The Predictive Value of Preoperative C-Reactive Protein to Albumin Ratio (CAR), Neutrophil to Lymphocyte Ratio (NLR), and Platelet to Lymphocyte Ratio (PLR) for Early Postoperative Complications Following PEG
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Data Collection
2.3. Definitions and Assessments
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauderer, M.W.L.; Ponsky, J.L.; Izant, R.J., Jr. Gastrostomy without laparotomy: A percutaneous endoscopic technique. J. Pediatr. Surg. 1980, 15, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Löser, C.; Aschl, G.; Hébuterne, X.; Mathus-Vliegen, E.M.; Muscaritoli, M.; Niv, Y.; Rollins, H.; Singer, P.; Skelly, R.H. ESPEN guidelines on artificial enteral nutrition–percutaneous endoscopic gastrostomy (PEG). Clin. Nutr. 2005, 24, 848–861. [Google Scholar]
- Figueiredo, F.A.; da Costa, M.C.; Pelosi, A.D.; Martins, R.N.; Machado, L.; Francioni, E. Predicting outcomes and complications of percutaneous endoscopic gastrostomy. Endoscopy 2007, 39, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.; Lagergren, P.; Martin, L.; Mattsson, F.; Lagergren, J. Complications after percutaneous endoscopic gastrostomy in a prospective study. Scand. J. Gastroenterol. 2012, 47, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.D.; Rudberg, M.A.; Brody, J.A. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA 1998, 279, 1973–1976. [Google Scholar] [CrossRef]
- Callahan, C.M.; Haag, K.M.; Weinberger, M.; Tierney, W.M.; Buchanan, N.N.; Stump, T.E.; Nisi, R. Outcomes of percutaneous endoscopic gastrostomy among older adults in a community setting. J. Am. Geriatr. Soc. 2000, 48, 1048–1054. [Google Scholar] [CrossRef]
- Zopf, Y.; Maiss, J.; Konturek, P.; Rabe, C.; Hahn, E.G.; Schwab, D. Predictive factors of mortality after PEG insertion: Guidance for clinical practice. JPEN J. Parenter. Enter. Nutr. 2011, 35, 50–55. [Google Scholar] [CrossRef]
- Tominaga, N.; Shimoda, R.; Iwakiri, R.; Tsuruoka, N.; Sakata, Y.; Hara, H.; Hayashi, S.; Morita, S.; Hamasaki, Y.; Matsushima, T.; et al. Low serum albumin level is a risk factor for patients with percutaneous endoscopic gastrostomy. Intern. Med. 2010, 49, 2283–2288. [Google Scholar] [CrossRef]
- Blomberg, J.; Lagergren, P.; Martin, L.; Mattsson, F.; Lagergren, J. Albumin and C-reactive protein levels predict short-term mortality after percutaneous endoscopic gastrostomy. Gastrointest. Endosc. 2011, 73, 29–36. [Google Scholar] [CrossRef]
- Barbosa, M.; Magalhães, J.; Marinho, C.; Cotter, J. Predictive factors of early mortality after PEG placement: The importance of C-reactive protein. Clin. Nutr. ESPEN 2016, 14, 19–23. [Google Scholar] [CrossRef]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts—Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar] [PubMed]
- Guthrie, G.J.K.; Charles, K.A.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Sener, R.; Ozbilgec, S.; Akkus, F.; Serin, E.C.; Günenc, O. Neutrophil Percentage-to-Albumin Ratio in the Diagnosis of Ovarian Torsion-Predictive Role. Clin. Exp. Obstet. Gynecol. 2025, 52, 33516. [Google Scholar] [CrossRef]
- Moreno-Alfonso, J.C.; Molina Caballero, A.; Pérez Martínez, A.; Yárnoz Irazábal, M.C. Diagnostic value of the derived neutrophil-to-lymphocyte ratio for acute appendicitis. ANZ J. Surg. 2025, 95, 423–429. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Izuegbuna, O. Inflammation-Based Markers of Nutrition in Cancer Patients; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Pih, G.Y.; Na, H.K.; Ahn, J.Y.; Jung, K.W.; Kim, D.H.; Lee, J.H.; Choi, K.D.; Song, H.J.; Lee, G.H.; Jung, H.-Y. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy insertion. BMC Gastroenterol. 2018, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.W.; Xu, L.; Gong, C.X.; Yang, F.; Han, Y.D.; Chen, H.Z.; Li, C.G. Preoperative neutrophil-to-lymphocyte ratio predicts complications after esophagectomy. Front. Surg. 2022, 9, 897716. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Su, M.C.; Wang, Y.W.; Che, G.W. Postoperative Elevations of Neutrophil-to-lymphocyte and Platelet-to-lymphocyte Ratios Predict Postoperative Pulmonary Complications in Non-small Cell Lung Cancer Patients: A Retrospective Cohort Study. Curr. Med. Sci. 2020, 40, 339–347. [Google Scholar] [CrossRef]
- Topçu, H.; Sezikli, İ.; Tutan, D.; Köseoğlu, H.; Topçu, R. Yoğun bakım ünitesinde PEG uygulanan hastalarda mortaliteyi etkileyen faktörlerin değerlendirilmesi. J. Med. Palliat. Care 2021, 2, 103–109. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Aziret, M.; Karaman, K.; Ercan, M.; Celebi, F.; Akdeniz, Y.; Ebiloglu, T.; Tomak, Y.; Oter, V.; Firat, N.; Yirgin, H. Assessment of risk factors on morbidity and mortality in patients undergoing percutaneous endoscopic gastrostomy. Ann. Med. Res. 2018, 25, 545–551. [Google Scholar] [CrossRef]
- Lee, C.; Im, J.P.; Kim, J.W.; Kim, S.-E.; Ryu, D.Y.; Cha, J.M.; Kim, E.Y.; Chang, D.K.; Small Intestine Research Group of the Korean Association for the Study of Intestinal Disease (KASID). Risk factors for complications and mortality of percutaneous endoscopic gastrostomy: A multicenter, retrospective study. Surg. Endosc. 2013, 27, 3806–3815. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, S.H.; Lee, C.; Seo, J.Y.; Kang, H.Y.; Yang, J.I.; Chung, S.J.; Kim, J.S. Thirty-day mortality after percutaneous gastrostomy by endoscopic versus radiologic placement: A systematic review and meta-analysis. Intest. Res. 2016, 14, 333–342. [Google Scholar] [CrossRef]
- Anderloni, A.; Di Leo, M.; Barzaghi, F.; Semeraro, R.; Meucci, G.; Marino, R.; Amato, L.; Frigerio, M.; Saladino, V.; Toldi, A.; et al. Complications and early mortality in percutaneous endoscopic gastrostomy placement in Lombardy: A multicenter prospective cohort study. Dig. Liver Dis. 2019, 51, 1380–1387. [Google Scholar] [CrossRef]
- Duzenli, T.; Ketenci, M.; Akyol, T.; Koseoglu, H.; Tanoglu, A.; Kaplan, M.; Yazgan, Y. Predictive factors of complications and 30-day mortality in patients undergoing percutaneous endoscopic gastrostomy: The utility of C-reactive protein to albumin ratio. Acta Gastro-Enterol. Belg. 2021, 84, 283–288. [Google Scholar] [CrossRef]
- Madhoun, M.F.; Blankenship, M.M.; Ahmed, A. Predictors of early mortality after percutaneous endoscopic gastrostomy. Eur. J. Gastroenterol. Hepatol. 2011, 23, 826–830. [Google Scholar]
- Wollman, B.; D’Agostino, H.B.; Walus-Wigle, J.R.; Easter, D.W.; Beale, A. Radiologic, endoscopic, and surgical gastrostomy: An institutional evaluation and meta-analysis of the literature. Radiology 1995, 197, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef]
- Luman, W.; Scott, J.; Lowe, D.; Francis, N.; Naguiat, E.; Ledgerwood, S. Percutaneous endoscopic gastrostomy: Clinic outcome in 314 consecutive patients. Endoscopy 2001, 33, 204–208. [Google Scholar]
- Pulkkinen, J.; Rekola, J.; Asanti, M.; Grénman, R. Prophylactic percutaneous endoscopic gastrostomy in head and neck cancer patients: Results of tertiary institute. Eur. Arch. Otorhinolaryngol. 2014, 271, 1755–1758. [Google Scholar] [CrossRef]
- Lima, D.L.; Miranda, L.E.C.; Lima, R.N.C.L.; Romero-Velez, G.; Chin, R.; Shadduck, P.P.; Sreeramoju, P. Factors Associated with Mortality after Percutaneous Endoscopic Gastrostomy. J. Soc. Laparoendosc. Surg. 2023, 27, e00005. [Google Scholar] [CrossRef]
- Jafri, N.S.; Mahid, S.S.; Minor, K.S.; Idstein, S.R.; Hornung, C.A.; Galandiuk, S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment. Pharmacol. Ther. 2007, 25, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Farrer, K.; Blackett, B.; Lloyd, H.; Paine, P.; Lal, S. Reduced 30-day gastrostomy placement mortality following the introduction of a multidisciplinary nutrition support team: A cohort study. J. Hum. Nutr. Diet. 2018, 31, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Lipp, A.; Lusardi, G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst Rev. 2013, 11, CD008968. [Google Scholar] [CrossRef]
- Arvanitakis, M.; Gkolfakis, P.; Despott, E.J.; Ballarin, A.; Beyna, T.; Boeykens, K.; Elbe, P.; Gisbertz, I.; Hoyois, A.; Mosteanu, O.; et al. Endoscopic management of enteral tubes in adult patients—Part 1: ESGE Clinical Guideline. Endoscopy 2021, 53, 81–92. [Google Scholar] [PubMed]
- Kirby, D.F.; DeLegge, M.H.; Fleming, C.R. American Gastroenterological Association technical review on tube feeding for enteral nutrition. Gastroenterology 1995, 108, 1282–1301. [Google Scholar] [CrossRef]
- Rustom, I.K.; Jebreel, A.; Tayyab, M.; England, R.J.; Stafford, N.D. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: A comparison study in head and neck cancer patients. J. Laryngol. Otol. 2006, 120, 463–466. [Google Scholar] [CrossRef]
| Variable | OR (Odds Ratio) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| CAR | 2.88 | 1.62–5.13 | <0.001 |
| NLR | 1.34 | 0.96–1.87 | 0.08 |
| PLR | 1.02 | 1.01–1.42 | 0.06 |
| Age | 0.97 | 0.89–1.05 | 0.60 |
| Charlson Comorbidity Index | 0.91 | 0.68–1.23 | 0.52 |
| Sex (Male) | 1.18 | 0.52–2.67 | 0.69 |
| Indication (Malignancy) | 0.85 | 0.28–2.54 | 0.77 |
| Indication (Other) | 0.92 | 0.31–2.74 | 0.88 |
| Variable | No Complication (n = 158) | With Complication (n = 26) | p-Value |
|---|---|---|---|
| Age (years) | 71.6 ± 5.9 | 70.9 ± 5.9 | 0.54 |
| Gender (Male/Female) | 94 (59.5%)/64 (40.5%) | 14 (53.8%)/12 (46.2%) | 0.67 |
| Body Mass Index (kg/m2) | 22.9 ± 2.2 | 23.2 ± 1.6 | 0.52 |
| Charlson Comorbidity Index | 5.56 ± 1.07 | 5.38 ± 1.13 | 0.48 |
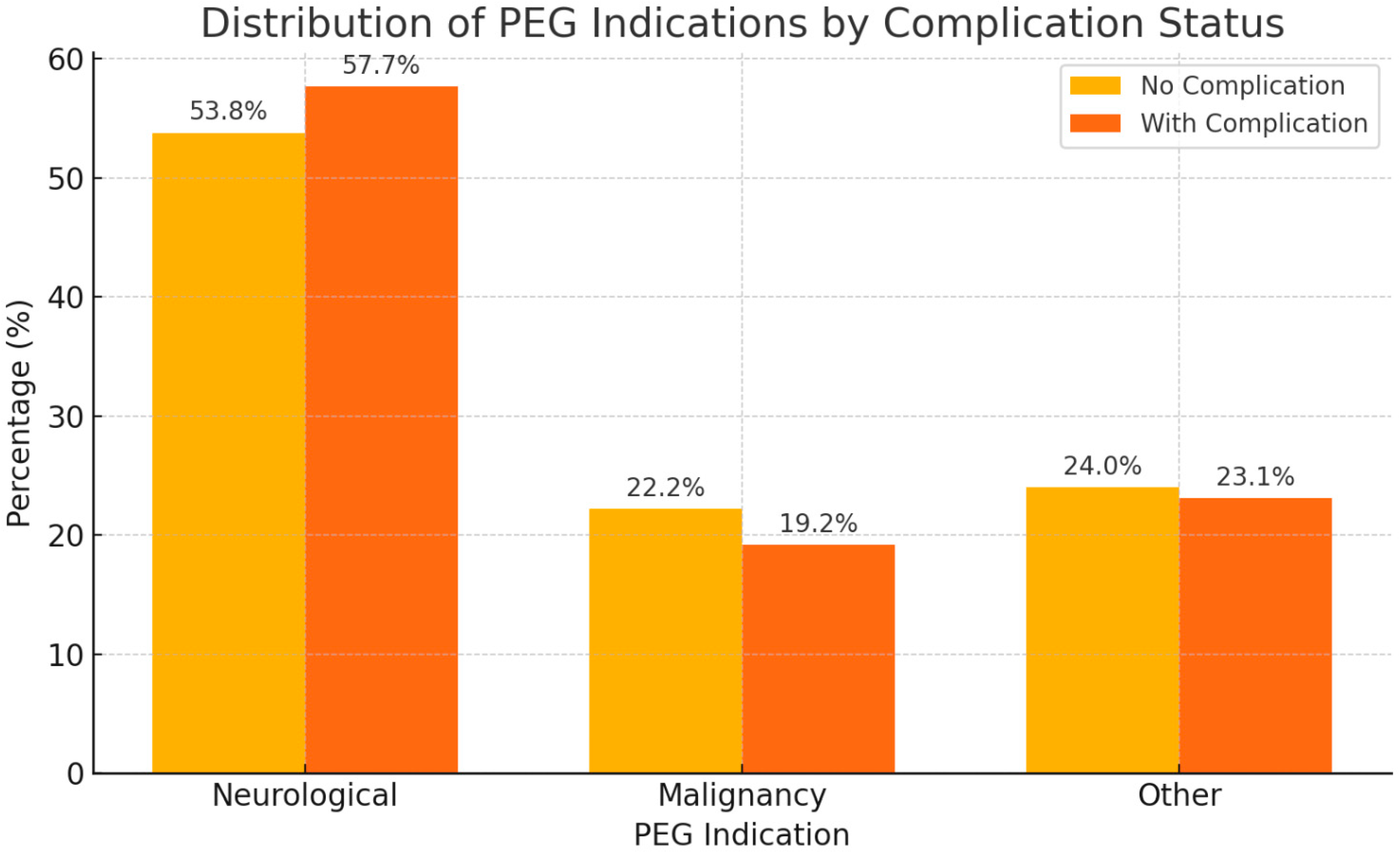
| PEG Indication—Neurological | 85 (53.8%) | 15 (57.7%) | 0.93 1 |
| PEG Indication—Malignancy | 35 (22.2%) | 5 (19.2%) | |
| PEG Indication—Other | 38 (24.0%) | 6 (23.1%) | |
| Albumin (g/dL) | 2.85 ± 0.31 | 2.78 ± 0.35 | 0.29 |
| CRP (mg/L) | 60.1 ± 30.7 | 53.5 ± 35.3 | 0.54 |
| CRP/Albumin Ratio (CAR) | 0.62 ± 0.16 | 1.89 ± 0.44 | <0.001 |
| Neutrophil-to-Lymphocyte Ratio | 4.61 ± 1.13 | 5.56 ± 1.10 | <0.001 |
| Platelet-to-Lymphocyte Ratio | 186.7 ± 34.9 | 231.3 ± 43.5 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evirgen, S.; Cetin, S. The Predictive Value of Preoperative C-Reactive Protein to Albumin Ratio (CAR), Neutrophil to Lymphocyte Ratio (NLR), and Platelet to Lymphocyte Ratio (PLR) for Early Postoperative Complications Following PEG. Complications 2025, 2, 16. https://doi.org/10.3390/complications2030016
Evirgen S, Cetin S. The Predictive Value of Preoperative C-Reactive Protein to Albumin Ratio (CAR), Neutrophil to Lymphocyte Ratio (NLR), and Platelet to Lymphocyte Ratio (PLR) for Early Postoperative Complications Following PEG. Complications. 2025; 2(3):16. https://doi.org/10.3390/complications2030016
Chicago/Turabian StyleEvirgen, Suat, and Sirin Cetin. 2025. "The Predictive Value of Preoperative C-Reactive Protein to Albumin Ratio (CAR), Neutrophil to Lymphocyte Ratio (NLR), and Platelet to Lymphocyte Ratio (PLR) for Early Postoperative Complications Following PEG" Complications 2, no. 3: 16. https://doi.org/10.3390/complications2030016
APA StyleEvirgen, S., & Cetin, S. (2025). The Predictive Value of Preoperative C-Reactive Protein to Albumin Ratio (CAR), Neutrophil to Lymphocyte Ratio (NLR), and Platelet to Lymphocyte Ratio (PLR) for Early Postoperative Complications Following PEG. Complications, 2(3), 16. https://doi.org/10.3390/complications2030016






