Chemical Fingerprinting and Quantification of Chinese Cinnamomi Cortex by Ultra High Performance Liquid Chromatography Coupled with Chemometrics Methods
Abstract
1. Introduction
2. Results and Discussion
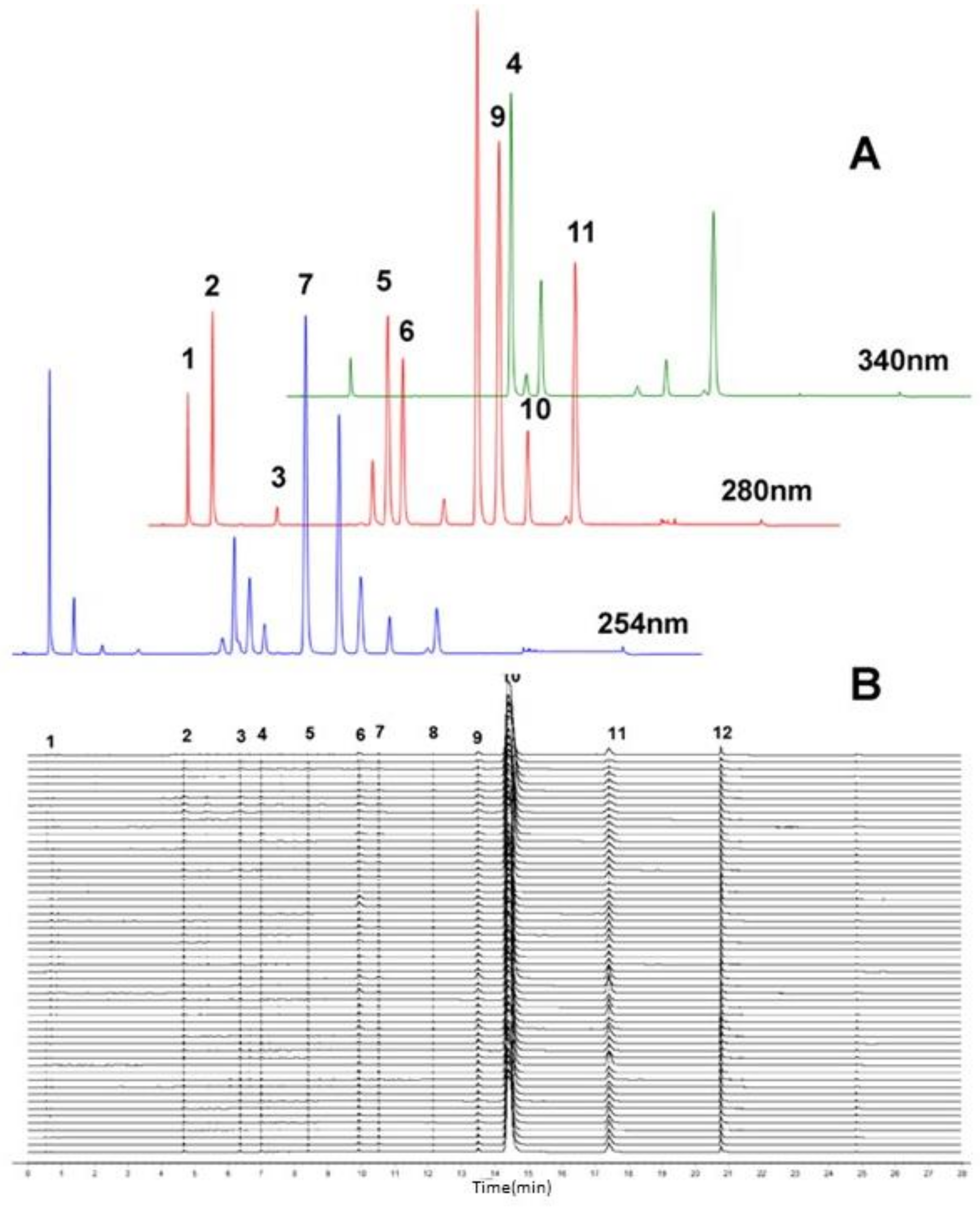
2.1. Optimum Conditions for HPLC-DAD Analysis
2.2. Similarity Evaluation
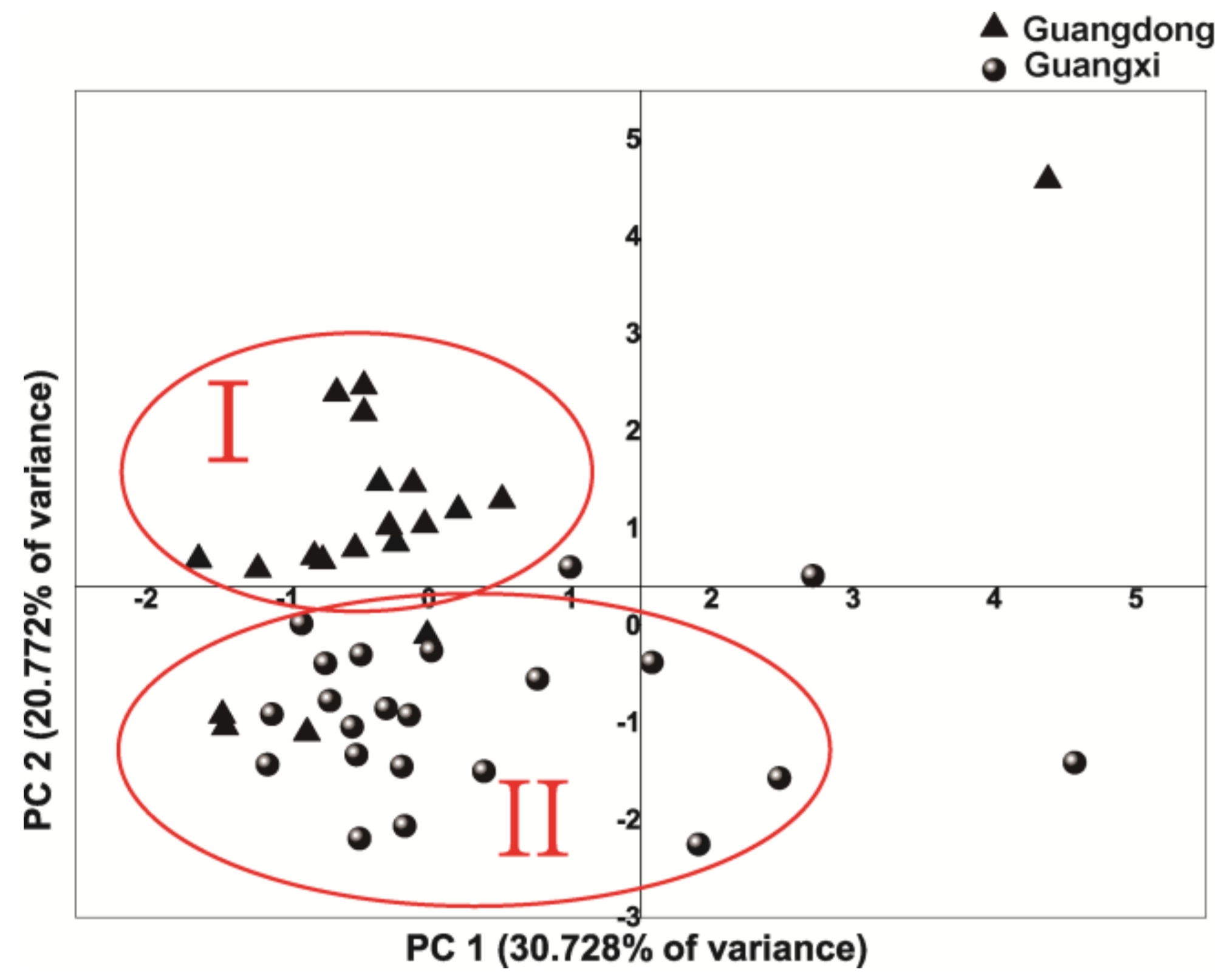
2.3. Principal Component Analysis (PCA)
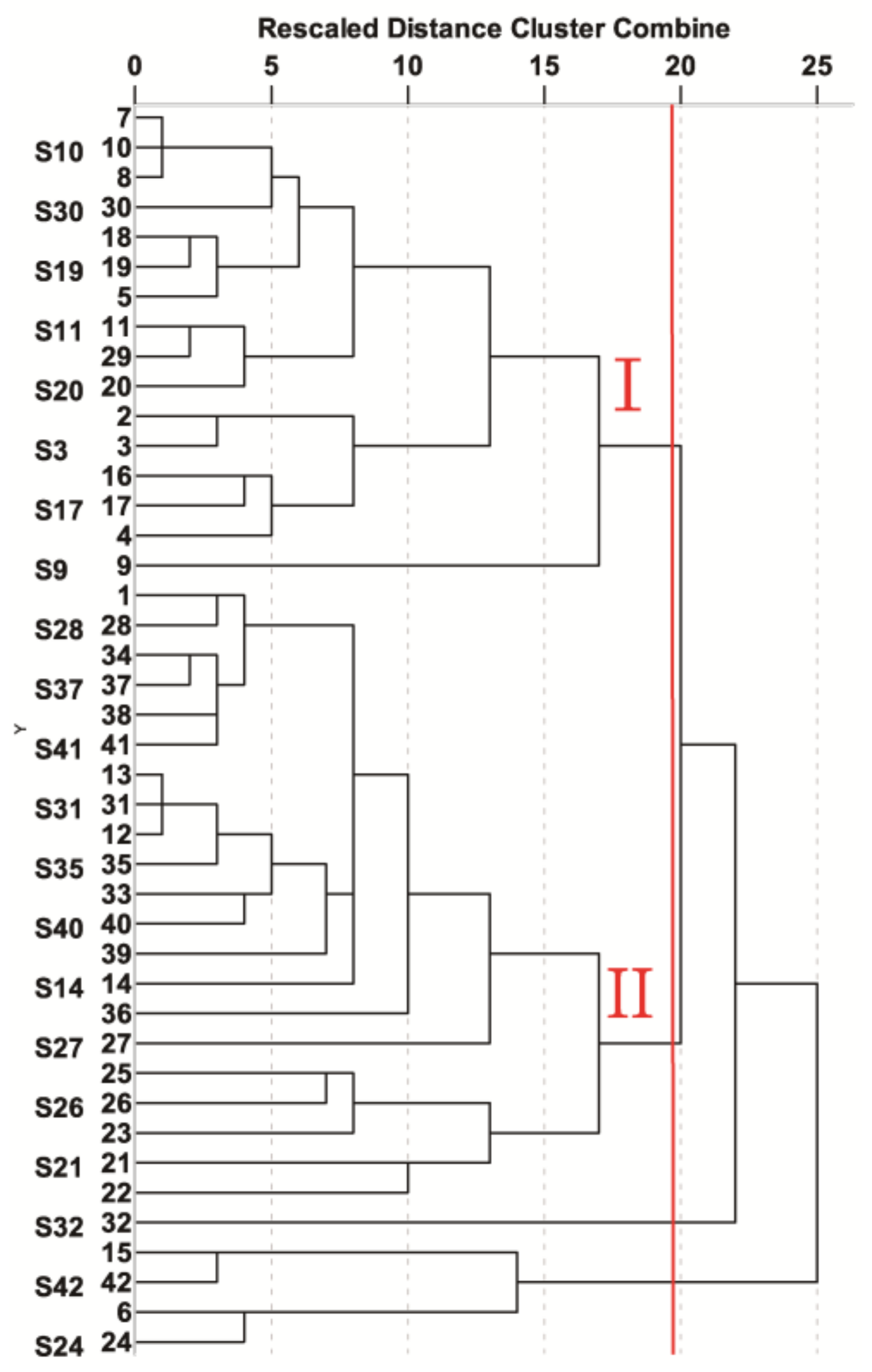
2.4. Hierarchical Clustering Analysis (HCA)
2.5. Structural Characterization of the Major Active Ingredients by HPLC-DAD-ESI-MS
2.6. Method Validation
2.6.1. Calibration Curves, Limits of Detection and Repeatability
2.6.2. Precision and Stability
2.6.3. Recovery
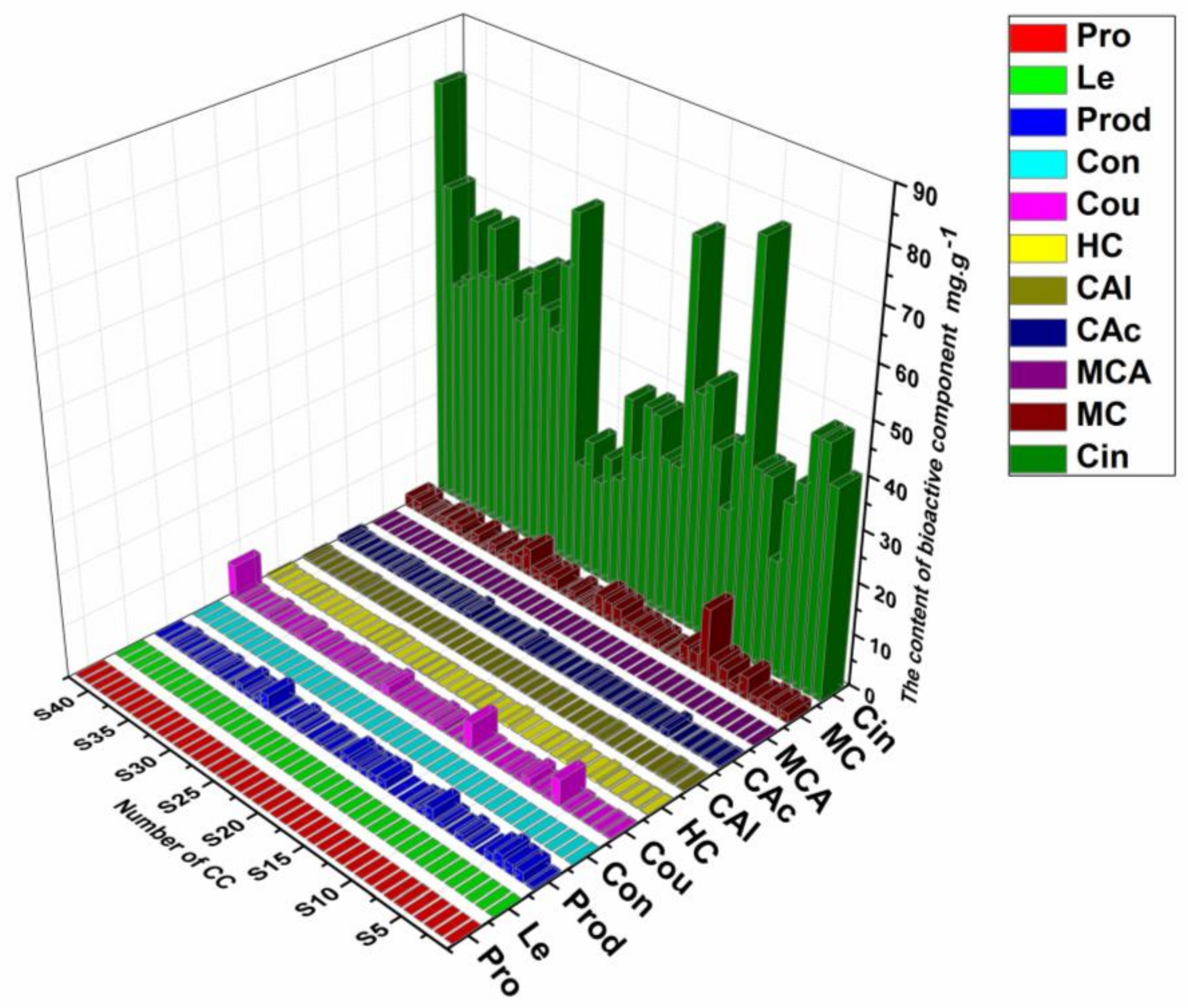
2.7. Quantitative Application
2.8. Comprehensive Evaluation of Different Cinnamomi Cortex Growth Stages
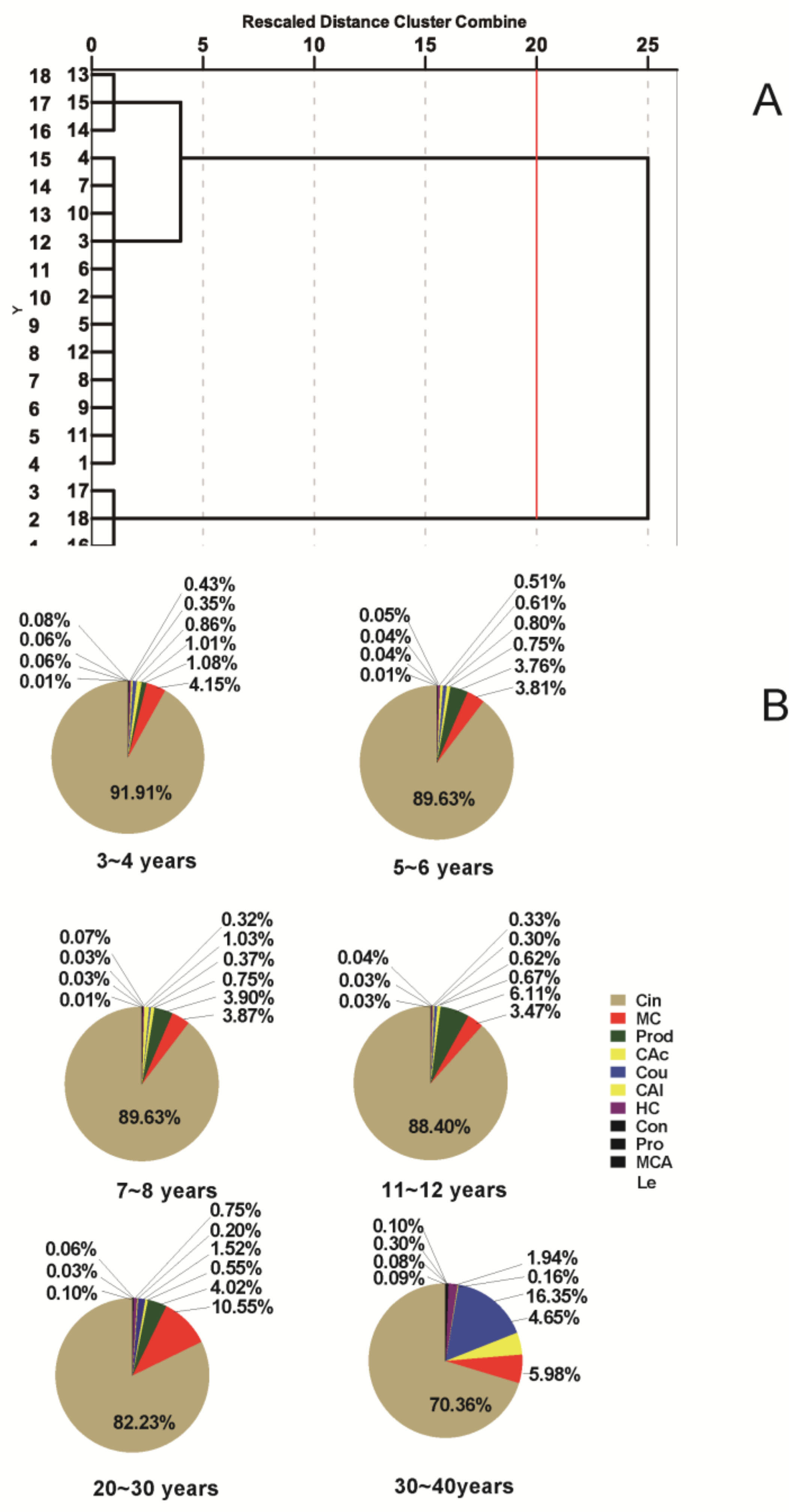
2.8.1. Hierarchical Clustering Analysis (HCA) of Different Growth Stages Cinnamomi Cortex
2.8.2. Quantitative Application of Different Cinnamomi Cortex Growth Stages
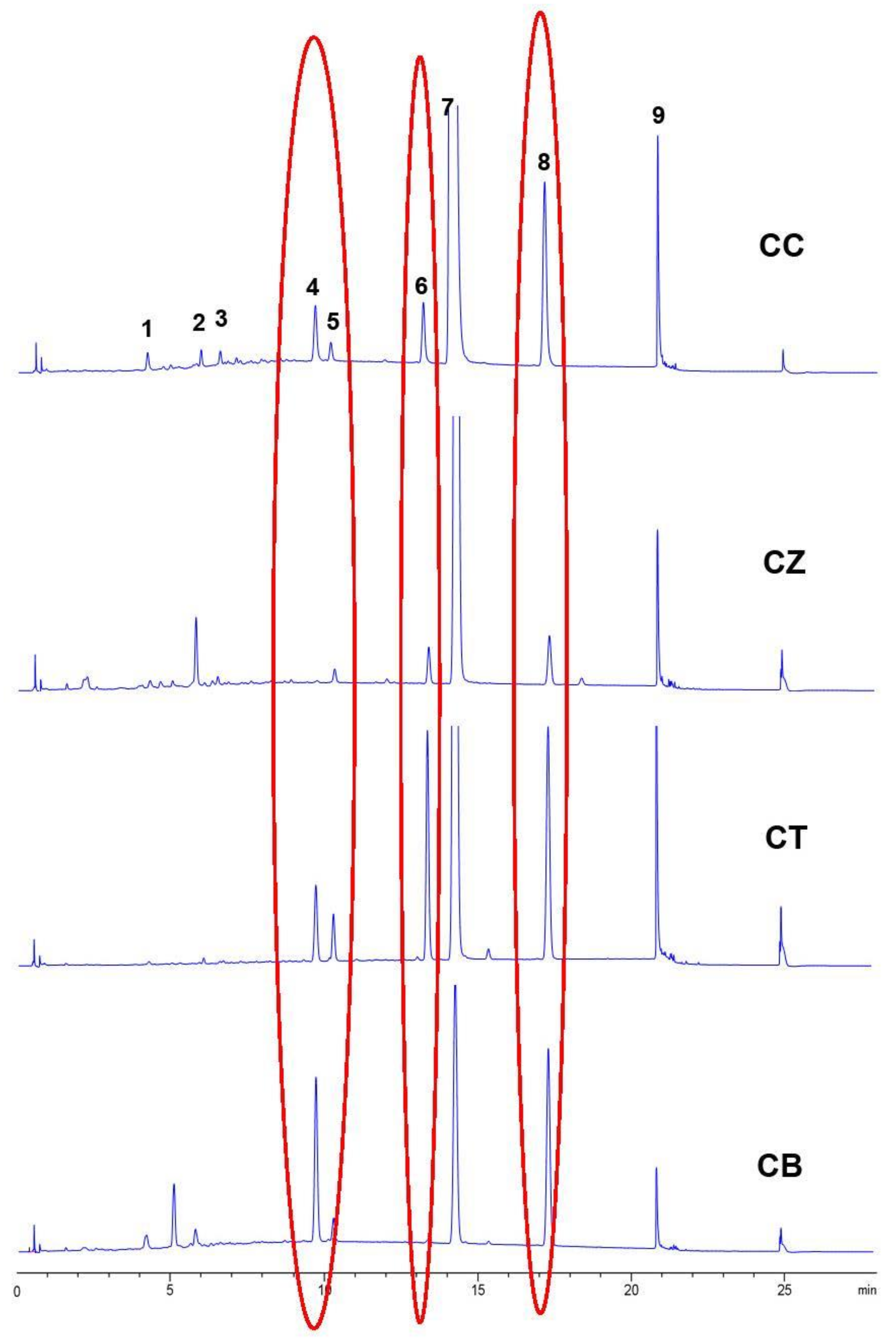
2.9. Indentication of CC and Its Adulterants
3. Materials and Methods
3.1. Reagents and Materials
3.2. Apparatus
3.3. Chromatographic Conditions
3.4. Preparation of Reference and Sample Solutions
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dartora, N.; De Souza, L.M.; Santana-Filho, A.P. UPLC-PDA–MS evaluation of bioactive compounds from leaves of ilex paraguariensis with different growth conditions, treatments and ageing. Food Chem. 2011, 129, 1453–1461. [Google Scholar] [CrossRef]
- Rasheed, A.; Satyanarayana, K.V.; Gulabi, P.S. Standardization: Accessing key for herbal drug safety assurance. J. Pharm. Res. 2011, 4, 1322–1325. [Google Scholar]
- Ni, Y.; Lai, Y.; Brandes, S.; Kokot, S. Multi-wavelength HPLC fingerprints from complex substances: An exploratory chemometrics study of the Cassia seed example. Anal. Chim. Acta 2009, 647, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.R; Tang, Y.H.; Sun, Z.L.; Ji, Y.B.; Huang, C.G. Determination of Cinnamaldehyde and Cinnamic Acid in Cortex Cinnamomi from Different Habits by RP-HPLC. Mod. Chin. Med. 2008, 4, 9–11. [Google Scholar]
- Zhu, H.; Wang, C.; Qi, Y.; Song, F.; Liu, Z.; Liu, S. Fingerprint analysis of Radix Aconiti using ultra-performance liquid chromatography-electrospray ionization/tandem mass spectrometry (UPLC-ESI/MSn) combined with stoichiometry. Talanta 2013, 103, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, L.; Wang, Y. Determination of Cinnamic Aldehyde in Cinnamomum cassia Presl after Compatibility with Coptis chinensis Franch. Chin. J. Pharm. Anal. 2002, 22, 11–14. [Google Scholar]
- Alaerts, G.; Pieters, S.; Logie, H.; Erps, J.V.; Merino-Arévalo, M.; Dejaegher, B.; Smeyers-Verbeke, J.; Heyden, Y.V. Exploration and classification of chromatographic fingerprints as additional tool for identification and quality control of several Artemisia species. J. Pharm. Biomed. Anal. 2014, 95, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ma, Y.; Su, D.; Shang, M.; Xu, F.; Liu, G.; Iranmanesh, N.; Li, L.; Jiang, T.; Cai, S. Quantification of seven phenylpropanoid compounds in Chinese Cinnamomi Cortex and Ramulus by HPLC. J. Chin. Pharm. Sci. 2015, 24, 369–392. [Google Scholar]
- Gad, H.A.; Bouzabata, A. Application of chemometrics in quality control of Turmeric (Curcuma longa) based on Ultra-violet, Fourier transform-infrared and 1H NMR spectroscopy. Food Chem. 2017, 237, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, K.H.; Lee, M.-H.; Kim, H.-T.; Seo, W.D.; Kim, J.Y.; Baek, I.-Y.; Jang, D.S.; Ha, T.J. Identification, characterisation, and quantification of phenolic;compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013, 136, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-W.; Zhu, M.; Shao, Y.-D.; Shen, Z.; Weng, C.-C.; Yan, W.-D. Determination and quality evaluation of green tea extracts through qualitative and quantitative analysis of multi-components by single marker (QAMS). Food Chem. 2016, 197, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Zu, Y.; Zhao, C.; Zhang, L.; Zhang, Y.; Zhang, Z.; Wang, W. Development of an ionic liquid-based microwave-assisted method for simultaneous extraction and distillation for determination of proanthocyanidins and essential oil in Cortex cinnamomi. Food Chem. 2012, 135, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Mok, D.K.W.; Chau, F.T. Chemical information of Chinese medicines: A challenge to chemist. Chemom. Intell. Lab. Syst. 2006, 82, 210–217. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Wang, T.-M.; Chu, Z.-Y.; Yu, W.-J.; Zhai, Y.-J. Determination of Cinimaldehyde in 5 Different Standards of Cinnamomum Cassia and Two Kinds of Adulterants by HPLC-DAD. Chin. J. Exp. Traditional Med. Formulae 2014, 20, 90–93. [Google Scholar]
- Li, J.-K.; He, X.-Y.; Li, M.-Y.; Zhao, W. Chemical fingerprint and quantitative analysis for quality control of polyphenols extracted from pomegranate peel by HPLC. Food Chem. 2015, 176, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-K.; Kim, E.-K.; Jeong, E.-S.; Lee, Y.-H.; Lee, Y.-R.; Park, J.-W.; Ryu, D.-G.; Park, B.-H. Cortex cinnamomi extract prevents streptozotocin- and cytokine-induced β-cell damage by inhibiting NF-κβ. World J. Gastroenterol. 2006, 12, 4331. [Google Scholar]
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Du, L. Diabetes is an inflammatory disease: Evidence from traditional Chinese medicines. Diabetes Obes. Metab. 2015, 13, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Maurya, S.; DeLampasona, M.-P.; Catalan, C.-A.-N. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liang, T.; Fu, Q.; Xiao, Y.-S.; Feng, J.-T.; Ke, Y.-X.; Liang, X.-M. Fingerprint analysis of Ligusticum chuanxiong using hydrophilic interaction chromatography and reversed-phase liquid chromatography. J. Chromatogr. A 2009, 1216, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-J.; Qi, L.-W.; Li, P.; Luo, H.-W.; Yi, L.; Sheng, L.-H. Improved quality control method for Fufang Danshen preparations through simultaneous determination of phenolic acids, saponins and diterpenoid quinones by HPLC coupled with diode array and evaporative light scattering detectors. J. Pharm. Biomed. Anal. 2007, 45, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Da, J.; Wu, W.-Y.; Hou, J.-J.; Long, H.-L.; Yao, S.; Yang, Z.; Cai, L.-Y.; Yang, M.; Jiang, B.-H.; Liu, X.; et al. Comparison of two officinal Chinese pharmacopoeia species of Ganoderma based on chemical research with multiple technologies and chemometrics analysis. J. Chromatogr. A 2012, 1222, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, S.-B.; Xie, M.-Y.; Nie, S.-P.; Liu, W.; Li, C.; Gong, X.-F.; Wang, Y.-X. Quality control and original discrimination of Ganoderma lucidum, based on high-performance liquid chromatographic fingerprints and combined chemometrics methods. Anal. Chim. Acta 2008, 623, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.-P.; Huang, W.-H.; Yang, F.-Q.; Li, J.; Li, S.-P. Pressurized liquid extraction and GC-MS analysis for simultaneous determination of seven components in Cinnamomum cassia and the effect of sample preparation. J. Sep. Sci. 2015, 33, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Wu, S.-J.; Chang, C.-H.; Ng, L.-T. Antioxidant activity of Cinnamomum cassia. Phytother. Res. 2010, 17, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Li, R.-X.; Chuang, L.-Y. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules 2012, 17, 7294–7304. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, L.-H.; Zhang, Q.; Lai, M.-X.; Wang, Q. Quality assessment of Cortex cinnamomi by HPLC chemical fingerprint, principle component analysis and cluster analysis. J. Sep. Sci. 2015, 30, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-Z.; Xie, P.; Chan, K. Quality control of herbal medicines. J. Chromatogr. B 2004, 812, 53–70. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Pro, Le, Prod, Con. Cou, Hc, Cal, Cac, Cin, Mca, Mc are available from the authors. |
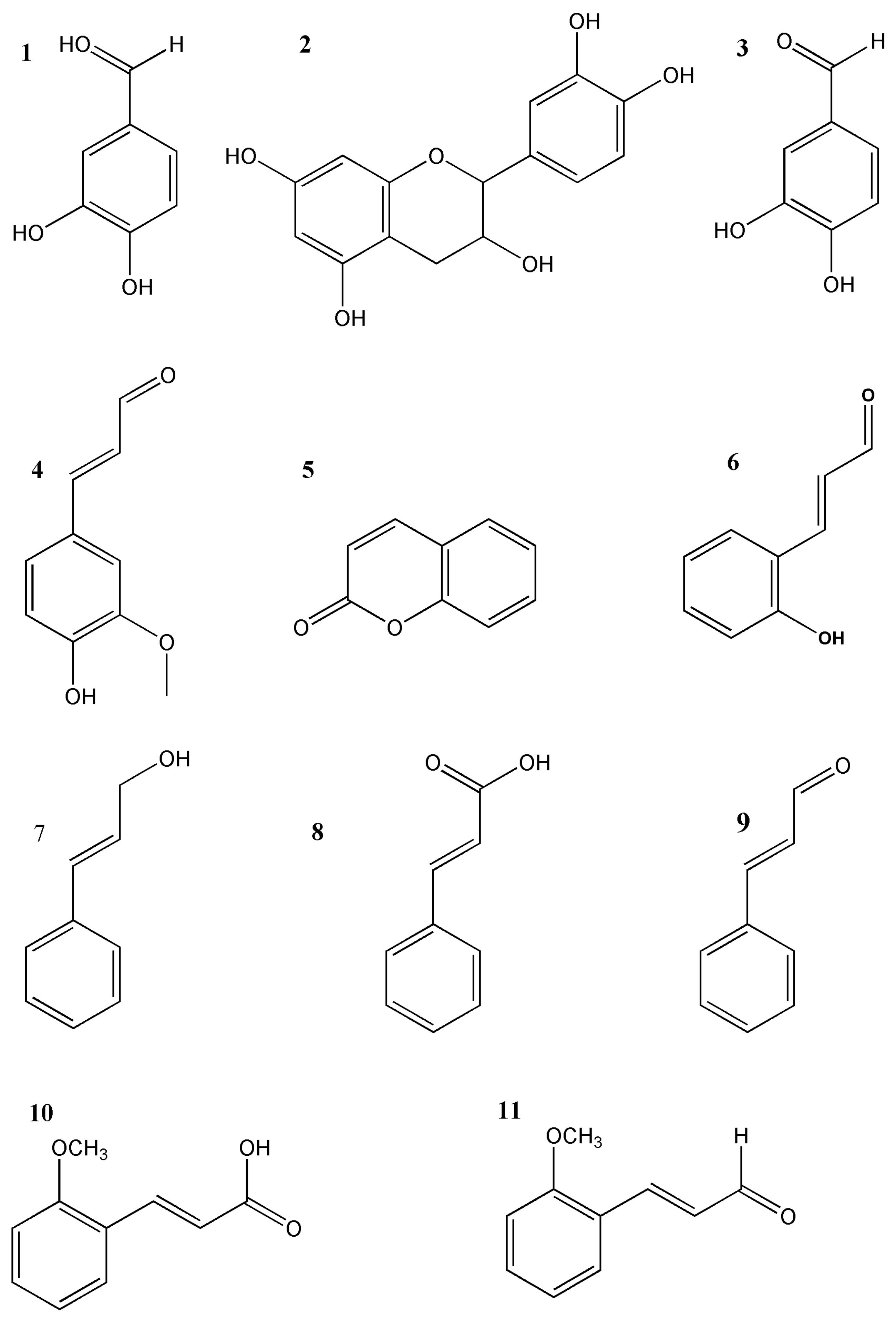
| Peak No. | tR (min) | λmax (nm) | [M + H]+ (m/z) | MS2 (m/z) | Reference |
|---|---|---|---|---|---|
| 1 | 1.598 | 260 (sh), 294 | 155.1 | 137.1, 111.1, 93.2, 81.2, 65.3 | Pro |
| 2 | 2.590 | 230 (sh), 280, 310 | 139.2 | 111.1, 93.2, 65.3 | Le |
| 3 | 5.193 | 279 (sh) | 291.2 | 207.2, 165.2, 147.2, 139.2, 123.2 | Prod |
| 4 | 9.092 | 340 (sh) | 179.2 | 161.3, 147.2, 133.2, 119.2, 105.2, 91.2, 55.2 | Con |
| 5 | 9.710 | 279 (sh), 310 | 147.2 | 103.2, 91.2, 77.3, 65.3 | Cou |
| 6 | 10.312 | 289 (sh), 340 | 149.2 | 131.2, 121.2, 103.0, 91.2, 77.3, 55.3 | Hc |
| 7 | 11.987 | 250 (sh) | 117.2 | 115.2, 91.2, 77.2, 65.3 | Cal |
| 8 | 13.353 | 278 (sh) | 149.2 | 131.2, 103.2, 77.3 | Cac |
| 9 | 14.228 | 292 (sh) | 133.2 | 115.1, 105.0, 103.1, 79.1, 77.2, 55.2 | Cin |
| 10 | 15.394 | 277 (sh), 324 | 179.1 | 161.2, 146.2, 118.2, 107.2, 103.2, 79.3, 77.3 | Mca |
| 11 | 17.310 | 288 (sh), 338 | 163.2 | 145.2, 135.2, 115.2,107.2, 105.2, 103.2, 91.2, 79.3, 77.3, 57.3, 55.3 | Mc |
| Analyte | Liner Range (μg·mL−1) | Calibration Equation | r | LOD (μg·mL−1) | LOQ (μg·mL−1) | Intra-Day RSD (%, n = 6) | Inter-Day RSD (%, n = 6) | Repeatability RSD (%, n = 6) | Recovery Mean and RSD (%, n = 6) |
|---|---|---|---|---|---|---|---|---|---|
| Pro | 0.99–126.2 | Y = 6.62X − 1.7136 | 1.0000 | 0.08 | 0.26 | L 1.0 | L 4.3 | 4.7 | 100.2 |
| M 1.0 | M 1.6 | ||||||||
| H 0.7 | H 1.2 | 3.3 | |||||||
| Le | 0.76–97.2 | Y = 20.10X − 3.8607 | 1.0000 | 0.04 | 0.14 | L 1.8 | L 4.8 | 4.9 | 101.0 |
| M 0.8 | M 1.6 | ||||||||
| H 0.5 | H 1.4 | 2.2 | |||||||
| Prod | 0.78–99.4 | Y = 3.23X − 0.8066 | 0.9999 | 0.23 | 0.78 | L 4.0 | L 2.1 | 3.6 | 100.6 |
| M 0.6 | M 1.1 | ||||||||
| H 0.3 | H 1.9 | 2.1 | |||||||
| Con | 0.97~123.6 | Y = 33.69X − 7.2236 | 1.0000 | 0.04 | 0.12 | L 0.4 | L 4.0 | 4.3 | 101.0 |
| M 0.3 | M 0.6 | ||||||||
| H 0.1 | H 0.9 | 3.3 | |||||||
| Cou | 1.33–169.6 | Y = 20.01X − 5.7422 | 1.0000 | 0.07 | 0.23 | L 0.5 | L 3.0 | 1.5 | 100.1 |
| M 0.4 | M 0.8 | ||||||||
| H 0.2 | H 0.9 | 3.9 | |||||||
| Hc | 0.80–101.8 | Y = 28.842X − 3.5346 | 1.0000 | 0.05 | 0.18 | L 0.3 | L 4.5 | 1.5 | 101.2 |
| M 0.3 | M 0.8 | ||||||||
| H 0.1 | H 0.8 | 2.6 | |||||||
| Cal | 1.26–161.6 | Y = 33.65X − 7.9961 | 1.0000 | 0.10 | 0.33 | L 0.1 | L 2.0 | 2.7 | 101.3 |
| M 0.3 | M 0.6 | ||||||||
| H 0.3 | H 0.9 | 2.8 | |||||||
| Cac | 1.61–205.6 | Y = 31.00X − 28.599 | 0.9999 | 0.04 | 0.12 | L 0.2 | L 4.9 | 1.9 | 100.9 |
| M 0.2 | M 0.7 | ||||||||
| H 0.3 | H 0.9 | 3.8 | |||||||
| Cin | 2.04–260.6 | Y = 23.31X − 2.5395 | 0.9998 | 0.05 | 0.18 | L 0.8 | L 1.0 | 0.5 | 98.0 |
| M 1.8 | M 1.0 | ||||||||
| H 0.5 | H 1.2 | 1.2 | |||||||
| Mca | 0.85–108.6 | Y = 25.65X − 26.201 | 1.0000 | 0.08 | 0.25 | L 0.5 | L 4.4 | 3.9 | 102.6 |
| M 0.2 | M 1.4 | ||||||||
| H 0.2 | H 1.0 | 0.9 | |||||||
| Mc | 1.72–219.6 | Y=25.56X − 26.201 | 0.9999 | 0.08 | 0.26 | L 0.7 | L 3.3 | 0.4 | 101.3 |
| M 1.3 | M 0.8 | ||||||||
| H 0.2 | H 1.0 | 2.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, N.; Ding, Y.; Zhang, Y.; Zhang, T.; Yi, Y.; Wang, B. Chemical Fingerprinting and Quantification of Chinese Cinnamomi Cortex by Ultra High Performance Liquid Chromatography Coupled with Chemometrics Methods. Molecules 2018, 23, 2214. https://doi.org/10.3390/molecules23092214
Ma N, Ding Y, Zhang Y, Zhang T, Yi Y, Wang B. Chemical Fingerprinting and Quantification of Chinese Cinnamomi Cortex by Ultra High Performance Liquid Chromatography Coupled with Chemometrics Methods. Molecules. 2018; 23(9):2214. https://doi.org/10.3390/molecules23092214
Chicago/Turabian StyleMa, Ninghui, Yue Ding, Yong Zhang, Tong Zhang, Yaxiong Yi, and Bing Wang. 2018. "Chemical Fingerprinting and Quantification of Chinese Cinnamomi Cortex by Ultra High Performance Liquid Chromatography Coupled with Chemometrics Methods" Molecules 23, no. 9: 2214. https://doi.org/10.3390/molecules23092214
APA StyleMa, N., Ding, Y., Zhang, Y., Zhang, T., Yi, Y., & Wang, B. (2018). Chemical Fingerprinting and Quantification of Chinese Cinnamomi Cortex by Ultra High Performance Liquid Chromatography Coupled with Chemometrics Methods. Molecules, 23(9), 2214. https://doi.org/10.3390/molecules23092214





