Non-Traditional Natural Stabilizers in Drug Nanosuspensions
Abstract
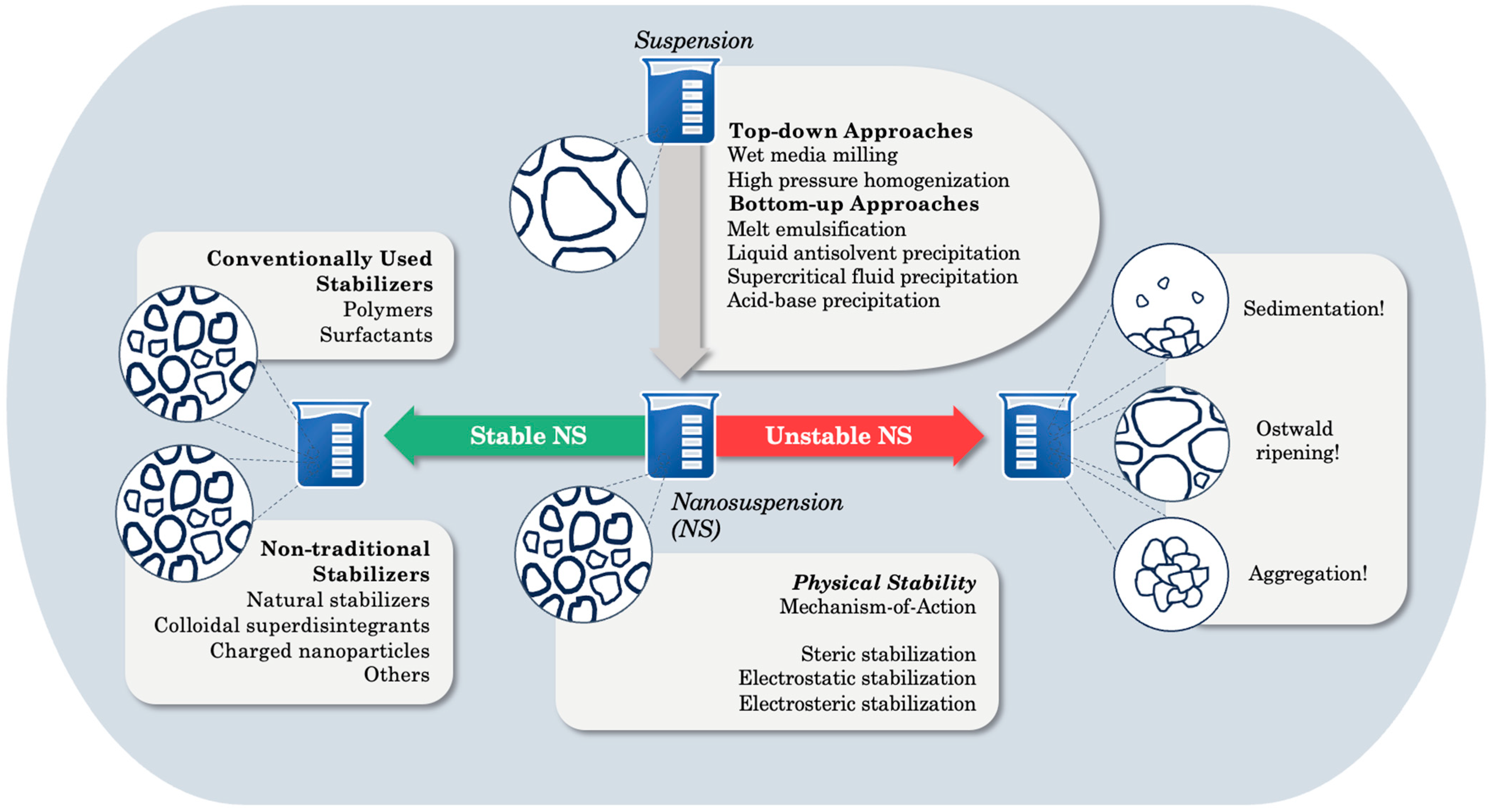
1. Introduction
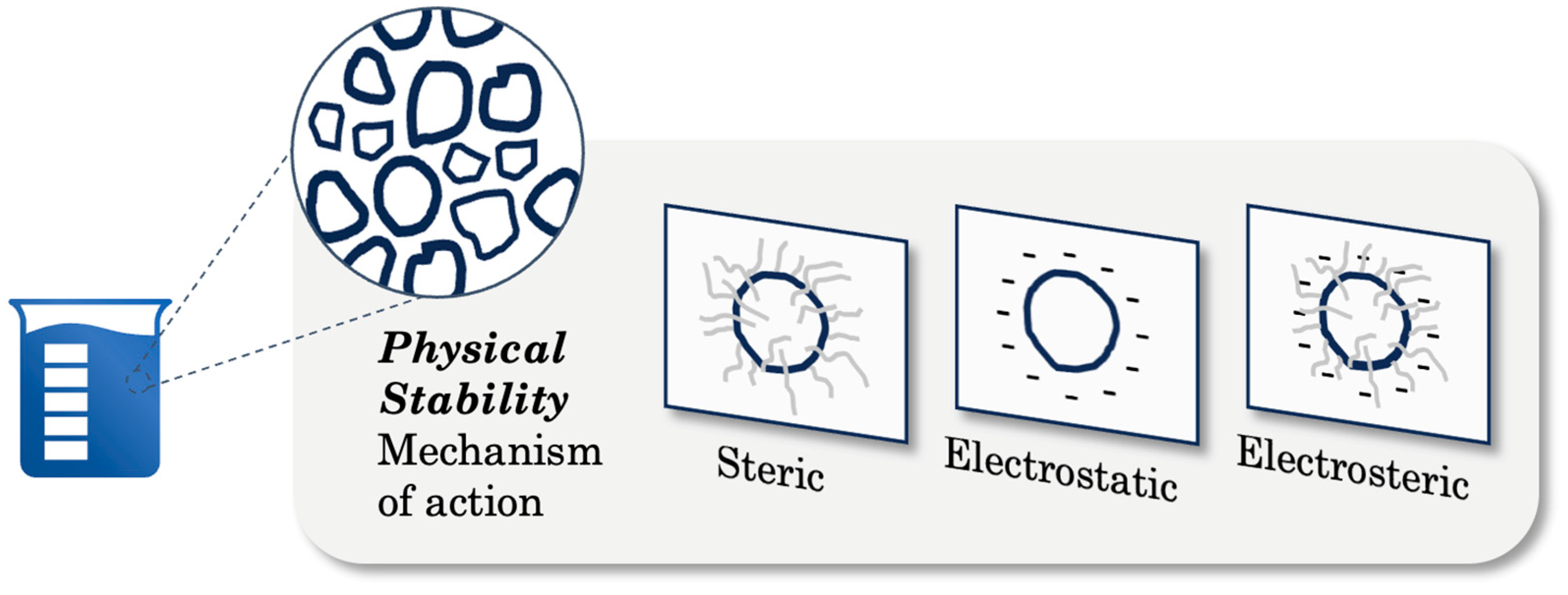
2. Physical Instability and Stabilization Mechanisms
3. In-Depth Exploration of Non-Traditional Natural Stabilizers
3.1. Natural Stabilizers and Their Stabilization Potential
3.1.1. Glycyrrhizin
| Stabilizer (s) a | Stabilizer Concentration (%) b | Drug c | Drug Concentration (%) b | Process d | Particle Size (nm) | Zeta Potential (mV) | Stability Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| Glycyrrhizin | 0.1 | AGE | 1 | HPH | 487 | −43.6 | Aggregation was effectively prevented. | [61] |
| P-188 | 0.1 | 1 | 550 | −15.7 | Compared to glycyrrhizin-stabilized nanosuspensions, they were relatively less stable. | |||
| Tween 80 | 0.1 | 1 | 482 | −13.4 | ||||
| TPGS | 0.1 | 1 | 659 | −16.5 | ||||
| Glycyrrhizin | N/A h | HPE | 10 e | WMM | 457 | N/A h | Significant aggregation and sedimentation were observed in both nanosuspensions. | [63] |
| P-407 | N/A h | 10 e | 442 | N/A h | ||||
| Panax notoginseng | 0.1 | BCL | 1 | HPH | 156 | −40.1 | Aggregation was effectively inhibited. | [65] |
| PVP-K30 | 0.1 | 1 | 145 | −31.7 | During one-month storage, NS stabilized with Tween 80 and HPMC were less stable in comparison to those stabilized with PNS and PVP-K30. | |||
| Tween 80 | 0.1 | 1 | 144 | −33.4 | ||||
| HPMC | 0.1 | 1 | 149 | −29.1 | ||||
| Panax notoginseng | 10 e | BVP | 1 | HPH | 141–160 | −47.9 | Panax notoginseng-stabilized nanosuspensions were relatively stable at 25 °C during one-month storage period. | [66] |
| RH40 | 10 e | 1 | −43.8 | |||||
| Tea saponins | 0.1 | DSN | 5 f | HPH | 525 g | −26.0 g | Exhibited good stabilization even in low doses. | [67] |
| Glycyrrhizin | 0.1 | 5 f | 728 g | −19.0 g | Layered notably after one-week storage due to sedimentation. | |||
| P-188 | 0.1 | 5 f | 911 g | −14.0 g | Displayed a higher polydispersity index, increased particle size, and lower stability relative to tea saponins-stabilized nanosuspensions. | |||
| PEG 6000 | 0.1 | 5 f | 818 g | −12.0 g | ||||
| HPMC | 0.1 | 5 f | 674 g | −15.0 g | ||||
| PVP-K30 | 0.1 | 5 f | 670 g | −13.0 g | ||||
| SDS | 0.1 | 5 f | 610 g | −23.0 g | ||||
| CMC-Na | 0.1 | 5 f | 905 g | −52.0 g | ||||
| Tea saponins | 0.05 | HDN | 0.8 | HPH | 356 g | N/A h | Even at very low concentrations, tea saponins were able to maintain stability. Ostwald ripening was prevented. Stable nanosuspensions were obtained through steric and electrostatic effects. Effective stabilization was attributed to the good interfacial properties of tea saponins. | [54] |
| 0.10 | 255 g | N/A h | ||||||
| 0.15 | 274 g | N/A h | ||||||
| 0.20 | 270 g | N/A h | ||||||
| 0.25 | 267 g | N/A h | ||||||
| 0.30 | 264 g | N/A h | ||||||
| 0.35 | 277 g | N/A h | ||||||
| 0.40 | 285 g | N/A h | ||||||
| 0.50 | 286 g | N/A h | ||||||
| Glycyrrhizin | 0.10 | 360 g | N/A h | Larger particle size and polydispersity index was observed compared to tea saponins-stabilized nanosuspensions. Overall considered, they are less stable than nanosuspensions stabilized with tea saponins. | ||||
| P-188 | 0.10 | 385 g | N/A h | |||||
| PEG 400 | 0.10 | 312 g | N/A h | |||||
| HPMC | 0.10 | 580 g | N/A h | |||||
| PVP-K30 | 0.10 | 409 g | N/A h | |||||
| SDS | 0.10 | 354 g | N/A h | |||||
| Gypenosides | N/A h | QUE | N/A h | HPH | 462 | N/A h | Exhibited strong stability within the pH range 6–8. | [68] |
| Gypenosides | 0.05 | QUE | 0.8 | HPH | 485 g | −27.0 g | Gypenosides-stabilized nanosuspensions had the narrowest size distribution (PDI < 0.1) and were highly stable. Effective stabilization was attributed to negative surface potential of these nanosuspensions. The main stabilization mechanism was hypothesized to be electrostatic repulsion. In general, all nanosuspensions, except HPMC, were able to maintain stability. Sedimentation was observed in HPMC-stabilized nanosuspensions due to presence of large particles. | [69] |
| 0.10 | 494 g | −28.0 g | ||||||
| 0.15 | 475 | −28.4 | ||||||
| 0.20 | 484 g | −28.3 g | ||||||
| 0.25 | 496 g | −27.9 g | ||||||
| 0.30 | 518 g | −25.3 g | ||||||
| 0.40 | 507 g | −24.9 g | ||||||
| Tea saponins | 0.15 | 474 g | −27.2 g | |||||
| Glycyrrhizin | 0.15 | 474 g | −29.7 g | |||||
| Soybean lecithin | 0.15 | 463 g | −38.4 g | |||||
| P-188 | 0.15 | 465 g | −30.6 g | |||||
| SDS | 0.15 | 471 g | −34.3 g | |||||
| Tween 80 | 0.15 | 479 g | −26.4 g | |||||
| HPMC | 0.15 | 790 g | −6.23 g | |||||
| PVP-K30 | 0.15 | 542 g | −25.8 g | |||||
| Alginate | 0.5 e | LOV | N/A h | HPH | 420 | −37.6 | Weak stabilization occurred. | [53] |
| 1 e | N/A h | 370 | −45.9 | Particle size remained stable during storage. | ||||
| 5 e | N/A h | 370 | −47.0 | |||||
| 10 e | N/A h | 466 g | N/A h | Use of high stabilizer concentrations led to higher viscosity. This resulted in weak impact force and less effective particle size reduction. | ||||
| 20 e | N/A h | 494 g | N/A h | |||||
| 30 e | N/A h | 605 g | N/A h | |||||
| 40 e | N/A h | 650 g | N/A h | |||||
| HPMC 2910 | 1 e | N/A h | 600 g | N/A h | In comparison to commonly used stabilizers, alginate-stabilized nanoparticles exhibited smaller particle size with narrow distribution, even at very low concentrations. No information was provided regarding the short-term stability of nanosuspensions containing commonly used stabilizers. | |||
| 20 e | N/A h | 431 g | N/A h | |||||
| PVP-K30 | 20 e | N/A h | 370 g | N/A h | ||||
| PVP-K17 | 20 e | N/A h | 360 g | N/A h | ||||
| PVP-K12 | 20 e | N/A h | 390 g | N/A h | ||||
| PVA | 20 e | N/A h | 470 g | N/A h | ||||
| P188 | 20 e | N/A h | 415 g | N/A h | ||||
| P127 | 20 e | N/A h | 442 g | N/A h | ||||
| SDS | 20 e | N/A h | 489 g | N/A h | ||||
| Alginate | 0.5 | LT | 1 f | P + US | 590 | −34.9 | Achieved smallest particle, greatest absolute zeta potential, highest stability performance. Both steric and electrostatic effects contributed to stability performance. | [70] |
| 1 | 1 f | 504 | −41.7 | |||||
| 2 | 1 f | 468 | −30.9 | |||||
| P127 | 0.5 | 1 f | 783 | −19.6 | Steric effects contributed to stability performance. The change in zeta potential with increasing concentrations was minimal. | |||
| 1 | 1 f | 617 | −22.6 | |||||
| 2 | 1 f | 837 | −20.8 | |||||
| Tween 80 | 0.5 | 1 f | 1020 | −17.9 | ||||
| 1 | 1 f | 933 | −16.2 | |||||
| 2 | 1 f | 987 | −15.3 | |||||
| HPMC | 0.5 | 1 f | 801 | −20.9 | ||||
| 1 | 1 f | 794 | −19.7 | |||||
| 2 | 1 f | 784 | −21.2 | |||||
| Lentinan | 0.05 | RG | 1 f | P | 302 g | N/A h | Lentinan generated steric hindrance on the surface of the drug, which prevented aggregation and growth of drug crystals. A weak electrostatic repulsion was observed due to low absolute zeta potential. The interaction between LNT-RG occurred through hydrogen bonding and hydrophobic forces. | [71] |
| 0.10 | 1 f | 309 g | N/A h | |||||
| 0.15 | 1 f | 239 g | N/A h | |||||
| 0.20 | 1 f | 222 g | N/A h | |||||
| 0.25 | 1 f | 191 g | N/A h | |||||
| 0.25 | 0.1 | 217 g | N/A h | |||||
| 0.25 | 0.2 | 249 g | N/A h | |||||
| 0.25 | 0.3 | 289 g | N/A h | |||||
| 0.25 | 0.4 | 348 g | N/A h | |||||
| 0.25 | 0.5 | 403 g | N/A h | |||||
| Soybean lecithin | 0.25 | DAI | 0.6 f | P + HPH | 425 | −56.9 | The stabilization mechanism is electrostatic. Formulations with SDS and soybean lecithin displayed good stability. Suspensions including chitosan and CMC-Na had large aggregates. | [72] |
| Chitosan | 0.25 | 0.6 f | 1600 | −56.5 | ||||
| CMC-Na | 0.25 | 0.6 f | 2300 | −62.3 | ||||
| SDS | 0.25 | 0.6 f | 460 | −52.1 | ||||
| P188 | 0.25 | 0.6 f | 379 | −28.0 | The stabilization mechanism is steric. Formulations with steric stabilization showed smaller particle sizes compared to those with electrostatic stabilization. Among all, PEG 600 demonstrated excellent stability, due to its high (absolute) zeta potential and the lowest PDI. | |||
| Tween 80 | 0.25 | 0.6 f | 456 | −22.0 | ||||
| PEG 600 | 0.25 | 0.6 f | 294 | −24.0 | ||||
| HPMC E3 | 0.25 | 0.6 f | 363 | −14.0 | ||||
| HPMC E5 | 0.25 | 0.6 f | 399 | −13.0 | ||||
| PVP-K30 | 0.25 | 0.6 f | 484 | −10.7 | ||||
| Soybean lecithin | 2 | MYR | 10 f | P + HPH | 419 g | −41.4 g | All nanosuspensions were physically stable during two-week storage. Nanosuspension including soybean lecithin displayed a slight decrease in particle size. | [73] |
| HPMC E3 | 2 | 10 f | 291 g | −17.8 g | ||||
| HP-β-CD | 2 | 10 f | 373 g | −30.1 g | ||||
| TPGS | 2 | 10 f | 386 g | −12.4 g | ||||
| SLS | 2 | 10 f | 400 g | −41.4 g | ||||
| P-188 | 2 | 10 f | 430 g | −29.5 g | ||||
| Gum arabic | 3 f | CUR | 0.5 f | HPH | 852 | N/A h | Maintained stability during one-week storage. | [74] |
| Native SPI | 0.80 | IND | 30 f | P + US | 304 g | N/A h | Protein-stabilized nanosuspensions achieved consistent stability through electrosteric stabilization mechanism. Among all, denatured proteins exhibited the best stability performance in comparison to others. | [75] |
| Native WPI | 0.80 | 30 f | 153 g | N/A h | ||||
| Native β-lg | 0.80 | 30 f | 907 g | N/A h | ||||
| Denatured SPI | 0.80 | 30 f | 131 g | −23.7 | ||||
| Denatured WPI | 0.80 | 30 f | 103 g | −30.8 | ||||
| Denatured β-lg | 0.80 | 30 f | 210 g | −25.9 | ||||
| PVP | 0.80 | 30 f | 412 g | N/A h | In general, traditional stabilizers displayed higher particle size. This is attributed to the impact of Ostwald ripening. | |||
| HPMC | 0.80 | 30 f | 390 g | N/A h | ||||
| PEG 6000 | 0.80 | 30 f | 308 g | N/A h | ||||
| EPC | 0.80 | 30 f | 414 g | N/A h | ||||
| Tween 80 | 0.80 | 30 f | 402 g | N/A h | ||||
| P188 | 0.80 | 30 f | 290 g | N/A h | ||||
| Denatured STE, denatured SPI | 0.00, 0.5 | RES | 60 f | P + US | 309 | −24.1 | The resultant mixture was unstable. | [76] |
| 0.10, 0.5 | 60 f | 276 | −24.1 | The low amount of the STE addition showed stability improvement. | ||||
| 0.25, 0.5 | 60 f | 196 | −22.3 | RES nanosuspensions displayed remarkable storage stability. | ||||
| 0.50, 0.5 | 60 f | 193 | −22.7 | |||||
| 1.00, 0.5 | 60 f | 312 | −22.1 | High STE concentrations resulted in decreased stability due to formation of aggregates. | ||||
| 2.00, 0.5 | 60 f | 361 | −20.2 | |||||
| β-lg(3.4) | 2 f | CUR | 4 f | P + US | 150 | +51.0 | Native β-lg stabilized nanosuspensions were stable overall. | [77] |
| β-lg (7.04) | 2 f | 4 f | 153 | −53.0 | ||||
| β-lg (5.5) | 2 f | 4 f | 1960 | N/A h | Severe aggregation. | |||
| Denatured β-lg(3.4) | 2 f | 4 f | 142 | +45.0 | Authors claimed curcumin sedimentation occurred during storage. | |||
| Denatured β-lg(7.04) | 2 f | 4 f | 171 | −51.0 | ||||
| Denatured β-lg(5.5) | 2 f | 4 f | 2740 | N/A h | Severe aggregation. | |||
| Denatured WPI | 0.25 | CAR | 7 f | P + US | 277 | −23.7 | All nanosuspensions exhibited less than 10% particle size increase during a three-month storage period. | [78] |
| P-188 | 0.70 | 2 f | 640 | −29.6 | ||||
| SDS | 0.50 | 4 f | 225 | −8.50 | ||||
| Chitosan, P-407 | 0.3, 0.2 | SPAR | 0.3 | P + US | 459 | −38.0 | The individual effects of P-407 and P-188 were less effective compared to combined use. | [79] |
| Chitosan, P-188 | 0.3, 0.2 | 0.3 | 498 | −40.0 | ||||
| Chitosan, P-407, P-188 | 0.3, 0.1, 0.1 | 0.3 | 400 | −39.0 | After six months, the nanosuspension remained stable. | |||
| HPMC, P-407 | 0.5, 0.2 | 0.3 | 137 | −34.0 | Nanosuspensions displayed particle size lower than 300 nm, and high entrapment efficiency (exceeding 90%), indicating good stability performance. | |||
| HPMC, P-188 | 0.5, 0.2 | 0.3 | 147 | −20.0 | ||||
| HPMC, P-407, P-188 | 0.5, 0.1 0.1 | 0.3 | 85.0 | −31.0 | ||||
| Chitosan, HPMC, P-407 | 0.15, 0.25, 0.2 | 0.3 | 267 | −42.0 | ||||
| Chitosan, HPMC, P-188 | 0.15, 0.25, 0.2 | 0.3 | 285 | −12.0 | ||||
| Chitosan, HPMC, P-407, P-188 | 0.15, 0.25, 0.1, 0.1 | 0.3 | 209 | −34.0 | ||||
| Human serum albumin | 2 f | PTX | 3 f | P + US | 443 | N/A h | Due to strong adsorption on the surface of drug nanoparticles, aggregation was effectively prevented. Higher concentration of albumin led to a further reduction in particle size. | [80] |
| 10 f | 400 | N/A h | ||||||
| 20 f | 383 | N/A h | ||||||
| 40 f | 352 | N/A h | ||||||
| 50 f | 326 | N/A h | ||||||
| Transferrin | 3 f | 400 | N/A h | Transferrin-stabilized formulations displayed highest stabilization performance. | ||||
| 4 f | 304 | N/A h | ||||||
| Immunoglobulin G | 3 f | 1540 | N/A h | Immunoglobulins promoted particle aggregation. | ||||
| 10 f | 1820 | N/A h | ||||||
| Immunoglobulin G (4.7) | 10 f | 534 | N/A h | Aggregation is intended to be prevented by reducing pH and adding organic osmolytes. | ||||
| Immunoglobulin G, 10% sucrose | 10 f | 360 | N/A h | |||||
| Human serum albumin | 0.3 f | PTX | 9.2 | HPH | 137 | −43.6 | Both nanosuspensions could maintain stability. | [81] |
| Human serum albumin, PEG | 0.3 f | 9.1 | 123 | −39.9 | ||||
| Neem gum | 0.03 f | ETO | 0.03 f | P + US | 89.0 | N/A h | Neem gum-stabilized nanosuspensions displayed lower stabilization performance compared to carboxymethyl neem gum-stabilized nanosuspensions. | [82] |
| 0.03 f | 0.04 f | 104 | N/A h | |||||
| 0.03 f | 0.05 f | 153 | N/A h | |||||
| 0.04 f | 0.03 f | 131 | N/A h | |||||
| 0.04 f | 0.04 f | 312 | N/A h | |||||
| 0.04 f | 0.05 f | 333 | N/A h | |||||
| 0.05 f | 0.03 f | 344 | N/A h | |||||
| 0.05 f | 0.04 f | 444 | N/A h | |||||
| 0.05 f | 0.05 f | 500 | N/A h | |||||
| g-Am Neem gum | 0.03 f | 0.03 f | 151 | N/A h | Acrylamide grafted neem gum-stabilized nanosuspensions displayed lower Stabilization performance compared to carboxymethyl neem gum-stabilized nanosuspensions. | |||
| 0.03 f | 0.04 f | 178 | N/A h | |||||
| 0.03 f | 0.05 f | 403 | N/A h | |||||
| 0.04 f | 0.03 f | 296 | N/A h | |||||
| 0.04 f | 0.04 f | 325 | N/A h | |||||
| 0.04 f | 0.05 f | 498 | N/A h | |||||
| 0.05 f | 0.03 f | 365 | N/A h | |||||
| 0.05 f | 0.04 f | 592 | N/A h | |||||
| 0.05 f | 0.05 f | 641 | N/A h | |||||
| Carboxymethyl Neem gum | 0.03 f | 0.03 f | 73.0 | N/A h | Carboxymethyl neem gum-stabilized nanosuspensions displayed smaller particle size, along with greatest stabilization performance. There was no significant change in particle size. | |||
| 0.03 f | 0.04 f | 84.0 | N/A h | |||||
| 0.03 f | 0.05 f | 136 | N/A h | |||||
| 0.04 f | 0.03 f | 177 | N/A h | |||||
| 0.04 f | 0.04 f | 293 | N/A h | |||||
| 0.04 f | 0.05 f | 366 | N/A h | |||||
| 0.05 f | 0.03 f | 272 | N/A h | |||||
| 0.05 f | 0.04 f | 464 | N/A h | |||||
| 0.05 f | 0.05 f | 499 | N/A h | |||||
| C. pulcherrima gum (1000 rpm, 1:10) | 0.1 | DRO | 20 f | P | 559 | N/A h | Increasing stirrer speed, solvent-to-antisolvent ratio and stabilizer concentration contributed to a reduction in particle size. C. pulcherrima gum served as an effective stabilizer; however, it could not prevent aggregation. | [83] |
| 0.3 | 20 f | 541 | N/A h | |||||
| C. pulcherrima gum (1500 rpm, 1:10) | 0.1 | 20 f | 441 | N/A h | ||||
| 0.3 | 20 f | 416 | N/A h | |||||
| C. pulcherrima gum (1000 rpm, 1:20) | 0.1 | DRO | 20 f | P | 490 | N/A h | ||
| 0.3 | 20 f | 453 | N/A h | |||||
| C. pulcherrima gum (1500 rpm, 1:20) | 0.1 | 20 f | 375 | N/A h | ||||
| 0.3 | 20 f | 346 | N/A h |
3.1.2. Panax Notoginseng
3.1.3. Tea Saponins
3.1.4. Gypenosides
3.1.5. Alginates
3.1.6. Lentinan
3.1.7. Lecithins
3.1.8. Gums
3.1.9. Food Proteins
3.1.10. Chitosan
3.1.11. Serum Proteins
3.1.12. Other Non-Traditional Natural Stabilizers
3.2. Pharmaceutical Safety, Acceptability and Health Concerns
3.3. Other Limitations, Challenges, and Advantages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, T.; Fleisher, D.; Kaddoumi, A. Overcoming poor aqueous solubility of drugs for oral delivery. In Prodrugs: Challenges and Rewards Part 1; Springer: New York, NY, USA, 2007; pp. 157–215. [Google Scholar] [CrossRef]
- Patel, D.; Zode, S.S.; Bansal, A.K. Formulation aspects of intravenous nanosuspensions. Int. J. Pharm. 2020, 586, 119555. [Google Scholar] [CrossRef] [PubMed]
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.M.; Stella, V.J. When can cyclodextrins be considered for solubilization purposes? J. Pharm. Sci. 2003, 92, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Vimalson, D.C. Techniques to enhance solubility of hydrophobic drugs: An overview. Asian J. Pharm. (AJP) 2016, 10, S67–S75. [Google Scholar]
- Jermain, S.V.; Brough, C.; Williams, R.O., III. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Da Silva, F.L.O.; Marques, M.B.D.F.; Kato, K.C.; Carneiro, G. Nanonization techniques to overcome poor water-solubility with drugs. Expert Opin. Drug Discov. 2020, 15, 853–864. [Google Scholar] [CrossRef]
- Geetha, G.; Poojitha, U.; Khan, K.A.A. Various techniques for preparation of nanosuspension—A review. Int. J. Pharma Res. Rev. 2014, 3, 30–37. [Google Scholar]
- Patravale, V.; Date, A.A.; Kulkarni, R. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, J.; Zhou, Y.; Wang, Y. Safety of nanosuspensions in drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: Formulation–Processing aspects and challenges. Pharmaceutics 2018, 10, 86. [Google Scholar] [CrossRef]
- Salazar, J.; Ghanem, A.; Müller, R.H.; Möschwitzer, J.P. Nanocrystals: Comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur. J. Pharm. Biopharm. 2012, 81, 82–90. [Google Scholar] [CrossRef]
- Jadhav, S.P.; Singh, S.K.; Chawra, H.S. Review on Nanosuspension as a Novel Method for Solubility and Bioavailability Enhancement of Poorly Soluble Drugs. Adv. Pharmacol. Pharm. 2023, 11, 117–130. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Pınar, S.G.; Oktay, A.N.; Karaküçük, A.E.; Çelebi, N. Formulation strategies of nanosuspensions for various administration routes. Pharmaceutics 2023, 15, 1520. [Google Scholar] [CrossRef]
- Chin, W.W.L.; Parmentier, J.; Widzinski, M.; Tan, E.H.; Gokhale, R. A brief literature and patent review of nanosuspensions to a final drug product. J. Pharm. Sci. 2014, 103, 2980–2999. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Taylor, K.M.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, A.; Hirvonen, J.; Peltonen, L. Stabilizing agents for drug nanocrystals: Effect on bioavailability. Pharmaceutics 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Ahire, E.; Thakkar, S.; Darshanwad, M.; Misra, M. Parenteral nanosuspensions: A brief review from solubility enhancement to more novel and specific applications. Acta Pharm. Sin. B 2018, 8, 733–755. [Google Scholar] [CrossRef] [PubMed]
- Tundisi, L.; Mostaço, G.B.; Carricondo, P.C.; Petri, D.F.S. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, X.; Zhao, H.; Zhou, Y.; Wang, L.; Tian, S.; Wang, Y. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int. J. Pharm. 2015, 495, 738–749. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, P.; Zhang, D.; Zhang, Q. A mini review of nanosuspensions development. J. Drug Target. 2012, 20, 209–223. [Google Scholar] [CrossRef]
- Elsebay, M.T.; Eissa, N.G.; Balata, G.F.; Kamal, M.A.; Elnahas, H.M. Nanosuspension: A Formulation Technology for Tackling the Poor Aqueous Solubility and Bioavailability of Poorly Soluble Drugs. Curr. Pharm. Des. 2023, 29, 2297–2312. [Google Scholar] [CrossRef]
- Kumbhar, P.S.; Nadaf, S.; Manjappa, A.S.; Jha, N.K.; Shinde, S.S.; Chopade, S.S.; Shete, A.S.; Disouza, J.I.; Sambamoorthy, U.; Kumar, S.A. D-α-tocopheryl polyethylene glycol succinate: A review of multifarious applications in nanomedicines. OpenNano 2022, 6, 100036. [Google Scholar] [CrossRef]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of drugs for bioavailability enhancement: A holistic formulation-process perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric plant-derived excipients in drug delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.K.; Wadhawan, J.; Bansal, A.K. Pharmaceutical nanocrystals: A promising approach for improved topical drug delivery. Drug Discov. Today 2021, 26, 2329–2349. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kumar, S.; Gokhale, R.; Burgess, D.J. Physical stability of nanosuspensions: Investigation of the role of stabilizers on Ostwald ripening. Int. J. Pharm. 2011, 406, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Michniak-Kohn, B. Influence of critical parameters of nanosuspension formulation on the permeability of a poorly soluble drug through the skin—A case study. Aaps Pharmscitech 2013, 14, 1108–1117. [Google Scholar] [CrossRef]
- Aldeeb, M.M.E.; Wilar, G.; Suhandi, C.; Elamin, K.M.; Wathoni, N. Nanosuspension-based drug delivery systems for topical applications. Int. J. Nanomed. 2024, 19, 825–844. [Google Scholar] [CrossRef]
- Yadav, G.V.; Singh, S.R. Nanosuspension: A promising drug delivery system. Pharmacophore 2012, 3, 217–243. [Google Scholar]
- Knieke, C.; Azad, M.; Davé, R.; Bilgili, E. A study of the physical stability of wet media-milled fenofibrate suspensions using dynamic equilibrium curves. Chem. Eng. Res. Des. 2013, 91, 1245–1258. [Google Scholar] [CrossRef]
- Li, M.; Alvarez, P.; Orbe, P.; Bilgili, E. Multi-faceted characterization of wet-milled griseofulvin nanosuspensions for elucidation of aggregation state and stabilization mechanisms. Aaps Pharmscitech 2018, 19, 1789–1801. [Google Scholar] [CrossRef]
- Azad, M.; Afolabi, A.; Bhakay, A.; Leonardi, J.; Davé, R.; Bilgili, E. Enhanced physical stabilization of fenofibrate nanosuspensions via wet co-milling with a superdisintegrant and an adsorbing polymer. Eur. J. Pharm. Biopharm. 2015, 94, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, E.; Afolabi, A. A combined microhydrodynamics–polymer adsorption analysis for elucidation of the roles of stabilizers in wet stirred media milling. Int. J. Pharm. 2012, 439, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, E.; Li, M.; Afolabi, A. Is the combination of cellulosic polymers and anionic surfactants a good strategy for ensuring physical stability of BCS Class II drug nanosuspensions? Pharm. Dev. Technol. 2016, 21, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Zhu, Z.; Margulis-Goshen, K.; Magdassi, S.; Talmon, Y.; Macosko, C.W. Polyelectrolyte stabilized drug nanoparticles via flash nanoprecipitation: A model study with β-carotene. J. Pharm. Sci. 2010, 99, 4295–4306. [Google Scholar] [CrossRef]
- Jassim, Z.E.; Rajab, N.A. Review on preparation, characterization, and pharmaceutical application of nanosuspension as an approach of solubility and dissolution enhancement. J. Pharm. Res. 2018, 12, 771–774. [Google Scholar]
- Jacobs, C.; Müller, R.H. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm. Res. 2002, 19, 189–194. [Google Scholar] [CrossRef]
- Mishra, P.R.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Production and characterization of Hesperetin nanosuspensions for dermal delivery. Int. J. Pharm. 2009, 371, 182–189. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C. Buparvaquone mucoadhesive nanosuspension: Preparation, optimisation and long-term stability. Int. J. Pharm. 2002, 237, 151–161. [Google Scholar] [CrossRef]
- Panmai, S.; Deshpande, S. Development of nanoformulations: Selection of polymeric stabilizers based on adsorption isotherm. In Abstracts of Papers of the American Chemical Society; Amer Chemical Soc: Washington, DC, USA, 2003; pp. U532–U533. [Google Scholar]
- Guan, J.; Zhang, Y.; Liu, Q.; Zhang, X.; Chokshi, R.; Mao, S. Exploration of alginates as potential stabilizers of nanosuspension. AAPS PharmSciTech 2017, 18, 3172–3181. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Song, J.; Zhang, X.; Deng, M.; Xie, L.; Zhang, L.; Li, X. Tea saponins as natural stabilizers for the production of hesperidin nanosuspensions. Int. J. Pharm. 2020, 583, 119406. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Juhnke, M.; John, E. Wet-Media Milling of Colloidal Drug Suspensions Stabilized by Means of Charged Nanoparticles. Chem. Eng. Technol. 2012, 35, 1931–1940. [Google Scholar] [CrossRef]
- Azad, M.A.; Afolabi, A.; Patel, N.; Davé, R.; Bilgili, E. Preparation of stable colloidal suspensions of superdisintegrants via wet stirred media milling. Particuology 2014, 14, 76–82. [Google Scholar] [CrossRef]
- Singh, P.; Mishra, G.; Dinda, S.C. Natural excipients in pharmaceutical formulations. In Evidence Based Validation of Traditional Medicines: A comprehensive Approach; Springer: Singapore, 2021; pp. 829–869. [Google Scholar]
- Nakach, M.; Authelin, J.-R.; Perrin, M.-A.; Lakkireddy, H.R. Comparison of high pressure homogenization and stirred bead milling for the production of nano-crystalline suspensions. Int. J. Pharm. 2018, 547, 61–71. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xu, J.; Xie, Y.; Zheng, Q.; Yue, P.; Yang, M. A natural triterpenoid saponin as multifunctional stabilizer for drug nanosuspension powder. Aaps Pharmscitech 2017, 18, 2744–2753. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Hang, L.; Hu, F.; Shen, C.; Shen, B.; Zhu, W.; Yuan, H. Development of herpetrione nanosuspensions stabilized by glycyrrhizin for enhancing bioavailability and synergistic hepatoprotective effect. Drug Dev. Ind. Pharm. 2021, 47, 1664–1673. [Google Scholar] [CrossRef]
- Ralla, T.; Salminen, H.; Braun, K.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Investigations into the structure-function relationship of the naturally-derived surfactant glycyrrhizin: Emulsion stability. Food Biophys. 2020, 15, 288–296. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, Y.; Xu, J.; Liu, Y.; Yue, P.; Zheng, Q.; Hu, P.; Yang, M. Panax notoginseng saponins as a novel nature stabilizer for poorly soluble drug nanocrystals: A case study with baicalein. Molecules 2016, 21, 1149. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Luo, Y.; Chen, Y.; Ma, Y.; Yue, P.; Yang, M. Novel breviscapine nanocrystals modified by panax notoginseng saponins for enhancing bioavailability and synergistic anti-platelet aggregation effect. Colloids Surf. B Biointerfaces 2019, 175, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Cai, C.; Cao, Y.; Li, X. Tea saponins as novel stabilizers for the development of diosmin nanosuspensions: Optimization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2023, 90, 105118. [Google Scholar] [CrossRef]
- Chen, H.-J.; Li, X.-F.; Deng, M.; Xie, L.; Liu, K.; Zhang, X.-M. Preparation and in vitro evaluation of quercetin nanosuspension stabilized by gypenosides. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2022, 47, 4365–4371. [Google Scholar]
- Chen, H.; Deng, M.; Xie, L.; Liu, K.; Zhang, X.; Li, X. Preparation and characterization of quercetin nanosuspensions using gypenosides as novel stabilizers. J. Drug Deliv. Sci. Technol. 2022, 67, 102962. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Al-Sanea, M.M.; Hendawy, O.M.; Salama, A.; Ibrahim, M.F.; Ghoneim, M.M. Influence of stabilizer on the development of luteolin nanosuspension for cutaneous delivery: An in vitro and in vivo evaluation. Pharmaceutics 2021, 13, 1812. [Google Scholar] [CrossRef]
- Suo, Z.; Sun, Q.; Peng, X.; Zhang, S.; Gan, N.; Zhao, L.; Yuan, N.; Zhang, Y.; Li, H. Lentinan as a natural stabilizer with bioactivities for preparation of drug–drug nanosuspensions. Int. J. Biol. Macromol. 2021, 184, 101–108. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Y.; Wang, H.; Sang, Z.; Han, X.; Ren, S.; Du, R.; Shi, X.; Xie, Y. Development of daidzein nanosuspensions: Preparation, characterization, in vitro evaluation, and pharmacokinetic analysis. Int. J. Pharm. 2019, 566, 67–76. [Google Scholar] [CrossRef]
- Hong, C.; Dang, Y.; Lin, G.; Yao, Y.; Li, G.; Ji, G.; Shen, H.; Xie, Y. Effects of stabilizing agents on the development of myricetin nanosuspension and its characterization: An in vitro and in vivo evaluation. Int. J. Pharm. 2014, 477, 251–260. [Google Scholar] [CrossRef]
- Duong, B.H.; Truong, H.N.; Phan Nguyen, Q.A.; Nguyen Phu, T.N.; Hong Nhan, L.T. Preparation of curcumin nanosuspension with gum arabic as a natural stabilizer: Process optimization and product characterization. Processes 2020, 8, 970. [Google Scholar] [CrossRef]
- He, W.; Lu, Y.; Qi, J.; Chen, L.; Hu, F.; Wu, W. Food proteins as novel nanosuspension stabilizers for poorly water-soluble drugs. Int. J. Pharm. 2013, 441, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.-L.; Wang, L.-Y.; Yang, X.-Q.; Wang, J.-M.; Wang, L.-J. Controlled formation and stabilization of nanosized colloidal suspensions by combination of soy protein and biosurfactant stevioside as stabilizers. Food Hydrocoll. 2016, 52, 317–328. [Google Scholar] [CrossRef]
- Aditya, N.; Yang, H.; Kim, S.; Ko, S. Fabrication of amorphous curcumin nanosuspensions using β-lactoglobulin to enhance solubility, stability, and bioavailability. Colloids Surf. B Biointerfaces 2015, 127, 114–121. [Google Scholar] [CrossRef]
- Geng, T.; Banerjee, P.; Lu, Z.; Zoghbi, A.; Li, T.; Wang, B. Comparative study on stabilizing ability of food protein, non-ionic surfactant and anionic surfactant on BCS type II drug carvedilol loaded nanosuspension: Physicochemical and pharmacokinetic investigation. Eur. J. Pharm. Sci. 2017, 109, 200–208. [Google Scholar] [CrossRef]
- Ambhore, N.P.; Dandagi, P.M.; Gadad, A.P. Formulation and comparative evaluation of HPMC and water soluble chitosan-based sparfloxacin nanosuspension for ophthalmic delivery. Drug Deliv. Transl. Res. 2016, 6, 48–56. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.-h.; Li, T.; McNally, H.; Park, K.; Sturek, M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J. Control. Release 2014, 176, 76–85. [Google Scholar] [CrossRef]
- Yin, T.; Cai, H.; Liu, J.; Cui, B.; Wang, L.; Yin, L.; Zhou, J.; Huo, M. Biological evaluation of PEG modified nanosuspensions based on human serum albumin for tumor targeted delivery of paclitaxel. Eur. J. Pharm. Sci. 2016, 83, 79–87. [Google Scholar] [CrossRef]
- Malviya, R.; Sharma, P.K.; Dubey, S.K. Stability facilitation of nanoparticles prepared by ultrasound assisted solvent-antisolvent method: Effect of neem gum, acrylamide grafted neem gum and carboxymethylated neem gum over size, morphology and drug release. Mater. Sci. Eng. C 2018, 91, 772–784. [Google Scholar] [CrossRef]
- Yeole, B.; Patil, R.; Lone, K.; Tekade, A. Preparation of nanoparticles of poorly water soluble dronedarone by antisolvent addition technique using natural polymer as a stabilizer. J. Pharm. Res. Clin. Pract. 2016, 6, 8–16. [Google Scholar]
- Wang, T.; Guo, R.; Zhou, G.; Zhou, X.; Kou, Z.; Sui, F.; Li, C.; Tang, L.; Wang, Z. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) FH Chen: A review. J. Ethnopharmacol. 2016, 188, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, W.; Wang, B.; Zhang, T.; Cui, X.; Pu, Y.; Li, N. Analytical methods and biological activities of Panax notoginseng saponins: Recent trends. J. Ethnopharmacol. 2019, 236, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-L.; He, Y. Tea saponins: Effective natural surfactants beneficial for soil remediation, from preparation to application. RSC Adv. 2018, 8, 24312–24321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Lee, Y.Z.; Kow, A.S.F.; Lee, Q.L.; Lim, L.W.C.; Yusof, R.; Tham, C.L.; Ho, Y.-C.; Tatt, L.M. Neuroprotective Effects of Gypenosides: A Review on Preclinical Studies in Neuropsychiatric Disorders. Eur. J. Pharmacol. 2024, 978, 176766. [Google Scholar] [CrossRef]
- Jain, D.; Bar-Shalom, D. Alginate drug delivery systems: Application in context of pharmaceutical and biomedical research. Drug Dev. Ind. Pharm. 2014, 40, 1576–1584. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential food and nutraceutical applications of alginate: A review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Kumar, A.; Paliwal, R.; Gulbake, A. Lentinan: An unexplored novel biomaterial in drug and gene delivery applications. J. Control. Release 2023, 356, 316–336. [Google Scholar] [CrossRef]
- Chung, C.; Sher, A.; Rousset, P.; Decker, E.A.; McClements, D.J. Formulation of food emulsions using natural emulsifiers: Utilization of quillaja saponin and soy lecithin to fabricate liquid coffee whiteners. J. Food Eng. 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? J. Sci. Food Agric. 2016, 96, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tikekar, R.V.; Nitin, N. Effect of antioxidant properties of lecithin emulsifier on oxidative stability of encapsulated bioactive compounds. Int. J. Pharm. 2013, 450, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.; Rai, P.; Tiwary, A.K.; Singh, R.S.; Kennedy, J.F.; Knill, C.J. Modified gums: Approaches and applications in drug delivery. Carbohydr. Polym. 2011, 83, 1031–1047. [Google Scholar] [CrossRef]
- Choudhary, P.D.; Pawar, H.A. Recently investigated natural gums and mucilages as pharmaceutical excipients: An overview. J. Pharm. 2014, 2014, 204849. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of natural polymer gum arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Gum arabic. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 627–652. [Google Scholar]
- Glicksman, M. Gum arabic (Gum acacia). In Food Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2019; pp. 7–29. [Google Scholar]
- Chen, L.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- Teimouri, S.; Kasapis, S.; Dokouhaki, M. Diffusional characteristics of food protein-based materials as nutraceutical delivery systems: A review. Trends Food Sci. Technol. 2022, 122, 201–210. [Google Scholar] [CrossRef]
- Kumar, M.R.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Klinkesorn, U. The role of chitosan in emulsion formation and stabilization. Food Rev. Int. 2013, 29, 371–393. [Google Scholar] [CrossRef]
- Kratz, F.; Elsadek, B. Clinical impact of serum proteins on drug delivery. J. Control. Release 2012, 161, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.; Booth, B.W. Biomedical applications of tannic acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Salama, A.H.; Asfour, M.H. Tannic acid coated nanosuspension for oral delivery of chrysin intended for anti-schizophrenic effect in mice. Int. J. Pharm. 2024, 656, 124085. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bang, J.-B.; Na, Y.-G.; Lee, J.-Y.; Cho, C.-W.; Baek, J.-S.; Lee, H.-K. Development and evaluation of tannic acid-coated nanosuspension for enhancing oral bioavailability of curcumin. Pharmaceutics 2021, 13, 1460. [Google Scholar] [CrossRef]
- López-Castejón, M.L.; Bengoechea, C.; Espinosa, S.; Carrera, C. Characterization of prebiotic emulsions stabilized by inulin and β-lactoglobulin. Food Hydrocoll. 2019, 87, 382–393. [Google Scholar] [CrossRef]
- Qin, Y.-Q.; Wang, L.-Y.; Yang, X.-Y.; Xu, Y.-J.; Fan, G.; Fan, Y.-G.; Ren, J.-N.; An, Q.; Li, X. Inulin: Properties and health benefits. Food Funct. 2023, 14, 2948–2968. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Froyen, L.; Van Humbeeck, J.; Martens, J.A.; Augustijns, P.; Van Den Mooter, G. Alternative matrix formers for nanosuspension solidification: Dissolution performance and X-ray microanalysis as an evaluation tool for powder dispersion. Eur. J. Pharm. Sci. 2008, 35, 344–353. [Google Scholar] [CrossRef]
- Tadros, T.F.; Vandamme, A.; Levecke, B.; Booten, K.; Stevens, C. Stabilization of emulsions using polymeric surfactants based on inulin. Adv. Colloid Interface Sci. 2004, 108, 207–226. [Google Scholar] [CrossRef]
- Exerowa, D.; Gotchev, G.; Kolarov, T.; Kristov, K.; Levecke, B.; Tadros, T. Oil-in-water emulsion films stabilized by polymeric surfactants based on inulin with different degree of hydrophobic modification. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 87–91. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Smistad, G.; Bøyum, S.; Alund, S.J.; Samuelsen, A.B.C.; Hiorth, M. The potential of pectin as a stabilizer for liposomal drug delivery systems. Carbohydr. Polym. 2012, 90, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-based gums and mucilages applications in pharmacology and nanomedicine: A review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.B.; Chawla, P.; Walasek-Janusz, M. A comprehensive review on plant-derived mucilage: Characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers 2021, 13, 1066. [Google Scholar] [CrossRef]
- Chowdhury, M.; Sengupta, A.; Datta, L.; Chatterjee, S. Role of mucilage as pharmaceutical additives and cytoprotective agent. J. Innov. Pharm. Biol. Sci. 2017, 4, 46–52. [Google Scholar]
- Thakur, L.; Ghodasra, U.; Patel, N.; Dabhi, M. Novel approaches for stability improvement in natural medicines. Pharmacogn. Rev. 2011, 5, 48. [Google Scholar] [CrossRef]
- Muller, R.E.; Morris, J.R.J. Sucrose-Ammoniated Glycyrrhizin Sweetening Agent. U.S. Patent No 3,282,706, 1966. [Google Scholar]
- El-Lahot, A.; El-Razek, A.; Amal, M.; Massoud, M.I.; Gomaa, E. Utilization of glycyrrhizin and licorice extract as natural sweetener in some food products and biological impacts. J. Food Dairy Sci. 2017, 8, 127–136. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of glycyrrhizic acid ammoniated (chemical group 30, miscellaneous substances) when used as a flavouring for all animal species. EFSA J. 2015, 13, 3971. [Google Scholar] [CrossRef]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A review on phytochemicals, pharmacological activities, drug interactions, and associated toxicities of licorice (Glycyrrhiza sp.). Food Front. 2021, 2, 449–485. [Google Scholar] [CrossRef]
- Isbrucker, R.; Burdock, G. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Mirhaghparast, S.K.; Zibaee, A.; Hajizadeh, J.; Ramzi, S. Toxicity and physiological effects of the tea seed saponin on Helicoverpa armigera. Biocatal. Agric. Biotechnol. 2020, 25, 101597. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, J.-Y.; Jeong, E.T.; Choi, T.H.; Yoon, T.M. Preservative effect of Camellia sinensis (L.) Kuntze seed extract in soy sauce and its mutagenicity. Food Res. Int. 2018, 105, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, C.; Hu, W.; Abdel-Samie, M.A.; Cui, H.; Lin, L. An overview of tea saponin as a surfactant in food applications. Crit. Rev. Food Sci. Nutr. 2023, 64, 12922–12934. [Google Scholar] [CrossRef]
- Nguyen, N.-H.; Ha, T.K.Q.; Yang, J.-L.; Pham, H.T.T.; Oh, W.K. Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021, 268, 113574. [Google Scholar] [CrossRef]
- Chen, Z.; Shu, G.; Taarji, N.; Barrow, C.J.; Nakajima, M.; Khalid, N.; Neves, M.A. Gypenosides as natural emulsifiers for oil-in-water nanoemulsions loaded with astaxanthin: Insights of formulation, stability and release properties. Food Chem. 2018, 261, 322–328. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current status of alginate in drug delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lindtner, O.; et al. Re-evaluation of lecithins (E 322) as a food additive. EFSA J. 2017, 15, e04742. [Google Scholar]
- Szuhaj, B.F.; Yeo, J.; Shahidi, F. Lecithins. Bailey’s Ind. Oil Fat Prod. 2005, 1–86. [Google Scholar] [CrossRef]
- Alhajj, M.J.; Montero, N.; Yarce, C.J.; Salamanca, C.H. Lecithins from vegetable, land, and marine animal sources and their potential applications for cosmetic, food, and pharmaceutical sectors. Cosmetics 2020, 7, 87. [Google Scholar] [CrossRef]
- Prasad, N.; Thombare, N.; Sharma, S.; Kumar, S. Gum arabic–A versatile natural gum: A review on production, processing, properties and applications. Ind. Crops Prod. 2022, 187, 115304. [Google Scholar] [CrossRef]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Phadke, C.; Mewada, A.; Dharmatti, R.; Thakur, M.; Pandey, S.; Sharon, M. Biogenic synthesis of fluorescent carbon dots at ambient temperature using Azadirachta indica (Neem) gum. J. Fluoresc. 2015, 25, 1103–1107. [Google Scholar] [CrossRef]
- Mankotia, P.; Choudhary, S.; Sharma, K.; Kumar, V.; Bhatia, J.K.; Parmar, A.; Sharma, S.; Sharma, V. Neem gum based pH responsive hydrogel matrix: A new pharmaceutical excipient for the sustained release of anticancer drug. Int. J. Biol. Macromol. 2020, 142, 742–755. [Google Scholar] [CrossRef]
- Buriti, F.C.; dos Santos, K.M.; Sombra, V.G.; Maciel, J.S.; Sá, D.M.T.; Salles, H.O.; Oliveira, G.; de Paula, R.C.; Feitosa, J.P.; Moreira, A.C.M. Characterisation of partially hydrolysed galactomannan from Caesalpinia pulcherrima seeds as a potential dietary fibre. Food Hydrocoll. 2014, 35, 512–521. [Google Scholar] [CrossRef]
- Senarathna, S.; Navaratne, S.; Wickramasinghe, I.; Coorey, R. Development and characterization of Caesalpinia pulcherrima seed gum-based films to determine their applicability in food packaging. J. Consum. Prot. Food Saf. 2022, 17, 65–72. [Google Scholar] [CrossRef]
- Pellegrini, A. Antimicrobial peptides from food proteins. Curr. Pharm. Des. 2003, 9, 1225–1238. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704S–712S. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Walker, D.A. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J. Nutr. 2006, 136, 319S–323S. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A.; Davis, J.P. Food protein functionality: A comprehensive approach. Food Hydrocoll. 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Perelman, M.; Hinchcliffe, M. Chitosan: A promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccines Immunother. 2014, 10, 797–807. [Google Scholar] [CrossRef]
- Kratz, F.; Beyer, U. Serum proteins as drug carriers of anticancer agents: A review. Drug Deliv. 1998, 5, 281–299. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Coussement, P.A. Inulin and oligofructose: Safe intakes and legal status. J. Nutr. 1999, 129, 1412S–1417S. [Google Scholar] [CrossRef]
- Kaur, N.; Gupta, A.K. Applications of inulin and oligofructose in health and nutrition. J. Biosci. 2002, 27, 703–714. [Google Scholar] [CrossRef]
- Song, H.; Chen, F.; Cao, Y.; Wang, F.; Wang, L.; Xiong, L.; Shen, X. Innovative applications of pectin in lipid management: Mechanisms, modifications, synergies, nanocarrier systems, and safety considerations. J. Agric. Food Chem. 2024, 72, 20261–20272. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Goksen, G.; Demir, D.; Dhama, K.; Kumar, M.; Shao, P.; Xie, F.; Echegaray, N.; Lorenzo, J.M. Mucilage polysaccharide as a plant secretion: Potential trends in food and biomedical applications. Int. J. Biol. Macromol. 2023, 230, 123146. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.S. Addressing excipient variability in formulation design and drug development. In Excipient Applications in Formulation Design and Drug Delivery; Springer: Cham, Switzerland, 2015; pp. 541–567. [Google Scholar]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- FSIS; USDA. Food Standards and Labeling Policy Book; US Department of Agriculture Food Safety and Inspection Service: Washington, DC, USA, 2005.
- Food and Drug Administration. Use of the term “natural” in the labeling of human food products; Requests for information and comments. Fed. Regist. 2015, 80, 69905–69909. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozsoysal, S.; Bilgili, E. Non-Traditional Natural Stabilizers in Drug Nanosuspensions. J. Pharm. BioTech Ind. 2024, 1, 38-71. https://doi.org/10.3390/jpbi1010005
Ozsoysal S, Bilgili E. Non-Traditional Natural Stabilizers in Drug Nanosuspensions. Journal of Pharmaceutical and BioTech Industry. 2024; 1(1):38-71. https://doi.org/10.3390/jpbi1010005
Chicago/Turabian StyleOzsoysal, Simay, and Ecevit Bilgili. 2024. "Non-Traditional Natural Stabilizers in Drug Nanosuspensions" Journal of Pharmaceutical and BioTech Industry 1, no. 1: 38-71. https://doi.org/10.3390/jpbi1010005
APA StyleOzsoysal, S., & Bilgili, E. (2024). Non-Traditional Natural Stabilizers in Drug Nanosuspensions. Journal of Pharmaceutical and BioTech Industry, 1(1), 38-71. https://doi.org/10.3390/jpbi1010005








