Abstract
The olive tree is currently cultivated worldwide, with higher incidence in Mediterranean Basin. Its climate is highly favorable to the synthesis of phenolic compounds, stored in olive leaves; their consumption has been linked to a lower incidence of cancer and cardiovascular disorders for which the research interest upon this feature has increased in last decade. This study aimed (i) to review evidence about the importance of olive leaf extract (OLE) on human health and the physiological effect of its major compounds; (ii) to update the state of the art of studies conducted on the health and technological usage of olive leaf extract; (iii) to report potential uses of OLE in pharmaceuticals, food production, and cosmetics; and (iv) to prospect the future of clinical applications of OLE from diverse cultivars, especially in metabolic inflammatory conditions such as polycystic ovary syndrome (PCOS). Overall, cultivars richer in TPC, including TFC, αT, omega-3 and omega-9, present a main research target for supplementation alone or in conjunction with vitaminic compounds, due to their nutraceutical value in metabolic disorders, chronic inflammatory diseases, and anti-aging treatments, whereas cultivars with less water content might be useful as substrates for food preservation. With regard to future prospects, it would be of great interest to clarify the specific mechanisms underlying the beneficial effects of OLE on neuro-immune and cardiovascular health to design safer and healthier nature-based medicine for a wide array of costly and highly prevalent chronic diseases, such as inflammatory and metabolic-related syndromes, namely, PCOS.
1. Introduction
1.1. Brief Historical Overview of Olea europaea L.
Olea europaea L., typically mentioned as olive tree, has been part of the Mediterranean landscape from prehistoric times, though its true origin remains unknown. This plant thrives in hot and dry summers; by convention, it represents regality, wisdom, health, peace, and victory [1,2]. Wild olive trees, mostly found in the countryside of the Middle East, began to be domesticated c. 5000–6000 years ago, developing fewer bushy branches and fruits richer in an oil and becoming a precious commodity [3]. Current research with genetic markers crossed with paleo-botanical and archaeological data diverge into three different theories about the dispersion of olive culture [4]: (1) starting point from Northern Levant with spreading to the Mediterranean Basin (Figure 1, part A) [5]; (2) simultaneous dissemination from two different starting points, Eastern and Central parts or Mediterranean area (Figure 1, part B) [6], and (3) a later proposal of a beginning of cultivation in Southern Levant with diversification of olive culture to other points (Figure 1, part C) [7].
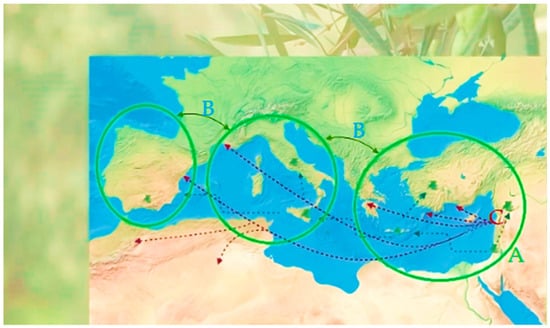
Figure 1.
Diagram of the current proposed theories for the expansion of olive tree cultivation in the Mediterranean Basin (adapted from Refs. [7,8]). Part A—olive tree expansion from the South Levant. Part B—simultaneous expansion of olive tree from two starting points. Part C—olive tree expansion from the North Levant. Credits: biorender.com.
With the beginning of the Aegean Iron Age (1200 B.C. to 600 B.C.), the Greek empire highly invested in domestic olive culture, holding the monopoly until the Etruscans drew that expertise to Italy and the Phoenicians to Malta and Northern Africa [9,10]. Centuries later, the Moorish introduced some olive cultivars in Southern Iberia [11], and from here, olive cultivation expanded to every part of the globe, especially during the European colonization period, by farmers [12]. An international germoplasm data bank of olive seeds is compiled online in a database (http://www.oleadb.it/, last accessed on 28 August 2024), though it still has scarce information if we take into consideration that from 47 olive growing countries, thousands of varieties have been identified so far [12]. Today’s olive production is mostly for oil manufacture, especially in Spain, Greece, Italy, and Portugal. The properties of O. europaea L. are naturally influenced by numerous factors, such as soil, olive variety, olive ripening, and postharvest processing [13]. Different cultivars emerge from diverse morphological characteristics and display distinct qualities of commercial and therapeutic value in their products [14,15].
1.2. Cardinal Derivates of O. europaea L.
The main exploits of O. europaea L. have always been end products like olive oil and table olives, vital commodities regarding dietetic, cultural, social, and economic aspects (Figure 2).
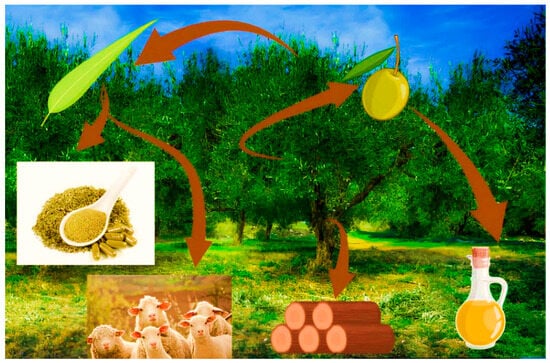
Figure 2.
Graphical abstract of the many derivates of olive tree. Image credits: biorender.com.
However, olive tree byproducts might serve other purposes: olive wood for making everyday use tools; the olive fruit for culinary purposes; olive leaves for traditional infusions to alleviate inflammatory and infection conditions; and even for cosmetics production [16,17]. Tree pruning delivers large volumes of wasted leaves [18,19] associated with economic and environmental costs [20], often used as a local food source for small ruminants, and more recently recycled in the food, medicine, and cosmetic industries, and notably valued for their biological properties and their organoleptic characteristics [21,22]. The Mediterranean Basin prone, to semi-arid climatic and soil conditions, promotes the synthesis of phenolic compounds in olives and olive leaves under drought stress, particularly of flavonoids as main responders to UV-B stress conditions [23]. The ingestion of phenolic compounds through olives and extra virgin olive oil (EVOO) was linked to a lower incidence of cancer and cardiovascular disorders (CVD), which drove researchers to further identify and understand the constituents of olive tree products [24] such as olive leaves, especially during the last decade [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. This study aims (i) to review and update evidence about the importance of olive leaf extract (OLE) on human health and the physiological effect of its major compounds; (ii) to review studies conducted on the health and technological usage of OLE; (iii) to report potential uses of OLE in pharmaceuticals, food production, and cosmetics; and (iv) to prospect the value of future research with OLE, especially in inflammatory metabolic clinical conditions such as polycystic ovary syndrome (PCOS).
2. Phytochemistry Overview of O. europaea L. folium
2.1. Nutritional Features
In the past, folk medicine used olive leaves to address fever, malaria, and hypertension [46]. Recently, for their nutritional characteristics, they proved valuable for a wide array of health conditions, turning into a very appreciated functional dietetic and health enhancement good [18]. While olives, among other macro and micronutrients (Figure 3), are known to be rich in fat, especially monounsaturated fatty acids (MUFAs), olive leaves have a considerable content of carbohydrates, phenolic compounds, vitamins, and minerals [47,48,49,50].
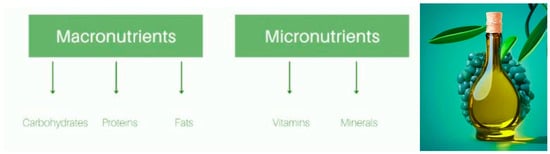
Figure 3.
Graphical abstract of nutritional differentiation between macro and micronutrients from olive leaves. Image credits: crayon.com.
Nutritional differences among profiles may vary according to soil, climate, and cultivation conditions, as well as genetic predisposition of the plant itself. For example, as seen in Figure 4, the Portuguese varieties cultivated in a grove from Valpaços display a higher content in carbohydrates and less water. Meanwhile, Tunisian Chemchali displays a higher water content and lower carbohydrates, and the Spanish Arbequina variety shows a good balance between carbohydrate, protein, and lipidic composition. Its relatively low carbohydrate composition (most likely nondigestible) makes Spanish Arbequina an interesting prospective target for supplementation research (Figure 4).
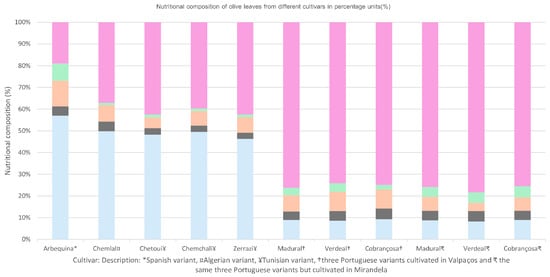
Figure 4.
Nutritional composition of olive leaves from different cultivars in percentage units (%), according to Refs. [50,51,52].
2.2. Biochemical Blueprint of O. europaea L. folium
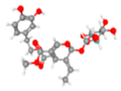
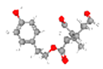
Olive leaves are praised for their extensive application in preventing and treating several disorders, namely, metabolic issues such as diabetes and CVD [53,54,55,56,57,58,59,60]. These benefits derive from their strong anti-inflammatory and antioxidant properties. So, research focus has been not only upon the antioxidant power of polyphenols, but also upon vitamin E, minerals, and fatty acids (FAs), among other constituents that have been identified in olive leaves [50,52]. OLE can be divided into aqueous and fatty fractions, where polar compounds like phenols would belong to the aqueous portion while lipophilic structures, including fatty acids and tocopherols, would remain in the fatty fraction. Most studies with olive leaves focus on the phenolic content, especially on the secoiridoid OLEP and its derivates (Figure 5), and very little research has paid attention at the study of fatty acids, namely on polyunsaturated fatty acids (PUFAs) and MUFAs.
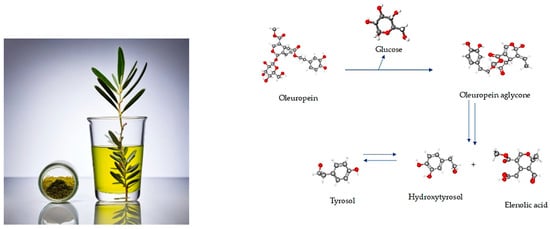
Figure 5.
Graphical abstract of the conversion of oleuropein in derivate polyphenols. Image credits: crayon.com; https://pubchem.ncbi.nlm.nih.gov.
It is interesting to understand how geographical features, including soil and climatic conditions as well as agricultural practices, have influence upon the phenolic composition and so determine the most adequate purpose for each variant [49]. Figure 6 shows the analysis of the relative composition in percentage of classes of polyphenols of some olive leaves referred to by the literature. According to literature, most of the polyphenols found in olive leaves belong to secoiridoids, simple phenols, phenolic acids, and flavonoids, and so these were considered the main groups of classification, with the remnant compounds being considered “other phenols”. The results of different studies confirmed that the phenolic composition varies not only by cultivar genotype (main factor), but also by the location of cultivation, season of harvest, level of leaf development/ripening, the implemented agriculture practice (ecological or conventional), and also the type of solvent used for extraction (Figure 6 and Table S1 in Supplementary) [14,19,49,50,58,59,60,61,62,63]. As shown in Figure 6 and Table S1, Italian cultivars harvested in Tuscan farms between September and October 2022 and extracted with methanolic solvent, showed higher levels of phenolic acids [63] compared to the same cultivars harvested in October 2021 in the farms from Tuscan and Emilia-Romagna extracted with hydroethanolic mix in 2021 [59]. These Italian samples from Emilia Romagna, as well as Croatian Oblica, cultivated in Pag and Marina Island, ref. [59] showed a higher level of secoiridoids, followed by flavonoids, while both Croatian cultivars Lastovka and Levatinka, also from Pag and Marina, had higher flavonoids, followed by secoiridoids [59]. On the other hand, hydromethanolic olive leaf extracts of both Italian and Croatian cultivars from Krk island, all had a profile higher in secoiridoids, very much like Croatian Oblica and Italian Moraiolo, Frantoio, and Nostrana di Brisighela cultivated in the island of Pag and Marina, despite the different solvents used in the extraction process [61]. Despite the age and local differences, and that the extractant was different for the Krk samples (methanolic mix), a common ground for the same phenolic class was still found, meaning that genotyping might have a heavier weight on younger samples. Tunisian Chetoui leaves cultivated at diverse altitudes presented a differential in their poliphenolic profile: altitude > 500 m showed high levels of secoiridoids and simple phenols; altitude between 500–300 m and altitude < 300 m showed higher levels in flavonoids [49]. Discriminant factorial analysis (DFA) made by the authors, associated Moraiolo to a pool of flavonoids, luteolin-7-glucoside and apigenin with hydroxytyrosol glucoside, secoiridoid ligstroside and maslinic acid, while Leccino and Frantoio showed a higher correlation to a mix of phenolic acids and derivates such as maslinic acid and caffeoyl-secologanoside. Regarding geographical origin identification, ligstroside and luteolin were the most adequate markers, although the flavonoid apigenin also serves as a biomarker to discriminate cultivation between the Siena and Grosseto provinces, while TPC together with the hydroxytyrosol acetate and ligstroside successfully biomarked between clusters of samples from very close areas to Siena province [63]. These results demonstrated that targeted metabolomic studies of differential phenolic composition are quite useful to identify the geo-origin of olive-related products on small geographic scales. Among the individual polyphenols identified for cultivars from Emilia-Romagna and Oblica from Pag and Merina, OLEP was the major compound, accounting for 40–50% of the total phenolics [59].
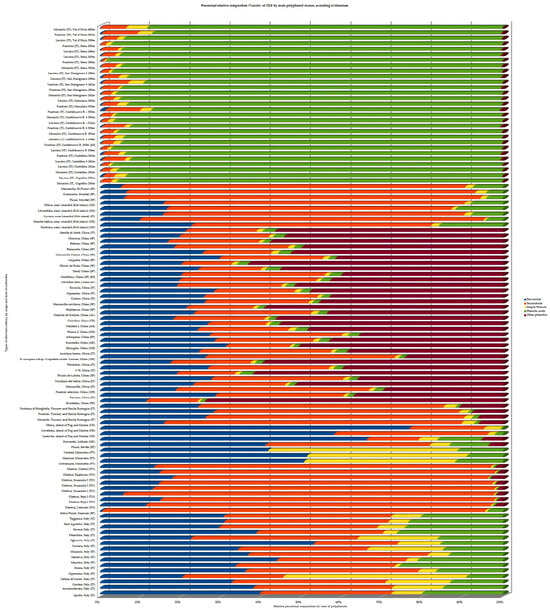
Figure 6.
Percentage of relative content by class of phenols identified in different cultivars, according to the literature [14,19,49,50,58,59,60,61,62,63].
OLEP has been used as a marker by type of cultivar, climate, and geographical parameters, sampling and drying techniques, and extraction parameters [64]. Despite some contradictory results in the literature, the season of harvest seems to influence the polyphenol profile of olive leaves, besides the drought stress [65]. With regards to TPC, Croatian Oblica and Italian Nostrana di Brisighella presented higher values, while Croatian Levantinka planted in Pag and Marina displayed the lowest results. These authors also tested the correlation between the phenolic content and antioxidant activity through DPPH radical inhibition and FRAP testing. While DPPH showed no correlation with TPC, the FRAP and ORAC results were correlated with higher TPC of Croatian Oblica and Italian Nostrana di Brisighella extracts and the lowest for Croatian Levantinka (highest level of flavonoids followed by secoiridoids) [59]. Pasković et al., 2020 also studied the phenolic profile of samples harvested by October 2017, January 2018 and March 2018, with an overall highest phenolic content, especially OLEP, reached by March 2018. Antioxidant activity for DPPH and FRAP were higher for Croatian Istarska Bjelica, particularly in samples harvested by spring/March 2018, in correlation with the concentration peak of the OLEP secoiridoid for the leaves of this cultivar by March of 2018 [61]. Simple phenols like TY, followed a similar variation. On the other hand, the phenolic acid verbascoside was higher during the colder temperatures of October and January [61]. Flavonoids like rutin remained unaltered through the leaf cycle, whereas catechin and luteolin-7-O-glucoside followed a pattern already noted for the other phenols, with similar levels in October and January, and the highest concentration reached by spring; the flavonol apigenin-7-O-glucoside was higher by October and March [61]. Overall, results showed that low values of temperature about 15 days before the spring lead to higher OLEP values and lower HT values in spring, especially for Italian Leccino and Croatian Oblica, Drobnica, and Levantinka cultivars. The high antioxidant property of OLEP, by lowering the level of cellular reactive oxygen species (ROS) during cold stress, is appointed as the reason for the higher values of this secoiridoid in those conditions, and so, when looking for samples richer in OLEP, it would be more interesting to harvest from locations with this kind of climatic conditions during the olive tree spring pruning. On the other hand, the Croatian Istarska bjelica cultivar maintained similar levels of the main phenols the whole cycle, leading to the possibility of its high resistance to low-temperature stress. Also, Istarska bjelica showed a specific correlation of phenols with mineral components, suggesting a differential response to environmental factors, compared to other cultivars. These authors also performed PCA analysis and assessed a correlation between FRAP and DPPH results; OLEP and verbascoside (Leccino, Levantinka, and Oblica cultivars harvested in March); Ty and boron (higher in Drobnica harvested in October); higher levels of luteolin by January for Drobnica and the lowest concentration by October and January for Istarska bjelica [61]. The Spanish variant of Picual cultivated in Montiel [62] and Granada [19], as well as Cornicabra from Montiel [62], were all higher in secoridoids compared to the cultivation from Sevilla [58]. Ronca et al., 2024 also analyzed phenolic variation according to the increase in sample size, concluding that higher concentrations of leaf extract do not necessarily contribute to a higher extraction of polyphenols, and so do not translate into a higher antioxidant activity [19]. Also, these authors verified the availability for polyphenols and minerals in leaf extract, in microparticles, and within a functional yogurt, where the secoiridoid OLEP was the popyphenol with the best retained value through the process, which means that some micronutrients and minerals might have an influence on improving polyphenol preservation as a supplement or food preservation agent; as well, microencapsulation helps to preserve the easy loss of OLEP through gastrointestinal tract [19]. Martínez-Navarro et al., 2021 studied the variation in polyphenols and minerals for a whole year and concluded that all the analyzed weather parameters had influence over polyphenol content, where absolute minimum temperature was much correlated with the verbascoside content in all cultivars regardless of the location, and also the flavonoid content [62]. Especially in Picual, absolute maximum temperature and relative humidity also had impact on verbacoside content as well on flavonoids [62]. Rainwater showed a negative correlation with the secoiridoid OLEP concentration in the Manzanilla [62]. Sevilla’s Picual had a higher content of flavonoids compared to Montiel’s production, though the value of TPC was lower, with higher OLEP as an individual poplyphenol, compared to the Koroneiki cultivar from Lefkada [58]. Also, the DPPH radical scavenging was higher for Greek Koroneiki than Sevillla’s Picual [58]; even the phenolic quantity is about half of the other. An equivalent ratio of flavonoids/secoiridoids was found for almost all the studied cultivars by Zhang et al., 2022 [60]. Despite the differences regarding the phenolic classes between the 32 cultivars analyzed by these authors, no correlation was found between the TPC and antioxidant activity. Instead, they found a correlation between antioxidant activity and the concentrations of individual phenolics such as kaempferol-7-O-glucoside, luteolin-7-O-glucoside, luteolin-3′,7-di-O-glucoside, and luteolin-4′-O-glucoside, and underlined the reinforcement by flavonoids upon the antioxidant power of the olive leaf [62]. Also, secoiridoids including OLEP and others, esculin, and HT concentrations showed positive correlation with antioxidant activity, with higher performance for HT compared to OLEP. PCA studies by those authors demonstrated a division between cultivars with high oil content characterized by the lowest polyphenol concentrations and high phenolic acids, and low-oil-content cultivar leaves marked by the highest glycosylated flavonoids, OLEP, and HT [62].
Most studies concerning fatty acid content are about the properties of olive oils or olives. According to the literature found in Cochrane and PubMed databases, the content of total lipids varies between 1.05 ± 0.11% and 8.14 ± 0.24% in different cultivars, and the most prominent FAs are usually PUFAs, followed by saturated fatty acids (SFAs) (Table 1 and Figure 7), in the Portuguese varieties cultivated in the hotter region of Mirandela, especially Cobrançosa’s cultivar, where SFAs come first, followed by MUFA content [50]. Research has proven that olive leaf PUFAs have health benefits regarding metabolic status and cardiovascular and neurofunction, as well as against carcinogenic conditions [1,66,67,68,69,70,71,72], while vegetable MUFAs may improve or be innocuous to CDV health and show inherent antimicrobial value [50,73]. However, most studies focus on phenolic and vitamin content as their antioxidant capacity, while fewer studies consider the FAs and mineral content, a gap that should be filled soon [74,75,76].

Table 1.
Composition of the total percentage of fatty acids (FAs) in olive leaf samples of different cultivars, (adapted from Refs. [50,51,52]).
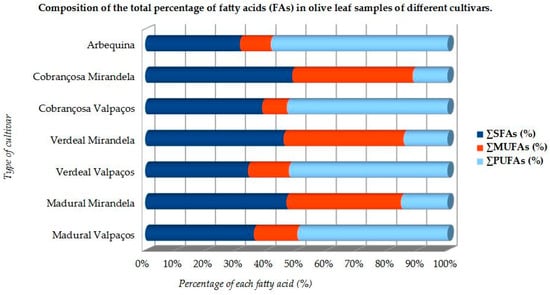
Figure 7.
Relative composition of fatty acids in olive leaves of some cultivars (adapted from Refs. [50,51,52].
Amongst PUFAs, omega-3 linolenic acid (ALA) is the major compound, followed by omega-6 linoleic acid (LA), while oleic acid (OA) is the most prominent of MUFAs [50,52]. O. europaea L. has also been widely accepted as one of the species with the highest antioxidant activity via its phenolic components, whether obtained through olive oil, fruits, or leaves [77,78]. Phenolic compounds found in olive leaves belong to different classes: from phenolic acids, such as caffeic, gallic, vanillic, and coumaric acids; phenolic alcohols, including tyrosol (TY) and hydroxytyrosol (HT) (Table 2); to more complex molecules, such as secoiridoids (OLEP and ligstroside), lignans (acetoxypinoresinol and pinoresinol), flavonoids, and hydroxyl-isochrons [78]. The leading bioactive is OLEP, a phenylethanoid credited with several therapeutic properties (Table 2) [79]. Olive leaves contain 1–14% OLEP compared to a scarce 0.005–0.12% in olive oil [56]. The OLEP molecule can be split into three distinct units: HT, elenolic acid, and glucose. HT (Table 2) is responsible for most biological qualities of OLEP and for its super antioxidant potential [79]. Experiments have demonstrated that OLEP, TY, and HT (Table 2) promote a wide range of pharmacologic and health-related effects, including immune-protective and antimicrobial power [80,81], cardio and neuroprotective [82,83,84,85,86,87,88,89], anti-osteoporotic [90], anti-diabetic [91], hypotensive and anti-atherosclerotic [92], weight control [93,94], anti-inflammatory [95], antioxidant [85,96,97], anti-carcinogenic [41,92,98,99,100], and antithrombotic action [101,102,103].

Table 2.
Physiological actions of the main polyphenols found in olive leaves, according to the literature. Figures from https://pubchem.ncbi.nlm.nih.gov (accessed on 28 August 2024).
A profile of vitamin E: α-tocopherol (α-T) > γ-tocopherol (γ-T) > β-tocopherol (β-T) > δ-tocopherol (δ-T) can be found in leaves from different cultivars [52], although O. europaea Madural, Verdeal and Cobrançosa cultivated in specific conditions in Mirandela showed no significant presence of both tocopherols and tocotrienols [50]. According to the literature, α-T has the ability to protect cell integrity [111,112], boost the immune response [113], regulate platelet aggregation [66], mitigate cancer cell proliferation [114,115,116] and atheroma formation [117,118], control triglyceride (TG) and cholesterol levels [119], and to help to preserve hippocampal response [120,121,122,123,124].
2.3. Mineral Arrangement of Olea europaea L. Folium
The therapeutic responses of olive leaves can also be influenced by their specific mineral composition (Figure 8) which varies with the soil, climate/season, type of cultivar, and phenolic composition as well as response to biotic and abiotic stress [125,126].

Figure 8.
Brief summary of macro and microminerals generally assessed in olive leaf extracts (adapted from Refs. [49,50,51,52,62]). Image credits: crayon.com.
Recent studies indicate a similar composition of macrominerals and microminerals between cultivars produced in similar soils and climates (Figure 9 and Figure 10). For example, in the Portuguese cultivars from Mirandela grove, calcium (Ca) > potassium (K) > phosphorus (P) > sulfur (S) > sodium (Na); Ca occupied the higher ranking for O. europaea Cobrançosa and Verdeal, whereas K came first in O. europaea Madural [50]. The same varieties cultivated in Valpaços presented a profile richer in calcium (Ca), potassium (K), phosphorus (P), magnesium (Mg), and silicon (Si) [78], as well as for Chetoui olive leaves in Tunisia [49]. These are in line with the literature, which reports that Na, Mg, P, K, Ca, Mn, Fe, Cu, Zn, and Pb are more often found in the whole [19,49,50,52,61,62,79]. Olive leaves can be handy to verify atmospheric trace element deposition and soil toxicity [58]. For example, toxic levels of As element identified in O. europaea Verdeal’s leaves from Mirandela [50], not being found in the same cultivar produced in Valpaços [52], underlines the differential of the cultivated soil and cultivar. Metals can also form metallophenolomics interacting with plant phenols. Metal chelation by polyphenols helps to understand the resistance of olive leaf to the toxic stress induced by the environmental pollution, and the adaptability in various acidic environments. For example, Seville’s Picual [58] showed a higher DPPH scavenging activity compared to what was expected according to the sample’s result for the TPC values, because metallocomplexes with free phenolic compounds also display antioxidant power, even if they carry a lesser number of –OH groups [58]. Even if the assessment of metal complexes of an extract is complicated due to high possibilities of chelation of several metals by different ligands, current research is focused on inorganic potential of plant leaves for the benefits of flavonoid metal complexes—for example, integration of Al3+ with 3-hydroxyflavone provides photostability to the medium besides its neuroprotective power [58]. Besides forming metallocomplexes, the mineral elements of plants also play a role in polyphenol biosynthesis. Higher levels of sodium (Na) in Chetoui olive leaves from site Bouarada 1 (Figure 9) pose a possible contribution of a plant to mild salt stress [49]. The same authors also found a negative correlation between (1) Na levels and luteolin-4-glucoside and (2) the concentrations of P in leaves and oleacein; (3) a positive correlation between Zn in soil and oleuropein synthesis; (4) a negative correlation between levels of HT and levels of luteolin-7-O-glucoside, luteolin, and apigenin; (5) a positive correlation between CaCO3 and apigenin; (6) levels of Mn, Ca, and B in the leaves and the level of Zn in the soil showed correlation with the abundance of OLEP and luteolin-7-O-glucoside [49]. Pasković et al., 2020 also reported a specific inorganic profile for each cultivar, suggesting a role of the mineral composition in their interplay with phenolic compounds [61]. Also, according to Martínez-Navarro et al., 2021, macromineral Mg and micromineral Fe can be both suggested as markers for differentiating locations [62]; verbascoside and HT were also affected by the location of olive cultivation and respective agricultural practice [62].
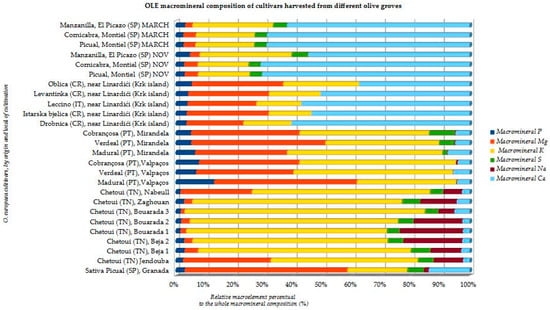
Figure 9.
OLE macromineral composition of several O. europaea cultivars cultivated in diverse olive groves (units in relative % of each element to the whole macromineral content) (adapted from Refs. [19,49,50,52,61,62]).
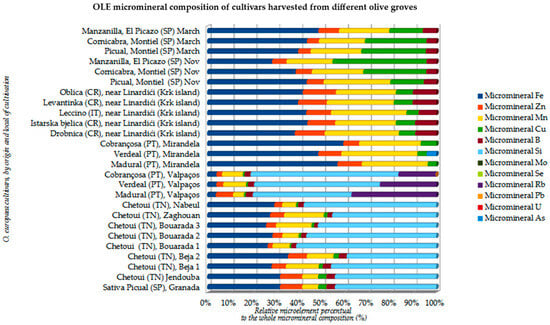
Figure 10.
OLE micromineral composition of several O. europaea cultivars cultivated in diverse olive groves (units in relative % of each microelement to the whole micromineral composition) (adapted from Refs. [19,49,50,52,61,62].
3. Therapeutic Prospective of Bioactives in Extracts of Olive Leaf
3.1. Anticarcinogenic Competence
OLEP, oleocanthaland and HT are considered the major candidates for pharmacological and industrial employment, either as a single drug or as food enrichment [127,128,129,130]. HT has been found to have a possible enactment against cancer through several mechanisms, such as ERK1/2 pathway inhibition, antagonism of G-protein coupled estrogen receptor, and apoptotic induction through ROS pathway, as well as through the inhibition of Akt, NFκB, STAT3, and EGFR signaling [127]. The latest literature points to several pathways possibly interfered by both HT and OLEP bioactives [101,107], as shown in Figure 11.
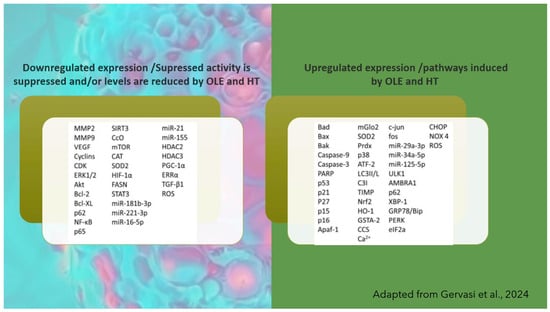
Figure 11.
Factors and pathways involved in tumor cells’ suppression by the main olive leaf bioactives OLE and HT, according to literature (adapted from Ref. [106]).
Studies also found that co-treatment with HT or OLEP reversed LC3 II/I and Beclin-1 downregulation as well as p62 upregulation [128]. Findings obtained with oleuropein aglycone or other semisynthetic compounds suggest a possibility to boost pharmacological action and thus widen its utility in pharmacology [128,130]. Oleocanthal also seems to play an active role regarding both tumor proliferation trough inhibition of ERK1/2, HGF, STAT3, and p90 ribosomal S6 kinase; and the arrest of the cell cycle at the G1/S phase and cell proliferation and/or tumor survival through inactivation of the AKT pathway, phosphorylation of p53 and apoptotic induction via caspase, poly-adenosine diphosphate-ribose polymerase and the mitochondrial pathway [127,131]. On the other hand, omega-9 oleic acid (OA) seems to be a shielding force against tumoral cells in both epidemiological and animal studies by (1) downregulating cellular multiplication and quelling the spreading potential of esophageal cancer cells through amplified expression of tumor suppressors [131], (2) downregulating COX2 expression and favoring apoptosis in colorectal cancer cell lines [132,133], and (3) showing chemo preventive properties in human glioblastoma cells [134], as well as significant anticancer reports in other human cell lines [131]. In addition, a novel nutraceutical approach to chemotherapy elicits innovative dietary-drug synergistic defense arrangements against both the progression of cancer itself and systemic toxicity (such as protection against Adriamycin cardiotoxicity by OLEP), which is of utmost importance in multifactorial etiology pathologies [49]. Beyond the preventive capacity of vitamin E against CVD and teratogenic effects described before, some papers also reported (1) a role in osteoporosis improvement, despite the controversial results of Ilesanmi-Oyelere et al., 2019 [85,135]; its combination with OA and polyphenols appears linked to bone mass recovery with less danger of osteoporotic fracture by hindering prostaglandins [85,136]; (2) a prospective benefit in periodontitis management through the adjustment of redox status and wound restoration [137,138]; and (3) a successful therapy for chronic inflammatory conditions such as vitiligo, psoriasis, atopic dermatitis, and acne [139]. Results from randomized control trials (RCTs) with vitamin E in the context of cancer have been communicated [140,141,142,143,144,145,146,147,148]. They follow different methodologies, including administration of vitamin E alone or combined with other treatments, and so, this lack of uniformity hinders the understanding of true benefits of vitamin E in cancer therapeutics. Larger and prospective consensually designed RCTs with pure isoforms of vitamin E are urgently required to clear the way [149]. Overall, this panoply of bioactives extracted from cultivated olive leaves –polar aromatic ring compounds such as OLE and HT, lipophilic polyphenolic compounds with a hydrocarbon side chain, and a phenolic aromatic ring such as tocopherols and fatty components such as OA—makes olive leaf a highly prospective anticarcinogenic synergistic force [150].
3.2. Cardioprotective Hallmarks of Olive Leaf: Antioxidant and Anti-Inflammatory Resource
Innumerous reports demonstrated benefits of OLE in CVD, such as blood and renal hypertension, heart failure, myocardial infarction, and diabetes [1,104,151,152,153,154,155,156,157]. Furthermore, several studies [153,158,159,160] identified a decrease in TG and plasmatic protein levels, with an overall correction of the lipid profile and blood pressure (BP). Relative to others, olive leaf infusion showed a higher antioxidant power against plasma oxidation [30,161], supporting evidence of the benefits alleged by Mediterranean folk medicine. Separate mechanisms appear involved: (1) management of glucose metabolism in humans and control of starch breakdown and absorption in animal models [162], (2) enhancement of hemoglobin and hematocrit counting [163], and (3) lipid-lowering action upon the concentrations of TG, LDL-C, and fasting plasma glucose [163]. Studies showed, compared to statin trials, that total cholesterol (TC) and LDL-C reductions by the administration of olive leaf infusion represent an all-around cut in CVD risk of 4.2% and 9.75%, respectively [164]. Also, OLE showed favorably modified BP beside plasma lipid profiles in a dose-dependent way, acting as an overall risk reductant of cardiovascular factors (BP, TC, LDL-C, and TG) [1,164]. For all this evidence, and similar to other extracts such as quince leaf [165], OLE has been proposed as a latent, prime quality phytochemical complement/alternative to the use of statins, currently prescribed in the treatment of hypercholesteremia and known for their risks of painful chronic myopathy [165,166,167]. In further detail, previous observational studies with OLE intake suggested that 2 mmHg drops in systolic blood pressure (SBP) and diastolic blood pressure (DBP) are associated with (1) 6% of declined risk in coronary heart disease (CHD); (2) 10% and 15% cutback in stroke and heart attack, respectively; (3) 9% and 14% saving in CHD risk; and (4) 20% and 22.5% fall in the risk of stroke and heart attack, respectively [164]. An OLEP dosage of 136 mg/day, compared to 200 mg/day, administrated to prehypertensive monozygotic twins confirmed (1) a mean reduction in SBP and DBP of 13 and 5 mmHg, respectively, and (2) a mean reduction, like that of Captopril®, of 12 and 5 mmHg respectively, in hypertensive patients [1,56]. Currently, physicians combine, for example, a drug to inhibit renin directly, and another to block the conversion of angiotensin I (AI) to angiotensin II and to block the main downstream targets of angiotensin II. Despite the dual treatment benefits, side harmful effects burdened the health of treated individuals: hyperkalemia, symptomatic hypotension, and a decline in estimated glomerular filtration rate [167,168]. Unconventional prophylactic solutions such as the pharmacological application of OLE, could be a response to alleviate the side effects of classic prescriptions, and recent studies identified candidates such as O. europaea Madural leaf extract from Mirandela grove, with best inhibition of ACE and renin, and so it is a potential dual activity BP modulator [52]. Besides blood pressure modulation, OLEP showed other cardiovascular benefits such as endothelial protection of polyphenols through L-type calcium channel regulation, nitric oxide (NO) production, reduction of ROS lipoprotein oxidation; as well, oleocanthal and oleacein stimulate the anti-inflammatory gene CD163 expression (Figure 12) [18,106].
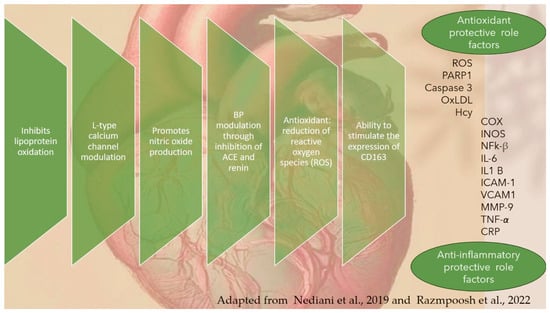
Figure 12.
Cardioprotective effects of olive leaf polyphenols and unsaturated fatty acids, including involved in their modulation, according to literature (adapted from Refs. [1,106]).
3.3. Metabolic Health Regulation Properties
Nonalcoholic steatohepatitis (NASH) is an aggressive form of nonalcoholic fatty liver disease (NAFLD), a precursor to hepatic fibrosis, cirrhosis, and portal hypertension, and finally to primary liver cancer. A therapeutic approach with PUFAs and vitamin E reinforcement of insulin sensitizers with a focus on pioglitazone and statins has been hypothesized. On one hand, supporting evidence reports that α-T, a well-known antioxidant, with a main role against lipid peroxidation in NASH pathogenesis, has achieved good results when combined with ascorbic acid [58]. PUFAs, either alone or associated with other supplements, also might play a key role in treating metabolic conditions. Studies so far indicate that PUFAs along with MUFAs have profits in CVD: (1) SBP and DBP diminution, where MUFAs act more favorably upon nocturnal SBP; (2) high LDL-C and TG level correction; and (3) induced higher energy expenditure, especially by MUFAs [168]. Overall, PUFAs seem to be a better candidate for fighting liver steatosis, especially in children [169]. On the other hand, previous literature also showed that (1) omega-3 supplementation brings down some types of blood fat while increasing others, such as LDL-C, which is not beneficial to CVD [170,171]; (2) higher PUFA intake slightly reduces the risk of CVD associated events [155,172]; and (3) there is a rather reduced risk of CHD/stroke but little or no impact upon all-cause CVD mortality, possibly acting via TG reduction [151]. So, we still lack cohesive evidence about the total benefits of nutritional supplementation compared to no additional interventions; drugs can act upon different pathways, targeting either insulin resistance (IR) such as tiazolidinediones (TZDs) and metformin, or insulin secretion like sulfonylureas, glucagon-like peptide 1 (GLP-1) agonists, and dipeptidyl peptide-4 (DPP-4) inhibitors [173]. IR-targeting drugs also facilitate fat redistribution from liver and skeletal muscle to adipocytes [101,133] including metformin, the most prescribed drug for diabetes [169,174,175,176,177]. The ability of OLE to maintain euglycemia may be factual and dose-related, although, a study using 264 mg OLEP had no impact on elevated insulin and no significant postprandial glucose levels, on all clinical outcomes for patients with primary NAFLD [178]. Further testing OLE with regards to (1) PUFA amount, (2) omega-6/omega-3 ratio, (3) total α-T composition, and (4) and total phenolic content (TPC) as a potential supplement/complement for treating metabolic conditions, such as NAFLD, should be part of the next prospective steps because these four features of OLE may help to mimic statins or pioglitazone action without their adverse side effects [179,180,181,182,183,184,185]. According to the World Health Organization (WHO), the number of diagnosed diabetics rose over 400% within the last 20 years, with a 5% surging in premature mortality for diabetes between 2000 and 2016. The world occurrence of diabetes is forecasted to about 700 million cases by 2045, which, associated with a fast-growing prevalence of metabolic disease, translates to serious economic and public health concerns [186]. Contemporary with type 2 diabetes mellitus (T2DM) decline [187], in agreement with Kerimi et al., 2019 [188], a significant postprandial glucose reduction by a 160 mg dosage of OLEP with a sucrose load was identified. Furthermore, the olive leaf was previously demonstrated to be a potential insulin secretagogue suitable for hyperglycemic individuals with pancreatic β-cell dysfunction [159,188]. One of the mechanisms thought to be behind the protective effect of phenolic compounds concerning T2DM is that OLE may increase glucagon-like-peptide-1 (GLP-1) secretion under both in vivo and in vitro conditions. Also, a systematic review concluded that OLE significantly increased insulin levels (4.83 μIU/mL) and decreased blood glucose levels (4.21 mg/dL) in diabetic rats [173,189,190], exhibiting a similar effect to the GLP-1 agonists that target the insulin secretagogue ability by β-cells. This effect benefits the risk reduction of glucotoxicity but does not prevent disease progression. Other studies have highlighted more mechanisms of olive tree polyphenols acting upon glucose regulation through the inhibition of enzymes and glucose transportation instead of insulin secretagogue agents. Kerimi et al., 2019 concluded that OLE did not affect postprandial glucose derived from bread intake but attenuated postprandial blood glucose after consumption of 25 g sucrose [188]. Previously, de Bock et al., 2013 had reported that olive leaf polyphenols improved both insulin action and secretion, comparable to classic drug prescription for diabetes [50,178]. Impressively, recent studies show that OLEs extracted from cultivars richer in TPC improve both insulin sensitivity and pancreatic β-cell secretory capacity, translating an equivalent clinical meaning to metformin therapeutics. Also, considering OLE, as a potential source of omega-9, OA may contribute to reducing the risk of thrombotic disease [50,191], whereas molecules such as HT and derivates [16], as well as maslinic acid, aid platelet functional regulation [50,191]. Overall, evidence shows OLE able to protect the heart and blood vessels from plaques and ischemic injuries [50], despite SFA content. Mostly, this protective effect is attributed to a considerate synergic presence of omega-3 (ALA), omega-9, vitamin E, and potent bioactives such as polyphenols, particularly HT. HT has been shown to exert a protective anti-atherogenic function, not only through antioxidant molecular mechanisms such as free radical (O2−, OH∙, ONOOH) scavenging, Fe chelating, and inducing of antioxidant enzymes like catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and transcription factor Nrf2, but also through anti-inflammatory mechanisms from which we can underline (1) the reduction of tumor necrosis factor-alpha (TNF-α), (2) chemokines like C-C motif chemokine ligand 2 (CCL2) or C-X-C motif chemokine ligand 10 (CXCL10), (3) reduction of adhesion molecules by the endothelium, (4) inhibition of COX-2 activity, (5) reduction of inducible nitric oxide synthase (iNOS) activity and nitric oxide (NO) production, and (6) modulation of NF-κB signaling [2,107,128,158]. HT can also be obtained from TY in the liver, through the enzymatic activity of cytochrome P450 (CYP), CYP2A6 and CYP2D6 [128,158]. A study in a murine model with OA showed that it reduced platelet aggregation, granule release, and calcium mobilization, probably through the Syk-PLCγ2 and CaMKKβ/AMPKα/VASP pathways [191]. The synergic action of OA with polyphenols (Figure 13) can be beneficial against detrimental metabolic conditions; OA improves insulin sensitivity by lowering ER stress, leukotriene B4 levels, IL-6, TNF-α but eliciting IL-10 and adiponectin [192].
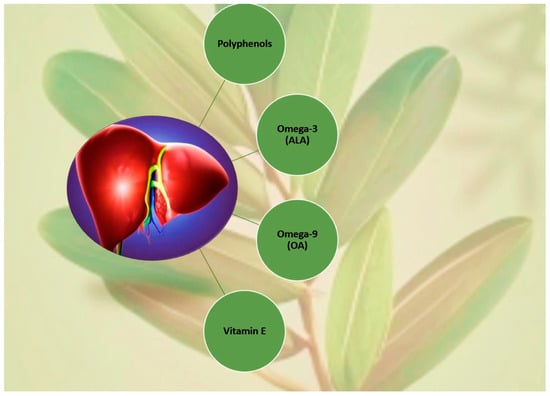
Figure 13.
Synergy from the OLE compounds generally involved in metabolic status (adapted and simplified from Refs. [50,52]). Image credits: crayon.com.
The olive leaf content in omega-3 can also contribute to better insulin signaling through reduction in lipid and ROS buildup by reduced ER stress and improved mitochondrial FA β-oxidation [192]. There is no doubt that OLE, as a nutraceutical within a diverse, complete, and balanced diet not only improves cardiovascular and endothelial function, but is also an aid to regulate metabolic status.
3.4. Neuroprotective Aspects
Aqueous polyphenols such as HT can have a beneficial impact on neuronal diseases such as Parkinson Disease (PD) by reducing toxic dopamine oxidation products like 5-S-cysteinyl-dopamine [193,194]. Oleuropein aglycones, derived from OLEP, have shown in vitro and animal in vivo anti-amyloid mechanisms related to mTOR pathway inhibition by the p70S6 protein kinase phosphorylation decrease [106], and increased expression of NAD-dependent deacetylase sirtuin-1 (SIRT-1) responsible for deacetylation of p53, NF-κB, and FOXO, by transferring the acetyl group to NAD+ and by attenuating phospho-NF-κB and phospho-p53 [106]. It is acknowledged that PUFAs supplementation (1) may improve the attention and behavior issues associated with attention deficit hyperactivity disorder (ADHD), (2) might play a possible role associated with mainstream therapy in managing dry-eye symptoms and signs, although the evidence is uncertain and inconsistent [195], (3) has been linked to a decreased risk of multiple sclerosis (MS) [179,188], (4) might assist in weight and appetite regulation as well as in the resolution of inflammation, relevant in anorexia nervosa [52], and (5) might help to balance mood disorders by regulating membrane fluidity and by modulating synaptogenesis, neurotrophic factor expression, neurogenesis, and neurotransmission [52]. Overall, low omega-3 levels are a feature associated with central nervous system-linked disorders such as poor cognition, depression, anxiety disorders, poor anger control, ADHD, and accelerated neurodegeneration in elderly individuals, for which OLE prove to be a valuable vegetable source for efficacy and bioavailability studies regarding neurobiological-based ailments [196]. Low levels of docosahexaenoic acid (DHA), an omega-3 derived form of ALA, is associated with poor cognitive and learning performance. DHA has shown to aid better neurofunction and survival by inhibiting neuronal apoptosis through translocation and activation of Raf and Akt, as well as DHA-derived resolvin (Rv) D1, neuroprotectin D1, and maresin-1 deliver neuroprotective and anti-inflammatory effects against cognitive impairment and dementia [197]. Olive leaf cultivars, whose properties include high content of PUFAs plus α-T to preserve the PUFAs structure, might be of interest for a prolonged effect on treating diseases related to low omega-3 bioavailability, namely, developmental and mood or emotional disorders [198,199], especially the recently identified cultivar O. europaea Madural from the Mirandela grove [50]. Moreover, the current prescription for resistant depressive/manic symptomatology of bipolar disorder (BD) includes mood stabilizers, antipsychotics, and antidepressants, or their combination, which have been associated with increased levels of TNFα and IL-6 shown to be controlled through omega-3 supplementation, among others [199,200]. On the other hand, low levels of omega-9 have been associated with major depressive disorders and Alzheimer’s disease, which is worrying, since OA is a great component of membrane phospholipids in the brain, and has a remarkable myelin content. OA neuroprotective action has been associated with the reductive power of ROS, IL-8, IL-6, and TNFα in the HIF-1α deacetylate pathway in PD and with an increase of monoamino release, dendrites, and axon development in the PPARγ pathway of hypothalamus neurons [132].
3.5. Immunomodulatory Activity
OLE, as a precious source of α-T, shows intriguing properties to address immunological conditions. Vitamin E supplementation is supported as a worthy treatment, as it reduces inflammation associated with asthma and allergic conditions by adding omega-3 and dwindling omega-6 FAs. Also, vitamin E regulates vascular cell adhesion patch-1-dependent leukocyte migration through its oxidant and nonantioxidant properties [201]. OLE and its phytoconstituents have demonstrated immunomodulatory activity by reducing the expression of proinflammatory intercessors (IL-1β, IL-6, IL-8, TNF-α, and iNOS, Figure 14) [58].
Figure 14.
Summary of the immunomodulatory capacity of OLE (adapted from Refs. [50,58]).
This immunomodulatory influence of OLE (0.1–100 μg/mL) was studied in different cell types and in ex vivo culture organs of mucosal explants from both healthy individuals and Crohn’s disease cases [202,203]. Immunomodulation has been often linked to phenolic action rather than that of flavonoids; however, cultivars with high reducing power, such as O. europaea Madural cultivated in Mirandela grove, seem to owe it to its total flavonoid content [50]. Thus, enzymatic and biochemical profiling of as many cultivars as possible would be very profitable to understand the key behind the immune protective and anti-carcinogenic effects of olive leaf extract.
3.6. Antimicrobial Power of Olive Leaf Compounds
Antimicrobial properties of olive leaf include a wide antibacterial scope with potent in vitro activity against several strains of bacteria responsible for intestinal and respiratory infections [204]. So far, the antimicrobial power of the OLE seems to be the result of the synergy of the phenolic antioxidant power in combination with the work of fatty acid (Figure 15).
Figure 15.
Synergy of the main OLE compounds that have been shown to contribute to antimicrobial power (adapted and simplified from Refs. [14,44,46,50,205,206,207,208,209,210,211,212,213]). Credits: crayon.com.
There are not many studies regarding the antimicrobial effect of olive leaves, and most of them focus on the relation with the total phenolic content (TPC), including total flavonoid content (TFC) [14,44,46,50,205,206,207,208,209,210,211,212,213], and less on the antimicrobial action of other compounds such as fatty acids [50,52]. Individual phenolic compounds show high antimicrobial application against pathogenic bacteria, with a synergistic effect and dose-dependent pro-antioxidant labor [58,214,215]. Their antibacterial action shows them as promising preservatives concerning the food business [214,215,216]. They add importance for an ability to reduce the growth of, among other bacteria, the Helicobacter pylori, an epidemiologic burden associated with peptic ulcers and gastric cancer [67,213]. It is identified that OLE polyphenols also stimulate the growth and metabolism of probiotic bacteria, particularly B. infantis and L. acidophilus [215,217]. Hence, the incorporation of OLE into the human diet might bring desirable factors upon the intestinal microflora. According to Marhamatizadeh et al., 2013, milk and yogurt set off with L. acidophilus and B. bifidum in OLE significantly lowered the pH compared to the control, suggesting a considerable outcome of lactic acid [218,219]. The authors attributed this pH reduction to the phenolic composites of OLE, which are known to serve as oxygen scavengers, and hence, are able to promote probiotic bacterial growth. In this work, some recent literature regarding the antioxidant power of phenolic compounds of olive leaves has been discussed, and some of those authors also studied a possible relation between the phenolic constitution and the antimicrobial action of those compounds. De Oliveira et al., 2024 found that hydroethanolic leaf extracts of an olive grove from Mirandela had the ability to inhibit bacterial growth of Gram (−) and Gram (+) bacteria. Both O. europaea Madural and Verdeal showed efficacy against the Gram (−) P. aeruginosa (ATCC 10145) and the Gram (+) B. cereus (ATCC 14579), with Verdeal cultivar also acting against Gram (+) S. aureus (ATCC 25923), besides B. cereus (ATCC 14579) [50]. Meanwhile, O. europaea Cobrançosa was more efficient against Gram (−) S. Enteritis. Both cultivars Cobrançosa and Madural showed a similar phenolic profile, with a predominance of HT, flavonoids, and verbascoside. This phenolic composition associated with a considerable amount of MUFAs may be behind their efficiency against Gram (−) microbiota, but further studies are needed to test this possibility [50]. Magyari-Pavel et al., 2024 also found a relation between the antioxidant capacity of both extracts of Sevilla’s Picual and Lefkanda’s Koroneiki and their antimicrobial effect, namely against Gram (+) bacteria like Streptococcus pyogenes, attributing this efficacy to the antioxidant power of metallocomplexes [58]. Considering the inorganic composition of the Portuguese Madural and Verdeal cultivars and their ability to successfully inhibit some Gram (+) strains [50], it would be also interesting to understand if antioxidant metalphenolic complexes might be involved in this mechanism. Šimat et al., 2022 found that despite a marked antioxidant capacity by Italian Moraiolo, Frantoio, Nostrana di Brisighella from Emilia-Romagna and Croatian Lastovka, Levantinka and Oblica, they showed no activity against Gram (−) E. coli and S. typhimurium. Lastovka, Oblica, and Nostrana di Brisighella cultivars presented weak activity against Gram (+) S. aureus, B. cereus, and Listeria innocua [59]. Even so, inhibitory action was found against Gram (−) Campilobacter jejuni by all cultivars, especially Croatian Oblica. The interest in olive derivates phytochemicals as therapeutic answers has grown, especially in cultivars notable for their high content in secoiridoids, particularly in OLEP, known to be involved in strong antimicrobial action [59]. On the other hand, the antimicrobial activity of cultivars such as Lastokvka, richer in flavonoids, might be related also to the possibility of antioxidant power of metallocomplexes derived of flavonol origin; however, more studies are needed to test this possibility. With respect to their antiviral virtues, OLE polyphenols have displayed (1) in vitro antiviral function [96] against HIV-1 [220] and influenza virus [221]; (2) in vivo recovery from labialis herpes simplex virus and genital herpes [222,223]. OLEs have also displayed antiprotozoal performance, including both anti-trypanossomal and antileishmanial action [224], probably due to surfactant action reported for these medium-chain tyrosyl FA esters [225,226]. OLE was used in some countries where SARS-CoV-2 infection was widespread. The hyperinflammation due to the associated cytokine storm, especially IL-6, carried out in acute respiratory suffering syndrome is appointed as a main reason for most of the deaths from SARS-CoV-2 [227,228]. In addition, one of the most dangerous complications of this syndrome is related to an increased rate of thrombotic cases [227]. So far, to the best of our knowledge, there is still no successful broad-spectrum antiviral countermeasures to fight SARS-CoV-2 or potential CoV pandemics, and so, redirecting interest in their affordability and minimal toxicity [229]. Several OLE metabolites have been tested in silico as viral targets against SARS-CoV-2 where verbascoside, maslinic acid, rutin and epicatechin achieved higher results [220]. Moreover, HT and HT-rich olive pulp extract have shown a strong ability to evoke structural changes in SARS-CoV-2 spike proteins and even to disrupt the viral genome, being considered an option for hand and surface hygiene, therefore, posing the potential and so desired phytochemical answer to the lack of response from the classic pharmaceutical route [230]. Moreover, a recent study suggests that OLE downregulates the expression of certain proteins related to the SARS-CoV-2 mechanism in a dose-dependent manner [231]. It is known that phenols have their antimicrobial activity through peroxidation inhibition and free radical scavenging activity, and so extraction with solvents that potentiate the acquisition of these compounds such as ethanol and ethyl acetate is recommended, instead of only the aqueous fraction, which has lower reduced power [232]. Khan et al., 2020 concluded that besides antibacterial action, both ethanolic and ethyl acetate extracts from a wild native variety showed antifungal power against Aspergillus parasiticus, Fusarium solani, and Candida glaberata [233]. A notable recent study brings to the table a possibility that young, wild olive genotypes derived from wild olive trees growing in natural locations along the Adriatic coast, might display a greater ability to withstand salt and drought stress than cultivated olive trees, and so appear more adequate an olive genotype for a breeding program adapted to a changing climate [234]. Moreover, another recent study about the phytochemical composition of a wild olive tree variety from the Tarsus district in Turkey, O. europaea L. subs. Oleaster, concluded that this plant is high in phenolic content, namely in flavonoids, and so shows antioxidant activity through ABTS and CUPRAC tests, as well as exhibiting metal chelation capacity [235]. It is known that the success of an extraction depends on the kind of solvent and its ratio, pH, temperature, and the time gap of the extractive process. Opting for new extraction methods that avoid phenolic loss, such as ultrasonic solvent extraction and microwave-assisted extraction, besides MeOH/water (4:1, v/v), supercritical CO2 extraction, seems to be also effective [235]. According to Gonçalves et al., 2024, OLE phenolic composites drop their bioavailability after digestion [236] and so, the antibacterial and antioxidant power of OLE are compromised after gastrointestinal digestion because an incomplete intestinal absorption allied to a quick elimination through urinary excretion [237]. For that, biological potential of OLEP as a dietary supplement or olive leaf extract or infusion should be protected by the use of microencapsulation techniques [238].
3.7. Clinical Value of OLE in the Therapeutics of Polycystic Ovary Syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in reproductive-aged women [239] and implicates endocrine and metabolic issues mirroring high comorbidity in addition to hormonal, fertility, and emotional imbalances [240]. Heterogeneous clinical conditions require that patients endure long-term treatments ranging from lifestyle modifications to precise medical or surgical methods [241]. One well-known etiologic factor of PCOS is oxidative stress, which increases free radical levels, causing elevated lipid peroxidation, dyslipidemia, and IR, usually present in this clinical pattern [242]. A retrospective study reported that PCOS women have higher TG, lower HDL-C, and higher non-HDL-C levels relative to healthy women [243]. Lifestyle measures, including diet modification, show positive effects on CVD, CHD, obesity, hepatic steatosis, and other metabolic comorbidities in women with PCOS [244,245]. In this context, it has been reported that replacing SFAs with PUFAs can reduce IR and enhance the lipid profile with positive effects on PCOS-related issues: (1) overweight regulation [246], (2) improved glycemic, hormonal, inflammation and adipokine production status, ref. [245] and (3) improved pregnancy rate in inducted-ovulation PCOS women [247]. A recent study assessed the effects of FA pattern intake on lipid profile and fatty liver severity, where a 10-week (about two-and-a-half month) intervention including 72 PCOS women who received 25 g/day canola oil (CA), olive, or sunflower oils disclosed that, compared to olive and sunflower oils, CA ingestion induced significant corrections in the lipid profile (Table 3, Figure 16), liver function, and homeostasis model evaluation for the insulin resistance index (HOMA-IR) [248]. These benefits are accredited to its elevated concentration of omega-3 and lower ratio of n-6/n-3 PUFAs. The literature reports that elevated consumption of omega-3 improves insulin sensitivity and lipoprotein lipase activity with serum TG reduction, which could justify the significant TG reduction in the patients treated with CA compared to the group treated with olive oil. Nevertheless, the effect of various oils on blood lipids is a controversial research area, and the literature also reports that higher daily PUFA ingestion could not decrease serum TG and HDL-C despite reducing LDL-C and TC [249].

Table 3.
Comparison of the total amount of FAs (%) and ratio (omega-6/omega-3) in samples of different vegetable oils and OLEs of several cultivars reported by recent studies.
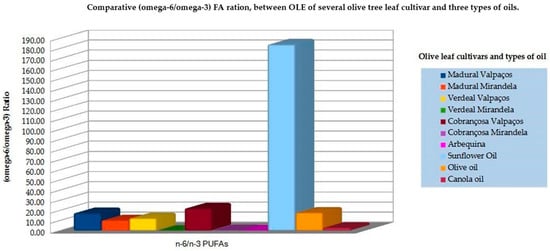
Figure 16.
Bar graph of the comparative (omega-6/omega-3) FA ratio distribution between OLE of several olive tree cultivars and three types of vegetable oil (adapted from Refs. [50,51,52,248]).
On the other hand, Yahay et al., 2021 reported that olive oil ingestion led to a significant decline in fatty liver severity without effects on the lipid profile, contradicting some previous literature [248]. Venturini et al., 2015 found that 10 mL/day of EVOO combined with 3 g/day of fish oil reduced serum levels of TC, TC/HDL-C, and LDL-C/HDL-C ratios [250]. Violante et al., 2009 found that the intake of 4 g/day of EVOO by mildly hypocholesterolemic participants induced favorable modifications in the plasma lipid profile [251]. Finally, a meta-analysis by Ghobadi et al., 2019 demonstrated that the reduction in TC by olive oil was significantly lower compared to oils richer in omega-3 [249]. This discrepancy may be explained by the type of olive oil used in different studies, but from the literature, it seems to be clear that a high concentration of omega-3 and a lower (n-6/n-3) ratio is important to modulate lipid profile [182,195]. The ratio of n-6 to n-3 PUFAs is known to influence the inflammatory state in PCOS women, with the literature illustrating that higher omega-6 PUFA intake aggravates CVD risk, which is independently associated with elevated serum TC and TG levels. As seen in Table 3 the OLE composition of FAs presents a higher presence of PUFAs, especially ALA [52] and lower (n-6/n-3) FAs, compared with different types of oils. Therefore, it would be interesting to pursue future studies with OLE, namely, from the Verdeal’s Sample cultivar, to understand the possible aid of its supplementation to manage lipidic and metabolic dysfunction in women with PCOS. Besides the clinical trials with cancer patients [252] and others [222,253], only a few studies have been done about the benefits of OLE on the CVD markers [153,254], and without significant conclusions, except for the potential to downgrade inflammatory biomarkers [158], as well as lipidic values in adults with prediabetes [156,255]. This urges the design of optimized microenpasulated extracts with stable shelf storage and able to address pro-inflammatory conditions with as few collateral effects as possible.
4. Potential of Olive Leaves in Pharmaceuticals, the Food Industry, and Cosmetics
It is known that α-T is a safe food additive [256], and thus, the relative content of this metabolite in OLE makes the extract an affordable option for use in the food industry. It is also known that to evaluate individual vitamin E status, the analytical procedures used for detecting and quantifying vitamins and their metabolites are of utmost importance [257], so it is crucial to understand the different metabolic pathways in which vitamin E is involved. In particular, the low bioavailability of tocotrienols remains a serious limitation for clinical applications of vitamin E. Nano formulations such as nanovesicles, solid–lipid nanoparticles, nanostructured lipid carriers, nano emulsions, and polymeric nanoparticles have shown promising outcomes in improving their efficacy and bioavailability [258]. Regarding protein transport of vitamin E, α-T transfer protein (α-TTP), relative to other transport proteins, had the highest affinities for all isomers except β-tocotrienols (β-T3). Improving the bioavailability of the adequate isomers may enhance their beneficial effects to human gain [259], namely, for cosmetic products [259]. Overall, the content of vitamin E in O. europaea L. folium might be an indicator that this substrate is a potential low-cost solution to produce food preservatives, cosmetic products, and supplementation, either pure or reinforced with OA and/or polyphenols, depending on the therapeutic target. Leaves from cultivars endowed with a higher absolute content of α-T would be more indicated for a nutraceutical approach to treat ailments such as (1) allergies; (2) bone mass reduction; and (3) inflammatory skin diseases, such as vitiligo, psoriasis, atopic dermatitis, and acne. Leaves with less water content, such as those from the Verdeal’s cultivar, might be suggested as a research substrate of vitamin E for food preservation. Other cultivars rich in α-T and TPC appear to be valuable substrates because of their nutraceutical properties against metabolic and chronic inflammatory diseases and anti-aging treatments. In this context, nanoemulsion encapsulation of E vitamers with other therapeutic agents, such as vitamins C and A and other antioxidants, acting upon a synergetic anti-inflammatory capacity, is a possible route to efficient supplement manufacture.
5. Conclusions and Future Perspectives
The main conclusion of this overview is that olive leaves offer a myriad of therapeutic benefits, and that each cultivar can be regarded as a specific therapeutic target to address totally diverse conditions according to its own organic and inorganic architecture, comparable to a rainbow in the sky of natural pharmacy. For example, the inorganic profile from different cultivars shows potential for O. europaea Madural’s leaves from Mirandela to address hypocalcemia and hypomagnesemia; Verdeal’s leaf sprouts from Valpaços to address hypocupremia, and Verdeal’s leaves from Mirandela grove to address Mn deficiency, if the As element or other toxic element is properly removed and controlled; O. europaea Cobrançosa’s leaves, also from Mirandela olive grove, to address hypocupremia and sideropenia. On other hand, the particular inorganic combination of micro- and macrominerals of each cultivar potentiates the antioxidant power of olive leaves by the metal coordination of certain phenolic compounds, namely flavonoids. Given these phenolic and mineral properties, olive leaves from different cultivars, including from wild genotypes, can be used as extracts to target: (1) hematologic disorders, including thrombotic issues, (2) cardiovascular aspects such hypercholesteremia and hypertension, (3) glycemic status regulation, (4) possible synergy with other anticancer treatments, (5) improvement of neuroinflammation and anti-aging processes, (6) antipathogenic efficiency, and (7) promotion of healthy gut flora. However, the phenolic metabolism and absorption in the human body requires the development of extraction and encapsulation techniques adapted to a design that optimizes blood-barrier absorption, as well as the erythrocyte membrane translocation, and more studies regarding that aspect are urgent. Also, there is no information about the long-term toxicity of OLEP in the human body, so more studies are needed to address a secure storage system. Storing the extract at 4 °C and −20 °C [260], helps to slow the polyphenol loss; as well, the liquid extract seems to have better performance at preserving TPC, mainly of verbascoside and luteolin-7-O-glucoside, compared to powder extract [261]. Besides phenolic content, some cultivars derive an OLE with a predominance of α-T allied to essential PUFAs, particularly the Verdeal’s cultivar identified in a grove of the Valpaços region [52], which has potential to address (1) bone and cardiovascular health, (2) hydration for dry skin and eye issues, (3) control of symptomatology in neurological conditions such as MS, ADHD, and neurodegenerative disorders, as well as in behavioral and mood disorders, and (4) allergy management. On the other hand, the presence of MUFAs such as oleic acid plus powerful antioxidants like polyphenols [50], found in the Portuguese cultivars from Mirandela, opens a proxy also with a potential for prophylaxis against atheroma formation and ischemic injuries. Attending these results and other literature regarding the benefits of OLE over platelet function [191,261], we are designing a new study to understand better the potential of selected national cultivars regarding this important feature for CDV health. It must be considered that OLE polyphenol efficacy is dependent on its bioavailability, and so new approaches for extract optimization [236], including metabolome studies [262,263], are currently being developed to identify the most suitable cultivar by geographic location also according to the prediction of bioclimatic changes. The phenolic plus flavonoid action of MUFAs identified in certain cultivars seem linked to antimicrobial power, in particular, of O. europaea Verdeal’s leaf extract against Gram (+) S. aureus; O. europaea Madural’s leaves against the Gram-negative P. aeruginosa; and O. europaea Cobrançosa against Salmonella enteritidis. It would be interesting to understand if these cultivars, or others with similar composition, could be of benefit in the treatment of periodontitis, due to the large health cost this condition brings to national health systems [264]. In addition, it has shown great potential as a natural candidate to control SARS-CoV-2 symptoms, mainly when related to exacerbated inflammatory processes [34,101,230,265,266]. Moreover, given the content of omega-9, OLE could be a research target to enhance vascular disease symptoms. Also, for the ability to inhibit both AChE, BuChE, and MAO-A, O. europaea Madural’s from Mirandela seems a natural multitarget treatment for Alzheimer’s disease, Parkinson’s disease, and depression. In the near future, it would be interesting to understand better the molecular mechanisms behind the benefits of OLE on inflammatory, neuro-immune, and cardiovascular physiology, and so identify the most adequate inorganic and organic profiles of as many cultivars as possible. This would allow the formulation of natural supplements based on local olive leaf extracts that could provide (1) an economic and environmentally sustainable solution free of the side effects, (2) careful and knowledgeable tailoring as a complement or treatment of symptoms associated with costly and highly prevalent chronic diseases, such as inflammatory and metabolic-related syndromes, including PCOS and neurodegenerative conditions such as PD and AD. Moreover, it would be beneficial to invest in further knowledge of types of cultivation as well as how the season–type of cultivar–soil interaction can influence and optimize the extraction of high-quality custom-made nutraceuticals while still respecting a sustainable and environmentally friendly economy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applbiosci3030026/s1, Table S1: Resume of the type of cultivar, month and year of leaf harvest, as wells as of the sites of cultivation, and the kind of solved used for extraction in the studies mentioned analyzed in this work [14,19,49,50,58,59,60,61,62,63].
Author Contributions
Conceptualization, N.M.d.O.; methodology, N.M.d.O.; software, N.M.d.O.; formal analysis, N.M.d.O., M.B.C.; investigation, N.M.d.O.; resources, N.M.d.O., J.M., L.L.; data curation, N.M.d.O., M.H.C.; writing—original draft preparation, N.M.d.O., J.M.; writing—review and editing, M.B.C., N.M.d.O.; supervision, N.M.d.O.; project administration, M.B.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no funding for this research.
Acknowledgments
The authors are grateful to the Biomedical Science Institute Abel Salazar, Porto (ICBAS-UP), for welcoming and supporting scientific knowledge regarding the therapeutic potential of local flora and its application in the pharmaceutical and health industries.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Razmpoosh, E.; Abdollahi, S.; Mousavirad, M.; Clark, C.C.T.; Soltani, S. The effects of olive leaf extract on cardiovascular risk factors in the general adult population: A systematic review and meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2022, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Vijakumaran, U.; Shanmugam, J.; Heng, J.W.; Azman, S.S.; Yazid, M.D.; Abdullah, N.A.H.; Sulaiman, N. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules 2023, 28, 1861. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Macciola, V.; Iftikhar, A. Present and Future Perspectives on the Use of Olive-Oil Mill Wastewater in Food Applications. In Wastewater from Olive Oil Production: Environmental Impacts, Treatment and Valorisation, 1st ed.; Souabi, S., Anouzla, A., Eds.; Springer: Cham, Switzerland, 2023; pp. 85–105. [Google Scholar]
- Barazani, O.; Dag, A.; Dunseth, Z. The history of olive cultivation in the southern Levant. Front. Plant Sci. 2023, 14, 1131557. [Google Scholar] [CrossRef]
- Medda, S.; Fadda, A.; Mulas, M. Influence of Climate Change on Metabolism and Biological Characteristics in Perennial Woody Fruit Crops in the Mediterranean Environment. Horticulturae 2022, 8, 273. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bartolini, G.; Ganino, T.; Zelasco, S.; Lombardo, L.; Perri, E.; Durante, M.; Bernardi, R. Cold Stress, Freezing Adaptation, Varietal Susceptibility of Olea europaea L.: A Review. Plants 2022, 11, 1367. [Google Scholar] [CrossRef]
- Langgut, D.; Cheddadi, R.; Carrión, J.S.; Cavanagh, M.; Colombaroli, D.; Eastwood, W.J.; Greenberg, R.; Litt, T.; Mercuri, A.M.; Miebach, A.; et al. The origin and spread of olive cultivation in the Mediterranean Basin: The fossil pollen evidence. Holocene 2019, 29, 902–922. [Google Scholar] [CrossRef]
- Diez, C.M.; Trujilo, I.; Martinez-Urdiroz, N.; Barranco, D.; Rallo, L.; Marfil, P.; Gaut, B.S. Olive domestication and diversification in the Mediterranean Basin. New Phytol. 2015, 206.1, 436–447. [Google Scholar] [CrossRef]
- Saddoud Debbabi, O.; Rahmani Mnasri, S.; Ben Amar, F.; Ben Naceur, M.; Montemurro, C.; Miazzi, M.M. Applications of Microsatellite Markers for the Characterization of Olive Genetic Resources of Tunisia. Genes 2021, 12, 286. [Google Scholar] [CrossRef]
- Passeri, V.; Sammut, C.; Mifsud, D.; Domesi, A.; Stanzione, V.; Baldoni, L.; Mousavi, S.; Mariotti, R.; Pandolfi, S.; Cinosi, N.; et al. The Ancient Olive Trees (Olea europaea L.) of the Maltese Islands: A Rich and Unexplored Patrimony to Enhance Oliviculture. Plants 2023, 12, 1988. [Google Scholar] [CrossRef]
- Gómez-Gálvez, F.J.; Ninot, A.; Rodríguez, J.C.; Compañ, S.P.; Andreva, J.U.; Rubio, J.A.G.; Aragón, I.P.; Viñuales-Andreu, J.; Casanova-Gascón, J.; Šatović, Z.; et al. New insights in the Spanish gene pool of olive (Olea europaea L.) preserved ex situ and in situ based on high-throughput molecular markers. Front. Plant Sci. 2024, 14, 1267601. [Google Scholar] [CrossRef]
- Khadari, B.; El Bakkali, A.; Essalouh, L.; Tollon, C.; Pinatel, C.; Besnard, G. Cultivated Olive Diversification at Local and Regional Scales: Evidence From the Genetic Characterization of French Genetic Resources. Front. Plant Sci. 2019, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of phytochemical and antioxidant properties of 15 Italian Olea europaea L. cultivar leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Deiana, P.; Santona, M.; Dettori, S.; Culeddu, N.; Dore, A.; Molinu, M.G. Multivariate approach to assess the chemical composition of Italian virgin olive oils as a function of variety and harvest period. Food Chem. 2019, 300, 125243. [Google Scholar] [CrossRef]
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the usage of olive leaves, bioactive compounds, biological activities, and food applications: A comprehensive review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef]
- Ramírez, E.M.; Brenes, M.; Romero, C.; Medina, E. Olive Leaf Processing for Infusion Purposes. Foods 2023, 12, 591. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Ronca, C.L.; Duque-Soto, C.; Samaniego-Sánchez, C.; Morales-Hernández, M.E.; Olalla-Herrera, M.; Lozano-Sánchez, J.; Martínez, R.G. Exploring the Nutritional and Bioactive Potential of Olive Leaf Residues: A Focus on Minerals and Polyphenols in the Context of Spain’s Olive Oil Production. Foods 2024, 13, 1036. [Google Scholar] [CrossRef]
- Bernardi, B.; Falcone, G.; Stillitano, T.; Benalia, S.; Bacenetti, J.; De Luca, A.I. Harvesting system sustainability in Mediterranean olive cultivation: Other principal cultivar. Sci. Total. Environ. 2021, 766, 142508. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertini, S.; Macchia, M.; Digiacomo, M. Enhanced Nutraceutical Properties of Extra Virgin Olive Oil Extract by Olive Leaf Enrichment. Nutrients 2023, 15, 1073. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Parri, S.; Cai, G.; Romi, M.; Cantini, C.; Pinto, D.C.G.A.; Silva, A.M.S.; Dias, M.C.P. Comparative metabolomics of leaves and stems of three Italian olive cultivars under drought stress. Front. Plant Sci. 2024, 15, 1408731. [Google Scholar] [CrossRef] [PubMed]
- Giudice, V.L.; Faraone, I.; Bruno, M.R.; Ponticelli, M.; Labanca, F.; Bisaccia, D.; Massarelli, C.; Milella, L.; Todaro, L. Olive Trees By-Products as Sources of Bioactive and Other Industrially Useful Compounds: A Systematic Review. Molecules 2021, 26, 5081. [Google Scholar] [CrossRef]
- Giacometti, J.; Grubić-Kezele, T. Olive Leaf Polyphenols Attenuate the Clinical Course of Experimental Autoimmune Encephalomyelitis and Provide Neuroprotection by Reducing Oxidative Stress, Regulating Microglia and SIRT1, and Preserving Myelin Integrity. Oxidative Med. Cell. Longev. 2020, 2020, 6125638. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Jones, G.L. From Petri Dish to Patient: Bioavailability Estimation and Mechanism of Action for Antimicrobial and Immunomodulatory Natural Products. Front. Microbiol. 2020, 10, 2470. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Xu, J.; Xue, C.; Mao, Z. Plant-derived compounds for treating autosomal dominant polycystic kidney disease. Front. Nephrol. 2023, 3, 1071441. [Google Scholar] [CrossRef]
- Jung, Y.C.; Kim, H.W.; Min, B.K.; Cho, J.Y.; Son, H.J.; Lee, J.Y.; Kim, J.Y.; Kwon, S.B.; Li, Q.; Lee, H.W. Inhibitory Effect of Olive Leaf Extract on Obesity in High-fat Diet-induced Mice. In Vivo 2019, 33, 707–715. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Navarro-Hortal, M.D.; Quirantes-Piné, R.; Grosso, G.; Giampieri, F.; Lipari, V.; Sánchez-González, C.; Battino, M.; Quiles, J.L. Molecular Mechanisms of the Protective Effects of Olive Leaf Polyphenols against Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4353. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; García, P.; Brenes, M. Characterization of bioactive compounds in commercial olive leaf extracts, and olive leaves and their infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef]
- Topalović, D.; Dekanski, D.; Spremo-Potparević, B.; Pirković, A.; Borozan, S.; Bajić, V.; Stojanović, D.; Giampieri, F.; Gasparrini, M.; Živković, L. Dry olive leaf extract attenuates DNA damage induced by estradiol and diethylstilbestrol in human peripheral blood cells in vitro. Mutat. Res. Toxicol. Environ. Mutagen. 2019, 845, 402993. [Google Scholar] [CrossRef]
- Pang, K.L.; Lumintang, J.N.; Chin, K.Y. Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review. Nutrients 2021, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarem, H.M.; El-Sherif, M.A.; Gomma, S.B.; Kassem, S.S.; Abdelkader, M.M. Olive Leaf Powder Modulate Insulin Production and Circulating Adipokines in Streptozotocin Induced Diabetic Rats. J. Diet. Suppl. 2022, 19, 550–565. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Forbes-Hernández, T.Y.; Varela-López, A.; Puentes, J.G.; Del Pino-García, R.; Sánchez-González, C.; Elio, I.; Battino, M.; García, R.; et al. Exploring the Antioxidant, Neuroprotective, and Anti-Inflammatory Potential of Olive Leaf Extracts from Spain, Portugal, Greece, and Italy. Antioxidants 2023, 12, 1538. [Google Scholar] [CrossRef]
- Losada-Echeberría, M.; Naranjo, G.; Malouche, D.; Taamalli, A.; Barrajón-Catalán, E.; Micol, V. Influence of Drying Temperature and Harvesting Season on Phenolic Content and Antioxidant and Antiproliferative Activities of Olive (Olea europaea) Leaf Extracts. Int. J. Mol. Sci. 2022, 24, 54. [Google Scholar] [CrossRef]
- De Cicco, P.; Ercolano, G.; Tenore, G.C.; Ianaro, A. Olive leaf extract inhibits metastatic melanoma spread through suppression of epithelial to mesenchymal transition. Phytother. Res. 2022, 36, 4002–4013. [Google Scholar] [CrossRef]
- Katsiani, A.; Panailidou, P.; Mathioudakis, M.; Katis, N.; Maliogka, V.I. Identification and complete genome sequencing of a divergent olive virus T isolate and an olive leaf yellowing-associated virus isolate naturally infecting olive trees in Greece. Virus Genes 2022, 58, 560–569. [Google Scholar] [CrossRef]
- Khwaldia, K.; Attour, N.; Matthes, J.; Beck, L.; Schmid, M. Olive byproducts and their bioactive compounds as a valuable source for food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1218–1253. [Google Scholar] [CrossRef]
- Majewski, G.P.; Singh, S.; Bojanowski, K. Olive leaf-derived PPAR agonist complex induces collagen IV synthesis in human skin models. Int. J. Cosmet. Sci. 2021, 43, 662–676. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Ali, H.A.; Mutar, T.F. Protective effects of olive leaf extract against reproductive toxicity of the lead acetate in rats. Environ. Sci. Pollut. Res. 2021, 28, 63102–63110. [Google Scholar] [CrossRef]
- Rishmawi, S.; Haddad, F.; Dokmak, G.; Karaman, R. A Comprehensive Review on the Anti-Cancer Effects of Oleuropein. Life 2022, 12, 1140. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, C.; Liu, L.; Xu, Z.; Chen, T.; Zhou, L.; Yuan, M.; Li, T.; Ding, C. Comparison of Phenolic Compounds in Olive Leaves by Different Drying and Storage Methods. Separations 2021, 8, 156. [Google Scholar] [CrossRef]
- Cör Andrejč, D.; Butinar, B.; Knez, Ž.; Tomažič, K.; Knez Marevci, M. The Effect of Drying Methods and Extraction Techniques on Oleuropein Content in Olive Leaves. Plants 2022, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Khelouf, I.; Karoui, I.J.; Lakoud, A.; Hammami, M.; Abderrabba, M. Comparative chemical composition and antioxidant activity of olive leaves Olea europaea L. of Tunisian and Algerian varieties. Heliyon 2023, 9, e22217. [Google Scholar] [CrossRef] [PubMed]
- Maestri, D.; Barrionuevo, D.; Bodoira, R.; Zafra, A.; Jiménez-López, J.; Alché, J.D.D. Nutritional profile and nutraceutical components of olive (Olea europaea L.) seeds. J. Food Sci. Technol. 2019, 56, 4359–4370. [Google Scholar] [CrossRef] [PubMed]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef]
- Seçmeler, Ö.; Galanakis, C.M. Olive fruit and olive oil. In Innovations in Traditional Foods; Woodhead Publishing: Sawston, UK, 2019; pp. 193–220. [Google Scholar]
- Mir-Cerdà, A.; Granados, M.; Saurina, J.; Sentellas, S. Olive tree leaves as A great source of phenolic compounds: Comprehensive profiling of NADES extracts. Food Chem. 2024, 456, 140042. [Google Scholar] [CrossRef]
- Zakraoui, M.; Hannachi, H.; Pasković, I.; Vidović, N.; Pasković, M.P.; Palčić, I.; Major, N.; Ban, S.G.; Hamrouni, L. Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves. Foods 2023, 12, 2565. [Google Scholar] [CrossRef]
- de Oliveira, N.M.; Machado, J.; Chéu, M.H.; Lopes, L.; Barroso, M.F.; Silva, A.; Sousa, S.; Domingues, V.F.; Grosso, C. Potential Therapeutic Properties of Olea europaea Leaves from Selected Cultivars Based on Their Mineral and Organic Profiles. Pharmaceuticals 2024, 17, 274. [Google Scholar] [CrossRef]
- Cavalheiro, C.V.; Rosso, V.D.; Paulus, E.; Cichoski, A.J.; Wagner, R.; de Menezes, C.R.; Barin, J.S. Chemical composition of olive leaves (Olea europaea L.) from the region of cacapava do Sul, RS, Brazil/Composicao quimica de folhas de oliveira (Olea europaea L.) da regiao de cacapava do Sul, RS. Ciência Rural 2014, 44, 1874–1880. [Google Scholar] [CrossRef]
- Ferreira, D.M.; de Oliveira, N.M.; Chéu, M.H.; Meireles, D.; Lopes, L.; Oliveira, M.B.; Machado, J. Updated Organic Composition and Potential Therapeutic Properties of Different Varieties of Olive Leaves from Olea europaea. Plants 2023, 12, 688. [Google Scholar] [CrossRef]
- Gulisano, M.; Consoli, V.; Sorrenti, V.; Vanella, L. Red Oranges and Olive Leaf Waste-Derived Bioactive Extracts Promote Adipocyte Functionality In Vitro. Nutrients 2024, 16, 1959. [Google Scholar] [CrossRef] [PubMed]
- Chavanelle, V.; Langhi, C.; Michaux, A.; Ripoche, D.; Otero, Y.F.; Le Joubioux, F.; Maugard, T.; Guigas, B.; Giera, M.; Peltier, S.; et al. A novel polyphenol-rich combination of 5 plant extracts prevents high-fat diet-induced body weight gain by regulating intestinal macronutrient absorption in mice. Nutr. Res. 2023, 118, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Abulnaja, K.; Bakkar, A.; Kannan, K.; Al-Manzlawi, A.M.; Kumosani, T.; Qari, M.; Moselhy, S. Olive leaf (Olea europaea L. folium) extract influences liver microsomal detoxifying enzymes in rats orally exposed to 2-amino-l-methyI-6-phenyI-imidazo pyridine (PhIP). Environ. Sci. Pollut. Res. 2023, 30, 16346–16354. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Norhayati, M.N.; Mohamad, N. Olive leaf extract effect on cardiometabolic profile among adults with prehypertension and hypertension: A systematic review and meta-analysis. PeerJ 2021, 9, e11173. [Google Scholar] [CrossRef]
- Álvares, A.A.; Garcêz, A.; Silva, L.T.; Averbuch, N.; Garavaglia, J. Olive leaf extract effect on cardiometabolic risk factors: A systematic review and meta-analysis of randomized clinical trials. Nutr. Rev. 2024, nuad164. [Google Scholar] [CrossRef]
- Magyari-Pavel, I.Z.; Moacă, E.-A.; Avram, Ș.; Diaconeasa, Z.; Haidu, D.; Ștefănuț, M.N.; Rostas, A.M.; Muntean, D.; Bora, L.; Badescu, B.; et al. Antioxidant Extracts from Greek and Spanish Olive Leaves: Antimicrobial, Anticancer and Antiangiogenic Effects. Antioxidants 2024, 13, 774. [Google Scholar] [CrossRef]
- Šimat, V.; Skroza, D.; Tabanelli, G.; Čagalj, M.; Pasini, F.; Gómez-Caravaca, A.M.; Fernández-Fernández, C.; Sterniša, M.; Možina, S.S.; Ozogul, Y.; et al. Antioxidant and Antimicrobial Activity of Hydroethanolic Leaf Extracts from Six Mediterranean Olive Cultivars. Antioxidants 2022, 11, 1656. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, X.; Zhang, J.; Zhu, S.; Niu, E.; Zhou, Z.; Liu, D. Comparative Evaluation of the Phytochemical Profiles and Antioxidant Potentials of Olive Leaves from 32 Cultivars Grown in China. Molecules 2022, 27, 1292. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Alonso, G.L.; Salinas, M.R. Determination of the Variability of Bioactive Compounds and Minerals in Olive Leaf along an Agronomic Cycle. Agronomy 2021, 11, 2447. [Google Scholar] [CrossRef]
- Borghini, F.; Tamasi, G.; Loiselle, S.A.; Baglioni, M.; Ferrari, S.; Bisozzi, F.; Costantini, S.; Tozzi, C.; Riccaboni, A.; Rossi, C. Phenolic Profiles in Olive Leaves from Different Cultivars in Tuscany and Their Use as a Marker of Varietal and Geographical Origin on a Small Scale. Molecules 2024, 29, 3617. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarro, E.M.; Cebrián-Tarancón, C.; Moratalla-López, N.; Lorenzo, C.; Alonso, G.L.; Salinas, R.M. Development and validation of an HPLC-DAD method for determination of oleuropein and other bioactive compounds in olive leaf by-products. J. Sci. Food Agric. 2021, 101, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Kabbash, E.M.; Abdel-Shakour, Z.T.; El-Ahmady, S.H.; Wink, M.; Ayoub, I.M. Comparative metabolic profiling of olive leaf extracts from twelve different cultivars collected in both fruiting and flowering seasons. Sci. Rep. 2023, 13, 612. [Google Scholar] [CrossRef]
- Hadrich, F.; Chamkha, M.; Sayadi, S. Protective effect of olive leaves phenolic compounds against neurodegenerative disorders: Promising alternative for Alzheimer and Parkinson diseases modulation. Food Chem. Toxicol. 2022, 159, 112752. [Google Scholar] [CrossRef]
- Villalva, M.; Silvan, J.M.; Guerrero-Hurtado, E.; Gutierrez-Docio, A.; del Hierro, J.N.; Alarcón-Cavero, T.; Prodanov, M.; Martin, D.; Martinez-Rodriguez, A.J. Influence of In Vitro Gastric Digestion of Olive Leaf Extracts on Their Bioactive Properties against H. pylori. Foods 2022, 11, 1832. [Google Scholar] [CrossRef]
- Antoniou, C.; Hull, J. The Anti-cancer Effect of Olea europaea L. Products: A Review. Curr. Nutr. Rep. 2021, 10, 99–124. [Google Scholar] [CrossRef]
- Morandi, F.; Bensa, V.; Calarco, E.; Pastorino, F.; Perri, P.; Corrias, M.V.; Ponzoni, M.; Brignole, C. The Olive Leaves Extract Has Anti-Tumor Effects against Neuroblastoma through Inhibition of Cell Proliferation and Induction of Apoptosis. Nutrients 2021, 13, 2178. [Google Scholar] [CrossRef]
- Albogami, S.; Hassan, A.M. Assessment of the Efficacy of Olive Leaf (Olea europaea L.) Extracts in the Treatment of Colorectal Cancer and Prostate Cancer Using In Vitro Cell Models. Molecules 2021, 26, 4069. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Farràs, M.; Almanza-Aguilera, E.; Hernáez, Á.; Agustí, N.; Julve, J.; Fitó, M.; Castañer, O. Beneficial effects of olive oil and Mediterranean diet on cancer physio-pathology and incidence. Semin. Cancer Biol. 2021, 73, 178–195. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, M.Z. Omega-9 fatty acids: Potential roles in inflammation and cancer management. J. Genet. Eng. Biotechnol. 2022, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Batçıoğlu, K.; Küçükbay, F.; Alagöz, M.A.; Günal, S.; Yilmaztekin, Y. Antioxidant and antithrombotic properties of fruit, leaf, and seed extracts of the Halhalı olive (Olea europaea L.) native to the Hatay region in Turkey. Health 2023, 1, 3. [Google Scholar] [CrossRef]
- Mirsoleimani, A.; Najafi-Ghiri, M.; Amin, H. Comparison of leaf and root nutrients concentration in twenty olive cultivars grown on a calcareous soil. J. Plant Nutr. 2024, 1–15. [Google Scholar] [CrossRef]
- del Pilar Fernández-Poyatos, M.; Ruiz-Medina, A.; Llorent-Martínez, E.J. Phytochemical profile and mineral content of Royal variety olive fruits. Influence of the ripening stage. J. Food Compos. Anal. 2021, 95, 103671. [Google Scholar] [CrossRef]
- de Oliveira, N.M.; Lopes, L.; Chéu, M.H.; Soares, E.; Meireles, D.; Machado, J. Updated Mineral Composition and Potential Therapeutic Properties of Different Varieties of Olive Leaves from Olea europaea. Plants 2023, 12, 916. [Google Scholar] [CrossRef]
- Kourti, M.; Skaperda, Z.; Tekos, F.; Stathopoulos, P.; Koutra, C.; Skaltsounis, A.L.; Kouretas, D. The Bioactivity of a Hydroxytyrosol-Enriched Extract Originated after Direct Hydrolysis of Olive Leaves from Greek Cultivars. Molecules 2024, 29, 299. [Google Scholar] [CrossRef]
- Ramírez, E.M.; Brenes, M.; Romero, C.; Medina, E. Chemical and Enzymatic Characterization of Leaves from Spanish Table Olive Cultivars. Foods 2022, 11, 3879. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. 18—Valorization of phenolic extracts from Olea europaea L. by membrane operations. In Membrane Engineering in the Circular Economy; Iulianelli, A., Cassano, A., Conidi, C., Petrotos, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 31, pp. 495–524. [Google Scholar]
- Difonzo, G.; Squeo, G.; Pasqualone, A.; Summo, C.; Paradiso, V.M.; Caponio, F. The challenge of exploiting polyphenols from olive leaves: Adition to foods to improve their shelf-life and nutritional value. J. Sci. Food Agric. 2021, 101, 3099–3116. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Mahmoudi, A.; Maalej, A.; Hadrich, F.; Isoda, H.; Sayadi, S. Contribution of Major Polyphenols to the Antioxidant Profile and Cytotoxic Activity of Olive Leaves. Anti-Cancer Agents Med. Chem. 2019, 19, 1651–1657. [Google Scholar] [CrossRef]
- Liang, J.; Bonvino, N.P.; Hung, A.; Karagiannis, T.C. In silico characterisation of olive phenolic compounds as potential cyclooxygenase modulators. Part 1. J. Mol. Graph. Model. 2020, 101, 107719. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, M.; Wu, Y.; Xia, T.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Promotes the Mitochondrial Function through Activating Mitophagy. Antioxidants 2022, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xu, J.; Cao, W.; Zhang, J.; Feng, Z.; Cao, K.; Liu, J. Hydroxytyrosol improves strenuous exercise-associated cardiac pathological changes via modulation of mitochondrial homeostasis. Food Funct. 2022, 13, 8676–8684. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Z.; Wei, F.; Hou, G.; You, Y.; Wang, X.; Cao, S.; Yang, X.; Liu, W.; Zhang, S.; et al. Hydroxytyrosol Ameliorates Intervertebral Disc Degeneration and Neuropathic Pain by Reducing Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2022, 2022, 2240894. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Kaddoumi, A. Extra-Virgin Olive Oil in Alzheimer’s Disease: A Comprehensive Review of Cellular, Animal, and Clinical Studies. Int. J. Mol. Sci. 2024, 25, 1914. [Google Scholar] [CrossRef]
- Calfio, C.; Gonzalez, A.; Singh, S.K.; Rojo, L.E.; Maccioni, R.B. The Emerging Role of Nutraceuticals and Phytochemicals in the Prevention and Treatment of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 77, 33–51. [Google Scholar] [CrossRef]
- Grubić Kezele, T.; Ćurko-Cofek, B. Neuroprotective Panel of Olive Polyphenols: Mechanisms of Action, Anti-Demyelination, and Anti-Stroke Properties. Nutrients 2022, 14, 4533. [Google Scholar] [CrossRef]
- Alabadi, B.; Civera, M.; Moreno-Errasquin, B.; Cruz-Jentoft, A.J. Nutrition-Based Support for Osteoporosis in Postmenopausal Women: A Review of Recent Evidence. Int. J. Women’s Health 2024, 16, 693–705. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Z.; Yang, X.; Liu, L.; Ahn, K.S. An updated review on the potential antineoplastic actions of oleuropein. Phytother. Res. 2022, 36, 365–379. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, A.; Martínez-Ortega, A.J.; Remón-Ruiz, P.J.; Piñar-Gutiérrez, A.; Pereira-Cunill, J.L.; García-Luna, P.P. Therapeutic Properties and Use of Extra Virgin Olive Oil in Clinical Nutrition: A Narrative Review and Literature Update. Nutrients 2022, 14, 1440. [Google Scholar] [CrossRef]
- Fytili, C.; Nikou, T.; Tentolouris, N.; Tseti, I.K.; Dimosthenopoulos, C.; Sfikakis, P.P.; Simos, D.; Kokkinos, A.; Skaltsounis, A.L.; Katsilambros, N.; et al. Effect of Long-Term Hydroxytyrosol Administration on Body Weight, Fat Mass and Urine Metabolomics: A Randomized DoubleBlind Prospective Human Study. Nutrients 2022, 14, 1525. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Le Sayec, M.; Diotallevi, C.; Teissier, A.; Deiana, M.; Corona, G. Conjugated Metabolites of Hydroxytyrosol and Tyrosol Contribute to the Maintenance of Nitric Oxide Balance in Human Aortic Endothelial Cells at Physiologically Relevant Concentrations. Molecules 2021, 26, 7480. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Trepiana, J.; Macarulla, M.T.; Gómez-Zorita, S.; Arellano-García, L.; Fernández-Quintela, A.; Portillo, M.P. Potential usefulness of Mediterranean diet polyphenols against COVID-19-induced inflammation: A review of the current knowledge. J. Physiol. Biochem. 2022, 79, 371–382. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Iacovino, S. Delivery Systems for Hydroxytyrosol Supplementation: State of the Art. Colloids Interfaces 2020, 4, 25. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, X.; Wu, N.; Hou, P.-C.P. Oleuropein inhibits pancreatic cancer through miR-190b-5p induction. STEMedicine 2022, 3, e125. [Google Scholar] [CrossRef]
- Panera, N.; Braghini, M.R.; Crudele, A.; Smeriglio, A.; Bianchi, M.; Condorelli, A.G.; Nobili, R.; Conti, L.A.; De Stefanis, C.; Lioci, G.; et al. Combination Treatment with Hydroxytyrosol and Vitamin E Improves NAFLD-Related Fibrosis. Nutrients 2022, 14, 3791. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Tsoumani, M.; Georgoulis, A.; Nikolaou, P.-E.; Kostopoulos, I.V.; Dermintzoglou, T.; Papatheodorou, I.; Zoga, A.; Efentakis, P.; Konstantinou, M.; Gikas, E.; et al. Acute administration of the olive constituent, oleuropein, combined with ischemic postconditioning increases myocardial protection by modulating oxidative defense. Free Radic. Biol. Med. 2021, 166, 18–32. [Google Scholar] [CrossRef]
- Mnafgui, K.; Ghazouani, L.; Hajji, R.; Tlili, A.; Derbali, F.; da Silva, F.I.; Araújo, J.L.; Schinoff, B.d.O.; Bachega, J.F.R.; Santos, A.L.d.S.; et al. Oleuropein Protects Against Cerebral Ischemia Injury in Rats: Molecular Docking, Biochemical and Histological Findings. Neurochem. Res. 2021, 46, 2131–2142. [Google Scholar] [CrossRef]
- Smith, D.K.; Lennon, R.P.; Carlsgaard, P.B. Managing Hypertension Using Combination Therapy. Am. Fam. Physician 2020, 101, 341–349. [Google Scholar]
- Sánchez-Gomar, I.; Benítez-Camacho, J.; Cejudo-Bastante, C.; Casas, L.; Moreno-Luna, R.; Mantell, C.; Durán-Ruiz, M.C. Pro-Angiogenic Effects of Natural Antioxidants Extracted from Mango Leaf, Olive Leaf and Red Grape Pomace over Endothelial Colony-Forming Cells. Antioxidants 2022, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, F.; Pojero, F. Use of Oleuropein and Hydroxytyrosol for Cancer Prevention and Treatment: Considerations about How Bioavailability and Metabolism Impact Their Adoption in Clinical Routine. Biomedicines 2024, 12, 502. [Google Scholar] [CrossRef]
- Moukham, H.; Lambiase, A.; Barone, G.D.; Tripodi, F.; Coccetti, P. Exploiting Natural Niches with Neuroprotective Properties: A Comprehensive Review. Nutrients 2024, 16, 1298. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Edeogu, C.O.; Offor, F.I.; Besong, E.E.; Akunna, G.G.; Maduagwuna, E.K. Downregulation of redox imbalance and iNOS/NF-ĸB/caspase-3 signalling with zinc supplementation prevents urotoxicity of cyclophosphamide-induced hemorrhagic cystitis in rats. Life Sci. 2021, 266, 118913. [Google Scholar] [CrossRef]
- Zorić, N.; Kosalec, I. The Antimicrobial Activities of Oleuropein and Hydroxytyrosol. In Promising Antimicrobials from Natural Products, 1st ed.; Rai, M., Kosalec, I., Eds.; Springer: Cham, Switzerland, 2022; pp. 75–89. [Google Scholar]
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef]
- Zhang, J.; Nugrahaningrum, D.A.; Marcelina, O.; Ariyanti, A.D.; Wang, G.; Liu, C.; Wu, S.; Kasim, V. Tyrosol Facilitates Neovascularization by Enhancing Skeletal Muscle Cells Viability and Paracrine Function in Diabetic Hindlimb Ischemia Mice. Front. Pharmacol. 2019, 10, 909. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Alkhatib, A. Antiviral Functional Foods and Exercise Lifestyle Prevention of Coronavirus. Nutrients 2020, 12, 2633. [Google Scholar] [CrossRef]
- Garg, A.; Lee, J.C. Vitamin E: Where Are We Now in Vascular Diseases? Life 2022, 12, 310. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.; Zulli, A. Revisiting the therapeutic potential of tocotrienol. BioFactors 2022, 48, 813–856. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Charalambous, C.; Kanakis, D. Vitamin E and cancer: An update on the emerging role of γ and δ tocotrienols. Eur. J. Nutr. 2020, 59, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.S. Antioxidant Activity of Polyphenols in Combating Atherosclerosis. Int. J. Res. Anal. Rev. 2022, 9, 905–924. [Google Scholar]
- Khan, Z.; Ahmad, S.; Ullah, H.; Khan, H. Vitamin E (tocopherols and tocotrienols) (natural-occurring antioxidant; bright and dark side). In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 547–560. [Google Scholar]
- Traber, M.G.; Leonard, S.W.; Vasu, V.T.; Morrissey, B.M.; Lei, H.; Atkinson, J.; Cross, C.E. α-Tocopherol Pharmacokinetics in Adults with Cystic Fibrosis: Benefits of Supplemental Vitamin C Administration. Nutrients 2022, 14, 3717. [Google Scholar] [CrossRef]
- Mishra, R.; Dutta, K.; Bharali, M.K. L-ascorbic acid and α-tocopherol treatment alleviates parabenzoquinone-induced hemato-biochemical and histopathological changes in Wistar rats. Toxicol. Environ. Health Sci. 2022, 14, 379–387. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress. Antioxidants 2022, 11, 539. [Google Scholar] [CrossRef]
- Sozen, E.; Demirel-Yalciner, T.; Aslı, E.C.E.; Ismicoglu, A.; Özer, N.K. Effect of High Cholesterol Diet and αTocopherol Supplementation on Endoplasmic Retüculum Stress and Apoptosis in Hippocampus Tissue. Clin. Exp. Health Sci. 2022, 12, 439–444. [Google Scholar]
- Pinto-Ribeiro, L.; Silva, C.; Andrade, N.; Martel, F. α-tocopherol prevents oxidative stress-induced proliferative dysfunction in first-trimester human placental (HTR-8/SVneo) cells. Reprod. Biol. 2022, 22, 100602. [Google Scholar] [CrossRef]
- Misra, D.; Dutta, W.; Jha, G.; Ray, P. Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus. Agronomy 2023, 13, 280. [Google Scholar] [CrossRef]
- Mkaouar, S.; Charfi, B.; Tounsi, L.; Bahloul, N.; Allaf, K.; Kechaou, N. Instant controlled pressure drop (DIC) effect on compositional analysis of olive leaves (Olea europaea L.). J. Food Meas. Charact. 2022, 16, 1494–1501. [Google Scholar] [CrossRef]
- Papakonstantinou, A.; Koumarianou, P.; Rigakou, A.; Diamantakos, P.; Frakolaki, E.; Vassilaki, N.; Chavdoula, E.; Melliou, E.; Magiatis, P.; Boleti, H. New Affordable Methods for Large-Scale Isolation of Major Olive Secoiridoids and Systematic Comparative Study of Their Antiproliferative/Cytotoxic Effect on Multiple Cancer Cell Lines of Different Cancer Origins. Int. J. Mol. Sci. 2022, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Marrero, A.D.; Quesada, A.R.; Martínez-Poveda, B.; Medina, M. Anti-Cancer, Anti-Angiogenic, and Anti-Atherogenic Potential of Key Phenolic Compounds from Virgin Olive Oil. Nutrients 2024, 16, 1283. [Google Scholar] [CrossRef] [PubMed]
- Nardi, M.; Brocchini, S.; Somavarapu, S.; Procopio, A. Hydroxytyrosol oleate: A promising neuroprotective nanocarrier delivery system of oleuropein and derivatives. Int. J. Pharm. 2023, 631, 122498. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, N.M.; Teiama, M.S.; Iskandar, S.S.; Osman, A.; Hammad, S.F. A Novel Nanoemulsion Formula for an Improved Delivery of a Thalidomide Analogue to Triple-Negative Breast Cancer; Synthesis, Formulation, Characterization and Molecular Studies. Int. J. Nanomed. 2023, 18, 1219–1243. [Google Scholar] [CrossRef] [PubMed]
- Moral, R.; Escrich, E. Influence of olive oil and its components on breast cancer: Molecular mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef]
- Shekari, S.; Fathi, S.; Roumi, Z.; Akbari, M.E.; Tajadod, S.; Afsharfar, M.; Ardekanizadeh, N.H.; Bourbour, F.; Keshavarz, S.A.; Sotoudeh, M.; et al. Association between dietary intake of fatty acids and colorectal cancer, a case-control study. Front. Nutr. 2022, 9, 856408. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; la Paz, S.M.-D.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Ghosh, A.; Chakraborty, D.; Mukerjee, N.; Baishya, D.; Chigurupati, S.; Felemban, S.G.; Almahmoud, S.A.; Almikhlafi, M.A.; Sehgal, A.; Singh, S.; et al. Target-based virtual screening and molecular interaction studies for lead identification of natural olive compounds against glioblastoma multiforme. Environ. Sci. Pollut. Res. 2023, 30, 6170–6191. [Google Scholar] [CrossRef]
- Ilesanmi-Oyelere, B.L.; Brough, L.; Coad, J.; Roy, N.; Kruger, M.C. The Relationship between Nutrient Patterns and Bone Mineral Density in Postmenopausal Women. Nutrients 2019, 11, 1262. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Tsatsakis, A.; Rocha, J.B.; Santamaria, A.; Spandidos, D.A.; Martins, A.C.; Lu, R.; Korobeinikova, T.V.; Chen, W.; et al. Role of vitamins beyond vitamin D3 in bone health and osteoporosis (Review). Int. J. Mol. Med. 2024, 53, 9. [Google Scholar] [CrossRef]
- Bas, N.; Kayar, N.A.; Baba, Z.F.; Avunduk, M.C.; Haliloğlu, S.; Alptekin, N. Systemic treatment with alpha-tocopherol and/or sodium selenite decreases the progression of experimental periodontitis. Clin. Oral Investig. 2021, 25, 2677–2688. [Google Scholar] [CrossRef]
- Shadisvaaran, S.; Chin, K.-Y.; Shahida, M.-S.; Ima-Nirwana, S.; Leong, X.-F. Effect of vitamin E on periodontitis: Evidence and proposed mechanisms of action. J. Oral Biosci. 2021, 63, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, G.; Luo, M.; Lan, Q.; Shi, X.; Deng, H.; Wang, N.; Xu, X.; Zhang, C. Serum vitamin E levels and chronic inflammatory skin diseases: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0261259. [Google Scholar] [CrossRef] [PubMed]
- Agha-Hosseini, F.; Pourpasha, M.; Amanlou, M.; Moosavi, M.-S. Mouthwash Containing Vitamin E, Triamcinolon, and Hyaluronic Acid Compared to Triamcinolone Mouthwash Alone in Patients with Radiotherapy-Induced Oral Mucositis: Randomized Clinical Trial. Front. Oncol. 2021, 11, 614877. [Google Scholar] [CrossRef]
- Andreyev, H.J.N.; Matthews, J.; Adams, C.; Gothard, L.; Lucy, C.; Tovey, H.; Boyle, S.; Anbalagan, S.; Musallam, A.; Yarnold, J.; et al. Randomised single centre double-blind placebo controlled phase II trial of Tocovid SupraBio in combination with pentoxifylline in patients suffering long-term gastrointestinal adverse effects of radiotherapy for pelvic cancer: The PPALM study. Radiother. Oncol. 2022, 168, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Heiba, M.A.; Ismail, S.S.; Sabry, M.; Bayoumy, W.A.E.; Kamal, K.A.-A. The use of vitamin E in preventing taxane-induced peripheral neuropathy. Cancer Chemother. Pharmacol. 2021, 88, 931–939. [Google Scholar] [CrossRef]
- Ashrafi, F.; Naeimi Tabiei, M.; Mousavi, S.; Nematbakhsh, M.; Sotoodehnasab, P.; Janbabaei, G. Does vitamin E mitigate cisplatin-induced nephrotoxicity in cancer patients: Results from a randomized placebo-controlled clinical trial. Middle East J. Cancer 2020, 11, 174–184. [Google Scholar]
- Sayed, R.; El Wakeel, L.; Saad, A.S.; Kelany, M.; El-Hamamsy, M. Pentoxifylline and vitamin E reduce the severity of radiotherapy-induced oral mucositis and dysphagia in head and neck cancer patients: A randomized, controlled study. Med. Oncol. 2020, 37, 8. [Google Scholar] [CrossRef]
- Keshavarzi, Z.; Janghorban, R.; Alipour, S.; Tahmasebi, S.; Jokar, A. The effect of vitamin D and E vaginal suppositories on tamoxifen-induced vaginal atrophy in women with breast cancer. Support. Care Cancer 2019, 27, 1325–1334. [Google Scholar] [CrossRef]
- Krejbich, P.; Birringer, M. The Self-Administered Use of Complementary and Alternative Medicine (CAM) Supplements and Antioxidants in Cancer Therapy and the Critical Role of Nrf-2—A Systematic Review. Antioxidants 2022, 11, 2149. [Google Scholar] [CrossRef]
- Lledó, I.; Ibáñez, B.; Melero, A.; Montoro, A.; Merino-Torres, J.F.; Onofre, N.S.; Soriano, J.M. Vitamins and Radioprotective Effect: A Review. Antioxidants 2023, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Coelho, M.D.P.S.; Pereira, I.C.; de Oliveira, K.G.F.; Oliveira, I.K.F.; Dos Santos Rizzo, M.; de Oliveira, V.A.; Carneiro da Silva, F.C.; Torres-Leal, F.L.; de Castro ESousa, J.M. Chemopreventive and anti-tumor potential of vitamin E in preclinical breast cancer studies: A systematic review. Clin. Nutr. ESPEN 2023, 53, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Appathurai, A.; Yeoh, H.-L.; Driscoll, K.; Faisal, W. Vitamin E in Cancer Treatment: A Review of Clinical Applications in Randomized Control Trials. Nutrients 2022, 14, 4329. [Google Scholar] [CrossRef] [PubMed]
- Campo, C.; Gangemi, S.; Pioggia, G.; Allegra, A. Beneficial Effect of Olive Oil and Its Derivates: Focus on Hematological Neoplasm. Life 2024, 14, 583. [Google Scholar] [CrossRef]
- Menezes, R.C.R.; Peres, K.K.; Costa-Valle, M.T.; Faccioli, L.S.; Dallegrave, E.; Garavaglia, J.; Bosco, S.M.D. Oral administration of oleuropein and olive leaf extract has cardioprotective effects in rodents: A systematic review. Rev. Port. Cardiol. 2022, 41, 167–175. [Google Scholar] [CrossRef]
- Acar-Tek, N.; Ağagündüz, D. Olive Leaf (Olea europaea L. folium): Potential Effects on Glycemia and Lipidemia. Ann. Nutr. Metab. 2020, 76, 10–15. [Google Scholar] [CrossRef]
- Tikhonoff, V.; Casiglia, E.; Virdis, A.; Grassi, G.; Angeli, F.; Arca, M.; Barbagallo, C.M.; Bombelli, M.; Cappelli, F.; Cianci, R.; et al. Prognostic Value and Relative Cutoffs of Triglycerides Predicting Cardiovascular Outcome in a Large Regional-Based Italian Database. J. Am. Heart Assoc. 2024, 13, e030319. [Google Scholar] [CrossRef]
- Stevens, Y.; Winkens, B.; Jonkers, D.; Masclee, A. The effect of olive leaf extract on cardiovascular health markers: A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2021, 60, 2111–2120. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef]
- D’angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef]
- Araki, R.; Fujie, K.; Yuine, N.; Watabe, Y.; Nakata, Y.; Suzuki, H.; Isoda, H.; Hashimoto, K. Olive leaf tea is beneficial for lipid metabolism in adults with prediabetes: An exploratory randomized controlled trial. Nutr. Res. 2019, 67, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Fujie, K.; Nakata, Y.; Suzuki, H.; Matsui, K.; Uematsu, K.; Shibasaki, H.; Ando, T.; Ueyama, Y.; Isoda, H.; et al. An Exploratory Study of the Effects of Continuous Intake of Olive Leaf Tea on Physique and Glucose and Lipid Metabolism. Nippon. Eiyo Shokuryo Gakkaishi 2018, 71, 121–131. [Google Scholar] [CrossRef]
- Javadi, H.; Yaghoobzadeh, H.; Esfahani, Z.; Reza Memarzadeh, M.; Mehdi Mirhashemi, S. Effects of Olive Leaf Extract on Metabolic Response, Liver and Kidney Functions and Inflammatory Biomarkers in Hypertensive Patients. Pak. J. Biol. Sci. 2019, 22, 342–348. [Google Scholar] [CrossRef]
- Yaghoobzadeh, H.; Mehravar, S.; Javadi, H.; Memarzadeh, M.R.; Mirhashemi, S.M. Determining cardiometabolic and antioxidant effects of olive leaf extract in patients with essential hypertension. J. Inflamm. Dis. 2019, 23, 372–381. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Saftić, L.; Klisović, D.; Pavelić, S.K. Polyphenol-Based Design of Functional Olive Leaf Infusions. Food Technol. Biotechnol. 2019, 57, 171–182. [Google Scholar] [CrossRef]
- Mancak, M.; Çalışkan, U.K. What do people prefer to support diabetes treatment in Turkiye? A study on olive lead and diabetes. J. Fac. Pharm. Ank. Univ. 2024, 48, 4. [Google Scholar]
- Ferdousi, F.; Araki, R.; Hashimoto, K.; Isoda, H. Olive leaf tea may have hematological health benefit over green tea. Clin. Nutr. 2019, 38, 2952–2955. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2016, 56, 1421–1432. [Google Scholar] [CrossRef]
- Ferreira, D.M.; de Oliveira, N.M.; Lopes, L.; Machado, J.; Oliveira, M.B. Potential Therapeutic Properties of the Leaf of Cydonia Oblonga Mill. Based on Mineral and Organic Profiles. Plants 2022, 11, 2638. [Google Scholar] [CrossRef]
- Turner, R.M.; Pirmohamed, M. Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef]
- Sohn, I.S.; Ihm, S.-H.; Kim, G.H.; Park, S.M.; Hong, B.-K.; Lee, C.H.; Lee, S.H.; Chang, D.I.; Joo, S.P.; Lee, S.C.; et al. Real-world evidence on the strategy of olmesartan-based triple single-pill combination in Korean hypertensive patients: A prospective, multicenter, observational study (RESOLVE-PRO). Clin. Hypertens. 2021, 27, 21. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, R.; Kavanagh, J.; Li, L.; Zeng, X.; Li, Y.; Fu, P. Efficacy and Safety of Dual Blockade of the Renin-Angiotensin-Aldosterone System in Diabetic Kidney Disease: A Meta-Analysis. Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2019, 19, 259–286. [Google Scholar]
- Sheashea, M.; Xiao, J.; Farag, M.A. MUFA in metabolic syndrome and associated risk factors: Is MUFA the opposite side of the PUFA coin? Food Funct. 2021, 12, 12221–12234. [Google Scholar] [CrossRef] [PubMed]
- Fiore, G.; Pascuzzi, M.C.; Di Profio, E.; Corsello, A.; Agostinelli, M.; La Mendola, A.; Milanta, C.; Campoy, C.; Calcaterra, V.; Zuccotti, G.; et al. Bioactive compounds in childhood obesity and associated metabolic complications: Current evidence, controversies and perspectives. Pharmacol. Res. 2023, 187, 106599. [Google Scholar]
- Djuricic, I.; Calder, P.C. Omega-3 (n-3) Fatty Acid–Statin Interaction: Evidence for a Novel Therapeutic Strategy for Atherosclerotic Cardiovascular Disease. Nutrients 2024, 16, 962. [Google Scholar] [CrossRef] [PubMed]
- Jaca, A.; Durão, S.; Harbron, J. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. South Afr. Med. J. 2020, 110, 1158–1159. [Google Scholar]
- Abunab, H.; Dator, W.L.; Hawamdeh, S. Effect of olive leaf extract on glucose levels in diabetes-induced rats: A systematic review and meta-analysis. J. Diabetes 2017, 9, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Żyrek, L.; Latocha, M. [A multidirectional effect of metformin]. Pol. Merkur. Lek. 2022, 50, 62–64. [Google Scholar]
- Wasner, H.K. Metformin’s Mechanism of Action Is Stimulation of the Biosynthesis of the Natural Cyclic AMP Antagonist Prostaglandylinositol Cyclic Phosphate (Cyclic PIP). Int. J. Mol. Sci. 2022, 23, 2200. [Google Scholar] [CrossRef]
- Love, K.M.; Barrett, E.J.; Horton, W.B. Metformin’s Impact on the Microvascular Response to Insulin. Endocrinology 2022, 163, bqac162. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Wang, Z.; Pang, H. Role of metformin in inflammation. Mol. Biol. Rep. 2022, 16, 5545–5564. [Google Scholar] [CrossRef]
- Cao, G.; Gong, T.; Du, Y.; Wang, Y.; Ge, T.; Liu, J. Mechanism of metformin regulation in central nervous system: Progression and future perspectives. Biomed. Pharmacother. 2022, 156, 113686. [Google Scholar] [CrossRef]
- De Bock, M.; Derraik, J.G.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) Leaf Polyphenols Improve Insulin Sensitivity in Middle-Aged Overweight Men: A Randomized, Placebo-Controlled, Crossover Trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Parks, N.E.; Jackson-Tarlton, C.S.; Vacchi, L.; Merdad, R.; Johnston, B.C. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst. Rev. 2020, 2020, CD004192. [Google Scholar] [CrossRef]
- Sokal-Dembowska, A.; Jarmakiewicz-Czaja, S.; Ferenc, K.; Filip, R. Can Nutraceuticals Support the Treatment of MASLD/MASH, and thus Affect the Process of Liver Fibrosis? Int. J. Mol. Sci. 2024, 25, 5238. [Google Scholar] [CrossRef] [PubMed]
- Reiner, M.F.; Bertschi, D.A.; Werlen, L.; Wiencierz, A.; Aeschbacher, S.; Lee, P.; Rodondi, N.; Moutzouri, E.; Bonati, L.; Reichlin, T.; et al. Omega-3 Fatty Acids and Markers of Thrombosis in Patients with Atrial Fibrillation. Nutrients 2024, 16, 178. [Google Scholar] [CrossRef]
- Tutor, A.; O’Keefe, E.L.; Lavie, C.J.; Elagizi, A.; Milani, R.; O’Keefe, J. Omega-3 fatty acids in primary and secondary prevention of cardiovascular diseases. Prog. Cardiovasc. Dis. 2024, 84, 19–26. [Google Scholar] [CrossRef]
- Komolafe, O.; Buzzetti, E.; Linden, A.; Best, L.M.; Madden, A.M.; Roberts, D.; Chase, T.J.; Fritche, D.; Freeman, S.C.; Cooper, N.J.; et al. Nutritional supplementation for nonalcohol-related fatty liver disease: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 2021, CD013157. [Google Scholar] [CrossRef]
- Guerra, J.V.S.; Dias, M.M.G.; Brilhante, A.J.V.C.; Terra, M.F.; García-Arévalo, M.; Figueira, A.C.M. Multifactorial Basis and Therapeutic Strategies in Metabolism-Related Diseases. Nutrients 2021, 13, 2830. [Google Scholar] [CrossRef]
- Kajal, K.; Singh, G.; Pradhan, T.; Bhurta, D.; Monga, V. The medicinal perspective of 2,4-thiazolidinediones based ligands as antimicrobial, antitumor and antidiabetic agents: A review. Arch. Pharm. 2022, 355, e2100517. [Google Scholar] [CrossRef]
- DeMarsilis, A.; Reddy, N.; Boutari, C.; Filippaios, A.; Sternthal, E.; Katsiki, N.; Mantzoros, C. Pharmacotherapy of type 2 diabetes: An update and future directions. Metabolism 2022, 137, 155332. [Google Scholar] [CrossRef]
- Lim, W.X.J.; Gammon, C.S.; Von Hurst, P.R.; Chepulis, L.; Mugridge, O.; Page, R.A. Hypoglycemic effects of antioxidant-rich plant extracts on postprandial glycemic responses in participants with prediabetes (GLARE study). Funct. Foods Health Dis. 2021, 11, 604–626. [Google Scholar] [CrossRef]
- Kerimi, A.; Nyambe-Silavwe, H.; Pyner, A.; Oladele, E.; Gauer, J.S.; Stevens, Y.; Williamson, G. Nutritional implications of olives and sugar: Attenuation of post-prandial glucose spikes in healthy volunteers by inhibition of sucrose hydrolysis and glucose transport by oleuropein. Eur. J. Nutr. 2019, 58, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Basuny, A.M.; Abdelaaty, L.N.; Zakhari, C.K.; Hussein, R.R.; Mohamed, M.R.A.; Ali, S.A.G. Efficacy of the traditional use of Olive Leaves decoction as Anti-diabetic Agent in Geriatrics. NILES J. Geriatr. Gerontol. 2023, 6.2, 420–431. [Google Scholar]
- Zhou, X.; Zhou, X.; Zhu, R.; Ming, Z.; Cheng, Z.; Hu, Y. The mechanism of oleic acid inhibiting platelet activation stimulated by collagen. Cell Commun. Signal. 2023, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.S.; Kumari, S. Fatty acids and their role in type-2 diabetes (Review). Exp. Ther. Med. 2021, 22, 706. [Google Scholar] [CrossRef] [PubMed]
- Bayram, S.Ş.; Kilzitan, G. The Role of Omega-3 Polyunsaturated Fatty Acids in Diabetes Mellitus Management: A Narrative Review. Curr. Nutr. Rep. 2024, 13, 527–551. [Google Scholar]
- Hartnett, K.B.; Ferguson, B.J.; Hecht, P.M.; Schuster, L.E.; Shenker, J.I.; Mehr, D.R.; Fritsche, K.L.; Belury, M.A.; Scharre, D.W.; Horwitz, A.J.; et al. Potential Neuroprotective Effects of Dietary Omega-3 Fatty Acids on Stress in Alzheimer’s Disease. Biomolecules 2023, 13, 1096. [Google Scholar] [CrossRef]
- Chen, C.; Ai, Q.-D.; Wei, Y.-H. Potential role of hydroxytyrosol in neuroprotection. J. Funct. Foods 2021, 82, 104506. [Google Scholar] [CrossRef]
- Downie, L.E.; Ng, S.M.; Lindsley, K.B.; Akpek, E.K. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst. Rev. 2019, 12, CD011016. [Google Scholar] [CrossRef]
- Longarzo, M.L.; Vázquez, R.F.; Bellini, M.J.; Zamora, R.A.; Redondo-Morata, L.; Giannotti, M.I.; Oliveira, O.N., Jr.; Fanani, M.L.; Maté, S.M. Understanding the effects of omega-3 fatty acid supplementation on the physical properties of brain lipid membranes. iScience 2024, 27, 110362. [Google Scholar] [CrossRef]
- Maliha, A.; Tahsin, M.; Fabia, T.Z.; Rahman, S.M.; & Rahman. Pro-resolving metabolites: Future of the fish oil supplements. J. Funct. Foods 2024, 121, 106439. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Natural Antioxidants: A Novel Therapeutic Approach to Autism Spectrum Disorders? Antioxidants 2020, 9, 1186. [Google Scholar] [CrossRef]
- Ruiz-Sastre, P.; Gómez-Sánchez-Lafuente, C.; Martín-Martín, J.; Herrera-Imbroda, J.; Mayoral-Cleries, F.; Santos-Amaya, I.; de Fonseca, F.R.; Guzmán-Parra, J.; Rivera, P.; Suárez, J. Pharmacotherapeutic value of inflammatory and neurotrophic biomarkers in bipolar disorder: A systematic review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 134, 111056. [Google Scholar] [CrossRef]
- Therdyothin, A.; Prokopidis, K.; Galli, F.; Witard, O.C.; Isanejad, M. The effects of omega-3 polyunsaturated fatty acids on muscle and whole-body protein synthesis: A systematic review and meta-analysis. Nutr. Rev. 2024, nuae055. [Google Scholar]
- Fujitaka, Y.; Hamada, H.; Uesugi, D.; Kuboki, A.; Shimoda, K.; Iwaki, T.; Kiriake, Y.; Saikawa, T. Synthesis of Daidzein Glycosides, α-Tocopherol Glycosides, Hesperetin Glycosides by Bioconversion and Their Potential for Anti-Allergic Functional-Foods and Cosmetics. Molecules 2019, 24, 2975. [Google Scholar] [CrossRef] [PubMed]
- Baky, M.H.; Elshahed, M.S.; Wessjohann, L.A.; Farag, M.A. Interactions between dietary flavonoids and the gut microbiome: A comprehensive review. Br. J. Nutr. 2022, 128, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Roberto, M.; Di Risola, D.; Mosca, L.; Di Pietro, M.; Sessa, R. Olea europaea L.-derived secoiridoids: Beneficial health effects and potential therapeutic approaches. Pharmacol. Ther. 2024, 254, 108595. [Google Scholar]
- Alghamdi, S.Q.; Alotaibi, N.; Al-Ghamdi, S.N.; Alqarni, L.S.; Amna, T.; Moustafa, S.M.; Alsohaimi, I.H.; Alruwaili, I.; Nassar, A. High Antiparasitic and Antimicrobial Performance of Biosynthesized NiO Nanoparticles via Wasted Olive Leaf Extract. Int. J. Nanomed. 2024, 19, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Medfai, W.; Oueslati, I.; Dumas, E.; Harzalli, Z.; Viton, C.; Mhamdi, R.; Gharsallaoui, A. Physicochemical and Biological Characterization of Encapsulated Olive Leaf Extracts for Food Preservation. Antibiotics 2023, 12, 987. [Google Scholar] [CrossRef]
- Alowaiesh, B.F.; Alhaithloul, H.A.S.; Saad, A.M.; Hassanin, A.A. Green Biogenic of Silver Nanoparticles Using Polyphenolic Extract of Olive Leaf Wastes with Focus on Their Anticancer and Antimicrobial Activities. Plants 2023, 12, 1410. [Google Scholar] [CrossRef]
- Famiglietti, M.; Savastano, A.; Gaglione, R.; Arciello, A.; Naviglio, D.; Mariniello, L. Edible Films Made of Dried Olive Leaf Extract and Chitosan: Characterization and Applications. Foods 2022, 11, 2078. [Google Scholar] [CrossRef]
- Baysal, G.; Kasapbaşı, E.E.; Yavuz, N.; Hür, Z.; Genç, K.; Genç, M. Determination of theoretical calculations by DFT method and investigation of antioxidant, antimicrobial properties of olive leaf extracts from different regions. J. Food Sci. Technol. 2021, 58, 1909–1917. [Google Scholar] [CrossRef]
- Martiny, T.R.; Raghavan, V.; de Moraes, C.C.; da Rosa, G.S.; Dotto, G.L. Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation. Foods 2020, 9, 1759. [Google Scholar] [CrossRef]
- Saleh, E.; Morshdy, A.E.; El-Manakhly, E.; Al-Rashed, S.; FHetta, H.; Jeandet, P.; Yahia, R.; El-Saber Batiha, G.; Ali, E. Effects of Olive Leaf Extracts as Natural Preservative on Retailed Poultry Meat Quality. Foods 2020, 9, 1017. [Google Scholar] [CrossRef]
- Moura-Alves, M.; Souza, V.G.L.; Silva, J.A.; Esteves, A.; Pastrana, L.M.; Saraiva, C.; Cerqueira, M.A. Characterization of Sodium Alginate-Based Films Blended with Olive Leaf and Laurel Leaf Extracts Obtained by Ultrasound-Assisted Technology. Foods 2023, 12, 4076. [Google Scholar] [CrossRef]
- Allegretta, C.; Difonzo, G.; Caponio, F.; Tamma, G.; Laselva, O. Olive Leaf Extract (OLE) as a Novel Antioxidant That Ameliorates the Inflammatory Response in Cystic Fibrosis. Cells 2023, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Arismendi Sosa, A.C.; Mariani, M.L.; Vega, A.E.; Penissi, A.B. Extra virgin olive oil inhibits Helicobacter pylori growth in vitro and the development of mice gastric mucosa lesions in vivo. Front. Microbiol. 2022, 13, 961597. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; De Feo, V.; Cruz, A.G.; D’acierno, A. Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition. Microorganisms 2019, 7, 321. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Nieto, G.; Pateiro, M.; Lorenzo, J.M. Phenolic Compounds Obtained from Olea europaea By-Products and Their Use to Improve the Quality and Shelf Life of Meat and Meat Products—A Review. Antioxidants 2020, 9, 1061. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Žugčić, T.; Abdelkebir, R.; Alcantara, C.; Collado, M.C.; Garcia-Perez, J.V.; Meléndez-Martínez, A.J.; Režek Jambrak, A.; Lorenzo, J.M.; Barba, F.J. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Technol. 2019, 83, 63–77. [Google Scholar] [CrossRef]
- Barukčić, I.; Filipan, K.; Jakopović, K.L.; Božanić, R.; Blažić, M.; Repajić, M. The Potential of Olive Leaf Extract as a Functional Ingredient in Yoghurt Production: The Effects on Fermentation, Rheology, Sensory, and Antioxidant Properties of Cow Milk Yoghurt. Foods 2022, 11, 701. [Google Scholar] [CrossRef]
- Majrashi, T.A.; El Hassab, M.A.; Mahmoud, S.H.; Mostafa, A.; Wahsh, E.A.; Elkaeed, E.B.; Hassan, F.E.; Eldehna, W.M. Abdelgawad SM. In vitro biological evaluation and in silico insights into the antiviral activity of standardized olive leaves extract against SARS-CoV-2. PLoS ONE 2024, 19, e0301086. [Google Scholar] [CrossRef]
- Salamanca, A.; Almodóvar, P.; Jarama, I.; González-Hedström, D.; Prodanov, M.; Inarejos-García, A.M. Anti-influenza virus activity of the elenolic acid rich olive leaf (Olea europaea L.) extract Isenolic®. Antivir. Chem. Chemother. 2021, 29, 20402066211063391. [Google Scholar] [CrossRef]
- Toulabi, T.; Delfan, B.; Rashidipour, M.; Yarahmadi, S.; Ravanshad, F.; Javanbakht, A.; Almasian, M. The efficacy of olive leaf extract on healing herpes simplex virus labialis: A randomized double-blind study. Explore 2022, 18, 287–292. [Google Scholar] [CrossRef]
- Lorzadeh, N.; Kazemirad, Y.; Kazemirad, N. Treatment of genital herpes using olive leaf extract. Clin. Case Rep. 2021, 9, 986–989. [Google Scholar] [CrossRef]
- Lafi, O.; Essid, R.; Lachaud, L.; Jimenez, C.; Rodríguez, J.; Ageitos, L.; Mhamdi, R.; Abaza, L. Synergistic antileishmanial activity of erythrodiol, uvaol, and oleanolic acid isolated from olive leaves of cv. Chemlali. 3 Biotech 2023, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- Floris, B.; Galloni, P.; Conte, V.; Sabuzi, F. Tailored Functionalization of Natural Phenols to Improve Biological Activity. Biomolecules 2021, 11, 1325. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Silva, A.F.R.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Strategies to Broaden the Applications of Olive Biophenols Oleuropein and Hydroxytyrosol in Food Products. Antioxidants 2021, 10, 444. [Google Scholar] [CrossRef]
- Zawawi, A.; Naser, A.Y.; Alwafi, H.; Minshawi, F. Profile of Circulatory Cytokines and Chemokines in Human Coronaviruses: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 666223. [Google Scholar] [CrossRef]
- Investigators, T.R.-C.; Derde, L.P.G. Effectiveness of Tocilizumab, Sarilumab, and Anakinra for critically ill patients with COVID-19 The REMAP-CAP COVID-19 Immune Modulation Therapy Domain Randomized Clinical Trial. MedRxiv 2021. [Google Scholar] [CrossRef]
- Abdelgawad, S.M.; El Hassab, M.A.; Abourehab, M.A.S.; Elkaeed, E.B.; Eldehna, W.M. Olive Leaves as a Potential Phytotherapy in the Treatment of COVID-19 Disease; A Mini-Review. Front. Pharmacol. 2022, 13, 879118. [Google Scholar] [CrossRef]
- Boadu, A.; Agoni, C.; Karpoormath, R.; Soliman, M.; Nlooto, M. Repurposing antiviral phytochemicals from the leaf extracts of Spondias mombin (Linn) towards the identification of potential SARSCOV-2 inhibitors. Sci. Rep. 2022, 12, 10896. [Google Scholar] [CrossRef]
- Kocyigit, A.; Kanımdan, E.; Yenigun, V.B.; Ozman, Z.; Balıbey, F.B.; Durmuş, E.; Yasar, O. Olive Leaf Extract Downregulates the Protein Expression of Key SARS-CoV-2 Entry Enzyme ACE-2, TMPRSS2, and Furin. Chem. Biodivers. 2024, 21, e202400717. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative Extraction Technologies for Development of Functional Ingredients Based on Polyphenols from Olive Leaves. Foods 2021, 11, 103. [Google Scholar] [CrossRef]
- Khan, H.; Ahmad, W.; Hussain, I.; Imran, M.; Afridi, M.S.; Ullah, S. Phytochemical composition, antioxidant and antimicrobial activities of leaves of Olea europaea wild variety. J. Food Meas. Charact. 2020, 14, 640–648. [Google Scholar] [CrossRef]
- Tadić, J.; Dumičić, G.; Veršić Bratinčević, M.; Vitko, S.; Liber, Z.; Radić Brkanac, S. Comparative analysis of cultivated and wild olive genotypes to salinity and drought stress. Front. Plant Sci. 2024, 15, 1423761. [Google Scholar] [CrossRef] [PubMed]
- Argon, H.; Banu, K.; Zeliha, Ü.; Süleyman, D.; Turan, A. Phenolic Content and In-vitro Antioxidant Activity of Olea europaea L. subs. oleaster Leaves by Supercritical CO2 Extraction. Ereğli Tarım Bilim. Derg. 2023, 3, 75–85. [Google Scholar]
- Gonçalves, M.; Aiello, A.; Rodríguez-Pérez, M.; Accardi, G.; Burgos-Ramos, E.; Silva, P. Olive Oil Components as Novel Antioxidants in Neuroblastoma Treatment: Exploring the Therapeutic Potential of Oleuropein and Hydroxytyrosol. Nutrients 2024, 16, 818. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Crupi, P.; Desantis, A.; Rondanelli, M.; Corbo, F.; Clodoveo, M.L. Olive Oil Polyphenols Improve HDL Cholesterol and Promote Maintenance of Lipid Metabolism: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Metabolites 2023, 13, 1187. [Google Scholar] [CrossRef]
- Soldo, B.; Bilušić, T.; Giacometti, J.; Ljubenkov, I.; Čikeš Čulić, V.; Bratanić, A.; Bošković, P.; Šola, I.; Ilić, K. A Comparative Study of Oleuropein Extraction from Wild Olive Leaves (Olea europea subsp. oleaster, Hoffmanns. & Link), Its Gastrointestinal Stability, and Biological Potential. Appl. Sci. 2024, 14, 869. [Google Scholar] [CrossRef]
- de Oliveira, N.M.; Machado, J.; Huang, Z.; Criado, M.B. Acupuncture in Women with Human Polycystic Ovary/Ovarian Syndrome: Protocol for a Randomized Controlled Trial. Healthcare 2022, 10, 1999. [Google Scholar] [CrossRef]
- Vine, D.; Ghosh, M.; Wang, T.; Bakal, J. Increased Prevalence of Adverse Health Outcomes Across the Lifespan in Those Affected by Polycystic Ovary Syndrome: A Canadian Population Cohort. CJC Open 2023, 6 Pt B, 314–326. [Google Scholar] [CrossRef]
- de Oliveira, N.M.; Machado, J.; Lopes, L.; Criado, M.B. A Review on Acupuncture Efficiency in Human Polycystic Ovary/Ovarian Syndrome. J. Pharmacopunct. 2023, 26, 105–123. [Google Scholar] [CrossRef]
- Wen, X.; Wang, L.; Bai, E. Metabolic characteristics of different phenotypes in reproductive-aged women with polycystic ovary syndrome. Front. Endocrinol. 2024, 15, 1370578. [Google Scholar] [CrossRef]
- Omma, T.; Gokce, A.; Celik, M.; Karahan, I.; Culha, C.; Gulcelik, N.E. A New Predictor for Insulin Resistance in Polycystic Ovary Syndrome: InsuTAG. Curr. Women S Health Rev. 2023, 20, 61–67. [Google Scholar] [CrossRef]
- Herbert, S.; Woolf, K. Moving beyond Weight: A Narrative Review of the Dietary and Lifestyle Management for Reducing Cardiometabolic Risk in Polycystic Ovary Syndrome (PCOS). Nutrients 2023, 15, 5069. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, X.; Lv, L.; Xu, Y.; Wu, B.; Huang, C. Associations between omega-3 fatty acids and insulin resistance and body composition in women with polycystic ovary syndrome. Front. Nutr. 2022, 9, 1016943. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, Y.; Lv, Y.; Xu, Y.; Bai, X.; Zhang, H.; Wang, Y.; Song, X. The association between serum fatty acids and pregnancy in PCOS women undergoing ovulation induction. Gynecol. Endocrinol. 2022, 38, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Yahay, M.; Heidari, Z.; Allameh, Z.; Amani, R. The effects of canola and olive oils consumption compared to sunflower oil, on lipid profile and hepatic steatosis in women with polycystic ovarian syndrome: A randomized controlled trial. Lipids Health Dis. 2021, 20, 7. [Google Scholar] [CrossRef]
- Ghobadi, S.; Hassanzadeh-Rostami, Z.; Mohammadian, F.; Nikfetrat, A.; Ghasemifard, N.; Raeisi Dehkordi, H.; Faghih, S. Comparison of blood lipid-lowering effects of olive oil and other plant oils: A systematic review and meta-analysis of 27 randomized placebo-controlled clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2110–2124. [Google Scholar] [CrossRef]
- Venturini, D.; Simão, A.N.C.; Urbano, M.R.; Dichi, I. Effects of extra virgin olive oil and fish oil on lipid profile and oxidative stress in patients with metabolic syndrome. Nutrition 2015, 31, 834–840. [Google Scholar] [CrossRef]
- Violante, B.; Gerbaudo, L.; Borretta, G.; Tassone, F. Effects of extra virgin olive oil supplementation at two different low doses on lipid profile in mild hypercholesterolemic subjects: A randomised clinical trial. J. Endocrinol. Investig. 2009, 32, 794–796. [Google Scholar]
- Ahmed, K.M. The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer. A randomized clinical trial. Saudi Dent. J. 2013, 25, 141–147. [Google Scholar] [CrossRef]
- Zamanifard, M.; Nasiri, M.; Yarahmadi, F.; Zonoori, S.; Razani, O.; Salajegheh, Z.; Imanipour, M.; Mohammadi, S.M.; Jomehzadeh, N.; Asadi, M. Healing of diabetic foot ulcer with topical and oral administrations of herbal products: A systematic review and meta-analysis of randomized controlled trials. Int. Wound J. 2024, 21, e14875. [Google Scholar] [CrossRef]
- Sánchez Macarro, M.; Martínez Rodríguez, J.P.; Bernal Morell, E.; Pérez-Piñero, S.; Victoria-Montesinos, D.; García-Muñoz, A.M.; Cánovas García, F.; Castillo Sánchez, J.; López-Román, F.J. Effect of a Combination of Citrus Flavones and Flavanones and Olive Polyphenols for the Reduction of Cardiovascular Disease Risk: An Exploratory Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Nutrients 2020, 12, 1475. [Google Scholar] [CrossRef]
- Haidari, F.; Shayesteh, F.; Mohammad-Shahi, M.; Jalali, M.-T.; Ahmadi-Angali, K. Olive Leaf Extract Supplementation Combined with Calorie-Restricted Diet on Reducing Body Weight and Fat Mass in Obese Women: Result of a Randomized Control Trial. Clin. Nutr. Res. 2021, 10, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaffarin, A.S.; Ng, S.-F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef] [PubMed]
- Zulkiflee, N.S.; Awang, S.A.; Ming, W.X.; Kamilan, M.F.W.; Mariappan, M.Y.; Kit, T.J. In Silico Docking of Vitamin E Isomers on Transport Proteins. Curr. Comput. Aided-Drug Des. 2020, 16, 467–472. [Google Scholar] [CrossRef]
- Jaberi, N.; Anarjan, N.; Jafarizadeh-Malmiri, H. Optimization the formulation parameters in preparation of α-tocopherol nanodispersions using low-energy solvent displacement technique. Int. J. Vitam. Nutr. Res. 2020, 90, 5–16. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A. Effect of storage, temperature, and pH on the preservation of the oleuropein content of olive leaf extracts. Sustain. Food Technol. 2024, 2, 750–759. [Google Scholar]
- Gonzalez-Ortega, R.; Di Mattia, C.D.; Pittia, P.; Natasa, P.U. Effect of heat treatment on phenolic composition and radical scavenging activity of olive leaf extract at different pH conditions: A spectroscopic and kinetic study. J. Sci. Food Agric. 2023, 103, 2047–2056. [Google Scholar] [CrossRef]
- Lemonakis, N.; Mougios, V.; Halabalaki, M.; Dagla, I.; Tsarbopoulos, A.; Skaltsounis, A.-L.; Gikas, E. Effect of Supplementation with Olive Leaf Extract Enriched with Oleuropein on the Metabolome and Redox Status of Athletes’ Blood and Urine—A Metabolomic Approach. Metabolites 2022, 12, 195. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Leyva-Jiménez, F.J.; Quirantes-Piné, R.; López-Bascón, M.A.; Lozano-Sánchez, J.; Borrás-Linares, I. Evaluation of Olive Leaf Phenolic Compounds’ Gastrointestinal Stability Based on Co-Administration and Microencapsulation with Non-Digestible Carbohydrates. Nutrients 2023, 16, 93. [Google Scholar] [CrossRef]
- Pattamatta, M.; Chapple, I.; Listl, S. The value-for money of preventing and managing periodontitis: Opportunities and challenges. Periodontology 2024. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Toulabi, T.; Yadegarinia, D.; Yarahmadi, S.; Mohammadi, R.; Keyvanfar, A. Efficacy of olive leaves extract on the outcomes of hospitalized COVID-19 patients: A randomized, triple-blinded clinical trial. Explore 2023, 19, 536–543. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Irban, A.; Kiran, B.; Atayoglu, A.T. Assessment of Association Between the Potential Immunomodulatory Activity and Drinking Olive Leaf Tea in the Coronavirus Disease-2019 Pandemic: An Observational Study. J. Integr. Complement. Med. 2022, 28, 940–947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).