Biological Characterization of 3D-Printed, Sintered Hydroxyapatite Scaffolds Obtained by Fused Filament Fabrication: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cell Culture
2.3. Scaffold Design and Fabrication
2.4. Experimental Assays
2.4.1. Morphological Characteristics (Scanning Electron Microscopy—SEM)
2.4.2. Cell Viability (Confocal Scanning Laser Microscopy—CSLM)
2.4.3. Cell Proliferation (Tetrazolium Assay WST-1)
2.4.4. Cytotoxicity (Tetrazolium Assay WST-1)
2.4.5. Gene Expression (Reverse-Transcription–Quantitative Polymerase Chain Reaction)
2.4.6. Protein Synthesis (Multiplex Immunoassay)
2.5. Data Analysis
2.5.1. Outcome Variables
2.5.2. Statistical Analysis
3. Results
3.1. Morphological Characteristics (Scanning Electron Microscopy—SEM)
3.2. Cell Viability (Confocal Scanning Laser Microscopy—CSLM)
3.3. Cell Proliferation (Tetrazolium Assay WST-1)
3.4. Cytotoxicity (Tetrazolium Assay WST-1)
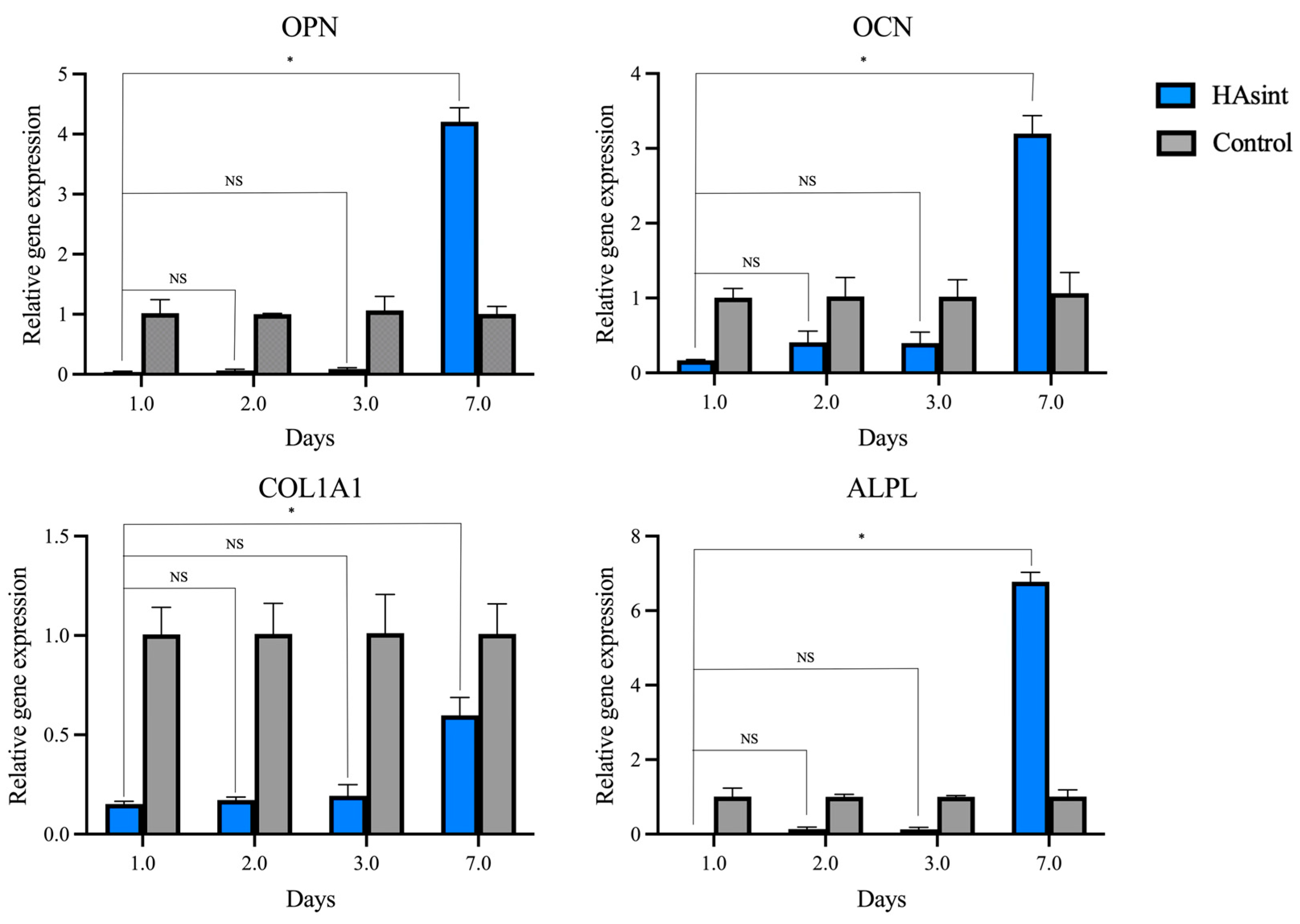
3.5. Gene Expression (Reverse-Transcription–Quantitative Polymerase Chain Reaction)
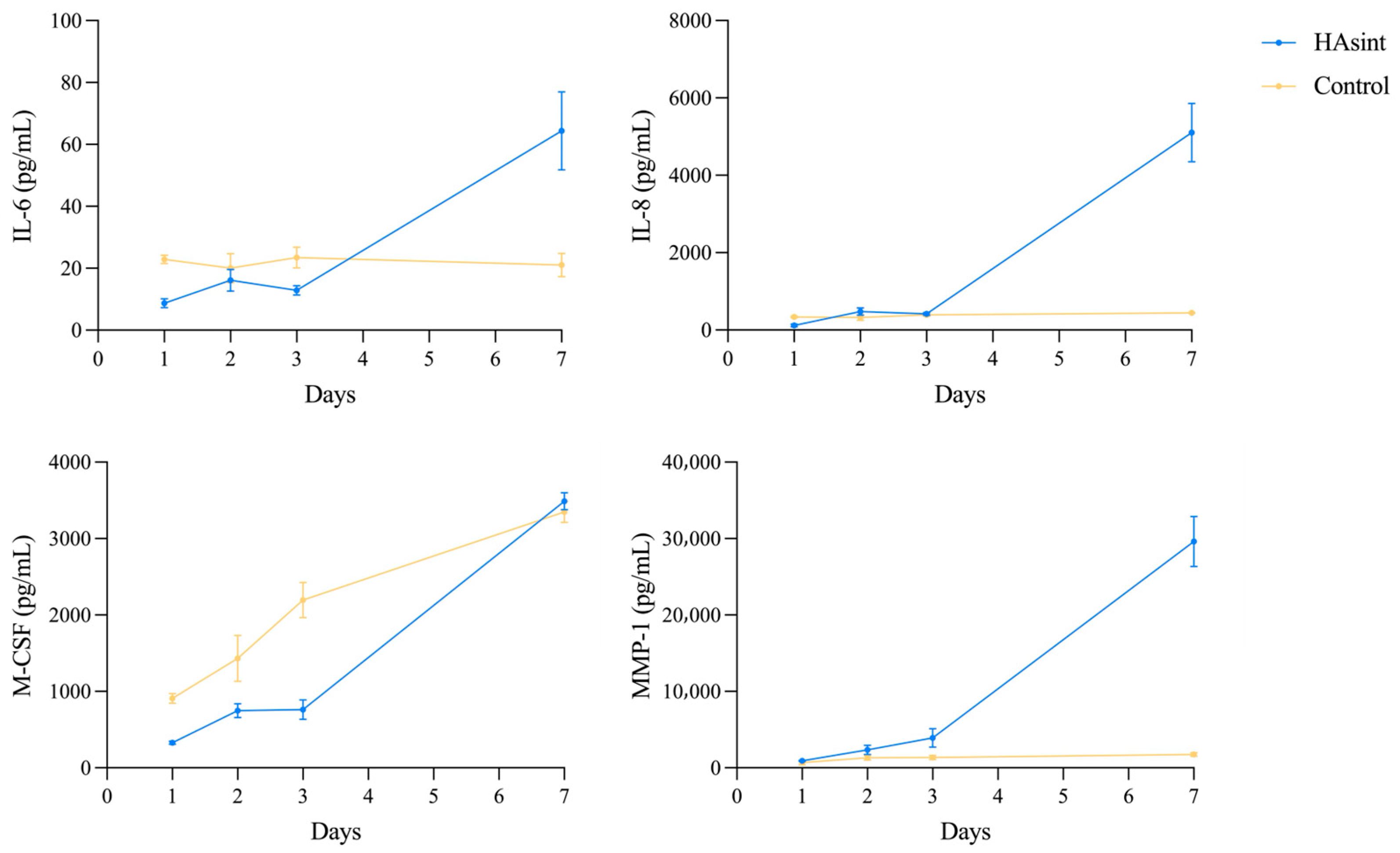
3.6. Protein Synthesis (Multiplex Immunoassay)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLSM | Confocal scanning laser microscopy |
| COL1A1 | Collagen type 1 |
| FFF | Fused filament fabrication |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HA | Hydroxyapatite |
| HAsint | Sintered hydroxyapatite |
| IL | Interleukin |
| M-CSF | Macrophage colony-stimulating factor |
| MMP | Matrix metalloproteinase |
| PLA | Polylactic acid |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| RT-qPCR | Reverse transcription–quantitative polymerase chain reaction |
| SEM | Scanning electron microscopy |
| TBP | TATA-box binding protein |
| WST | Water-soluble tetrazolium |
Appendix A
| Gene | Primer | Sequence |
|---|---|---|
| TBP | Forward | 5’-TGTATCCACAGTGAATCTTGGTTG-3’ |
| Reverse | 5’-GGTTCGTGGCTCTCTTATCCTC-3’ | |
| GAPDH | Forward | 5’-GTCTCCTCTGACTTCAACAGCG-3’ |
| Reverse | 5’-ACCACCCTGTTGCTGTAGCCAA-3’ | |
| OPN | Forward | 5’-CGAGGTGATATAGTGTGGTTTATGG-3’ |
| Reverse | 5’-GCACCATTCAACTCCTCGCTTTC-3’ | |
| OCN | Forward | 5’-CGCTACCTGTATCAATGGCTGG-3’ |
| Reverse | 5’-CTCCTGAAAGCCGATGTGGTCA-3’ | |
| COL1A1 | Forward | 5’-GATTCCCTGGACCTAAAGGTGC-3’ |
| Reverse | 5’-AGCCTCTCCATCTTTGCCAGCA-3’ | |
| ALPL | Forward | 5’-GCTGTAAGGACATCGCCTACCA-3’ |
| Reverse | 5’-CCTGGCTTTCTCGTCACTCTCA-3’ |
| Gene Expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Infill | N | Relative Gene Expression (Mean (SD)) | p Value (Between Timepoints) | |||||
| 24 h | 48 h | 72 h | 7 D | 24 h vs. 48 h | 24 h vs. 72 h | 24 h vs. 7 D | |||
| OPN | HAsint | 3 | 0.04 (0.01) | 0.07 (0.02) | 0.09 (0.02) | 4.21 (0.23) | 0.764 | 0.337 | 0.005 |
| Control | 3 | 1.02 (0.22) | 1.00 (0.01) | 1.06 (0.23) | 1.00 (0.12) | 1.000 | 1.000 | 1.000 | |
| p value (between groups) | 0.090 | <0.001 | 0.096 | 0.002 | |||||
| OCN | HAsint | 3 | 0.17 (0.01) | 0.41 (0.15) | 0.40 (0.15) | 3.20 (0.24) | 1.000 | 1.000 | <0.001 |
| Control | 3 | 1.00 (0.12) | 1.02 (0.26) | 1.02 (0.23) | 1.07 (0.27) | 1.000 | 1.000 | 1.000 | |
| p value (between groups) | 0.002 | 0.043 | 0.039 | <0.001 | |||||
| COL1A1 | HAsint | 3 | 0.15 (0.01) | 0.17 (0.02) | 0.19 (0.06) | 0.60 (0.09) | 1.000 | 1.000 | 0.009 |
| Control | 3 | 1.00 (0.14) | 1.00 (0.15) | 1.01 (0.20) | 1.00 (0.15) | 1.000 | 1.000 | 1.000 | |
| p value (between groups) | <0.001 | <0.001 | <0.001 | 0.019 | |||||
| ALPL | HAsint | 3 | 0.00 (0.00) | 0.13 (0.06) | 0.13 (0.05) | 6.77 (0.26) | 0.283 | 0.283 | 0.049 |
| Control | 3 | 1.01 (0.22) | 1.00 (0.07) | 1.00 (0.03) | 1.01 (0.18) | 1.000 | 1.000 | 1.000 | |
| p value (between groups) | 0.281 | 0.030 | 0.002 | 0.015 | |||||
| Protein Synthesis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Infill | N | Concentration pg/mL (Mean (SD)) | p Value (Between Timepoints) | |||||
| 24 h | 48 h | 72 h | 7 D | 24 h vs. 48 h | 24 h vs. 72 h | 24 h vs. 7 D | |||
| IL-6 | HAsint | 4 | 8.68 (1.44) | 16.16 (3.53) | 12.87 (1.49) | 64.35 (12.57) | 0.165 | 0.092 | 0.024 |
| Control | 3 | 22.91 (1.35) | 20.03 (4.72) | 23.43 (3.33) | 21.05 (3.77) | 0.985 | 1.000 | 0.998 | |
| p value (between groups) | <0.001 | 0.973 | 0.135 | 0.038 | |||||
| IL-8 | HAsint | 4 | 115.82 (31.02) | 475.85 (95.66) | 417.52 (32.70) | 4970.39 (756.44) | 0.029 | <0.001 | 0.008 |
| Control | 3 | 338.10 (20.06) | 326.54 (77.52) | 392.99 (37.52) | 444.07 (9.67) | 1.000 | 0.613 | 0.034 | |
| p value (between groups) | 0.001 | 0.570 | 0.998 | 0.010 | |||||
| M-CSF | HAsint | 4 | 327.62 (20.14) | 748.23 (91.14) | 762.60 (129.08) | 3488.53 (109.91) | 0.020 | 0.015 | <0.001 |
| Control | 3 | 908.80 (63.56) | 1431.56 (299.87) | 2194,41 (231.74) | 3347.19 (137.61) | 0.010 | <0.001 | <0.001 | |
| p value (between groups) | 0.002 | <0.001 | <0.001 | 1.000 | |||||
| MMP-1 | HAsint | 4 | 910.23 (71.25) | 2350.36 (593.97) | 3921.86 (1209.48) | 29,626.07 (3271.42) | 0.259 | 0.249 | 0.023 |
| Control | 3 | 679.47 (79.49) | 1332.80 (319.91) | 1341.54 (288.40) | 1745.65 (254.07) | 0.490 | 0.434 | 0.112 | |
| p value (between groups) | 0.950 | 0.482 | 0.316 | 0.024 | |||||
References
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Craniomaxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Wang, X.; Mu, M.; Yan, J.; Han, B.; Ye, R.; Guo, G. 3D printing materials and 3D printed surgical devices in oral and maxillofacial surgery: Design, workflow and effectiveness. Regen. Biomater. 2024, 11, rbae066. [Google Scholar] [CrossRef]
- Ivanovski, S.; Breik, O.; Carluccio, D.; Alayan, J.; Staples, R.; Vaquette, C. 3D printing for bone regeneration: Challenges and opportunities for achieving predictability. Periodontol. 2000 2023, 93, 358–384. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Naghib, S.M. 3D and 4D printing hydroxyapatite-based scaffolds for bone tissue engineering and regeneration. Heliyon 2023, 9, e19363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Ma, Y.; Han, B.; Niu, X.; Liu, J.; Gao, L.; Wang, J.; Zhai, X.; Chu, K.; et al. Preparation of poly(lactic acid)/sintered hydroxyapatite composite biomaterial by supercritical CO2. Biomed. Mater. Eng. 2018, 29, 67–79. [Google Scholar] [CrossRef]
- Shan, E.; Chamorro, C.; Ferrández-Montero, A.; Martin-Rodriguez, R.M.; Ferrari, B.; Sanchez-Herencia, A.J.; Virto, L.; Marín, M.J.; Figuero, E.; Sanz, M. In Vitro Biological Properties Assessment of 3D-Printed Hydroxyapatite-Polylactic Acid Scaffolds Intended for Bone Regeneration. J. Funct. Biomater. 2025, 16, 218. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Ortega-Columbrans, P.; Eguiluz, A.; Sanchez-Herencia, A.J.; Detsch, R.; Boccaccini, A.R.; Ferrari, B. Biocompatible colloidal feedstock for material extrusion processing of bioceramic-based scaffolds. Polym. Compos. 2024, 45, 7237–7255. [Google Scholar] [CrossRef]
- Mathiazhagan, N.; Palaniyappan, S.; Sivakumar, N.K. Effect of fused filament fabrication parameters on crashworthiness studies of hydroxyapatite particle reinforced PLA composite thin-walled tubes. J. Mech. Behav. Biomed. Mater. 2023, 138, 105611. [Google Scholar] [CrossRef]
- Winarso, R.; Anggoro, P.W.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Application of fused deposition modeling (FDM) on bone scaffold manufacturing process: A review. Heliyon 2022, 8, e11701. [Google Scholar] [CrossRef]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2017, 9, 012001. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Montagna, F.; Maiullaro, T.; Sannino, A.; Maffezzoli, A. 3D printing of hydroxyapatite polymer-based composites for bone tissue engineering. J. Polym. Eng. 2017, 37, 741–746. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, F.; Politi, S.; Ul Haq, A.; De Matteis, F.; Tamburri, E.; Terranova, M.L.; Teodori, L.; Pasquo, A.; Di Nardo, P. From Soft to Hard Biomimetic Materials: Tuning Micro/Nano-Architecture of Scaffolds for Tissue Regeneration. Micromachines 2022, 13, 780. [Google Scholar] [CrossRef]
- Cordell, J.M.; Vogl, M.L.; Wagoner Johnson, A.J. The influence of micropore size on the mechanical properties of bulk hydroxyapatite and hydroxyapatite scaffolds. J. Mech. Behav. Biomed. Mater. 2009, 2, 560–570. [Google Scholar] [CrossRef]
- Wang, S.; Kowal, T.J.; Marei, M.K.; Falk, M.M.; Jain, H. Nanoporosity significantly enhances the biological performance of engineered glass tissue scaffolds. Tissue Eng. Part A 2013, 19, 1632–1640. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Bhattacharya, I.; Guerrero, J.; Ozcan, M.; Weber, F.E. 3D-Printed HA-Based Scaffolds for Bone Regeneration: Microporosity, Osteoconduction and Osteoclastic Resorption. Materials 2022, 15, 1433. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, J.; Slane, J.; Nay, R.; Simpson, A.; Ploeg, H.L. The effect of sintering temperature on the microstructure and mechanical properties of a bioceramic bone scaffold. J. Mech. Behav. Biomed. Mater. 2011, 4, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Agarwal, A.K.; Rai, K.N.; Garg, A. Development of high strength hydroxyapatite by solid-state-sintering process. Ceram. Int. 2007, 33, 419–426. [Google Scholar] [CrossRef]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Zhou, C.; Zhang, X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9, 045008. [Google Scholar] [CrossRef]
- Bertone, P.M.; Olevsky, L.M.; Kathir, K.; Agnew, S.A.; Scheideler, W.J.; Hixon, K.R. Sintering 3D-Printed Hydroxyapatite-Wollastonite Lattices Improve Bioactivity and Mechanical Integrity for Bone Composite Scaffolds. bioRxiv 2025. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.W.; Heo, J.H.; Park, C.; Kim, D.H.; Yi, G.S.; Kang, H.C.; Jung, H.S.; Shin, H.; Lee, J.H. Natural bone-mimicking nanopore-incorporated hydroxyapatite scaffolds for enhanced bone tissue regeneration. Biomater. Res. 2022, 26, 7. [Google Scholar] [CrossRef]
- Patel, P.P.; Buckley, C.; Taylor, B.L.; Sahyoun, C.C.; Patel, S.D.; Mont, A.J.; Mai, L.; Patel, S.; Freeman, J.W. Mechanical and biological evaluation of a hydroxyapatite-reinforced scaffold for bone regeneration. J. Biomed. Mater. Res. Part A 2019, 107, 732–741. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Wu, B.; Huang, W. Effects of microwave sintering on the properties of porous hydroxyapatite scaffolds. Ceram. Int. 2013, 39, 2389–2395. [Google Scholar] [CrossRef]
- Ferrandez-Montero, A.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L.; Ferrari, B. New approach to improve polymer-Mg interface in biodegradable PLA/Mg composites through particle surface modification. Surf. Coat. Technol. 2020, 383, 125285. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Chirico, C.; Ferrández-Montero, A.; Eguiluz, Á.; Ortega-Columbrans, P.; Sanchez-Herencia, A.J.; Ferrari, B. Colloidal approach to fabricate high-loaded feedstocks for material extrusion of dense sintered Al2O3 structures for biomedical applications. Bol. Soc. Espa. Cerám. Vidr. 2025, 64, 102–121. [Google Scholar] [CrossRef]
- Schliephake, H.; Neukam, F.W.; Klosa, D. Influence of pore dimensions on bone ingrowth into porous hydroxylapatite blocks used as bone graft substitutes: A histometric study. Int. J. Oral Maxillofac. Surg. 1991, 20, 53–58. [Google Scholar] [CrossRef]
- Wilson, C.E.; de Bruijn, J.D.; van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W.J. Design and fabrication of standardized hydroxyapatite scaffolds with a defined macro-architecture by rapid prototyping for bone-tissue-engineering research. J. Biomed. Mater. Res. Part A 2004, 68, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Miranda, G.; Silva, F.; Pinto, P.; Carvalho, Ó. Review on current limits and potentialities of technologies for biomedical ceramic scaffolds production. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Bogala, M.R. Three-dimensional (3D) printing of hydroxyapatite-based scaffolds: A review. Bioprinting 2022, 28, e00244. [Google Scholar] [CrossRef]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive Manufacturing Methods for Producing Hydroxyapatite and Hydroxyapatite-Based Composite Scaffolds: A Review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Butscher, A.; Bohner, M.; Roth, C.; Ernstberger, A.; Heuberger, R.; Doebelin, N.; von Rohr, P.R.; Müller, R. Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater. 2012, 8, 373–385. [Google Scholar] [CrossRef]
- Gogolewski, S.; Mainil-Varlet, P. The effect of thermal treatment on sterility, molecular and mechanical properties of various polylactides: I. Poly(l-lactide). Biomaterials 1996, 17, 523–528. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2019, 43, 22–32. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, N. Effect of biomimetic 3D environment of an injectable polymeric scaffold on MG-63 osteoblastic-cell response. Mater. Sci. Eng. C 2010, 30, 1118–1128. [Google Scholar] [CrossRef]
- Chatree, K.; Sriboonaied, P.; Phetkong, C.; Wattananit, W.; Chanchao, C.; Charoenpanich, A. Distinctions in bone matrix nanostructure, composition, and formation between osteoblast-like cells, MG-63, and human mesenchymal stem cells, UE7T-13. Heliyon 2023, 9, e15556. [Google Scholar] [CrossRef]
- Docheva, D.; Padula, D.; Popov, C.; Mutschler, W.; Clausen-Schaumann, H.; Schieker, M. Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. J. Cell Mol. Med. 2008, 12, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, H.; Shi, T.; Xie, D.; Chen, R.; Han, X.; Shen, L.; Wang, C.; Tian, Z. Additive manufacturing of hydroxyapatite bone scaffolds via digital light processing and in vitro compatibility. Ceram. Int. 2019, 45, 11079–11086. [Google Scholar] [CrossRef]
- Retegi-Carrión, S.; Ferrandez-Montero, A.; Eguiluz, A.; Ferrari, B.; Abarrategi, A. The Effect of Ca2+ and Mg2+ Ions Loaded at Degradable PLA Membranes on the Proliferation and Osteoinduction of MSCs. Polymers 2022, 14, 2422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Lu, Q.Z.; Pei, R.; Ji, H.J.; Zhou, G.S.; Zhao, X.L.; Tang, R.K.; Zhang, M. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: Implication for bone tissue engineering. Biomed. Mater. 2009, 4, 025004. [Google Scholar] [CrossRef]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. “False” cytotoxicity of ions-adsorbing hydroxyapatite—Corrected method of cytotoxicity evaluation for ceramics of high specific surface area. Mater. Sci. Eng. C 2016, 65, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kikuchi, M.; Koyama, Y.; Takakuda, K. Osteogenic activity of MG63 cells on bone-like hydroxyapatite/collagen nanocomposite sponges. J. Mater. Sci. Mater. Med. 2010, 21, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filova, E.; Novak, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Born, A.K.; Rottmar, M.; Lischer, S.; Pleskova, M.; Bruinink, A.; Maniura-Weber, K. Correlating cell architecture with osteogenesis: First steps towards live single cell monitoring. Eur. Cell Mater. 2009, 18, 49–60, 1–2; discussion. [Google Scholar]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- Mangano, C.; Giuliani, A.; De Tullio, I.; Raspanti, M.; Piattelli, A.; Iezzi, G. Case Report: Histological and Histomorphometrical Results of a 3-D Printed Biphasic Calcium Phosphate Ceramic 7 Years After Insertion in a Human Maxillary Alveolar Ridge. Front. Bioeng. Biotechnol. 2021, 9, 614325. [Google Scholar] [CrossRef]


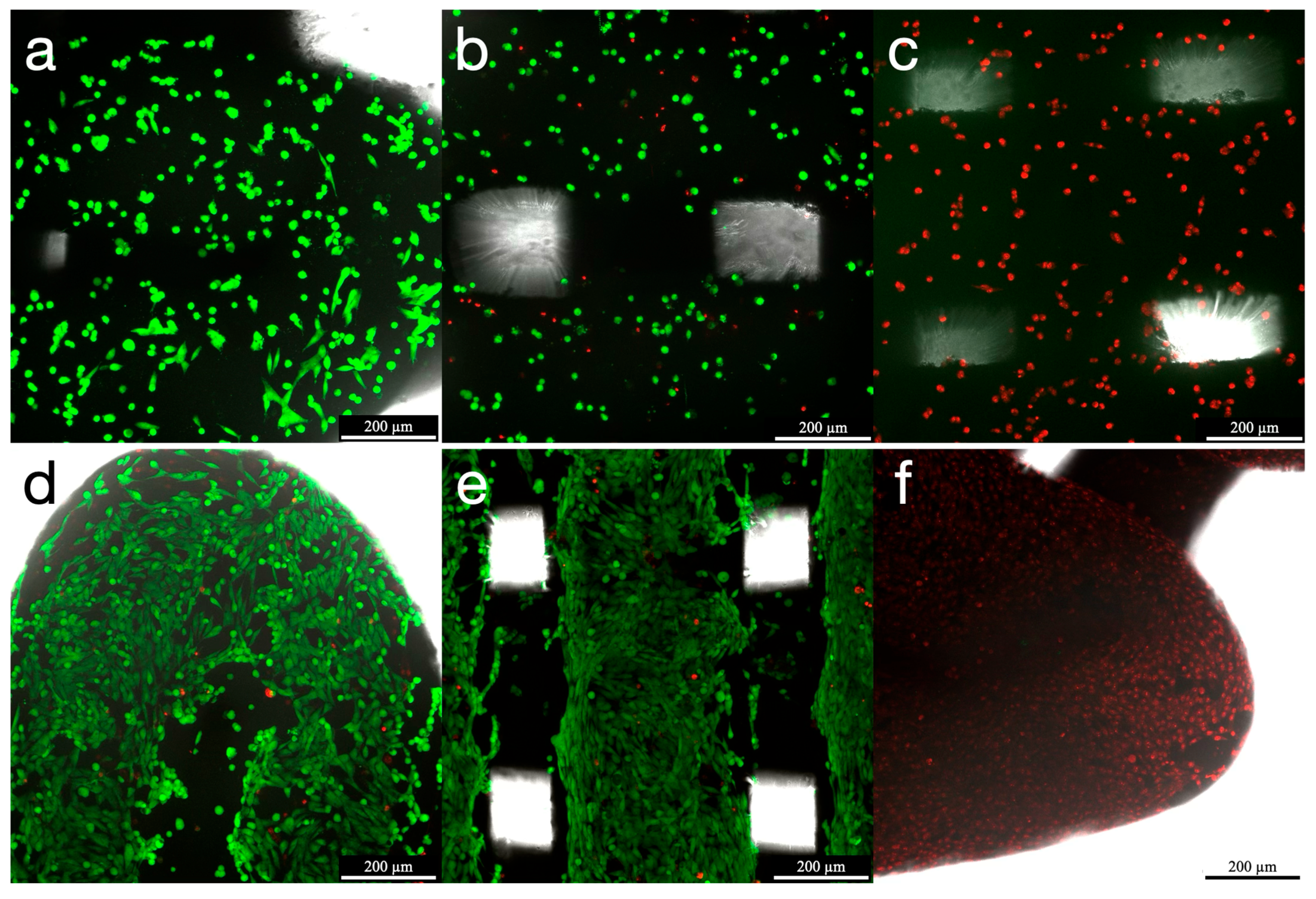
| Cell Proliferation Assay | ||||||||
|---|---|---|---|---|---|---|---|---|
| Infill | N | Absorbance (Mean SD) | p Value (Between Timepoints) | |||||
| 24 h | 48 h | 72 h | 7 D | 24 h vs. 48 h | 24 h vs. 72 h | 24 h vs. 7 D | ||
| HAsint | 6 | 0.42 (0.05) | 0.88 (0.23) | 1.40 (0.17) | 2.87 (0.23) | 0.049 | <0.001 | 0.001 |
| Control | 3 | 1.32 (0.04) | 2.47 (0.14) | 3.30 (0.06) | 4.78 (0.15) | 0.017 | <0.001 | 0.002 |
| p value (between groups) | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Indirect Cytotoxicity Assay | ||||||||
|---|---|---|---|---|---|---|---|---|
| Infill | N | Absorbance (Mean (SD)) | p Value (Between Timepoints) | |||||
| 24 h | 48 h | 72 h | 7 D | 24 h vs. 48 h | 24 h vs. 72 h | 24 h vs. 7 D | ||
| HAsint | 6 | 0.23 (0.02) | 0.21 (0.01) | 0.19 (0.01) | 0.22 (0.02) | 0.242 | 0.001 | 1.000 |
| Control | 3 | 0.28 (0.01) | 0.27 (0.02) | 0.27 (0.01) | 0.32 (0.02) | 1.000 | 1.000 | 0.235 |
| p value (between groups) | 0.002 | <0.001 | <0.001 | <0.001 | ||||
| Direct Cytotoxicity Assay | ||||||||
|---|---|---|---|---|---|---|---|---|
| Infill | N | Absorbance (Mean (SD)) | p Value (Between Timepoints) | |||||
| 24 h | 48 h | 72 h | 7 D | 24 h vs. 48 h | 24 h vs. 72 h | 24 h vs. 7 D | ||
| HAsint | 6 | 2.42 (0.22) | 3.62 (0.21) | 3.65 (0.26) | 1.72 (0.27) | <0.001 | <0.001 | <0.001 |
| Control | 3 | 3.95 (0.17) | 4.64 (0.18) | 4.59 (0.22) | 5.16 (0.07) | 0.020 | 0.043 | <0.001 |
| p value (between groups) | <0.001 | <0.001 | <0.001 | <0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, E.; Chamorro, C.; Ferrández-Montero, A.; Martin-Rodriguez, R.M.; Virto, L.; Marín, M.J.; Ferrari, B.; Sanchez-Herencia, A.J.; Figuero, E.; Sanz, M. Biological Characterization of 3D-Printed, Sintered Hydroxyapatite Scaffolds Obtained by Fused Filament Fabrication: An In Vitro Study. J. Funct. Biomater. 2025, 16, 392. https://doi.org/10.3390/jfb16100392
Shan E, Chamorro C, Ferrández-Montero A, Martin-Rodriguez RM, Virto L, Marín MJ, Ferrari B, Sanchez-Herencia AJ, Figuero E, Sanz M. Biological Characterization of 3D-Printed, Sintered Hydroxyapatite Scaffolds Obtained by Fused Filament Fabrication: An In Vitro Study. Journal of Functional Biomaterials. 2025; 16(10):392. https://doi.org/10.3390/jfb16100392
Chicago/Turabian StyleShan, Eddy, Cristina Chamorro, Ana Ferrández-Montero, Rosa M. Martin-Rodriguez, Leire Virto, María José Marín, Begoña Ferrari, Antonio Javier Sanchez-Herencia, Elena Figuero, and Mariano Sanz. 2025. "Biological Characterization of 3D-Printed, Sintered Hydroxyapatite Scaffolds Obtained by Fused Filament Fabrication: An In Vitro Study" Journal of Functional Biomaterials 16, no. 10: 392. https://doi.org/10.3390/jfb16100392
APA StyleShan, E., Chamorro, C., Ferrández-Montero, A., Martin-Rodriguez, R. M., Virto, L., Marín, M. J., Ferrari, B., Sanchez-Herencia, A. J., Figuero, E., & Sanz, M. (2025). Biological Characterization of 3D-Printed, Sintered Hydroxyapatite Scaffolds Obtained by Fused Filament Fabrication: An In Vitro Study. Journal of Functional Biomaterials, 16(10), 392. https://doi.org/10.3390/jfb16100392











