Abstract
Carbon disulfide (CS2) is a highly toxic and flammable solvent extensively used in chemical processes and laboratory analyses. This review examines both air and biological monitoring methods for assessing the exposure of laboratory workers to CS2. Emphasis is placed on the measurement of airborne CS2 concentrations and the monitoring of 2-thiothiazolidine-4-carboxylic acid (TTCA) in urine, a key biomarker of exposure. By analysing case studies and practical applications, the paper outlines the effectiveness and limitations of current monitoring techniques. Additionally, the review addresses key challenges such as CS2 volatility, dietary influence on biomarker levels, and the sufficiency of protective measures, including nitrile gloves. It also proposes best practices to mitigate exposure, such as improved ventilation, the use of polyvinyl alcohol gloves, and the substitution of CS2 with less hazardous solvents. This comprehensive review underscores the need for continuous vigilance in managing chemical exposure and offers insights into how laboratories can better protect their workers by integrating air and biological monitoring strategies.
1. Introduction
Carbon disulfide (CS2) is a highly toxic and flammable solvent with intensive applications in industrial and laboratory environments. Historically, CS2 has been used since the 19th century, predominantly in the production of rayon, rubber, and various sulphur-containing compounds [1,2]. In laboratory environments, CS2 is commonly used as a solvent, particularly in gas chromatography and other analytical procedures [3]. Despite its functional utility, CS2 poses considerable occupational health hazards, including neurotoxic, cardiotoxic, and reproductive toxicity effects [2,4,5].
Exposure to CS2 occurs primarily through inhalation and dermal contact, with acute exposure causing symptoms such as headaches, dizziness, and confusion. Chronic exposure, however, leads to more severe conditions, including polyneuropathy—characterised by motor and sensory dysfunction—and cardiovascular diseases such as atherosclerosis and hypertension. The compound’s toxicological profile has prompted regulatory bodies, including the American Conference of Governmental Industrial Hygienists (ACGIH) and the National Institute for Occupational Safety and Health (NIOSH), to establish strict exposure limits. The ACGIH recommends a threshold limit value (TLV) of 1 ppm for airborne CS2, while the biological exposure index (BEI) for 2-thiothiazolidine-4-carboxylic acid (TTCA), a key biomarker of CS2 metabolism, is set at 0.5 mg/g creatinine [6].
Although numerous studies on CS2 exposure monitoring have been conducted in industrial settings—particularly in the rayon, rubber, and sulphur chemical industries where large-scale handling occurs—comparatively little research has focused on laboratory environments [7,8,9,10,11,12]. These studies have provided valuable insights into controlling exposure in manufacturing environments. However, there is comparatively little research focused on CS2 exposure in laboratory settings [3], despite its widespread use in chemical analysis. The risks faced by laboratory personnel are equally significant, as they may be exposed to CS2 through inhalation and dermal contact during routine procedures. This gap in research underscores the need for more detailed evaluations of CS2 exposure in laboratory environments, where workers may be at risk even with smaller-scale usage of the solvent.
The reduction of CS2 exposure in laboratories has necessitated the implementation of both engineering and administrative controls. Engineering controls, such as fume hoods, are essential in minimising inhalation risks by providing adequate ventilation. Personal protective equipment, particularly gloves, plays a crucial role in preventing dermal absorption. However, studies [3] have shown that single-layer nitrile gloves may not provide sufficient protection during prolonged exposure, and the use of double-layered or polyvinyl alcohol gloves is recommended for extended handling of CS2. In addition to these protective measures, recent efforts to replace CS2 with less hazardous solvents in laboratory processes have shown promise [13,14]. Accelerated solvent extraction (ASE) using acetone, for example, offers a safer alternative for desorption processes in gas chromatography without compromising analytical performance [13]. Such innovations are critical in reducing reliance on hazardous solvents like CS2, contributing to a safer working environment in chemical laboratories.
This review critically evaluates current air and biological monitoring techniques for CS2 exposure, offering practical recommendations for laboratory practices. It offers a comparative analysis of methods and presents real-world applications, aiming to enhance occupational health standards. Lessons learned from the laboratory management of CS2 are applicable to other hazardous chemicals used in similar settings.
2. Chemical Properties and Toxicology of CS2
CS2 is a colourless, volatile liquid with a distinct odour. It is highly flammable and exhibits moderate solubility in water. CS2 has a boiling point of 46.3 °C and high vapour pressure, leading to rapid evaporation and significant inhalation exposure risks in laboratory environments. Due to its lipid solubility, CS2 can readily penetrate biological membranes, posing a significant risk for systemic toxicity.
2.1. Routes of Exposure
The primary route of CS2 exposure is inhalation, where it is absorbed into the bloodstream through the respiratory tract. Dermal contact also presents a significant exposure route, particularly when the skin is damaged or exposed for extended periods. Although ingestion is less common in laboratory settings, it can occur via contaminated food or inadvertent hand-to-mouth contact.
2.2. Health Effects
Acute exposure to elevated concentrations of CS2 can cause neurological symptoms, including headaches, dizziness, and confusion, reflecting its neurotoxic properties. Chronic exposure results in more severe conditions, such as polyneuropathy, which is characterised by motor and sensory dysfunction. Additionally, CS2 exposure adversely affects the cardiovascular system, increasing the risk of atherosclerosis and hypertension [2]. Reproductive toxicity is also associated with CS2, with evidence linking exposure to decreased fertility and negative pregnancy outcomes [5]. The overall toxicological profile of CS2 underscores the critical need for stringent exposure controls, especially in laboratory environments.
3. Regulatory Standards and Occupational Exposure Limits
3.1. Current Standards
Regulatory standards for CS2 in occupational settings primarily emphasise air monitoring, with several agencies providing complementary guidance on biological monitoring (Table 1). In the United States, OSHA has set a permissible exposure limit (PEL) for CS2 at 20 ppm, measured as an 8 h time-weighted average (TWA), to minimise airborne exposure in the workplace. In contrast, the NIOSH recommends a more stringent exposure limit of 1 ppm, reflecting a more conservative approach to the health risks posed by CS2. The ACGIH aligns with NIOSH, proposing a threshold limit value (TLV) of 1 ppm [6]. In the UK, the Health and Safety Executive (HSE) enforces a workplace exposure limit (WEL) of 10 ppm (TWA) [15], and Safe Work Australia has set similar limits, recognising the need for stringent control in occupational environments [16].

Table 1.
Comparison of exposure limits from different government bodies.
Biological monitoring, which evaluates the internal dose of CS2 or its metabolites, is also acknowledged by these agencies as an essential component of exposure assessment. ACGIH has established a BEI for CS2, recommending the measurement of TTCA in urine at the end of the shift, with a BEI of 5 mg/g creatinine. This measure is particularly valuable in assessing systemic exposure and ensuring that internal exposure levels remain within safe limits. NIOSH also supports this biological monitoring approach, while the HSE and Safe Work Australia provide guidance rather than mandatory limits, encouraging biological monitoring as a supplemental tool alongside air monitoring.
3.2. Comparison of International Standards
Each jurisdiction implements the monitoring of standards with some variation, influenced by local practices and compliance frameworks. For example, labs in the U.S. often adhere to OSHA’s higher PEL of 20 ppm, while European and Australian labs generally follow the more conservative NIOSH and ACGIH recommendations [17,18], focusing on the 1 ppm threshold. In practice, these jurisdictions tend to prioritise air monitoring due to its direct measurement of airborne CS2 levels, yet biological monitoring is also implemented selectively. ACGIH’s BEI offers a standardised reference for biological monitoring, which some labs use in conjunction with air monitoring for a more comprehensive exposure assessment. Jurisdictions like the UK and Australia provide guidelines on biological monitoring but do not mandate specific thresholds, allowing flexibility for labs to tailor monitoring based on their specific risk levels and operational demands.
3.3. Challenges in Implementing Standards
Implementing both airborne and biological monitoring in chemical analysis laboratories presents several challenges. The volatility of CS2 necessitates robust ventilation systems and continuous air monitoring to prevent overexposure. Additionally, the adoption of biological monitoring may be hindered by the requirement for specialised analytical techniques, resource constraints, and variability in individual biological responses. Despite these obstacles, integrating both monitoring approaches is crucial for providing a comprehensive assessment of CS2 exposure. This integration not only enhances worker protection but also ensures compliance with regulatory standards.
4. Air Monitoring Method for CS2
The Safe Work Australia [19] Model Work Health and Safety (WHS) Regulations mandate that no worker is exposed to CS2 at concentrations exceeding the Workplace Exposure Standard (WES) or Workplace Exposure Limit (WEL) and mandates air monitoring to determine workers’ CS2 exposure concentrations where there is uncertainty about whether the concentration exceeds the WES/WEL, or where monitoring will inform the determination of risk to workers’ health. Health risks associated with CS2 exposure are best assessed by measuring airborne concentrations through air monitoring. The measurement of CS2 typically requires occupational hygiene skills to sample the air in the worker’s breathing zone over several hours, followed by laboratory analysis of the collected sample.
The Regulations [19] note that air monitoring must not be used as an alternative to controlling exposure and is best conducted after control measures have been implemented. Air monitoring involves both sampling and analysis (Table 2), and the precision and accuracy of CS2 exposure measurements depend on both components.

Table 2.
Standard methods for determining CS2.
4.1. Sampling Techniques for Air Monitoring
Personal monitoring should be conducted when a workplace assessment by an occupational hygienist or another competent person indicates a potential health risk due to CS2 exposure. The commonly used standard methods for sampling CS2 in air are presented in Table 2. For personal monitoring during full shifts or tasks involving only the gaseous component, workers are to be equipped with a sampler (tube or passive badge) placed in the breathing zone. Tubes are connected to a portable sampling pump, whereas badges sample the air by diffusion. At the end of the sampling period, the tube or badge is sealed and transferred to a laboratory, where the chemical is liberated from the absorbent and quantified using separate analytical methods. If the sample cannot be sent to the laboratory immediately, it is important to ensure that the samples are stored properly while awaiting dispatch.
Recent advancements in portable GC-MS and sensor networks have enabled more precise, real-time CS2 monitoring in laboratory and industrial settings. For example, Duff et al. [23] demonstrated the use of a portable GC-MS for the in-field screening of organic pollutants, which provided rapid, actionable data to aid emergency responses in pollution incidents [23]. Similarly, Bocos-Bintintan et al. [24] employed ion mobility spectrometry (IMS) for sensitive, real-time detection of CS2 in air, showing how portable IMS devices can trace toxic gases like CS2 at low concentrations suitable for industrial hygiene applications [24]. Liao et al. [25] illustrated the effectiveness of sensor networks in continuously monitoring volatile organic compounds in a wastewater treatment setting, where CS2 and other odorants are tracked for compliance and environmental impact mitigation [25]. These technologies, when aligned with specific application requirements, improve monitoring accuracy and support compliance with safety standards across diverse environments, enhancing both response speed and worker protection.
For an in-depth evaluation of the advantages and limitations of direct-reading instruments and sample collection techniques, see Section 4.3, where each method’s strengths and limitations in the context of CS2 exposure monitoring are discussed.
4.2. Analysis Techniques for Air Monitoring
The analysis of collected CS2 samples should be performed by a laboratory accredited by the National Association of Testing Authorities (NATA). The methods mentioned in Table 2, which involve desorbing the CS2 from the charcoal using toluene, were also followed for sample analysis. In the early years, gas chromatography with a GC-FPD was used [20], with a limit of detection (LOD) of 20 µg per sample. Recently, gas chromatography–mass spectrometry (GC-MS) with single ion monitoring (SIM) mode has been employed, improving the LOQ to 0.01 µg per sample [3]. This analytical advantage is valuable, as CS2 vapour measurements in an analytical laboratory are expected to be very low compared to levels found in the viscose industry.
Beyond the analytical techniques discussed in this section, laboratory-specific risks include potential vapour buildup, dermal absorption during handling, and surface residue, which may lead to low-level, repeated exposures even at lower concentrations.
4.3. Comparison and Integration of Methods
The comparison of the two personal sampling methods in a field study using linear regression demonstrated with statistical certainty an excellent concordance of the methods. The study shows that the method for determining air levels of CS2 by passive sampling is particularly associated with high systematic errors [26].
Passive sampling offers simplicity and suitability for long-term studies, providing time-integrated exposure data without requiring continuous power. While generally less sensitive than active sampling, passive methods remain valuable for cumulative exposure assessments in environments with consistent pollutant levels [27]. Real-time monitors offer the advantage of immediate feedback and the ability to respond quickly, but they are prone to false positives and require frequent calibration. In terms of analytical precision, MS detectors provide greater sensitivity and selectivity compared to FPD. It is suggested to integrate different methods for monitoring CS2 exposure, such as using active or passive samplers for long-term monitoring and real-time instruments for immediate detection, to provide comprehensive monitoring.
4.4. Future Trends in Air Monitoring
Advancements in air monitoring technology are rapidly progressing, with emerging technologies such as sensor networks and advanced portable GC-MS systems offering potential enhancements for CS2 air monitoring. These advancements are likely to lead to more accurate and efficient monitoring, with sensor networks providing real-time data and portable GC-MS devices allowing for on-site analysis, reducing the time lag between sampling and results.
Nevertheless, several research gaps remain. For instance, there is a need to develop more sensitive passive samplers and to improve the accuracy of real-time detectors, which are essential for enhancing long-term monitoring and reducing false positives, respectively.
5. Biological Monitoring Method for CS2
Biological monitoring in occupational safety entails detecting specific biomarkers in workers’ biological samples and comparing the results to established reference values. CS2 is metabolised into TTCA, which can be measured in post-shift urine samples, making it a key biomarker for CS2 exposure [28]. The ACGIH sets a BEI of 0.5 mg/g creatinine in urine. However, TTCA levels may also increase due to dietary intake, particularly after consuming brassica vegetables. Traditional methods for monitoring CS2 exposure via TTCA in urine are often complex and time-consuming, which has led to the development of more efficient methods such as hydrophilic interaction liquid chromatography (HILIC) [3].
5.1. Sampling Techniques for Biological Monitoring
Urine samples should be collected at the end of the shift, and ideally at the end of the working week. It is essential to use the correct type of contamination-free container and ensure the required volume is collected. Employees should change out of their work clothes and wash their hands before providing a sample to prevent the possibility of inadvertent contamination.
TTCA is not a specific indicator solely for CS2 exposure. TTCA can also be detected in the urine of individuals taking disulfiram (Antabuse) or those exposed to dithiocarbamate pesticides and rubber accelerators. Dietary cabbage (or other cruciferous vegetables) may also give rise to significant quantities of TTCA in urine. Any exposure to these substances should be noted [3]. If the sample cannot be sent to the laboratory immediately, it is important to ensure that the samples are stored properly while awaiting dispatch.
5.2. Analysis Techniques for Biological Monitoring
The analysis of TTCA in urine typically involves two steps: either taking an aliquot of the extracted urine or diluting it with acetonitrile, followed by injection into a high-performance liquid chromatography system with a UV-visible detector (LC-UV) or an ultra-high-performance liquid chromatography–tandem mass spectrometer (UPLC-MS/MS). In Table 3, the method [3] demonstrates a commendable balance between simplicity and effectiveness. The use of direct dilution with acetonitrile, combined with LC-MS/MS, yields a solid LOD of 50 µg/L, offering a reliable approach with less procedural complexity compared to other methods. Although the techniques [12,29] achieve a lower LOD of 1 µg/L, the method [3] remains advantageous due to its straightforward preparation process, making it a practical and efficient option in many scenarios.

Table 3.
The summary of developed analytical methods for the determination of TTCA in urine.
To ensure the accuracy of these methods, it is recommended that laboratories participate in an interlaboratory proficiency programme for toxicological analyses of biological materials. One such programme is organised by the Institute and Outpatient Clinic for Occupational, Social, and Environmental Medicine at the University of Erlangen-Nuremberg, Germany (GEQUAS). This scheme includes 2-Thio-thiazolidine-4-carboxylic acid (TTCA), a metabolite of carbon disulfide (CS2), as analytical parameter 41 for evaluation purposes.
5.3. Future Trends in Biological Monitoring
Future trends in biological monitoring for CS2 exposure will likely focus on the automation and enhancement of analytical techniques, making processes faster and more accurate. There will be a push towards developing more specific biomarkers that better distinguish CS2 exposure from other factors. Additionally, wearable technology for real-time monitoring and personalised assessment approaches will emerge, allowing for more precise and immediate evaluations of worker exposure, ultimately improving occupational health and safety.
6. Case Studies and Real-World Applications
6.1. Laboratory Operations
CS2 is routinely used in analytical and environmental laboratories worldwide. These laboratories provide specialised occupational health analytical services, focusing on the detection of hazardous substances in workplace environments. In most cases, charcoal tubes or passive samplers are submitted to the laboratory by occupational hygienists from various industries where volatile organic compounds (VOCs) are monitored in workplace air. These sampling devices contain activated charcoal, which traps VOCs through adsorption. The laboratory procedure involves desorbing the VOCs from the charcoal tubes or passive samplers using CS2 as a solvent, followed by injection of the extract into a GC for analysis. Calibration standards are also prepared using CS2 and injected into the GC for comparison.
The sample preparation processes for CS2 exposure monitoring are rigorously standardised, adhering to established methodologies such as NIOSH methods 1600 and HSE MDHS 97 that are discussed in Section 4. These methods ensure accuracy and reproducibility in the measurement of airborne concentrations of CS2.
To preserve the integrity of samples, charcoal tubes or passive samplers are stored at a temperature of 4 °C until analysis. This controlled environment prevents any premature volatilisation of the solvent, which could lead to inaccurate results. During analysis, charcoal tubes are carefully opened within a fume hood to minimise the release of CS2 vapours. The granulated charcoal is placed into vials containing 1 mL of CS2 solvent. The mixture is then allowed to equilibrate for 30 min, ensuring that the adsorbed CS2 is fully desorbed into the solvent. Following this step, the vials are subjected to GC for quantification.
A slightly different protocol is followed for passive samplers. CS2 is added to the sampler, which is then agitated and left undisturbed for 60 min. This extended period allows for complete extraction of the analyte from the sampling medium. Calibration standards prepared using similar procedures are analysed alongside the samples to ensure accurate quantification. All waste generated during the analysis, including solvents and used charcoal, is safely disposed of in accordance with hazardous waste management guidelines to prevent environmental contamination and exposure to laboratory personnel.
6.2. Current Controls (Engineering, Administrative, PPE)
The primary engineering control for safely handling CS2 is the use of a fume cupboard, also known as a fume hood. This ventilation system is designed to continuously draw air away from the operator and out of the laboratory, thereby preventing the accumulation of hazardous airborne contaminants. In Australia, laboratory fume cupboards must undergo annual maintenance by a company accredited by the NATA to meet accreditation requirements. These cupboards typically operate at an average face velocity of 0.71 m/s, in accordance with AS/NZS 2243.8 [35], ensuring optimal performance and adequate protection.
In addition to engineering controls, a combination of administrative controls and personal protective equipment (PPE) is used to further minimise the risk of exposure. Laboratory personnel are required to wear laboratory coats, safety glasses, and nitrile disposable gloves when handling CS2. These protective measures form a barrier between the skin and the chemical, thereby reducing the potential for dermal absorption.
Inhalation and skin contact are the two primary routes through which CS2 can enter the body. Monitoring the levels of TTCA, a metabolite of CS2, in urine provides a comprehensive indicator of exposure, as it reflects absorption through all potential exposure routes. Air monitoring, especially in the worker’s breathing zone, is particularly useful in assessing exposure through inhalation, which is often the most significant route of exposure in laboratory settings.
6.3. Case Studies and Real-World Applications
To demonstrate the effectiveness of the implemented controls, a series of case studies were conducted in a laboratory in Sydney, Australia, comparing data from different time periods and analysing the impact of various safety measures on exposure levels [3].
The first set of studies in Table 4, conducted in 1998, focused solely on air monitoring. The results indicated that the mean exposure of laboratory staff handling CS2 was 0.043 ppm, as measured in their breathing zone over a TWA. Interestingly, the non-exposed control group, consisting of analytical staff not involved in CS2 handling, showed a mean exposure of 0.041 ppm. These findings suggest that the laboratory’s existing ventilation controls were somewhat effective in maintaining low levels of airborne CS2, although there was little distinction between exposed and non-exposed individuals.

Table 4.
Comparison of air and biological monitoring studies in different years.
In 2014, additional safety measures were implemented in the laboratory, including the installation of local exhaust ventilation (LEV) over the gas chromatography sample carousel and the use of thicker nitrile gloves for prolonged handling of CS2. The subsequent study included both air and biological monitoring, providing a more comprehensive assessment of exposure.
The air monitoring results from the 2014 study in Table 4 showed a significant reduction in CS2 levels, with the geometric mean concentrations recorded at 0.0033 ppm for exposed analysts and 0.0022 ppm for non-exposed controls. A t-test analysis confirmed no statistically significant difference between the two groups, indicating that the implemented ventilation controls were highly effective in minimising inhalation exposure.
Biological monitoring, which was introduced in the 2014 study, revealed that the pre-shift TTCA levels in urine for exposed workers were 1.0 µmol/mol creatinine, rising to 4.1 µmol/mol creatinine post-shift. The increase in TTCA levels was statistically significant (t = 4.8, p = 0.003), highlighting a mild but measurable exposure to CS2. Notably, all TTCA levels were below the ACGIH’s BEI of 350 µmol/mol creatinine, suggesting that the overall exposure was well controlled.
6.3.1. Biological Monitoring Insights
Biological monitoring has emerged as a crucial tool for assessing chemical exposure, offering several advantages over air monitoring alone. While air monitoring provides valuable information on inhalation risks, biological monitoring accounts for all potential routes of entry into the body, including inhalation, dermal absorption, and ingestion. This holistic approach enables a more accurate assessment of the total body burden of CS2.
The 2014 study’s biological monitoring data in Table 4 demonstrated that, even though air monitoring results were well below occupational exposure limits, there was still evidence of mild exposure through non-inhalation routes [3]. The significant post-shift increase in TTCA levels indicated the potential contribution of dermal absorption or inadvertent ingestion to overall CS2 exposure.
6.3.2. Glove Permeation Study
Nitrile gloves, commonly used in laboratories for their chemical resistance and dexterity, were subjected to a permeation study to evaluate their effectiveness in preventing dermal exposure to CS2. Nitrile is commonly chosen for its chemical resistance and dexterity, but the study revealed that it may not provide sufficient protection during extended contact with CS2 [3].
A glove permeation study was conducted to assess CS2 exposure during laboratory work [3]. Nitrile gloves were tested using 3M OVMs placed inside single, double, and quadruple glove layers. The gloves were exposed to a simulated atmosphere containing 50 ppm of CS2 for 110 min. Results showed that a single glove layer had a permeation level of 2.4 ppm, while double and quadruple layers reduced permeation to 1.2 and 0.6 ppm, respectively. These findings suggest that increasing the number of glove layers can effectively reduce CS2 exposure. However, a recent study [36] emphasised the need for careful evaluation of both the advantages and potential risks associated with the use of protective gloves and creams in workplace settings. In contrast, another study [37] raised concerns about the reliability of these conclusions, suggesting that further evidence is needed to substantiate the recommendations.
To enhance protection, it is recommended that laboratory personnel wear double gloves or use thicker gloves, especially during tasks involving prolonged exposure to CS2. While thicker gloves may reduce dexterity, the added protection is critical for minimising dermal exposure. Additionally, the glove manufacturer recommends frequent glove changes throughout the workday to further reduce the risk of permeation.
6.3.3. Dietary Influence on TTCA Levels
One often-overlooked factor in biological monitoring is the influence of diet on biomarker levels. The consumption of brassica vegetables, such as cabbage, broccoli, and Brussels sprouts, can significantly elevate TTCA levels in urine. These vegetables contain natural compounds that are metabolised into TTCA, leading to a potential confounding of exposure assessment results [11,34].
A dietary study conducted alongside the 2014 biological monitoring programme [3] investigated the impact of consuming 200 g of coleslaw on urinary TTCA levels. Participants provided urine samples before and after lunch, with post-lunch samples collected up to 6 h later. The results showed a substantial increase in TTCA levels following the consumption of coleslaw, although all levels remained below the ACGIH’s BEI guideline.
In the context of CS2 exposure assessment, it is crucial to control dietary factors by advising participants to refrain from consuming brassica vegetables on the day of the exposure assessment. This precaution ensures that the measured TTCA levels accurately reflect occupational exposure rather than dietary intake.
In the 2014 study [3], the three analysts handling CS2 maintained a dietary diary, documenting their intake of brassica vegetables. The data revealed that a normal meal including these vegetables could lead to a significant rise in TTCA levels, with a geometric mean increase from 1.7 µmol/mol creatinine to 29.9 µmol/mol creatinine. As a result, these data were excluded from the final analysis of CS2 exposure, underscoring the importance of dietary control in biological monitoring studies.
6.3.4. Correlation Between Airborne CS2 and TTCA in Urine
Lauwerys [38] summarised several studies that used linear regression equations to correlate CS2 air concentrations with end-of-shift TTCA urinary concentrations. These equations predict urinary TTCA levels at 5 ppm and 10 ppm CS2 exposure. The regression coefficients (r), ranging from 0.80 to 0.95, indicate strong correlations between airborne CS2 exposure and urinary biomarker levels, further validating TTCA as a reliable biomarker for monitoring CS2 exposure.
As previously discussed, the study [3] involving both air and biological monitoring of CS2 exposure in a chemical laboratory showed significantly lower exposure levels compared to a study [36] conducted in the viscose industry. The latter study [36] reported a median ambient air CS2 exposure of 2.77 ppm, with a median internal exposure of 644.6 µmol TTCA/mol creatinine. In contrast, the ACGIH threshold values for CS2 are set at 1 ppm (TWA) and 350 µmol TTCA/mol creatinine (BEI). Data from both studies [3,36], alongside the ACGIH threshold values, demonstrated a strong correlation (R2 = 0.96) between airborne CS2 concentrations and TTCA levels in urine, reinforcing the reliability of TTCA as an exposure biomarker.
These findings support recommendations to reduce the TWA for CS2 to 1 ppm, as established by the ACGIH, and suggest that health and safety authorities, such as the HSE and Safe Work Australia, consider adopting similar limits. While air monitoring provides direct evidence of inhalation exposure, biological monitoring offers a more comprehensive assessment by capturing total exposure through all routes, including inhalation, dermal absorption, and ingestion.
The combined use of air and biological monitoring in environments where CS2 is present can act as an early warning system for detecting and mitigating exposure risks. This integrated approach enables laboratory managers and safety officers to develop more targeted control measures, ensuring that both inhalation and dermal exposures are effectively managed.
7. Control Measures and Best Practices
Several control measures were evaluated to address PPE, odour, handling, and storage issues related to CS2 in the laboratory (Figure 1).
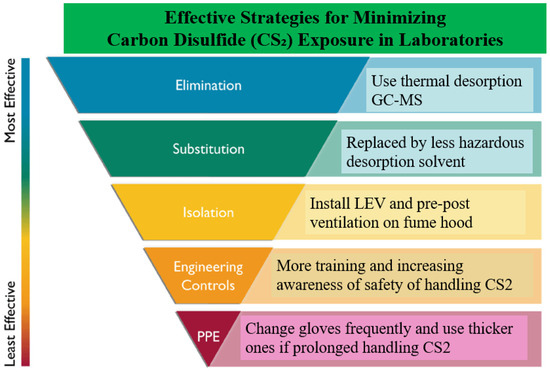
Figure 1.
Effective strategies for minimising CS2 exposure in laboratories.
Regarding PPE, laboratory personnel typically wear laboratory coats, safety glasses, and nitrile gloves, the latter offering moderate protection against CS2. Although nitrile gloves are not the most effective option for this solvent, they are considered satisfactory under controlled conditions. For better protection, polyvinyl alcohol gloves are recommended due to their excellent resistance to degradation and permeation.
Static sampling conducted around the laboratory revealed no detectable CS2 outside the building, but low background levels were detected indoors [3]. The highest concentrations were found in front of a switched-off fume hood and above a GC autosampler after injecting CS2 solutions. These levels are close to the odour threshold of 0.1 ppm, indicating that odours are noticed only when the fume hood is off.
Storage practices involve keeping CS2 in the fume hood, reducing the risk of accidental spillage. Although not ideal, this practice minimises handling. A worst-case scenario simulation indicated that dropping a one-litre bottle of CS2 in the laboratory could result in vapour concentrations as high as 700 ppm in the lab and over 5000 ppm in the storeroom, far exceeding the NIOSH Immediately Dangerous to Life or Health (IDLH) limit of 500 ppm. These risks underscore the importance of cautious handling and proper storage to prevent hazardous exposures.
In recent years, researchers worldwide have sought alternatives to hazardous CS2 by developing less harmful desorption solvents to control and reduce workplace exposure. A desorption study of 57 VOCs has been conducted by use of accelerated solvent extraction (ASE) and gas chromatography–mass spectrometry [13]. The findings demonstrated that the use of safer solvents, such as acetone, in conjunction with ASE, could replace CS2 for the chemical removal of VOCs from activated charcoal. ASE has several advantages over traditional solvent-extraction methods, including shorter extraction time, minimum sample manipulation, high reproducibility, and less extraction discrimination. No loss of sensitivity occurs and there is also a salutary effect on bench workers’ health and on the smell of laboratory air.
More new desorption methods were investigated to replace CS2 [14]. The efficient desorption of organic solvents from activated carbon was achieved with an anionic surfactant solution, focusing on its washing and emulsion.
The ultimate control measure is to eliminate the use of CS2 altogether. The NIOSH Method [39] employs a hyphenated thermal desorption–gas chromatography/mass spectrometry (TD-GC/MS) instrumental technique with thermal desorption tubes as the medium for analysing a wide range of volatile and semi-volatile compounds. This method does not use solvent extraction methods for analysis. While both methods have certain limitations, benefits such as sensitivity gains related to pre-concentration (thermal desorption) techniques along with the added benefit of control via the elimination of solvent support a review of standing methods for many VOCs.
Finally, the Work Health and Safety Regulations in many countries across the world prescribe that health monitoring is carried out by or supervised by a registered medical practitioner with experience in health monitoring if the worker is carrying out work with hazardous chemicals including CS2. Health monitoring before starting work in a CS2 process is essential to detect any health changes in workers. It includes discussing possible health effects, recognising and reporting symptoms, and determining the frequency and type of testing. Baseline kidney and liver function tests are recommended if exposure is expected. Continuous monitoring is necessary, especially after spills or symptoms. Urinary TTCA levels are used for biological monitoring, with specific guidance values for exposure assessment. Removal from work may be required if adverse health effects are detected. Final medical examinations should focus on the respiratory system and skin.
8. Conclusions
The combined use of air and biological monitoring methods provides a comprehensive approach to assessing CS2 exposure in laboratories, ensuring effective occupational health management. While air monitoring remains essential for identifying inhalation risks, biological monitoring complements it by capturing all routes of exposure, including inhalation, dermal absorption, and ingestion. Data from case studies conducted in laboratories demonstrate the effectiveness of control measures such as LEV and PPE in reducing exposure levels to well below established occupational exposure limits. Despite low airborne concentrations of CS2, the findings indicate that dermal absorption and dietary factors can still contribute to overall exposure, as reflected in elevated TTCA levels in urine.
To further reduce risks, increasing emphasis is being placed on replacing CS2 with less hazardous solvents in laboratory environments. Techniques such as ASE using acetone provide safer alternatives for desorbing VOCs from activated charcoal. Moreover, the adoption of TD-GC/MS methods can entirely eliminate the use of solvents, thereby contributing to a safer working environment.
In conclusion, the integration of air and biological monitoring should be a key component of laboratory safety programmes to ensure comprehensive exposure assessments. The continuous improvement of control measures, including the adoption of advanced technologies and the replacement of hazardous substances like CS2 with safer alternatives, is critical to maintaining a safe and sustainable laboratory environment.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Beauchamp, R.O., Jr.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Goldberg, L.A. Critical review of the literature on carbon disulfide toxicity. Crit. Rev. Tocicol 1983, 11, 169–278. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Youn, K.; Kim, K.; Park, K. Carbon disulfide exposure estimate and prevalence of chronic diseases after carbon disulfide poisoning-related occupational diseases. Ann. Occup. Environ. Med. 2017, 26, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tan, Z.; Mazereeuw, M.; O’Donnell, G.E. Evaluation of low-level carbon disulfide exposure in a chemical analysis laboratory using air and biological monitoring methods. J. Health Saf. Environ. 2014, 30, 185–195. [Google Scholar]
- Stetkiewicz, J.; Wronska-Nofer, T. Updating of hygiene standards for carbon disulfide based on health risk assessment. Int. J. Occup. Med. Environ. Health 1998, 11, 129–143. [Google Scholar] [PubMed]
- Sharma, A.; Choudhary, S.; Patel, B.; Kumar, S. Carbon Disulfide (CS2) Exposure and Human Reproductive Health—A Narrative Overview. J. Infertil. Reprod. Biol. 2022, 10, 22–27. [Google Scholar]
- ACGIH. 2024 TLVs and BEIs Based on the Documentation on the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2024. [Google Scholar]
- Woebkenberg, M.L. Carbon Disulfide NIOSH 1600. In NIOSH Manual of Analytical Methods; US Dept of Health & Human Services, Centers for Disease Control and Prevention: New York, NY, USA, 1994; pp. 1–4. [Google Scholar]
- van Doorn, R.; Delbressine, L.P.; Leijdekkers, C.M.; Vertin, P.G.; Henderson, P.T. Identification and determination of 2-thiothiazolidine-4-carboxylic acid in urine of workers exposed to carbon disulfide. Arch. Toxicol. 1981, 47, 51–58. [Google Scholar] [CrossRef]
- van Doorn, R.; Leijdekkers, C.M.; Nossent, S.M.; Henderson, P.T. Excretion of TTCA in human urine after administration of carbon disulfide. Toxicol. Lett. 1982, 12, 59–64. [Google Scholar] [CrossRef]
- Cox, C.; Hee, Q.S.S.; Tolos, W.P. Biological monitoring of workers exposed to carbon disulfide. Am. J. Ind. Med. 1998, 33, 48–54. [Google Scholar] [CrossRef]
- Kivistö, H. TTCA measurements in biomonitoring of low level exposure to carbon disulfide. Int. Arch. Occup. Environ. Health 2000, 73, 263–269. [Google Scholar]
- Vermeulen, R.; Jönsson, B.A.; Lindh, C.H.; Kromhout, H. Biological monitoring of carbon disulfide and phthalate exposure in the contemporary rubber industry. Int. Arch. Occup. Environ. Health 2005, 78, 663–669. [Google Scholar] [CrossRef]
- Fabrizi, G.; Fioretti, M.; Rocca, L.M. Occupational exposure to complex mixtures of volatile organic compounds in ambient air: Desorption from activated charcoal using accelerated solvent extraction can replace carbon disulfide? Anal. Bioanal. Chem. 2013, 405, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Hinoue, M.; Ishimatsu, S.; Fueta, Y.; Hori, H. A new desorption method for removing organic solvents from activated carbon using surfactant. J. Occup. Health 2017, 59, 194–200. [Google Scholar] [CrossRef] [PubMed]
- HSE. EH40/2005 Workplace Exposure Limits; Health and Safety Executive: London, UK, 2005. [Google Scholar]
- SWA 1. Workplace Exposure Standards for Airborne Contaminants; Safe Work Australia: Garran ACT, Australia, 2024. [Google Scholar]
- Domergue, J.; Lison, D.; Haufroid, V. No evidence of cardiovascular toxicity in workers exposed below 5 ppm carbon disulfide. Int. Arch. Occup. Environ. Health 2016, 89, 835–845. [Google Scholar] [CrossRef]
- Gelbke, H.P.; Göen, T.; Mäurer, M.; Sulsky, S.I. A review of health effects of carbon disulfide in viscose industry and a proposal for an occupational exposure limit. Crit. Rev. Toxicol. 2009, 39, 1–126. [Google Scholar] [CrossRef]
- SWA 2. Model Work Health and Safety Regulations; Safe Work Australia: Garran ACT, Australia, 2024. [Google Scholar]
- NIOSH 1600. NIOSH Manual of Analytical Methods (NMAM), 4th ed.; CDC Publisher: New York, NY, USA, 1994. [Google Scholar]
- MDHS 96. Methods for the Determination of Hazardous Substances; Health and Safety Laboratory: London, UK, 2000. [Google Scholar]
- 3M. Organic Vapor Monitor Sampling and Analysis Guide. In 3M Occupational Health and Environmental Safety Division; 3M: Saint Paul, MN, USA, 2019. [Google Scholar]
- Duff, D.; Lennard, C.; Li, Y.; Doyle, C.; Edge, K.J.; Ian Holland, J.; Lothridge, K.; Johnstone, P.; Beylerian, P.; Spikmans, V. Portable gas chromatography–mass spectrometry method for the in-field screening of organic pollutants in soil and water at pollution incidents. Environ. Sci. Pollut. Res. 2023, 30, 93088–93102. [Google Scholar] [CrossRef]
- Bocos-Bintintan, V.; Ratiu, I.A. Hunting for Toxic Industrial Chemicals: Real-Time Detection of Carbon Disulfide Traces by Means of Ion Mobility Spectrometry. Toxics 2020, 8, 121. [Google Scholar] [CrossRef]
- Liao, Z.; Gao, T.; Li, P.; Zheng, T.; Li, L.; Li, C.; Wang, W.; Wang, Y.; Liu, J. Diffusion behavior and environmental impact of odorants and TVOCs detected in a wastewater treatment plant for collaborative leachate treatment in Northwest China. Chemosphere 2024, 366, 143561–143576. [Google Scholar] [CrossRef]
- Göen, T.; Muller, J.; Angerer, J.; Drexler, H. Determination of carbon disulfide at the workplace by sampling on charcoal tubes: Problems and solutions. AIHA J. 2002, 63, 659–663. [Google Scholar] [CrossRef]
- Choi, H.; Seo, J.H.; Weon, S. Visualizing indoor ozone exposures via o-dianisidine based colorimetric passive sampler. J. Hazard. Mater. 2023, 460, 132510. [Google Scholar] [CrossRef]
- Daemen, E.; Risseghem, M.V.; Bacquer, D.D.; Bulat, P.; Braeckman, L.; Vanhoorne, M. Preliminary External Quality Assessment for the Biological Monitoring of Carbon Disulfide with Urinary 2-Thiothiazolidine-4-Carboxylic Acid. Ann. Occup. Hyg. 1999, 43, 125–130. [Google Scholar] [CrossRef]
- Jönsson, L.S.; Broberg, K.; Bergendorf, U.; Axmon, A.; Littorin, M.; Jönsson, B.A. Levels of 2-thiothiazolidine-4-carboxylic acid (TTCA) and effect modification of polymorphisms of glutathione-related genes in vulcanization workers in the southern Sweden rubber industries. Int. Arch. Occup. Environ. Health 2007, 80, 589–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chou, T.C.; Shih, T.S.; Sheu, H.M.; Chang, S.J.; Huang, C.C.; Chang, H.Y. The effect of personal factors on the relationship between carbon disulfide exposure and urinary 2-thiothiazolidine-4-carboxylic acid levels in rayon manufacturing workers. Sci. Total Environ. 2004, 322, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.S.; Chou, T.C.; Chang, H.Y.; Wu, C.C.; Wang, P.Y. Accumulation of urinary 2-thiothiazolidine-4-carboxylic acid (TTCA) among workers occupationally exposed to carbon disulfide for 1 week. Sci. Total Environ. 2003, 308, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Shin, T.S.; Li, C.C.; Chou, J.S. High performance liquid chromatographic determination of 2-thiothiazolidine-4-carboxylic acid as a marker of occupational exposure to carbon disulfide. Chromatographia 2002, 53, 665–668. [Google Scholar] [CrossRef]
- Jian, L. Alcohol and urinary 2-thiothiazolidine-4-carboxylic acid. Toxicol. Lett. 2002, 134, 277–283. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Uemura, T.; Yamauchi, T.; Takebayashi, T.; Nishiwaki, Y.; Yamada, K.; Sakurai, H.; Omae, K. Urinary Excretion of TTCA after Intake of brassica Vegetables. J. Occup. Health 2002, 44, 151–155. [Google Scholar] [CrossRef]
- AS/NZS 2243.8:2006; Safety in Laboratories Part 8: Fume Cupboards. Australian Standard: Sydney, Australia; New Zealand Standard: Wellington, New Zealand, 2006.
- Kilo, S.; Zonnur, N.; Uter, W.; Göen, T.; Drexler, T. Effect of Skin Protection and Skin Irritation on the Internal Exposure to Carbon Disulfide in Employees of the Viscose Industry. Ann. Work. Exp. Health 2015, 59, 972–981. [Google Scholar] [CrossRef]
- Cherrie, J.W. RE: Effect of Skin Protection and Skin Irritation on the Internal Exposure to Carbon Disulfide in Employees of the Viscose Industry. Ann. Work. Exp. Health 2016, 60, 525–527. [Google Scholar] [CrossRef]
- Lauwerys, R.; Hoet, P. Chapter 2: Exposure to Inorganic and Organometallic Substances, Industrial Chemical Exposure: Guidelines for Biological Monitoring, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 69–77. [Google Scholar]
- NIOSH 2549. Volatile Organic Compounds (Screening). In NIOSH Manual of Analytical Methods (NMAM), 4th ed.; CDC Publisher: New York, NY, USA, 1996. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).