight loss [421] |
| targeting macrophage dynamics | Macrophage function, adipocytes, inflammation within adipose tissue, ceramide accumulation, inflammatory kinases | Mitigates obesity-associated metaflammation, improves metabolic outcomes, reduces inflammation, improves adipose tissue health, restores catecholamine sensitivity, reverses metabolic dysfunctions associated with obesity | Targeting M1 to M2 macrophage polarisation [32,147,246,412,422,423], ceramide synthesis inhibitors, Amlexanox, Metformin [31], Thiazolidinediones [31], adipose tissue-targeting ultra-small hybrid nanoparticles [424], Interferon Tau [422] |
| Intervention | Main target | Systemic Effects | References |
|---|---|---|---|
| High-fibre, polyphenol-rich diet | gut microbiota | ↑ SCFA, ↓ Endotoxemia, ↓ Inflammation | [415,434] |
| Regular physical activity | adipose tissue, HPG/HPA axis | ↑ LEP sensitivity, ↓ Inflammation | [435,436,437,438] |
| IL-1β inhibitors | systemic inflammation | ↓ Inflammatory markers, better glycaemic control | [439,440,441,442,443,444,445,446] |
| weight loss surgery | adipose tissue, metabolic organs | ↓ Adipokines, improved insulin sensitivity | [29,447,448,449,450,451,452,453,454,455,456,457,458,459,460] |
| GLP-1 Receptor Agonists | appetite regulation, satiety, gastric emptying, glucose-dependent insulin secretion, glucagon release | Significant weight loss, improved glucose metabolism, enhanced insulin sensitivity, cardiovascular protection, anti-inflammatory and anti-atherogenic actions, improved blood pressure and lipid profile | [461,462,463,464,465,466,467,468,469,470,471,472,473,474,475] |
| microbiota modulation | gut microbiota composition and function, intestinal barrier integrity | ↓ Systemic inflammation, improved metabolic signalling, improved metabolic balance, weight loss, increased SCFA production, reduced metabolic endotoxemia | [415,434] |
| targeting macrophage dynamics | macrophage function, adipocyte clearance, inflammatory pathways | ↓ Adipose tissue inflammation, improved metabolic outcomes, restored M2 macrophage phenotype, improved insulin sensitivity, inhibition of ceramide synthesis | [246,348,422,476] |
| leptin sensitising/adiponectin-based therapies | leptin resistance, adiponectin signalling | Overcome LEP resistance, improved insulin resistance, positive effects on fatty liver and other organ disturbances, enhanced energy expenditure, reduced weight gain | [447] (for LEP to adiponectin ratio), [437] (for adipokine regulation by exercise) |
| epigenetic modulation | epigenetic modifications | Reversal of unfavourable gene expression patterns, improved metabolic profiles, restored energy balance, reduced inflammation, potential for transgenerational impact reversal | [447,448,449,450,451,452,453,454,455,456,457,458,459,460,461,462,463,464,465,466,467,468,469,470,471,472,473,474,475,476,477,478,479,480] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crasan, I.-M.; Tanase, M.; Delia, C.E.; Gradisteanu-Pircalabioru, G.; Cimpean, A.; Ionica, E. Metaflammation’s Role in Systemic Dysfunction in Obesity: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 10445. https://doi.org/10.3390/ijms262110445
Crasan I-M, Tanase M, Delia CE, Gradisteanu-Pircalabioru G, Cimpean A, Ionica E. Metaflammation’s Role in Systemic Dysfunction in Obesity: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(21):10445. https://doi.org/10.3390/ijms262110445
Chicago/Turabian StyleCrasan, Ioana-Maria, Matei Tanase, Corina Elena Delia, Gratiela Gradisteanu-Pircalabioru, Anisoara Cimpean, and Elena Ionica. 2025. "Metaflammation’s Role in Systemic Dysfunction in Obesity: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 21: 10445. https://doi.org/10.3390/ijms262110445
APA StyleCrasan, I.-M., Tanase, M., Delia, C. E., Gradisteanu-Pircalabioru, G., Cimpean, A., & Ionica, E. (2025). Metaflammation’s Role in Systemic Dysfunction in Obesity: A Comprehensive Review. International Journal of Molecular Sciences, 26(21), 10445. https://doi.org/10.3390/ijms262110445







