Pigment Extracts of Tetradesmus obliquus, Phaeodactylum tricornutum and Desmodesmus armatus Exert Anti-Adipogenic Effects on Maturing 3T3-L1 Pre-Adipocytes
Abstract
1. Introduction
2. Results
2.1. Characterisation of Microalgae Extracts
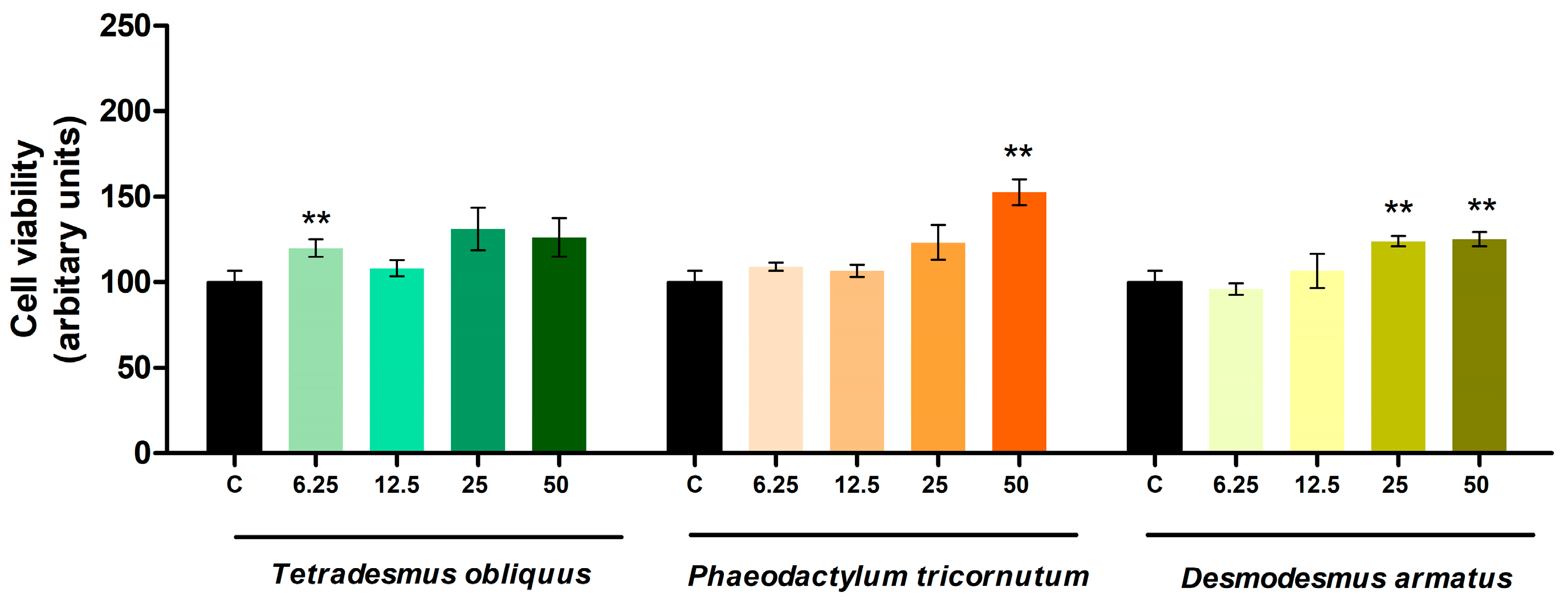
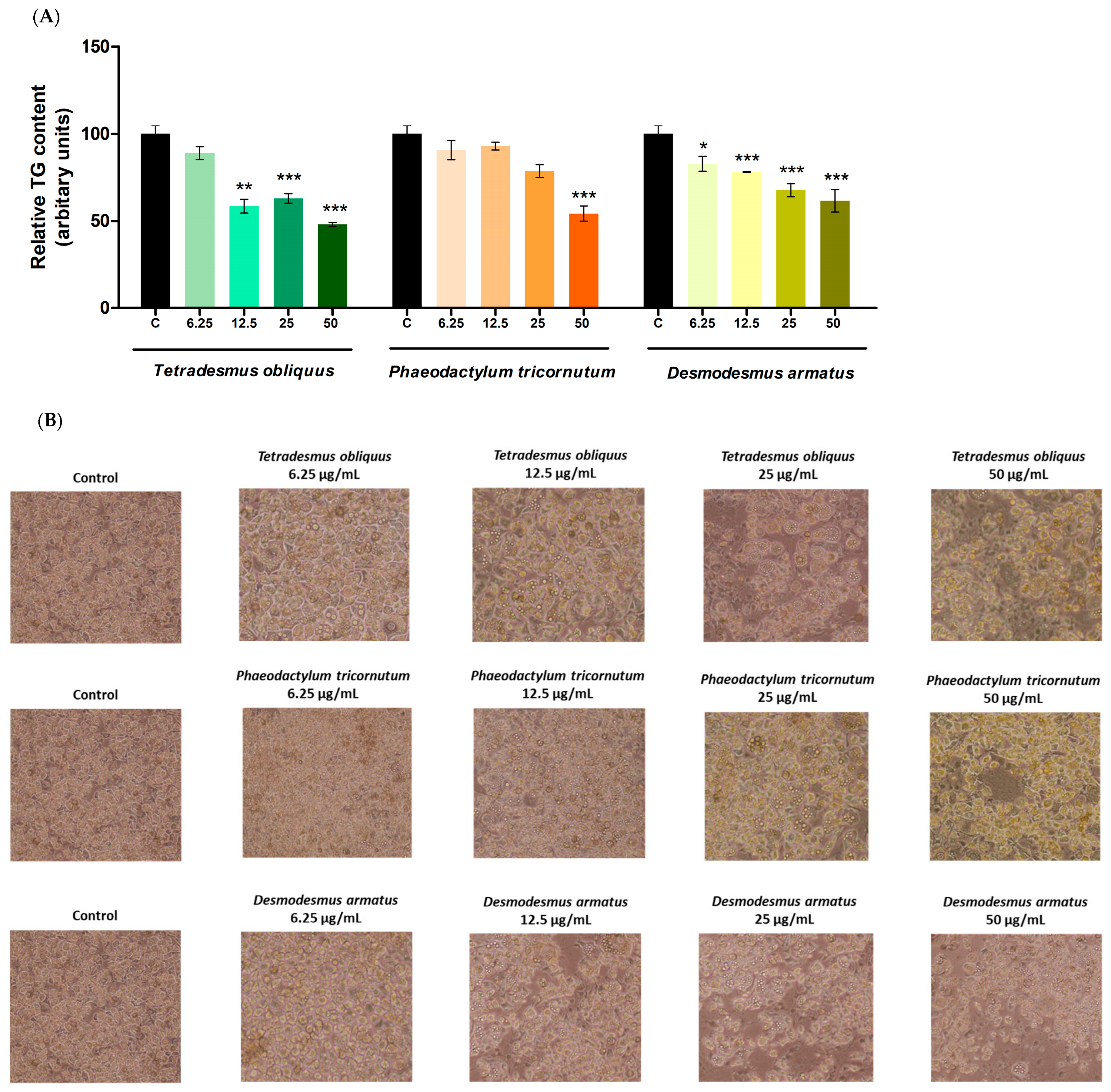
2.2. Effects on Cell Viability and Triglyceride Content
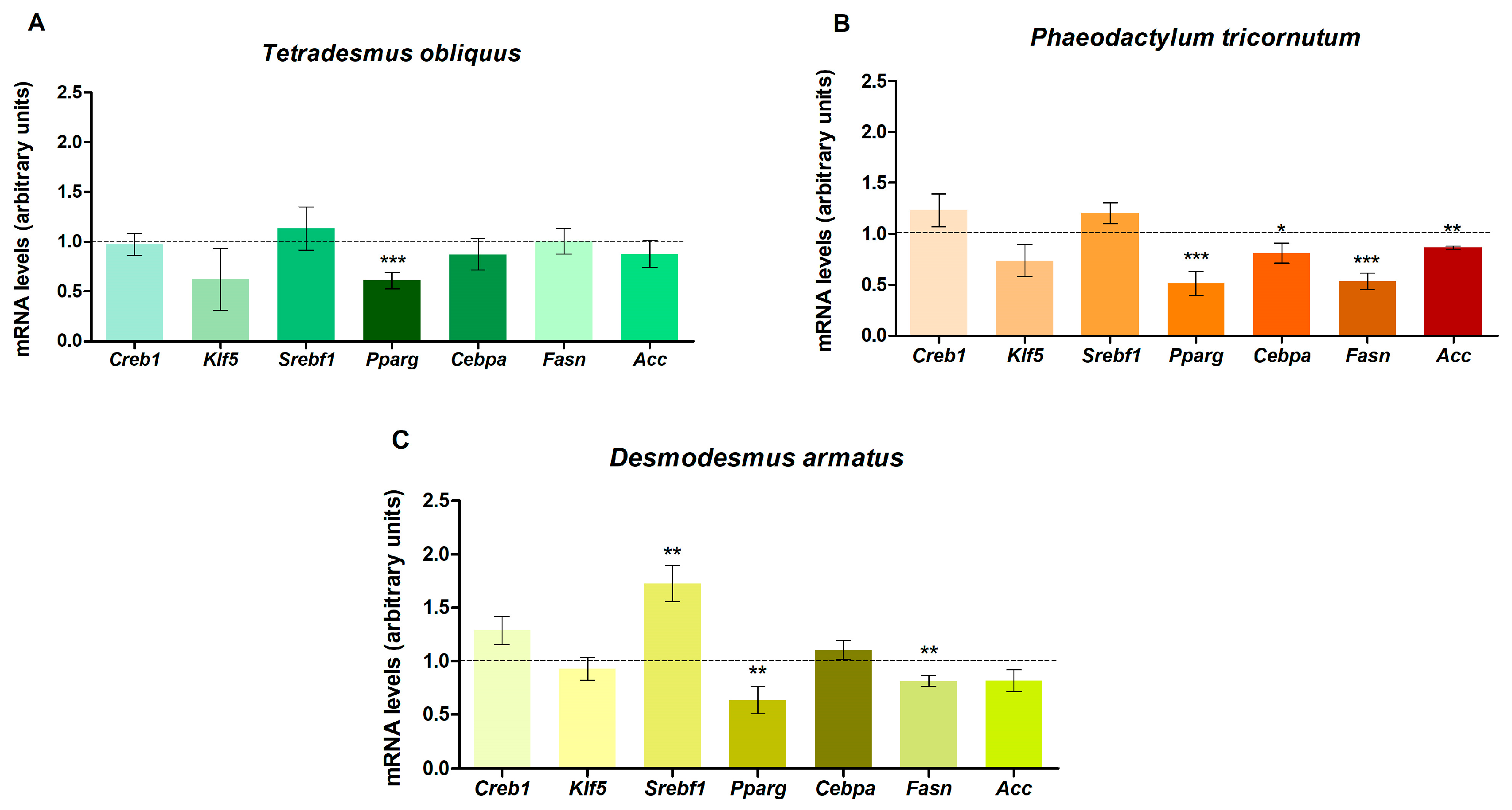
2.3. Effect of Microalgae Pigment Extracts on Gene Expression in Maturing Pre-Adipocytes
3. Discussion
4. Materials and Methods
4.1. Microalgae Production
4.2. Microalgae Extraction Preparation
4.3. Determination of Carotenoid and Chlorophyll Profiles by LC-DAD-QTOF-MS/MS
4.4. Experimental Design
4.5. Cell Treatments
4.6. Cell Viability Assay
4.7. Determination of Triglyceride Content
4.8. Analysis of Gene Expression by Real-Time PCR
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 May 2025).
- Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Eseberri, I.; Fernández-Quintela, A.; Portillo, M.P. Anti-Obesity Effects of Microalgae. Int. J. Mol. Sci. 2019, 21, 41. [Google Scholar] [CrossRef]
- Bolton, J.J.; Cyrus, D.M.; Brand, M.J.; Joubert, M.; Macey, B.M. Why grow Ulva? Its potential role in the future of aquaculture. Perspect. Phycol. 2016, 3, 113–120. [Google Scholar] [CrossRef]
- Marques, F.; Lopes, D.; Conde, T.; Melo, T.; Silva, J.; Abreu, M.H.; Domingues, P.; Domingues, M.R. Lipidomic Characterization and Antioxidant Activity of Macro- and Microalgae Blend. Life 2023, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Bárcenas-Pérez, D.; Střížek, A.; Hrouzek, P.; Kopecký, J.; Barradas, M.; Sierra-Ramirez, A.; Fernandez-Marcos, P.J.; Cheel, J. Production of Fucoxanthin from. Mar. Drugs 2021, 19, 517. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Seo, Y.J.; Pan, C.H.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Fucoxanthin Suppresses Lipid Accumulation and ROS Production During Differentiation in 3T3-L1 Adipocytes. Phytother. Res. 2016, 30, 1802–1808. [Google Scholar] [CrossRef]
- Lee, H.G.; Lu, Y.A.; Je, J.G.; Jayawardena, T.U.; Kang, M.C.; Lee, S.H.; Kim, T.H.; Lee, D.S.; Lee, J.M.; Yim, M.J.; et al. Effects of Ethanol Extracts from Grateloupia elliptica, a Red Seaweed, and Its Chlorophyll Derivative on 3T3-L1 Adipocytes: Suppression of Lipid Accumulation through Downregulation of Adipogenic Protein Expression. Mar. Drugs 2021, 19, 91. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Xie, J.; Xu, Q.; Pan, C.; Wang, J.; Wu, X.; Sanabil, S.; Zheng, M.; Liu, J. Anti-obesity effects of zeaxanthin on 3T3-L1 preadipocyte and high fat induced obese mice. Food Funct. 2017, 8, 3327–3338. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, M.; Cai, D.; Xie, J.; Jin, Z.; Liu, H.; Liu, J. Zeaxanthin promotes mitochondrial biogenesis and adipocyte browning via AMPKα1 activation. Food Funct. 2019, 10, 2221–2233. [Google Scholar] [CrossRef]
- Gopal, S.S.; Eligar, S.M.; Vallikannan, B.; Ponesakki, G. Inhibitory efficacy of lutein on adipogenesis is associated with blockage of early phase regulators of adipocyte differentiation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158812. [Google Scholar] [CrossRef]
- Martini, D.; Negrini, L.; Marino, M.; Riso, P.; Del Bo, C.; Porrini, M. What Is the Current Direction of the Research on Carotenoids and Human Health? An Overview of Registered Clinical Trials. Nutrients 2022, 14, 1191. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Shroff, M.R.; Chen, X.; Beydoun, H.A.; Wang, Y.; Zonderman, A.B. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J. Nutr. 2011, 141, 903–913. [Google Scholar] [CrossRef]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.; Greenfield, J.R.; Harats, D.; Shaish, A.; Samocha-Bonet, D. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J. Nutr. 2020, 150, 38–46. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]
- Truong, T.Q.; Park, Y.J.; Koo, S.Y.; Choi, J.-H.; Enkhbayar, A.; Song, D.-G.; Kim, S.M. Interdependence of fucoxanthin biosynthesis and fucoxanthin-chlorophyll a/c binding proteins in Phaeodactylum tricornutum under different light intensities. J. Appl. Phycol. 2023, 35, 25–42. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Nowicka, B.; Jakubowska, A.; Strzalka, W.; Burda, K.; Strzalka, K. The xanthophyll cycle in diatom Phaeodactylum tricornutum in response to light stress. Plant Physiol. Biochem. 2020, 152, 125–137. [Google Scholar] [CrossRef]
- Tamaki, S.; Shinomura, T.; Mochida, K. Illuminating the diversity of carotenoids in microalgal eyespots and phototaxis. Plant Signal. Behav. 2023, 18, 2257348. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic Pigments in Diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef] [PubMed]
- Greipel, E.; Kósa, A.; Böddi, B.; Bakony, M.; Bernát, G.; Felföldi, T.; Preininger, É.; Kutasi, J. Extraction of chlorophyll a from Tetradesmus obliquus—A method upgrade. Biol. Futur. 2024, 75, 243–250. [Google Scholar] [CrossRef]
- Kim, H.J.; Koo, K.A.; Park, W.S.; Kang, D.M.; Kim, H.S.; Lee, B.Y.; Goo, Y.M.; Kim, J.H.; Lee, M.K.; Woo, D.K.; et al. Anti-obesity activity of anthocyanin and carotenoid extracts from color-fleshed sweet potatoes. J. Food Biochem. 2020, 44, e13438. [Google Scholar] [CrossRef] [PubMed]
- Ranga Rao, A.; Raghunath Reddy, R.L.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J. Agric. Food Chem. 2010, 58, 8553–8559. [Google Scholar] [CrossRef]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kim, S.M. Anti-Obesity Effect of Standardized Extract of Microalga. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef]
- Kang, S.I.; Ko, H.C.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Lee, N.H.; Kim, S.J. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes. Biochem. Biophys. Res. Commun. 2011, 409, 769–774. [Google Scholar] [CrossRef]
- Seo, Y.J.; Kim, K.J.; Choi, J.; Koh, E.J.; Lee, B.Y. Extract Reduces Obesity through Suppression of Adipogenesis and Activation of Browning in 3T3-L1 Cells and High-Fat Diet-Induced Obese Mice. Nutrients 2018, 10, 712. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Hu, X.; Liao, X.; Zhang, Y. Chlorophyll Supplementation in Early Life Prevents Diet-Induced Obesity and Modulates Gut Microbiota in Mice. Mol. Nutr. Food Res. 2019, 63, e1801219. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Lu, F.; Wang, X.; Liao, X.; Hu, X.; Zhang, Y. Beneficial effects of a chlorophyll-rich spinach extract supplementation on prevention of obesity and modulation of gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2019, 60, 103436. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, H.J.; Han, J.S. Pheophorbide A isolated from Gelidium amansii inhibits adipogenesis by regulating adipogenic transcription factors and AMPK in 3T3-L1 adipocytes. Nutr. Res. 2022, 107, 187–194. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lu, Y.L.; Chang, Y.C.; Chyuan, J.H.; Lee, T.H.; Hou, W.C. Anti-adipogenic activities of pheophorbide a and pyropheophorbide a isolated from wild bitter gourd (Momordica charantia L. var. abbreviata Seringe) in vitro. J. Sci. Food Agric. 2022, 102, 6771–6779. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes. Dev. 2000, 14, 1293–1307. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Li, X.; Zeng, S.; Chen, L.; Zhang, Y.; Zhang, B.; Su, D.; Du, Q.; Zhang, J.; Wang, H.; Zhong, Z.; et al. An intronic enhancer of Cebpa regulates adipocyte differentiation and adipose tissue development via long-range loop formation. Cell Prolif. 2024, 57, e13552. [Google Scholar] [CrossRef]
- Payne, V.A.; Au, W.S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215–223. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, A.; Qi, W. The Potential to Fight Obesity with Adipogenesis Modulating Compounds. Int. J. Mol. Sci. 2022, 23, 2299. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Zingg, J.M.; Fassini, P.G.; Suen, V.M.M. Bioactive dietary components-Anti-obesity effects related to energy metabolism and inflammation. Biofactors 2023, 49, 297–321. [Google Scholar] [CrossRef]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- do Carmo Cesário, C.; Soares, J.; Cossolin, J.F.S.; Almeida, A.V.M.; Bermudez Sierra, J.J.; de Oliveira Leite, M.; Nunes, M.C.; Serrão, J.E.; Martins, M.A.; Dos Reis Coimbra, J.S. Biochemical and morphological characterization of freshwater microalga Tetradesmus obliquus (Chlorophyta: Chlorophyceae). Protoplasma 2022, 259, 937–948. [Google Scholar] [CrossRef]
- Gillies, R.J.; Didier, N.; Denton, M. Determination of cell number in monolayer cultures. Anal. Biochem. 1986, 159, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
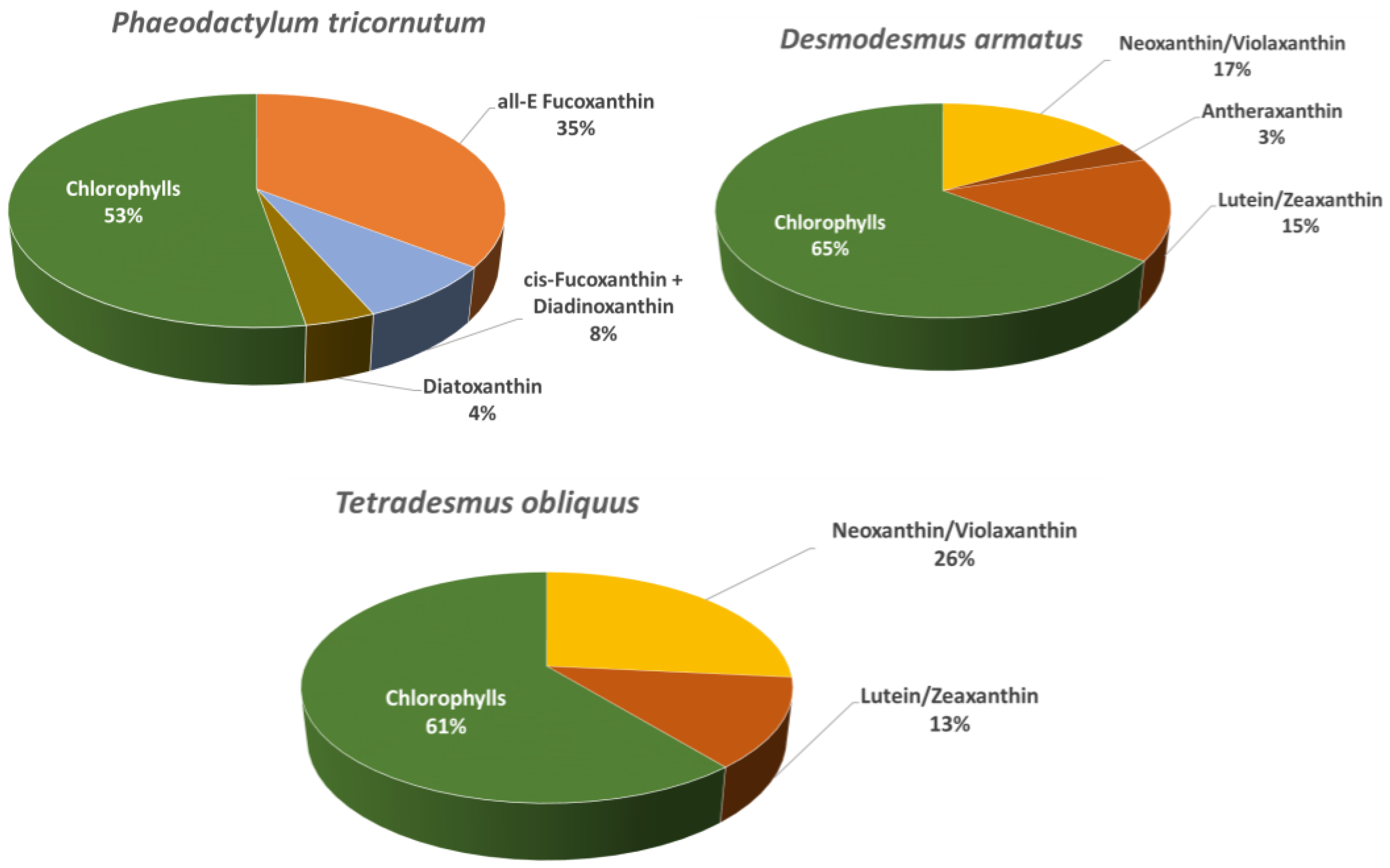

| Peak No | RT (min) | Identification | Molecular Formula | m/z [M + H]+ (Theoretical) | Error (ppm) | MS/MS Product Ions | UV–Vis Maxima (nm) |
|---|---|---|---|---|---|---|---|
| 1 | 0.724 | Chlorophyll | - | - | - | 400, 435, 470 | |
| 2 | 0.847 | all-E Fucoxanthin | C42H58O6 | 659.4306 | 2.6 | 641, 623, 581, 109 | 420 s, 445, 466 s |
| 3 | 0.891 | Neoxanthin-/Violaxanthin | C40H56O4 | 601.4251 | 2.8 | 583, 565, 167,116 | 316 s, 436, 464 |
| 4 | 0.998 | Pheophorbide a | C35H36N4O5 | 593.2759 | 2.6 | 533, 505 | 410 |
| 5 | 1.0891 | cis-Fucoxanthin | C42H58O6 | 659.4306 | 1.3 | 641, 623, 581, 109 | 330, 420 s, 445, 470 s |
| 6 | 1.120 | Diadinoxanthin | C40H54O3 | 583.4145 | −0.8 | 565, 527, 283 | 424 s, 445, 474 s |
| 7 | 1.214 | Pheophorbide a-like | - | - | - | 410 | |
| 8 | 1.271 | Antheraxanthin | C40H56O3 | 585.4302 | −0.4 | 567, 493, 337, 231 | 380 s, 410, 455 s |
| 9 | 1.333 | Diatoxanthin | C40H54O2 | 567.4196 | 3.1 | 285, 217, 119 | 424 s, 450, 476 |
| 10 | 1.353 | Lutein/Zeaxanthin | C40H56O2 | 569.4353 | 2.8 | 551, 337, 251 | 420 s, 444, 472 |
| 11 | 1.693 | Chlorophyll | - | - | - | 450 | |
| 12 | 1.698 | Echinenone | C40H54O | 551.4247 | 0.3 | 533, 429, 175, 121 | 410 |
| 15 | 2.360 | Chlorophyll b’ | C55H70MgN4O6 | 907.5219 | 1.8 | 567, 476, 133 | 417, 440, 467 |
| 16 | 2.544 | Chlorophyll b | C55H70MgN4O6 | 907.5219 | 2.4 | 583, 333, 167 | 417, 440, 467 |
| 17 | 3.338 | Chlorophyll a’ | C55H72MgN4O5 | 893.5426 | 4.5 | 593, 533 | 408 |
| 18 | 3.644 | Chlorophyll a | C55H72MgN4O5 | 893.5426 | 4.5 | 593, 533 | 408 |
| 19 | 5.684 | Pheophytin a’ | C55H74N4O5 | 871.5732 | −2.3 | 615, 583, 555 | 339, 390 s, 420 s, 432 |
| 20 | 5.904 | Pheophytin a | C55H74N4O5 | 871.5732 | −1.4 | 615, 583, 555 | 339, 390 s, 420 s, 432 |
| Gene | Sense Primer 5′-3′ | Antisense Primer 5′-3′ |
|---|---|---|
| Acc | GGA CCA CTG CAT GGA ATG TTA A | TGA GTG ACT GCC GAA ACA TCT C |
| Cebpa | TGG ACA AGA ACA GCA ACG AG | TCA CTG GTC AAC TCC AGC AC |
| Creb1 | TTT GTC CTT GCT TTC CGA AT | CAC TTT GGC TGG ACA TCT TG |
| Fasn | AGC CCC TCA AGT GCA CAG TG | TGC CAA TGT GTT TTC CCT GA |
| Klf5 | GGT CCA GAC AAG ATG TGA AAT GG | TTT ATG CTC TGA AAT TAT CGG AAC TG |
| Pparg | TCG CTG ATG CAC TGC CTA TG | GAG AGG TCC ACA GAG CTG ATT |
| Srebf1 | GCT GTT GGC ATC CTG CTA TC | TAG CTG GAA GTG ACG GTG GT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carr-Ugarte, H.; Aguirre, L.; Portillo, M.P.; Álvarez-Rivera, G.; Seoane, S.; Aramendi, P.; Eseberri, I. Pigment Extracts of Tetradesmus obliquus, Phaeodactylum tricornutum and Desmodesmus armatus Exert Anti-Adipogenic Effects on Maturing 3T3-L1 Pre-Adipocytes. Int. J. Mol. Sci. 2025, 26, 10314. https://doi.org/10.3390/ijms262110314
Carr-Ugarte H, Aguirre L, Portillo MP, Álvarez-Rivera G, Seoane S, Aramendi P, Eseberri I. Pigment Extracts of Tetradesmus obliquus, Phaeodactylum tricornutum and Desmodesmus armatus Exert Anti-Adipogenic Effects on Maturing 3T3-L1 Pre-Adipocytes. International Journal of Molecular Sciences. 2025; 26(21):10314. https://doi.org/10.3390/ijms262110314
Chicago/Turabian StyleCarr-Ugarte, Helen, Leixuri Aguirre, María P. Portillo, Gerardo Álvarez-Rivera, Sergio Seoane, Pablo Aramendi, and Itziar Eseberri. 2025. "Pigment Extracts of Tetradesmus obliquus, Phaeodactylum tricornutum and Desmodesmus armatus Exert Anti-Adipogenic Effects on Maturing 3T3-L1 Pre-Adipocytes" International Journal of Molecular Sciences 26, no. 21: 10314. https://doi.org/10.3390/ijms262110314
APA StyleCarr-Ugarte, H., Aguirre, L., Portillo, M. P., Álvarez-Rivera, G., Seoane, S., Aramendi, P., & Eseberri, I. (2025). Pigment Extracts of Tetradesmus obliquus, Phaeodactylum tricornutum and Desmodesmus armatus Exert Anti-Adipogenic Effects on Maturing 3T3-L1 Pre-Adipocytes. International Journal of Molecular Sciences, 26(21), 10314. https://doi.org/10.3390/ijms262110314










