Abstract
Clarifying the function of approximately 20,000 proteins encoded by the human genome is a key challenge in the fields of medicine and biology. However, many proteins remain uncharacterized. In this review, we introduce a challenge that uses adult T-cell leukemia/lymphoma (ATL) and proteomics to study human proteins of unknown function (PUFs). The characteristic properties of ATL cells are as follows: ATL cells (1) are infected with virus, (2) are derived from CD4+ T cells, (3) are generated via multi-stage carcinogenesis, (4) have flower-like nuclei, and (5) are highly infiltrative in the aggressive type. Given that ATL cells have contributed to impressive basic research, such as the discovery of HTLV-1 as a human cancer virus and interleukin-2 (IL-2) receptor α chain (IL-2Rα)/CD25, which is used for identifying regulatory T (Treg) cells, ATL cell lines could still be considered an attractive research tool. Furthermore, the “Unknome database” is useful for examining function-unknown degrees of proteins of interest using known scores based on Gene Ontology (GO) annotations and protein analysis through evolutionary relationships (PANTHER). Combining ATL proteomic data obtained by us with the “Unknome database” is expected to contribute not only to investigating the pathogenetic mechanism of ATL but also to clarifying the functions of PUFs.
1. Introduction
Since the first draft of the human genome sequence was released in 2000 [1,2], there has been an attractive challenge in clarifying the functions of approximately 20,000 proteins encoded by the human genome. The Human Genome Project has revealed thousands of open reading frames that encode proteins whose functions have not yet been clarified, and omics analysis has accelerated the functional clarification of these new proteins [3]. However, despite extensive efforts to clarify their functions over the past 20 years, many proteins still have unknown functions [4,5]. In addition to clinical and biological necessity, the development of experimental systems, including model cells and animals, plays important roles in clarifying protein function.
Adult T-cell leukemia/lymphoma (ATL) is a hematologic cancer caused by the infection of CD4+ T cells with human T-cell leukemia virus type-1 (HTLV-1) [6]. Approximately 5–10 million individuals are infected with HTLV-1 worldwide [7]. ATL has four subtypes: acute, lymphoma, chronic, and smoldering [8]. Among these subtypes, the first two types and the chronic type with one or more unfavorable prognostic factors (high lactate dehydrogenase, high blood urea nitrogen, and low albumin), which are defined as aggressive ATL [9], are subjected to medical treatment but have a very poor prognosis, with a median overall survival of less than 1 year [10,11]. Despite over 40 years since the discovery of HTLV-1, ATL still requires new drug target molecules for therapies [12,13], and basic findings on ATL, including the developmental mechanism, characterization of ATL cell lines, and the molecular mechanism underlying the HTLV-1 infection, are still lacking.
Previously, we studied the prevention of ATL and hepatitis C virus (HCV)-related hepatocellular carcinoma (HCC) using functional food ingredients and made use of proteomics [14,15,16,17]. In HCV-related HCC, proteomic analysis using sera from HCC patients established a decision tree for early diagnosis [14], and chemical biology identified hnRNP A2/B1 as a target protein of proanthocyanidin from blueberry leaves, which suppresses HCV replication [16]. In ATL, we identified a complementary component, C3f, as a biomarker by analyzing patient sera [15]. In addition, we reported that carnosol, a compound found in rosemary, induces apoptosis in ATL cells dependent on oxidative stress [17]. In the course of these studies, we noticed that ATL cells are a useful tool for basic biological research, in particular, function-clarifying human proteins of unknown function (PUFs). Furthermore, a series of our studies on RNA degradation complex made us realize the requirement of focused proteomics and of increasing comprehensiveness of proteome [18,19,20].
In this review, we introduce a challenge to make use of ATL and proteomics to study human PUFs.
2. Overview of Human PUFs
The human genome encodes approximately 20,000 proteins, and transcriptomics and proteomics have revealed that most proteins whose functions have not yet been clarified are expressed in various organs/tissues/cells, including cultured cells, under certain conditions, such as healthy or disease status [3]. However, there are still many proteins with unknown functions [4,5], and clarifying the functions of these PUFs is important for understanding organisms and diseases, followed by the development of new therapies [21].
In 2001, when the two papers on the human genome sequence were published, functional characteristics were reported to be either known or predictable for approximately 40% [1] and 58% [2] of human protein-coding genes. To date, over 20 years of extensive efforts have advanced the accumulation of knowledge on protein function, and databases constructed from literature and individual experimental data have made it possible to obtain various kinds of information about the proteins of interest [22,23,24,25,26,27,28,29]. A recent paper by the Gene Ontology (GO) Consortium reported that the coverage is greater, with 82% of the human genes associated with at least one functional characteristic, but those with primary GO annotations remain at 67% [30]. Approximately 30% of human genes remain without fully clarified functions.
Despite the presence of many human PUFs, publication trend analysis has revealed that research interests focus on genes/proteins that have already been characterized with these annotations [4,31,32]. This is probably due to research limitations, such as funding and peer review that support research on proteins with some evidence, elimination of project risks, and convenience of analysis tools such as antibodies, inhibitors, and model organisms [32,33,34]. However, the biological and medical significance of human PUFs is likely to be enhanced by the fact that many of them are conserved among organisms [5] and are also attractive as drug target molecules [35]. In addition, it has been reported that a significant number of them do not contain particular functional domains of known proteins [31]. This is also a factor for being removed from analysis objects because clues for clarifying protein function are poor.
Since the principal purpose of the above databases is to accumulate protein-functional information followed by its supply for contribution to the next breakthroughs, researchers naturally tend to focus on well-studied proteins with detailed annotations but not on the PUFs. To highlight these ignored proteins, it is important for researchers to evaluate the degree of “unknown” proteins of interest.
“Unknome database” has been developed to score known degree of each protein from a particular organism [36]. The term “Unknome” is presumed to be a neologism derived from function-unknown and genome/proteome. In the Unknome database, a known score is assigned to proteins based on the widely used GO annotations [22] and protein analysis through evolutionary relationships (PANTHER) [23], which makes it possible to examine the unknown degree of proteins of interest for humans or a chosen model organism. For instance, while human proteins with top 10 recognition, such as β-catenin and transforming growth factor β1, show scores of 121.3 to 174.1, those of proteins with little or no annotations are 2 or less, accounting for 23% of human proteins in 2022. Among the 260 unknown genes conserved between humans and flies, knockdown of some genes in Drosophila affects viability, fertility, development, locomotion, protein quality control, and resilience to stress. This suggests the biological importance of the remaining PUFs.
The Unknome database is useful for selecting PUFs for analysis. We plan to apply the Unknome database to select PUFs from the ATL proteome.
3. ATL Cell Line Is Good Tool for Basic Biology
3.1. Leukemogenesis Process and Cellular Characteristics of ATL
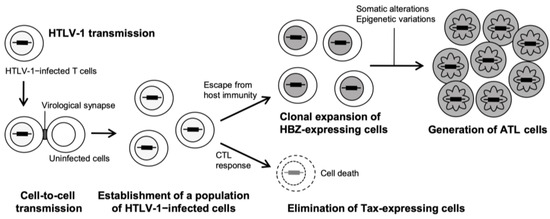
Figure 1.
Natural history of HTLV-1 infection to ATL development.
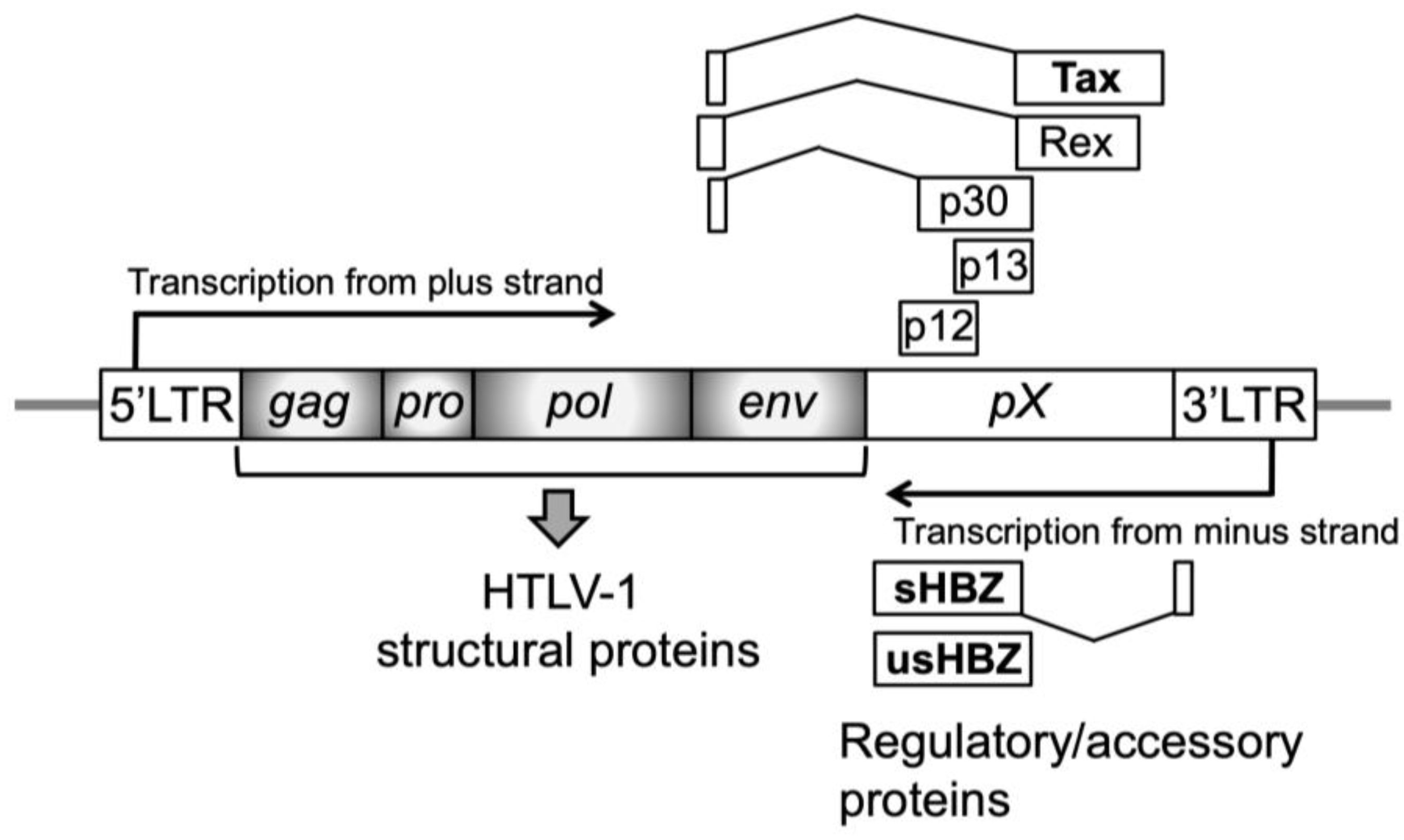
After an individual is infected with HTLV-1 via three primary routes: mother-infant (mainly through breastfeeding), sexual contact, and parenteral transmission, the viral genome RNA is reverse-transcribed into double-stranded DNA and then integrated as a provirus into the host chromosomal DNA [39,40]. While HTLV-1 can infect various cells, such as B cells and macrophages, in addition to T cells, transformation is induced almost exclusively in CD4+ T-cells. A population of infected cells is established in the body by de novo infection from infected cells to uninfected cells (virological synapse formation) and proliferation of the infected cells themselves. The HTLV-1 provirus has four structural genes (gag, pro, pol, and env) necessary for virion formation and several regulatory (Tax and Rex) and accessory (p12, p13, and p30) proteins located in the pX region, all of which are derived from plus-strand transcripts (Figure 2) [41].
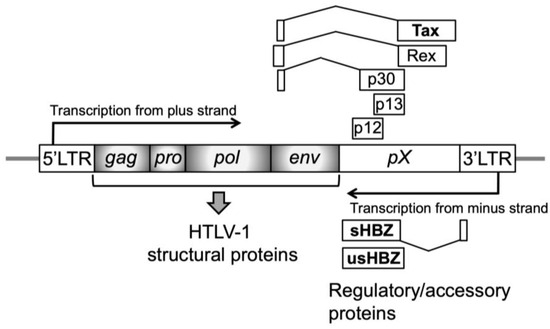
Figure 2.
Structure of the HTLV-1 provirus. The positions and relative sizes of open reading frames for viral-specific proteins are shown as boxes. Viral structural proteins for progeny virions are encoded by four genes (gag, pro, pol, and env; shaded gray boxes), and most amino acid sequences of viral regulatory proteins (Tax and Rex) and accessory proteins (p12, p13, and p30) are encoded by the pX region. These proteins are synthesized from the plus-strand transcript. In contrast, another regulatory protein, HTLV-1 bZIP factor (HBZ), which has spliced (sHBZ) and unspliced (usHBZ) forms, is synthesized from the minus-strand transcript. These coding regions are flanked by a 5′-long terminal region (5′-LTR) and 3′-LTR.
Among these viral proteins, Tax plays a central role in establishing an infected cell population. Tax enhances the transcription of HTLV-1 structural genes by being recruited to the 5′ long terminal repeat (LTR), contributing to cell–cell contact site formation by structural proteins. As Tax can also immortalize T cells, the infected cells clonally expand. In contrast, Tax-expressing cells are eliminated by the host cytotoxic T lymphocyte (CTL) response owing to their high immunogenicity. In addition to Tax, p12, p13, p30, and Rex contribute to the establishment of persistent viral infections.
The HTLV-1 bZIP factor (HBZ) is essentially associated with leukemogenesis in ATL development (Figure 2) [42,43,44]. HBZ, synthesized from the minus strand of the viral genome, is a regulatory protein with two isoforms: spliced (sHBZ) and unspliced (usHBZ). HBZ’s key function is that it regulates in opposition to viral and host cellular events influenced by Tax [44,45]. For example, HBZ inhibits Tax-dependent viral transcription [46]. As previously reported, HBZ suppresses the activation of NF-κB by Tax, which plays a crucial role in HTLV-1–induced transformation [47], and HBZ acts against Tax in diverse Tax-dependent cellular signal transduction pathways [44,45]. Similarly to the opposing functions of Tax and HBZ, analysis of clinical specimens reports that while the infected cells from asymptomatic carriers express both Tax and HBZ, the expression in the cells from ATL patients is maintained with regard to HBZ, in contrast to the silence or inactivation of Tax expression. Studies on the association with immunity suggest that Tax-expressing cells are eliminated by the host cytotoxic T lymphocyte (CTL) due to their high degree of immunogenicity [48,49], whereas HBZ is not a target antigen for HTLV-1–specific CTL (Figure 1) [50]. Therefore, it is supposed that the infected cells that express HBZ but not Tax escape from the immune system and clonally expand [51,52], although clear evidence has not yet been shown. The finding that HBZ plays a critical role in ATL development is supported by the fact that conditional transgenic mice that specifically express HBZ in CD4+ T-cells exhibit pathology of T-cell lymphoma [43]. Recently, the nuclear localization of HBZ from the cytoplasm has been proposed to be required for development [53,54]. Epigenetic events (e.g., DNA methylation and histone modifications) and gene mutations and fusions have also been reported to play important roles in development [55,56,57,58].
Leukemia cells from acute-type ATL typically exhibit a hallmark morphology called “flower cell” with a deeply lobulated, petal-like nucleus (Figure 1) [59,60,61]. Although flower cells are present in the peripheral blood in all types except for the lymphoma type, the abnormality of the nuclear shape is generally milder in the indolent types of ATL, smoldering, and chronic types. Since the appearance of flower cells characterizes ATL, its diagnostic value is high, as is the detection of HTLV-1 antibodies in the serum and monoclonal integration of HTLV-1 provirus into the genome [62]. In particular, the degree of lobulation of flower cells is highest in the acute type with high malignancy, and clarifying the underlying mechanism of lobulation may be the key to identifying treatment target molecules against ATL.
As described above, some HTLV-1 carriers develop ATL after multiple cellular stages during a long latency period of at least 20–30 years following viral infection. Basic research on ATL is crucial for developing treatments for ATL due to its intractability. Furthermore, the process of ATL leukemogenesis has been reported to be consistent with the general multi-stage theory of carcinogenesis [63,64]. Therefore, ATL cell line has also been used as one of the interesting and attractive research tools for clarifying the molecular mechanism underlying cancer in general by many researchers, including us. While the underlying pathogenic factors in most other cancers are multiple and complex, the pathogen of ATL is clearly a single virus, namely HTLV-1. This is the benefit of ATL in cancer research.
3.2. Contribution of ATL Cell to Basic Research
The biological characteristics of ATL cells are as follows: ATL cells (1) are infected with virus, (2) are derived from CD4+ T cells, (3) are generated via multi-stage carcinogenesis, (4) have flower-like nuclei, and (5) are highly infiltrative in the aggressive type. These properties may contribute to clarifying the mechanisms of viral infection and immunity in virology, the functions of T-cells in immunology, carcinogenesis and metastasis/invasion in cancer biology, and the morphogenesis of nuclei in cell biology.
Table 1 shows principal examples of basic research in which ATL cells play important roles.

Table 1.
Principal examples of basic research validating the important roles of ATL cells.
The first impressive example in basic research on ATL is the discovery of HTLV-1 as its pathogen [6,39]. The significance of the HTLV-1 discovery in history of basic biology, including virology, lies in its identification as the first human cancer virus. The existence of ATL, a type of leukemia unique to adults in southern Kyushu and Shikoku, Japan, was reported in 1977 [71]. In 1980, Gallo’s research group reported that type C retrovirus particles were detected and isolated from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma (mycosis fungoides) [39]. In 1981, Hinuma et al. reported the existence of viral antigens by immunofluorescent staining using sera from ATL patients, accompanied by type C retrovirus particles observed in the cells using an electron microscope [6]. This was the first study to clearly reveal the association between retroviruses and ATL. Subsequently, both viruses were reported to be the same species of human retrovirus and were named HTLV-1 [72,73]. Considering the circumstances at the levels of these original papers, it was Gallo’s group in 1980 that first discovered HTLV-1. Additionally, Hinuma’s group in 1981 first supplied accurate findings that HTLV-1 is a human cancer virus, discovered for the first time, based on data on ATL. Furthermore, experimental procedures cultivated through studies on ATL, such as T-cell culture and HTLV-1 genome sequencing, were of great use in AIDS studies [74]. Although there was controversy among the researchers involved in the discovery of HTLV-1 [75,76,77,78], in any case, studies on ATL contributed to this major biological discovery. The first discovery of HTLV-1 as a cancer virus marked the beginning of subsequent research into oncogenic viruses [79].
Next example is the discovery of the interleukin-2 (IL-2) receptor α chain (IL-2Rα)/CD25. Molecular cloning of IL-2Rα/CD25 was reported by two independent research groups in 1984 [66,80]. Since the cloning technique without sequence information requires purifying the protein coded by the cDNA followed by partial amino acid sequence, purification was performed using ATL cells [66], based on the findings that the protein is highly expressed in these cells [65]. In later years, IL-2Rα/CD25 contributed to the identification of regulatory T (Treg) cells [81,82], and its soluble form has also been utilized as a biomarker for clinical diagnosis of various diseases, including ATL [83,84,85].
In recent years, oxidative stress and reduction–oxidation (redox) status in living organisms have been the focus of medical attention [86,87]. Redox changes are a common feature of aging, and their disruption is a common factor in disease [88]. In ATL, proliferation of HTLV-1–infected T-cells and leukemogenesis involves oxidative stress, of which cellular redox-related proteins and viral proteins (HBZ, Tax, p30, and p13) regulate the generation and elimination [89,90,91,92]. Although thioredoxin plays a central role in redox regulation and signaling [86,88], the human counterpart of this factor was discovered from the conditioned medium of ATL cells as a factor that induces IL-2Rα/CD25 expression (termed ATL-derived factor, ADF) [67], and was revealed to have high homology with the prokaryotic disulfide-reducing enzyme thioredoxin [68,69,93], followed by being termed thioredoxin 1 [94]. Recently, the importance of thioredoxin 1 in health and disease is also reported [95]. ATL cells play a crucial role in oxidative stress research.
The final example is the development of the molecular-targeted drug mogamulizumab for certain types of leukemia/lymphoma, including ATL [96,97]. From the late 1980s to the 1990s, the cloning and characterization of chemokine (C-C motif) ligands (CCLs) and chemokine (C-C motif) receptors (CCRs) progressed, and the potential of drug development by regulating CCLs and CCRs was suggested [98,99]. Although mogamulizumab, the first molecular-targeted drug approved for ATL treatment in Japan in 2012, is a humanized antibody against CCR4, ATL cells contributed to its development. In the course of cloning cDNAs coding novel membrane/secretory proteins, a chemokine ligand CCL17/TARC was identified as a secretory protein with T-cell chemotactic activity, and CCR4 as its receptor by Yoshie’s research group [100,101]. In contrast, Matsushima’s research group has been conducting research focusing on the chemokine family since the 1980s [102]. Collaboration between the two groups revealed that CCR4 is a chemokine receptor preferentially expressed in a subset of helper T (Th) cells, Th2 cells, using a mouse monoclonal antibody against human CCR4 (KW2160) that was generated collaboratively with Kyowa Hakko Kogyo [97,103]. Further expression analysis in human T-cell lines revealed that ATL cells and HTLV-1–transformed cells highly express CCR4, which was confirmed in peripheral blood mononuclear cells (PBMCs) from ATL patients [70]. Next, KM2160 was converted into a chimeric antibody with human IgG1, and antibody-dependent cellular cytotoxicity (ADCC) activity was enhanced by the defucosylation of the sugar chain in the Fc region of the antibody molecule, which generated KM2760 [104]. As the final form for clinical application, the defucosylated and fully humanized anti CCR4 monoclonal antibody KW-0761 (mogamulizumab) was developed and approved by the Japanese PMDA in March 2012 for the treatment of relapsed/refractory ATL [96,97,105]. Recently, the combination therapy of mogamulizumab with chemotherapies such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) has been reported to be efficient for older patients with aggressive ATL with no standard care [106,107].
To date, ATL cells have contributed to obtaining fundamental and important biological insights across fields ranging from basic research to clinical practice. In conclusion, ATL cell line is a useful tool for studying basic biology.
4. Challenges to PUFs in ATL Research
ATL cell lines are still considered attractive research tools in the basic research field, such as molecular cell biology. The following reasons can be cited: (1) globally, few researchers conduct studies using ATL cells; (2) since research interest tends to focus on diagnostic and therapeutic applications, there is not sufficient accumulation of fundamental findings on ATL; (3) ATL possesses the unique pathogen HTLV-1; (4) ATL cells exhibit unique morphology, such as flower-like nuclei; (5) since ATL still requires new drug target molecules for the therapies, it is speculated that ATL cells possess unclarified signal pathways underlying leukemogenesis, including the molecules.
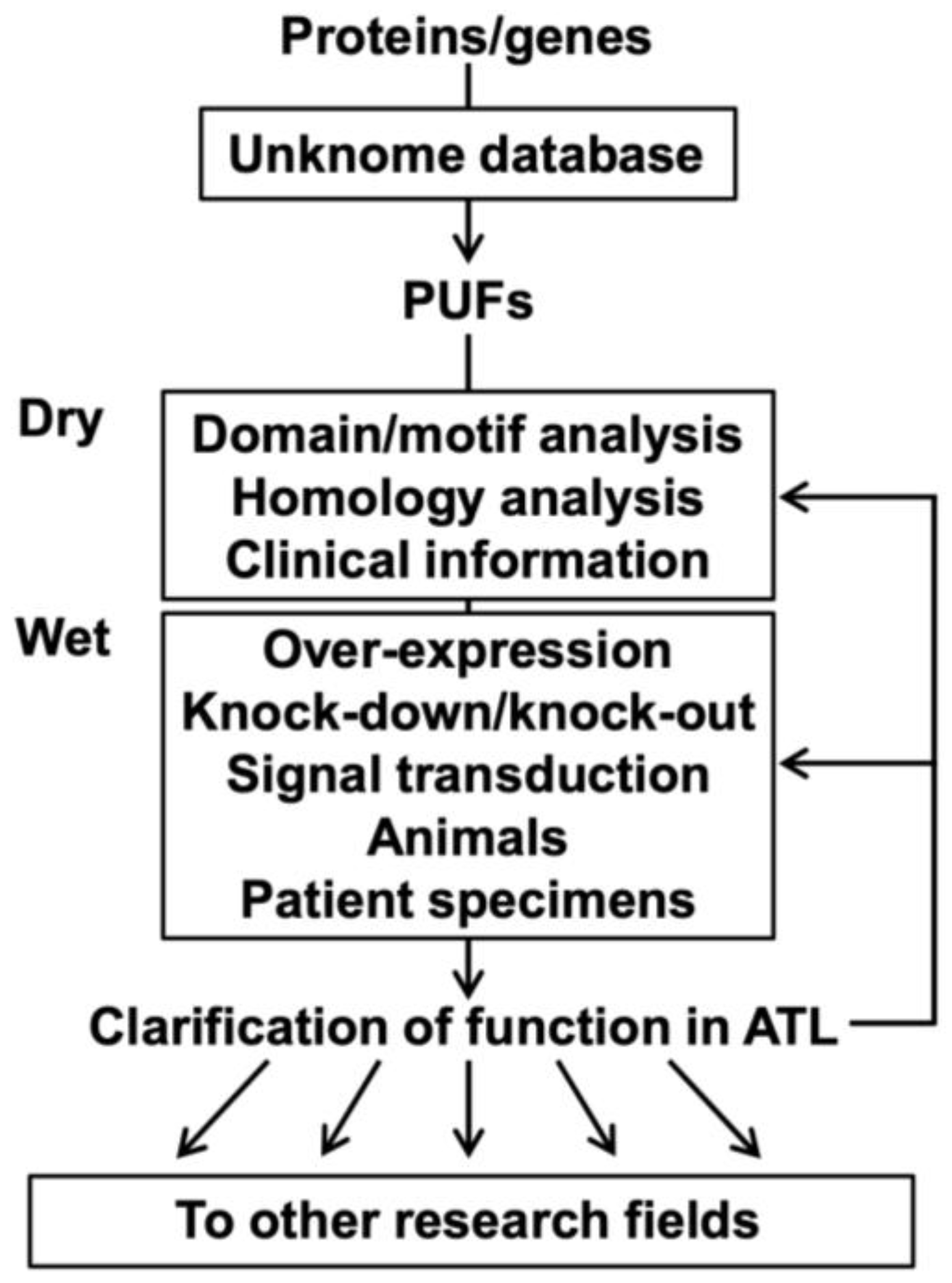
To discover unidentified factors associated with ATL development, focusing on human PUFs may be reasonable and useful as a research approach. Figure 3 shows the research flow of PUFs in ATL.
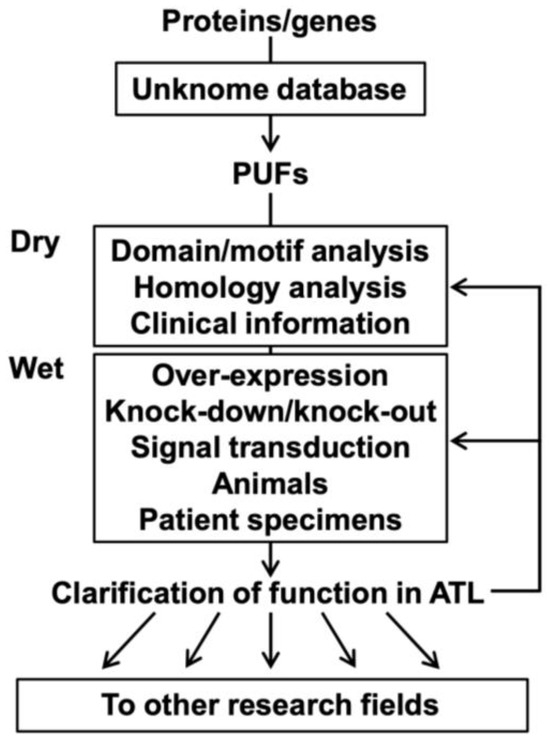
Figure 3.
Research flow of PUFs in ATL.
Researchers interested in proteins/genes can examine these known scores using the Unknome database and select the PUFs for analysis. Selected PUFs are subjected to dry experiments (e.g., domain/motif analysis, homology analysis, and clinical information on ATL) and wet experiments (e.g., overexpression, knockdown/knockout, signal transduction experiments), including experiments using ATL model animals and clinical specimens from ATL patients. A series of analyses using ATL cells could provide findings not only on ATL and T-cells but also on general functions and other types of diseases/organs/tissues/cells. Clarifying the functions of PUFs in ATL cells would enable further deepening of ATL research and expansion to other research fields, such as drug discovery.
Proteomic and transcriptomic data on ATL would undoubtedly be attractive as a source of proteins/genes. In general, transcriptomic analysis is superior to proteomic analysis in terms of comprehensiveness and convenience and is capable of detecting changes in protein expression via transcripts. Thus, the application of transcriptomic analysis in protein expression analysis is limited to proteins with a quantitative correlation between mRNAs and translated products. While many protein biomarkers have been identified using transcriptomic analysis, as shown in the example of TSLC1/CADM1 in ATL [108], the analysis cannot theoretically capture quantitative changes in proteins whose expression is regulated at the step of translation and degradation. mRNA expression only weakly correlates with protein expression [109]. Transcriptome analysis can be performed relatively simply owing to its standardized protocols and is more widely adapted than proteomics, which requires skilled expertise in sample preparation and maintenance of nano-flow liquid chromatography and mass spectrometry. Collectively, the analysis of PUFs identified by proteomic, “unknomic,” and transcriptomic analyses provides novel findings on ATL.
Therefore, we established a biomarker identification strategy based on the proteomics of cultured ATL cells to search for novel ATL biomarkers [110]. Our proteomic analysis identified 24 and 27 proteins that were significantly increased (ratio ≥ 2.0, p < 0.05) and decreased (ratio ≤ 0.5, p < 0.05), respectively, in the ATL cell group compared to the HTLV-1–infected and HTLV-1–negative cell groups. Furthermore, changing the ratio thresholds from 2.0 (increased) and 1/2.0 (decreased) to 1.5 and 1/1.5, respectively, increased the number of candidates to approximately 250 proteins.
To focus on the PUFs in the research field of ATL, we used the Unknome database as follows [36]. In the Unknome database (https://unknome.mrc-lmb.cam.ac.uk/), each protein is placed in a cluster of orthologs based on the PANTHER database, and the known score is determined based on the number of GO terms that have been assigned to the cluster and its members. Accordingly, researchers can investigate known scores as the cluster to which each protein belongs and as the protein simply by entering the Uniprot ID, gene name, and so on. Since our proteomic analysis identified ATL-specific proteins using amino acid sequences from the Uniprot database, their known scores can be easily investigated in the Unknown database. Our preliminary analysis of ANKRD22 with the highest ratio in the ATL group compared with the HTLV-1–infected cell group using the Unknome database showed that the known scores of ANKRD22 and its cluster (Cluster ID: UKP09284) were both “0.0”, namely a PUF. Although PubMed searches have found ANKRD22-related papers in diseases such as lung cancer [111], its general and concrete cellular functions have not been clarified apart from intracellular localization [112]. The PUFs such as ANKRD22 are expected to be found in our ATL proteomic data.
While the above is an instance of using cultured ATL cell lines, another anticipated application is the use of the Unknome database for clinical specimens from ATL patients. It is still technically difficult to cultivate primary cultured cells from patients, and in vivo phenotypes, including gene/protein expression, may not be maintained ex vivo. Thus, ATL cells need to be directly subjected to proteomic and transcriptomic analyses immediately after separation from the living body. PBMCs from ATL patients, HTLV-1–infected asymptomatic carriers (ACs: control), or healthy controls (HC) were sorted by fluorescence-activated cell sorting (FACS), and it would be preferable for separated CD4+ IL-2Rα/CD25+ T cells to be subjected to extraction of proteins and RNA, followed by proteomics and transcriptomics, respectively. CD4+ T-cells from ACs and HC have been widely adopted as controls in this type of differential analysis [108]. IL-2Rα/CD25, which is highly expressed in ATL and Treg cells, may be an efficient additional marker for analysis, as used in the expression analysis of Foxp3, a master gene of Treg cells [113]. The Unknome database is useful for selecting genes/proteins of unknown function from ATL-specific genes/proteins identified by omics analysis of clinical specimens.
5. Conclusions
ATL is a hematologic cancer with poor prognosis, and new drug target molecules for therapy are required. Function clarification of PUFs as a research tool using ATL cells may provide new insights into the development of ATL treatments and is also significant in basic research. The ATL cell line is a useful tool for studying basic biology.
Author Contributions
Conceptualization, Y.-i.I.; Investigation, Y.-i.I. and Y.T.; writing—original draft preparation, Y.-i.I.; writing—review and editing, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
To Takashi Morita (National Institute of Infectious Diseases in Japan) for evaluation of research ideas.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PUFs | Proteins of unknown function |
| ATL | Adult T-cell leukemia/lymphoma |
| HTLV-1 | Human T-cell leukemia virus type 1 |
| IL-2 | Interleukin-2 |
| IL-2Rα | IL-2 receptor α chain |
| Treg | Regulatory T |
| GO | Gene Ontology |
| PANTHER | Protein analysis through evolutionary relationships |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| CTL | Cytotoxic T lymphocyte |
| LTR | Long terminal region |
| HBZ | HTLV-1 bZIP factor |
| sHBZ | Spliced HBZ |
| usHBZ | Unspliced HBZ |
| ADF | ATL-derived factor |
| CCL | Chemokine (C-C motif) ligand |
| CCR | Chemokine (C-C motif) receptor |
| PBMC | Peripheral blood mononuclear cell |
| ADCC | Antibody-dependent cellular cytotoxicity |
References
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef]
- Adhikari, S.; Nice, E.C.; Deutsch, E.W.; Lane, L.; Omenn, G.S.; Pennington, S.R.; Paik, Y.K.; Overall, C.M.; Corrales, F.J.; Cristea, I.M.; et al. A high-stringency blueprint of the human proteome. Nat. Commun. 2020, 11, 5301. [Google Scholar] [CrossRef]
- Sinha, S.; Eisenhaber, B.; Jensen, L.J.; Kalbuaji, B.; Eisenhaber, F. Darkness in the Human Gene and Protein Function Space: Widely Modest or Absent Illumination by the Life Science Literature and the Trend for Fewer Protein Function Discoveries Since 2000. Proteomics 2018, 18, e1800093. [Google Scholar] [CrossRef]
- Wood, V.; Lock, A.; Harris, M.A.; Rutherford, K.; Bahler, J.; Oliver, S.G. Hidden in plain sight: What remains to be discovered in the eukaryotic proteome? Open Biol. 2019, 9, 180241. [Google Scholar] [CrossRef]
- Hinuma, Y.; Nagata, K.; Hanaoka, M.; Nakai, M.; Matsumoto, T.; Kinoshita, K.I.; Shirakawa, S.; Miyoshi, I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 1981, 78, 6476–6480. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–1987). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, M. Chemotherapy of ATL; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Katsuya, H.; Ishitsuka, K.; Utsunomiya, A.; Hanada, S.; Eto, T.; Moriuchi, Y.; Saburi, Y.; Miyahara, M.; Sueoka, E.; Uike, N.; et al. Treatment and survival among 1594 patients with ATL. Blood 2015, 126, 2570–2577. [Google Scholar] [CrossRef]
- Sekine, M.; Kameda, T.; Shide, K.; Maeda, K.; Toyama, T.; Kawano, N.; Takeuchi, M.; Kawano, H.; Sato, S.; Ishizaki, J.; et al. Higher average chemotherapy dose intensity improves prognosis in patients with aggressive adult T-cell leukemia/lymphoma. Eur. J. Haematol. 2021, 106, 398–407. [Google Scholar] [CrossRef]
- Weterings, D.A.; Rowan, A.G.; Cook, L.B. Immunological aspects of HTLV-1 persistence; for the prevention and treatment of Adult T-cell leukaemia-lymphoma (ATL). Leuk. Res. 2025, 148, 107635. [Google Scholar] [CrossRef] [PubMed]
- Epstein-Peterson, Z.D.; Gurumurthi, A.; Horwitz, S.M. New treatments for adult T-cell leukemia/lymphoma. Leuk. Res. 2025, 149, 107642. [Google Scholar] [CrossRef]
- Kanmura, S.; Uto, H.; Kusumoto, K.; Ishida, Y.; Hasuike, S.; Nagata, K.; Hayashi, K.; Ido, A.; Stuver, S.O.; Tsubouchi, H. Early diagnostic potential for hepatocellular carcinoma using the SELDI ProteinChip system. Hepatology 2007, 45, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Yamashita, K.; Sasaki, H.; Takajou, I.; Kubuki, Y.; Morishita, K.; Tsubouchi, H.; Okayama, A. Activation of complement system in adult T-cell leukemia (ATL) occurs mainly through lectin pathway: A serum proteomic approach using mass spectrometry. Cancer Lett. 2008, 271, 167–177. [Google Scholar] [CrossRef]
- Takeshita, M.; Ishida, Y.; Akamatsu, E.; Ohmori, Y.; Sudoh, M.; Uto, H.; Tsubouchi, H.; Kataoka, H. Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J. Biol. Chem. 2009, 284, 21165–21176. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Yamasaki, M.; Yukizaki, C.; Nishiyama, K.; Tsubouchi, H.; Okayama, A.; Kataoka, H. Carnosol, rosemary ingredient, induces apoptosis in adult T-cell leukemia/lymphoma cells via glutathione depletion: Proteomic approach using fluorescent two-dimensional differential gel electrophoresis. Hum. Cell 2014, 27, 68–77. [Google Scholar] [CrossRef]
- Hiraishi, N.; Ishida, Y.; Nagahama, M. AAA-ATPase NVL2 acts on MTR4-exosome complex to dissociate the nucleolar protein WDR74. Biochem. Biophys. Res. Commun. 2015, 467, 534–540. [Google Scholar] [CrossRef]
- Hiraishi, N.; Ishida, Y.I.; Sudo, H.; Nagahama, M. WDR74 participates in an early cleavage of the pre-rRNA processing pathway in cooperation with the nucleolar AAA-ATPase NVL2. Biochem. Biophys. Res. Commun. 2018, 495, 116–123. [Google Scholar] [CrossRef]
- Ishida, Y.I.; Miyao, S.; Saito, M.; Hiraishi, N.; Nagahama, M. Interactome analysis of the Tudor domain-containing protein SPF30 which associates with the MTR4-exosome RNA-decay machinery under the regulation of AAA-ATPase NVL2. Int. J. Biochem. Cell Biol. 2021, 132, 105919. [Google Scholar] [CrossRef]
- Kang, H.; Lee, C.J. Transmembrane proteins with unknown function (TMEMs) as ion channels: Electrophysiological properties, structure, and pathophysiological roles. Exp. Mol. Med. 2024, 56, 850–860. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Thomas, P.D.; Campbell, M.J.; Kejariwal, A.; Mi, H.; Karlak, B.; Daverman, R.; Diemer, K.; Muruganujan, A.; Narechania, A. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res. 2003, 13, 2129–2141. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Mathias, S.; Bologa, C.; Brunak, S.; Fernandez, N.; Gaulton, A.; Hersey, A.; Holmes, J.; Jensen, L.J.; Karlsson, A.; et al. Pharos: Collating protein information to shed light on the druggable genome. Nucleic Acids Res. 2017, 45, D995–D1002. [Google Scholar] [CrossRef] [PubMed]
- Duek, P.; Gateau, A.; Bairoch, A.; Lane, L. Exploring the Uncharacterized Human Proteome Using neXtProt. J. Proteome Res. 2018, 17, 4211–4226. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef]
- Feuermann, M.; Mi, H.; Gaudet, P.; Muruganujan, A.; Lewis, S.E.; Ebert, D.; Mushayahama, T.; Gene Ontology, C.; Thomas, P.D. A compendium of human gene functions derived from evolutionary modelling. Nature 2025, 640, 146–154. [Google Scholar] [CrossRef]
- Pena-Castillo, L.; Hughes, T.R. Why are there still over 1000 uncharacterized yeast genes? Genetics 2007, 176, 7–14. [Google Scholar] [CrossRef]
- Edwards, A.M.; Isserlin, R.; Bader, G.D.; Frye, S.V.; Willson, T.M.; Yu, F.H. Too many roads not taken. Nature 2011, 470, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.A.; Tomczak, A.; Khatri, P. Gene annotation bias impedes biomedical research. Sci. Rep. 2018, 8, 1362. [Google Scholar] [CrossRef]
- Stoeger, T.; Gerlach, M.; Morimoto, R.I.; Nunes Amaral, L.A. Large-scale investigation of the reasons why potentially important genes are ignored. PLoS Biol. 2018, 16, e2006643. [Google Scholar] [CrossRef]
- Oprea, T.I.; Bologa, C.G.; Brunak, S.; Campbell, A.; Gan, G.N.; Gaulton, A.; Gomez, S.M.; Guha, R.; Hersey, A.; Holmes, J.; et al. Unexplored therapeutic opportunities in the human genome. Nat. Rev. Drug Discov. 2018, 17, 317–332. [Google Scholar] [CrossRef]
- Rocha, J.J.; Jayaram, S.A.; Stevens, T.J.; Muschalik, N.; Shah, R.D.; Emran, S.; Robles, C.; Freeman, M.; Munro, S. Functional unknomics: Systematic screening of conserved genes of unknown function. PLoS Biol. 2023, 21, e3002222. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Jeang, K.T. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 2007, 7, 270–280. [Google Scholar] [CrossRef]
- Satou, Y.; Matsuoka, M. Molecular and Cellular Mechanism of Leukemogenesis of ATL: Emergent Evidence of a Significant Role for HBZ in HTLV-1-Induced Pathogenesis. Leuk. Res. Treat. 2012, 2012, 213653. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef]
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 1982, 79, 2031–2035. [Google Scholar] [CrossRef]
- Barbeau, B.; Mesnard, J.M. Does the HBZ gene represent a new potential target for the treatment of adult T-cell leukemia? Int. Rev. Immunol. 2007, 26, 283–304. [Google Scholar] [CrossRef]
- Gaudray, G.; Gachon, F.; Basbous, J.; Biard-Piechaczyk, M.; Devaux, C.; Mesnard, J.M. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J. Virol. 2002, 76, 12813–12822. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Yasunaga, J.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011, 7, e1001274. [Google Scholar] [CrossRef] [PubMed]
- Romerio, F. Origin and functional role of antisense transcription in endogenous and exogenous retroviruses. Retrovirology 2023, 20, 6. [Google Scholar] [CrossRef]
- Matsuoka, M.; Yasunaga, J. Human T-cell leukemia virus type 1: Replication, proliferation and propagation by Tax and HTLV-1 bZIP factor. Curr. Opin. Virol. 2013, 3, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, I.; Lewis, M.R.; Polakowski, N.; Hivin, P.; Cavanagh, M.H.; Thebault, S.; Barbeau, B.; Nyborg, J.K.; Mesnard, J.M. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J. Virol. 2007, 81, 1543–1553. [Google Scholar] [CrossRef]
- Zhao, T.; Yasunaga, J.; Satou, Y.; Nakao, M.; Takahashi, M.; Fujii, M.; Matsuoka, M. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-kappaB. Blood 2009, 113, 2755–2764. [Google Scholar] [CrossRef]
- Kannagi, M.; Harada, S.; Maruyama, I.; Inoko, H.; Igarashi, H.; Kuwashima, G.; Sato, S.; Morita, M.; Kidokoro, M.; Sugimoto, M.; et al. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int. Immunol. 1991, 3, 761–767. [Google Scholar] [CrossRef]
- Goon, P.K.; Biancardi, A.; Fast, N.; Igakura, T.; Hanon, E.; Mosley, A.J.; Asquith, B.; Gould, K.G.; Marshall, S.; Taylor, G.P.; et al. Human T cell lymphotropic virus (HTLV) type-1-specific CD8+ T cells: Frequency and immunodominance hierarchy. J. Infect. Dis. 2004, 189, 2294–2298. [Google Scholar] [CrossRef]
- Suemori, K.; Fujiwara, H.; Ochi, T.; Ogawa, T.; Matsuoka, M.; Matsumoto, T.; Mesnard, J.M.; Yasukawa, M. HBZ is an immunogenic protein, but not a target antigen for human T-cell leukemia virus type 1-specific cytotoxic T lymphocytes. J. Gen. Virol. 2009, 90, 1806–1811. [Google Scholar] [CrossRef]
- Gazon, H.; Chauhan, P.; Hamaidia, M.; Hoyos, C.; Li, L.; Safari, R.; Willems, L. How Does HTLV-1 Undergo Oncogene-Dependent Replication Despite a Strong Immune Response? Front. Microbiol. 2017, 8, 2684. [Google Scholar] [CrossRef]
- El Hajj, H.; Bazarbachi, A. Interplay between innate immunity and the viral oncoproteins Tax and HBZ in the pathogenesis and therapeutic response of HTLV-1 associated adult T cell leukemia. Front. Immunol. 2022, 13, 957535. [Google Scholar] [CrossRef]
- Raval, G.U.; Bidoia, C.; Forlani, G.; Tosi, G.; Gessain, A.; Accolla, R.S. Localization, quantification and interaction with host factors of endogenous HTLV-1 HBZ protein in infected cells and ATL. Retrovirology 2015, 12, 59. [Google Scholar] [CrossRef]
- Accolla, R.S. The Road to HTLV-1-Induced Leukemia by Following the Subcellular Localization of HTLV-1-Encoded HBZ Protein. Front. Immunol. 2022, 13, 940131. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, D.; Nakagawa, S.; Hori, M.; Kurokawa, N.; Soejima, A.; Nakano, K.; Yamochi, T.; Nakashima, M.; Kobayashi, S.; Tanaka, Y.; et al. Polycomb-dependent epigenetic landscape in adult T-cell leukemia. Blood 2016, 127, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, H.; Ishitsuka, K. Treatment advances and prognosis for patients with adult T-cell leukemia-lymphoma. J. Clin. Exp. Hematop. 2017, 57, 87–97. [Google Scholar] [CrossRef]
- Letafati, A.; Mehdigholian Chaijani, R.; Edalat, F.; Eslami, N.; Askari, H.; Askari, F.; Shirvani, S.; Talebzadeh, H.; Tarahomi, M.; MirKhani, N.; et al. Advances in epigenetic treatment of adult T-cell leukemia/lymphoma: A comprehensive review. Clin. Epigenetics 2025, 17, 39. [Google Scholar] [CrossRef]
- Liu, M.M.; Furusato, E.; Cao, X.; Shen, D.; Chan, C.C. Ocular manifestations and pathology of adult T-cell leukemia/lymphoma associated with human T-lymphotropic virus type 1. Rare Tumors 2010, 2, e63. [Google Scholar] [CrossRef]
- Tezuka, K.; Xun, R.; Tei, M.; Ueno, T.; Tanaka, M.; Takenouchi, N.; Fujisawa, J. An animal model of adult T-cell leukemia: Humanized mice with HTLV-1-specific immunity. Blood 2014, 123, 346–355. [Google Scholar] [CrossRef]
- Fuji, S.; Muta, M.; Hisakata, T.; Kawano, N.; Ikeda, E.; Kouno, H.; Tanabe, Y.; Nakanishi, K. Comprehensive review of morphological diagnosis of adult T-cell leukemia-lymphoma. Expert. Rev. Hematol. 2025, 18, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, M.; Minato, K.; Tobinai, K.; Nagai, M.; Setoya, T.; Takenaka, T.; Ishihara, K.; Watanabe, S.; Hoshino, H.; Miwa, M.; et al. Atypical adult T-cell leukemia-lymphoma: Diverse clinical manifestations of adult T-cell leukemia-lymphoma. Jpn. J. Clin. Oncol. 1983, 13 (Suppl. S2), 165–187. [Google Scholar] [PubMed]
- Okamoto, T.; Ohno, Y.; Tsugane, S.; Watanabe, S.; Shimoyama, M.; Tajima, K.; Miwa, M.; Shimotohno, K. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn. J. Cancer Res. 1989, 80, 191–195. [Google Scholar] [CrossRef]
- Koya, J.; Saito, Y.; Kameda, T.; Kogure, Y.; Yuasa, M.; Nagasaki, J.; McClure, M.B.; Shingaki, S.; Tabata, M.; Tahira, Y.; et al. Single-Cell Analysis of the Multicellular Ecosystem in Viral Carcinogenesis by HTLV-1. Blood Cancer Discov. 2021, 2, 450–467. [Google Scholar] [CrossRef]
- Hattori, T.; Uchiyama, T.; Toibana, T.; Takatsuki, K.; Uchino, H. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood 1981, 58, 645–647. [Google Scholar] [CrossRef]
- Nikaido, T.; Shimizu, A.; Ishida, N.; Sabe, H.; Teshigawara, K.; Maeda, M.; Uchiyama, T.; Yodoi, J.; Honjo, T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature 1984, 311, 631–635. [Google Scholar] [CrossRef]
- Teshigawara, K.; Maeda, M.; Nishino, K.; Nikaido, T.; Uchiyama, T.; Tsudo, M.; Wano, Y.; Yodoi, J. Adult T leukemia cells produce a lymphokine that augments interleukin 2 receptor expression. J. Mol. Cell Immunol. 1985, 2, 17–26. [Google Scholar] [PubMed]
- Tagaya, Y.; Maeda, Y.; Mitsui, A.; Kondo, N.; Matsui, H.; Hamuro, J.; Brown, N.; Arai, K.; Yokota, T.; Wakasugi, H.; et al. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 1989, 8, 757–764, Erratum in EMBO J. 1994, 13, 2244. [Google Scholar] [CrossRef]
- Wakasugi, N.; Tagaya, Y.; Wakasugi, H.; Mitsui, A.; Maeda, M.; Yodoi, J.; Tursz, T. Adult T-cell leukemia-derived factor/thioredoxin, produced by both human T-lymphotropic virus type I- and Epstein-Barr virus-transformed lymphocytes, acts as an autocrine growth factor and synergizes with interleukin 1 and interleukin 2. Proc. Natl. Acad. Sci. USA 1990, 87, 8282–8286. [Google Scholar] [CrossRef]
- Yoshie, O.; Fujisawa, R.; Nakayama, T.; Harasawa, H.; Tago, H.; Izawa, D.; Hieshima, K.; Tatsumi, Y.; Matsushima, K.; Hasegawa, H.; et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood 2002, 99, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Yodoi, J.; Sagawa, K.; Takatsuki, K.; Uchino, H. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood 1977, 50, 481–492. [Google Scholar] [CrossRef]
- Watanabe, T.; Seiki, M.; Yoshida, M. Retrovirus terminology. Science 1983, 222, 1178. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Seiki, M.; Yoshida, M. HTLV type I (U. S. isolate) and ATLV (Japanese isolate) are the same species of human retrovirus. Virology 1984, 133, 238–241. [Google Scholar] [CrossRef]
- Gallo, R.C.; Montagnier, L. The discovery of HIV as the cause of AIDS. N. Engl. J. Med. 2003, 349, 2283–2285. [Google Scholar] [CrossRef]
- Hinuma, Y. Retrovirus etiology of adult T-cell leukemia. Leuk. Res. 1993, 17, 379–381. [Google Scholar] [CrossRef]
- de The, G. The history of HTLV-I. Leuk. Res. 1993, 17, 383–384. [Google Scholar] [CrossRef]
- Blattner, W.A. HTLV-I and adult T-cell leukemia. Leuk. Res. 1993, 17, 385–386. [Google Scholar] [CrossRef]
- Aoki, T. Discovery of HTLV-1. Leuk. Res. 1993, 17, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Hatano, Y.; Ideta, T.; Hirata, A.; Hatano, K.; Tomita, H.; Okada, H.; Shimizu, M.; Tanaka, T.; Hara, A. Virus-Driven Carcinogenesis. Cancers 2021, 13, 2625. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.J.; Depper, J.M.; Crabtree, G.R.; Rudikoff, S.; Pumphrey, J.; Robb, R.J.; Kronke, M.; Svetlik, P.B.; Peffer, N.J.; Waldmann, T.A.; et al. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature 1984, 311, 626–631. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef]
- Wood, K.J.; Sakaguchi, S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 2003, 3, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Bien, E.; Balcerska, A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: A review. Biomarkers 2008, 13, 1–26. [Google Scholar] [CrossRef]
- Yasuda, N.; Lai, P.K.; Ip, S.H.; Kung, P.C.; Hinuma, Y.; Matsuoka, M.; Hattori, T.; Takatsuki, K.; Purtilo, D.T. Soluble interleukin 2 receptors in sera of Japanese patients with adult T cell leukemia mark activity of disease. Blood 1988, 71, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Lokau, J.; Petasch, L.M.; Garbers, C. The soluble IL-2 receptor alpha/CD25 as a modulator of IL-2 function. Immunology 2024, 171, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Brandl, N.; Seitz, R.; Sendtner, N.; Muller, M.; Gulow, K. Living on the Edge: ROS Homeostasis in Cancer Cells and Its Potential as a Therapeutic Target. Antioxidants 2025, 14, 1002. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Silic-Benussi, M.; Cavallari, I.; Vajente, N.; Vidali, S.; Chieco-Bianchi, L.; Di Lisa, F.; Saggioro, D.; D’Agostino, D.M.; Ciminale, V. Redox regulation of T-cell turnover by the p13 protein of human T-cell leukemia virus type 1: Distinct effects in primary versus transformed cells. Blood 2010, 116, 54–62. [Google Scholar] [CrossRef]
- Takahashi, M.; Higuchi, M.; Makokha, G.N.; Matsuki, H.; Yoshita, M.; Tanaka, Y.; Fujii, M. HTLV-1 Tax oncoprotein stimulates ROS production and apoptosis in T cells by interacting with USP10. Blood 2013, 122, 715–725. [Google Scholar] [CrossRef]
- Romeo, M.; Hutchison, T.; Malu, A.; White, A.; Kim, J.; Gardner, R.; Smith, K.; Nelson, K.; Bergeson, R.; McKee, R.; et al. The human T-cell leukemia virus type-1 p30(II) protein activates p53 and induces the TIGAR and suppresses oncogene-induced oxidative stress during viral carcinogenesis. Virology 2018, 518, 103–115. [Google Scholar] [CrossRef]
- Rushing, A.W.; Rushing, B.; Hoang, K.; Sanders, S.V.; Peloponese, J.M., Jr.; Polakowski, N.; Lemasson, I. HTLV-1 basic leucine zipper factor protects cells from oxidative stress by upregulating expression of Heme Oxygenase I. PLoS Pathog. 2019, 15, e1007922. [Google Scholar] [CrossRef]
- Wollman, E.E.; d’Auriol, L.; Rimsky, L.; Shaw, A.; Jacquot, J.P.; Wingfield, P.; Graber, P.; Dessarps, F.; Robin, P.; Galibert, F.; et al. Cloning and expression of a cDNA for human thioredoxin. J. Biol. Chem. 1988, 263, 15506–15512. [Google Scholar] [CrossRef]
- Powis, G.; Mustacich, D.; Coon, A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic. Biol. Med. 2000, 29, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Oberacker, T.; Kraft, L.; Schanz, M.; Latus, J.; Schricker, S. The Importance of Thioredoxin-1 in Health and Disease. Antioxidants 2023, 12, 1078. [Google Scholar] [CrossRef]
- Ishii, T.; Ishida, T.; Utsunomiya, A.; Inagaki, A.; Yano, H.; Komatsu, H.; Iida, S.; Imada, K.; Uchiyama, T.; Akinaga, S.; et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin. Cancer Res. 2010, 16, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef]
- Baggiolini, M.; Moser, B. Blocking chemokine receptors. J. Exp. Med. 1997, 186, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Sherry, B.; Cerami, A. Small cytokine superfamily. Curr. Opin. Immunol. 1991, 3, 56–60. [Google Scholar] [CrossRef]
- Imai, T.; Yoshida, T.; Baba, M.; Nishimura, M.; Kakizaki, M.; Yoshie, O. Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J. Biol. Chem. 1996, 271, 21514–21521. [Google Scholar] [CrossRef]
- Imai, T.; Baba, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Yoshie, O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J. Biol. Chem. 1997, 272, 15036–15042. [Google Scholar] [CrossRef]
- Matsushima, K. Chemokines. Introduction. Springer Semin. Immunopathol. 2000, 22, 321–328. [Google Scholar] [CrossRef]
- Imai, T.; Nagira, M.; Takagi, S.; Kakizaki, M.; Nishimura, M.; Wang, J.; Gray, P.W.; Matsushima, K.; Yoshie, O. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int. Immunol. 1999, 11, 81–88. [Google Scholar] [CrossRef]
- Niwa, R.; Shoji-Hosaka, E.; Sakurada, M.; Shinkawa, T.; Uchida, K.; Nakamura, K.; Matsushima, K.; Ueda, R.; Hanai, N.; Shitara, K. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004, 64, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Ishida, T.; Hatake, K.; Taniwaki, M.; Ando, K.; Tobinai, K.; Fujimoto, K.; Yamamoto, K.; Miyamoto, T.; Uike, N.; et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol. 2014, 32, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Yoshimitsu, M. Targeted antibody therapy as a treatment strategy for aggressive adult T-cell leukemia/lymphoma. Leuk. Res. 2025, 149, 107653. [Google Scholar] [CrossRef]
- Yoshimitsu, M.; Choi, I.; Kusumoto, S.; Shimokawa, M.; Utsunomiya, A.; Suehiro, Y.; Hidaka, T.; Nosaka, K.; Sasaki, H.; Rai, S.; et al. A phase 2 trial of CHOP with anti-CCR4 antibody mogamulizumab for older patients with adult T-cell leukemia/lymphoma. Blood 2025, 146, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Nishikata, I.; Shiraga, T.; Akamatsu, E.; Fukami, T.; Hidaka, T.; Kubuki, Y.; Okayama, A.; Hamada, K.; Okabe, H.; et al. Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute-type adult T-cell leukemia. Blood 2005, 105, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Maes, E.; Mertens, I.; Valkenborg, D.; Pauwels, P.; Rolfo, C.; Baggerman, G. Proteomics in cancer research: Are we ready for clinical practice? Crit. Rev. Oncol. Hematol. 2015, 96, 437–448. [Google Scholar] [CrossRef]
- Sudo, H.; Tonoyama, Y.; Ikebe, E.; Hasegawa, H.; Iha, H.; Ishida, Y.I. Proteomic analysis of adult T-cell leukemia/lymphoma: A biomarker identification strategy based on preparation and in-solution digestion methods of total proteins. Leuk. Res. 2024, 138, 107454. [Google Scholar] [CrossRef]
- Yin, J.; Fu, W.; Dai, L.; Jiang, Z.; Liao, H.; Chen, W.; Pan, L.; Zhao, J. ANKRD22 promotes progression of non-small cell lung cancer through transcriptional up-regulation of E2F1. Sci. Rep. 2017, 7, 4430. [Google Scholar] [CrossRef]
- Utsumi, T.; Hosokawa, T.; Shichita, M.; Nishiue, M.; Iwamoto, N.; Harada, H.; Kiwado, A.; Yano, M.; Otsuka, M.; Moriya, K. ANKRD22 is an N-myristoylated hairpin-like monotopic membrane protein specifically localized to lipid droplets. Sci. Rep. 2021, 11, 19233. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Uchihashi, K.; Kazuto, T.; Osaka, A.; Yanagihara, K.; Tsukasaki, K.; Hasegawa, H.; Yamada, Y.; Kamihira, S. Foxp3 expression on normal and leukemic CD4+CD25+ T cells implicated in human T-cell leukemia virus type-1 is inconsistent with Treg cells. Eur. J. Haematol. 2008, 81, 209–217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).