An Analysis of the mRNA Expression of Peripheral-Blood Stem and Progenitor Cell Markers in Pancreatic Neoplastic Disorders
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Assessment of mRNA in Peripheral Blood
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Expression of mRNAs Encoding Stem-Cell and Progenitor Pancreatic Genes in PB Patients with Pancreatic Neoplastic Disorders
3.3. Correlations
3.4. Multivariable Analysis of PDAC Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Dougan, S.K. The Pancreatic Cancer Microenvironment. Cancer J. 2017, 23, 321–325. [Google Scholar] [CrossRef]
- Mercanti, L.; Sindaco, M.; Mazzone, M.; Di Marcantonio, M.C.; Piscione, M.; Muraro, R.; Mincione, G. PDAC, the Influencer Cancer: Cross-Talk with Tumor Microenvironment and Connected Potential Therapy Strategies. Cancers 2023, 15, 2923. [Google Scholar] [CrossRef]
- Bulle, A.; Lim, K.H. Beyond just a tight fortress: Contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct. Target. Ther. 2020, 5, 249. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Vega, A.; Maldonado-Lagunas, V.; Mitre-Aguilar, I.B.; Melendez-Zajgla, J. Tumor Microenvironment Role in Pancreatic Cancer Stem Cells. Cells 2023, 12, 1560. [Google Scholar] [CrossRef]
- Kopp, J.L.; von Figura, G.; Mayes, E.; Liu, F.F.; Dubois, C.L.; Morris, J.P.T.; Pan, F.C.; Akiyama, H.; Wright, C.V.; Jensen, K.; et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 22, 737–750. [Google Scholar] [CrossRef]
- Gidekel Friedlander, S.Y.; Chu, G.C.; Snyder, E.L.; Girnius, N.; Dibelius, G.; Crowley, D.; Vasile, E.; DePinho, R.A.; Jacks, T. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 2009, 16, 379–389. [Google Scholar] [CrossRef]
- Sun, J. Pancreatic neuroendocrine tumors. Intractable Rare Dis. Res. 2017, 6, 21–28. [Google Scholar] [CrossRef]
- Ro, C.; Chai, W.; Yu, V.E.; Yu, R. Pancreatic neuroendocrine tumors: Biology, diagnosis, and treatment. Chin. J. Cancer 2013, 32, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Dumlu, E.G.; Karakoç, D.; Özdemir, A. Nonfunctional Pancreatic Neuroendocrine Tumors: Advances in Diagnosis, Management, and Controversies. Int. Surg. 2015, 100, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.W.; Bennett, J.J. Clinical Presentation and Diagnosis of Pancreatic Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2016, 25, 363–374. [Google Scholar] [CrossRef]
- Alexovič, M.; Uličná, C.; Sabo, J.; Davalieva, K. Human peripheral blood mononuclear cells as a valuable source of disease-related biomarkers: Evidence from comparative proteomics studies. Proteom. Clin. Appl. 2024, 18, e2300072. [Google Scholar] [CrossRef]
- Vaz, A.P.; Ponnusamy, M.P.; Seshacharyulu, P.; Batra, S.K. A concise review on the current understanding of pancreatic cancer stem cells. J. Cancer Stem Cell Res. 2014, 2, e1004. [Google Scholar] [CrossRef]
- MacLean, M.R.; Walker, O.L.; Arun, R.P.; Fernando, W.; Marcato, P. Informed by Cancer Stem Cells of Solid Tumors: Advances in Treatments Targeting Tumor-Promoting Factors and Pathways. Int. J. Mol. Sci. 2024, 25, 4102. [Google Scholar] [CrossRef]
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.; Rigoli, E.; Uccella, S.; Novario, R.; Capella, C. Prognostic and biological significance of cytokeratin 19 in pancreatic endocrine tumours. Histopathology 2007, 50, 597–606. [Google Scholar] [CrossRef]
- Marui, S.; Nishikawa, Y.; Shiokawa, M.; Yokode, M.; Matsumoto, S.; Muramoto, Y.; Ota, S.; Nakamura, T.; Yoshida, H.; Okada, H.; et al. Context-Dependent Roles of Hes1 in the Adult Pancreas and Pancreatic Tumor Formation. Gastroenterology 2022, 163, 1613–1629.e1612. [Google Scholar] [CrossRef]
- Ezeh, U.I.; Turek, P.J.; Reijo, R.A.; Clark, A.T. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer 2005, 104, 2255–2265. [Google Scholar] [CrossRef]
- Wang, D.; Lu, P.; Zhang, H.; Luo, M.; Zhang, X.; Wei, X.; Gao, J.; Zhao, Z.; Liu, C. Oct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget 2014, 5, 10803–10815. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Cai, H.; Xu, Z.; Xu, T.; Zou, Q.; Gu, M. Expressions of stem cell transcription factors Nanog and Oct4 in renal cell carcinoma tissues and clinical significance. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Paterson, C.; Kilmister, E.J.; Brasch, H.D.; Bockett, N.; Patel, J.; Paterson, E.; Purdie, G.; Galvin, S.; Davis, P.F.; Itinteang, T.; et al. Cell Populations Expressing Stemness-Associated Markers in Lung Adenocarcinoma. Life 2021, 11, 1106. [Google Scholar] [CrossRef]
- Sodja, E.; Rijavec, M.; Koren, A.; Sadikov, A.; Korošec, P.; Cufer, T. The prognostic value of whole blood SOX2, NANOG and OCT4 mRNA expression in advanced small-cell lung cancer. Radiol. Oncol. 2016, 50, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Skubisz, K.; Dąbkowski, K.; Samborowska, E.; Starzyńska, T.; Deskur, A.; Ambrozkiewicz, F.; Karczmarski, J.; Radkiewicz, M.; Kusnierz, K.; Kos-Kudła, B.; et al. Serum Metabolite Biomarkers for Pancreatic Tumors: Neuroendocrine and Pancreatic Ductal Adenocarcinomas-A Preliminary Study. Cancers 2023, 15, 3242. [Google Scholar] [CrossRef]
- Dąbkowski, K.; Łabędź-Masłowska, A.; Dołęgowska, B.; Safranow, K.; Budkowska, M.; Zuba-Surma, E.; Starzyńska, T. Evidence of Stem Cells Mobilization in the Blood of Patients with Pancreatitis: A Potential Link with Disease Severity. Stem Cells Int. 2022, 2022, 5395248. [Google Scholar] [CrossRef]
- Starzynska, T.; Dabkowski, K.; Blogowski, W.; Zuba-Surma, E.; Budkowska, M.; Salata, D.; Dolegowska, B.; Marlicz, W.; Lubikowski, J.; Ratajczak, M.Z. An intensified systemic trafficking of bone marrow-derived stem/progenitor cells in patients with pancreatic cancer. J. Cell. Mol. Med. 2013, 17, 792–799. [Google Scholar] [CrossRef]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef]
- Amaral, M.J.; Oliveira, R.C.; Donato, P.; Tralhão, J.G. Pancreatic Cancer Biomarkers: Oncogenic Mutations, Tissue and Liquid Biopsies, and Radiomics—A Review. Dig. Dis. Sci. 2023, 68, 2811–2823. [Google Scholar] [CrossRef]
- Roy, S.; Dukic, T.; Keepers, Z.; Bhandary, B.; Lamichhane, N.; Molitoris, J.; Ko, Y.H.; Banerjee, A.; Shukla, H.D. SOX2 and OCT4 mediate radiation and drug resistance in pancreatic tumor organoids. Cell Death Discov. 2024, 10, 106. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, C.; Zhao, B.; Wang, Y.; Li, Z.; Li, T.; Yang, X.; Wang, W. Pancreatic cancer stemness: Dynamic status in malignant progression. J. Exp. Clin. Cancer Res. 2023, 42, 122. [Google Scholar] [CrossRef]
- Safa, A.R. Epithelial-mesenchymal transition: A hallmark in pancreatic cancer stem cell migration, metastasis formation, and drug resistance. J. Cancer Metastasis Treat. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, Z.; Zhu, Y.; Chen, J.; Li, W. The Role and Specific Mechanism of OCT4 in Cancer Stem Cells: A Review. Int. J. Stem Cells 2020, 13, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Menz, A.; Bauer, R.; Kluth, M.; Marie von Bargen, C.; Gorbokon, N.; Viehweger, F.; Lennartz, M.; Völkl, C.; Fraune, C.; Uhlig, R.; et al. Diagnostic and prognostic impact of cytokeratin 19 expression analysis in human tumors: A tissue microarray study of 13,172 tumors. Human. Pathol. 2021, 115, 19–36. [Google Scholar] [CrossRef]
- Ferrara, B.; Pignatelli, C.; Cossutta, M.; Citro, A.; Courty, J.; Piemonti, L. The Extracellular Matrix in Pancreatic Cancer: Description of a Complex Network and Promising Therapeutic Options. Cancers 2021, 13, 4442. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Gaggar, S.; Tajik, A.; Orhan, A.; Gögenur, I. The Matrix Reloaded-The Role of the Extracellular Matrix in Cancer. Cancers 2023, 15, 2057. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Kodama, Y.; Shiokawa, M.; Matsumori, T.; Marui, S.; Kuriyama, K.; Kuwada, T.; Sogabe, Y.; Kakiuchi, N.; Tomono, T.; et al. Hes1 plays an essential role in Kras-driven pancreatic tumorigenesis. Oncogene 2019, 38, 4283–4296. [Google Scholar] [CrossRef]
- Oliver-Krasinski, J.M.; Stoffers, D.A. On the origin of the beta cell. Genes. Dev. 2008, 22, 1998–2021. [Google Scholar] [CrossRef]
- Zhang, A.M.Y.; Chu, K.H.; Daly, B.F.; Ruiter, T.; Dou, Y.; Yang, J.C.C.; de Winter, T.J.J.; Chhuor, J.; Wang, S.; Flibotte, S.; et al. Effects of hyperinsulinemia on pancreatic cancer development and the immune microenvironment revealed through single-cell transcriptomics. Cancer Metab. 2022, 10, 5. [Google Scholar] [CrossRef]
- Dabkowski, K.; Bogacka, B.; Tarnowski, M.; Starzynska, T. Pancreatic cancer microenvironment. Pol. Merkur. Lek. Organ. Pol. Tow. Lek. 2016, 41, 296–302. [Google Scholar]
- Gao, S.; Pan, Y.; Song, L.; Dong, L.; Weng, L.I.; Wang, P.; Hua, Y.; Chen, Z.; Liu, L. Nanog Predicts Poor Prognosis in Human Pancreatic Cancer and Is Downregulated by QingyihuaJi Formula in Pancreatic Cancer Stem Cells. Evid. Based Complement. Altern. Med. 2016, 2016, 7028289. [Google Scholar] [CrossRef]
- Baine, M.J.; Chakraborty, S.; Smith, L.M.; Mallya, K.; Sasson, A.R.; Brand, R.E.; Batra, S.K. Transcriptional profiling of peripheral blood mononuclear cells in pancreatic cancer patients identifies novel genes with potential diagnostic utility. PLoS ONE 2011, 6, e17014. [Google Scholar] [CrossRef]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep. Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology 2016, 5, e1093722. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.; Stoicov, C.; Nomura, S.; Rogers, A.B.; Carlson, J.; Li, H.; Cai, X.; Fox, J.G.; Goldenring, J.R.; Wang, T.C. Gastric cancer originating from bone marrow-derived cells. Science 2004, 306, 1568–1571. [Google Scholar] [CrossRef]
- Dabkowski, K.; Labedz-Maslowska, A.; Zuba-Surma, E.; Starzynska, T. Role of the bone marrow derived stem cells in pancreatic inflammatory disorders. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2017, 42, 137–141. [Google Scholar]
- Nurmi, A.M.; Mustonen, H.K.; Stenman, U.H.; Seppänen, H.E.; Haglund, C.H. Combining CRP and CA19-9 in a novel prognostic score in pancreatic ductal adenocarcinoma. Sci. Rep. 2021, 11, 781. [Google Scholar] [CrossRef]
- Sollie, S.; Michaud, D.S.; Sarker, D.; Karagiannis, S.N.; Josephs, D.H.; Hammar, N.; Santaolalla, A.; Walldius, G.; Garmo, H.; Holmberg, L.; et al. Chronic inflammation markers are associated with risk of pancreatic cancer in the Swedish AMORIS cohort study. BMC Cancer 2019, 19, 858. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.Y.; Kim, D.S.; Kang, E.J.; Kim, J.S.; Choi, Y.J.; Oh, S.C.; Seo, J.H.; Kim, J.S. Inflammatory markers as prognostic indicators in pancreatic cancer patients who underwent gemcitabine-based palliative chemotherapy. Korean J. Intern. Med. 2020, 35, 171–184. [Google Scholar] [CrossRef]
- Zhou, Q.; Lu, X.; Qian, L.; Yu, C.; Xie, J.; Kong, D. Procalcitonin, C-reactive protein, and white blood cell count levels in end-stage cancer patients: A retrospective study on inflammatory markers and their prognostic value. Medicine 2024, 103, e40792. [Google Scholar] [CrossRef]
- Feng, L.; Gu, S.; Wang, P.; Chen, H.; Chen, Z.; Meng, Z.; Liu, L. White Blood Cell and Granulocyte Counts Are Independent Predictive Factors for Prognosis of Advanced Pancreatic Caner. Gastroenterol. Res. Pract. 2018, 2018, 8096234. [Google Scholar] [CrossRef] [PubMed]
- Blogowski, W.; Deskur, A.; Budkowska, M.; Sałata, D.; Madej-Michniewicz, A.; Dąbkowski, K.; Dołęgowska, B.; Starzyńska, T. Selected cytokines in patients with pancreatic cancer: A preliminary report. PLoS ONE 2014, 9, e97613. [Google Scholar] [CrossRef]
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Potempa, L.A. C-Reactive Protein and Cancer-Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835. [Google Scholar] [CrossRef]
- Li, X.; Gulati, M.; Larson, A.C.; Solheim, J.C.; Jain, M.; Kumar, S.; Batra, S.K. Immune checkpoint blockade in pancreatic cancer: Trudging through the immune desert. Semin. Cancer Biol. 2022, 86 Pt 2, 14–27. [Google Scholar] [CrossRef]
- Shadhu, K.; Xi, C. Inflammation and pancreatic cancer: An updated review. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2019, 25, 3–13. [Google Scholar] [CrossRef]
- Hausmann, S.; Kong, B.; Michalski, C.; Erkan, M.; Friess, H. The role of inflammation in pancreatic cancer. Adv. Exp. Med. Biol. 2014, 816, 129–151. [Google Scholar] [CrossRef]
- Cen, D.; Chen, J.; Li, Z.; Zhao, J.; Cai, X. Prognostic significance of cytokeratin 19 expression in pancreatic neuroendocrine tumor: A meta-analysis. PLoS ONE 2017, 12, e0187588. [Google Scholar] [CrossRef]
- Han, X.; Zhao, J.; Ji, Y.; Xu, X.; Lou, W. Expression of CK19 and KIT in resectable pancreatic neuroendocrine tumors. Tumour Biol. 2013, 34, 2881–2889. [Google Scholar] [CrossRef]
- Rex, J.; Lutz, A.; Faletti, L.E.; Albrecht, U.; Thomas, M.; Bode, J.G.; Borner, C.; Sawodny, O.; Merfort, I. IL-1β and TNFα Differentially Influence NF-κB Activity and FasL-Induced Apoptosis in Primary Murine Hepatocytes During LPS-Induced Inflammation. Front. Physiol. 2019, 10, 117. [Google Scholar] [CrossRef]
- Ling, S.; Feng, T.; Jia, K.; Tian, Y.; Li, Y. Inflammation to cancer: The molecular biology in the pancreas (Review). Oncol. Lett. 2014, 7, 1747–1754. [Google Scholar] [CrossRef]
- Lüttges, J.; Klöppel, G. Precancerous conditions of pancreatic carcinoma. J. Hepatobiliary Pancreat. Surg. 2000, 7, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef]
- Cotterill, A.M.; Holly, J.M.; Wass, J.A. The regulation of insulin-like growth factor binding protein (IGFBP)-1 during prolonged fasting. Clin. Endocrinol. 1993, 39, 357–362. [Google Scholar] [CrossRef]
- Yang, J.; Waldron, R.T.; Su, H.Y.; Moro, A.; Chang, H.H.; Eibl, G.; Ferreri, K.; Kandeel, F.R.; Lugea, A.; Li, L.; et al. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G675–G687. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, T.; Lin, C.; Zhao, B.; Li, Z.; Zhao, Y.; Wang, W. The systematic role of pancreatic cancer exosomes: Distant communication, liquid biopsy and future therapy. Cancer Cell Int. 2024, 24, 264. [Google Scholar] [CrossRef]
- Mutgan, A.C.; Besikcioglu, H.E.; Wang, S.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Insulin/IGF-driven cancer cell-stroma crosstalk as a novel therapeutic target in pancreatic cancer. Mol. Cancer 2018, 17, 66. [Google Scholar] [CrossRef]
- Tang, Z.C.; Chu, Y.; Tan, Y.Y.; Li, J.; Gao, S. Pancreatic and duodenal homeobox-1 in pancreatic ductal adenocarcinoma and diabetes mellitus. Chin. Med. J. 2020, 133, 344–350. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, X.; Wei, J.; Miao, R.; Wu, H.; Ma, K.; Tian, J. PDX-1: A Promising Therapeutic Target to Reverse Diabetes. Biomolecules 2022, 12, 1785. [Google Scholar] [CrossRef]
- Ebrahim, N.; Shakirova, K.; Dashinimaev, E. PDX1 is the cornerstone of pancreatic β-cell functions and identity. Front. Mol. Biosci. 2022, 9, 1091757. [Google Scholar] [CrossRef]


| PDAC N = 37 | PNETs N = 12 | Controls N = 34 | |
|---|---|---|---|
| Age (years) | 64 (57–69) | 51.5 (38–58.5) * | 61 (49–73) |
| Height (cm) | 168 (160–175) | 171.5 (165.5–178.5) | 166.5 (162–173) |
| Weight (kg) | 65 (55–74) * | 70 (60–75.5) | 75 (63–80) |
| Female/Male | 23/14 | 5/7 | 25/9 |
| BMI (kg/m2) | 22.5 (20–25.8) * | 23.3 (20.7–25.7) | 25 (23.4–28.6) |
| WBC (K/μL) | 8.2 (6.5–10.2) * | 6.47 (4.7–7.4) | 6.2 (5.2–7.3) |
| Plt (K/μL) | 233 (167–276) | 220 (198–327) | 229.5 (185–267) |
| Ery (mln/μL) | 4.3 (3.7–4.7) * | 4.5 (4.3–4.9) | 4.7 (4.3–4.9) |
| CRP (mg/L) | 11 (3.46–74) * | 2 (1–4.7) | 2.5 (1–5) |
| Ca 19-9 | 482.2 (114.8–5890) * | 6.1 (3.5–9.0) | 4.2 (2–8.9) |
| Control N = 34 | PNETs N = 12 | PDAC N = 34 | |
|---|---|---|---|
| POU5F1 | 0.002 (0.0005–0.006) | 0.0009 (0.0002–0.004) | 0.003 (0.001–0.01) |
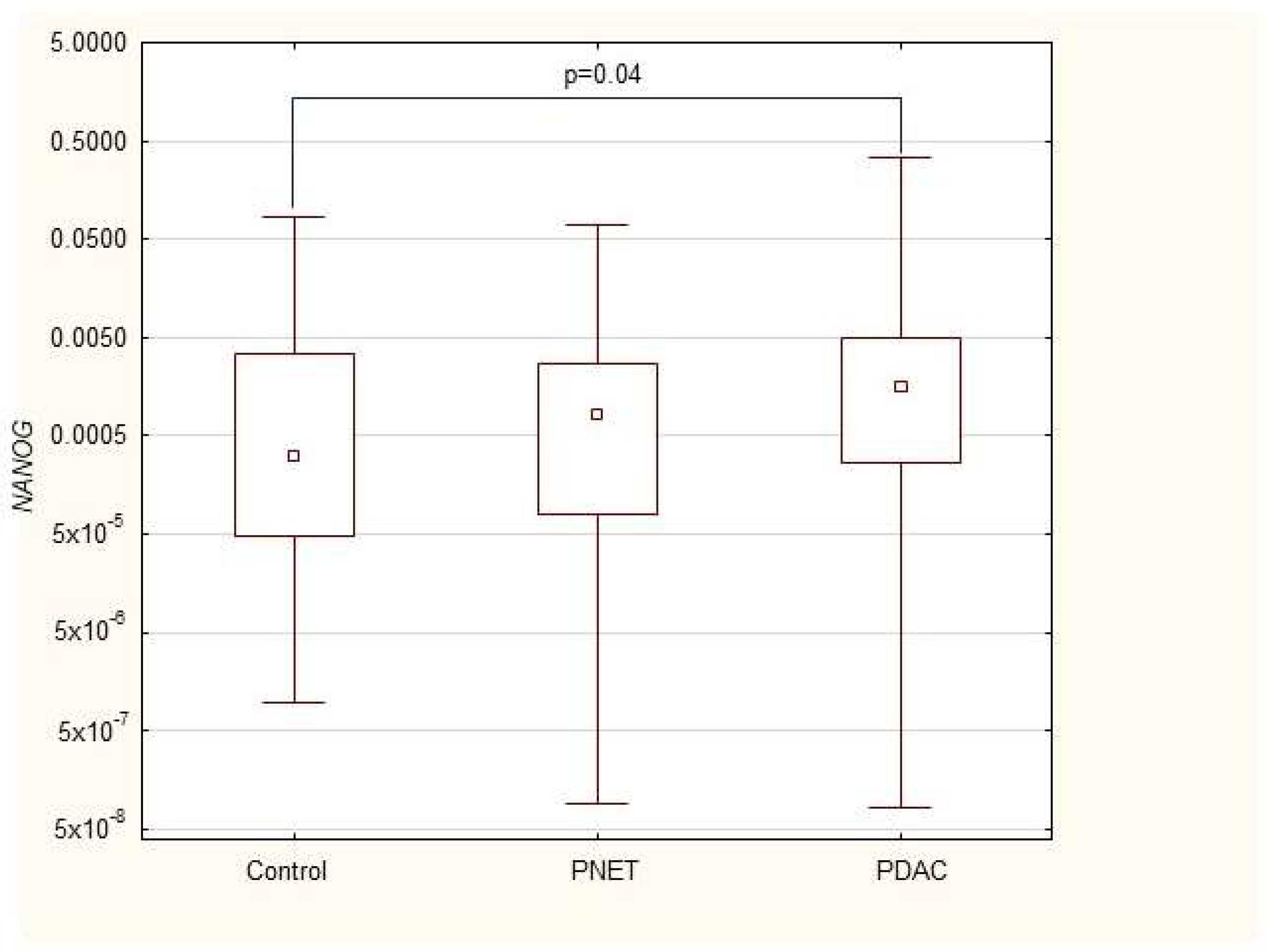
| NANOG | 0.0003 (0.00005–0.004) | 0.0008 (0.00008–0.003) | 0.002 (0.0003–0.005) * |
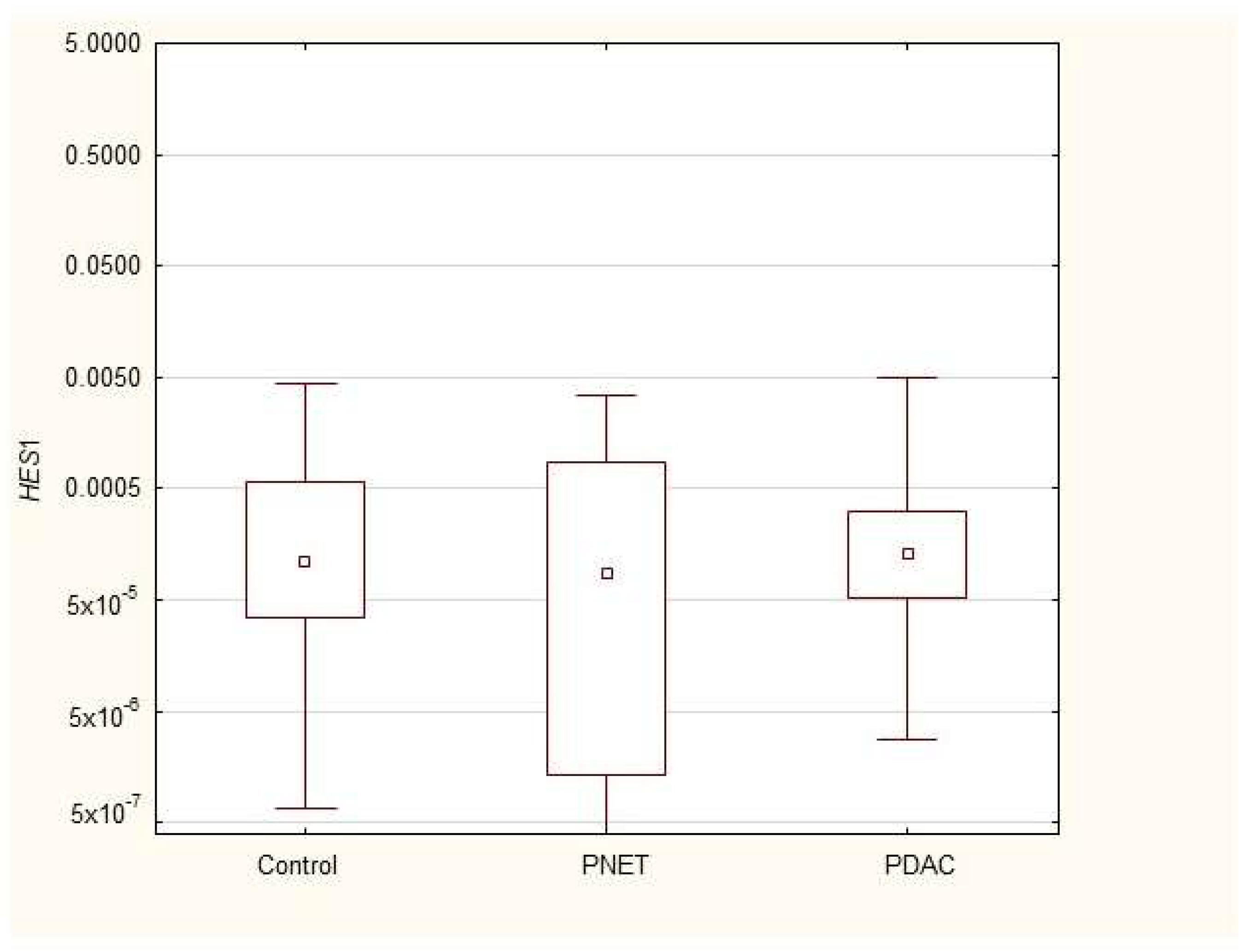
| HES1 | 0.00011 (0.00003–0.0006) | 0.00009 (1.3 × 10−6–0.0009) | 0.0001 (0.0003–0.0003) |
| PDX | 0.00003 (6.5 × 10−6–0.0001) | 0.00003 (3.7 × 10−6–0.0005) | 0.00008 (0.00002–0.0002 |
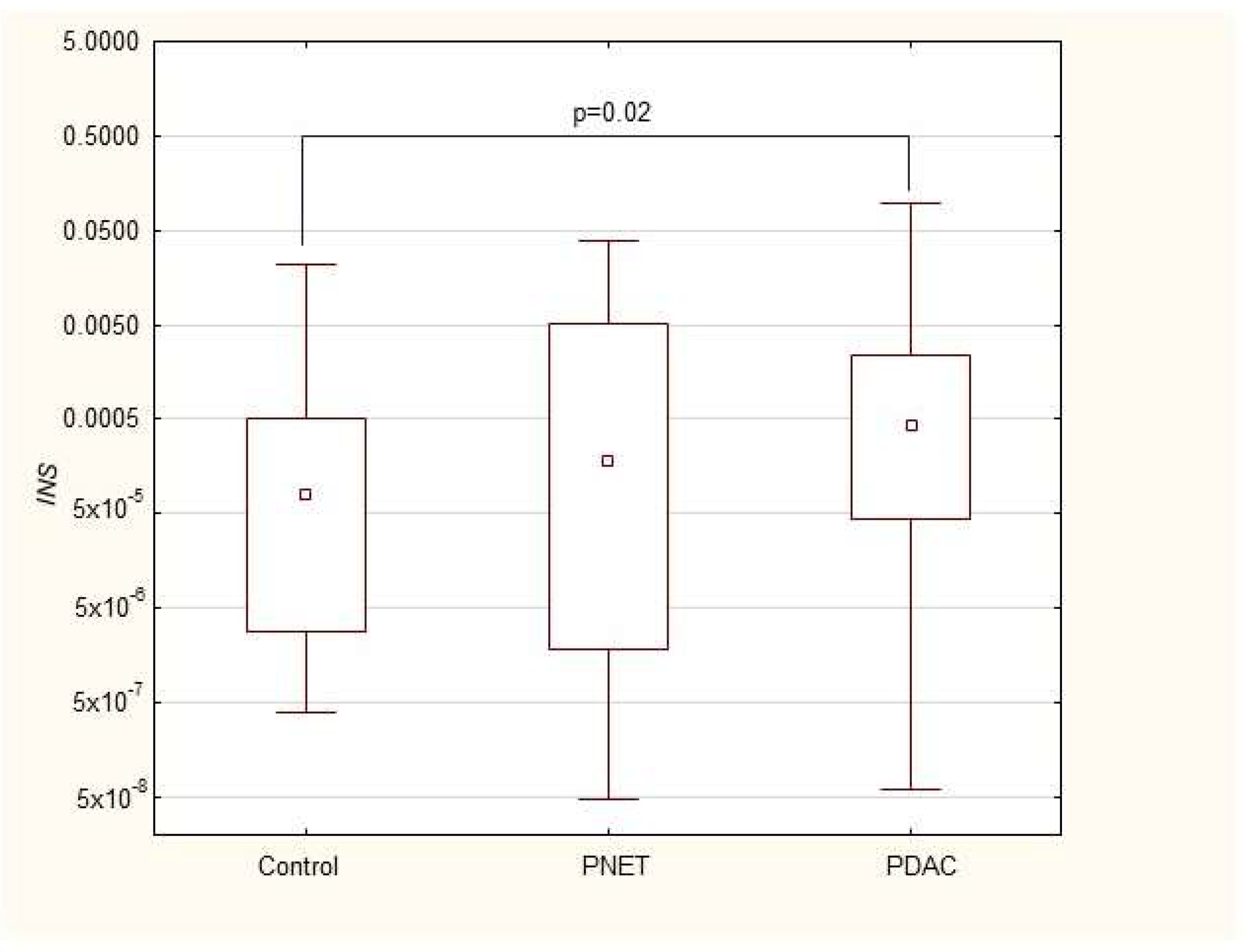
| INS | 0.00008 (2.7 × 10−6–0.0005) | 0.0002 (1.8 × 10−6–0.005) | 0.0004 (0.00004–0.002) * |
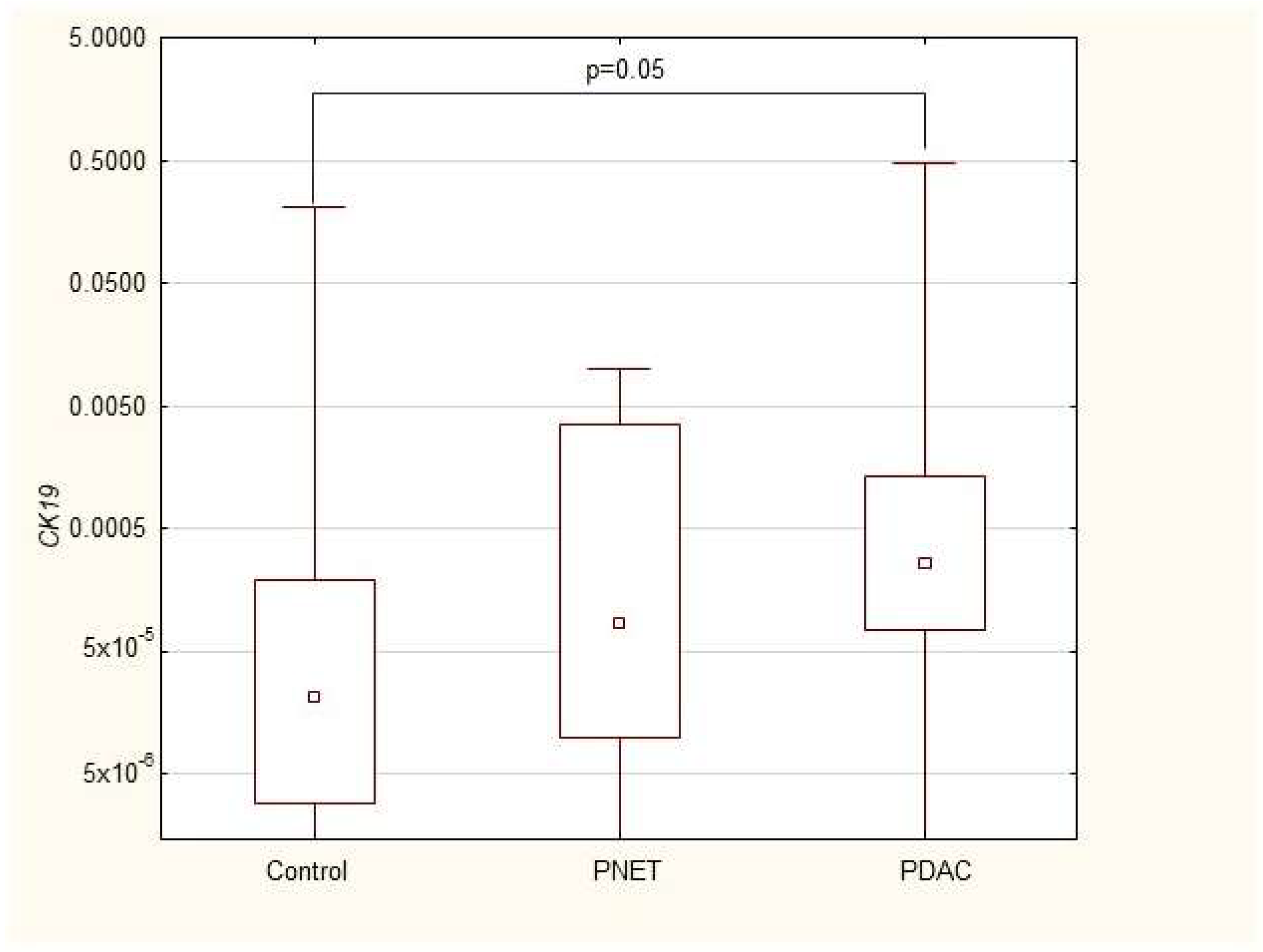
| CK19 | 0.000022 (2.9 × 10−6–0.0002) | 0.00008 (9.7 × 10−6–0.004) | 0.0003 (0.00007–0.001) * |
| Locally Advanced N = 23 | Metastatic N = 14 | |
|---|---|---|
| Age | 61 (55–66) * | 67.7 (63–74) * |
| POU5F1 | 0.003 (0.0007–0.009) | 0.003 (0.001–0.03) |
| NANOG | 0.002 (0.0002–0.005) | 0.002 (0.0004–0.006) |
| HES1 | 0.0001 (0.00005–0.0003) | 0.00009 (0.00003–0.0005) |
| PDX | 0.00008 (0.00001–0.0001) | 0.00009 (0.00005–0.0006) |
| INS | 0.0004 (0.00004–0.002) | 0.0007 (0.00004–0.004) |
| CK19 | 0.0002 (0.00008–0.001) | 0.0003 (0.00001–0.002) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbkowski, K.; Tarnowski, M.; Safranow, K.; Dąbkowska, M.; Kosiorowska, A.; Pukacka, K.; Starzyńska, T. An Analysis of the mRNA Expression of Peripheral-Blood Stem and Progenitor Cell Markers in Pancreatic Neoplastic Disorders. Curr. Issues Mol. Biol. 2025, 47, 236. https://doi.org/10.3390/cimb47040236
Dąbkowski K, Tarnowski M, Safranow K, Dąbkowska M, Kosiorowska A, Pukacka K, Starzyńska T. An Analysis of the mRNA Expression of Peripheral-Blood Stem and Progenitor Cell Markers in Pancreatic Neoplastic Disorders. Current Issues in Molecular Biology. 2025; 47(4):236. https://doi.org/10.3390/cimb47040236
Chicago/Turabian StyleDąbkowski, Krzysztof, Maciej Tarnowski, Krzysztof Safranow, Maria Dąbkowska, Alicja Kosiorowska, Kamila Pukacka, and Teresa Starzyńska. 2025. "An Analysis of the mRNA Expression of Peripheral-Blood Stem and Progenitor Cell Markers in Pancreatic Neoplastic Disorders" Current Issues in Molecular Biology 47, no. 4: 236. https://doi.org/10.3390/cimb47040236
APA StyleDąbkowski, K., Tarnowski, M., Safranow, K., Dąbkowska, M., Kosiorowska, A., Pukacka, K., & Starzyńska, T. (2025). An Analysis of the mRNA Expression of Peripheral-Blood Stem and Progenitor Cell Markers in Pancreatic Neoplastic Disorders. Current Issues in Molecular Biology, 47(4), 236. https://doi.org/10.3390/cimb47040236






