1. Introduction
Aluminum (Al) toxicity represents a serious limitation to plant production in acid soils worldwide, as approximately 40–50% of the world’s total potential arable land consists of acidic soils [
1] Acid soils (pH 5.5 or lower) are globally distributed and comprise approximately 30% of the total area of the earth [
2]. Hence, soil acidification is a natural process which occurs mostly in tropical and subtropical regions. Several natural and/or anthropogenic inputs are responsible for accelerating soil acidification [
3]. The important causes of soil acidification on agricultural land are acidic precipitation (H
+ ions in precipitation), input of acidifying gasses or particles (i.e., SO
2; NO
3), contribution of nitric and hydrochloric acids (i.e., HNO
3; HCl) from the atmosphere, application of elemental sulfur (S), ammonium-based fertilizer (NH
4+), nutrient uptake by leguminous crops, and mineralization of organic matter [
4,
5,
6].
Impacts of soil acidification have been critically recorded on agricultural soil as well as pH level. The replacement of exchangeable base cations such as calcium (Ca
2+), magnesium (Mg
2+) and potassium (K
+) by H
+ and Al
3+, and the dissolution of Al-bearing and Mn minerals, and the dissolution of Fe-bearing minerals are the most significant consequences of soil acidifications. These three processes buffer the soil pH at approximately 5–6, 4, and 3, respectively [
5]. Consequently, metal toxicity (i.e., Mn, Fe, and Al) and nutrient imbalance (i.e., P) are found to occur in acid soils, wherein Al toxicity is the most significant threat to plant survival in acid soils [
3].
Al is the most abundant metal on earth; it is ubiquitously distributed as the third most abundant element in the earth’s crust that comprising 7–8% of its mass after oxygen and silicon [
3,
6]. However, the specific biological function of Al is still to be disclosed. The presence of Al could be marked easily in all forms of life as it is an integral component of mineral soil. Country specific soil acidity and concentration of Al in soils has been widely distribution worldwide (
Table 1). Al in the soil is mainly found incorporated in the form of minerals such as aluminum oxides or harmless aluminosilicates [
7]. However, solubilization and speciation of Al depend on the chemical environment and the pH of the soil solution [
8]. In acid soil at low pH (4.3), Al is solubilized into [Al(H
2O)
6]
3+ that usually referred as to Al
3+. Al also formulates other species such as Al(OH)
2+, Al(OH)
2+, Al(OH)
3−, and Al(OH)
4−, wherein Al
3+ is considered as the most toxic form that has a huge impact on plant growth and development [
6,
7,
9]. One of the major consequences and the most obvious symptom of Al toxicity is the root growth inhibition in plants [
10]. Excessive Al inhibits roots cell division–elongation, root hair formation, and enhances the development of swollen roots apices [
11]. Concurrently, toxic Al inhibits the uptake of water and nutrients by plants [
3]. Several reports have provided indications that toxic Al
3+ alters nutrient levels such as N, K, Ca, Mg, and P, and reduces the photosynthetic rate (
PN), stomatal conductance (gs), and leaf transpiration (
E) rate in plants [
7,
12,
13]. Surprisingly, the initial response to Al toxicity marked to be induced within a few minutes even at micro-molar (µM) concentrations of toxic Al in plant cells [
14]. The phytotoxic Al leads to generate excess reactive oxygen species (ROS) such as H
2O
2 and O
2, as those ROS were detected in the root tips of
Glycine max [
15], the leaves of
Oryza sativa [
16], and cells of
Nicotiana tabacum [
17]. Other phytotoxic effects of Al have been described in different cellular organelles; such as toxic Al-induced disruption of free cytosolic Ca
2+ [
18], callose deposition at the plasmodesmata [
19], and respiration inhibition in mitochondria [
20].
Several studies have shown the impact of Al toxicity on crop plants based on their sensitivity threshold to acid soils; wherein most of them mainly focused on roots and their growth [
21,
22,
23]. Other issues are included like Al protection, tolerance, and/or resistance mechanisms [
13,
24,
25,
26], Al effects on plant metabolism [
7], Al speciation and detoxification in plants [
9], the role of mitochondria in the Al response [
27], the link between Al chemistry and biology [
8], inhibition of auxins synthesis, and transportation inhibition by Al toxicity [
23], have been extensively studied in plants. Recently, it has been elucidated both toxic and beneficial impacts of Al in plants [
3], also clarified the accumulation, localization, and impacts of Al on various levels of the plant organs [
6], and identified the Al-induced genes in the root apex of buckwheat [
26]. Molecular and physiological mechanisms related to Al resistance and/or tolerance are extremely complex phenomena. As a part of natural selection, plants have evolved some specific mechanism to cope with Al toxicity. Exclusion was extensively mentioned as it the key approach to detoxifying Al toxicity in plants [
25]. Exudation chelating ligands, speciation of pH barriers at the root apoplasm or at the rhizosphere, immobilization of cell walls, selective permeability of the plasma membrane (PM), and Al efflux have been widely suggested as the mechanisms of Al-exclusion [
13,
21,
24,
25]. However, these resistance and tolerance mechanisms in plants are not mutually exclusive; rice (
Oriza sativa) and buckwheat (
Fagopyrum esculentum) for example benefit from both mechanisms [
28]. Al is a non-biodegradable metal that widely distributed in soil environmentglobally [
29]. Al is able to be transmitted through the food cycle. The impact of Al toxicity has been manifested not only in plants but also in animals and humans; thus, Al represents a critical threat to the whole system. For example, Al toxicity was reported to be involved in poor quality forage and fodder production, consequently it negatively impacts on grazing animals, cow milk and overall livestock production [
30]. Chaneysuggested that 1000 mg Al·kg
−1 should be the maximum in animal diet, although no results have been given for the time course analysis [
31]. At the beginning of the 21st century, soil acidity along with Al-toxicity related damage in crops resulted in huge economic losses of more than 600 million USD in the agricultural sector in Australia [
32]. Al is also recognized as a risk factor for human health. For instance, tea leaves appear to accumulate substantial amounts of Al [
33], and one-third of total Al is able to be transferred easily during the tea leaf infusion process, this Al can cause potential health problems for humans [
34]. Although, a trace amount of Al is reported to be available for absorption across the gastro-intestinal tract [
35], tea-drinkers should be warned about this health risk. In contrast, high levels of Al can induce chronic renal failure in humans [
36]. Based on several investigations, it has been hypothesized that high levels of Al in the human body are related to several diseases including osteomalacia fractures, encephalopathy, Parkinsonism dementia, and Alzheimer’s disease [
37,
38,
39].
Several conventional strategies for farmers have been proposed to ameliorate Al toxicity and/or decrease Al-accumulation through liming, P fertilizer, and the production of low Al-accumulating cultivars through genetic manipulation [
13]. In addition, developing a variety with new traits may take 5–10 years, and the entire process involves in a considerable amount of time and expense [
40]. Moreover, continuous application of P fertilizer and lime in soil is not only expensive but also environmentally risky [
41]. Therefore, low-cost effective and environmentally friendly approaches are in high demand. In this regard, the application of mineral nutrition would be a suitable strategy for minimizing Al toxicity in plants to acid soils.
Many crop plants have a range of susceptibility to acidic soils, and overall their performance is highly influenced by Al toxicity [
42]. Therefore, Al toxicity has emerged as the major limitation to agronomic performance in acidic soils. We updated here the information of several literature reviews provide the evidence on the exogenous application of mineral nutrients mitigating Al toxicity in plants. Consequently, the application of mineral nutrients to mitigate Al toxicity in plants exposed to acid soils is now the most recent and important research topic in this field.
The application of mineral nutrition provides several benefits such as (i) a cost-effective approach relative to other organic and inorganic amendments, (ii) little or low environmental risk of application, (iii) high availability of nutrients, (iv) few skills are needed to sustain crop yield, and (v) crop plants can easily take up mineral nutrition throughout the entire year. Therefore, more attention has been given to study plant–nutrient–soil interactions as well as to minimize Al toxicity in plants exposed to acid soil by nutritional amendments. The main objectives of this review were to: (a) present a comprehensive discussion of several factors affecting soil acidification, Al toxicity, and Al accumulation in plants; (b) understated how plant nutrition can be an effective strategy to minimize Al toxicity; (c) analyze how plants develop suitable strategies to enhance Al toxicity tolerance in the presence of mineral nutrition; and (d) explore the best nutrition management practice for minimizing Al toxicity in plants. This review updates the knowledge concerning the influence and distribution of Al toxicity in crop plants grown in acidic conditions, factors affecting Al toxicity and nutritional imbalance and homeostasis, and overall mechanisms related to the efficiency of different mineral nutrition strategies to mitigate Al toxicity in plants. Captivating materials have been distributed in the current literature pinning down Al toxicity at various levels of plant cells and organisms, the application of mineral nutrition would be an effective strategy to counteract Al toxicity. These updated findings might disclose new avenues for minerals leading to physiological, molecular and agricultural inquiries into Al toxicity that markedly advances our understanding.
2. Multiple Forms of Aluminum in the Soil Environment Relevant to Toxicity
Al represents approximately 7–8% of the total solid matter in the earth’s crusts, it ubiquitously distributed as different forms in soil environments [
3,
6]. Different forms of Al may exist in the soil such as inorganic, soluble, and/or organic forms. Inorganic forms of Al are exchangeable and are primarily bound to silicate clays, hydrous oxides, phosphates, and sulfates [
60]. A significant correlation has been found between soil pH and phytotoxicity of Al species. Hence, these multiple forms of Al, their concentrations, speciation, and toxicity in the soil environment depend on pH level and the chemistry of the soil solution [
8].
Several soluble forms of Al species such as Al
3+, Al(OH)
2+, Al(OH)
2+, and Al(OH)
4− have been shown to occur when the soil pH drops below 5 [
8,
61]. In acidic soil (pH < 5), Al is solubilized into [Al(H
2O)
6]
3+, usually referred to as Al
3+. The solubilization of Al occurs due to the inception of soil acidification which leads to the release of phytotoxic Al
3+ [
6]. This trivalent Al
3+, which is the most abundant form and very toxic, has the greatest impact on plant growth at pH < 4.3. In contrast, solution pH level increases and reduces the Al
3+ concentration by repeated deprotonation [
8]. At pH > 5–6, mononuclear species are reported to be formed, including Al(OH)
2+ and Al(OH)
2+, which are toxic to the dicotyledonous plants but not toxic as Al
3+ is initiated [
3]. At pH 7, the formation of gibbsite [Al(OH)
3], occurs; however, it is non-toxic in nature and relatively insoluble [
62]. At alkaline pH (>7), aluminate (Al(OH)
4−) was reported to be formed [
63]. Al(OH)
4− is not always toxic; for example, at a concentration of 25 micromolar it was shown to be non-toxic in red clover whereas in wheat it showed at pH 8–8.9 [
64]. Despite the above-mentioned speciation of Al, a highly toxic polynuclear Al species that is identified as “Al
13”, is reported to be toxic ten-fold higher than Al
3+ [
61]. Surprisingly, the soluble or exchangeable aluminum (Al
3+) is able to associate with a variety of organic and inorganic ligands. However, organic Al is formed when Al
3+ binds to several ligands (i.e., SO
42−, PO
43−, F
− etc.) in the soil solution to generate stable complexes [
62]. Exchangeable Al
3+ cations and assimilable Al can participate in the formation of the above types of complexes. Briefly, the above-mentioned facts suggest that Al speciation, level of Al toxicity, availability of Al in the soil, alteration of pH level, and complexation of ligands are influenced by the chemistry of soil environment.
3. Aluminum Uptake, Accumulation, and Toxicity Responses in Plants
The uptake of Al from the soil by vascular plants is a complex process that is highly influenced by the soil pH, and the chemical environment of the rhizosphere. Due to the lack of conclusive evidence on precise mechanisms regarding Al uptake, some questions about this issue have still to be answered. For example, in which form and process Al are taken up? Which is the most active cellular site for Al uptake? Where is the location of Al-loading into the xylem, and how the process is mediated? Al ions are taken up by plants mostly through the root system, and only to a limited extent do they penetrate the leaves. A body of knowledge indicates that Al is taken up as Al
3+ by an active process wherein root apices play a vital role in Al toxicity perception and response [
9,
62]. Though it is still a matter of debate whether Al uptake at the root surface occurs symplastically or apoplastically? Lazof et al. [
29] elucidated that a sizable amount of Al efficiently enters the root symplasm, but later it can affect the growth of the membrane on the cytosolic side. Surprisingly, the highest ratio of symplastic Al to total Al has been found at the root apex in buckwheat [
22]. Rengel [
65] suggested that the entrance pathway of Al
3+ is the root apoplast, as he found that approximately 30–90% of the total Al that is present in the root apoplasm could be acquired by a plant. Recently, an apoplasmic lesion caused by Al, wherein it is primarily bound to the outer cell wall after immediate exposure has been explored [
66].
Based on the Al concentration and organic acid exudation in root systems, several researchers have tried to determine the most active site for Al uptake in plants [
9]. The mature elongation zone above the root apex has been suggested as the Al uptake site in buckwheat, as the highest level of oxalic acid exudated from the apex [
67]. Klug et al. [
22] detected a high proportion of total Al at the root apex, thus, they suggested that the root apex was the most active site of Al uptake in plants. Transporters are often associated with the uptake and transport of metal into the plant system. Broadly, aquaporin (AQP) family members are responsible for Al transport in plants [
68].
Plants are capable of accumulating Al in above-ground tissues. Chenery, [
69,
70] classified plants as Al accumulators or non-accumulators after an investigation of 1000 plant species, plants that accumulate Al greater than 1000 mg Al·kg
−1 in their leaves or roots were marked as Al accumulators and those with less than 1000 mg Al kg
−1 were Al non-accumulators. Moreover, the author found that high Al accumulators were mostly woody plant species, wherein cereals were marked as lesser Al accumulators. For example,
Oriza sativa,
Glycine max, and
Zia mays were found to accumulate less than 500 mg Al·kg
−1 in the leaves. Rice accumulates less than 200 mg Al·kg
−1 in shoots and is reported as a potential Al excluder [
71]. In contrast,
Camellia sinensis was recognized as the highest accumulator species that accumulated 13,500 mg Al kg
−1 in the leaves. This result suggests that woody plants that normally thrive in soil with high concentrations of free Al have evolved internal mechanisms to cope with Al toxicity.
Al toxicity has dramatic impacts on plant growth and development that lead to significant yield reductions [
9]. One of the clearest signs of Al toxicity is the inhibition of root growth in plants. Direct impacts of Al toxicity can be estimated by the evaluation of plant parameters based short and long term responses. Based on the result of several investigations, a variety of physiological, molecular, and economical occurrences resulting from Al toxicity have been detected in plants (
Table 2). Moreover, it has been shown that the toxic Al tends to fix phosphorus (P) in a less available form which leads to a severe limitation of P availability for plant growth [
13].Toxic Al leads to the production of ROS in the root apex, decreases root respiration, reduces polysaccharide deposition in the cell wall, reduces DNA replication by enhancing the rigidity of the double helix, induces programmed cell death, and results in a decline in photosynthetic efficiency [
7,
25]. These impacts of Al toxicity in plants can be induced within a few minutes to hours [
14]. Rengel, [
65] demonstrated that the first 15 min is the shortest critical time for the detection of measurable symptoms of Al-toxicity in intact root cells. The first symptoms of Al toxicity resulting in root growth inhibition were detected in wheat after 1 h of Al exposure [
72]. Additionally, Al toxicity-induced disturbance of Ca
2+ ion passing across the plasma membrane has been recognized as one of the earliest responses in wheat root apical cells.
The responses of plants to Al toxicity can vary across the species, variation can also be found among the different genotypes of the same species. Cereals along with other cultivated crop plants which diversely responded under Al stress can be classified as sensitive or moderate sensitive to Al toxicity. According to several investigations, the responses of various plants to Al exposure have been presented in
Table 3. Among cereals,
Hordeum vulgare and
Triticum durum are considered the most sensitive crops to Al toxicity while
Oryza sativa is reported as a potential Al excluder that significantly prevents Al toxicity compared to other cereals [
71]. Notably,
Fagopyrum esculentum and
Camellia sinensis were able to store Al in above-ground tissue without symptoms of Al toxicity [
9].
Al tolerance has been reported in a number of plants species enabling them to grow on acid soil. These tolerance mechanisms are mainly involved with the chelation strategy in roots by means of organic acids, such as citrate, malate, etc. Among the members,
ALMT1 located in root plasma membrane, was associated with Al tolerance through the exudation of malate into the rhizosphere in few plant species [
73]. In addition,
NIP1;2 assists the Al-malate in
Arabidopsis [
68]. Yokosho et al. [
74] demonstrated that
FRDL4 gene responsible for citrate efflux, was involved in citrate secretion from rice roots. Further, Al toxicity was alleviated in transgenic
Arabidopsis roots while
Brassica oleracea MATE (
BoMATE) gene expression was more abundant in roots compared with wild-type plants. This gene was related to the of citrate exudation that confers Al tolerance in
Arabidopsis [
75].
Several reports have provided indications regarding the level of Al sensitivity in plants. It has been found that the inhibition of root growth occurs within in a few minutes to hours with a low concentration (µM) of toxic Al
3+ [
14]. Though in some cases low doses of Al have been reported to stimulate root and shoot growth of plants [
3]. This might be due to Al enhancing capability of meristematic regions. Concentration-dependent Al toxicity and its effects have been widely manifested in various plants. For example, Al toxicity-induced rhizodermal cracks were observed in ahipa roots following exposure of roots to Al (11 µM) stress. In cowpea (
Vigna unguiculata) the sensitivity threshold was observed at 0.1 µM Al wherein complete growth inhibition occurred at 40 µM Al [
93]. Several sensitive and tolerant cereals and legumes with their responses to Al toxicity have been explored whereas the rice was marked as tolerant compared to other cereals [
25]. Recently, nodulated legumes including common bean (
Phaseolus vulgaris), soybean (
Glycine max), and pea (
Pisum sativum) has been reported to be sensitive to Al toxicity wherein their sensitivity doses were greater than 25 µM, 4.7 µM, and 50 µM, respectively, though soybean growth was inhibited at 10 µM Al [
60].
4. Factors Affecting Aluminum Toxicity and Nutrient Imbalance
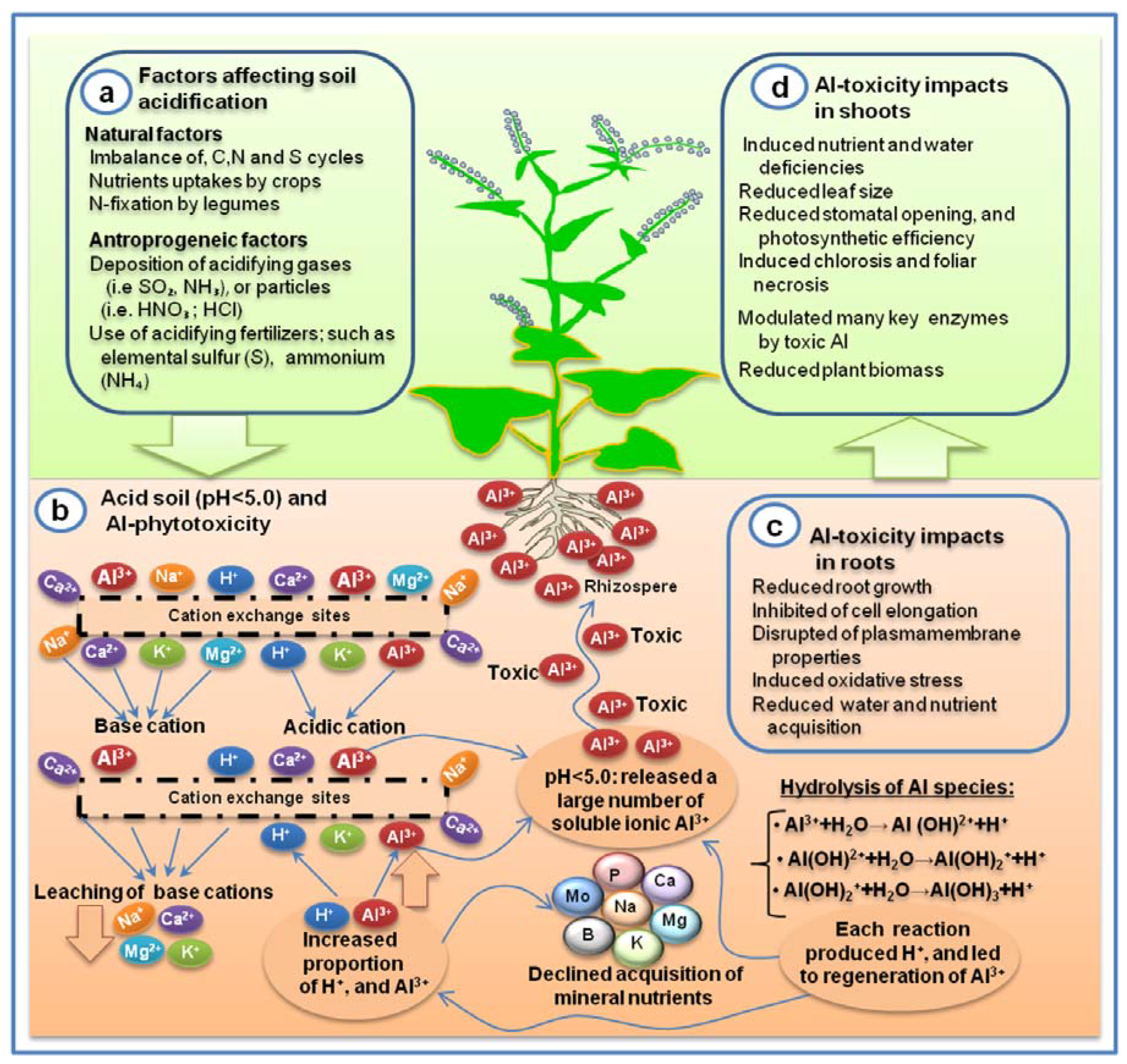
Soil acidification is an important factor that influences Al toxicity on agricultural land. The acidification of soil is caused by a number of natural and/or anthropogenic processes (
Figure 1). Deposition of atmospheric gases or particles such as SO
2, NH
3, HNO
3, and HCl; and application of acidifying fertilizer including elemental sulfur (S) or ammonium (NH
4) salt accelerated the soil acidification process that led to increased soluble Al
3+ concentrations in the soil solution [
5]. Moreover, the imbalance of N, S, and C cycles, uptake of N by legumes, and intensified leaching of base cations (BC) were responsible for increasing H
+ ions and decreasing soil pH level [
94]. Over 100 years ago, it was noted for the first time that the concentration of soluble Al
3+ increased in soils [
95], this Al
3+ was able to create phytotoxicity in the rhizosphere when the pH was below 5, and the most prominent sign of Al toxicity was considered the inhibition of root growth [
62].
Acid rain/deposition has dramatic impacts on leachability of essential nutrient cations, mobility of toxic element (Al
3+), and acidity development in soil [
96]. Basic and acidic cations are available at soil exchange sites or in the soil solution. Cation exchange sites that hold cations in the soil are negatively charged. Soil is buffered during acidification by a series of chemical processes resulting in the replacement of exchangeable base cations (Ca
2+, Mg
2+, K
+, and Na
+) by H
+ and Al
3+ at the cation exchange sites [
5]. Concurrently, the proportion of acidic cations such as H
+ and Al
3+ in the soil solution increases. Often, acid rain stimulates the leaching of base cations such as Ca
2+, Mg
2+, K
+, and Na
+ from soil. As a consequence, the essential nutrient cations such as Ca
2+, Mg
2+, and K
+ are leached resulting in the depletion of base cations (BC) from the soil. The significant losses of these nutrient cations from soil solution or soil exchange sites result in nutrient imbalance in the soil. However, acid soils contain high amounts of Al
3+ and a low amount of BC that are linked to deficiencies of important plant nutrients. Soil toxicity is known to be induced by the excess cations such as Mn
2+, Fe
3+, H
+ and Al
3+ [
3]. Among these, Al
3+ is the most critical cation that leads to rhizotoxicity and severely impairs plant growth in acid soil. A ratio of BC/Al
3+ less than 1 in soil solution was considered as a potential index for the adverse effect of soluble Al
3+ and nutrient imbalance for plant growth [
97]. Hence, the availability of BC and toxic Al
3+ in acid soil depends on acid rain pH, soil properties, cation exchange capacity (CEC), soil texture and initial base content in soil [
98].
Soil pH is often considered a master variable as it controls solubility, bioavailability, mobility, ionic speciation, and ultimately toxicity of any metal in the soil [
99]. Ion availability in soil solution is influenced by low-pH. For example, Mn oxide solubilizes at soil pH below 5.5, and releases Mn
2+ ions; at pH 4.3 a large amount of soluble Al
3+ is released; at pH < 3.8 Fe becomes the most exchangeable ion in the soil solution [
13,
62]. The pH-dependent metal toxicity is quite complex; acid deposition to soil promotes soil acidity wherein more soluble ions are released into the soil solution. Consequently, potential phytotoxicity of metal ions was found to be enhanced due to their increased availability and concentration in soil solution [
99]. The Al
3+ and Al(OH)
4− are often described as the major rhizotoxic Al species at low and high-Ph levels, respectively. At low pH (about 4.3) soluble ionic aluminum (Al
3+) is the most dominant form that is toxic for plant growth [
3]. Surprisingly, it appears that toxic Al species not only inhibit root grow at pH 4.3 but also at pH 8.0, though the concentration of Al species at pH 8.0 was lower compared to at pH 4.3 [
100]. Conversely, Al acquisition of root apices was higher at pH 8.0 compared to at pH 4.3. Calose (1,3-glucan) formation is often considered as the most perceptible indicator for Al toxicity that induced at pH 8.0, whereas the mobilization of callose was highly induced at pH 4.3 in the cortical region [
100,
101]. In addition, several nutrients such as P, K, Ca, Mg, Mo, and B contents were altered at low pH with Al toxicity [
6,
42]. So, the important note is that plants adapting to grow on low pH acid soils are threatened by the combination of Al toxicity and nutrient imbalance and/or deficiency.
The solubility of Al is an important factor that influences Al-availability, mobility, and toxicity in the environment [
102]. Al was reported to highly soluble at more acidic (pH < 6.0) and at more alkaline (pH > 8.0) conditions but relatively insoluble at pH 6.0–8.0 [gibbsite; Al(OH)
3] [
103]. This insoluble form of Al is considered less toxic compared to soluble Al
3+ [
104]. Hence, Al species existing in clay fractions are mainly in a less toxic form such as alumino-silicate or aluminum oxide. Soil acidification promotes the process of its solubilization and mobilization which lead to potential phytotoxicity [
105]. In acidic soil, when the pH drops (about 4.3) a large number of soluble Al ions (mostly Al
3+) are released, this toxic Al
3+ rapidly inhibits root elongation [
62,
100]. At neutral pH (7.0), Al hydroxide species (e.g., AlOH
3) are relatively insoluble but at pH above 7.5, Al species are formed as Al(OH)
4− and solubilized again [
61]. However, a relationship has been observed between pH and Al solubility, wherein the Al solubility increases when pH is below 4.5 in acid soil [
3]. In addition, Al toxicity is also known to be stimulated by the pH-dependent hydrolysis intensity of several Al species in soil solution [
6]. Hydrolysis of ions occurred when the charge/radius (z/r) ratio is large enough to break down the bond (H–O), and releases H
+ into the soil solution. pH-dependent hydrolysis of mononuclear Al species are presented by simple equations (
Figure 1), wherein each chemical reaction indicated the production of H
+ that leads to the generation of more soluble Al
3+ Hence, the hydrolysis of these Al species occurs at low pH (<5.0).
Toxic-Al interferes in the acquisition, accumulation, localization, and utilization of most of the mineral elements. For instance, the uptake of mineral nutrients such as Ca
2+ (69%), Mg
2+, K
+ (13%), and NH
4+ (40%) were inhibited by Al toxicity, and Al was known to be enhanced influx of the specific anions such as HN
3− (44%), and PO
43− [
42]. Al toxicity-induced nutritional imbalances have been widely manifested in several plant species. For instance, a distinct Al accumulating pattern with nutritional imbalance (e.g., Ca, Mg, P, and K) has been detected in eleven pteridophytes families [
106]. Al toxicity generally inhibits to uptake up macro-and micro elements plants, whereas tolerant cultivars found to be exhibited several macro elements including Ca and Mg [
12]. In wheat, both sensitive and tolerant genotypes manifested a marked reduction of K and Mg content whereas the concentration of Ca, Al and Si increased in roots [
78]. Hence, the sensitive genotypes exhibited higher Al accumulation and nutritional imbalance in both roots and shoots than the tolerant one.
Al-toxicity reduced K, Mg, Ca, and P accumulation in two contrasting rice cultivars, whereas the utilization of P, Ca and Mg increased more in tolerant cultivar than a sensitive plant [
107]. Simon et al. [
108] reported that Al exposure was involved in the reduction of Ca, K, Mg, Mn, Fe and Zn contents in tomato. Zobel et al. [
109] observed that the changes in fine root diameter with changes in nutrient such as N, P, and Al in plants. Additionally, the
specific absorption rate of B (SAR
B) was significantly influenced by Al-toxicity. Poschenrieder et al. [
110] detected a correlation between the Al-induced reduction of B absorption and the root growth inhibition in maize. From the above-mentioned literature it appears that Al toxicity induces the imbalance of nutrient uptake and acquisition in plants
6. Conclusions and Prospects
This extensive review clarified that Al toxicity can be effectively mitigated in plants by the application of nutrient elements in optimum quantities. Exogenous phosphorus (P) application is more beneficial during plants were found to be affected rigorously by soil acidification, Al toxicity and P deficiency. P was able to repair P-deficiency in acid soil, increased root respiration, plant growth, chlorophyll content, and dry matter yield. Ca amendment (liming) is more effective for correcting soil acidity and alleviating Al toxicity, but it takes more time to solubilize, and neutralize soil acidity. Moreover, continuous input of P-fertilizer, and lime in soil is expensive and environmentally risky. Gypsum (G) is attributed with better solubility (approximately 5-fold higher than lime), supplied dual benefits simultaneously as an alleviator of Al toxicity, and inducer of nutrients content. Magnesium (Mg) is effective for decreasing Al-activity at plasma membrane of root apex, and able to prevent Al toxicity-induced Ca cytotoxicity. Sulfur (S) can be used as several metals (Al, As) toxicity alleviator. The beneficial impact of exogenous Si-application has been widely recognized against Al toxicity in plants though it is still to be reconsidered as an essential element. Despite these above-mentioned facts, less attention has been given to clarify the potential roles of exogenous application of K, Mg, Zn, and Mo toward Al toxicity alleviation in plants to acidic soils.
Some other promising approaches have been applied considering miscellaneous mineral elements to ameliorate Al toxicity in plants to acid soils. For example, exogenous application of several low cost effective industrial byproducts (alkaline slag, coal fly ash, and red mud), hormones (auxin; IAA), organic acids (OAs; citrate, oxalate, and malate), polyamines (putrescine), biofertilizers (e.g., micorrhizal fungi, growth promoting bacteria), biochars (e.g., agricultural wastes, organic wastes) mitigated Al toxicity in plants to acidic soils. However, this review presents existing knowledge concerning the application of mineral nutrition on Al toxicity mitigation in plants that could be a potential approach for further crop plants improvement under Al toxicity and soil acidity worldwide.