Exposure to Ambient Particulate Matter during Pregnancy: Implications for Infant Telomere Length
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Procedures
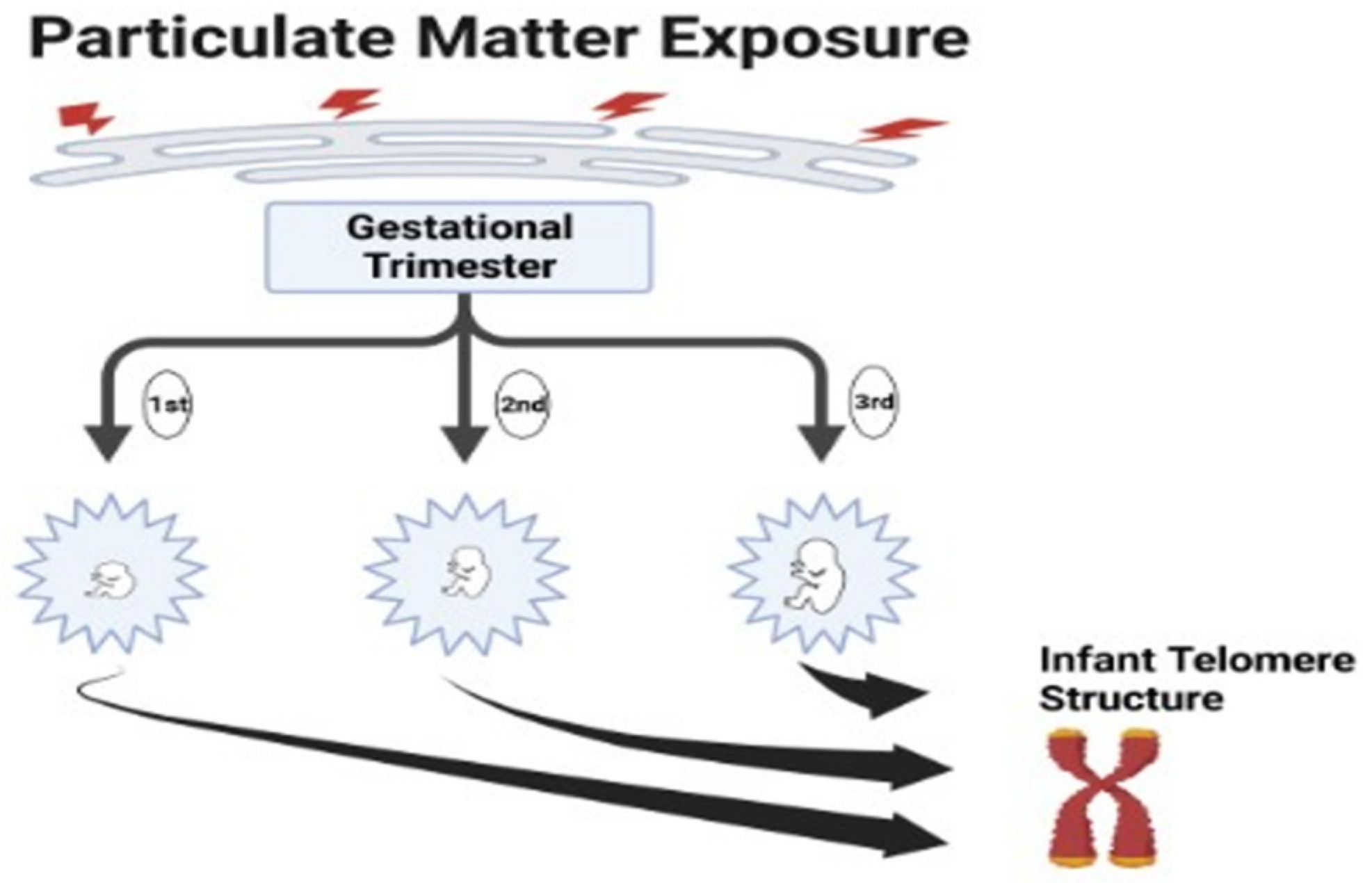
2.2. Air Pollutant (PM2.5) Exposure during Pregnancy
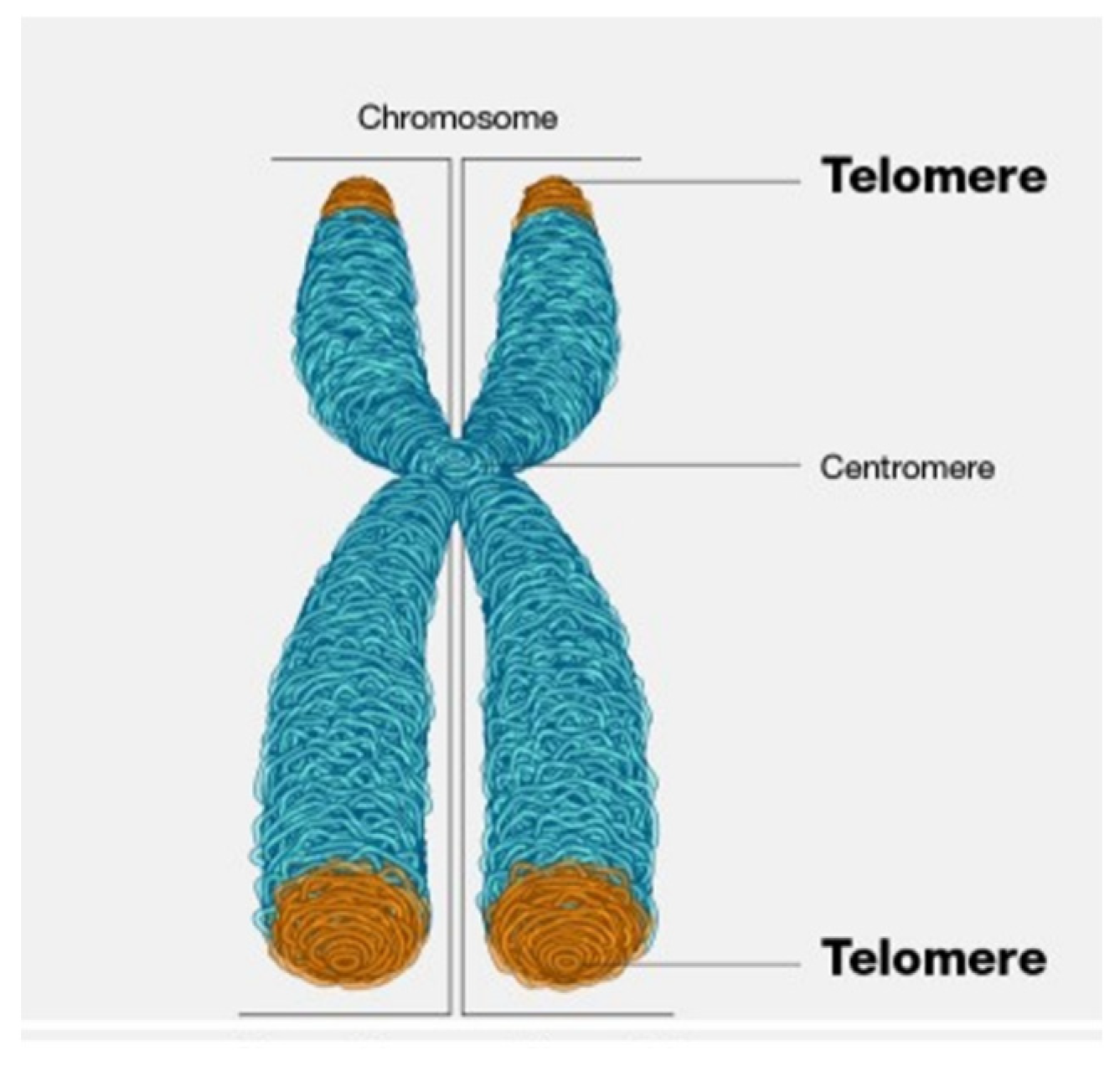
2.3. Infant Telomere Length
2.4. Covariates
2.5. Analysis
3. Results
3.1. Sample Characteristics
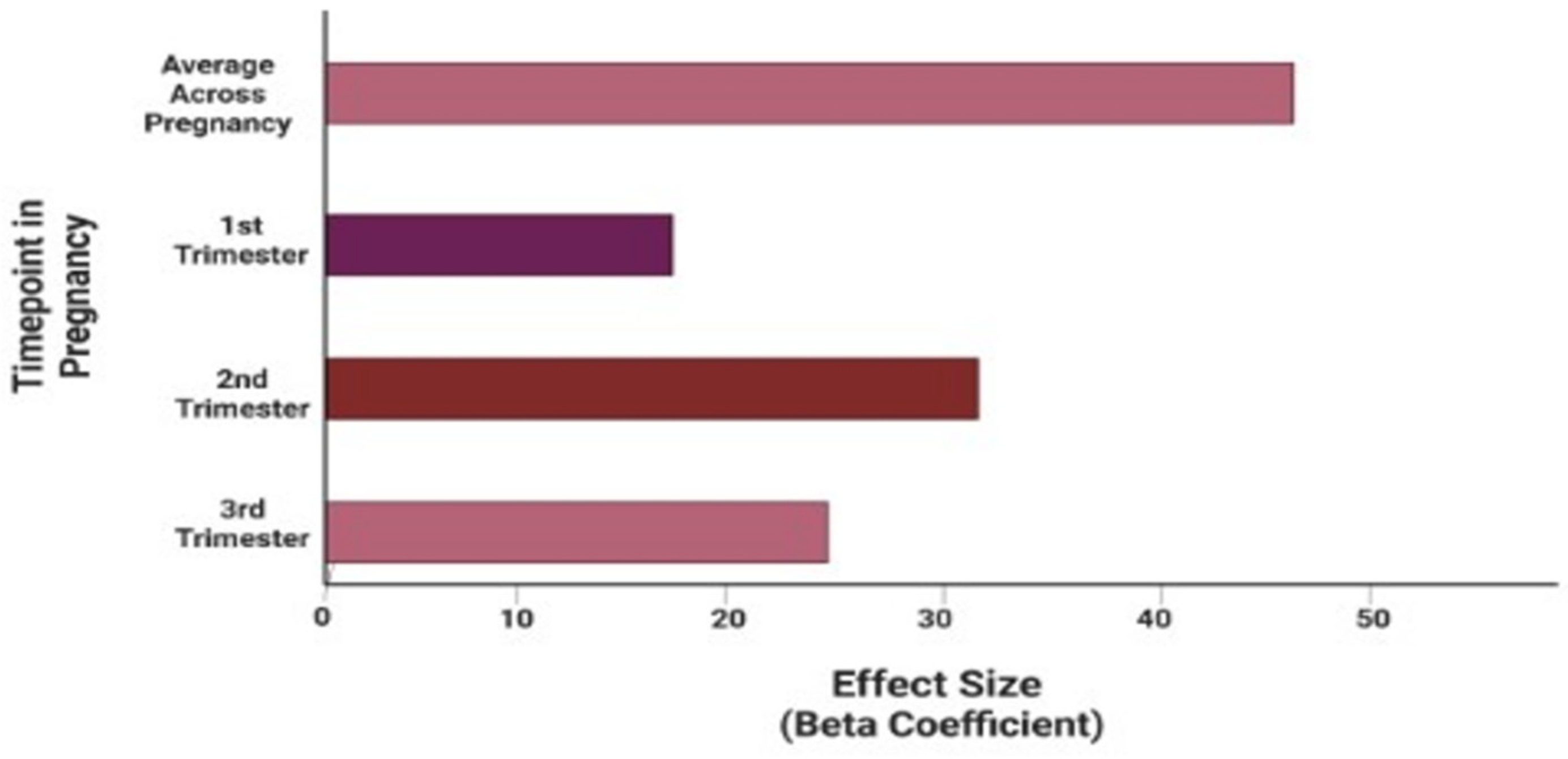
3.2. Relationships between Prenatal PM2.5 Exposures and Infant Telomere Length
4. Discussion
4.1. Exposure to PM2.5 during Pregnancy and Infant Telomere Length
4.2. Associations of Covariates with Infant Telomere Length
4.3. Implications
4.4. Strengths and Limitations
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominski, F.H.; Lorenzetti Branco, J.H.; Buonanno, G.; Stabile, L.; Gameiro da Silva, M.; Andrade, A. Effects of air pollution on health: A mapping review of systematic reviews and meta-analyses. Environ. Res. 2021, 201, 111487. [Google Scholar] [CrossRef]
- Johnson, N.M.; Hoffmann, A.R.; Behlen, J.C.; Lau, C.; Pendleton, D.; Harvey, N.; Shore, R.; Li, Y.; Chen, J.; Tian, Y.; et al. Air pollution and children’s health-a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ. Health Prev. Med. 2021, 26, 72. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Li, Y.; Niu, Y.; Ding, Y.; Yu, X.; Zhu, B.; Duan, R.; Duan, H.; Kou, C.; Li, Y.; et al. Association between ambient air pollution and pregnancy complications: A systematic review and meta-analysis of cohort studies. Environ. Res. 2020, 185, 109471. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Röösli, M.; Frey, U.; Latzin, P. Air pollution during pregnancy and neonatal outcome: A review. J. Aerosol. Med. Pulm. Drug Deliv. 2013, 26, 9–23. [Google Scholar] [CrossRef]
- Yan, M.; Liu, N.; Fan, Y.; Ma, L.; Guan, T. Associations of pregnancy complications with ambient air pollution in China. Ecotoxicol. Environ. Saf. 2022, 241, 113727. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Vázquez, L.; Binter, A.C.; Canals, J.; Hernández-Martínez, C.; Voltas, N.; Ambròs, A.; Fernández-Barrés, S.; Pérez-Crespo, L.; Guxens, M.; Arija, V. Maternal exposure to air pollution during pregnancy and child’s cognitive, language, and motor function: ECLIPSES study. Environ. Res. 2022, 212, 113501. [Google Scholar] [CrossRef] [PubMed]
- Lubczyńska, M.J.; Muetzel, R.L.; El Marroun, H.; Hoek, G.; Kooter, I.M.; Thomson, E.M.; Hillegers, M.; Vernooij, M.W.; White, T.; Tiemeier, H.; et al. Air pollution exposure during pregnancy and childhood and brain morphology in preadolescents. Environ. Res. 2021, 198, 110446. [Google Scholar] [CrossRef] [PubMed]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2019, 14, 47–50. [Google Scholar] [CrossRef]
- Swanson, J.M.; Entringer, S.; Buss, C.; Wadhwa, P.D. Developmental origins of health and disease: Environmental exposures. Semin. Reprod. Med. 2009, 27, 391–402. [Google Scholar] [CrossRef]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef]
- Kim, D.; Chen, Z.; Zhou, L.F.; Huang, S.X. Air pollutants and early origins of respiratory diseases. Chronic Dis. Transl. Med. 2018, 4, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.M.; Miyashita, L.; Maher, B.A.; McPhail, G.; Jones, C.J.P.; Barratt, B.; Thangaratinam, S.; Karloukovski, V.; Ahmed, I.A.; Aslam, Z.; et al. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci. Total Environ. 2021, 751, 142235. [Google Scholar] [CrossRef] [PubMed]
- Hermanova, B.; Riedlova, P.; Dalecka, A.; Jirik, V.; Janout, V.; Sram, R.J. Air pollution and molecular changes in age-related diseases. Int. J. Environ. Health Res. 2022, 32, 772–790. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ha, V.; Johnston, F.; Negishi, K. Air pollution and telomere length: A systematic review of 12,058 subjects. Cardiovasc. Diagn. Ther. 2018, 8, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, Y.; Song, X.; Lazar, L.; Li, Z.; Zhao, J. Impact of ambient pm 2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol. Environ. Saf. 2019, 169, 248–254. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef]
- Lee, A.G.; Cowell, W.; Kannan, S.; Ganguri, H.B.; Nentin, F.; Wilson, A.; Coull, B.A.; Wright, R.O.; Baccarelli, A.; Bollati, V.; et al. Prenatal particulate air pollution and newborn telomere length: Effect modification by maternal antioxidant intakes and infant sex. Environ. Res. 2020, 187, 109707. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Panfoli, I.; Candiano, G.; Malova, M.; De Angelis, L.; Cardiello, V.; Buonocore, G.; Ramenghi, L.A. Oxidative Stress as a Primary Risk Factor for Brain Damage in Preterm Newborns. Front. Pediatr. 2018, 6, 369. [Google Scholar] [CrossRef]
- Bosquet Enlow, M.; Sideridis, G.; Bollati, V.; Hoxha, M.; Hacker, M.R.; Wright, R.J. Maternal cortisol output in pregnancy and newborn telomere length: Evidence for sex-specific effects. Psychoneuroendocrinology 2019, 102, 225–235. [Google Scholar] [CrossRef]
- Entringer, S.; de Punder, K.; Buss, C.; Wadhwa, P. The fetal programming of telomere biology hypothesis: An update. Phil. Trans. R. Soc. 2018, 373, 20170151. [Google Scholar] [CrossRef]
- Shalev, I.; Entringer, S.; Wadhwa, P.D.; Wolkowitz, O.M.; Puterman, E.; Lin, J.; Epel, E.S. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology 2013, 38, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Schaetzlein, S.; Lucas-Hahn, A.; Lemme, E.; Kues, W.A.; Dorsch, M.; Manns, M.P.; Niemann, H.; Rudolph, K.L. Telomere length is reset during early mammalian embryogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 8034–8038. [Google Scholar] [CrossRef] [PubMed]
- Factor-Litvak, P.; Susser, E. The importance of early life studies of telomere attrition. Paediatr. Perinat. Epidemiol. 2015, 29, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere length in the newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef]
- Rosa, M.J.; Hsu, H.L.; Just, A.C.; Brennan, K.J.; Bloomquist, T.; Kloog, I.; Pantic, I.; García, A.M.; Wilson, A.; Coull, B.A.; et al. Association between prenatal particulate air pollution exposure and telomere length in cord blood: Effect modification by fetal sex. Environ. Res. 2019, 172, 495–501. [Google Scholar] [CrossRef]
- Martens, D.S.; Cox, B.; Janssen, B.G.; Clemente, D.; Gasparrini, A.; Vanpoucke, C.; Lefebvre, W.; Roels, H.A.; Plusquin, M.; Nawrot, T.S. Prenatal Air Pollution and Newborns’ Predisposition to Accelerated Biological Aging. JAMA Pediatr. 2017, 171, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Scholten, R.; Møller, P.; Andersen, Z.; Dehlendorff, C.; Khan, J.; Brandt, J.; Ketzel, M.; Knudsen, L.; Mathiesen, L. Telomere length in newborns is associated with exposure to low levels of air pollution during pregnancy. Environ. Int. 2021, 146, 106202. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, B.; Liu, B.; Wu, M.; Zhang, L.; Wang, L.; Xu, S.; Cao, Z.; Wang, Y. Effects of maternal exposure to ambient air pollution on newborn telomere length. Environ. Int. 2019, 128, 254–260. [Google Scholar] [CrossRef]
- Knoderer, C.; Nguyen, D.; Alrick, D.; Hoag, K. 2016 Air Monitoring Network plan; Bay Area Air Quality Management District: San Francisco, CA, USA, 2019. [Google Scholar]
- Kim, D.; Sass-Kortsak, A.; Purdham, J.; Dales, R.; Brook, J. Associations between personal exposures and fixed-site ambient measurements of fine particulate matter, nitrogen dioxide, and carbon monoxide in Toronto, Canada. J. Expo. Sci. Environ. Epidemiol. 2005, 16, 172–183. [Google Scholar] [CrossRef]
- Hu, Y.; Ehli, E.A.; Nelson, K.; Bohlen, K.; Lynch, C.; Huizenga, P.; Kittlelsrud, J.; Soundy, T.J.; Davies, G.E. Genotyping Performance between Saliva and Blood-Derived Genomic DNAs on the DMET Array: A Comparison. PLoS ONE 2012, 7, e33968. [Google Scholar] [CrossRef][Green Version]
- Tomiyama, A.J.; O’Donovan, A.; Lin, J.; Puterman, E.; Lazaro, A.; Chan, J.; Dhabhar, F.S.; Wolkowitz, O.; Kirschbaum, C.; Blackburn, E.; et al. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol. Behav. 2012, 106, 40–45. [Google Scholar] [CrossRef]
- Van den Bergh, B.R.H.; van den Heuvel, M.I.; Lahti, M.; Braeken, M.; de Rooij, S.R.; Entringer, S.; Hoyer, D.; Roseboom, T.; Räikkönen, K.; King, S.; et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2017, 117, 26–64. [Google Scholar] [CrossRef]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Wikgren, M.; Maripuu, M.; Karlsson, T.; Nordfjall, K.; Bergdahl, J.; Hultdin, J.; Del-Favero, J.; Roos, G.; Nilsson, L.-G.; Adolfsson, R.; et al. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol. Psychiatry 2012, 71, 294–300. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E.; Cheon, J.; Kroenke, C.; Sinclair, E.; Bigos, M.; Wolkowitz, O.; Mellon, S.; Blackburn, E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. J. Immunol. Methods. 2010, 352, 71–80. [Google Scholar] [CrossRef]
- Smith, D.L.; Wu, C.; Gregorich, S.; Dai, G.; Lin, J. Impact of DNA Extraction Methods on Quantitative PCR Telomere Length Assay Precision in Human Saliva Samples. Int. J. Methodol. 2022, 1, 44–57. [Google Scholar] [CrossRef]
- Berry, C.; Shalowitz, M.; Quinn, K.; Wolf, R. Validation of the Crisis in Family Systems-Revised, a contemporary measure of life stressors. Psychol. Rep. 2001, 88 Pt 1, 713–724. [Google Scholar] [CrossRef]
- Berry, C.A.; Quinn, K.A.; Portillo, N.; Shalowitz, M.U. Reliability and validity of the Spanish version of the Crisis in Family Systems—Revised. Psychol. Rep. 2006, 98, 123–132. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; and Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.; Williams, J. The PHQ-15: Validity of a New Measure for Evaluating the Severity of Somatic Symptoms. Psychosom. Med. 2002, 64, 258–266. [Google Scholar] [CrossRef]
- National Ambient Air Quality Standards (NAAQS) for, PM. Available online: https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm (accessed on 20 January 2020).
- Walton, R.T.; Mudway, I.S.; Dundas, I.; Marlin, N.; Koh, L.C.; Aitlhadj, L.; Vulliamy, T.; Jamaludin, J.B.; Wood, H.E.; Barratt, B.M.; et al. Air pollution, ethnicity and telomere length in east London schoolchildren: An observational study. Environ. Int. 2016, 96, 41–47. [Google Scholar] [CrossRef]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.A.; Zisch, A.; Krug, H.F.; von Mandach, U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef]
- Risom, L.; Møller, P.; Loft, S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res. 2005, 592, 119–137. [Google Scholar] [CrossRef]
- Hawkins, S.J.; Crompton, L.A.; Sood, A.; Saunders, M.; Boyle, N.T.; Buckley, A.; Minogue, A.M.; McComish, S.F.; Jiménez-Moreno, N.; Cordero-Llana, O.; et al. Nanoparticle-induced neuronal toxicity across placental barriers is mediated by autophagy and dependent on astrocytes. Nat. Nanotechnol. 2018, 13, 427–433. [Google Scholar] [CrossRef]
- van den Hooven, E.H.; de Kluizenaar, Y.; Pierik, F.H.; Hofman, A.; van Ratingen, S.W.; Zandveld, P.Y.; Lindemans, J.; Russcher, H.; Steegers, E.A.; Miedema, H.M.; et al. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: The Generation R Study. Environ. Health Perspect. 2012, 120, 746–751. [Google Scholar] [CrossRef]
- Dioni, L.; Hoxha, M.; Nordio, F.; Bonzini, M.; Tarantini, L.; Albetti, B.; Savarese, A.; Schwartz, J.; Bertazzi, P.A.; Apostoli, P.; et al. Effects of short-term exposure to inhalable particulate matter ontelomere length, telomerase expression, and telomerase methylation in steel workers. Environ. Health Perspect. 2011, 119, 622–627. [Google Scholar] [CrossRef]
- Hodes, R.J.; Hathcock, K.S.; Weng, N.P. Telomeres in T and B cells. Nat. Rev. Immunol. 2002, 2, 699–706. [Google Scholar] [CrossRef]
- Weng, N.P.; Granger, L.; Hodes, R.J. Telomere lengthening and telomerase activation during human B celldifferentiation. Proc. Natl. Acad. Sci. USA 1997, 94, 10827–10832. [Google Scholar] [CrossRef]
- Thiede, C.; Prange-Krex, G.; Freiberg-Richter, J.; Bornhäuser, M.; Ehninger, G. Buccal swabs but not mouthwash samples can be used to obtain pretransplant DNA fingerprints from recipients of allogeneic bone marrow transplants. Bone Marrow Transplant. 2000, 25, 575–577. [Google Scholar] [CrossRef]
- Vasu, V.; Turner, K.J.; George, S.; Greenall, J.; Slijepcevic, P.; Griffin, D.K. Preterm infants have significantly longer telomeres than their term born counterparts. PLoS ONE 2017, 12, e0180082. [Google Scholar] [CrossRef]
- Wang, C.; Wolters, P.J.; Calfee, C.S.; Liu, S.; Balmes, J.R.; Zhao, Z.; Koyama, T.; Ware, L.B. Long-term ozone exposure is positively associated with telomere length in critically ill patients. Environ. Int. 2020, 141, 105780. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L.; Wu, M.; Bi, J.; Wang, L.; Liu, Q.; Xiong, C.; Cao, Z.; Xu, S.; Wang, Y. Association between rare earth element exposure during pregnancy and newborn telomere length. Environ. Sci. Pollut. Res. Int. 2023, 30, 38751–38760. [Google Scholar] [CrossRef]
- Fragkiadaki, P.; Tsoukalas, D.; Fragkiadoulaki, I.; Psycharakis, C.; Nikitovic, D.; Spandidos, D.A.; Tsatsakis, A.M. Telomerase activity in pregnancy complications (Review). Mol. Med. Rep. 2016, 14, 16–21. [Google Scholar] [CrossRef]
- Gielen, M.; Hageman, G.; Pachen, D.; Derom, C.; Vlietinck, R.; Zeegers, M.P. Placental telomere length decreases with gestational age and is influenced by parity: A study of third trimester live-born twins. Placenta 2014, 35, 791–796. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Beedle, A.S. Early life events and their consequences for later disease: A life history and evolutionary perspective. Am. J. Hum. Biol. 2007, 19, 1–19. [Google Scholar] [CrossRef]
- Bosquet Enlow, M.; Petty, C.R.; Hacker, M.R.; Burris, H.H. Maternal psychosocial functioning, obstetric health history, and newborn telomere length. Psychoneuroendocrinology 2021, 123, 105043. [Google Scholar] [CrossRef]
- Ridout, K.K.; Syed, S.A.; Kao, H.; Porton, B.; Rozenboym, A.V.; Tang, J.; Fulton, S.; Perera, T.; Jackowski, A.P.; Kral, J.G.; et al. Relationships between telomere length, plasma glucagon-like peptide 1, and insulin in early-life stress–exposed nonhuman primates. Biol. Psychiatry Glob. Open Sci. 2022, 2, 54–60. [Google Scholar] [CrossRef]
- Ameer, S.S.; Xu, Y.; Engström, K.; Li, H.; Tallving, P.; Nermell, B.; Boemo, A.; Parada, L.A.; Peñaloza, L.G.; Concha, G.; et al. Exposure to Inorganic Arsenic Is Associated with Increased Mitochondrial DNA Copy Number and Longer Telomere Length in Peripheral Blood. Front. Cell Dev. Biol. 2016, 4, 87. [Google Scholar] [CrossRef]
- Bassig, B.A.; Zhang, L.; Cawthon, R.M.; Smith, M.T.; Yin, S.; Li, G.; Hu, W.; Shen, M.; Rappaport, S.; Barone-Adesi, F.; et al. Alterations in leukocyte telomere length in workers occupationally exposed to benzene. Environ. Mol. Mutagen. 2014, 55, 673–678. [Google Scholar] [CrossRef]
- Mitro, S.D.; Birnbaum, L.S.; Needham, B.L.; Zota, A.R. Cross-sectional Associations between Exposure to Persistent Organic Pollutants and Leukocyte Telomere Length among, U.S. Adults in NHANES, 2001–2002. Environ. Health Perspect. 2016, 124, 651–658. [Google Scholar] [CrossRef]
- Jimenez Villarreal, J.; Murillo Ortiz, B.; Martinez Garza, S.; Rivas Armendáriz, D.I.; Boone Villa, V.D.; Carranza Rosales, P.; Betancourt Martínez, N.D.; Delgado Aguirre, H.; Morán Martínez, J. Telomere length analysis in residents of a community exposed to arsenic. J. Biochem. Mol. Toxicol. 2018, 33, e22230. [Google Scholar] [CrossRef]
- Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; Willeit, P.; et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef]
- Zhang, E.; Bell, A.J.; Wilkie, G.S.; Suárez, N.M.; Batini, C.; Veal, C.D.; Armendáriz-Castillo, I.; Neumann, R.; Cotton, V.E.; Huang, Y.; et al. Inherited Chromosomally Integrated Human Herpesvirus 6 Genomes Are Ancient, Intact, and Potentially Able To Reactivate from Telomeres. J. Virol. 2017, 91, e01137-17. [Google Scholar] [CrossRef]
- Ämmälä, A.J.; Vitikainen, E.I.K.; Hovatta, I.; Paavonen, J.; Saarenpää-Heikkilä, O.; Kylliäinen, A.; Pölkki, P.; Porkka-Heiskanen, T.; Paunio, T. Maternal stress or sleep during pregnancy are not reflected on telomere length of newborns. Sci. Rep. 2020, 10, 13986. [Google Scholar] [CrossRef]
- Wojcicki, J.M.; Heyman, M.B.; Elwan, D.; Shiboski, S.; Lin, J.; Blackburn, E.; Epel, E. Telomere length is associated with oppositional defiant behavior and maternal clinical depression in Latino preschool children. Transl. Psychiatry 2015, 5, e581. [Google Scholar] [CrossRef]
- Bosquet Enlow, M.; Bollati, V.; Sideridis, G.; Flom, J.D.; Hoxha, M.; Hacker, M.R.; Wright, R.J. Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn telomere length. Psychoneuroendocrinology 2018, 95, 74–85. [Google Scholar] [CrossRef]
- García-Martín, I.; Penketh, R.; Garay, S.; Jones, R.; Grimstead, J.; Baird, D.; John, R. Symptoms of Prenatal Depression Associated with Shorter Telomeres in Female Placenta. Int. J. Mol. Sci. 2021, 22, 7458. [Google Scholar] [CrossRef]
- Eisenberg, D.T.A.; Kuzawa, C.W. The paternal age at conception effect on offspring telomere length: Mechanistic, comparative and adaptive perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160442. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tan, K.M.L.; Gong, M.; Chong, M.F.F.; Tan, K.H.; Chong, Y.S.; Meaney, M.J.; Gluckman, P.D.; Eriksson, J.G.; Karnani, N. Variability in newborn telomere length is explained by inheritance and intrauterine environment. BMC Med. 2022, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Hande, P.; Yeo, G.S.; Tan, E.C. Correlation of cord blood telomere length with birth weight. BMC Res. Notes 2017, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Verner, G.; Epel, E.; Lahti-Pulkkinen, M.; Kajantie, E.; Buss, C.; Lin, J.; Blackburn, E.; Räikkönen, K.; Wadhwa, P.D.; Entringer, S. Maternal Psychological Resilience During Pregnancy and Newborn Telomere Length: A Prospective Study. Am. J. Psychiatry 2021, 178, 183–192. [Google Scholar] [CrossRef]
- Herlin, M.; Broberg, K.; Igra, A.M.; Li, H.; Harari, F.; Vahter, M. Exploring telomere length in mother-newborn pairs in relation to exposure to multiple toxic metals and potential modifying effects by nutritional factors. BMC Med. 2019, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J. Mol. Med. 2002, 80, 689–695. [Google Scholar] [CrossRef]
- Revesz, D.; Verhoeven, J.E.; Milaneschi, Y. Penninx BW Depressive and anxiety disorders and short leukocyte telomere length: Mediating effects of metabolic stress and lifestyle factors. Psychol. Med. 2016, 46, 2337–2349. [Google Scholar] [CrossRef]
- Colatto, B.N.; de Souza, I.F.; Schinke, L.A.A.; Noda-Nicolau, N.M.; da Silva, M.G.; Morceli, G.; Menon, R.; Polettini, J. Telomere Length and Telomerase Activity in Foetal Membranes from Term and Spontaneous Preterm Births. Reprod. Sci. 2020, 27, 411–417. [Google Scholar] [CrossRef]
- Houminer-Klepar, N.; Bord, S.; Epel, E.; Baron-Epel, O. Are pregnancy and parity associated with telomere length? A systematic review. BMC Pregnancy Childbirth 2023, 23, 733. [Google Scholar] [CrossRef]
- Cheng, S.-B.; Davis, S.; Sharma, S. Maternal-fetal cross talk through cell-free fetal, D.N.A.; telomere shortening, microchimerism, and inflammation. Am. J. Reprod. Immunol. 2018, 79, e12851. [Google Scholar] [CrossRef]
- Lazarides, C.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Voelkle, M.C.; Buss, C.; Simhan, H.N.; Wadhwa, P.D.; Entringer, S. Maternal pro-inflammatory state during pregnancy and newborn leukocyte telomere length: A prospective investigation. Brain Behav. Immun. 2019, 80, 419–426. [Google Scholar] [CrossRef]
- Balan, E.; Decottignies, A.; Deldicque, L. Physical Activity and Nutrition: Two Promising Strategies for Telomere Maintenance? Nutrients 2018, 10, 1942. [Google Scholar] [CrossRef] [PubMed]
- Lis, N.; Lamnisos, D.; Bograkou-Tzanetakou, A.; Hadjimbei, E.; Tzanetakou, I.P. Preterm Birth and Its Association with Maternal Diet, and Placental and Neonatal Telomere Length. Nutrients 2023, 15, 4975. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.E.; Mage, D.T.; Grant, L.D. Estimating separately personal exposure to ambient and nonambient particulate matter for epidemiology and risk assessment: Why and how. J. Air Waste Manag. Assoc. 2000, 50, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
| Variable | Unit or Category | Mean (Range) or N (%) |
|---|---|---|
| Maternal age | Years | 33 (21–44) |
| Maternal education | ||
| Elementary school | 3 (4%) | |
| High school or GED | 15 (20%) | |
| Some college | 15 (20%) | |
| Bachelor’s degree | 16 (22%) | |
| Master’s degree Advanced degree | 10 (14%) 15 (20%) | |
| Household income | ||
| Less than USD 15,000 | 14(19%) | |
| USD 15,000–30,999 | 14 (19%) | |
| USD 31,000–50,999 | 3 (4%) | |
| USD 51,000–100,999 | 2 (3%) | |
| USD 101,000–149,999 | 10 (13%) | |
| USD 150,000+ | 31 (42%) | |
| Stressors | Number of events | 6.8 (0–39) |
| Perceived stress | Continuous score | 14.6 (3–31) |
| Maternal depression | Continuous score | 5.8 (0–18) |
| Infant sex | ||
| Male | 38 (51%) | |
| Female | 36 (49%) | |
| Infant gestational age | Weeks | 36.4 (27.3–41.6) |
| Infant birth weight | Grams | 2771.6 (700–4650) |
| Infant telomere length | T/S ratios * | 2.71 (2.07–3.63) |
| PM2.5 exposure | ||
| First trimester exposure | μg/m3 | 9.4 (4.2–22.7) |
| Second trimester exposure | μg/m3 | 8.8 (3.9–21.1) |
| Third trimester exposure | μg/m3 | 8.3 (4.2–19.7) |
| Average pregnancy exposure | μg/m3 | 8.8 (5.5–14.4) |
| Effects on Infant Telomere Length | |||
|---|---|---|---|
| Model Covariates | β (95% CI) | Standard Error | p-Value |
| Average Prenatal PM2.5 | 0.466 (0.275–0.657) | 0.096 | 0.000 |
| Depressive Symptoms | 0.225 (0.029–0.421) | 0.098 | 0.025 |
| Maternal Age | 0.218 (0.020–0.417) | 0.099 | 0.032 |
| Infant Male Sex | −0.258 (−0.451–−0.065) | 0.097 | 0.010 |
| Effects on Infant Telomere Length | |||
|---|---|---|---|
| Model Covariates | β (95% CI) | Standard Error | p-Value |
| T1 PM2.5 | 0.184 (−0.015–0.382) | 0.099 | 0.069 |
| T2 PM2.5 | 0.310 (0.110–0.509) | 0.099 | 0.003 |
| T3 PM2.5 | 0.241 (0.038–0.443) | 0.101 | 0.021 |
| Depressive Symptoms | 0.207 (0.004–0.410) | 0.102 | 0.046 |
| Maternal Age | 0.211 (−0.011–0.412) | 0.101 | 0.039 |
| Infant Male Sex | −0.245 (−0.442–−0.049) | 0.098 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahlers, N.E.; Lin, J.; Weiss, S.J. Exposure to Ambient Particulate Matter during Pregnancy: Implications for Infant Telomere Length. Air 2024, 2, 24-37. https://doi.org/10.3390/air2010002
Ahlers NE, Lin J, Weiss SJ. Exposure to Ambient Particulate Matter during Pregnancy: Implications for Infant Telomere Length. Air. 2024; 2(1):24-37. https://doi.org/10.3390/air2010002
Chicago/Turabian StyleAhlers, Nina E., Jue Lin, and Sandra J. Weiss. 2024. "Exposure to Ambient Particulate Matter during Pregnancy: Implications for Infant Telomere Length" Air 2, no. 1: 24-37. https://doi.org/10.3390/air2010002
APA StyleAhlers, N. E., Lin, J., & Weiss, S. J. (2024). Exposure to Ambient Particulate Matter during Pregnancy: Implications for Infant Telomere Length. Air, 2(1), 24-37. https://doi.org/10.3390/air2010002





