The Effect of Ultrasound, Oxygen and Sunlight on the Stability of (−)-Epigallocatechin Gallate
Abstract
1. Introduction
2. Results
2.1. Method Validation
2.1.1. Selectivity and Specificity
2.1.2. Linearity and Lower Limit of Quantification (LLOQ) of EGCG in Water
2.1.3. Precision and Accuracy
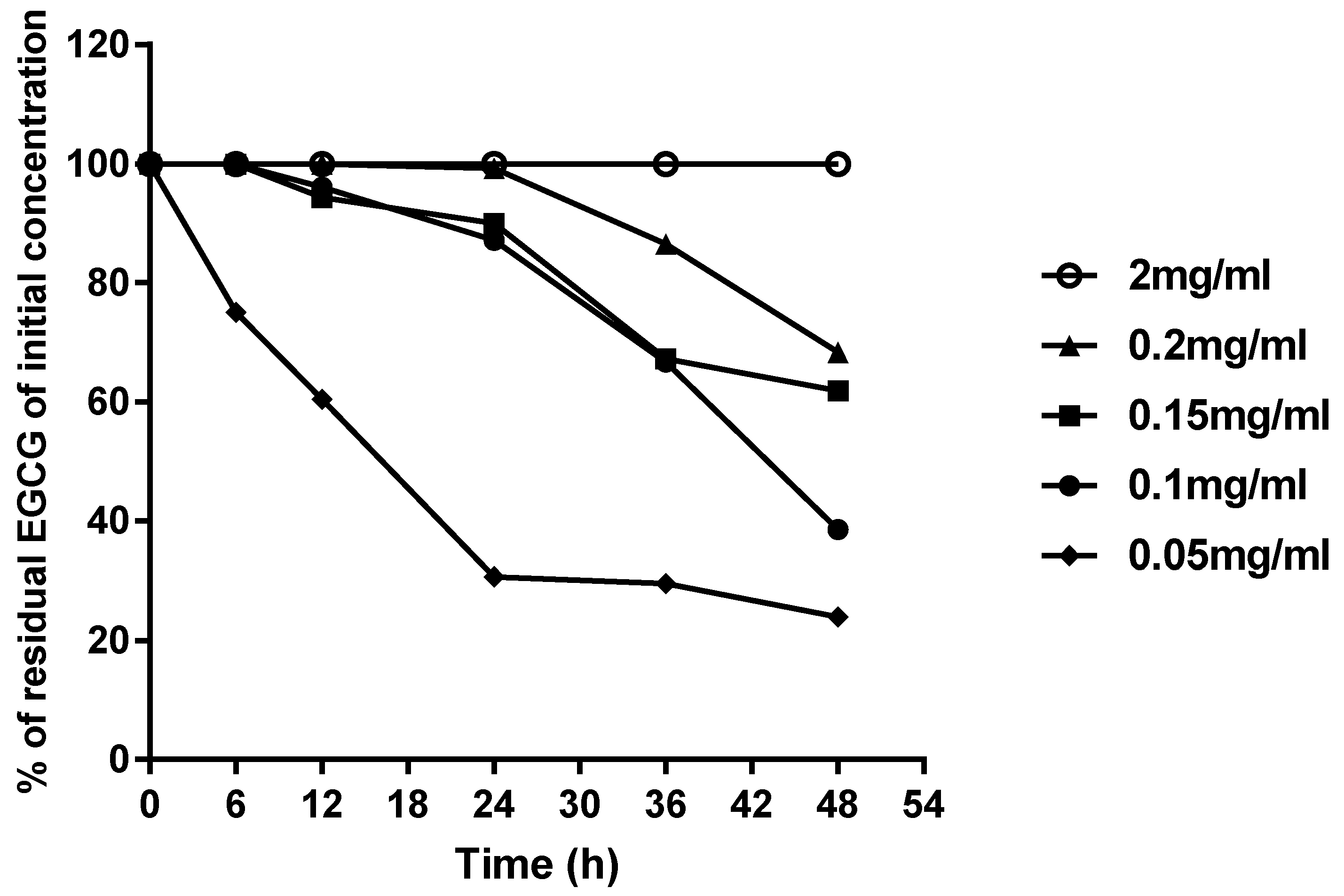
2.2. Analysis of Stability of EGCG Aqueous Solution under Different Storage Conditions
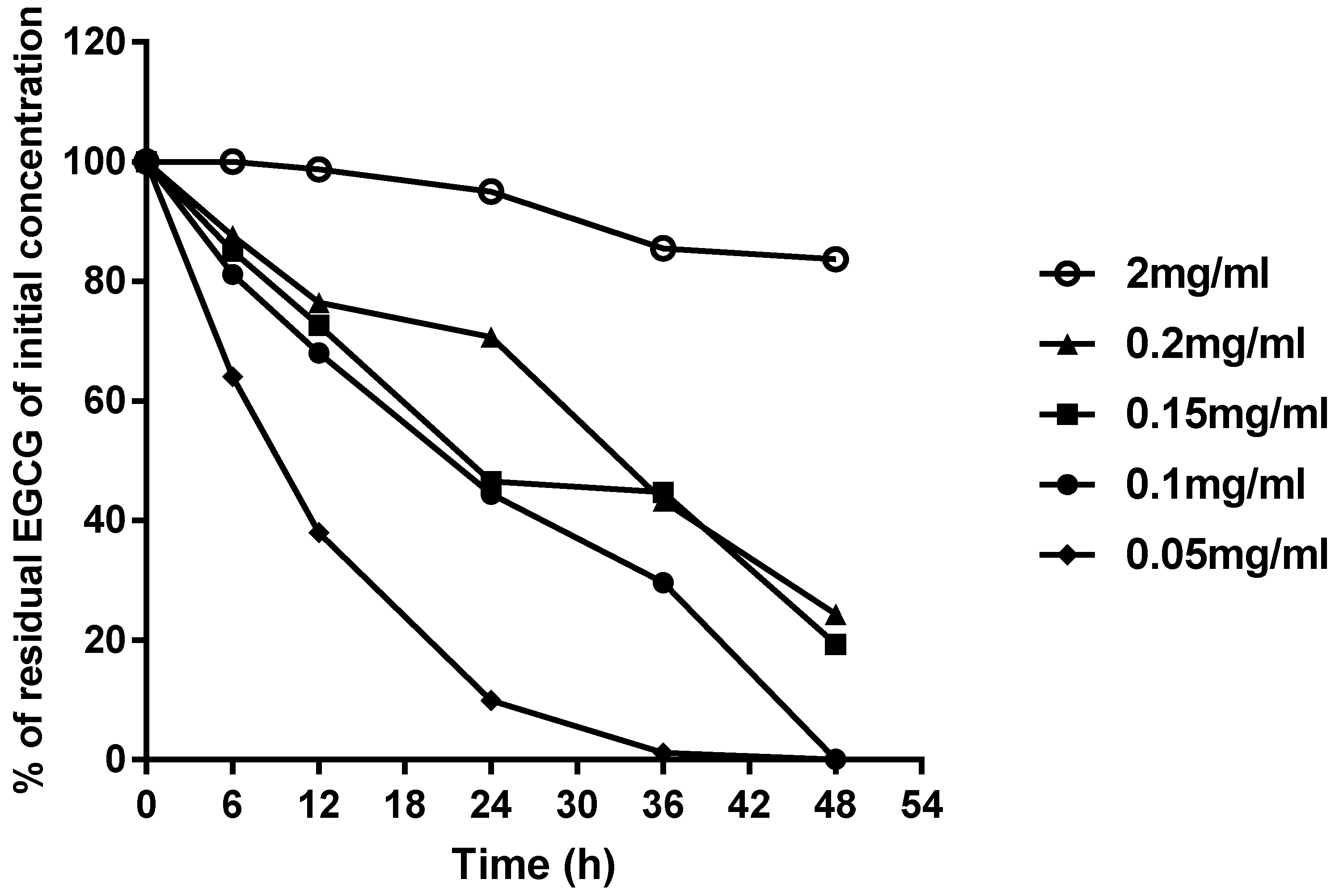
2.3. Analysis of Stability of EGCG Solution Atomized by the Ultrasonic Dental Scaler
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. High Performance Liquid Chromatography (HPLC) Analysis
4.3. Stability of EGCG Aqueous Solution under Different Storage Conditions
4.4. Stability of EGCG Aqueous Solution Atomized by the Ultrasonic Dental Scaler
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.E.; Harrington, K.L.; Vollmer, T.R.; Zhang, J.Z. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011, 17, 533–542. [Google Scholar] [PubMed]
- Zhong, Y.; Chiou, Y.S.; Pan, M.H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ma, C.M.; Shahidi, F. Antioxidant and antiviral activities of lipophilic epigallocatechin gallate (EGCG) derivatives. J. Funct. Foods 2012, 4, 87–93. [Google Scholar] [CrossRef]
- Yang, C.S.; Maliakal, P.; Meng, X. Inhibition of carcinogenesis by tea. Nature 1997, 389, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. CRC Crit. Rev. Food Technol. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Lee, M.J.; Lu, H.; Meng, X.; Ju, J.; Hong, J.; Seril, D.N.; Sturgill, M.G.; Yang, C.S. Epigallocatechin-3-Gallate Is Absorbed but Extensively Glucuronidated Following Oral Administration to Mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Ferguson, S.J.; Wuertzkozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yamazaki, S.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta. Gen. Subj. 2002, 1569, 35–44. [Google Scholar] [CrossRef]
- Bianchi, A.; Marchetti, N.; Scalia, S. Photodegradation of (−)-epigallocatechin-3-gallate in topical cream formulations and its photostabilization. J. Pharm. Biomed. Anal. 2011, 56, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Marchetti, N.; Bianchi, A. Comparative evaluation of different co-antioxidants on the photochemical- and functional-stability of epigallocatechin-3-gallate in topical creams exposed to simulated sunlight. Molecules 2013, 18, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Sang, S.; You, H.; Lee, M.J.; Hong, J.; Chin, K.V.; Yang, C.S. Mechanism of Action of (−)-Epigallocatechin-3-Gallate: Auto-oxidation–Dependent Inactivation of Epidermal Growth Factor Receptor and Direct Effects on Growth Inhibition in Human Esophageal Cancer KYSE 150 Cells. Cancer Res. 2005, 65, 8049–8056. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, Q.Y.; Tsang, D.; Huang, Y. Degradation of green tea catechins in tea drinks. J. Agric. Food Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lee, M.J.; Hou, Z.; Ho, C.T.; Yang, C.S. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, Cellular Uptake, Biotransformation, and Efflux of Tea Polyphenol (−)-Epigallocatechin-3-Gallate in HT-29 Human Colon Adenocarcinoma Cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar] [PubMed]
- Severino, J.F.; Goodman, B.A.; Kay, C.W.; Stolze, K.; Tunega, D.; Reichenauer, T.G.; Pirker, K.F. Free radicals generated during oxidation of green tea polyphenols: electron paramagnetic resonance spectroscopy combined with density functional theory calculations. Free Radical Biol. Med. 2009, 46, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Proniuk, S.; Liederer, B.M.; Blanchard, J. Preformulation study of epigallocatechin gallate, a promising antioxidant for topical skin cancer prevention. J. Pharm. Sci. 2002, 91, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Parra, A.; Silva, A.M.; Egea, M.A.; Souto, E.B.; Garcia, M.L.; Calpena, A.C. Validation of a high performance liquid chromatography method for the stabilization of epigallocatechin gallate. Int. J. Pharm. 2014, 475, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Lai, K.L.; Huang, Y.; Chen, Z.Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar]
- Wang, R.; Zhou, W.; Jiang, X. Reaction Kinetics of Degradation and Epimerization of Epigallocatechin Gallate (EGCG) in Aqueous System over a Wide Temperature Range. J. Agric. Food Chem. 2008, 56, 2694. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, W.; Wen, R.A. Kinetic study of the thermal stability of tea catechins in aqueous systems using a microwave reactor. J. Agric. Food Chem. 2006, 54, 5924–5932. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sano, M.; Yoshida, R.; Degawa, M.; Miyase, T.; Maeda-Yamamoto, M. Epimerization of tea catechins and O-methylated derivatives of (−)-epigallocatechin-3-O-gallate: relationship between epimerization and chemical structure. J. Agric. Food Chem. 2003, 51, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Z.; Yeung, S.Y.; Chang, Q.; Huang, Y.; Chen, Z.Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004, 91, 873–881. [Google Scholar] [PubMed]
- Ikeda, I.; Kobayashi, M.; Hamada, T.; Tsuda, K.; Goto, H.; Imaizumi, K.; Nozawa, A.; Sugimoto, A.; Kakuda, T. Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate. J. Agric. Food Chem. 2003, 51, 7303–7307. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.L.; Kim, C.W.; Kim, J.K.; Shin, H.J.; Baik, J.H. GCG-Rich Tea Catechins are Effective in Lowering Cholesterol and Triglyceride Concentrations in Hyperlipidemic Rats. Lipids 2008, 43, 419–429. [Google Scholar]
- Ramasamy, C. Potential natural antioxidants: adjuvant effect of green tea polyphenols in periodontal infections. Infect. Disord. Drug Targets 2015, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kroes, I.; Lepp, P.W.; Relman, D.A. Bacterial diversity within the human subgingival crevice. Proc. Natl. Acad. Sci. USA 1999, 96, 14547–14552. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Jain, S.; Kaur, A. Comparative evaluation of the antiplaque effectiveness of green tea catechin mouthwash with chlorhexidine gluconate. J. Indian Soc. Periodontol. 2014, 18, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Moghbel, A.; Farjzadeh, A.; Aghel, N.; Agheli, H.; Raisi, N. Formulation and Evaluation of Green Tea Antibacterial Mouthwash Effect on the Aerobic Mouth Bacterial Load. Jundishapur Sci. Med. J. 2010, 9, 56–60. [Google Scholar]
- Chava, V.K.; Vedula, B.D. Thermo-Reversible Green Tea Catechin Gel for Local Application in Chronic Periodontitis: A 4-Week Clinical Trial. J. Periodontol. 2013, 84, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Hattarki, S.A.; Pushpa, S.P.; Bhat, K. Evaluation of the efficacy of green tea catechins as an adjunct to scaling and root planing in the management of chronic periodontitis using PCR analysis: A clinical and microbiological study. J. Indian Soc. Periodontol. 2013, 17, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kudva, P.; Tabasum, S.T.; Shekhawat, N.K. Effect of green tea catechin, a local drug delivery system as an adjunct to scaling and root planing in chronic periodontitis patients: A clinicomicrobiological study. J. Indian Soc. Periodontol. 2011, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic Study of Catechin Stability: Effects of pH, Concentration, and Temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, H. Formulation and stability testing of photolabile drugs. Int. J. Pharm. 2001, 225, 1–14. [Google Scholar] [CrossRef]
- Asahi, Y.; Noiri, Y.; Miura, J.; Maezono, H.; Yamaguchi, M.; Yamamoto, R.; Azakami, H.; Hayashi, M.; Ebisu, S. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J. Appl. Microbiol. 2014, 116, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, M.; Takada, K.; Makimura, M.; Otake, S. Improvement of periodontal status by green tea catechin using a local delivery system: A clinical pilot study. J. Periodontal. Res. 2010, 37, 433–438. [Google Scholar] [CrossRef]
- Determination of Polyphenols and Catechins Content in Tea; GB/T 8313-2008; China National Standards: Shenzhen, China, 2008.
Sample Availability: Samples of the compounds are available from the corresponding author Qingxian Luan. |
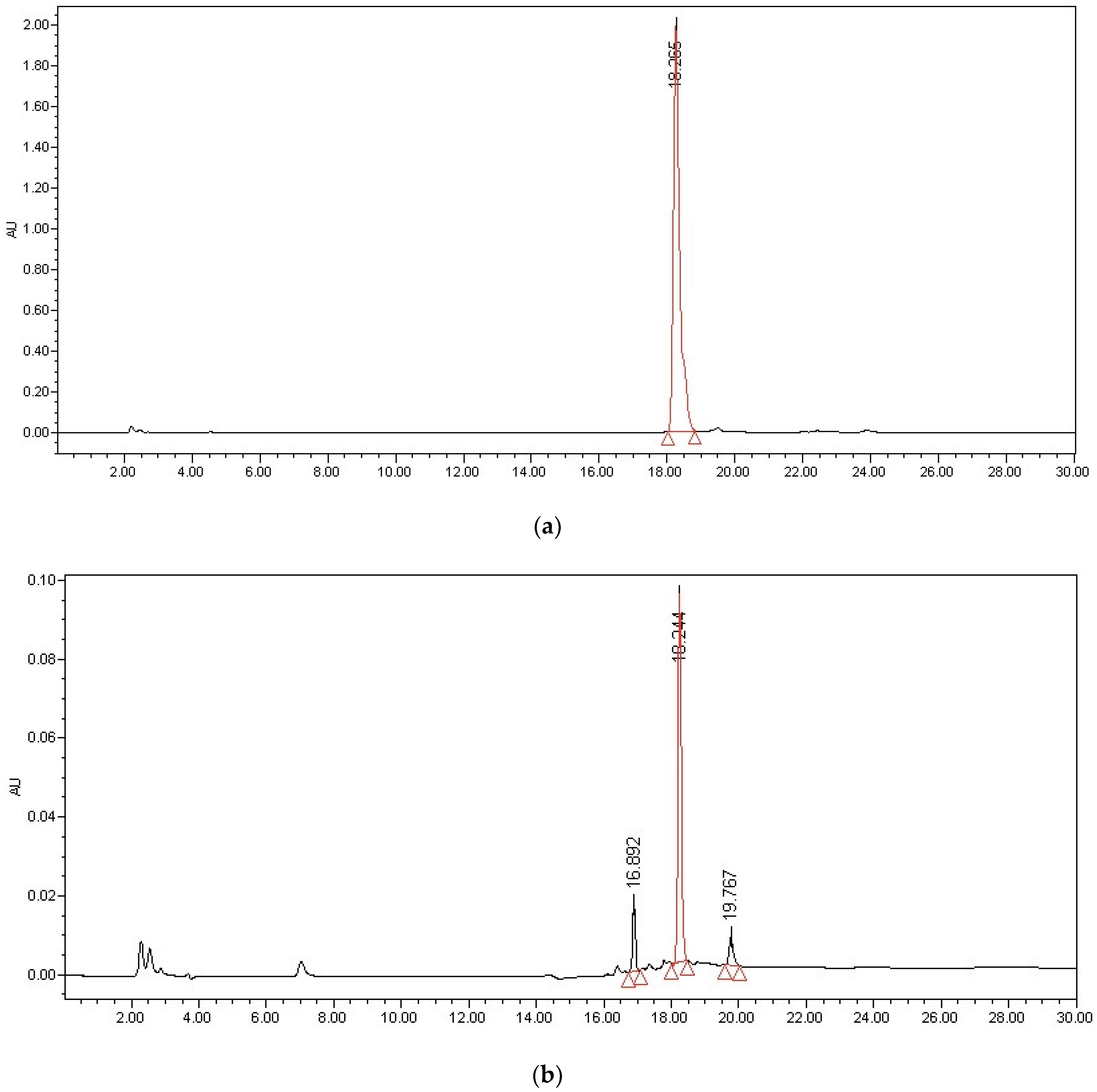
| Analyte | Calibration Curve | R2 | Linear Range (mg/mL) | LLOQ (mg/mL) |
|---|---|---|---|---|
| EGCG | Y = 12215845.6X + 9542.815 | 0.999 | 0.005–2.5 | 0.005 |
| Nominal Concentration (mg/mL) | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|
| Measured Concentration (mg/mL) | RSD (%) | Accuracy (%) | Measured Concentration (mg/mL) | RSD (%) | Accuracy (%) | |
| 0.203 | 0.201 ± 0.003 | 1.73 | 98.88 ± 1.71 | 0.189 ± 0.008 | 4.19 | 93.14 ± 3.90 |
| 2.109 | 2.099 ± 0.040 | 1.93 | 99.51 ± 1.92 | 2.071 ± 0.040 | 1.93 | 99.85 ± 1.90 |
| Initial Concentration (mg/mL) | Condition Name | Sunlight | Oxygen | Time (h) | ||||
|---|---|---|---|---|---|---|---|---|
| 6 h (mg/mL) | 12 h (mg/mL) | 24 h (mg/mL) | 36 h (mg/mL) | 48h (mg/mL) | ||||
| 0.05 | (A) | No | No | 0.038 ± 0.003 | 0.031 ± 0.004 | 0.015 ± 0.001 | 0.015 ± 0.001 | 0.012 ± 0.001 |
| (B) | Yes | No | 0.036 ± 0.006 | 0.027 ± 0.001 | 0.013 ± 0.000 | 0.009 ± 0.001 | 0.008 ± 0.001 | |
| (C) | No | Yes | 0.033 ± 0.009 | 0.020 ± 0.006 | 0.006 ± 0.001 | N | N | |
| (D) | Yes | Yes | 0.032 ± 0.004 | 0.019 ± 0.002 | 0.005 ± 0.001 | N | N | |
| 0.10 | (A) | No | No | 0.100 ± 0.006 | 0.090 ± 0.007 | 0.086 ± 0.005 | 0.070 ± 0.004 | 0.038 ± 0.002 |
| (B) | Yes | No | 0.093 ± 0.005 | 0.088 ± 0.003 | 0.071 ± 0.003 | 0.065 ± 0.003 | 0.028 ± 0.001 | |
| (C) | No | Yes | 0.082 ± 0.004 | 0.074 ± 0.004 | 0.055 ± 0.005 | 0.041 ± 0.002 | N | |
| (D) | Yes | Yes | 0.082 ± 0.009 | 0.068 ± 0.008 | 0.044 ± 0.007 | 0.030 ± 0.004 | N | |
| 0.15 | (A) | No | No | 0.150 ± 0.011 | 0.141 ± 0.004 | 0.135 ± 0.003 | 0.104 ± 0.003 | 0.093 ± 0.006 |
| (B) | Yes | No | 0.136 ± 0.005 | 0.136 ± 0.007 | 0.118 ± 0.004 | 0.089 ± 0.005 | 0.087 ± 0.001 | |
| (C) | No | Yes | 0.128 ± 0.007 | 0.121 ± 0.009 | 0.107 ± 0.002 | 0.072 ± 0.006 | 0.058 ± 0.003 | |
| (D) | Yes | Yes | 0.128 ± 0.010 | 0.109 ± 0.004 | 0.070 ± 0.003 | 0.068 ± 0.010 | 0.029 ± 0.002 | |
| 2.00 | (A) | No | No | 2.015 ± 0.031 | 2.000 ± 0.020 | 2.000 ± 0.040 | 1.998 ± 0.025 | 1.997 ± 0.031 |
| (B) | Yes | No | 2.004 ± 0.041 | 2.001 ± 0.039 | 2.000 ± 0.028 | 1.999 ± 0.050 | 1.997 ± 0.056 | |
| (C) | No | Yes | 2.003 ± 0.024 | 2.002 ± 0.018 | 2.000 ± 0.022 | 1.997 ± 0.046 | 1.991 ± 0.039 | |
| (D) | Yes | Yes | 1.947 ± 0.010 | 1.900 ± 0.028 | 1.893 ± 0.032 | 1.806 ± 0.036 | 1.786 ± 0.060 | |
| Effect Factor | Correlation Coefficient | p-Value |
|---|---|---|
| Sunlight | –0.091 | 0.425 |
| Oxygen | –0.266 | 0.017 * |
| Time | –0.522 | 0.000 * |
| Concentration | 0.706 | 0.000 * |
| Initial Concentration (mg/mL) | Sunlight | Oxygen | Test Group Concentration (mg/mL) | Control Group Concentration (mg/mL) | p-Value |
|---|---|---|---|---|---|
| 0.05 | No | No | 0.047 ± 0.002 | 0.052 ± 0.002 | 0.057 |
| Yes | No | 0.046 ± 0.005 | 0.224 | ||
| 0.10 | No | No | 0.099 ± 0.003 | 0.099 ± 0.002 | 0.803 |
| Yes | No | 0.099 ± 0.009 | 0.990 | ||
| 0.15 | No | No | 0.151 ± 0.002 | 0.149 ± 0.002 | 0.230 |
| Yes | No | 0.150 ± 0.001 | 0.157 | ||
| 2.00 | No | No | 2.010 ± 0.018 | 2.003 ± 0.040 | 0.592 |
| Yes | No | 2.005 ± 0.014 | 0.864 |
| Initial Concentration (mg/mL) | Sunlight | Oxygen | Test Group Concentration (mg/mL) | Intra-Group p-Value | Control Group Concentration (mg/mL) | Between Group p-Value |
|---|---|---|---|---|---|---|
| 0.05 | No | Yes | 0.036 ± 0.001 | 0.010* | 0.052 ± 0.002 | 0.001 * |
| Yes | Yes | 0.033 ± 0.001 | <0.001 * | |||
| 0.10 | No | Yes | 0.098 ± 0.009 | 0.484 | 0.099 ± 0.002 | 0.879 |
| Yes | Yes | 0.084 ± 0.005 | 0.028 * | |||
| 0.15 | No | Yes | 0.146 ± 0.002 | 0.053 | 0.099 ± 0.002 | 0.117 |
| Yes | Yes | 0.138 ± 0.004 | 0.040 * | |||
| 2.00 | No | Yes | 1.997 ± 0.049 | 0.882 | 2.003 ± 0.040 | 0.875 |
| Yes | Yes | 1.944 ± 0.032 | 0.094 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Xu, H.; Cai, Y.; Xuan, Y.; Liu, J.; Gao, Y.; Luan, Q. The Effect of Ultrasound, Oxygen and Sunlight on the Stability of (−)-Epigallocatechin Gallate. Molecules 2018, 23, 2394. https://doi.org/10.3390/molecules23092394
Zeng J, Xu H, Cai Y, Xuan Y, Liu J, Gao Y, Luan Q. The Effect of Ultrasound, Oxygen and Sunlight on the Stability of (−)-Epigallocatechin Gallate. Molecules. 2018; 23(9):2394. https://doi.org/10.3390/molecules23092394
Chicago/Turabian StyleZeng, Jiajun, Huanhua Xu, Yu Cai, Yan Xuan, Jia Liu, Yue Gao, and Qingxian Luan. 2018. "The Effect of Ultrasound, Oxygen and Sunlight on the Stability of (−)-Epigallocatechin Gallate" Molecules 23, no. 9: 2394. https://doi.org/10.3390/molecules23092394
APA StyleZeng, J., Xu, H., Cai, Y., Xuan, Y., Liu, J., Gao, Y., & Luan, Q. (2018). The Effect of Ultrasound, Oxygen and Sunlight on the Stability of (−)-Epigallocatechin Gallate. Molecules, 23(9), 2394. https://doi.org/10.3390/molecules23092394




