Fulminant Necrotizing Soft Tissue Infection Following Abdominal Liposuction: Comprehensive Literature Review and Case Report
Abstract
1. Introduction
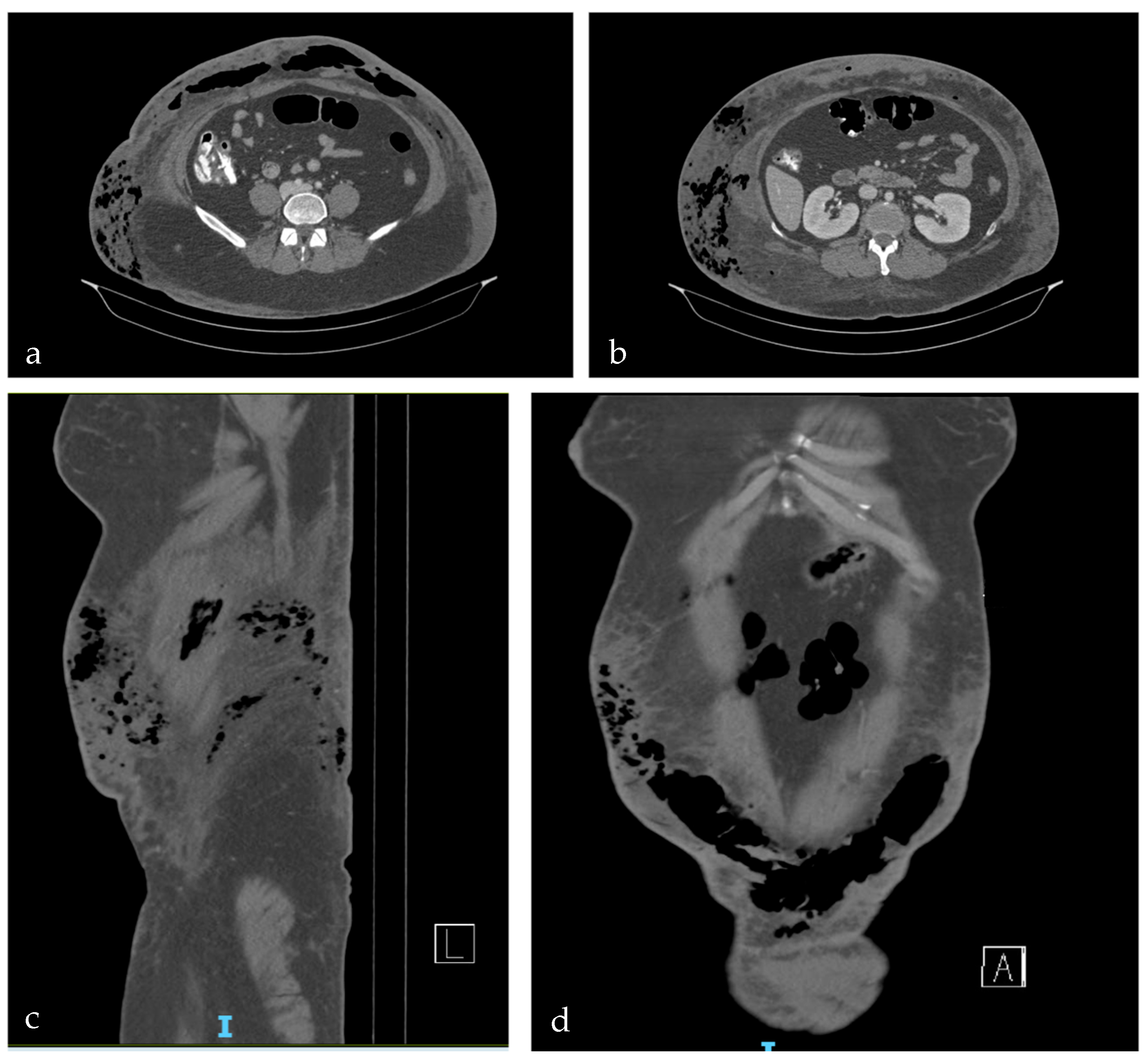
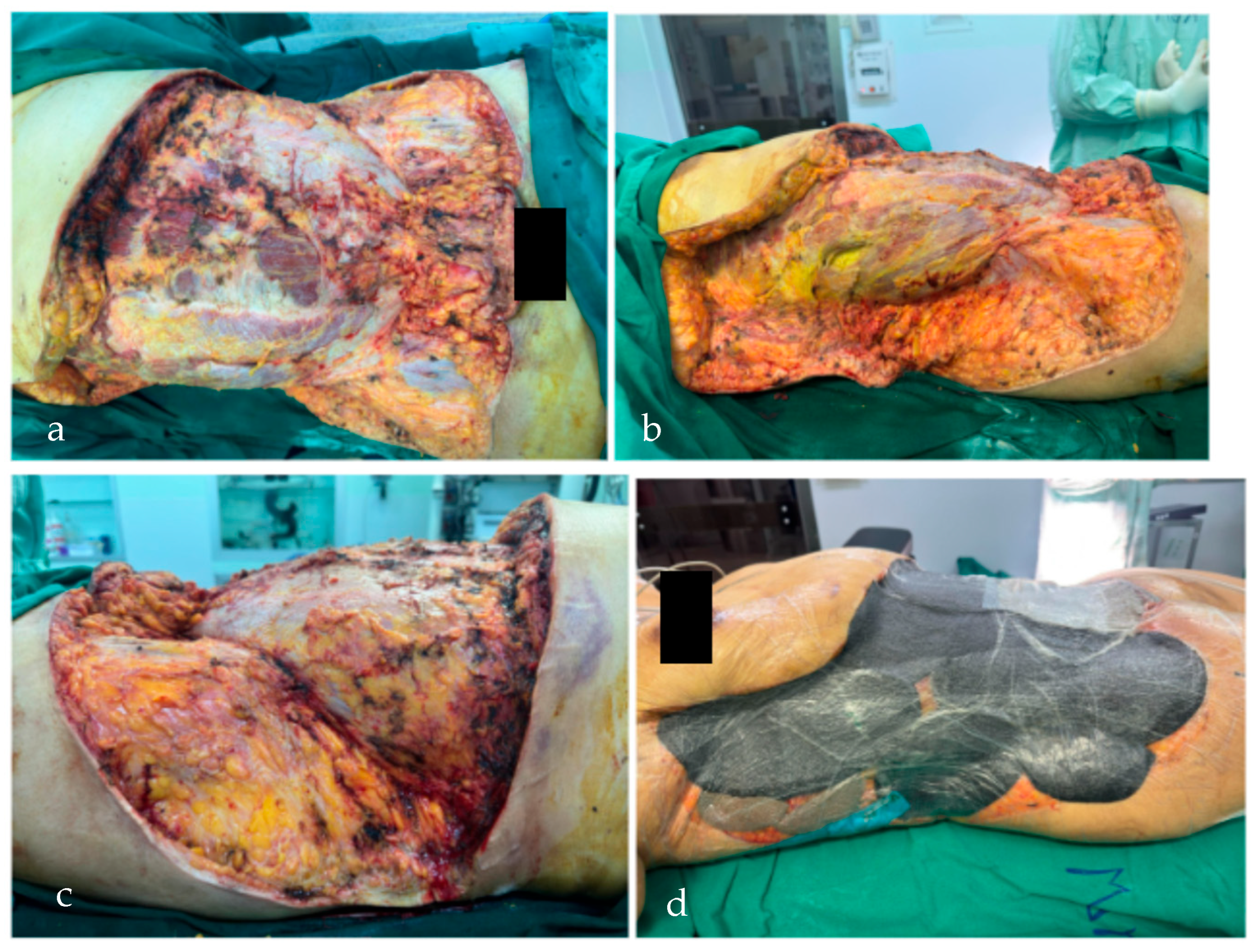
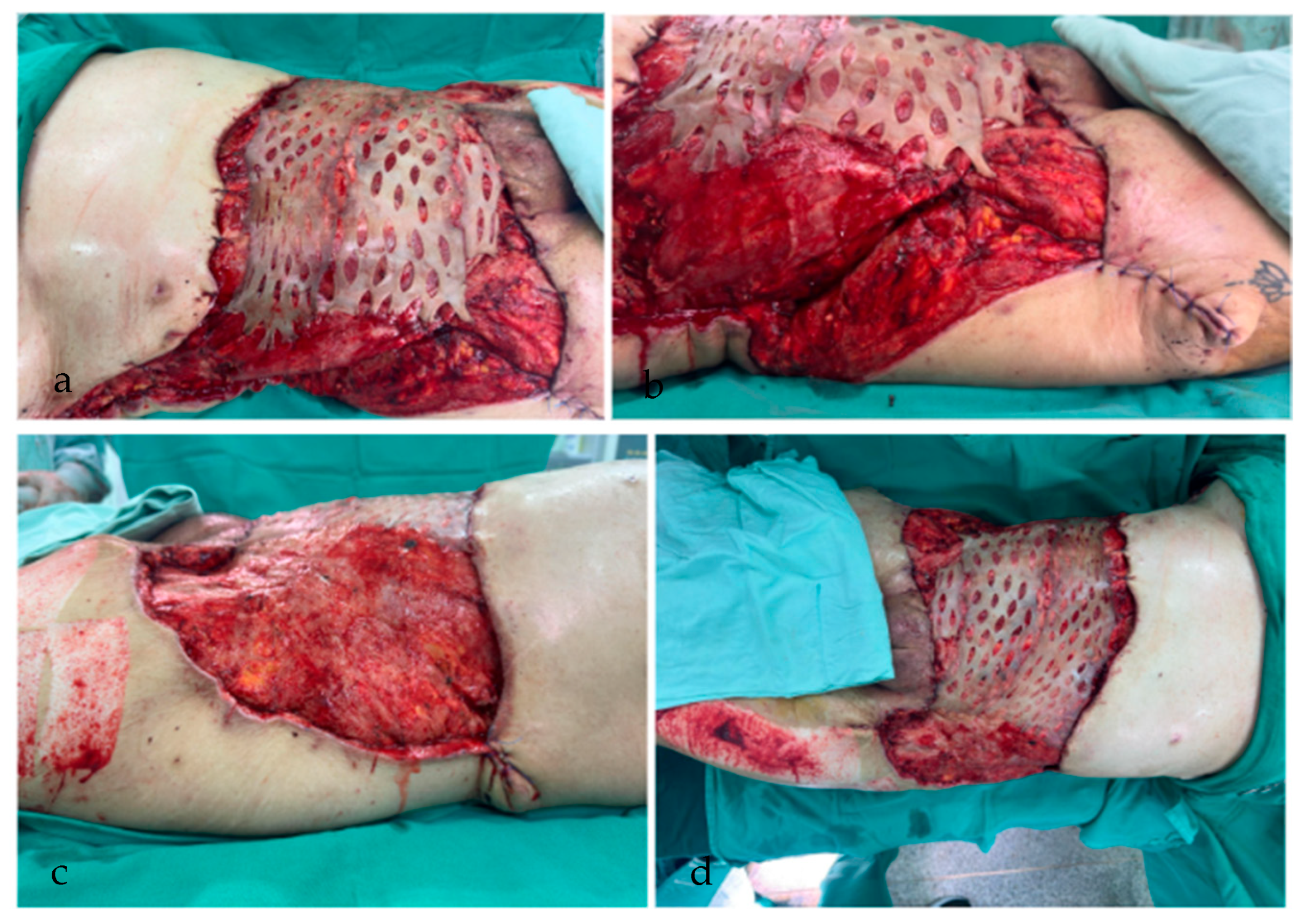
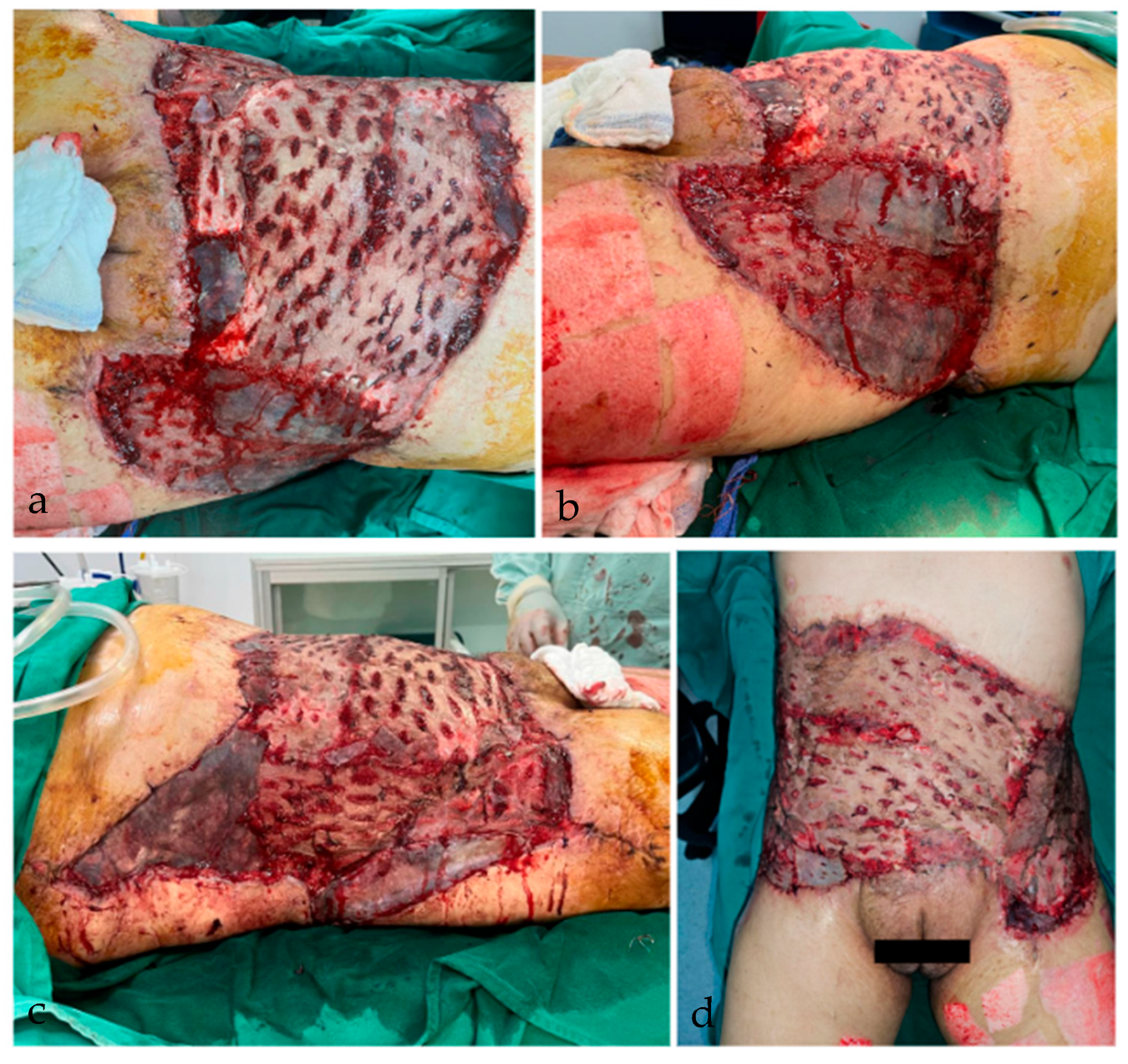
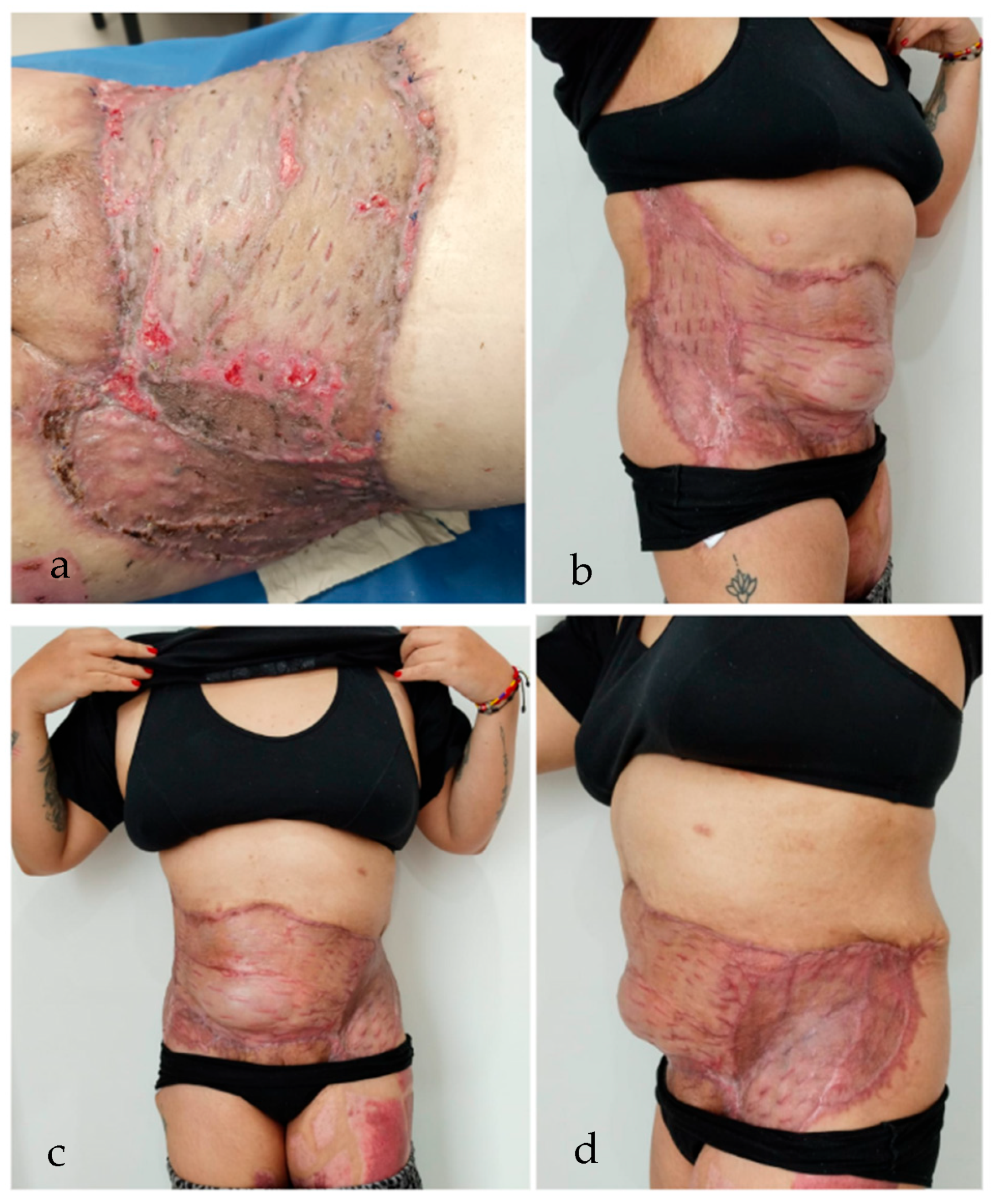
2. Case Report
3. Postoperative Necrotizing Soft Tissue Infection
3.1. Epidemiology and Risk Factors
3.2. Microbial Etiology and Pathophysiology
3.3. Diagnostic Challenges
3.4. Treatment Strategies
3.5. Reported Cases of NSTI Following Liposuction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSTI | Necrotizing soft tissue infection |
| NF | Necrotizing Fasciitis |
| ICU | Intensive Care Unit |
| CT | Computed Tomography |
| NPWT | Negative Pressure Wound Therapy |
| PNSTI | Postoperative Necrotizing soft tissue infection |
| SIRS | Systemic Inflammatory Response Syndrome |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| GAS | Group A Streptococcus |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| LRINEC | Laboratory Risk Indicator for Necrotizing Fasciitis |
| HBOT | Hyperbaric Oxygen Therapy |
References
- Surgical Demand in Aesthetic Surgery. Ann. Plast. Surg. 2023, 91, 206–210. [CrossRef]
- Comerci, A.J.; Arellano, J.A.; Alessandri-Bonetti, M.; Mocharnuk, J.W.; Marangi, G.F.; Persichetti, P.; Rubin, J.P.; Egro, F.M. Risks and Complications Rate in Liposuction: A Systematic Review and Meta-Analysis. Aesthet Surg. J. 2024, 44, NP454–NP463. [Google Scholar] [CrossRef]
- Montrief, T.; Bornstein, K.; Ramzy, M.; Koyfman, A.; Long, B.J. Plastic Surgery Complications: A Review for Emergency Clinicians. West. J. Emerg. Med. 2020, 21, 179–189. [Google Scholar] [CrossRef]
- Gravante, G.; Caruso, R.; Araco, A.; Cervelli, V. Infections after plastic procedures: Incidences, etiologies, risk factors, and antibiotic prophylaxis. Aesthetic Plast. Surg. 2008, 32, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Di Grezia, M.; Tropeano, G.; Altieri, G.; Brisinda, G. Necrotizing soft tissue infections: A surgical narrative review. Updates Surg. 2025, 77, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Urbina, T.; Bosc, R.; Parks, T.; Sriskandan, S.; de Prost, N.; Chosidow, O. Necrotising soft-tissue infections. Lancet Infect. Dis. 2023, 23, e81–e94. [Google Scholar] [CrossRef]
- Bodansky, D.M.S.; Begaj, I.; Evison, F.; Webber, M.; Woodman, C.B.; Tucker, O.N. A 16-year Longitudinal Cohort Study of Incidence and Bacteriology of Necrotising Fasciitis in England. World J. Surg. 2020, 44, 2580–2591. [Google Scholar] [CrossRef]
- Miller, A.T.; Saadai, P.; Greenstein, A.; Divino, C.M. Postprocedural Necrotizing Fasciitis: A 10-year Retrospective Review. Am. Surg. 2008, 74, 405–409. [Google Scholar] [CrossRef]
- Lehnhardt, M.; Homann, H.H.; Daigeler, A.; Hauser, J.; Palka, P.; Steinau, H.U. Major and Lethal Complications of Liposuction: A Review of 72 Cases in Germany Between 1998 and 2002. Plast Reconstr. Surg. 2008, 121, 396–403. [Google Scholar] [CrossRef]
- Gaston, R.G.; Kuremsky, M.A. Postoperative Infections: Prevention and Management. Hand Clin. 2010, 26, 265–280. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bryant, A.E. Necrotizing Soft-Tissue Infections. N. Engl. J. Med. 2017, 377, 2253–2265. [Google Scholar] [CrossRef] [PubMed]
- Allaw, F.; Wehbe, S.; Kanj, S.S. Necrotizing Fasciitis: An Update on Epidemiology, Diagnostic Methods, and Treatment. Curr. Opin. Infect. Dis. 2024, 37, 105–111. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.; Kao, L.S.; Keeley, J.A.; Grigorian, A.; Neville, A.; de Virgilio, C. Necrotizing Soft Tissue Infections: A Review. JAMA Surg. 2024, 159, 1308–1315. [Google Scholar] [CrossRef]
- You, J.S.; Chung, Y.E.; Baek, S.E.; Chung, S.P.; Kim, M.J. Imaging Findings of Liposuction with an Emphasis on Postsurgical Complications. Korean J. Radiol. 2015, 16, 1197–1206. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef]
- Chiang, I.H.; Chang, S.C.; Wang, C.H. Management of Necrotising Fasciitis Secondary to Abdominal Liposuction Using a Combination of Surgery, Hyperbaric Oxygen and Negative Pressure Wound Therapy in a Patient with Burn Scars. Int. Wound J. 2017, 14, 989–992. [Google Scholar] [CrossRef]
- Marchesi, A.; Marcelli, S.; Parodi, P.C.; Perrotta, R.E.; Riccio, M.; Vaienti, L. Necrotizing Fasciitis in Aesthetic Surgery: A Review of the Literature. Aesthetic Plast. Surg. 2017, 41, 352–358. [Google Scholar] [CrossRef]
- Barillo, D.J.; Cancio, L.C.; Kim, S.H.; Shirani, K.Z.; Goodwin, C.W. Fatal and Near-Fatal Complications of Liposuction. South. Med. J. 1998, 91, 487–492. [Google Scholar] [CrossRef]
- Anwar, U.M.; Ahmad, M.; Sharpe, D.T. Necrotizing Fasciitis After Liposculpture. Aesthetic Plast. Surg. 2004, 28, 426–427. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, X.; Huang, H.; Tang, L.; Zou, X.; Mao, H.; Liu, H. Infectious shock after liposuction. BMC Infect. Dis. 2022, 22, 6178. [Google Scholar] [CrossRef]
- Sacks, B.; Dela Cruz, G.; Capstick, R.; Seifman, M. Clinical concern for necrotising fasciitis: A review of referrals to plastic surgery units at two tertiary centres. ANZ J. Surg. 2021, 91, 1724–1732. [Google Scholar] [CrossRef]
- Eming, S.A.; Murray, P.J.; Pearce, E.J. Metabolic orchestration of the wound healing response. Cell Metab. 2021, 33, 1726–1743. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Management of Burns. N. Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N.; Jeschke, M.G. The hypermetabolic response to burn injury and interventions to modify this response. Clin. Plast Surg. 2009, 36, 583–596. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaimes Gonzalez, C.V.; Barrera Guaca, J.S.; Gomez Martinez, M.A.; Caballero Paz, F.; Alvarez Molina, L.F. Fulminant Necrotizing Soft Tissue Infection Following Abdominal Liposuction: Comprehensive Literature Review and Case Report. Complications 2025, 2, 23. https://doi.org/10.3390/complications2030023
Jaimes Gonzalez CV, Barrera Guaca JS, Gomez Martinez MA, Caballero Paz F, Alvarez Molina LF. Fulminant Necrotizing Soft Tissue Infection Following Abdominal Liposuction: Comprehensive Literature Review and Case Report. Complications. 2025; 2(3):23. https://doi.org/10.3390/complications2030023
Chicago/Turabian StyleJaimes Gonzalez, Claudia Viviana, Joan Sebastian Barrera Guaca, Maria Angela Gomez Martinez, Felipe Caballero Paz, and Luis Fernando Alvarez Molina. 2025. "Fulminant Necrotizing Soft Tissue Infection Following Abdominal Liposuction: Comprehensive Literature Review and Case Report" Complications 2, no. 3: 23. https://doi.org/10.3390/complications2030023
APA StyleJaimes Gonzalez, C. V., Barrera Guaca, J. S., Gomez Martinez, M. A., Caballero Paz, F., & Alvarez Molina, L. F. (2025). Fulminant Necrotizing Soft Tissue Infection Following Abdominal Liposuction: Comprehensive Literature Review and Case Report. Complications, 2(3), 23. https://doi.org/10.3390/complications2030023





