Abstract
During the last decade, artificial intelligence (AI) has enabled key technological innovations within the modern dementia and frailty healthcare and prevention landscape. This has boosted the impact of technology in the clinical setting, enabling earlier diagnosis with improved specificity and sensitivity, leading to accurate and time-efficient support that has driven the development of preventative interventions minimizing the risk and rate of progression. Background/Objectives: The rapid ageing of the European population places a substantial strain on the current healthcare system and imposes several challenges. COMFORTage is the joint effort of medical experts (i.e., neurologists, psychiatrists, neuropsychologists, nurses, and memory clinics), social scientists and humanists, technical experts (i.e., data scientists, AI experts, and robotic experts), digital innovation hubs (DIHs), and living labs (LLs) to establish a pan-European framework for community-based, integrated, and people-centric prevention, monitoring, and progression-managing solutions for dementia and frailty. Its main goal is to introduce an integrated and digitally enabled framework that will facilitate the provision of personalized and integrated care prevention and intervention strategies on dementia and frailty, by piloting novel technologies and producing quantified evidence on the impact to individuals’ wellbeing and quality of life. Methods: A robust and comprehensive design approach adopted through this framework provides the guidelines, tools, and methodologies necessary to empower stakeholders by enhancing their health and digital literacy. The integration of the initial information from 13 pilots across 8 European countries demonstrates the scalability and adaptability of this approach across diverse healthcare systems. Through a systematic analysis, it aims to streamline healthcare processes, reduce health inequalities in modern communities, and foster healthy and active ageing by leveraging evidence-based insights and real-world implementations across multiple regions. Results: Emerging technologies are integrated with societal and clinical innovations, as well as with advanced and evidence-based care models, toward the introduction of a comprehensive global coordination framework that: (a) improves individuals’ adherence to risk mitigation and prevention strategies; (b) delivers targeted and personalized recommendations; (c) supports societal, lifestyle, and behavioral changes; (d) empowers individuals toward their health and digital literacy; and (e) fosters inclusiveness and promotes equality of access to health and care services. Conclusions: The proposed framework is designed to enable earlier diagnosis and improved prognosis coupled with personalized prevention interventions. It capitalizes on the integration of technical, clinical, and social innovations and is deployed in 13 real-world pilots to empirically assess its potential impact, ensuring robust validation across diverse healthcare settings.
1. Introduction
The rapid ageing of the European population places a substantial strain on the current healthcare systems and imposes an emerging need for improving older adults’ wellbeing and active ageing. Modern societies should prioritize innovation and research focused on enhancing the longevity and quality of life (QoL) of individuals, as they are also considered fundamental human rights. Population ageing is a major challenge for European societies and economies, as relatively high rates of chronic illness, mental health conditions, disability, and frailty are observed in older people with high individual and social costs [1]. Recent epidemiological surveys and studies indicate the increasing prevalence of dementia across Europe [2,3,4]. Dementia continues to pose significant public health challenges, with approximately 9.78 million people living with dementia across Europe (EU27 and non-EU27) countries [5]. Evidence from large European longitudinal studies examining prevalence trends from the age group 60–64 onwards through to 80+ indicates a progressive increase in dementia prevalence with advancing age [6,7,8]. Alzheimer’s disease (AD) remains the most common cause of dementia in older adults, with incidence rates rising with age and with an estimated number of 7 million people in Europe that currently live with AD. Projections even indicate that this number could double to 14 million by 2030 due to the rapidly ageing population of Europe [9,10]. However, AD-related neuropathological changes may be present decades before the onset of clinical symptoms [11], and AD has been also related to early-onset dementia with specific needs and experiences [12].
In addition, individuals at risk of dementia constitute a significant under-recognized sub-group, with latent risks to progress to severe cognitive impairment later in their life [13]. Subjective cognitive decline (SCD) refers to self-reported cognitive decline in the absence of objective cognitive impairment [14]. It is considered a potential risk factor for progression to mild cognitive impairment (MCI), which represents a transitional stage between normal ageing and dementia, characterized by objective impairment in cognitive functioning that goes beyond normal age-related changes yet does not significantly impact daily activities [15,16]. Studies have shown that individuals with SCD have an increased risk of developing MCI and dementia, ranging from 5% to 59% in the case of amyloid pathology and 57–65% in case of a full AD profile [17]. The latter highlights the fact that older adults with expressed SCD can progress to more severe types of dementia within the upcoming years. Therefore, timely and accurate diagnosis is crucial in dementia, as early detection enables personalized and effective planning, facilitating the implementation of suitable interventions and treatments to enhance the QoL of affected individuals and potentially slow disease progression. Thus, targeted prevention programs at a younger age prior to the progression of ageing will reduce future dementia risk. This is particularly important given that dementia is a progressive condition, and primary prevention and intervention strategies tend to be most effective when implemented in its early stages. Early identification of individuals at risk, both biologically and clinically, relies on healthcare professionals (HCPs) to possess sufficient and integrated knowledge, age-appropriate care programs, and community-based approaches toward specialized, multidisciplinary services both in terms of prevention and diagnosis of diverse dementia related factors. Highly linked with the notion of dementia is also the physical decline of the individuals or otherwise frailty, which is commonly defined as a clinical syndrome characterized by reduced physiological capacity, increased vulnerability to stressors, and declines in strength, endurance, and functional capacity. Recent studies have indicated that frailty is one of the risk factors associated with the development of dementia [18,19,20]. Individuals with pre-frailty and frailty are at a higher risk of dementia incidence, even after adjusting for a wide range of confounding factors. On top of this, a recent report highlights the permanent important role of cognitive and physical resilience that is empowered across the life course and how reducing vascular damage, low-density lipoprotein (LDL) cholesterol, and other vital risk factors can reduce the development of dementia [9]. Hence, early detection of and interventions in physical frailty, vascular threats, and vision loss, could be also translated into prevention or delayed onset of dementia.
In this direction, emerging technologies also offer new opportunities to enhance personalized care approaches. For instance, patients’ digital twins (PDTs) are used to create dynamic virtual replicas of individuals to simulate health conditions and care scenarios, as well as to predict outcomes and impact of the recommendations. Moreover, ambient assistive living (AAL) systems use smart sensors and devices that further support daily living and monitor health in real-time, and serious games and gamification (SGG) approaches are used to engage users in therapeutic and educational activities through interactive digital tools. Finally, clinical decision support systems (CDSSs) provide clinicians with intelligent recommendations based on integrated patient data and health determinants. The utilization of such advanced technologies will foster and enhance the delivery of personalized recommendations, prevention and intervention measures, advanced visualization analytics, and the introduction of long-lasting behavioral and lifestyle changes. Integrated also with the use of Internet of Things (IoT), mobile, and wearable devices, they can result in cost-effective solutions that can complement HCPs toward delivering targeted and personalized recommendations. The latter can also improve health and digital awareness, healthcare advice, behavioral nudges, training, psychological support, and consultation, leading to the creation of more age-friendly settings (e.g., home, hospital, and working) and empowerment of the health and digital literacy of patients, formal/informal caregivers, and all relevant stakeholders.
This research work initially explores and structures the different stages of dementia prevention, i.e., primary, secondary, and tertiary, within a homogenized coordination framework, COMFORTage, by leveraging the eight identified drivers of dementia research as introduced by the World Health Organization (WHO) [21]. COMFORTage is an EU-funded Horizon Europe project that seeks to introduce a pan-European framework that integrates medical innovation, cutting-edge AI, digital innovation hubs (DIHs), and social initiatives. It started on January 2024 and will last until December 2027. In its context, the framework seeks to provide a structured, multidisciplinary approach to facilitate significant advancements and create the conditions necessary for meaningful progress in understanding, preventing, and treating dementia. The current research work contributes to that aim by specifying and designing a digitally enabled, human-centric healthcare framework that leverages emerging technologies to enhance the prevention and management of dementia and frailty across primary, secondary, and tertiary care settings. The core research questions guiding this work include:
- How can a human-centric, AI-enabled framework be structured to support integrated, personalized prevention and care strategies for dementia and frailty across primary, secondary, and tertiary stages?
- What are the core clinical, technological, and societal components that constitute the COMFORTage framework, and how are these operationalized across the diverse pilot studies?
- In what ways can pilot studies across different European healthcare contexts be aligned under a unified methodological and architectural framework for personalized dementia and frailty care?
- What types of pilot studies are required to demonstrate the feasibility, scalability, and real-world impact of the proposed framework, and how can these be designed to meet regulatory, ethical, and clinical validation requirements across diverse healthcare settings?
- What are the essential data modalities (clinical, biological, behavioral, and digital) that need to be integrated for effective risk prediction and personalized intervention planning in older adults?
Addressing these research questions will be achieved through the analysis of the integration of clinical and social innovation, multimodal health data, AI-based predictive models, PDTs, SGG, and IoT-enabled solutions, and user-centered design principles into a virtualized AI-based healthcare platform (VHP) that supports personalized care strategies, empowers stakeholders, and promotes social and digital inclusion. Such an approach can result in improving individuals’ adherence to risk mitigation and prevention strategies, delivering targeted and personalized recommendations, supporting lifestyle changes, and providing better equality of access to health and care services. These technologies are planned to be utilized in the different prevention stages to enable the creation of a holistic and robust framework covering the whole spectrum of prevention in the modern healthcare domain. Clinical pilot studies will evaluate the real-world application of these technologies, focusing on their ability to address modifiable risk factors, deliver targeted recommendations, and promote lifestyle and behavioral changes. These innovations will be embedded into multidisciplinary, evidence-based care frameworks [12,22,23] toward empowering HCPs, optimizing clinical workflows, and creating scalable, age-friendly interventions that improve outcomes, longevity, and QoL for individuals at risk of or living with dementia.
The remainder of this research work is structured as follows. Section 2 describes the key challenges that the modern healthcare ecosystem faces due to the increase in the ageing population and toward the provision of personalized prevention solutions. In Section 3, the different prevention stages and care phases that should be considered toward the establishment of the framework are detailed. Subsequently, Section 4 presents the main pillars of the COMFORTage framework that are foreseen to be validated and evaluated through the design and implementation of thirteen real-world pilot studies. Finally, Section 5 states the expected wider impacts and how they will be realized, whereas Section 6 concludes this article and states the future work that should be conducted.
2. Challenges of Ageing and Personalized Prevention in the Era of Digital Healthcare Innovation
One of the primary hurdles in the modern healthcare domain is the fact that many treatment protocols for dementia prioritize life prolongation while overlooking patient-centered outcomes, such as longevity, QoL, and wellbeing as perceived by the patients themselves [24]. Existing models for dementia and frailty prognosis, prediction, and treatment often struggle to remain current due to fragmented data sources and the lack of integrated knowledge bases. This fragmentation poses a negative effect on clinical decision-making processes and limits the understanding of underlying causal risk factors for these conditions [25].
Addressing the latter also results in overpassing the current one-size-fits-all approaches in healthcare delivery and addressing the increasing need for personalized care. Personalization aims to treat the individual rather than the disease, accounting for comorbidities, genetic predispositions, and environmental factors [26]. While person-centered AI and assistive technologies hold tremendous potential for improving patient QoL and wellbeing, several challenges occur. Ensuring data privacy and security, increasing accessibility, addressing cost concerns, and resolving ethical and clinical issues are essential steps toward realizing the full potential of personalized healthcare for dementia and frailty care [27]. In parallel, advanced assistive technologies for dementia patients often feature complex interfaces or fail to adapt to individual needs, limiting their effectiveness in enhancing patient QoL [28]. Despite their increasing adoption, there remains limited clinical evidence to validate these technologies’ impacts on individual outcomes. Also, scalability and acceptability among users remain scattered and the adoption of these strategies among stakeholders and healthcare providers is limited. Hence, additional research into and validation under real-world clinical settings is essential to establish their efficacy in improving the personalization and longevity of older adults [29].
Another challenge that affects the digital transformation of the healthcare domain and hinders inclusiveness and equitable healthcare access is the digital divide that is observed in modern societies, which reflects disparities in access to and proficiency with technology [30]. Older adults often face difficulties due to limited digital literacy, leading very often to the development of stigmatization, resistance to technology, and unwanted loneliness [31]. These aspects can prevent them from fully utilizing telehealth services, health apps, or remote monitoring devices [32]. Hence, this divide is particularly critical when integrating digital technologies to address the needs of the ageing population. Recent studies show that an increasing number of older adults are embracing digital technologies, with tailored digital literacy programs boosting their confidence in using healthcare tools [33,34]. However, these initiatives remain insufficiently widespread to close the gap comprehensively. Physical limitations such as poor vision, hearing impairments, and reduced motor skills further complicate interactions with digital platforms, underscoring the need for accessible user interfaces.
Ageing is also intrinsically linked to age-related conditions, such as cognitive decline, dementia, physical disabilities, and frailty. Since effective treatments for these conditions remain limited, prevention emerges as a crucial strategy. However, as noted in the WHO’s Global Status Report on the Public Health Response to Dementia, research into dementia lags significantly behind that into other non-communicable diseases (NCDs) such as cancer, heart disease, and diabetes [35]. In this context, the research output on these conditions is up to 14 times higher than that on dementia. This disparity underlines the urgent need for a comprehensive research agenda, including extensive literature reviews and the integration of data across various domains to support HCPs and healthcare organizations.
During the last decade, several longitudinal studies try to raise awareness, advance research, and improve our understanding of dementia and frailty and have sought to identify modifiable risk factors for these conditions [36,37]. However, most of them focused primarily on genetic predisposition, often neglecting critical modifiable risk factors related to lifestyle, nutrition, and behavioral habits [38]. In addition to this issue, many AI-based prevention and intervention models rely on limited patient data, often excluding critical health determinants such as social factors, real-world data (RWD), patient-reported outcomes, and genomics [39]. This underutilization of comprehensive data sources and integrated data undermines efforts to develop personalized, patient-centric clinical decisions. In alignment with this, a new initiative emerges within the European healthcare landscape, the European Health Data Space [40], that seeks to provide seamless data exchange between healthcare providers across Europe.
To overcome these challenges, a framework must emphasize on the aspects of inclusivity, accessibility, and adaptability while addressing the unique needs of the elderly population. Central to this is the integration of comprehensive data sources, merging clinical, genetic, behavioral, and social determinants of health into unified, interoperable knowledge bases to provide a holistic view of patient health. Dynamic personalization, powered by AI, plays a crucial role in delivering real-time, patient-centric recommendations that adapt to the evolving profiles of individuals. Equally important is the creation of enhanced user interfaces that are intuitive, adaptable, and accessible, specifically designed to accommodate the age-related limitations commonly faced by older adults. To ensure the effectiveness of interventions, the framework must incorporate rigorous evaluation metrics, establishing robust methods to assess improvements in QoL and overall wellbeing. Finally, fostering collaborative ecosystems is essential, involving healthcare professionals, policymakers, and patients in co-designing and implementing interventions. These combined elements form the foundation of a robust framework that can drive innovation in clinical studies, ensuring that they are patient-centered, adaptable, and impactful.
Following these mandates and specifications, the integrated framework introduced in this research work aims to lay the groundwork for digitally-enabled clinical studies that align to the specific needs of the ageing population and dementia and frailty care, ensuring a more holistic, person-centered approach to prevention, risk identification, and treatment. A detailed overview of this holistic and integrated approach is depicted in the figure below, Figure 1.
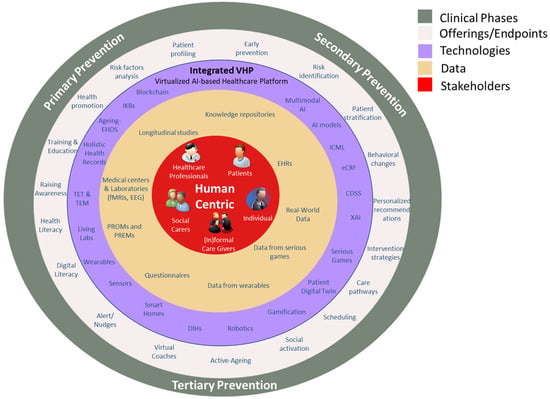
Figure 1.
Human-centric, AI-enabled framework for dementia and frailty prevention and care across primary, secondary, and tertiary stages.
In more detail, this figure presents the COMFORTage human-centric model for the establishment of an integrated, AI-enabled healthcare framework with a particular focus on personalized prevention and care strategies across the continuum of primary, secondary, and tertiary prevention. At its core (red center), it places the individual, patients, healthcare professionals, and formal/informal caregivers at the center of the ecosystem, emphasizing that all interventions and technologies must be specified around human needs and experiences. The next layer (beige) features the clinical data and feedback mechanisms, such as electronic health records (EHRs), real-world data, questionnaires, patient-reported outcome measures (PROMs), patient-reported experience measures (PREMs), genomic data, and data from wearables and serious games, toward enabling continuous monitoring, feedback loops, and decision-making support.
The outer rings represent progressively broader system-level enablers supporting personalized care across the three levels of prevention. The purple layer includes AI and data-driven technologies and tools (e.g., electronic case report forms (eCFRs), multimodal AI, CDSS, eXplainable AI (XAI), PDTs, training and educational marketplaces (TEMs), and training and educational toolkits (TETs)) that can translate data into actionable insights for risk identification, stratification, and intervention planning, particularly in secondary and tertiary prevention. The grey outer ring aligns these technologies and data processes with public health objectives at each prevention stage: from raising awareness and health literacy (primary), to early detection and behavioral change (secondary), and ongoing support for active ageing and social participation (tertiary). Each concentric layer shows how an integrated platform like the COMFORTage’s VHP can potentially harmonize human, technological, and systemic elements for proactive, adaptive, and personalized healthcare delivery.
3. Dementia and Frailty Care Prevention Stages
The specification of the prevention and care assessment stages that need to be addressed plays a vital role in the design of such a framework in the context of the digitalization of the healthcare pathways of dementia and frailty. Hence, there is a need for the establishment of a framework that considers the focus of the pilots on the different stages of prevention (i.e., primary, secondary, and tertiary) based on their individual characteristics, the target populations, and clinical innovations. To substantially help individuals and patients, it is essential to provide high-quality health and care services that are preventive, integrated, coordinated, evidence-based, and person-centered. These services should be both responsive and proactive and should prioritize placing individuals at the core of care delivery. Encapsulating this holistic approach in the context of a framework safeguards that the needs and preferences of all stakeholders (i.e., individuals, patients, caregivers, HCPs, etc.) are respected and addressed throughout the process by also improving outcomes across cognitive, physical, and emotional domains for the individuals that belong to any of the below prevention stages.
Primary Prevention Stage: In this first stage, leading primary data sources, biobanks, and long-term longitudinal observational studies are utilized and examined to better identify and understand the criteria and the key causal factors for developing dementia or frailty. This initial analysis and identification of the risk factors contributing to the development of these conditions seeks to improve early diagnosis and the provision of enhanced personalized preventions. For the establishment of a concrete primary prevention approach, there is the need to target the recognized risk factors and pathways from normal to cognitive/physical decline and finally to dementia/frailty. Methodologies such as stratification of risk by genomic profiling and other blood-based biomarkers, and the monitoring of lifestyle factors should be leveraged with the aim of identifying individuals at risk of dementia and frailty well before the onset of clinical symptoms. This emphasis on early detection and stratification of high-risk populations may allow for timely interventions in modifiable risk factors, thereby reducing the incidence of disease progression. Another challenge is the education of healthcare providers and the empowerment of older adults to take an active role in managing their health, ensuring a holistic and community-based prevention model that may reshape the primary care setting. An innovative approach with societal dimensions is the concept of the Living Labs (LLs) that are user-centered, open innovation ecosystems operating in real-life settings and aim to bring together stakeholders such as end-users, HCPs, researchers, public institutions, and private organizations [41]. Leveraging the LLs will foster the development and implementation of more personalized, inclusive, co-creative, and innovative solutions that are tailored to older adults’ needs.
Secondary Prevention Stage: At this stage, individuals may exhibit early signs of frailty, MCI, or functional vulnerabilities without yet having developed severe clinical conditions. The aim is to recognize these early deviations from baseline functioning and apply proactive measures to maintain autonomy, prevent complications, and delay the transition to more advanced stages of disease. Individuals that are diagnosed as at high-risk, are stratified to personalized care pathways and pipelines based on established clinical guidelines. During this stage, the suitable target and control groups are selected by HCPs based on specific characteristics and inclusion/exclusion criteria. Clinical studies should be conducted in a uniform and harmonized way and under a core minimal set and of clinical protocols that can be extended with pilot-specific adaptations. This approach ensures compliance and alignment with relevant ethical, security, and privacy requirements. In addition, the stratification of patients is followed by utilizing omics (molecular level data, such as genetic, epigenetic, transcriptomic, and gut microbiome metagenomics) and/or other technology-oriented diagnostic tests that are developed toward supporting clinical activities. For instance, a web-based mobile application can be utilized to collect bio-signals from a target group that has been assessed as individuals at high-risk (using the results of neurological and/or psychological tests, magnetic resonance imaging (MRI) scans, and/or electroencephalogram (EEG) tests). In addition, health indexes, lifestyle, and cognitive ability factors at the start of the prevention period are also assessed through on-line questionnaires and measures of both biological, immunological and brain age as assessed by combined methylation indexes using the biological sample provided. Using these test assessments, machine learning (ML) and AI models can be implemented and utilized to stratify the patients to different groups depending on their risk for developing dementia/frailty. Finally, the stratification of patients is based on the utilization of preliminary exam results (e.g., MRI, functional magnetic resonance imaging (fMRI), fluid biomarkers analysis, EEG and electrocardiogram (ECG)) and the selected patients are divided into the different dementia/frailty groups. These preventive efforts are supported by emerging technological infrastructures, such as AAL platforms, PDTs, and decision support systems. These technologies enable clinicians and caregivers to continuously monitor risk trajectories and make informed adjustments to care strategies even in real time.
The interplay between AI and clinical experts enables the development of these specific solutions that can be offered through mobile, IoT, and wearable platforms to engage with high-risk individuals and also patients with prodromal dementia and/or frailty, deliver personalized care plans, monitor their impact, promote long-lasting behavioral changes, and gather valuable feedback. The care plans are targeted to either mitigate the risk by bringing improvements to the medical conditions of the patients or improvements to their longevity and QoL. Based on the interactions between AI and clinical experts the health models are enhanced and calibrated. To this end, personalized and targeted care plans (comprising of recommendations and interventions) are delivered to individuals with specific conditions and through appropriate delivery, feedback, and behavioral change solutions. In that context, serious games can be utilized targeting the prevention of dementia and frailty by stimulating the cognitive function and improving the memory recall of the user. These games are digital applications that have been designed for educational purposes and for increasing the health and digital literacy of individuals [42]. They can track the individual’s performance and provide feedback to healthcare providers, while tracking the progress and performance of the individual. Through this integrated, holistic, and technology-supported model, secondary prevention becomes a more dynamic, personalized process designed to foster cognitive, physical, and emotional well-being in individuals at the earliest stages of decline.
Tertiary Prevention Stage: The focal point in this stage is the identification of personalized treatment and coping schemes for each patient and mitigating the impact of established conditions such as advanced frailty, cognitive impairment, and chronic comorbidities. The goal is to reduce complications, slow disease progression, and support functional recovery, longevity, and QoL through personalized and digitally-supported care pathways. Patients at this stage often require structured interventions that go beyond prevention and into active care management; thus improved and personalized monitoring is of vital importance. In that context, utilizing emerging technologies such as the AAL, SGG, AI-based CDSS, and PDT fosters the continuous observation of patients’ health trajectories and provides clinical decision support facilitating the timely adjustments in care plans. Through this approach, the potential of how these tools enhance current treatment protocols is assessed. Secondary care facilities should be equipped with decision-support tools and advanced monitoring technologies, ensuring that HCPs can make more informed and precise diagnoses, ultimately improving patient outcomes. Technology-driven advancements in treatment, monitoring, and personalized recommendations play a vital role at this stage. Treatment is categorized in three types: (a) medical, including any medication and body treatment (such as kinesiotherapy, physiotherapy, etc.); (b) cognitive, including tools designed for keeping the brain active or training the patient in such a way so as to slow down the evolution of cognitive and physical conditions and/or helping the patient improve their QoL by being able to provide self-service; and (c) societal, addressing the signs of social withdrawal and emotional isolation, which often underlie cognitive and psychological decline. It should be noted that these three types of treatment act complementary and synergistically depending on the patient case.
One of the central components of tertiary prevention is fall prevention, especially among older adults who show reduced mobility, instability, or musculoskeletal decline. Through targeted mobility exercises, environmental modifications, and monitoring technologies, the risk of falls is significantly reduced, thus preventing injury and subsequent physical deterioration. Highly related is also the prevention of sarcopenia, which is addressed through structured movement programs, strength training routines, and continuous guidance from HCPs and (in)formal caregivers. These approaches facilitate a better management of musculoskeletal health and power, as well as encourage functional autonomy and independence toward reducing caregiver burden. Furthermore, nutritional and dietary guidance is also an integral part of this third stage with the aim of maintaining the physical capacity of the patients and managing chronic symptoms. In that direction, the integration between clinical expertise and AI-supported systems leads to the development of personalized nutrition plans considering the individual’s metabolic profile, dietary preferences, and comorbid conditions. Such plans should foster diets rich in diverse and valuable nutrients that can potentially have a positive impact on the cognitive and physical resilience of individuals and in slowing disease progression [43]. For instance, the Mediterranean diet, which is characterized by high intake of fruits, vegetables, whole grains, legumes, nuts, seeds, olive oil, and fish, has been extensively associated with improved cognitive function and reduced risk of cognitive decline [44].
When it comes to addressing cognitive aspects, then this resorts to using graphical tools, serious games, gamification approaches, training tools and materials, etc., that are developed according to the patient case in hand and depending on the findings of the initial stage of individual stratification, and always guided by clinical experts. The incorporation of AI-optimized serious games exhibits great potential as a precision tool for tertiary dementia prevention by dynamically adapting to patients’ real-time engagement patterns [45]. Such systems address the need for the adoption of solutions that go beyond “one-size-fits-all” interventions, taking into consideration the divergent cognitive capacities of dementia patients, by optimizing adherence and mitigating disease progression through responsive and patient-centered stimulation [46]. Moreover, patients of this stage can also be monitored using various appropriate devices, such as wearables, smart devices in their different environments (e.g., home, work, and social) connected with IoT and integrated with AAL tools to help alleviate problems. The latter is also coupled with the utilization of robotics systems, especially in real home environments. These robotic systems base their functionality on enhanced knowledge and innovative capabilities through the combination of multi-sensorial networks and AI, covering the full lifecycle of robotics. Advanced manipulation and control (mobile robot navigation, arm manipulation and grasping, etc.), intuitive perception (semantic mapping, human tracking, activity recognition, etc.), and cognition (learning by demonstration, proactive engagement, adaptive task planning, decision making, etc.) are some of the functionalities that seek to be utilized and support patients in advanced stages of their disease. Meanwhile, the evaluation of DIHs, smart homes, and their accompanied virtual and AAL technologies results in the introduction of narrative interventions, scenario testing, and simulation-driven planning that further strengthen the personalization and responsiveness of tertiary prevention strategies. The latter can also support the integration of continuous learning and feedback loops across care stakeholders optimizing strategies and the healthcare continuum.
Addressing the challenges faced by older adults in a holistic manner also requires close attention to handling the societal dimensions of ageing and disease progression. Such challenges are mainly related to combating loneliness and social isolation, improving social participation, tackling the digital divide and lack of technological skills, and decreasing age-related stigma and resistance to technology. To this end, initiatives and advanced social learning techniques should be introduced to foster digital inclusion and social support, using user-centered collaborative approaches, and technologies that promote communication, interaction, and sustained participation in social networks. The involvement of individuals with lived experience in LLs and in the co-design and evaluation of technologies will ensure that the introduced solutions effectively reflect users’ needs, preferences, and everyday realities. This also includes the specification of measures to alleviate unwanted loneliness, physical and cognitive decline, and frailty in older adults, highlighting the need for comprehensive interventions that address physical and emotional well-being. Such interventions should take up and leverage the importance of social learning as a driver for the adoption of technologies and social improvements among older adults.
4. COMFORTage Framework and Its Pilot Studies
The COMFORTage framework is a comprehensive and digitally enabled model designed to foster transformative change in dementia and frailty research and care. It is built upon three core interconnected pillars, clinical, technological, and societal, as showcased in Table 1. These pillars will be piloted and validated across multiple and large-scale healthcare facilities and in the context of 13 different real life use cases and clinical studies ranging across 8 European countries. The table provides a structured overview of how the COMFORTage framework’s three core pillars align with the strategic goals and interventions across different prevention stages. It maps the pillars with the key endpoints and offerings associated with each stage of dementia prevention, i.e., primary, secondary, and tertiary, as well as primary prevention of frailty. For each stage, the table outlines the specific focus areas: for example, in the clinical domain, it highlights actions such as risk factor identification through longitudinal data and biobanks (primary), patient stratification using omics data (secondary), or tailored treatment schemes and fall prevention (tertiary). The technical pillar maps tools like AI-based risk prediction models, AAL, PDTs, and robotics to each stage, supporting monitoring, care planning, and intervention delivery. The societal dimension emphasizes empowerment through health literacy, behavioral change promotion, social participation, and digital inclusion.

Table 1.
COMFORTage Framework: Main pillars and prevention stages’ specification.
To address the challenges of dementia and frailty prevention across the different stages of care, a structured mapping, categorization, and analysis of pilot studies was included in the COMFORTage project and is further presented in Table 2. The selection of studies was guided by the main goal of the project, which is to establish a comprehensive, clinical, and digitally enabled framework that supports personalized prevention, early risk identification, and intervention. The pool of studies consists of those officially embedded within the COMFORTage pilot activities, led by academic, clinical, and research institutions across Europe. The selection criteria included relevance to dementia and/or frailty prevention, integration of digital tools or AI, level of personalization, and alignment with one or more of the prevention stages (primary, secondary, and tertiary). The table provided below illustrates the diversity and scope of these studies, categorizing them by organization, study rationale, methodological design, main focus area, and degree of personalization. Its purpose is to highlight how different research strands contribute to the validation and operationalization of the COMFORTage framework, demonstrating its multi-layered approach and applicability across clinical, technological, and social domains.

Table 2.
COMFORTage digitally enabled pilot studies.
The allocation of the pilot studies is an outcome of the objective to adopt a multidimensional approach to addressing dementia prevention, early diagnosis, and improved management, adopting broader societal and technological innovations. Specifically, four studies focus on identifying causal risk factors and implementing early detection strategies for dementia through the integration of genetic and biomarker data, lifestyle factors, and community-based primary prevention. One study explores the early identification of dementia among patients with early signs of frailty and sarcopenia. Two pilots are dedicated to designing and delivering improved, personalized interventions aimed at delaying the progression of dementia and providing relief for patients. Beyond dementia-specific studies, three pilots explore frailty and neuromechanical interventions, broadening the scope to fall prevention and postural control for the modern ageing population. Finally, three pilots address societal and environmental aspects, utilizing advanced technologies like IoT, mobile health (mHealth) applications and devices, and social learning mechanisms within LLs to enhance the QoL, wellbeing, and social interactions of individuals and caregivers. An overview and outline of these studies follows in the table below. It should be also noted that these pilot studies are planned to be conducted within a 38-month period initiated in October 2024 and running until December 2027 with varying duration between them, as indicated in the below table. The studies include both observational and interventional designs and all subjects will be followed longitudinally throughout the different study periods.
Although the pilots include different populations ranging from SCD to MCI to mild-to-moderate dementia, all of them share the aim of establishing a model of personalized intervention to reduce clinical progression in frailty and demented populations. In order to achieve a comparable standard in the outcome measures, a common set of scales and questionnaires has been defined to similarly measure among pilots cognitive, functional, and QoL domains. To ensure consistency and comparability, a co-design process was followed focused on applying standard diagnostic definitions and identifying shared variables, neurophysiological assessments, and questionnaires. This approach supported a unified methodological framework while allowing for pilot-specific adaptations.
Each pilot is recruiting participants who will undergo comprehensive baseline assessments, including demographic data, clinical history, neuropsychological evaluations, and quality of life questionnaires. The tools and questionnaires identified for use have been standardized and are presented in Table 3, though minor modifications may occur to accommodate language availability or context-specific constraints. Depending on the focus of the individual pilot, additional assessments may include blood and CSF sampling for biomarker analyses, physical strength evaluations, extended neuropsychological testing, and neuroimaging.

Table 3.
Key variables and neuropsychological assessments in the COMFORTage harmonized clinical protocol.
Concerning the common variables across all pilots, harmonized demographic data will be collected, including age, sex, education level, employment status, marital status, living situation, and ethnicity. Clinical information will include comorbidities, lifestyle habits, and current medical treatments, supported by standard measures such as blood pressure, as well as comorbidity scoring systems. Most pilots will also gather data on physical activity, nutrition, and frailty. All participants will undergo a baseline neuropsychological evaluation using the MMSE, CDR, and ADL scales. In selected pilots, extended neuropsychological batteries will be administered to assess domains such as memory, executive function, attention, and visuospatial abilities, using validated tools appropriate to each local context. Depression and behavioral symptoms will also be evaluated, and additional questionnaires may be used to assess sleep quality, cognitive reserve, and overall QoL. Furthermore, selected pilots will include routine blood tests, along with CSF and blood-based biomarker analyses for amyloidopathy, tauopathy, and neurodegeneration. Genetic analyses, such as determination of the ApoE genotype, will also be conducted in specific pilots. This harmonized data-driven approach supports the development and delivery of personalized prevention and intervention plans tailored to individual profiles and disease trajectories.
A more detailed positioning of the pilots follows in Figure 2, which depicts the different stages of prevention (x axis) in alignment with the focus area (y axis) of each pilot, giving a glance on how the different pilot studies integrate in the modern healthcare landscape. The latter can also act as a comprehensive coordination mechanism and guide for any future study involving digitally enabled clinical studies. The pilot studies cover diverse prevention stages and focus areas, and vary significantly in the prevention stage that they target on, reflecting their tailored approach to addressing dementia, frailty, and ageing-related challenges. More specifically, three pilots emphasize the early and primary prevention on healthy populations, as well as risk factors analysis, by integrating molecular, clinical, wearables, sensor-based, and other real-world data with advanced technologies to offer tailored prevention strategies. These pilots focus also on the introduction of personalized recommendations to individuals fostering behavioral and lifestyle changes. One pilot focuses on a mixed prevention stage between primary and secondary, as the risk identification will act as a basis for the stratification of the individuals toward providing personalized care strategies on the ones identified as of high risk. In parallel, five pilots clearly focus on the provision of secondary prevention measures and recommendations to patients that have already developed early signs of cognitive or physical decline. Two of these pilots seek to specifically leverage biomarkers and genetic data for enhancing the understanding of the key factors affecting the progression of dementia, while one more seeks to integrate emerging technologies such as PDTs and AAL applications toward this scope. Moreover, two of them specifically study the frailty symptoms and the management of physical decline. Finally, four pilots focus on the provision of solutions targeting tertiary prevention by using social learning, collective intelligence mechanisms, DIHs, and smart homes, as well as LLs to adapt interventions to individual needs while leveraging advanced technologies. These pilots adopt high integration with technology and social learning mechanisms, emphasizing community-based prevention, lifestyle adaptation, and early identification for larger population groups. The below allocation of pilot studies underscores the introduced framework’s adaptability in addressing the wide range of needs across various population groups.
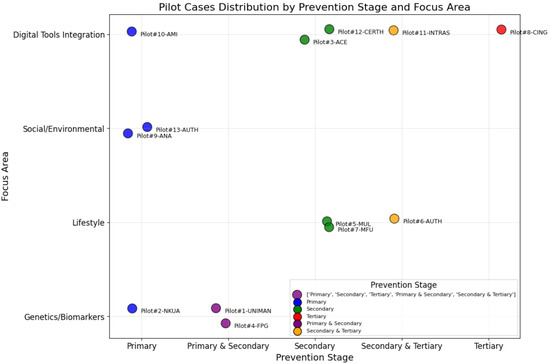
Figure 2.
Distribution of COMFORTage pilot studies in terms of prevention stage (x axis) and focus area (y axis).
5. Discussion and Expected Impacts
COMFORTage framework significantly impacts the ability of healthcare organizations, citizens and social scientists, and healthcare public authorities to take data-driven, evidence-based, and integrated healthcare policies in terms of prevention and intervention measures, while also older adults are empowered to take an active role in the management of their own physical and mental health. This impact is reflected in tangible and significant contributions and key propositions.
5.1. Clinical Pillar
COMFORTage introduces holistic and integrated healthcare models and digitally-enabled care pathways to foster personalized dementia and frailty preventions and interventions promoting individuals’ physical and mental health. These approaches are designed to improve individuals’ physical and mental health, leveraging expert clinical insights to develop evidence-based study protocols and personalized care plans. The combination of clinical expertise, behavioral science, community engagement, and civil society aspects fosters sustainable lifestyle changes that enhance individuals mental and physical health, wellbeing, and QoL. To this end, this framework facilitates the support of high-risk individuals and of people in the early stages of dementia and frailty and the delivery of personalized care plans and interventions. Through this, it enables more accurate, timelier, more automated, and personalized clinical decisions about dementia and frailty patients, by also utilizing integrated knowledge bases that result in the delivery of accurate, patient-centric predictions, which provide a sound basis for tailored care recommendations and will improve the patients’ QoL and wellbeing. These advances directly benefit patients while providing healthcare service providers, such as hospitals and geriatric clinics, effective tools and harmonized solutions.
Emerging technologies, including AI-driven CDSS, virtual coaches, and training systems, are pivotal in helping patients better understand their medical conditions, treatment options, and self-management strategies. Patients are monitored to evaluate their responses to treatment, symptom progression, independence levels, potential safety hazards, and communication abilities. Dedicated tools collect real-time data—from sensor readings (e.g., oxygen levels, pulse rates) to images and videos—and present this information through the CDSS, providing graphical insights, alerts, and notifications as needed. These tools enable the design of holistic digital care pathways tailored to individual needs, such as questionnaires and virtual coach guidance. HCPs also benefit from updated, evidence-based medical knowledge, enhancing their clinical decision-making and improving patient care delivery.
5.2. Technological Pillar
COMFORTage’s tools boost the development, deployment, and operation of people-centered age-related prevention and intervention measures enhancing security, trustworthiness, robustness, and safety of AI systems in healthcare sector. These tools contribute to the EU’s strategies and actions, such as the Green Paper on Ageing and the EU Long-Term Care Report [49], through (1) causal risk factors analysis, (2) prevention (i.e., personalized real-time recommendations based on RWD), (3) early diagnosis and accurate personalized prediction, (5) cost efficient and effective treatment for all through the introduction of a robust health technology assessment (HTA) methodology, (6) improved QoL through personalized post-treatment interventions, and (7) provision of evidence-based interventions introduced throughout the design and implementation of the framework and further scaled-up through large-scale piloting activities and relevant multipliers and networks. COMFORTage enables and demonstrates the use of cutting-edge AI technologies on dementia and frailty prevention and prediction, which provide clinicians and policymakers with the most advanced, transparent, and reliable AI technologies, coupled with AAL solutions for dementia and frailty prevention and delay of progression.
5.3. Societal Pillar
People-centered care, understood as the process of treating patients as unique individuals and users whose opinions are key in the design and development of healthcare services [50], lies at the core of COMFORTage framework to also reflect the societal impact of advancing inclusive, technology-driven solutions for ageing populations. Key areas of impact include empowering individuals to maintain autonomy and combat loneliness through user-friendly tools that foster social connections and provide accessible health information. These solutions will enhance early diagnosis and prevention of dementia and frailty, supported by initiatives to improve digital literacy. Although the intertwined missions of public health and primary health care evidence-based policies have long been identified, most health systems have found it challenging to fully take advantage of the potential synergy offered by collaborative programs. However, since demographic, social, and economic trends increase the pressure on health systems to deliver improved and efficient policies at a lower cost, effective collaboration between public health authorities, primary health care, and social scientists emerges as an essential ingredient for ensuring the sustainability of introduced evidence-based innovations and policies, as well of health systems.
Caregivers will benefit from solutions that streamline daily routines and health monitoring, reducing their burden while maintaining high care quality. Emotional and psychological support systems will also be integral, addressing the stress associated with caregiving. Access to reliable and updated information on dementia and frailty care will further enhance caregiving outcomes. In parallel, for HCPs, the focus on reducing workload while ensuring efficient and high-quality patient care will drive the adoption of tools that promote healthy lifestyles and provide patient education. Emotional and psychological support for healthcare providers will play a crucial role in addressing the challenges of demanding clinical environments.
Lastly, individuals are upon prevention and mitigation actions about lifestyle and behavioral changes and health-related risks, embracing the notion “know your risk, lower your risk”. To this end, they are empowered to take an active role in the management of their own physical and mental health, which results in improved adherence to treatment plans by providing to them with a better understanding of the importance of their adherence to proposed prevention and intervention measures and lifestyle changes.
COMFORTage serves as a holistic, digitally enabled reference framework for healthcare stakeholders and systems, designed to support the application of stratification strategies that more accurately identify individuals at risk and enable the planning of personalized interventions across the different stages of dementia prevention. Personalized interventions and preventions strategies will rely on the different variables considered in the various pilots following an harmonized clinical protocol to the greatest possible extent. Coupled with a multi-disciplinary HTA approach the outcomes and insights of this framework will be introduced and established in the real-world healthcare settings of the pilots in line with the framework’s reference model. The latter will foster the evaluation of these technologies determining their added value to all stakeholders of the healthcare domain. This approach will promote and enhance transparency and accountability in healthcare decision-making, by also providing evidence-based information about the value of digital healthcare technologies with the ultimate goal to ensure that decisions are based on the best available evidence rather than on subjective or vested interests.
6. Conclusions
This research work achieves a two-fold objective, as it acts as a comprehensive introduction of the holistic COMFORTage framework and as a coordination mechanism for future initiatives and projects toward the specification of the main instances and innovations that should drive their design and implementation. The COMFORTage framework aims to leverage advanced AI tools and a suite of specialized applications to enhance the study of dementia and frailty among elderly populations across multiple pilot clinical and research sites. The primary objective is to utilize data-driven insights to improve diagnostic accuracy, treatment efficacy, and overall patient care. The integration of AI-driven analytics with heterogeneous clinical and lifestyle data will result in the identification of new patterns, causal pathways, and predictors of dementia and frailty, thereby enabling early intervention and personalized treatment plans. This research work outlines this holistic framework and acts as a strategic blueprint aimed at revolutionizing dementia and ageing care through the coordination of integrated and digitally-enabled pilot studies. This will result in improved transparency, accountability, and evidence-based decision-making in the healthcare domain, ensuring also the cost-effectiveness of the healthcare resources and the improvement of the care, longevity, QoL, and wellbeing of older adults. In that direction, COMFORTage seeks to become a catalyst to help prevent, monitor, and manage progression of cognitive and physical decline based on high-end research and analysis of the utilization of AI-based applications and their medical, technological, and societal impacts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jdad2030030/s1, Document: COMFORTage Consortium Members List.
Author Contributions
Conceptualization, G.M., S.L., C.M. and D.K. (Dimosthenis Kyriazis); Data curation, D.T. (Dimitrios Tsolis), D.K. (Dimitrios Koutsomitropoulos), A.G., J.L., A.M.T., N.B. and M.S.; Formal analysis, E.A., G.T., A.G., P.V., D.V. and K.C.; Funding acquisition, G.M., S.L. and D.K. (Dimosthenis Kyriazis); Investigation, C.M., G.M.G., K.K.L., J.L., N.M. and A.M.T.; Methodology, E.A., E.C., P.V., W.-J.v.d.H. and COMFORTage Consortium; Project administration, G.M. and D.K. (Dimosthenis Kyriazis); Resources, A.A., D.T. (Dimitrios Tsolis), D.T. (Dimitrios Tsoukalos) and S.K.; Software, G.T. and D.T. (Dimitrios Tsoukalos); Supervision, D.K. (Dimitrios Koutsomitropoulos), N.B., M.S. and W.-J.v.d.H.; Validation, D.T. (Dimitrios Tsolis), G.T., D.K. (Dimitrios Koutsomitropoulos), K.K.L., P.V., S.K. and COMFORTage Consortium; Visualization, S.K., K.C., A.M.T. and W.-J.v.d.H.; Writing—original draft, G.M., S.L., E.A. and E.C.; Writing—review and editing, A.A., C.M., G.M.G., A.G., D.V. and N.M. For the complete list of authors contributions please advise the attached document. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon Europe research and innovation program under grant agreement no. 101137301 and is supported by the Innovative UK under grant agreement no. 10103541. Views and opinions expressed are however those of the authors only and do not necessarily reflect those of the European Union or European Health and Digital Executive Agency (HaDEA). Neither the European Union nor the granting authority can be held responsible for them.
Institutional Review Board Statement
The studies introduced in the context of this research work are in the design phase. Thus, this statement is not applicable.
Informed Consent Statement
The studies introduced in the context of this research work have not yet initiated or their results are not provided. Thus, this statement is not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The research leading to the results presented in this paper has received funding from the European Union’s Horizon Europe research and innovation program under grant agreement no. 101137301 and is supported by the Innovative UK under grant agreement no. 10103541. Views and opinions expressed are however those of the authors only and do not necessarily reflect those of the European Union or European Health and Digital Executive Agency (HaDEA). Neither the European Union nor the granting authority can be held responsible for them.
Conflicts of Interest
Authors Athos Antoniades, Emily Charalambous, Paris Vogazianos, Dimitris Vrachnos and Konstantinos Charilaou, were employed by the company Stremble Ventures Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Santini, Z.I.; Jose, P.E.; Koyanagi, A.; Meilstrup, C.; Nielsen, L.; Madsen, K.R.; Koushede, V. Formal social participation protects physical health through enhanced mental health: A longitudinal mediation analysis using three consecutive waves of the Survey of Health, Ageing and Retirement in Europe (SHARE). Soc. Sci. Med. 2020, 251, 112906. [Google Scholar] [CrossRef]
- World Health Organization. World failing to address dementia challenge. Available online: https://www.who.int/news/item/02-09-2021-world-failing-to-address-dementia-challenge (accessed on 28 March 2025).
- Meijer, E.; Casanova, M.; Kim, H.; Llena-Nozal, A.; Lee, J. Economic costs of dementia in 11 countries in Europe: Estimates from nationally representative cohorts of a panel study. Lancet Reg. Health 2022, 20, 100445. [Google Scholar] [CrossRef]
- OECD/European Union. Health at a Glance: Europe 2022: State of Health in the EU Cycle; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Alzheimer Europe. Prevalence of Dementia in Europe. Available online: https://www.alzheimer-europe.org/dementia/prevalence-dementia-europe (accessed on 8 April 2025).
- Solomon, A.; Handels, R.; Wimo, A.; Antikainen, R.; Laatikainen, T.; Levälahti, E.; Peltonen, M.; Soininen, H.; Strandberg, T.; Tuomilehto, J.; et al. Effect of a multidomain lifestyle intervention on estimated dementia risk. J. Alzheimer’s Dis. 2021, 82, 1461–1466. [Google Scholar] [CrossRef]
- Jönsson, L.; Tate, A.; Frisell, O.; Wimo, A. The costs of dementia in Europe: An updated review and meta-analysis. PharmacoEconomics 2023, 41, 59–75. [Google Scholar] [CrossRef]
- Veronese, N.; Koyanagi, A.; Dominguez, L.J.; Maggi, S.; Soysal, P.; Bolzetta, F.; Vernuccio, L.; Smith, L.; Matranga, D.; Barbagallo, M. Multimorbidity increases the risk of dementia: A 15 year follow-up of the SHARE study. Age Ageing 2023, 52, afad052. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- European Brain Council (EBC). Rethinking Alzheimer’s Disease. Available online: https://www.braincouncil.eu/projects/rethinking-alzheimers-disease (accessed on 18 March 2025).
- Hendriks, S.; Peetoom, K.; Bakker, C.; van der Flier, W.M.; Papma, J.M.; Koopmans, R.; Verhey, F.R.J.; de Vugt, M.; Köhler, S.; Young-Onset Dementia Epidemiology Study Group; et al. Global prevalence of young-onset dementia: A systematic review and meta-analysis. JAMA Neurol. 2021, 78, 1080–1090. [Google Scholar] [CrossRef]
- Logsdon, R.G.; McCurry, S.M.; Teri, L. Evidence-based interventions to improve quality of life for individuals with dementia. Alzheimer’s Care Today 2007, 8, 309–318. [Google Scholar] [PubMed]
- Banerjee, S.; Samsi, K.; Petrie, C.D.; Alvir, J.; Treglia, M.; Schwam, E.M.; del Valle, M. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int. J. Geriatr. Psychiatry 2009, 24, 15–24. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2024, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Gao, Y.; Huang, X.; Yang, Y.; Yang, C.; Wan, Q. Predictors of progression from subjective cognitive decline to objective cognitive impairment: A systematic review and meta-analysis of longitudinal studies. Int. J. Nurs. Stud. 2024, 149, 104629. [Google Scholar] [CrossRef] [PubMed]
- Rostamzadeh, A.; Bohr, L.; Wagner, M.; Baethge, C.; Jessen, F. Progression of subjective cognitive decline to MCI or dementia in relation to biomarkers for Alzheimer disease: A meta-analysis. Neurology 2022, 99, E1866–E1874. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Lewis, C.M.; Vassos, E. Polygenic risk scores: From research tools to clinical instruments. Genome Med. 2020, 12, 44. [Google Scholar] [CrossRef]
- Vernarelli, J.A. Impact of genetic risk assessment on nutrition-related lifestyle behaviours. Proc. Nutr. Soc. 2013, 72, 153–159. [Google Scholar] [CrossRef]
- WHO. A Blueprint for Dementia Research; WHO: Geneva, Switzerland. Available online: https://www.who.int/publications/i/item/9789240058248 (accessed on 28 March 2025).
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Sturge, J.; Klaassens, M.; Jones, C.A.; Légaré, F.; Elf, M.; Weitkamp, G.; Meijering, L. Exploring assets of people with memory problems and dementia in public space: A qualitative study. Wellbeing. Space Soc. 2021, 2, 100063. [Google Scholar] [CrossRef]
- Davies, M.; Zúñiga, F.; Verbeek, H.; Simon, M.; Staudacher, S.; Heyn, P. Exploring interrelations between person-centered care and quality of life following a transition into long-term residential care: A meta-ethnography. Gerontologist 2023, 63, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.-Y.; Yu, H.-W.; Goto, R.; Lai, W.-L.; Li, H.-C.; Tsai, E.-T.; Chen, Y.-M. From fragmentation toward integration: A preliminary study of a new long-term care policy in a fast-aging country. BMC Geriatr. 2019, 19, 159. [Google Scholar] [CrossRef]
- Yadav, S. Transformative Frontiers: A Comprehensive Review of Emerging Technologies in Modern Healthcare. Cureus 2024, 16, e56538. [Google Scholar] [CrossRef]
- Baker, S.; Xiang, W. Artificial Intelligence of Things for Smarter Healthcare: A survey of advancements, challenges, and opportunities. IEEE Commun. Surv. Tutorials 2023, 25, 1261–1293. [Google Scholar] [CrossRef]
- Dada, S.; Van der Walt, C.; May, A.A.; Murray, J. Intelligent assistive technology devices for persons with dementia: A scoping review. Assist. Technol. 2024, 36, 338–351. [Google Scholar] [CrossRef]
- Weck, M.; Afanassieva, M. Toward the adoption of digital assistive technology: Factors affecting older people’s initial trust formation. Telecommun. Policy 2023, 47, 102483. [Google Scholar] [CrossRef]
- Saeed, S.A.; Masters, R.M. Disparities in Health Care and the Digital Divide. Curr. Psychiatry Rep. 2021, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lim, J.-A.; Nam, H.-K. Effect of a Digital Literacy Program on Older Adults’ Digital Social Behavior: A Quasi-Experimental Study. Int. J. Environ. Res. Public Health 2022, 19, 12404. [Google Scholar] [CrossRef]
- Nikou, S.; Agahari, W.; Keijzer-Broers, W.; de Reuver, M. Digital healthcare technology adoption by elderly people: A capability approach model. Telemat. Inform. 2020, 53, 101315. [Google Scholar] [CrossRef]
- Bertolazzi, A.; Quaglia, V.; Bongelli, R. Barriers and facilitators to health technology adoption by older adults with chronic diseases: An integrative systematic review. BMC Public Health 2024, 24, 506. [Google Scholar] [CrossRef]
- Ambrens, M.; Stanners, M.; Valenzuela, T.; Razee, H.; Chow, J.; van Schooten, K.S.; Close, J.C.T.; Clemson, L.; Zijlstra, G.A.R.; Lord, S.R.; et al. Exploring Older Adults’ Experiences of a Home-Based, Technology-Driven Balance Training Exercise Program Designed to Reduce Fall Risk: A Qualitative Research Study Within a Randomized Controlled Trial. J. Geriatr. Phys. Ther. 2023, 46, 139–148. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on the Public Health Response to Dementia; World Health Organization: Geneva, Switzerland, 2021.
- Gao, L.; Tang, J.; Odden, M.C.; Wu, C. The influence of frailty: How the associations between modifiable risk factors and dementia vary. Ann. Epidemiol. 2024, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Booth, A.; Rockwood, K.; Peters, J.; D’eSte, C.; Anstey, K.J. Combining modifiable risk factors and risk of dementia: A systematic review and meta-analysis. BMJ Open 2019, 9, e022846. [Google Scholar] [CrossRef]
- Ward, D.D.; Ranson, J.M.; Wallace, L.M.K.; Llewellyn, D.J.; Rockwood, K. Frailty, lifestyle, genetics and dementia risk. J. Neurol. Neurosurg. Psychiatry 2022, 93, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, C.; Skene, N.; Bandres-Ciga, S.; Anderson, E.; Winchester, L.M.; Foote, I.F.; Schwartzentruber, J.; Botia, J.A.; Nalls, M.; Singleton, A.; et al. Artificial intelligence for dementia genetics and omics. Alzheimer’s Dement. 2023, 19, 5905–5921. [Google Scholar] [CrossRef]
- Hussein, R.; Scherdel, L.; Nicolet, F.; Martin-Sanchez, F. Towards the European Health Data Space (EHDS) ecosystem: A survey research on future health data scenarios. Int. J. Med. Inform. 2023, 170, 104949. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.L.; Jang, H.; Cho, M.; Lee, M.; Kim, J.; Lee, H. Living labs for health: An integrative literature review. Eur. J. Public Health 2020, 30, 55–63. [Google Scholar] [CrossRef]
- Laamarti, F.; Eid, M.; El Saddik, A. An overview of serious games. Int. J. Comput. Games Technol. 2014, 2014, 358152. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Peris-Ramos, H.C.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; David-Fernandez, S.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Personalizing nutrition strategies: Bridging research and public health. J. Pers. Med. 2024, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Saragih, I.D.; Everard, G.; Lee, B.-O. A systematic review and meta-analysis of randomized controlled trials on the effect of serious games on people with dementia. Ageing Res. Rev. 2022, 82, 101740. [Google Scholar] [CrossRef] [PubMed]
- Manera, V.; Ben-Sadoun, G.; Aalbers, T.; Agopyan, H.; Askenazy, F.; Benoit, M.; Bensamoun, D.; Bourgeois, J.; Bredin, J.; Bremond, F.; et al. Recommendations for the use of serious games in neurodegenerative disorders: 2016 Delphi Panel. Front. Psychol. 2017, 8, 1243. [Google Scholar] [CrossRef]
- Kyriazakos, S.; Pnevmatikakis, A.; Kostopoulou, K.; Ferrière, L.; Thibaut, K.; Giacobini, E.; Pastorino, R.; Gorini, M.; Fenici, P. Benchmarking the clinical outcomes of Healthentia SaMD in chronic disease management: A systematic literature review comparison. Front. Public Health 2024, 12, 1488687. [Google Scholar] [CrossRef] [PubMed]
- Eligence & Exercise Intelligence. Available online: https://eligence.eu/portal/index.php/en/home-english/ (accessed on 14 March 2025).
- European Commission. Green Paper on Ageing. Available online: https://commission.europa.eu/system/files/2021-06/green_paper_ageing_2021_en.pdf (accessed on 18 March 2025).
- Zhao, J.; Gao, S.; Wang, J.; Liu, X.; Hao, Y. Differentiation between two healthcare concepts: Person-centered and patient-centered care. Int. J. Nurs. Sci. 2016, 3, 398–402. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).