Abstract
Background/Objectives: Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, synaptic dysfunction, and neuronal loss. Although amyloid-β plaques and neurofibrillary tangles have been the historical hallmarks of AD pathology, growing evidence highlights microglial-mediated neuroinflammation as a central driver of disease onset and progression. This review aims to provide an updated overview of the dual roles of microglia in AD, from their protective functions to their contribution to chronic inflammation and neurodegeneration. Methods: This review synthesizes findings from recent experimental and clinical studies to examine the molecular mechanisms underlying microglial activation and dysfunction in AD. Key areas of focus include microglial signaling pathways, gut–brain axis interactions, and immunometabolic regulation. The review also evaluates emerging immunomodulatory therapeutic strategies designed to restore microglial homeostasis. Results: Recent studies reveal that microglia undergo a dynamic transition from a homeostatic to a reactive state in AD, contributing to sustained neuroinflammation and impaired clearance of pathological aggregates. Molecular mechanisms such as TREM2 signaling, NLRP3 inflammasome activation, and metabolic reprogramming play critical roles in this process. Additionally, gut microbiota alterations and systemic inflammation have been shown to influence microglial function, further exacerbating disease pathology. Conclusions: Targeting microglial dysfunction through immunomodulatory strategies holds promise as a disease-modifying approach in AD. Therapeutic avenues under investigation include natural compounds, synthetic modulators, immunotherapies, and microbiota-based interventions. A deeper mechanistic understanding of microglial regulation may open new translational pathways for the development of effective treatments for AD.
1. Introduction
Alzheimer’s disease (AD) represents the most prevalent cause of dementia worldwide, affecting more than 55 million individuals and contributing to significant healthcare and socioeconomic burdens. As global life expectancy continues to rise, the incidence of AD is projected to triple by 2050, underscoring an urgent need for effective disease-modifying therapies [1]. Clinically, AD manifests as progressive cognitive decline, memory loss, and functional impairment. Histopathologically, it is characterized by the accumulation of extracellular amyloid beta (Aβ) plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein, hallmarks that have dominated therapeutic development for decades [2].
Despite the centrality of the amyloid cascade hypothesis, the clinical outcomes of Aβ-targeting interventions have been largely disappointing. Monoclonal antibodies (mAbs) such as aducanumab and lecanemab demonstrated limited cognitive benefit, prompting a reassessment of the underlying mechanisms that drive disease progression [3,4]. In addition to mechanistic limitations associated with amyloid-beta targeting, mAbs used in AD therapy present several pharmacological and clinical drawbacks that may explain their limited cognitive efficacy. One major limitation is their suboptimal ability to cross the blood–brain barrier (BBB), thus reducing their bioavailability at target sites [5]. Moreover, repeated intravenous administration is often required due to their large molecular size and short central nervous system (CNS) half-life, posing logistical and economic challenges [6]. In addition, amyloid-targeting antibodies, including aducanumab and lecanemab, are associated with amyloid-related imaging abnormalities (ARIA), such as vasogenic edema and microhemorrhages, which may necessitate dose reduction or treatment discontinuation [7]. These limitations, when combined with the complexity of AD pathophysiology, may help explain the modest cognitive outcomes observed in clinical trials despite reductions in amyloid burden.
Microglia serve as the brain’s innate immune sentinels, maintaining homeostasis through debris clearance, synaptic remodeling, and neurotrophic support. Under pathological conditions, however, microglia undergo phenotypic transitions that may lead to maladaptive responses, including sustained cytokine release, oxidative stress, and impaired phagocytosis [8]. Advances in single-cell transcriptomics have revealed a remarkable heterogeneity in microglial states, including disease-associated microglia (DAM), a specialized microglial phenotype associated with amyloid plaque localization and phagocytic activation, as well as interferon-responsive subsets, and senescent phenotypes. Many of these reactive profiles are modulated by AD risk genes identified through genome-wide association studies (GWAS), such as triggering receptor expressed on myeloid cells 2 (TREM2), cluster of differentiation 33 (CD33), and apolipoprotein E (APOE) [9].
In addition to intrinsic genetic factors, microglial function is shaped by extrinsic influences, including systemic inflammation, metabolic dysregulation, and gut microbiota composition. The convergence of these signals governs the shift between neuroprotective and neurotoxic microglial roles [10,11,12].
Beyond AD, microglia play pivotal roles in the pathogenesis of various neurological and neurodegenerative disorders. For instance, in Parkinson’s disease (PD), activated microglia contribute to the progressive degeneration of dopaminergic neurons through sustained oxidative stress and pro-inflammatory cytokine release. Similarly, in multiple sclerosis (MS), microglia are key mediators of demyelination, lesion expansion, and axonal damage within the central nervous system. Moreover, in Huntington’s disease (HD), aberrant microglial activation is implicated in synaptic dysfunction, neuroinflammation, and the exacerbation of motor and cognitive deficits [13]. Collectively, these findings underscore the critical involvement of microglia in a broad spectrum of CNS pathologies, emphasizing their therapeutic relevance.
This review aims to integrate recent advances in microglial biology, focusing on their functional plasticity, molecular regulators, immunometabolic networks, and therapeutic tractability. Unlike prior reviews focused on individual pathways, it was aimed to provide a multidimensional synthesis of microglial responses in AD, with emphasis on translational strategies that reposition microglia as central targets in disease intervention.
2. Microglial Phenotypes in AD
Microglia display exceptional phenotypic plasticity, transitioning from a surveillant, homeostatic state to various reactive subtypes in response to aging, injury, or neurodegeneration [14]. In the healthy brain, microglia continuously monitor the microenvironment, participate in synaptic pruning, support neuronal viability, and contribute to immunological tolerance. However, under AD-related stressors such as Aβ deposition, tau pathology, and systemic inflammation, microglia undergo transcriptional and morphological reprogramming, adopting reactive profiles that may either mitigate or exacerbate neuropathology [15].
Recent advances in single-cell RNA sequencing identified a continuum of microglial states in the AD brain, each with distinct molecular signatures and functional consequences. These include homeostatic microglia, DAM, pro-inflammatory subtypes, senescent microglia, and interferon-responsive phenotypes. Understanding these distinct yet overlapping states provides critical insight into the dual role of microglia as both protectors and propagators of neurodegeneration [16].
2.1. Homeostatic Microglia
In the healthy central nervous system, microglia maintain a quiescent yet responsive surveillance role. This phenotype is defined by the expression of signature genes such as P2RY12, TMEM119, CX3CR1, SALL1, and TGFBR1, which regulate synaptic pruning, neuroimmune communication, and anti-inflammatory tone. Morphologically, these microglia exhibit a highly ramified structure with motile processes that scan the brain parenchyma [17].
The maintenance of homeostatic microglia is critically dependent on TGF-β signaling and neuron–microglia interactions mediated by CX3CL1–CX3CR1. A decline in this phenotype often precedes overt AD pathology and is considered an early event in disease progression [18]. Loss of homeostatic markers, particularly P2RY12 and TMEM119, serves as an indicator of microglial activation and may herald the transition toward disease-promoting states.
2.2. Disease-Associated Microglia
DAM represents a specialized reactive phenotype localized around Aβ plaques. This phenotype follows a biphasic activation trajectory. It begins with an initial phase that is independent of TREM2, during which homeostatic genes are suppressed. This is followed by a TREM2-dependent phase, characterized by the upregulation of genes associated with phagocytosis, lipid metabolism, and immune signaling. These genes include APOE, LPL, CST7, and TREM2 itself [19].
DAM exhibits enhanced metabolic activity and increased expression of lysosomal genes, facilitating the clearance of amyloid deposits and apoptotic debris. However, their sustained activation is also associated with increased inflammatory gene expression and antigen presentation, potentially contributing to chronic neuroinflammation. Although DAMs are initially neuroprotective, prolonged activity may inadvertently promote synaptic loss and glial scarring, reflecting their complex role in AD [20].
2.3. Pro-Inflammatory and Interferon-Responsive Microglia
Reactive microglia in AD often exhibit a pro-inflammatory phenotype resembling the classical “M1-like” macrophage profile. These cells secrete pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6, and are marked by elevated expression of iNOS, reactive oxygen species (ROS), and inflammasome components such as pyrin domain-containing protein 3 (NLRP3) [21]. Activation of the nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) and JAK/STAT signaling pathways sustains this inflammatory state, which contributes to neuronal dysfunction, blood–brain barrier disruption, and impaired neurogenesis.
A distinct but overlapping subset of interferon-responsive microglia is enriched in aging and AD-afflicted brains. These cells display elevated expression of interferon-inducible genes, including IFITM3, IRF7, and STAT1, and contribute to maladaptive antigen presentation and T-cell recruitment [22]. The sustained activation of interferon signaling may exacerbate synaptic loss and cognitive decline, particularly in late-stage disease.
2.4. Senescent and Aged Microglia
Microglial senescence is characterized by dystrophic morphology, impaired motility, and accumulation of intracellular waste products such as lipofuscin. These aged microglia exhibit downregulation of homeostatic genes, reduced phagocytic competence, and a persistent low-grade inflammatory profile termed “inflammaging.” Molecularly, they show increased expression of senescence-associated genes such as CDKN2A and GLB1, along with chronic activation of the NLRP3 inflammasome [23].
Senescent microglia fail to respond effectively to environmental cues and may contribute to the persistence of toxic aggregates and a hostile neuroinflammatory environment. Their accumulation in the aging brain poses a significant barrier to immune resolution and may accelerate disease progression [24].
These phenotypic transitions are not merely descriptive but have profound consequences for neuroinflammatory signaling, synaptic remodeling, and neuronal viability in the AD brain. Each phenotype exhibits distinct transcriptional and functional features, which may align with different AD stages, such as homeostatic microglia in early aging, DAM in plaque-rich regions, and pro-inflammatory phenotypes during late-stage neurodegeneration. These phenotypic states, summarized in Table 1, offer a dynamic framework for understanding the diverse and context-dependent roles of microglia across AD progression.

Table 1.
Overview of major microglial phenotypes observed in AD, including representative molecular markers, associated functional roles, and their relevance to different stages of disease progression.
3. Genetic and Molecular Regulators of Microglial Function in AD
The phenotypic diversity and functional plasticity of microglia in AD are governed by an intricate network of genetic variants, receptor–ligand interactions, transcriptional regulators, and intracellular signaling pathways. GWAS identified several AD risk genes, including TREM2, CD33, and APOE, that are preferentially or exclusively expressed in microglia [25]. These genes play critical roles in modulating microglial survival, lipid sensing, immune activation, and phagocytic clearance of pathological aggregates. Their dysregulation can tip the balance from neuroprotection to neurotoxicity, positioning them as both biomarkers of disease progression and promising therapeutic targets [26].
3.1. The Role of TREM2 in Microglia
TREM2 is a membrane-bound receptor of the immunoglobulin superfamily, selectively expressed by microglia. TREM2 recognizes a broad range of lipid ligands and damage-associated molecular patterns (DAMPs), including phospholipids, apoptotic bodies, and APOE-containing lipoprotein complexes. Upon ligand binding, TREM2 engages the adaptor protein DAP12 (TYROBP), initiating phosphorylation cascades involving spleen tyrosine kinase (SYK), PI3K-AKT, and ERK signaling. These downstream pathways regulate microglial survival, actin remodeling, lysosomal biogenesis, and metabolic reprogramming [27].
TREM2 signaling is essential for the transition from homeostatic microglia to DAM. This transition occurs in two sequential phases, including an initial TREM2-independent phase involving downregulation of homeostatic genes, and a subsequent TREM2-dependent phase characterized by induction of genes involved in phagocytosis and lipid metabolism, such as APOE, CST7, and LPL. Loss-of-function variants in TREM2, most notably the R47H mutation, are associated with a two- to four-fold increase in late-onset AD risk. These mutations impair ligand binding, hinder DAM differentiation, and reduce microglial capacity to contain amyloid pathology, thereby facilitating disease progression [28].
3.2. The Role of CD33 in Microglia
CD33, a sialic acid-binding immunoglobulin-like lectin (Siglec-3), functions as an inhibitory immune checkpoint in microglia. It harbors immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in its cytoplasmic domain that recruit Src homology region 2 domain-containing phosphatase (SHP)-1 and SHP-2 phosphatases. These phosphatases attenuate downstream signaling by dephosphorylating kinases critical for phagocytosis and inflammatory gene expression. In the AD brain, CD33 expression is upregulated and inversely correlates with amyloid clearance and cognitive function [29].
Splice isoforms of CD33 further influence AD susceptibility. The risk allele (rs3865444^C^) enhances the expression of the full-length isoform containing both ligand-binding and ITIM domains, which actively suppresses microglial activation. In contrast, the protective allele (rs3865444^A^) favors a truncated isoform lacking the sialic acid-binding domain, which permits greater microglial responsiveness [30]. Recent findings elucidated that splice isoforms of CD33—namely, hCD33M (the full-length isoform) and hCD33m (the truncated variant) exert opposing effects on microglial activation. While hCD33M suppresses Aβ clearance and promotes a pro-inflammatory phenotype through ITIM-mediated SHP-1/2 recruitment, hCD33m lacks the ligand-binding and inhibitory domains, thereby enabling a more homeostatic and phagocytic microglial state. The relative expression and interaction of these isoforms appear to critically shape microglial responses in AD models, suggesting that isoform balance may be a key regulator of neuroinflammation and disease progression [31].
3.3. The Role of APOE in Microglia
APOE is a multifunctional glycoprotein involved in cholesterol transport, neuronal repair, and lipid homeostasis in the central nervous system. Among the three common human isoforms, APOE2, APOE3, and APOE4, the last isoform, APOE4, confers the highest genetic risk for sporadic AD. While astrocytes are the primary source of APOE under physiological conditions, microglia can upregulate APOE expression in response to pathological stress and during the transition to the DAM phenotype [32]. This increase reflects elevated transcriptional activity relative to the quiescent microglial state, rather than a surpassing of astrocytic output. In disease contexts, however, microglial APOE may contribute disproportionately to local immune signaling and lipid dysregulation within plaque-associated regions [33,34].
Microglia expressing APOE4 exhibit impaired cholesterol efflux, intracellular lipid droplet accumulation, and heightened oxidative stress. These changes disrupt phagocytosis and promote a shift toward a pro-inflammatory phenotype marked by NF-κB activation and cytokine release. APOE4 also destabilizes TREM2-mediated lipid sensing and interferes with receptor interactions involving low-density lipoprotein receptor (LDLR) and sortilin-related receptor 1 (SORL1) [34]. In contrast, the APOE2 isoform is neuroprotective and may enhance anti-inflammatory microglial programs. The bidirectional interaction between TREM2 and APOE reinforces their cooperative role in microglial adaptation to neurodegeneration, and APOE-targeted therapeutics, including antisense therapies and small molecules, are being explored to modulate isoform-specific functions [35].
3.4. Transcriptional Control of Microglial Identity and Plasticity
Transcription factors play a pivotal role in defining microglial identity and their capacity to respond to environmental changes. Purine-rich box 1 (PU.1), encoded by the SPI1 gene, is a master regulator of microglial lineage and homeostasis. Risk variants near SPI1 affect PU.1 expression levels and influence susceptibility to AD. Reduction in PU.1 expression diminishes phagocytic capacity and impairs microglial resilience, whereas overexpression may lead to hyperactivation and inflammatory responses [36].
Interferon regulatory factor 8 (IRF8) is upregulated in reactive microglia and drives expression of interferon-stimulated genes and pro-inflammatory chemokines. It is particularly elevated in AD-affected regions with heavy plaque burden. Conversely, myocyte enhancer factor 2C (MEF2C) suppresses excessive inflammatory responses by recruiting histone deacetylases to pro-inflammatory loci [37]. MEF2C-deficient microglia display increased synaptic pruning and behavioral abnormalities, highlighting its role as a homeostatic regulator.
3.5. Epigenetic and Non-Coding RNA Regulation
Epigenetic modifications and post-transcriptional regulators provide additional layers of microglial control. In response to neurodegenerative stimuli, disease-associated microglia show altered histone modifications such as increased H3K27 acetylation and reduced H3K9 trimethylation, which remodel chromatin to favor the expression of inflammatory and metabolic genes [38]. These epigenetic changes often precede overt phenotypic shifts, suggesting a primed state that may persist across disease stages [39].
Non-coding RNAs, including long non-coding RNAs (lncRNAs) and microRNAs (miRNAs), modulate gene expression post-transcriptionally. For example, the lncRNA MEG3 is downregulated in AD and was implicated in suppressing inflammatory gene expression via interaction with p53 and chromatin remodeling complexes [40]. Among miRNAs, miR-155 promotes a pro-inflammatory microglial phenotype by targeting suppressors of cytokine signaling, while miR-124 exerts anti-inflammatory effects and supports synaptic integrity. These findings prompted interest in RNA-based therapeutics, including antagomirs and miRNA mimics, for precise reprogramming of microglial phenotypes [41].
The complexity of AD pathogenesis is further underscored by the temporal and causal ambiguity between microglial dysfunction and the classical pathological hallmarks, namely Aβ plaques and tau tangles. Although Aβ plaques and tau tangles have been viewed as the primary initiators of pathology, there is growing consideration that early microglial impairment may play a causative role by compromising clearance pathways and promoting an inflammatory environment. At the same time, the accumulation of pathological aggregates may drive microglial reprogramming toward maladaptive states, diminishing their protective capacity. These intertwined mechanisms highlight the difficulty in determining a single upstream trigger of disease progression. A reciprocal relationship likely exists, in which microglial dysfunction and proteinopathy reinforce each other. Therefore, therapeutic strategies targeting both aspects simultaneously, microglial dysfunction and classical protein aggregates, may yield greater benefit than approaches focused on either axis alone.
4. Neuroinflammatory Signaling Pathways in Microglia
Microglia orchestrate a broad spectrum of immune responses in the central nervous system through intricate signaling networks that detect, amplify, and resolve neuroinflammatory cues. In AD, chronic activation of these pathways contributes to neuronal injury, synaptic loss, and disease progression [42]. The inflammatory cascade in microglia is governed by pattern recognition receptors (PRRs), inflammasomes, and downstream transcriptional regulators that integrate signals from damaged neurons, amyloid plaques, and systemic insults. Understanding these molecular pathways is essential for identifying therapeutic targets that can interrupt the maladaptive cycle of neuroinflammation in AD [43]. Figure 1 summarizes the sequential transition of microglia from a resting to a hyperactivated state upon exposure to soluble Aβ oligomers, highlighting the resulting pro-inflammatory cascade and neuronal injury in AD.
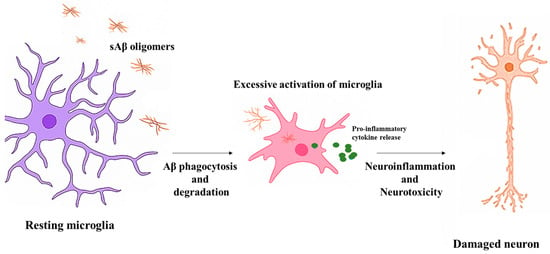
Figure 1.
Microglial activation and neuroinflammation in AD. Resting microglia respond to soluble Aβ oligomers through phagocytosis. Chronic exposure leads to excessive activation, cytokine release, and neuronal damage. Dendritic degeneration precedes axonal damage during neuroinflammation.
4.1. Toll-like Receptors and Pattern Recognition Signaling
Toll-like receptors (TLRs) are sentinel PRRs that recognize pathogen-associated molecular patterns (PAMPs) and DAMPs, triggering innate immune responses. Among the TLR family, TLR2 and TLR4 are prominently expressed in microglia and are upregulated in AD brains. These receptors detect misfolded proteins such as Aβ and heat shock proteins, initiating intracellular signaling cascades that converge on NF-κB cells [44].
Upon ligand binding, TLRs recruit adaptor proteins such as myeloid differentiation primary response 88 (MyD88) and TIR-domain-containing adapter-inducing interferon-β (TRIF), leading to the activation of interleukin-1 receptor-associated kinases (IRAK) and TNF receptor-associated factor 6 (TRAF6). This cascade culminates in the nuclear translocation of NF-κB and the transcription of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6. While transient activation of TLR signaling may aid in Aβ clearance, chronic stimulation leads to persistent cytokine release, microglial priming, and neuronal dysfunction. Inhibition of TLR4 signaling has been shown to reduce plaque burden and improve cognitive outcomes in AD mouse models, highlighting its therapeutic potential [45].
4.2. NLRP3 Inflammasome Activation and IL-1β Maturation
The nucleotide-binding domain, leucine-rich repeat, and NLRP3 inflammasome are a multiprotein complex that mediates caspase-1 activation and the maturation of interleukin-1 family cytokines. In microglia, NLRP3 is activated by a range of AD-related stimuli, including Aβ oligomers, lysosomal rupture, mitochondrial dysfunction, and potassium efflux [46]. Once assembled, the inflammasome cleaves pro-caspase-1 into its active form, which in turn processes pro-IL-1β and pro-IL-18 into their mature, bioactive forms.
Persistent activation of the NLRP3 inflammasome exacerbates neuroinflammation and neuronal injury. In AD models, pharmacological or genetic inhibition of NLRP3 has been shown to attenuate microgliosis, reduce Aβ deposition, and preserve cognitive function. The regulatory interplay between TREM2 and NLRP3 also suggests a convergence of immune-sensing and inflammatory effector pathways in DAM, where TREM2 signaling may temper inflammasome activation under physiological conditions [47]. Targeting NLRP3 thus represents a promising strategy to modulate maladaptive inflammation in AD.
4.3. NF-κB and MAPK Signaling Cascades
NF-κB is a central transcription factor that regulates the expression of pro-inflammatory genes in microglia. In its inactive form, NF-κB is sequestered in the cytoplasm by IκB inhibitors. Upon activation by TLRs, cytokines, or oxidative stress, IκB is phosphorylated and degraded, allowing NF-κB to translocate to the nucleus and initiate transcription [48]. In AD brains, NF-κB is persistently activated in microglia and correlates with increased cytokine production, impaired Aβ clearance, and neurotoxicity.
In parallel, mitogen-activated protein kinase (MAPK) pathways, including the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK branches, mediate microglial responses to extracellular stimuli. These kinases regulate gene expression, cytoskeletal remodeling, and cytokine secretion [49]. Chronic MAPK activation promotes microglial proliferation, reactive gliosis, and the secretion of matrix metalloproteinases that degrade the extracellular matrix and compromise synaptic integrity. Crosstalk between NF-κB and MAPK pathways amplifies the inflammatory milieu in AD, and dual inhibition of these pathways has shown additive effects in attenuating neuroinflammation in preclinical models [50].
4.4. JAK/STAT Pathway and Interferon Signaling
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway mediates responses to interferons, interleukins, and colony-stimulating factors. In microglia, this pathway is activated by type I and II interferons and regulates genes involved in antigen presentation, chemotaxis, and oxidative stress. In AD, a subset of microglia exhibits an interferon-responsive phenotype characterized by elevated expression of STAT1, IRF7, IFITM3, and other interferon-stimulated genes [51].
Chronic interferon signaling disrupts microglial homeostasis, enhances synapse elimination, and promotes immune cell infiltration into the brain parenchyma. JAK1/2 inhibitors, already approved for inflammatory disorders, have demonstrated the capacity to dampen microglial activation and improve memory performance in AD mouse models, suggesting a potential avenue for drug repurposing [52].
4.5. Complement Cascade and Synaptic Tagging
The complement system plays a critical role in synaptic pruning during development and is aberrantly reactivated in neurodegeneration. In AD, microglia overexpress complement components such as C1q, C3, and CR3 in response to Aβ and tau pathology. These proteins opsonize synapses, marking them for elimination by phagocytic microglia [53]. While this mechanism may initially serve to remove dysfunctional synapses, its chronic activation results in widespread synaptic loss and cognitive impairment [54].
C1q and C3 deposition are observed in early stages of AD, and their expression correlates with microglial activation and dendritic spine loss. Genetic deletion of C1q or C3 mitigates synaptic loss and rescues memory deficits in AD mouse models [55]. Pharmacological inhibition of complement receptors on microglia is currently under investigation as a neuroprotective strategy to preserve synaptic connectivity in AD.
Figure 2 provides a schematic overview of key neuroinflammatory pathways activated in microglia, illustrating the interconnected signaling cascades implicated in AD.
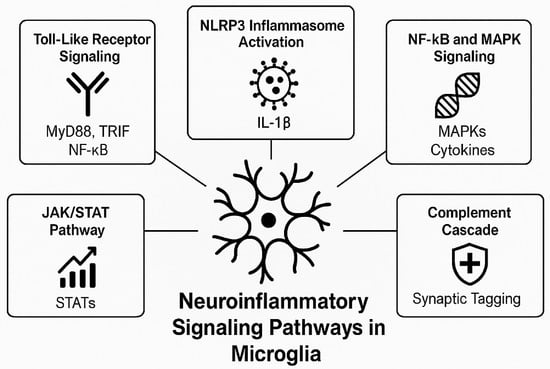
Figure 2.
Microglial neuroinflammatory signaling pathways in microglia during AD. Upon exposure to amyloid-beta and other danger signals, microglia activate multiple signaling pathways including Toll-like receptor signaling (via MyD88/TRIF and NF-κB), NLRP3 inflammasome activation (resulting in IL-1β release), JAK/STAT and MAPK cascades (modulating cytokine expression), and the complement pathway (leading to synaptic tagging and pruning). These pathways collectively drive the chronic inflammatory state observed in AD pathology.
5. Immunometabolism and Microglial Bioenergetics in AD
Microglial function is intimately linked to cellular metabolism, with distinct metabolic programs guiding phenotypic transitions between homeostasis, activation, and immune resolution. In the context of AD, metabolic dysregulation serves not only as a consequence of chronic inflammation but also as a driving force behind microglial dysfunction [56]. Emerging evidence indicates that impaired bioenergetics, mitochondrial dysfunction, and aberrant lipid handling contribute to the persistent inflammatory phenotype observed in AD microglia. Understanding these immunometabolic shifts provides critical insight into disease mechanisms and offers therapeutic opportunities to reprogram dysfunctional microglia toward homeostatic states [57].
In the healthy brain, homeostatic microglia rely predominantly on oxidative phosphorylation (OXPHOS) to sustain their surveillance and neuroprotective roles. This metabolic profile is supported by a well-organized mitochondrial network and regulated expression of genes involved in the tricarboxylic acid (TCA) cycle and electron transport chain [58]. When activated by pathological stimuli such as Aβ, microglia undergo a metabolic shift characterized by increased glycolysis and reduced mitochondrial respiration, similar to the Warburg effect seen in cancer cells. This glycolytic shift supports rapid energy production, ROS generation, and biosynthesis of pro-inflammatory mediators, facilitating the transition to an inflammatory phenotype [59].
However, sustained reliance on glycolysis impairs long-term microglial fitness and promotes a senescent, pro-inflammatory state. In AD, microglia accumulate lipid droplets and exhibit defective autophagy and mitophagy pathways, leading to the buildup of damaged mitochondria and impaired metabolic flexibility [60]. Single-cell transcriptomic analyses of AD brains revealed downregulation of genes associated with mitochondrial function and fatty acid oxidation in DAM, while genes involved in cholesterol metabolism and lipid uptake, such as APOE, LPL, and CD36, are markedly upregulated. This suggests an altered lipid-handling program that may exacerbate inflammation and hinder Aβ clearance [61].
Lipid metabolism, particularly cholesterol and phospholipid turnover, plays a crucial role in shaping microglial immune responses. The APOE4 isoform, a major genetic risk factor for sporadic AD, was associated with impaired cholesterol efflux, accumulation of intracellular lipid droplets, and heightened susceptibility to oxidative stress. APOE4-expressing microglia exhibit dysfunctional lysosomal activity and reduced expression of lipid transporters such as ABCA1 and ABCG1, contributing to intracellular lipid overload and compromised phagocytic capacity. Moreover, APOE4 disrupts lipid-sensing interactions with TREM2, further amplifying metabolic stress and inflammatory signaling [62].
Mitochondrial dysfunction is another hallmark of microglial impairment in AD. Aging and chronic neuroinflammation lead to reduced mitochondrial membrane potential, decreased ATP production, and elevated ROS levels. These changes activate the NLRP3 inflammasome and induce oxidative damage to proteins, lipids, and nucleic acids, perpetuating a feedforward cycle of inflammation and bioenergetic collapse [63]. Mitochondrial DNA released from damaged organelles acts as a DAMP, further exacerbating microglial activation through the TLR9 pathway.
Targeting microglial metabolism has emerged as a promising therapeutic strategy. Pharmacological agents that promote mitochondrial biogenesis, enhance fatty acid oxidation, or restore autophagic flux have shown beneficial effects in preclinical models. For instance, peroxisome proliferator-activated receptor gamma (PPARγ) agonists were reported to modulate glucose and lipid metabolism while reducing pro-inflammatory gene expression. Similarly, AMP-activated protein kinase (AMPK) activators improve mitochondrial function and reduce cytokine secretion in reactive microglia. Dietary interventions such as ketogenic diets and calorie restriction were also proposed to modulate brain immunometabolism, although translational evidence remains limited [64].
In summary, microglial metabolic reprogramming is a key driver of neuroinflammation in AD. Disruption of mitochondrial dynamics, lipid homeostasis, and energy sensing pathways fuels a chronic inflammatory phenotype that undermines microglial protective functions. Therapeutic strategies aimed at restoring metabolic balance may offer a viable means of attenuating microglial-mediated pathology and slowing AD progression [65].
6. The Gut–Brain–Microglia Axis in AD
The CNS and gastrointestinal tract are intricately connected through a bidirectional communication network known as the gut–brain axis, which integrates neural, endocrine, immune, and metabolic pathways. In recent years, this axis has garnered significant attention in the context of AD, particularly regarding its influence on neuroinflammation and microglial activation. Alterations in gut microbiota composition were associated with systemic inflammation, disruption of the BBB, and modulation of microglial function, all of which contribute to the pathogenesis of AD [66].
The gut microbiota plays a fundamental role in shaping the peripheral immune system and influencing CNS homeostasis. In healthy individuals, commensal bacteria produce short-chain fatty acids (SCFAs), such as butyrate, propionate, and acetate, which exert anti-inflammatory effects, maintain gut barrier integrity, and support microglial maturation via G-protein-coupled receptors (e.g., GPR41, GPR43) [67]. SCFAs also promote the differentiation of regulatory T cells (Tregs), which mitigate peripheral inflammation and modulate microglial priming. In contrast, dysbiosis reduces SCFA availability, disrupts immune tolerance, and permits the translocation of lipopolysaccharides (LPS) and bacterial metabolites into systemic circulation [68].
LPS is a potent activator of innate immunity and has been implicated in microglial activation through TLR4 signaling. Chronic peripheral exposure to LPS leads to increased expression of pro-inflammatory cytokines, enhanced NLRP3 inflammasome activation, and augmented production of ROS within the CNS. These events contribute to microglial priming, a state of exaggerated responsiveness to subsequent insults, and accelerate neurodegeneration [69]. Experimental models have shown that germ-free or antibiotic-treated mice display altered microglial morphology and transcriptional profiles, further supporting the notion that microbial-derived signals are essential for maintaining microglial homeostasis [70].
Moreover, gut-derived metabolites such as trimethylamine N-oxide (TMAO), indoles, and bile acids influence neuroimmune signaling. Elevated levels of TMAO, commonly observed in dysbiotic states and aging, correlate with cognitive decline and microglial activation. TMAO contributes to oxidative stress, endothelial dysfunction, and mitochondrial impairment, which are features that overlap with AD pathology [71]. Conversely, microbial metabolites such as indole-3-propionic acid exert neuroprotective effects by scavenging free radicals and inhibiting pro-inflammatory pathways in microglia.
The gut microbiota also impacts the permeability and integrity of the blood–brain barrier. Dysbiosis-induced systemic inflammation compromises tight junction proteins such as claudins and occludins, facilitating the entry of circulating cytokines, pathogens, and neurotoxic compounds into the brain parenchyma [72]. This breach exacerbates microglial activation and amplifies neuroinflammatory responses. Restoration of microbiota composition through probiotics, prebiotics, or dietary interventions was shown to reverse BBB disruption and attenuate microgliosis in animal models of AD [73].
Therapeutic modulation of the gut–brain axis represents a novel strategy for mitigating microglial-driven inflammation. Probiotics such as Lactobacillus and Bifidobacterium strains demonstrated the ability to reduce systemic and CNS inflammation, improve cognitive performance, and normalize microglial phenotypes in transgenic AD mice. Fecal microbiota transplantation (FMT) also emerged as a potential intervention, with early studies showing that transferring fecal material from young or healthy donors improves cognitive function and reduces plaque burden in aged or AD-prone mice. However, clinical translation of these approaches remains in its infancy, and further research is needed to elucidate the mechanistic underpinnings and long-term safety of gut-targeted therapies [74].
In summary, the gut microbiota exerts profound effects on microglial development, immune activation, and neuroinflammatory cascades in AD. Dysbiosis disrupts this equilibrium, contributing to chronic inflammation, BBB dysfunction, and neuronal damage. Targeting the gut–brain–microglia axis offers a promising avenue for therapeutic intervention and represents an expanding frontier in neurodegeneration research [75].
7. Pharmacological and Nutraceutical Modulation of Microglia in AD
Given the central role of microglia in AD pathogenesis, therapeutic strategies aimed at modulating microglial activation states have gained considerable momentum. Unlike previous approaches that primarily targeted Aβ accumulation or tau pathology, emerging interventions seek to rebalance microglial function, either by promoting protective phenotypes or dampening chronic inflammatory responses [76]. Both pharmacological agents and naturally derived compounds have shown promise in reprogramming microglial states and alleviating neuroinflammation in AD models.
One of the most actively explored pharmacological targets is TREM2, which governs microglial transition into disease-associated states and facilitates lipid sensing, phagocytosis, and Aβ containment [77]. TREM2-activating antibodies, such as AL002 (produced by Alector), demonstrated the ability to enhance plaque compaction and improve cognitive function in preclinical models. Clinical trials of TREM2-targeting therapies are ongoing, aiming to harness the neuroprotective potential of DAM without tipping the balance toward excessive inflammation. Similarly, antagonists of CD33, a negative regulator of microglial clearance, are being developed to release the inhibitory constraints on phagocytic function, restore amyloid clearance, and rejuvenate microglial homeostasis [78].
In parallel, inhibitors of the NLRP3 inflammasome garnered attention for their capacity to suppress IL-1β maturation and mitigate neuroinflammatory amplification. MCC950, a selective NLRP3 inhibitor, was shown to reduce microglial activation, Aβ load, and cognitive decline in transgenic AD mouse models [79]. Other inflammasome modulators, including caspase-1 inhibitors and IL-1 receptor antagonists, offer complementary approaches to interrupt the inflammatory feedback loop perpetuated by microglia. Importantly, these therapies must be carefully timed and dosed to preserve microglial capacity for surveillance and repair while preventing chronic activation [80].
Anti-inflammatory agents such as non-steroidal anti-inflammatory drugs (NSAIDs), although initially promising in epidemiological studies, have yielded inconclusive results in clinical trials. This may be due to their non-specific effects and the failure to target early disease stages when microglial priming occurs. In particular, large-scale clinical trials demonstrated that NSAIDs do not significantly slow cognitive decline or prevent AD in patients with established pathology. This may stem from the timing of intervention—once pathological hallmarks such as amyloid plaques and tau tangles are already present, microglial responses may have transitioned into a chronic pro-inflammatory state that is no longer responsive to NSAID modulation. Moreover, inter-individual variability in inflammatory profiles and drug responsiveness further complicates therapeutic efficacy [81]. More targeted immunomodulators, including PPARγ agonists like pioglitazone and rosiglitazone, have demonstrated the ability to promote anti-inflammatory phenotypes, enhance mitochondrial function, and reduce oxidative stress in microglia. Although large-scale trials of PPARγ agonists in AD have not met primary endpoints, their pleiotropic effects on metabolism and immunity warrant further investigation, particularly in combination therapies [82].
Natural products and dietary phytochemicals have also emerged as modulators of microglial activation, offering favorable safety profiles and multi-targeted mechanisms. Flavonoids such as quercetin, luteolin, and apigenin exhibit potent anti-inflammatory and antioxidant activities, suppressing NF-κB signaling, inhibiting NLRP3 activation, and restoring redox balance in reactive microglia [83]. Curcumin, derived from Curcuma longa, has demonstrated the ability to reduce pro-inflammatory cytokine release, improve mitochondrial function, and enhance Aβ clearance in vitro and in vivo. Similarly, resveratrol, a polyphenol found in grapes and berries, exerts neuroprotective effects by activating sirtuin 1 (SIRT1), enhancing autophagy, and mitigating microglial-mediated neurotoxicity [84].
Melatonin, an endogenously produced indoleamine with known circadian and antioxidant properties, was also shown to inhibit pro-inflammatory microglial polarization, suppress oxidative stress, and attenuate Aβ- and tau-induced toxicity [85]. Through modulation of clock genes, mitochondrial stabilization, and epigenetic regulation, melatonin exerts pleiotropic effects on neuroimmune function and has demonstrated efficacy in delaying cognitive decline in preclinical models. Its chronobiotic nature further positions it as a candidate for time-targeted interventions that align with the circadian fluctuations of neuroinflammation [86].
Dietary approaches such as the Mediterranean diet, rich in polyphenols, omega-3 fatty acids, and prebiotic fibers, were associated with reduced neuroinflammation and preservation of cognitive function. Emerging evidence underscores the role of the Mediterranean diet in modulating the gut–brain axis, where bioactive nutrients such as polyphenols, prebiotics, and omega-3 fatty acids help restore microbiota balance, reduce neuroinflammation, and ultimately delay cognitive decline in AD [87]. A recent meta-analysis of 23 prospective studies by Fekete et al. confirmed that high adherence to the Mediterranean diet is associated with a significant reduction in cognitive impairment (HR = 0.82), dementia (HR = 0.89), and AD (HR = 0.70), supporting its role in neuroprotective dietary interventions [88]. Another randomized controlled trial by Valls-Pedret et al. revealed that elderly individuals at high cardiovascular risk who followed a Mediterranean diet supplemented with either extra-virgin olive oil or nuts exhibited improved cognitive function compared to those on a low-fat control diet [89].
Furthermore, nutritional supplementation with docosahexaenoic acid (DHA), a major omega-3 fatty acid in the brain, enhances anti-inflammatory signaling, promotes synaptic plasticity, and supports microglial resolution states. While these nutraceuticals may not reverse established pathology, they offer valuable adjunctive strategies for early intervention and disease prevention [90,91].
An often-overlooked yet critical factor in the success of microglia-targeted interventions is the route of therapeutic delivery. Intravenous (IV) administration is commonly employed for monoclonal antibodies to ensure systemic bioavailability and BBB penetration [92]. Oral and peroral delivery routes, while less invasive, are more applicable for nutraceuticals like curcumin and melatonin, although they face challenges related to bioavailability and first-pass metabolism [93]. Intrathecal delivery is under exploration for direct CNS access in experimental therapies, and nanoparticle-assisted systems are increasingly used to facilitate targeted delivery and circumvent BBB restrictions [94,95]. Table 2 summarizes various pharmacological and naturally derived compounds targeting microglial pathways, highlighting their molecular targets, mechanisms of action, delivery methods, and current status in preclinical or clinical development.

Table 2.
Selected therapeutic agents targeting microglia in AD.
8. Nanomedicine and Targeted Drug Delivery for Microglial Modulation in AD
The BBB represents a formidable challenge in the development of central nervous system therapeutics, particularly for modulating microglial function in AD. Conventional drug delivery systems often fail to achieve sufficient CNS penetration or exhibit off-target effects that compromise efficacy and safety [96]. In this context, nanotechnology-based delivery platforms emerged as promising tools for enhancing drug bioavailability, targeting specificity, and therapeutic precision in neuroinflammatory conditions [97].
Nanocarriers—including liposomes, polymeric nanoparticles, solid lipid nanoparticles, dendrimers, and micelles—can be engineered to cross the BBB via receptor-mediated transcytosis, adsorptive-mediated transport, or modulation of tight junctions. Their physicochemical properties, such as size, charge, and surface functionalization, can be precisely tailored to optimize circulation time, CNS uptake, and release kinetics [98]. In AD, nanoparticle-based systems were designed to deliver anti-inflammatory agents, antioxidants, and gene modulators directly to microglia, to reprogram their phenotypic states and mitigate neurodegeneration [99].
Targeting moieties such as antibodies, peptides, and aptamers can be conjugated to nanoparticle surfaces to enhance microglial specificity. For example, nanoparticles functionalized with antibodies against TREM2 or CD11b demonstrated selective uptake by activated microglia, enabling localized delivery of immunomodulators or nucleic acid-based therapies. This approach minimizes systemic toxicity and allows for the sustained modulation of microglial responses within neuroinflammatory niches [100]. Similarly, nanocarriers encapsulating small interfering RNA (siRNA) or microRNA mimics were employed to silence pro-inflammatory genes such as NLRP3, TNF-α, or IL-1β, providing a mechanistically targeted approach to dampen microglial activation [101].
Liposomes remain among the most widely used nanocarriers due to their biocompatibility, ability to encapsulate both hydrophilic and lipophilic agents, and adaptability for surface modification. Curcumin-loaded liposomes, for instance, were shown to reduce microglial activation and Aβ burden in AD models more effectively than free curcumin [102]. Similarly, resveratrol or melatonin-loaded nanoparticles demonstrated enhanced antioxidant and anti-inflammatory efficacy compared to their unencapsulated forms. These formulations not only improve pharmacokinetic profiles but also enable chronotherapeutic delivery strategies aligned with circadian fluctuations in neuroinflammatory activity [103].
Dendrimers, highly branched, monodisperse macromolecules, offer another promising platform for brain-targeted delivery. Their multivalent surface allows for simultaneous conjugation of drugs, targeting ligands, and imaging agents. Polyamidoamine (PAMAM) dendrimers loaded with anti-inflammatory agents were used to modulate microglial activation and reduce pro-inflammatory cytokine production in models of neurodegeneration. Importantly, dendrimers can be designed to release their cargo in response to microenvironmental stimuli, such as pH changes or oxidative stress, enabling site-specific drug release in regions of active neuroinflammation [104].
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) offer the advantage of higher stability, controlled release, and lower cytotoxicity compared to traditional formulations. These carriers were explored for the delivery of bioactive lipids, omega-3 fatty acids, and anti-inflammatory phytochemicals with demonstrated benefits in attenuating microgliosis and improving cognitive outcomes. Moreover, their lipid-based composition closely mimics cellular membranes, facilitating endocytosis and intracellular trafficking within microglial cells [105].
Despite their promise, several challenges remain in the clinical translation of nanotherapeutics for AD. These include concerns about long-term toxicity, immunogenicity, scalability of production, and variability in BBB permeability across disease stages and patient populations. Regulatory hurdles also persist, given the complexity of nanocarrier systems and the need for robust safety and efficacy data. Nonetheless, advances in nanomedicine and microglial biology are converging to offer precision-targeted interventions that may overcome the limitations of current AD therapies [106].
In summary, nanotechnology-based delivery systems represent a transformative approach to modulating microglial activity in AD. By enabling targeted, controlled, and efficient delivery of therapeutic agents across the blood–brain barrier, these platforms hold substantial potential to enhance treatment specificity and efficacy [107]. Continued interdisciplinary efforts are needed to refine nanoparticle design, ensure biocompatibility, and validate therapeutic outcomes in translational models and clinical trials.
9. Clinical Translation and Future Perspectives
The recognition of microglia as central players in AD pathogenesis prompted a shift in therapeutic paradigms from targeting amyloid-β and tau aggregates exclusively to modulating neuroimmune dynamics [108]. Despite encouraging results from preclinical studies, the clinical translation of microglia-targeted therapies remains in its early stages. Several challenges, including patient heterogeneity, stage-specific microglial phenotypes, and the complexity of central immune signaling, impeded the success of immunomodulatory interventions in AD. Nonetheless, emerging clinical trials, innovative technologies, and biomarker strategies are paving the way toward more personalized and effective treatments [109].
Several clinical-stage therapeutics are now exploring direct or indirect modulation of microglial activity. For example, AL002, a humanized monoclonal antibody targeting TREM2, entered Phase II trials in patients with early-stage AD. This antibody is designed to promote TREM2-dependent microglial activation, enhance phagocytosis, and restore immune surveillance. Early data suggest favorable safety and biomarker profiles, but long-term cognitive outcomes remain to be established [110]. Similarly, CD33-targeting agents are being developed to suppress inhibitory signaling and rejuvenate microglial Aβ clearance capabilities. These agents underscore the clinical feasibility of modulating microglial checkpoints to rebalance immune homeostasis.
Anti-inflammatory therapies, including NLRP3 inflammasome inhibitors and selective cytokine blockers, are also undergoing evaluation for their capacity to attenuate chronic neuroinflammation. While broad-spectrum agents such as NSAIDs failed to show benefit in late-stage AD, more selective immunomodulators may offer better efficacy when administered during the prodromal or early symptomatic phases [111]. Ongoing studies are also exploring the use of immune tolerance-inducing strategies, including regulatory T cell–based therapies, to counteract excessive microglial activation without impairing host defense.
One of the major bottlenecks in clinical translation is the lack of robust, non-invasive biomarkers that reflect microglial activity in vivo. Advances in positron emission tomography (PET) imaging enabled the use of translocator protein (TSPO) ligands as proxies for microglial activation, though their specificity and reliability are limited. Novel radiotracers targeting TREM2, P2RY12, and other microglial surface markers are currently under development to improve imaging accuracy. In parallel, cerebrospinal fluid (CSF) and blood-based biomarkers, including soluble TREM2, YKL-40, and inflammatory cytokines, are being investigated as tools to stratify patients, monitor disease progression, and assess treatment response [112].
The advent of single-cell omics and spatial transcriptomics has revealed profound heterogeneity in microglial states across brain regions, disease stages, and patient genotypes. This complexity demands precision medicine approaches that consider individual immune signatures when designing interventions. For example, patients carrying the APOE4 allele may exhibit distinct microglial metabolic and inflammatory responses compared to non-carriers, necessitating tailored therapeutic strategies [113]. Integrating genomics, transcriptomics, and clinical phenotyping will be essential to identify responder subpopulations and optimize treatment timing.
Looking ahead, combinatorial therapies that target multiple aspects of microglial dysfunction may hold the greatest promise. Such approaches could include dual modulation of immune checkpoints, metabolic reprogramming to restore mitochondrial and lipid balance, and delivery of anti-inflammatory agents via nanocarriers. Moreover, coupling these strategies with lifestyle interventions, such as diet, circadian regulation, and microbiota modulation, may enhance therapeutic efficacy and prevent disease progression in at-risk populations [114].
Finally, ethical and regulatory considerations must keep pace with scientific advances. As microglial-targeted therapies move toward clinical application, long-term safety, off-target effects, and immune tolerance must be rigorously evaluated. Regulatory frameworks will need to adapt to the complexities of immunomodulation in the CNS, particularly for advanced delivery platforms and gene-editing technologies [115].
10. Conclusions
Microglial cells are central to the immune landscape of the brain and play a dualistic role in AD, acting as both defenders and drivers of neurodegeneration. Recent advances in transcriptomics, immunogenetics, and neuroimaging have reshaped our understanding of microglial biology, revealing a spectrum of activation states governed by genetic, metabolic, and environmental cues. Dysregulation of these finely tuned responses contributes to chronic inflammation, impaired clearance of pathological aggregates, and progressive neuronal damage.
Key molecular regulators such as TREM2, CD33, APOE, and the NLRP3 inflammasome highlight the therapeutic potential of targeting microglial signaling pathways. Likewise, disruptions in immunometabolism, gut–brain communication, and circadian homeostasis emerge as modifiable contributors to microglial dysfunction in AD. Pharmacological agents, nutraceuticals, and nanocarrier-based interventions offer multiple avenues to reprogram microglial phenotypes toward neuroprotective functions. However, the complexity of microglial responses, combined with patient heterogeneity and the challenges of CNS drug delivery, necessitates a precision medicine approach for successful clinical translation.
Future research should prioritize the development of reliable microglia-specific biomarkers, disease-stage-tailored interventions, and combinatorial strategies that integrate immune modulation with lifestyle and environmental factors. As our understanding of microglial heterogeneity deepens, targeting microglia may no longer be a peripheral strategy but rather a central pillar in the search for disease-modifying therapies in AD.
Author Contributions
E.E. and I.C.H.; writing—original draft preparation, E.E. and I.C.H.; writing—review and editing, E.E.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| APOE | Apolipoprotein E |
| ARIA | Amyloid-related imaging abnormalities |
| Aβ | Amyloid beta |
| BBB | Blood–brain barrier |
| CD33 | Cluster of Differentiation 33 |
| CNS | Central nervous system |
| CX3CL1 | Chemokine (C-X3-C motif) ligand 1 |
| CX3CR1 | Chemokine (C-X3-C motif) receptor 1 |
| DAM | Disease-associated microglia |
| DAMPs | Damage-associated molecular patterns |
| ERK | Extracellular signal-regulated kinase |
| GWAS | Genome-wide association studies |
| HD | Huntington’s disease |
| IFITM3 | Interferon-induced transmembrane protein 3 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IRAK | Interleukin-1 receptor-associated kinase |
| IRF7 | Interferon regulatory factor 7 |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LDLR | Low-density lipoprotein receptor |
| lncRNA | Long non-coding RNA |
| LPL | Lipoprotein lipase |
| mAbs | Monoclonal antibodies |
| MAPK | Mitogen-activated protein kinase |
| MEF2C | Myocyte enhancer factor 2C |
| miRNA | MicroRNA |
| MS | Multiple sclerosis |
| MyD88 | Myeloid differentiation primary response 88 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OXPHOS | Oxidative phosphorylation |
| PD | Parkinson’s disease |
| PET | Positron emission tomography |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PRRs | Pattern recognition receptors |
| PU.1 | Purine-rich box 1 |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SH2 | Src homology 2 domain |
| SHP | Src homology region 2 domain-containing phosphatase |
| Siglec | Sialic acid-binding immunoglobulin-type lectin |
| SIRT1 | Sirtuin 1 |
| SNP | Single-nucleotide polymorphism |
| SORL1 | Sortilin-related receptor 1 |
| STAT | Signal transducer and activator of transcription |
| SYK | Spleen tyrosine kinase |
| TCA | Tricarboxylic acid |
| TLRs | Toll-like receptors |
| TMAO | Trimethylamine N-oxide |
| TMEM119 | Transmembrane protein 119 |
| TRAF6 | TNF receptor-associated factor 6 |
| Tregs | Regulatory T cells |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| TSPO | Translocator protein |
| TYROBP | TYRO protein tyrosine kinase-binding protein |
References
- 2024 Alzheimer’s disease facts figures. Alzheimers Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. The controversy around anti-amyloid antibodies for treating Alzheimer’s disease: The European Medical Agency’s ruling against the latest anti-amyloid drugs highlights the ongoing debate about their safety and efficacy. EMBO Rep. 2024, 25, 5227–5231. [Google Scholar] [CrossRef]
- Haskologlu, I.C.; Erdag, E.; Sehirli, A.O.; Uludag, O.; Abacioglu, N. Beyond Conventional Therapies: Molecular Dynamics of Alzheimer’s Treatment through CLOCK/BMAL1 Interactions. Curr. Alzheimer Res. 2024, 20, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nature reviews. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Withington, C.G.; Turner, R.S. Amyloid-Related Imaging Abnormalities With Anti-amyloid Antibodies for the Treatment of Dementia Due to Alzheimer’s Disease. Front. Neurol. 2022, 13, 862369. [Google Scholar] [CrossRef]
- Haskologlu, I.C.; Erdag, E.; Sayiner, S.; Abacioglu, N.; Sehirli, A.O. Melatonin and REGN-CoV2 combination as a vaccine adjuvant for Omicron variant of SARS-CoV-2. Mol. Biol. Rep. 2022, 49, 4061–4068. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in neuroinflammation and synaptic dysfunction: A focus on Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 19. [Google Scholar] [CrossRef]
- Lewcock, J.W.; Schlepckow, K.; Di Paolo, G.; Tahirovic, S.; Monroe, K.M.; Haass, C. Emerging microglia biology defines novel therapeutic approaches for Alzheimer’s disease. Neuron 2020, 108, 801–821. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Rehman, S.A.; Subhani, A.; Khan, M.A.; Rahman, Z.; Iqubal, M.K.; Iqubal, A. Mechanism of microglia-mediated neuroinflammation, associated cognitive dysfunction, and therapeutic updates in Alzheimer’s disease. Hlife 2025, 3, 64–81. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurother. J. Am. Soc. Exp. 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef]
- Mirarchi, A.; Albi, E.; Arcuri, C. Microglia Signatures: A Cause or Consequence of Microglia-Related Brain Disorders? Int. J. Mol. Sci. 2024, 25, 10951. [Google Scholar] [CrossRef]
- Rao, C.; Semrau, S.; Fossati, V. Decoding microglial functions in Alzheimer’s disease: Insights from human models. Trends Immunol. 2025, 46, 310–323. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X. Different phenotypes of microglia in animal models of Alzheimer disease. Immun. Ageing 2022, 19, 44. [Google Scholar] [CrossRef]
- Li, L.; Sun, B.; Harris, O.A.; Luo, J. TGF-β Signaling in Microglia: A Key Regulator of Development, Homeostasis and Reactivity. Biomedicines 2024, 12, 2468. [Google Scholar] [CrossRef]
- Chen, K.; Li, F.; Zhang, S.; Chen, Y.; Ikezu, T.C.; Li, Z.; Martens, Y.A.; Qiao, W.; Meneses, A.; Zhu, Y. Enhancing TREM2 expression activates microglia and modestly mitigates tau pathology and neurodegeneration. J. Neuroinflammation 2025, 22, 93. [Google Scholar] [CrossRef]
- Valiukas, Z.; Tangalakis, K.; Apostolopoulos, V.; Feehan, J. Microglial activation states and their implications for Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2025, 12, 100013. [Google Scholar] [CrossRef]
- Erdag, E. Investigation of Some Phenolic Compounds as iNOS Inhibitors: An in silico Approach. Chem. Methodol. 2023, 7, 904–915. [Google Scholar] [CrossRef]
- Tastan, B.; Heneka, M.T. The impact of neuroinflammation on neuronal integrity. Immunol. Rev. 2024, 327, 8–32. [Google Scholar] [CrossRef]
- Erdag, E.; Kucuk, M.; Aksoy, U.; Abacioglu, N.; Sehirli, A.O. Docking Study of Ligands Targeting NLRP3 Inflammatory Pathway for Endodontic Diseases. Chem. Methodol. 2023, 7, 200–210. [Google Scholar] [CrossRef]
- Rim, C.; You, M.J.; Nahm, M.; Kwon, M.S. Emerging role of senescent microglia in brain aging-related neurodegenerative diseases. Transl. Neurodegener. 2024, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Au, N.P.B.; Ma, C.H.E. Functional and phenotypic diversity of microglia: Implication for microglia-based therapies for Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 896852. [Google Scholar] [CrossRef]
- Takalo, M.; Jeskanen, H.; Rolova, T.; Kervinen, I.; Hellén, M.; Heikkinen, S.; Koivisto, H.; Jokivarsi, K.; Müller, S.A.; Koivumäki, E.M.; et al. The protective PLCγ2-P522R variant mitigates Alzheimer’s disease-associated pathologies by enhancing beneficial microglial functions. J. Neuroinflammation 2025, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Lan, Z.; Li, C. The triggering receptor expressed on myeloid cells 2-apolipoprotein E signaling pathway in diseases. Chin. Med. J. 2023, 136, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, Y.; Grajales-Reyes, G.; Colonna, M. TREM2 dependent and independent functions of microglia in Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 84. [Google Scholar] [CrossRef]
- Tu, H.; Yuan, L.; Ni, B.; Lin, Y.; Wang, K. Siglecs-mediated immune regulation in neurological disorders. Pharmacol. Res. 2024, 210, 107531. [Google Scholar] [CrossRef]
- Javor, J.; Bucová, M.; Ďurmanová, V.; Radošinská, D.; Párnická, Z.; Čierny, D.; Kurča, E.; Čopíková-Cudráková, D.; Gmitterová, K.; Shawkatová, I. Alzheimer’s Disease Risk Variant rs3865444 in the CD33 Gene: A Possible Role in Susceptibility to Multiple Sclerosis. Life 2022, 12, 1094. [Google Scholar] [CrossRef]
- Eskandari-Sedighi, G.; Crichton, M.; Zia, S.; Gomez-Cardona, E.; Cortez, L.M.; Patel, Z.H.; Takahashi-Yamashiro, K.; St. Laurent, C.D.; Sidhu, G.; Sarkar, S.; et al. Alzheimer’s disease associated isoforms of human CD33 distinctively modulate microglial cell responses in 2024,5XFAD mice. Mol. Neurodegener. 2024, 19, 42. [Google Scholar] [CrossRef]
- Preman, P.; Moechars, D.; Fertan, E.; Wolfs, L.; Serneels, L.; Shah, D.; Lamote, J.; Poovathingal, S.; Snellinx, A.; Mancuso, R.; et al. APOE from astrocytes restores Alzheimer’s Aβ-pathology and DAM-like responses in APOE deficient microglia. EMBO Mol. Med. 2024, 16, 3113–3141. [Google Scholar] [CrossRef]
- Ulrich, J.D.; Ulland, T.K.; Mahan, T.E.; Nyström, S.; Nilsson, K.P.; Song, W.M.; Zhou, Y.; Reinartz, M.; Choi, S.; Jiang, H.; et al. ApoE facilitates the microglial response to amyloid plaque pathology. J. Exp. Med. 2018, 215, 1047–1058. [Google Scholar] [CrossRef]
- Haney, M.S.; Pálovics, R.; Munson, C.N.; Long, C.; Johansson, P.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.; et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s microglia. bioRxiv 2023, bioRxiv:2023.07.21.549930. [Google Scholar] [CrossRef]
- Popescu, S.A. Investigating the Effects of TREM2, APOE and PILRα Variants on Microglial Functions and Neuronal Loss. Ph.D. Thesis, University of Cambridge, Cambridge, UK, February 2023. [Google Scholar]
- Maurya, S.K.; Gupta, S.; Mishra, R. Transcriptional and epigenetic regulation of microglia in maintenance of brain homeostasis and neurodegeneration. Front. Mol. Neurosci. 2023, 15, 1072046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Schlecht, A.; Wolf, J.; Boneva, S.; Laich, Y.; Koch, J.; Ludwig, F.; Boeck, M.; Thien, A.; Härdtner, C. The role of interferon regulatory factor 8 for retinal tissue homeostasis and development of choroidal neovascularisation. J. Neuroinflammation 2021, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, Z.; Li, X. Epigenetic Explorations of Neurological Disorders, the Identification Methods, and Therapeutic Avenues. Int. J. Mol. Sci. 2024, 25, 11658. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Ren, B.; Fang, Y.; Ren, J.; Liu, X.; Wang, X.; Zhou, F.; Xiao, R.; Luo, X.; You, L.; et al. Epigenetic regulation in cancer. MedComm 2024, 5, e495. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Merighi, S.; Nigro, M.; Travagli, A.; Gessi, S. Microglia and Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 12990. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Ozverel, C.S.; Erdag, E. Investigation of the molecular interactions of vaccine adjuvants: Can a strategic trio of Toll-like receptor agonists enhance efficacy in a multifaceted approach? Biomed. Biotechnol. Res. J. 2024, 8, 27–36. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liu, S.; Sui, Y.X.; Yang, M. Nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 inflammasome: From action mechanism to therapeutic target in clinical trials. World J. Gastrointest. Oncol. 2025, 17, 100094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jha, D.; Bakker, E.N.; Kumar, R. Mechanistic and therapeutic role of NLRP3 inflammasome in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2024, 168, 3574–3598. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, S.; Wright-Jin, E. NF-κB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J.; Wang, B.; Sun, M.; Yang, H. Microglia in the Neuroinflammatory Pathogenesis of Alzheimer’s Disease and Related Therapeutic Targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Polyphenols targeting NF-κB pathway in neurological disorders: What we know so far? Int. J. Biol. Sci. 2024, 20, 1332–1355. [Google Scholar] [CrossRef]
- Sarapultsev, A.; Gusev, E.; Komelkova, M.; Utepova, I.; Luo, S.; Hu, D. JAK-STAT signaling in inflammation and stress-related diseases: Implications for therapeutic interventions. Mol. Biomed. 2023, 4, 40. [Google Scholar] [CrossRef]
- Tripathi, A.; Bartosh, A.; Mata, J.; Jacks, C.; Madeshiya, A.K.; Hussein, U.; Hong, L.E.; Zhao, Z.; Pillai, A. Microglial type I interferon signaling mediates chronic stress-induced synapse loss and social behavior deficits. Mol. Psychiatry 2025, 30, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arboledas, A.; Acharya, M.M.; Tenner, A.J. The Role of Complement in Synaptic Pruning and Neurodegeneration. ImmunoTargets Ther. 2021, 10, 373–386. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Reddy, P.H. Synaptic basis of Alzheimer’s disease: Focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res. Rev. 2021, 65, 101208. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.F.; Khan, K.A.; Papavergi, M.-T.; Lemere, C.A. The Importance of Complement-Mediated Immune Signaling in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2024, 25, 817. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Miao, J.; Chen, L.; Pan, X.; Li, L.; Zhao, B.; Lan, J. Microglial Metabolic Reprogramming: Emerging Insights and Therapeutic Strategies in Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2023, 43, 3191–3210. [Google Scholar] [CrossRef]
- Jung, E.S.; Choi, H.; Mook-Jung, I. Decoding microglial immunometabolism: A new frontier in Alzheimer’s disease research. Mol. Neurodegener. 2025, 20, 37. [Google Scholar] [CrossRef]
- Na, D.; Zhang, Z.; Meng, M.; Li, M.; Gao, J.; Kong, J.; Zhang, G.; Guo, Y. Energy Metabolism and Brain Aging: Strategies to Delay Neuronal Degeneration. Cell. Mol. Neurobiol. 2025, 45, 38. [Google Scholar] [CrossRef]
- Guha Ray, A.; Odum, O.P.; Wiseman, D.; Weinstock, A. The diverse roles of macrophages in metabolic inflammation and its resolution. Front. Cell Dev. Biol. 2023, 11, 1147434. [Google Scholar] [CrossRef]
- Yen, J.J.; Yu, I.I. The role of ApoE-mediated microglial lipid metabolism in brain aging and disease. Immunometabolism 2023, 5, e00018. [Google Scholar] [CrossRef]
- Peggion, C.; Calì, T.; Brini, M. Mitochondria Dysfunction and Neuroinflammation in Neurodegeneration: Who Comes First? Antioxidants 2024, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.L.; Huang, H.; Zeng, X.; Duan, C.Y. Targeting mitochondrial quality control: New therapeutic strategies for major diseases. Mil. Med. Res. 2024, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Lauro, C.; Limatola, C. Metabolic reprograming of microglia in the regulation of the innate inflammatory response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omaraf, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Mezzasoma, L.; Schmidt-Weber, C.B.; Fallarino, F. In Vitro Study of TLR4-NLRP3-Inflammasome Activation in Innate Immune Response. Methods Mol. Biol. 2023, 2700, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Mossad, O.; Erny, D. The microbiota-microglia axis in central nervous system disorders. Brain Pathol. 2020, 30, 1159–1177. [Google Scholar] [CrossRef]
- Shanmugham, M.; Bellanger, S.; Leo, C.H. Gut-Derived Metabolite, Trimethylamine-N-oxide (TMAO) in Cardio-Metabolic Diseases: Detection, Mechanism, and Potential Therapeutics. Pharmaceuticals 2023, 16, 504. [Google Scholar] [CrossRef]
- Kim, C.S.; Jung, S.; Hwang, G.S.; Shin, D.M. Gut microbiota indole-3-propionic acid mediates neuroprotective effect of probiotic consumption in healthy elderly: A randomized, double-blind, placebo-controlled, multicenter trial and in vitro study. Clin Nutr. 2023, 42, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Ayyanar, M.P.; Vijayan, M. A review on gut microbiota and miRNA crosstalk: Implications for Alzheimer’s disease. GeroScience 2025, 47, 339–385. [Google Scholar] [CrossRef]
- Elangovan, S.; Borody, T.J.; Holsinger, R.M.D. Fecal Microbiota Transplantation Reduces Pathology and Improves Cognition in a Mouse Model of Alzheimer’s Disease. Cells 2022, 12, 119. [Google Scholar] [CrossRef]
- Yang, J.; Liang, J.; Hu, N.; He, N.; Liu, B.; Liu, G.; Qin, Y. The Gut Microbiota Modulates Neuroinflammation in Alzheimer’s Disease: Elucidating Crucial Factors and Mechanistic Underpinnings. CNS Neurosci. Ther. 2024, 30, e70091. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Y.; Chen, S.; Liang, F. Glycometabolic Reprogramming of Microglia in Neurodegenerative Diseases: Insights from Neuroinflammation. Aging Dis. 2024, 15, 1155–1175. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Simmons, A.; Mayorga, A.; Burgess, B.; Nguyen, T.; Budda, B.; Rychkova, A.; Rhinn, H.; Tassi, I.; Ward, M.; et al. Preclinical and first-in-human evaluation of AL002, a novel TREM2 agonistic antibody for Alzheimer’s disease. Alzheimer’s Res. Ther. 2024, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Zheng, Y.; Yu, Q.; Zeng, M.; Bai, L.; Yang, L.; Guo, M.; Jiang, X.; Gan, J. Inhibitors of the NLRP3 inflammasome pathway as promising therapeutic candidates for inflammatory diseases (Review). Int. J. Mol. Med. 2023, 51, 35. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kwon, H.M.; Lee, E.; Kim, P.H.; Jeung, E.B.; Lee, G.S. Role of inflammasome regulation on immune modulators. J. Biomed. Res. 2018, 32, 401–410. [Google Scholar] [CrossRef]
- Yong, V.W. Microglia in multiple sclerosis: Protectors turn destroyers. Neuron 2022, 110, 3534–3548. [Google Scholar] [CrossRef]
- Ziesenitz, V.C.; Welzel, T.; van Dyk, M.; Saur, P.; Gorenflo, M.; van den Anker, J.N. Efficacy and safety of NSAIDs in infants: A comprehensive review of the literature of the past 20 years. Pediatr. Drugs 2022, 24, 603–655. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Luo, S.; Zhan, Y.; Lu, Q. The roles of PPARγ and its agonists in autoimmune diseases: A comprehensive review. J. Autoimmun. 2020, 113, 102510. [Google Scholar] [CrossRef]
- Taner, N.; Haskologlu, I.C.; Erdag, E.; Mercan, M.; Chuckwunyere, U.; Ulker, D.; Sehirli, A.O.; Abacioglu, N. Chronobiological Efficacy of Combined Therapy of Pelargonium Sidoides and Melatonin in Acute and Persistent Cases of COVID-19: A Hypothetical Approach. Adv. Exp. Med. Biol. 2023, 1412, 427–442. [Google Scholar] [CrossRef]
- Naik, R.A.; Rajpoot, R.; Koiri, R.K.; Bhardwaj, R.; Aldairi, A.F.; Johargy, A.K.; Faidah, H.; Babalghith, A.O.; Hjazi, A.; Alsanie, W.F.; et al. Dietary supplementation and the role of phytochemicals against the Alzheimer’s disease: Focus on polyphenolic compounds. J. Prev. Alzheimer’s Dis. 2025, 12, 100004. [Google Scholar] [CrossRef]
- Haskologlu, I.C.; Erdag, E.; Uludag, O.; Abacioglu, N. A chronobiological approach: The potential of photoswitchable drug derivatives in the treatment of Alzheimer’s disease. Chronobiol. Med. 2024, 6, 194–204. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Haskologlu, I.C.; Erdag, E. Molecular Links Between Circadian Rhythm Disruption, Melatonin, and Neurodegenerative Diseases: An Updated Review. Molecules 2025, 30, 1888. [Google Scholar] [CrossRef] [PubMed]
- Mafe, A.N.; Büsselberg, D. Could a Mediterranean Diet Modulate Alzheimer’s Disease Progression? The Role of Gut Microbiota and Metabolite Signatures in Neurodegeneration. Foods 2023, 14, 1559. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Varga, P.; Ungvari, Z.; Fekete, J.T.; Buda, A.; Szappanos, Á.; Lehoczki, A.; Mózes, N.; Grosso, G.; Godos, J.; et al. The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: A meta-analysis. GeroScience 2025, 47, 3111–3130. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Picone, P.; Girgenti, A.; Buttacavoli, M.; Nuzzo, D. Enriching the Mediterranean diet could nourish the brain more effectively. Front. Nutr. 2024, 11, 1489489. [Google Scholar] [CrossRef]
- Yassaghi, Y.; Nazerian, Y.; Ghasemi, M.; Nazerian, A.; Sayehmiri, F.; Perry, G.; Gholami Pourbadie, H. Microglial modulation as a therapeutic strategy in Alzheimer’s disease: Focus on microglial preconditioning approaches. J. Cell. Mol. Med. 2024, 28, e18554. [Google Scholar] [CrossRef]
- Desai, M.; Kundu, A.; Hageman, M.; Lou, H.; Boisvert, D. Monoclonal antibody and protein therapeutic formulations for subcutaneous delivery: High-concentration, low-volume vs. low-concentration, high-volume. MAbs 2023, 15, 2285277. [Google Scholar] [CrossRef]
- Gleeson, J.P.; Ryan, S.M.; Brayden, D.J. Oral delivery strategies for nutraceuticals: Delivery vehicles and absorption enhancers. Trends Food Sci. Technol. 2016, 53, 90–101. [Google Scholar] [CrossRef]
- De Andres, J.; Hayek, S.; Perruchoud, C.; Lawrence, M.M.; Reina, M.A.; De Andres-Serrano, C.; Rubio-Haro, R.; Hunt, M.; Yaksh, T.L. Intrathecal Drug Delivery: Advances and Applications in the Management of Chronic Pain Patient. Front. Pain Res. 2022, 3, 900566. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.; Saqib, F.; Mubarak, Z.; Latif, M.F.; Wahid, M.; Nasir, B.; Shahzad, H.; Sharifi-Rad, J.; Mubarak, M.S. Alzheimer’s disease and drug delivery across the blood-brain barrier: Approaches and challenges. Eur. J. Med. Res. 2024, 29, 313. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Zhao, N.; Francis, N.L.; Calvelli, H.R.; Moghe, P.V. Microglia-targeting nanotherapeutics for neurodegenerative diseases. APL Bioeng. 2020, 4, 030902. [Google Scholar] [CrossRef]
- Millozzi, F.; Milán-Rois, P.; Sett, A.; Delli Carpini, G.; De Bardi, M.; Gisbert-Garzarán, M.; Sandonà, M.; Rodríguez-Díaz, C.; Martínez-Mingo, M.; Pardo, I.; et al. Aptamer-conjugated gold nanoparticles enable oligonucleotide delivery into muscle stem cells to promote regeneration of dystrophic muscles. Nat. Commun. 2025, 16, 577. [Google Scholar] [CrossRef]
- Hou, J.; Deng, Q.; Deng, X.; Zhong, W.; Liu, S.; Zhong, Z. MicroRNA-146a-5p alleviates lipopolysaccharide-induced NLRP3 inflammasome injury and pro-inflammatory cytokine production via the regulation of TRAF6 and IRAK1 in human umbilical vein endothelial cells (HUVECs). Ann. Transl. Med. 2021, 9, 1433. [Google Scholar] [CrossRef]
- Abbasi, H.; Kouchak, M.; Mirveis, Z.; Hajipour, F.; Khodarahmi, M.; Rahbar, N.; Handali, S. What We Need to Know about Liposomes as Drug Nanocarriers: An Updated Review. Adv. Pharm. Bull. 2023, 13, 7–23. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Di Raimondo, D.; Salvaggio, G.; Tuttolomondo, A.; Comelli, A. Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals 2024, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.A.; Santos, S.D.; Leiro, V.; Pêgo, A.P. Dendrimers and Derivatives as Multifunctional Nanotherapeutics for Alzheimer’s Disease. Pharmaceutics 2023, 15, 1054. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Chen, H.-L.; Dong, J.-R. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Food-Grade Nanovehicles for Hydrophobic Nutraceuticals or Bioactives. Appl. Sci. 2023, 13, 1726. [Google Scholar] [CrossRef]
- Sharma, H.; Narayanan, K.B.; Ghosh, S.; Singh, K.K.; Rehan, P.; Amist, A.D.; Bhaskar, R.; Sinha, J.K. Nanotherapeutics for Meningitis: Enhancing Drug Delivery Across the Blood-Brain Barrier. Biomimetics 2025, 10, 25. [Google Scholar] [CrossRef]
- Dighe, S.; Jog, S.; Momin, M.; Sawarkar, S.; Omri, A. Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management. Pharmaceutics 2023, 16, 58. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; So, K.F.; Jiang, W.; Chiu, K. Targeting Microglia in Alzheimer’s Disease: Pathogenesis and Potential Therapeutic Strategies. Biomolecules 2024, 14, 833. [Google Scholar] [CrossRef]
- Balkhi, S.; Di Spirito, A.; Poggi, A.; Mortara, L. Immune Modulation in Alzheimer’s Disease: From Pathogenesis to Immunotherapy. Cells 2025, 14, 264. [Google Scholar] [CrossRef]
- Ma, Y.N.; Hu, X.; Karako, K.; Song, P.; Tang, W.; Xia, Y. The potential and challenges of TREM2-targeted therapy in Alzheimer’s disease: Insights from the INVOKE-2 study. Front. Aging Neurosci. 2025, 17, 1576020. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Lee, Y.; Park, Y.; Nam, H.; Lee, J.W.; Yu, S.W. Translocator protein (TSPO): The new story of the old protein in neuroinflammation. BMB Rep. 2020, 53, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Vo, S.; Brase, L.; Aladyeva, E.; Albanus, R.D.; Nallapu, A.; Fu, H.; Harari, O. Exploring Cellular Heterogeneity: Single-Cell and Spatial Transcriptomics of Alzheimer Disease Brains and iPSC-Derived Microglia. Res. Sq. 2024, rs.3.rs-5045715, Preprint. [Google Scholar] [CrossRef]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Kırkık, D.; Özadenç, H.M.; Taş, S.K. Immunomodulation by Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-Associated Protein 9. Hamidiye Med. J. 2025, 6, 1–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).