Abstract
Disease eponyms can be confusing, difficult to remember, scientifically non-robust, and lacking in implications on and relationships with cell lineage, histogenesis, and pathogenesis. This review is geared toward revisiting hematolymphoid diseases with eponyms in light of recent advances in technology and science by searching the past fifty years of the literature using Scopus and Google Scholar with the keywords “eponyms, hematolymphoid, diseases, lymphoma, benign, malignant, lymph node, spleen, liver, bone marrow, leukemia”. With advances in science and technology, there is accumulation of information on the morphologic nuances and immunologic, immunophenotypic, and genetic features of various hematolymphoid eponymic diseases, thus shedding light on important issues of etiology and pathogenesis with implications on therapy in various non-neoplastic (Castleman, Evans syndrome Kikuchi–Fujimoto, IgG4-related diseases) and neoplastic (Hodgkin, Burkitt, NK/T-cell lymphomas, dendritic/histiocytic neoplasms, and Sezary syndrome) diseases. This contributes to modern nomenclature, classification, subtyping, prognostication, and discoveries on new treatment strategies of hematolymphoid eponymic diseases.
1. Introduction
In the old days, due to the lack of clinical investigative methods and technology, many diseases were named after people, usually physicians who first identified the diseases. Less commonly, they were named after patients who first had the diseases, a fictional character depicted with the diseases, or places where the diseases were prevalent.
A meeting on classification and nomenclature of morphologic defects was held at the National Institute of Health, USA, with the proposal published in the Lancet in 1974 that eponyms be used only in the absence of a reasonable descriptive designation, be limited to one proper name, and until the disease basis is recognized [1]. Though eponyms are memorable of the credited persons or places, they are scientifically non-robust with no relevance or implications to cell lineage, etiology, histogenesis, pathogenesis, immunophenotypic, or genetic features. This compromises further investigations and the discovery of important prognostic and therapeutic attributes of the diseases. Furthermore, the same eponym may be used for different diseases (e.g., Murphy’s sign for acute cholecystitis and acute appendicitis), thus potentially causing confusion.
Eponyms have been used for many diseases in a plethora of organ systems. This review aims to comprehensively look into eponyms used in hematolymphoid neoplastic and non-neoplastic diseases. A literature search with Google Scholar and Scopus for the past 50 years using the keywords “eponyms, hematolymphoid, diseases, lymphoma, benign, malignant, lymph node, spleen, liver, bone marrow, leukemia” was performed. The various relevant eponymic hematolymphoid diseases were then reviewed in light of impactful advances in science and technology.
2. Hematolymphoid Non-Neoplastic Eponymic Diseases
Storage diseases represent a major disease group designated with eponyms. They are inborn errors of metabolism with specific enzyme deficiencies leading to the abnormal accumulation of substances ranging from glycogen to lipids. Patients often present with hepatosplenomegaly or blood cytopenias, thus masquerading as hematolymphoid diseases. Differential diagnosis from other hematolymphoid diseases is often not difficult due to early presentation in life, hereditary basis, clinical picture, characteristic histology, and electron microscopy features, with confirmation by enzyme studies. Due to the distinctive clinical and investigative features, they are not included in this review.
2.1. Castleman Disease (CD)
CD was first described by Benjamin Castleman in 1956 as localized enlarged mediastinal lymph nodes which he and colleagues in 1972 described pathologically as hyaline vascular (HV) type with plasma cell (PC) type [2,3]. As more CD cases were reported, it became increasingly recognized that HV and PC types often occur together as a “mixed” type, albeit the HV pattern is more common in unicentric (UC) and the PC pattern more common in multicentric (MC) CD [4,5].
2.1.1. Epidemiology and Pathogenesis
This is poorly understood, mostly because CD is heterogeneous, encompassing several lymphoproliferative disorders with distinct clinicopathologic entities [6,7]. CD is classified under “tumour-like lesions with B-cell predominance” in the 5th edition of the WHO Classification of Haematolymphoid Tumours [7]. In UCCD, a clonal neoplastic proliferation of stromal and possibly follicular dendritic cells with some harboring platelet-derived growth factor receptor beta mutations was demonstrated [5,7,8,9,10,11]. IL-6 is a critical driver in many idiopathic MCCD (iMCCD), as supported by the clinical effectiveness of using IL-6 inhibitors [4,5,7,12,13,14]. However, IL-6 is not universally elevated in iMCCD, and increased T-cell activation, elevated serum vascular endothelial growth factor (VEGF), immunoglobulin (Ig) gene rearrangement, chromatin remodeling gene mutations, PIK3/AKT/mTOR, MAK, JAK-STAT, and IL signaling pathway alterations may also be at play [5,7]. There is a key role of B-cells in the pathogenesis of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV8)+ MCCD [6,7,15], as demonstrated by the efficacy of rituximab treatment. IL-6 (including viral IL-6 and secreted human IL-6) is also important in driving KSHV/HHV8 MCC. Other KSHV/HHV8 gene products (vFLIP, vIRF3/LANA2, viral microRNA) are important in affecting infected cell survival and host/cytokine responses [7]. The role of autoimmunity, which has also been considered as autoantibodies, are present in one-third of iMCCD [5,16]. Monoclonal plasma cells also play a role in polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, skin changes (POEMS) syndrome-associated MCCD [5,6].
2.1.2. Classification of CD
Due to heterogeneity and lack of a unifying concept with regard to etiology or pathogenesis, the classification of CD is based on its centricity and known etiologic or syndromic associations [5,6,7].
- Unicentric CD (UCCD), when the disease is localized to one lymph node or a single lymph node station. This accounts for 50–70% of CD.
- Multicentric CD (MCCD), when the disease affects multiple lymph nodes or lymph node stations, including the following:
- (a)
- POEMS (polyneuropathy, organomegaly, endocrinopathy, M-protein and skin changes)-associated CD.
- (b)
- iMCCD.
- (i)
- iMCCD-TAFRO (thrombocytopenia, ascites, reticulin fibrosis, renal dysfunction, organomegaly). This is a severe form of MCCD.
- (ii)
- iMCCD, not otherwise specified (NOS).
- (c)
- KSHV/HHV8-associated MCCD.
- (i)
- Human immunodeficiency virus (HIV) negative.
- (ii)
- HIV positive.
2.1.3. Pathology
Excised lymph nodes are required for histopathologic diagnosis as small biopsies may not contain full diagnostic features. There are three histopathologic patterns [4,5,6,7].
- (a)
- Hyaline vascular (HV) pattern
This is most common in UCCD featuring atretic follicles with regressed germinal centers depleted of lymphoid cells, with follicular dendritic cell retention, frequent radial traversion by hyalinized sclerotic blood vessels to impart a “lollipop” appearance, and concentric rings of mantle or marginal lymphocytes to portray the characteristic “onion-skin” picture. Twining of two or more atretic follicles by rings of mantle lymphocytes is often present. The interfollicular region shows marked vascular proliferation with plump endothelial cells and inconspicuous or absent lymph node sinuses (Figure 1A–D). This pattern may also be present in the plasma cell type CD, where the interfollicular lymph node sinuses are patent. The follicular dendritic cells of the germinal centers and interfollicular region may show dysplastic changes.
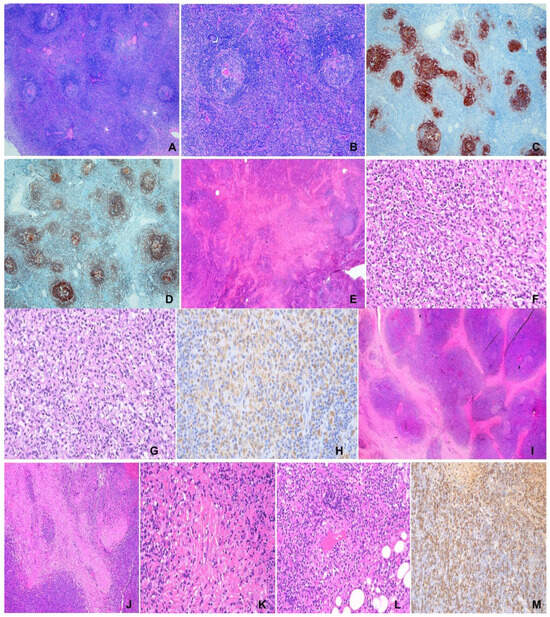
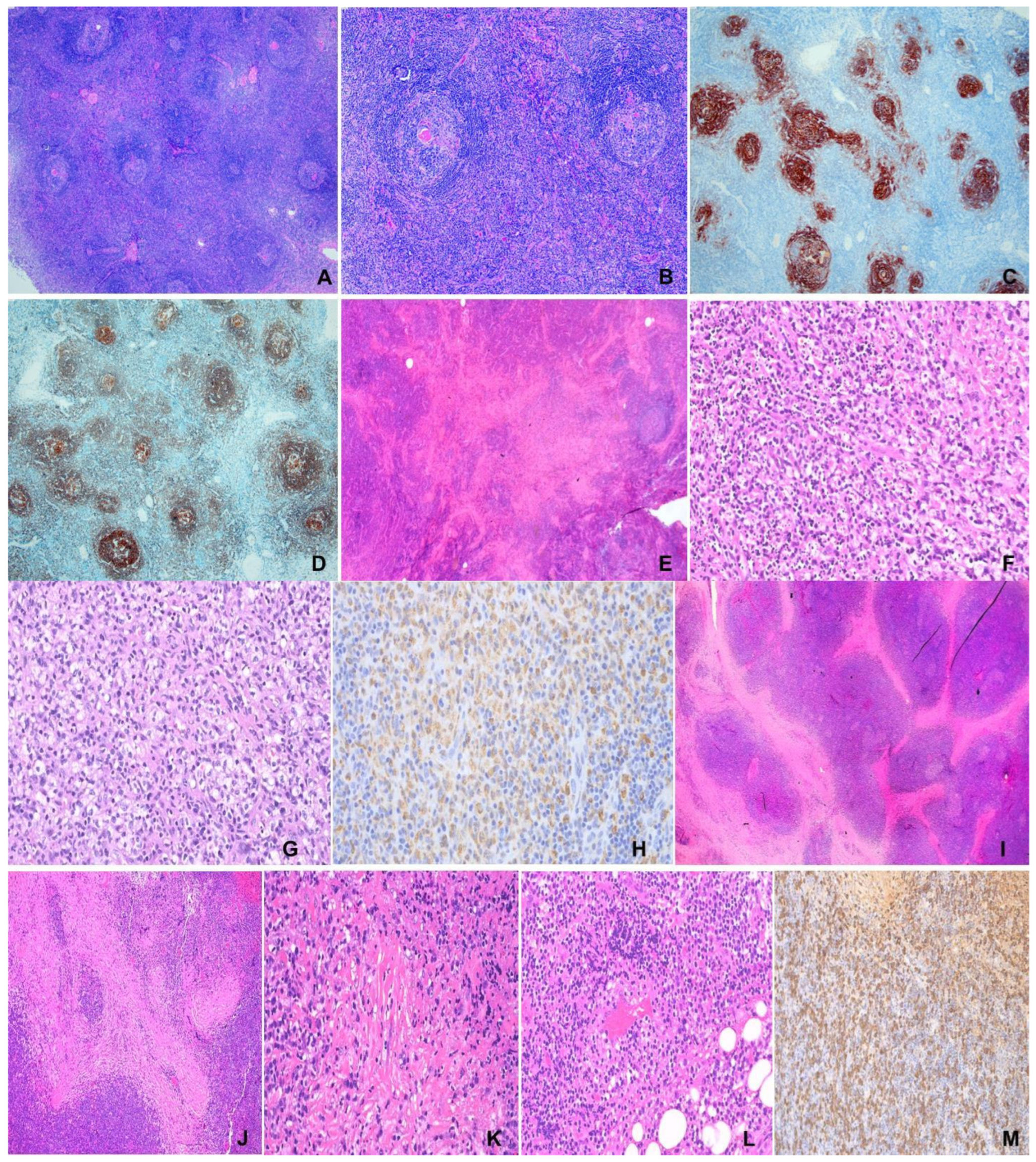
Figure 1.
(A–D) Unicentric Castleman disease. F/47, 10 cm mediastinal mass. (A) Hyperplastic follicles with prominent germinal centers and interfollicular lymphoplasmacytic cells. H&E ×100. (B) Hyperplastic follicles with radial vessels (lollipop appearance) and concentric rings of mantle lymphocytes (onion skinning). H&E ×400. (C,D) “Twining” of follicles. (C) CD21, (D) CD20 ×100. (E–H) Kikuchi-Fujimoto disease. M/13, left cervical lymph node. (E) Large pale “necrotic” area involving paracortex and cortex. H&E ×100. (F) Nuclear debris in “necrotic” area. H&E ×200. (G) Characteristic histiocytes in “necrotic” area. H&E ×400. (H) CD68 ×100. (I–M) IgG4-related disease. M/85, left orbital mass. (I) Extensive fibrosis in lymphoplasmacytic infiltrate. H&E ×100. (J) Storiform fibrosis. H&E ×200. (K) Obliterative phlebitis. H&E ×400. (L) Obliterated vein. H&E ×200. (M) IgG4-positive plasma cells ×200.
- (b)
- Plasma cell (PC) pattern
This is characterized by hyperplastic lymphoid follicles and sheets of PC in the interfollicular region. In KSHV/HHV8-associated MCCD, some follicles and mantle zones may contain clusters of large immunoblasts or plasmablasts, often expressing monotypic IgM-λ, though polyclonality is demonstrated on molecular analysis.
- (c)
- Mixed pattern
This shows both HV and PC patterns in varying proportions and may occur in UCCD and MCCD.
- (d)
- Histologic variants
This occurs in the HV pattern and mostly in UCCD. The interfollicular region is expanded with stromal, angiomyoid, or follicular dendritic cell proliferation. The latter may progress to follicular dendritic cell sarcoma [17,18,19,20]. The occurrence of Kikuchi lymphadenitis-like change in UCCD has also been reported [21].
2.1.4. Diagnosis and Differential Diagnosis
Diagnosis of CD requires correlation of histology, clinical findings, and exclusion of a myriad of reactive and neoplastic mimickers [5,6,21].
UCCD usually present as single site lymph node enlargement with or without local compression symptoms and no systemic symptoms. Histology is often adequate for diagnosis.
MCCD is characterized by multicentric lymphadenopathy, systemic clinical features, and laboratory abnormalities in 80% of cases (cytopenias and liver and kidney dysfunction). In addition, the following types of MCCD are diagnosed using specific tests and clinical/syndromic features.
- (i)
- KSHV/HHV8-associated MCCD—Positive HHV8 testing.
- (ii)
- iMCCD-TAFRO—Associated TAFRO signs and symptoms (thrombocytopenia, anarsaca, fever, renal insufficiency, organomegaly) and negative for KSHV/HHV8 and HIV.
- (iii)
- POEMS-MCCD—Associated POEM syndrome and monoclonal gammopathy, and negative for KSHV/HHV8 and HIV.
The differential diagnoses include autoimmune diseases, IgG4-related disease (Ig4RD), EBV infection, lymphoma, follicular dendritic cell sarcoma, and plasma cell neoplasm [5,6,7].
2.1.5. Treatment and Outcome
UCCD—Complete surgical excision usually yields excellent outcome. Radiotherapy, immunochemotherapy, or embolization may be used for unresectable disease or compressing symptoms. Anti-IL6, immunotherapy may be required in patients with inflammatory symptoms.
MCCD—Appropriate treatment is varied due to unclear etiology.
- -
- iMCCD: Anti-IL-6-directed therapy, with about one third of patients responding. Steroids, chemotherapy, rituximab, immunomodulators, intravenous immunoglobulins, and thalidomide applied singly or in combination may be required in anti-IL6 non-responders and very ill patients.
- -
- POEM-MCCD: When no bone lesions, iMCCD-like therapy.
- -
- When bone lesions present, myeloma type therapy.
- -
- HHV8 + MCCD: Anti-retrovirus therapy, rituximab.
The outcome is, in general, optimal for UCCD, but variable in MCCD and a proportion of UCCD [5,7,12,13,14].
2.1.6. Secondary Malignancies
There is a higher risk of developing secondary malignancies including follicular dendritic cell sarcoma, lymphoma (KSHV/HHV8-positive diffuse large B-cell lymphoma, primary effusion lymphoma), and Kaposi sarcoma [5,7,9,17,18,19,20].
2.2. Evans Syndrome (ES)
First described by Evans I 1951, acquired hemolytic anemia and primary thrombocytopenia were postulated to be autoimmune cytopenias [22]. ES is nowadays extended to include autoimmune neutropenia [23] and is diagnosed when immune cytopenias affect any two hematologic lineages [24]. Advances in molecular biology such as next generation and whole exome sequencing contributed to the identification of genetic defects associated with inborn errors of immunity in 40% of ES. Monogenic defects in genes involved in the immune regulatory pathway TNFRSF6, CTLA4, STAT3, PIK3CD, CBL, ADAR1, LRBA, RAG1, and KRAS have been discovered [24,25]. In the remaining ES, however, no genetic defects have been identified. Despite being regarded as an autoimmune cytopenic disease on the discovery of defects in immune regulatory genes, the underlying immunobiology of ES is not well known. In a cohort of 24 ES pediatric patients, Kumar et al. discovered broad immune anomalies including increased circulating T follicular helper (cTfh) cells, increased T-cell activation, decreased naïve CD4+ T-cells, and decreased class switched memory B-cells in ES, compared to patients with chronic immune thrombocytopenia and healthy controls [24]. These immune anomalies were also detected in ES patients without detectable genetic defects and irrespective of immunoglobulin levels. This may imply that patients with ES or immune cytopenias are at risk of developing broader immune complications. Recognition of these immune anomalies, expanded cTfh cells, immune activation, and exhaustion in ES also opens a horizon for novel therapeutic measures such as mTOR inhibitors, phospatidylinositol 3 kinase inhibitors, and JAK-STAT pathway inhibitors [23].
2.3. Kikuchi/Kikuchi–Fujimoto Disease (KFD)
KFD was described in 1972 independently by Kikuchi [26] and Fujimoto [27]. The greatest dimension of the involved lymph node could be large and up to 7cm [28]. Rarely, extranodal involvement of skin, liver, spleen, central nervous system, kidney, and heart has been reported [28]. KFD affects mostly Asians with female predominance, though non-Asians are also affected [29,30,31]. Patients present with fever, leukopenia, with self-limiting disease duration of up to 6 months. Recurrence may occur up to 2 years after the initial presentation [28,32]. The involved lymph nodes characteristically show cortical and paracortical variably sized discrete or confluent pale nodular areas constituted by many histiocytes, which are phagocytic or non-phagocytic with special morphologic types including crescentic histiocytes, signet ring histiocytes, and plasmacytoid dendritic cells (pDC). There are admixed variable quantities of immunoblasts, karyorrhectic and eosinophilic debris, and sometimes coagulative necrosis and focal fibrovascular organization. Neutrophils are conspicuously absent or very rare (Figure 1E–H). Kuo [28] recognized three histologic patterns, proliferative, necrotizing, and xanthomatous, which are not patterns of temporal progression but histologic variations related to individual host immune response. Immunohistochemically, the histiocytic cells are CD163+, CD68+, CD4+, and myeloperoxidase+. The T-cells are mostly CD8+, and with rarely CD4+ T-cells. The pDC are positive for CD123, CD4, and CD68. CD20+ B-cells are practically absent. DNA ploidy shows diploidy, supporting a reactive nature [28]. KFD is classified under “tumour-like lesion with T-cell predominance” in the fifth edition of the WHO Classification of Haematolymphoid Tumours, which is in line with the important role of exuberant T-cell-mediated response in the disease [7].
Fine needle aspiration (FNA) cytology yields characteristic histiocytes, a lack of neutrophils, karyorrhectic debris, and necrosis, corresponding to the histologic features. In the proper clinical context, FNA is diagnostic [33]. With regard to pathogenesis, recent studies demonstrated the involvement of cytokine and chemokine pathways of interferon (IF) γ, IL-18, MIG(CXCL9), IFγ-induced protein 10, and IF-1 [34]. The most important differential diagnosis is from lymphoma and systemic lupus erythematosus [28,32,35]. Etiology is unknown and autoimmune or infection-mediated mechanisms have not been substantiated [34]. Due to the frequent presence of two histologic features, histiocytes and necrosis, the term histiocytic necrotizing lymphadenitis has been recommended. However, in the fifth edition of the WHO Classification of Haematolymphoid Tumours, Kikuch–Fujimoto disease is classified under ”tumour-like lesions with T-cell predominance” and involves an exuberant T-cell response with CD-8+ cytotoxic cell-induced apoptosis in genetically predisposed subjects [7]. The disease is self-limiting, usually not requiring treatment.
2.4. Kuttner’s Disease—KT, Mikulicz’s Disease—MD, Ormond’s Disease—OD, Riedel’s Thyroiditis—RT (IgG4-Related Diseases)
KT, MD, OD, and RT are chronic fibroinflammatory diseases affecting salivary glands, lacrimal glands, retroperitoneum, and thyroid gland. MD was initially described by Mikulicz in 1888 being characterized by symmetrical bilateral lacrimal, parotid, and submandibular gland enlargement. However, MD was later described by Napp to be related to leukemia, lymphoma, sarcoidosis, and tuberculosis by Napp [36]. These entities are now recognized as IgG4-related diseases (IgG4RD) with local manifestations [37]. IgG4RD was initially recognized in 2001 as sclerosing pancreatitis associated with raised serum IgG4 or tissue IgG4-positive plasma cells [38,39]. With increased recognition, there is a proliferation of reports on IgG4RD [40], culminating in the consensus statement on the pathology of IgG4RD in 2012 [41]. In the latter, there are three possible characteristic histological features of IgG4RD: (1) dense lymphoplasmacytic infiltrate, (2) fibrosis, usually storiform in character, and (3) obliterative phlebitis. The IgG4+ PC count ranges from 10 to 200 cells/HPF, depending on the involved organ. An elevated IgG4+/IgG+ PC ratio of >40% (>50% for aorta specimens) is also necessary. Accordingly, there are three diagnostic categories: (1) highly suggestive of IgG4RD, with 2 of the 3 characteristic histologic features and fulfilled IgG4+ PC count; (2) probable IgG4RD with only one characteristic histologic feature and the required IgG4+ PC count; and (3) insufficient histopathological evidence of IgG4RD, when criteria of neither categories (1) or (2) are met (Figure 1I–M). A Japanese group further proposed the inclusion of clinical and serological criteria for IgG4RD diagnosis. The group also required the exclusion of cancers and inflammatory conditions known to be associated with increased tissue IgG4+ B-cells/PC or elevated serum IgG4 [42].
IgG4RD involves multiple organs and tissues, including superficial or deep, with synchronous or metachronous involvement of three organs on average. The affected deep sites include pancreas, hepatobiliary system, liver, retroperitoneum, mesentery, mediastinum, aorta, lung, pleura, kidney and urinary tract, central nervous system, thyroid, prostate, seminal vesicles, maxillary sinus, nasal septum, paranasal sinus, and pericardium. Lymph nodes are involved in 30–60% of cases manifesting five histologic patterns, with the pseudotumor-like pattern more specific for IgG4RD [40]. The involved superficial sites include orbit, lacrimal gland, salivary gland, skin, and breast [40,43]. In the pancreas, pancreatectomy and Whipple’s operation is not infrequently performed for tumor-like lesions caused by IgG4RD [44,45]. However, true malignancies including lymphoma, pancreatic ductal adenocarcinoma, salivary duct carcinoma, pulmonary adenocarcinoma, and gastrointestinal clear cell sarcoma have been described in a backdrop of IgG4RD, thus requiring their vigorous exclusion [40]. The patients are in good general condition with symptoms attributable to local mass lesions. Serum IgG4 level correlates with disease activity [41]. Treatment does not require radical surgery and steroids are usually effective [40,43].
Pathogenesis, Etiology, and Proposed Classification
The etiology of IgG4Rd is uncertain. There is evidence that it may be related to autoimmunity [46,47,48,49,50] or allergy [48,50]. Pathogenesis is immunologic involving the interaction of Tfh2, Th2, Tfh1, B-cells, macrophages, and fibroblasts, resulting in polarized Tfh cells and polarized IgG4 heavy chain isotype switching of B-cells [48,50]. This results in an abundance of tissue IgG4+ B-cells/PC and fibrosis. The cytokines involved include IL-4 and IL-10, which favor and tilt towards IgG4 isotype switching [48,50].
Based on the distribution of the involved organs, it was proposed by a Japanese group to delineate IgG4RD into four clinical phenotypic groups. Group 1 affects the pancreatico-biliary system and involves older subjects. Group 2 causes retroperitoneal and periaortic fibrosis in older males with higher incidence of associated malignancy. Group 3 involves the head and neck region. Group 4 features systemic Mikullicz disease. Groups 3 and 4 are more prevalent in middle-aged women with higher serum IgG4 levels. Group 2 is also referred to as the fibrotic phenotype, while Groups 1, 3, and 4 the proliferative phenotype. Increased understanding in the immunologic pathogenesis of IgG4RD also facilitates demonstration of immunologic differences among these four clinical phenotypic groups. CX3CR1+ cytotoxic T-cells are more prominent in Group 2, which contribute to fibrosis of the affected tissues [50].
IgG4RD is observed in conditions of allergy and a variety of malignancies and may also be classified into the allergy phenotype and the malignancy phenotype. In the allergy phenotype, IgG4 is considered to be a beneficial response in alleviating inflammation and inducing tolerance to allergies. In the malignancy phenotype, IgG4 is postulated to be induced by IL-4 and IL-10 produced by malignant tumor cells. The induced IgG4 competes with anti-tumor antibodies to bind tumor antigens, thus “protecting” the tumor cells from the latter antibodies. Increased IgG4 in the malignancy phenotype may be a mechanism of tumor immunologic evasion [50].
3. Hematolymphoid Neoplastic Eponymic Diseases
3.1. Histiocytic/Dendritic Cell Neoplasm
The eponymic diseases encompassed under this category include Hashimoto–Pritzker disease (HPD), Hand–Schuller–Christian disease (HSCD), Letterer–Sieve disease (LSD), Langerhans cell histiocytosis (LCH), Langerhans cell sarcoma (LCS), Erdheim–Chester disease (ECD), and Rosai–Dorfman disease (RDD).
3.1.1. Langerhans Cell Histiocytosis (LCH—Including HPD, HSCD and LSD)
Langerhans cells (LC) were named after Paul Langerhans, who discovered the LC as part of the peripheral nervous system in the epidermis [51]. The LC was however later confirmed to be a hematopoietic cell [52]. There are two forms of LCH. The primarily cutaneous HPD (also known as congenital self-healing Langerhans cell histiocytosis—CSHLCH) is mostly self-healing and causes vesicular papulonodular skin lesions in neonates, infants, and very young children [53,54]. CSHLCH (HPD) does not harbor BRAF mutations, in contrast to systemic LCH [53,54]. Epidermal LCs develop from yolk sac-derived primitive myeloid progenitors [55]. Mutated epidermal LCs are unable to replicate [54], which may be related to the self-healing behavior of CSHLCH. Systemic LCH is mostly a clonal neoplastic disease of bone marrow-derived immature myeloid dendritic cells and not from epidermal LCs [7,56,57]. There are recurrent BRAF V600E and MAP2K1 gene mutations [57,58,59] that activate the mitogen-activated protein kinase (MAPK) pathway causing phosphorylated MEK and ERK proteins [7,48,49,50,51,52,53,54,55]. Mutations in BRAF V600E and MAP2K1 are mutually exclusive [60,61]. Additional hematologic malignancy co-existing with LCH has been reported [57]. In the fifth edition of the WHO Classification of Haematolymphoid Tumours, LCH is classified as a histiocytic/dendritic cell neoplasm and a close relationship of LCH with bone marrow-derived myeloid dendritic cells is described [7].
LCH is classified as a single system (SS) or multisystem (MS) disease with either unifocal (UF) or multifocal (MF) involvement in SS. HSCD is MFSS-LCH and LSD is MS-LCH. SS-LCH commonly affects skin, pituitary, or bone while in MS-LCH, bone, skin, liver, spleen, lymph node, lungs, and bone marrow are involved. The involvement of bone marrow, spleen, or liver is high risk LCH. SS-LCH affects older children and adults while MS-LCH affects neonates and infants with systemic symptoms, cytopenias, disturbed liver functions hepatosplenomegaly, endocrine dysfunction, diabetes insipidus, and central nervous system involvement, with grave prognosis [7,61]. LCH is a rare disease with an annual incidence of 5–9 new cases per million in the pediatric population and 1–2 new cases per million in the adult population. There is a slight male predominance affecting, more commonly, Caucasians [7,61].
Pathology
There is characteristic accumulation of large oval to round histiocytic cells with irregular nuclei, often with nuclear grooves, abundant pale cytoplasm, and minimal nuclear atypia in a backdrop of giant cells, eosinophils, lymphocytes, and multinucleated histiocytes. LCH cells express CD1a, S100, CD207, and CD68 (Figure 2A–E). CD207 is a surrogate for ultrastructural Birbeck granules, which are tennis-racket or zipper-like structures. Staining for downstream markers of MAPK activation, cyclin D1 and p-ERK, is positive [7,61].
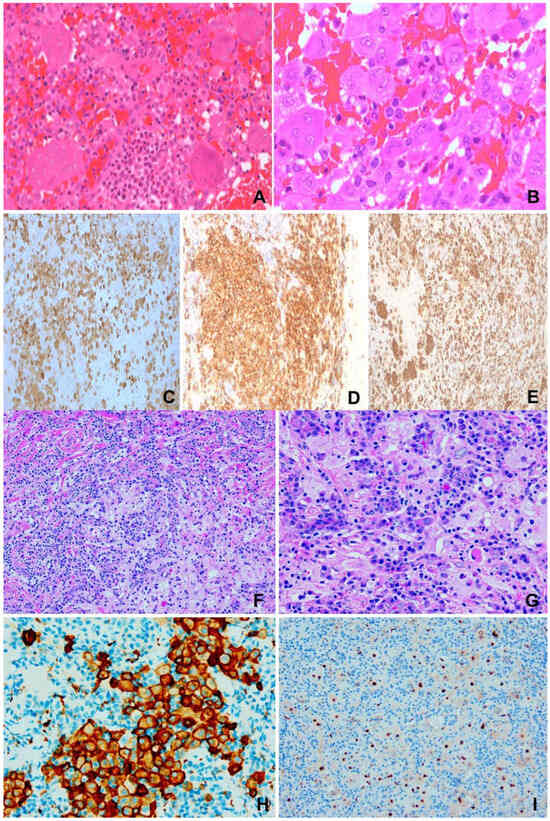
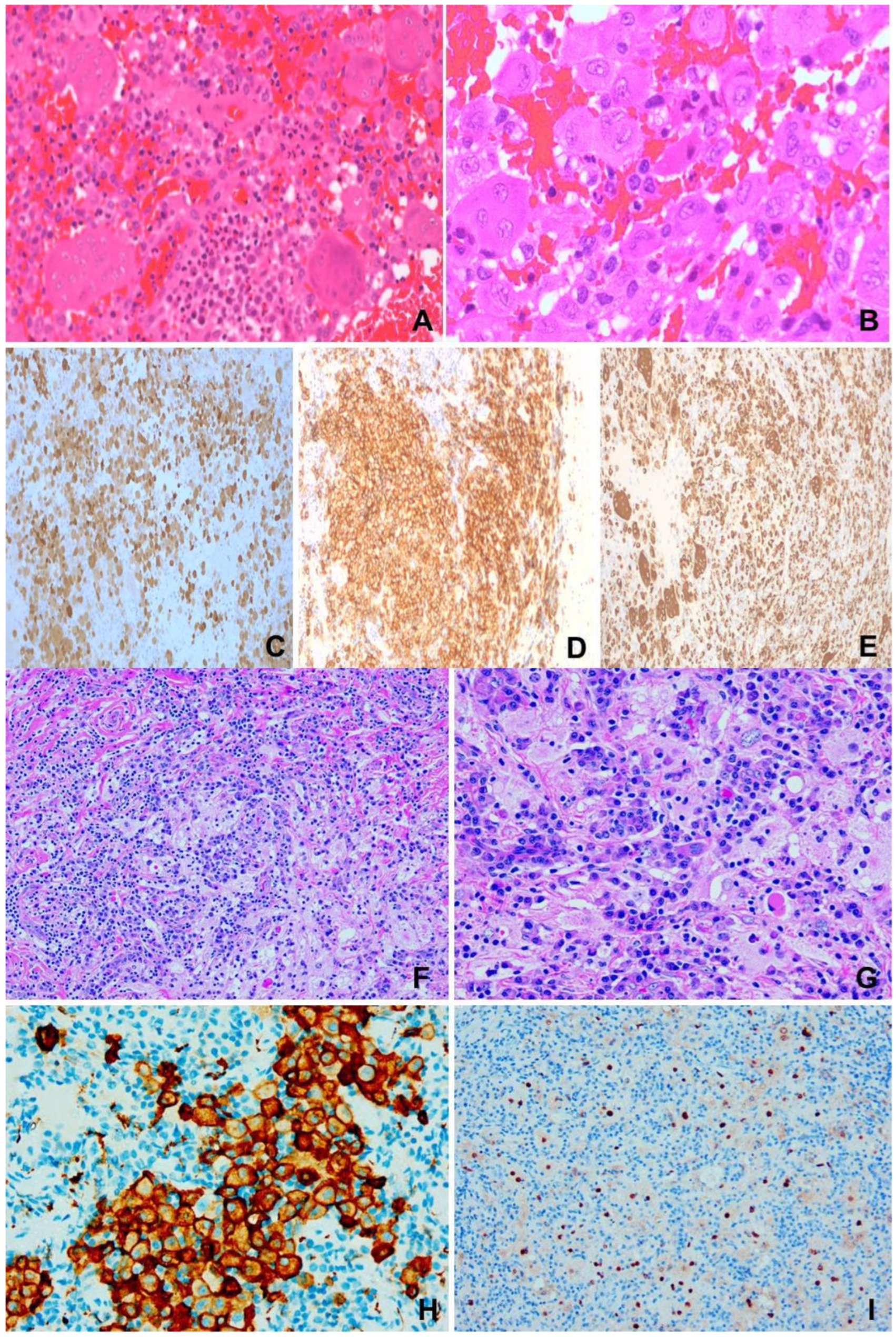
Figure 2.
(A–E) Langerhans cell histiocytosis. F/1 right orbital mass. (A) Infiltrates of eosinophils among histiocytes and giant cells. H&E ×200. (B) Characteristic irregular or grooved nuclei of histiocytes and giant cells. H&E ×400. (C) S100 ×200. (D) CD1a ×200. (E) CD68 ×200. (F–I) Rosai-Dorfman disease. F/31, 1.5 m lung mass. (F) “Sinusoidal” accumulation of large pale histiocytes in backdrop of plasma cells and lymphocytes. H&E ×100. (G) Large pale histiocytes exhibiting emperipolesis. H&E ×400. (H) CD163 ×200. (I) Cyclin D1 ×200.
Treatment and Prognosis
This is related to stage, SS, or MS involvement, and whether high risk organ involvement is present. SS-LCH usually requires only local control with a 90% survival rate. MS-LCH requires systemic therapy with variable clinical courses. About 60% achieve no evidence of disease after LCH-III therapy for 1 year and about 37% have a relapse rate of 1 year after vinblastine and prednisone. Targeted therapy may be useful [7,62,63].
3.1.2. Langerhans Cell Sarcoma (LCS)
LCS is a very rare highly aggressive neoplasm with high grade LC morphology. The incidence is 0.02 per million. It is a multisystem disease affecting skin, lung, bone, soft tissue, liver, spleen, and lymph nodes. A total of 45 percent is widespread disease and isolated lymph node involvement occurs in 20% of cases. LCS is a clonal neoplasm harboring mutations, including KRAS and BRAF V600E, in the MAPK pathway. Other implicated genes include CDKN2A, TP53, and PTEN. Secondary hematolymphoid malignancies (follicular lymphoma, chronic lymphocytic leukemia, B-lymphoblastic leukemia, and hairy cell leukemia) with shared clonality/molecular aberrations are reported, possibly by transdifferentiation from LCS [7,61,63].
Pathology
The neoplastic cells are markedly pleomorphic, with nuclear irregularities but no grooves, high mitotic activity, and atypical mitoses. Sinusoidal infiltration is prominent in lymph nodes. Ultrastructurally, Birbeck granules may be demonstrable. The tumor cells are CD1a+, S-100+, and CD207+ [7,61].
Treatment and Prognosis
This is a highly aggressive disease. Localized disease fares better. Targeted therapy or chemotherapy has produced responses in some cases [7].
3.1.3. Erdheim–Chester Disease (ECD)
ECD is a rare non-LC histiocytic neoplasm affecting adults between the fifth and seventh decades. It is a multisystem disease with the commonly involved sites including bone, central nervous system, cardiovascular system, lungs, kidneys, and skin. Lymph nodes, liver, and spleen, however, are generally spared. There is BRAF V600E mutation in 50% of patients. Patients may be asymptomatic but more often show systemic symptoms, bone pain, exophthalmos, xanthelasma, and diabetes insipidus. Moreover, 3–15% of ECD may be associated with myeloid neoplasms including acute myeloblastic leukemia, chronic myelomonocytic leukemia, and multiple myeloma [7,49]. There is a gain of function somatic alteration in BRAFV600E in 50–60% of cases, activating the MAPK signaling pathway. Other implicated mutations include BRAF, ARAF, NRAS, KRAS, MAPZK1, and PIK3CA. A relationship with bone marrow progenitors was proposed [7,57].
Pathology
There is infiltration by foamy, lipid-laden small histiocytic cells in a backdrop of Touton giant cells, plasma cells, lymphocytes, and neutrophils, but not eosinophils, mimicking xanthogranuloma. Fibrosis may be prominent. There may be co-existing LCH and Rosai-Dorfman disease (RDD). The tumor cells are positive for CD163, CD68, CD14, and CD4 but negative for CD1a and CD207. The expression of pERK is frequent and diffuse strong cytoplasmic staining with VE1 may reflect BRAF V600E mutation [7,64].
Treatment and Prognosis
Prognosis depends on disease extent and the involved sites. CNS involvement is a poor prognostic factor. Treatment by IF-α is usually beneficial. Targeted therapy with BRAF and/or MER inhibitors has been met with 43–100% response and almost no disease progression [7,64].
3.1.4. Rosai-Dorfman Disease (RDD)
RDD is classified as a histiocytic/macrophage neoplasm in the fifth edition (2024) of the WHO Classification of Haematolymphoid Tumours [7] and is a clonal histiocytic disease with nodal or extranodal accumulation of large S-100-positive histiocytes, commonly exhibiting emperipolesis [7,65,66]. Some RDD are familial and may be related to the inherited autoimmune lymphoproliferative syndrome with disordered immune functions and defects in Fas-mediated apoptosis with germline mutations in the TNFRSF6 gene-encoding Fas [67]. Classic sporadic RDD affects children and young adults with an average age of 21 years, more common in African subjects, with slight male predominance, massive bilateral painless cervical lymphadenopathy, fever, weight loss, and night sweats. Inguinal, retroperitoneal, and mediastinal lymph nodes, and skin, nasal cavity, and central nervous system involvement may occur. Cutaneous RDD exhibits higher female predominance and old age (44 years), more Asian and white subjects, with papulonodular skin lesions [7,65].
Pathology
There is extensive lymph nodal sinusoidal expansion or effacement of nodal architecture (advanced cases) by histiocytes with hyperchromatic nuclei, distinct nucleoli, pale crispy cytoplasm characteristically featuring hematolymphoid cells within vacuoles; a feature known as emperipolesis (Figure 2F–I). The cortex comprises B-cells, plasma cells, and some follicles, which, with the pale histiocytic cells, constitute an alternating dark and light zonal appearance. Many IgG4-positive plasma cells may be present, which may be confused with IgG4RD [42]. Marked fibrosis, sometimes storiform, may occur. Extranodal disease is similar, usually with more lymphoid follicles and less emperipolesis. There may be co-existing lymphoma, LCH, and ECD. The histiocytic cells are S-100+, CD68+, CD163+, CD1a−, and CD207 [7,65].
Pathogenesis and Etiology
Familial RDD may show Fas gene TNFRSF6 germline mutations [67]. Approximately 50% sporadic carry mutations in the MAPK/ERK pathway, including KRAS, NRAS, MAP2K1, ARAF, CSF1R, and BRAF V600E. Others include SNX24, CIC, INTS2, SFR1, BRD4, PHOX2B, PD55A, MUC4, ERCC2, LATS2, BRCA1, ATM, and USP25 [7,65,66], thus supporting the neoplastic nature of at least a proportion of RDD.
Classification
RDD may be sporadic or familial. Sporadic RDD is most common, including nodal, extranodal, neoplasia-associated, and immune disease-associated. Familial RDD includes H syndrome and autoimmune lymphoproliferative syndrome-associated RDD [65,66,67,68]. Sporadic RDD is classified under “R group” and cutaneous RDD under “C group” of histiocytosis by the Histiocyte Society [65,68].
Prognosis
Prognosis is good and most cases resolve spontaneously. Surgical treatment is required for organ decompression. MEK inhibitors may be helpful. Bad prognostic factors include immune dysregulation and extranodal involvement. Disease-specific death is reported to be 5–11% [7,65].
3.2. Hodgkin Lymphoma (HL)
Described by Thomas Hodgkin in 1832 [69], subsequent microscopic studies on reported cases that were diagnosed by macroscopy showed that the originally described HL may have included other lymphomas, tuberculosis, or syphilis [70]. HL is subsequently discovered to be a GC B-cell lymphoma [7,71,72,73,74], after uncovering a high load of somatic mutations in the rearranged immunoglobulin heavy chain variable region (IGHV) gene. It is categorized in the fifth edition of the WHO Classification of Haematolymphoid Tumours under B-cell lymphoid proliferations and lymphomas. There are two types of Hodgkin lymphoma: classic HL and nodular lymphocyte predominant HL [7].
3.2.1. Classic HL (CHL)
The characteristic and diagnostic Hodgkin and Reed–Sternberg (HRS) cells are the minority neoplastic cells in a reactive cell milieu, which accounts for a major component of the disease. CHL is primarily a lymph node disease with supradiaphragmatic predilection (cervical, supraclavicular, and mediastinal nodes). Less than 5% of CHL is infradiaphragmatic. The nodes are painless, and spread is typically predictable in a contiguous fashion. Primary extranodal involvement is extremely rare and when present (spleen, lungs, liver, bone marrow) usually represent Stage IV disease. Incidence is higher in socioeconomically developed countries (2.63 per 100,000 persons–year in USA) with slight male predominance (except nodular sclerosis subtype) [7].
Pathogenesis and Etiology
Derived from crippled pre-apoptotic GC B-cells, HRS cells lose B-lineage-specific gene expression program and B-cell identity [7,71,72,73,74]. Recurrent gene mutations of NF-KB, JAK/STAT, MAPK/ERK, AP1, PIK3/AKT, and NOTCH1 cause activation of the respective signaling pathways. CHL may be EBV+ (latency II) or EBV−. The reactive cell milieu and HRS cells are symbiotic [7,71,72,73,74].
Pathology
There are four subtypes: nodular sclerosis (NSCHL), mixed cellularity (MCCHL), lymphocyte-rich (LRCHL), and lymphocyte-depleted CHL (LDCHL, rare, <2% CHL). NSHL is characterized by capsular fibrosis, sclerotic bands, and neoplastic lacunar cells (Figure 3A–F). Sheets of neoplastic cells constitute the syncytial variant. MCCLH shows architectural effacement and more frequent HRS cells (Figure 3G–I). LRCHL is nodular (less commonly diffuse) and lymphocyte-rich. LDCHL exhibits diffuse infiltration of pleomorphic HRS cells with strong EBV association. The reactive milieu is exuberant in MCCHL, rare in eosinophils in LRCHL, and lymphocyte-sparse in LDCHL. HRS and lacunar cells are CD30+ (almost all cases), CD15+ (majority of cases), PAX5+, CD20+ (some cases), and CD45–, J chain–, IG heavy, and light chains–, Oct2–, BOB1–, PU1–, and BCL6–. Aberrant T-cell marker expression may occur. EBV staining is variable [7].
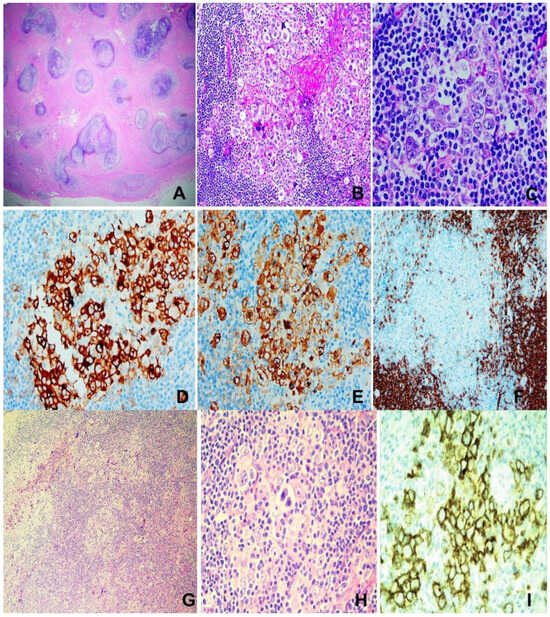
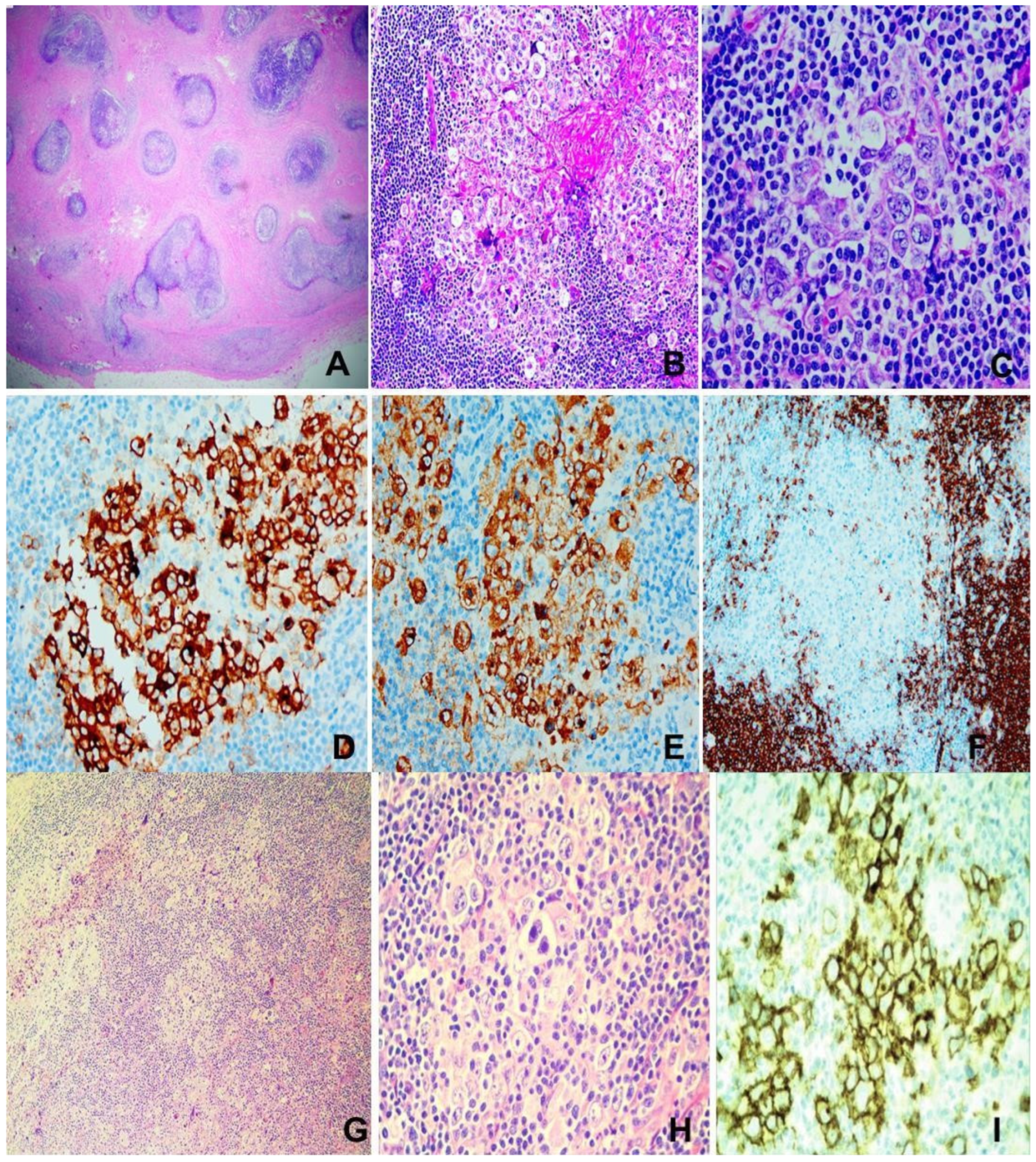
Figure 3.
Hodgkin lymphoma. (A–F) Nodular sclerosing type. M/14, neck mass. (A) Extensive fibrosis dissecting cellular islands of lymphoma tissue. H&E ×100. (B) Aggregates of “lacunar cells” in reactive cellular milieu. H&E ×200. (C) Large “lacunar cells”. H&E ×400. (D) CD30 ×200. (E) CD15 ×200. (F) CD20 showing rare positively stained “lacunar cells” ×100. (G–I) Mixed cellularity type. (G,H) Reed–Sternberg and Hodgkin cells in mixed cellular milieu. H&E ×200 (G), ×400 (H). (I) CD30 ×400.
Prognosis and Treatment
More than 80% of CHL can be cured with modern polychemotherapy. Prognosis is related to stage, B symptoms, and prognostic score [7].
3.2.2. Nodular Lymphocyte Predominant CHL (NLPHL)
NLPHL is a GC B-cell lymphoma [7,75,76] characterized by LP cells with multilobulated nuclei (popcorn appearance, thus nicknamed “popcorn cells”) arranged in nodules. This is mostly a nodal disease, with less supradiaphragmatic, bulky, and Stage IV disease compared to CHL. This is rare (0.11 per 100,000 person–years) with male predominance and wide age distribution (childhood to >80 years of age) [7].
Pathogenesis and Etiology
Etiology is unknown and there is no association with EBV. Genetic predisposition [75,76,77,78] and primary immunodeficiency may be contributory [79]. The neoplastic cells are clonal GC B-cells with ongoing somatic hypermutation (PIM1, RhoH, PAX5, MYC, SOCS1, JUNB, DUSP2, SGK1), intraclonal diversity in rearranged IG genes [7,74,75], and BCL6 translocations or amplifications [80]. Preceding bacterial infection may be a triggering event [81].
Pathology
Nodular, sometimes with diffuse areas, containing characteristic LP (popcorn) cells featuring multilobulated nuclei, variable nucleoli, and pale cytoplasm occurring singly or in small clusters. Reactive B-, T-, and histiocytic cells but no eosinophils or neutrophils constitute the backdrop microenvironment. Granulomas are sometimes present. LP cells are positive for B-cell markers (CD20, CD79a, OCT2, BOB1, PU1), GC markers (BCL6, LMO2, HGAL), rarely for CD15, and are negative for CD30. NLPHL can transform into large B-cell lymphoma, with infradiaphragmatic and splenic involvement and clonal IG rearrangement (at diagnosis) being risk factors [7,82,83].
Prognosis and Treatment
Prognosis is good (10-year survival >90%, progression-free survival >75%). Treatment may include excision, chemotherapy, immunotherapy, and radiation, which varies with patient age and stage [7,78].
3.3. Burkitt Lymphoma (BL)
Described by Dennis Burkitt in 1958 [84], BL is an aggressive mature B-cell lymphoma with GC phenotype and high proliferation and tumor burden [7], involving mostly extranodal tissues, and classified under “mature B-cell lymphoma” in the fifth edition of the WHO Classification of Haematolymphoid Tumours [7]. BL affects mostly extranodal sites. There are three epidemiologic types: endemic (equatorial Africa and Papua New Guinea), common in children causing facial bones and abdominal disease and associated with EBV in 90% cases; sporadic, in the USA and Western Europe, affecting children and adults causing commonly abdominal disease with EBV in 20% cases; and immunodeficiency (IDF)-associated, mostly associated with HIV infection (affecting those with higher CD4 count) than other causes of immunosuppression, and commonly causes lymph node disease. Leukemic picture and bone marrow involvement occurs in 20% of cases and is more common in IDF-associated BL. CNS involvement occurs in any of these types, with a frequency of 10–30% [85] and portends worse prognosis among high stage BL patients [86].
EBV (latency 1) is implicated in almost 100% endemic and 25–40% of IDF-associated BL, being involved in early pathogenesis by apoptosis evasion [87]. The EBNA1 protein is the most consistently detected transforming EBV protein in EBV-positive BL [7]. Therefore, EBV may be the defining feature of BL types, and EBV-positive and EBV-negative BL differ in their underlying biology and pathogenesis [88,89], despite conventional subtyping of BL into endemic, sporadic, and immunodeficiency-associated.
Malaria (particularly mixed P falciparum infection) is also pathogenetically related, probably causing chronic B-cell stimulation and T-cell suppression, and the induction of activation-induced cytidine deaminase, which is associated with MYC translocation [90]. In 70% of BL, there is IG::MYC translocation (involving genes of heavy t(8;14) or light chains t(8;22); t(2;8)). The MYC translocation is not sufficient for oncogenesis and other genetic changes including TCF3, ID3, CCND3, and TP53 alterations, resulting in BCR, PIK3, SWI/SNF, and GPCR signaling activation also being at play [7,91].
3.3.1. Pathology
Diffuse infiltrate of monomorphic medium-sized lymphoid cells with squared cell borders, round nuclei, basophilic paracentric nucleoli, basophilic cytoplasm, brisk mitoses, and frequent apoptosis, punctuated by tingible body macrophages portraying “starry sky” appearance. Focal granulomatous reactions may sometimes be present. The tumor cells are positive for B-cell markers (CD19, CD20, CD79a, CD22, PAX5), GC markers (CD10, BCL6, CD38, HGAL, MEF2B), IgM, Ki67 > 95%, and negative for BCL2 and TdT (Figure 4A–F) [7].
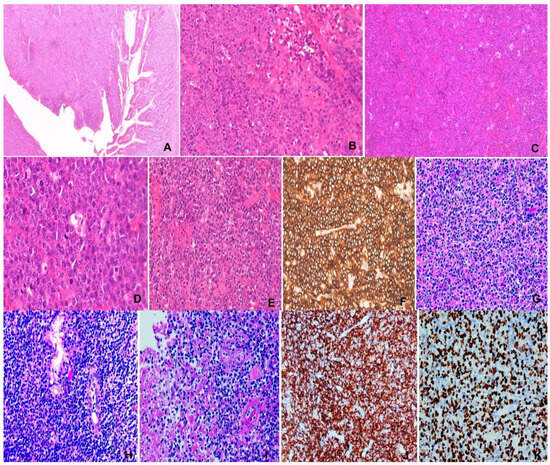
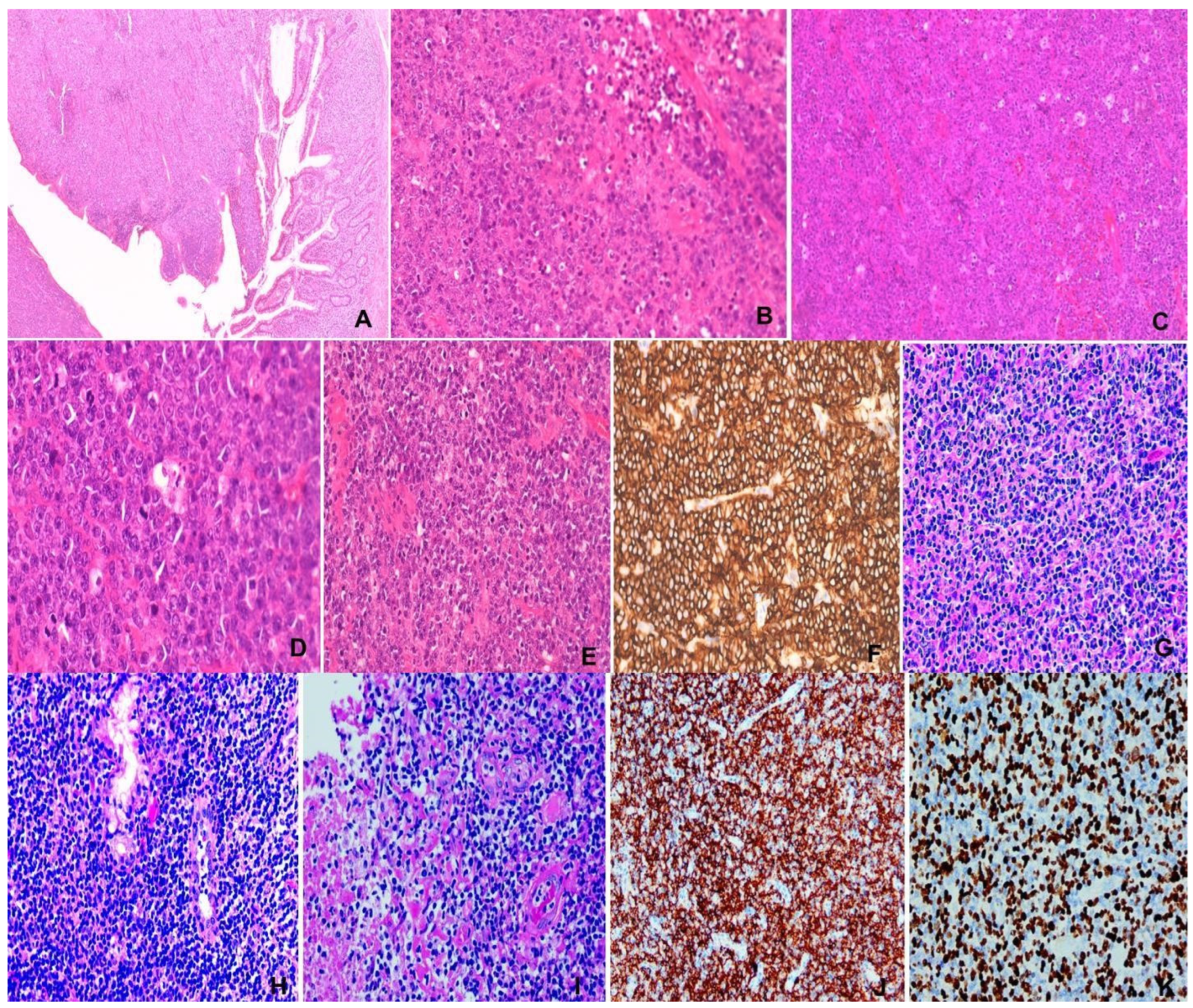
Figure 4.
(A–F) Burkitt lymphoma. M/74, small intestinal tumor. (A) Intestinal wall mural invasion. H&E ×20. (B,C) “Starry sky” appearance. Pale histiocytes in “dark” backdrop of tumor cells. H&E ×100&200. (D) Medium-sized tumor cells with squaring and brisk mitosis. H&E ×400. (E) Necrosis of tumor. H&E ×200. (F) CD20 immunostaining ×200. (G–K) NK/T-cell lymphoma, nasal type. M/47, nasal mass. (G) Polymorphic lymphoid infiltrate. H&E ×200, (H) Epithelial invasion. H&E ×400. (I) Angioinvasion and necrosis. H&E ×400. (J) CD56 ×200. (K) EBER ×200.
3.3.2. Prognosis and Treatment
Prognosis is good with contemporary immunochemotherapy. Overall survival is >80% in adults and 90% in children [7,92].
3.4. Stewart Granuloma (SG)
Stewart granuloma was first reported in 1897 by McBride as a “Case of rapid destruction of the nose and face” [93] and later in 1928 by Stewart [94], whose name became the disease eponym. This disease is characterized by midfacial disfiguring, extirpative and ulcerative disease of the nose, paranasal sinuses, nasopharynx, tonsils, palate, hypopharynx, and larynx, often accompanied by distant metastasis at presentation [7,93,94,95,96]. It has therefore attracted many descriptive names including lethal midline granuloma, malignant/mutilating granuloma of the nose, granuloma ganrenescens, reticulo-endothelial sarcoma, and later malignant histiocytosis or lymphoma [93,94,96]. The latter was a misnomer because the prominent presence of histiocytes represents reactive hemophagocytic lymphohistiocytosis [7,95,96]. The application of modern immunohistochemistry and molecular analysis unraveled the true nature of the disease, which was not possible in the past due to the need for functional studies to confirm natural killer cell lineage [96]. SG has been described as a T-cell lymphoma, which is confirmed by the expression of surface CD3 and T-cell receptor (TCR) and TCR gene rearrangement. However, the cases that are surface CD3−, TCR−, and lack TCR rearrangement, and express NK cell markers (often CD56) are of NK-cell lineage [7,96,97]. With this modern development, SG is now reclassified as extranodal NK/T-cell lymphoma, nasal type (ENNKTL). Most ENNKTL (60–90%) are of NK-lineage, with the remaining 10–40% being of T-cell lineage.
3.4.1. Etiology and Pathogenesis
There is prevalence in eastern Asia and Central and South America. Prevalence in haplotypes HLA-DPB1, HLA-DRB1, and IL18RAP in eastern Asia predisposes these populations to the disease. There is strong association with EBV, which occurs in clonal episomal forms in the tumor cells. EBV type A is usually involved, with type II latency [7,96,98,99,100,101]. Immunodeficiency may also be a predisposing factor [7]. Complex genetic changes have been documented, which involve genes of the JAK/STAT and NF-KB and DPGFR signaling pathways, epigenetic modifiers, tumor suppressor genes, RNA helicase gene DDX3X, and non-coding RNAs (microRNAs and long intervening/intergenic non-coding RNA) [7,96].
3.4.2. Pathology
There is infiltration by atypical lymphoid cells, which range from small, medium, to large size, usually occurring in a mixture. Typically, epithelial invasion leading to lymphoepithelial lesions, angiocentricity, angioinvasion, and extensive geographical necrosis are evident. The tumor cells are immunophenotyically surface CD3 and TCR+ in T-cell type and surface CD3− and TCR−, with CD56 positivity in the NK-cell type ENNKTL (Figure 4G–K). TCR rearrangement is present in the former and negative in the latter. Cytoplasmic CD3 may be present in the NK-cell type. Typically, both types express cytotoxic proteins. ENNKTL is positive for EBV, which is required for definitive diagnosis [7,96].
3.4.3. Treatment and Prognosis
Much advancement has been made in treating ENNKTL. Radiotherapy is the mainstay of treatment. For local disease, concurrent chemoradiotherapy and sequential chemoradiotherapy are used. The most recent protocol for advanced disease is combined radiotherapy and chemotherapy administered synchronously or metachronously. Chemotherapeutic agents include L-asparaginase, Aspa-Met-Dex, SMILE, DeVIC, ESHAP, DEP, VIPD, VIDL, DDGP, MESA, GELOX, and PGEMOX, and pegaspargase is used in patients allergic to Escherichia coli L-asparaginase [96,102,103,104,105,106]. Hemopoietic stem cell transplantation [99], immune checkpoint inhibitors [96,107,108], and EBV-targeted treatment [96,109] are also useful. Prognosis is related to disease stage, circulating EBV DNA levels, and Ki-67 rate in tumor cells [96,104,105,106,107,108].
3.5. Sezary Syndrome (SS)
Named after Albert Sezary [110], SS is a T-cell neoplasm featuring the triad erythroderma, generalized lymphadenopathy, and clonally proliferated T-cells featuring cerebriform nuclei. SS is rare, and affects adults aged >60 years, with male predominance, presenting as leukemia, with skin and lymph node involvement. Almost the whole skin (>80%) is affected, with extensive erythroderma. In advanced stages, lymph nodes and various viscera, more commonly oropharynx, lungs, and CNS, are affected. This is a rare disease with an incidence of 0.36/100,000 person–years and accounts for 2–3% of cutaneous T-cell lymphomas [7].
3.5.1. Etiology and Pathogenesis
Two genetic mutational signatures are identified. Signature 1 is indicative of age-related deamination that is prevalent among T-cell lymphomas and signature 7 is characteristic of UV exposure prevalent in cutaneous T-cell lymphomas [111]. Environmental UV exposure is causal in transforming T-cells in the skin. A high tumor mutation burden reflective of ultraviolet-induced mutations, gene mutations affecting T-cell signaling, and upregulation of NF-KB activity (PLCG1, CARD11, CD28, CARMIL2), mutations in the DNA damage response pathway (TP53, POT1, ATM), and mutations affecting JAK/STAT signaling (STAT5B, JAK3), and chromatin modifiers (ARID1A, TRRAP, DMNT3A, TET2) are detected [7,111,112,113].
3.5.2. Pathology
CD3+, CD4+, CD8−, PD1 (CD279)+, CLA (cutaneous lymphocyte antigen)+, and CCR4 (skin homing receptor)+ neoplastic medium-sized T-cells with cerebriform nuclei infiltrate involved tissues and circulate in the peripheral blood. In the skin, epidermotropism may be absent. In lymph nodes, partial or complete infiltration may occur. In bone marrow, there may be interstitial or sparse infiltration [7]. The major histologic differential diagnosis is Mycosis fungoides, which is distinguishable by being an indolent disease primarily affecting the skin with consistent epidermotropism [7,114].
3.5.3. Prognosis
Aggression and prognosis vary with stage. Median survival is 32 months and 5 year survival for 10–30%. Worse prognosis occurs with peripheral blood involvement at diagnosis [7,114].
4. Conclusions
Eponyms have fulfilled a meritorious service—assembling one or more similar/related diseases with comparable manifestations into disease entities for easier recognition and management. With scientific and technological advances in the past decades, the nature of many have been elucidated with regard to cell lineage, histogenesis, morphologic nuances, and immunophenotypic and genetic characteristics. New nomenclature, classification, prognostic predictions, and treatment strategies have thus been developed for these eponymic diseases. Eye-opening discoveries have uncovered GC B-cell lineage of Hodgkin lymphoma, GC B-cell phenotype in Burkitt lymphoma, and NK/T-cell lineage of Stewart granuloma, which is reclassified as NK/T-cell lymphoma, nasal type. Classic Hodgkin lymphoma is further found to originate from crippled GC B-cells, which have mostly lost their B-cell identity, thus explaining non-expression of many B-cell markers in HL. NK/T-cell lymphomas are discovered to be etiologically and pathogenetically EBV-related, and UV light exposure is causally related to cutaneous T-cell transformation in Sezary syndrome. Langerhans cell histiocytosis, Erdheim–Chester disease, and Rosai–Dorfman disease have also been discovered to be neoplasms of histiocytic/dendritic cell lineage. A classification system has been formulated for Castleman disease based on etiologic (KSHV/HHV8) and clinical syndromic (POEMS. TAFRO) findings. A number of fibroinflammatory diseases of various sites are reclassified as IgG4-related disease. Kikuchi–Fujimoto disease is discovered to be the sequel of a prominent T-cell response to some unknown antigens. Recent discoveries on hematolymphoid eponymic diseases are summarized in Table 1. While best avoided, many eponyms of these diseases are here to stay, and it remains to be seen if they could be renamed or reclassified as more findings in state of the art technology unfold.

Table 1.
Recent discoveries on hematolymphoid eponymic diseases.
Author Contributions
C.S.N.—Conception, conceptualization, literature review, curation of data, drafting manuscript, finalization and approval of manuscript. J.Q.—Retrieval and review of cases, image preparation of figures, review and approval of final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Yvonne Chan for preparing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anoymous. Classification and nomenclature of morphological defects. Lancet 1975, 305, 513. [Google Scholar] [CrossRef]
- Castleman, B.; Ivenon, L.; Menendez, V.P. Localized mediastinal lymph node hyperplasia resembling thymoma. Cancer 1956, 9, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.R.; Hochholzer, L.; Castleman, B. Hyaline vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer 1972, 29, 670–683. [Google Scholar] [CrossRef]
- van Rhee, F.; Stone, K.; Szmania, S.; Barlogie, B.; Singh, Z. Castleman disease in the 21st century: An update on diagnosis, assessment, and therapy. Clin. Adv. Hematol. Oncol. 2010, 8, 486–498. [Google Scholar] [PubMed]
- Dispenzieri, A.; Fajgenbaum, D. Overview of Castleman disease. Blood 2020, 135, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lim, M.S.; Jaffe, E.S. Pathology of Castleman disease. Hematol. Oncol. Clin. N. Am. 2018, 32, 37–52. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Haematolymphoid Tumours, 5th ed.; Beta version ahead of print; International Agency for Research on Cancer: Lyon, French, 2024; Volume 11, Available online: https://tumourclassification.iarc.who.int (accessed on 9 February 2025).
- Chang, K.C.; Wang, Y.C.; Hung, L.Y.; Huang, W.T.; Tsou, J.H.; Jones, D.M.; Song, H.L.; Yeh, Y.M.; Kao, L.Y.; Medeiros, S.L.J.; et al. Monoclonality and cytogenetic abnormality in hyaline vascular Castleman disease. Mod. Pathol. 2014, 27, 823–831. [Google Scholar] [CrossRef]
- Li, Z.; Lan, X.; Li, C.; Zhang, Y.; Wang, Y.; Xue, W.; Lu, L.; Jin, M.; Zhou, Z.; Wang, X.; et al. Recurrent PDGFRB mutations in unicentric Castleman disease. Leukemia 2019, 33, 1035–1038. [Google Scholar] [CrossRef]
- Butzmann, A.; Kumar, J.; Sridhar, K.; Gallapudi, S.; Ohgami, R.S. A review of genetic abnormalities in unicentric and multicentric Castleman disease. Biology 2021, 10, 251. [Google Scholar] [CrossRef]
- Goodman, A.M.; Jeong, A.; Philips, A.; Wang, H.Y.; Sokol, E.S.; Cohen, P.R.; Sicklick, J.; Fajgenbaum, D.C.; Kurzrock, R. Novel somatic alterations in unicentric and idiopathic multicentric Castleman disease. Eur. J. Haematol. 2021, 107, 642–649. [Google Scholar] [CrossRef]
- Nishimoto, N.; Karakura, Y.; Aozasa, K.; Johkoh, T.; Nakamura, M.; Nakano, S.; Nakano, N.; Ikeda, Y.; Saski, T.; Nishioka, K.; et al. Humanized anti-interleukin 6 receptor antibody treatment of multicentric Castleman disease. Blood 2005, 106, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- van Rhee, F.; Fayad, L.; Voorhee, P.; Furman, R.; Lonial, S.; Borghaei, H.; Sokol, L.; Crawford, J.; Cornfeld, M.; Qi, M.; et al. Situximab, a novel anti-interleukin 6 monoclonal antibody for Castleman disease. J. Clin. Oncol. 2010, 28, 3701–3708. [Google Scholar] [CrossRef]
- van Rhee, F.; Oksenhendler, E.; Sokalovic, G.; Voorhees, P.; Lim, M.; Dispenzieri, A.; Ide, M.; Parente, S.; Schey, S.; Streetly, M.; et al. International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood. Adv. 2020, 4, 6039–6050. [Google Scholar] [CrossRef]
- Suda, T.; Katano, H.; Delso, G.; Kakiuchi, C.; Nakamura, T.; Shiota, M.; Sata, T.; Higashihara, M.; Mori, S. HHV-8 infection status of AIDS-unrelated and AIDS-associated multicentric Castleman disease. Pathol. Int. 2001, 51, 671–679. [Google Scholar] [CrossRef]
- Liu, A.Y.; Nabel, C.S.; Finkelman, B.S.; Ruth, J.R.; Kurzrock, R.; van Rhee, F.; Krymskaya, V.P.; Kelleher, D.; Rubenstein, A.H.; Fajgenbaum, D.C. Idiopathic multicentric Castleman disease: A systematic literature review. Lancet Haematol. 2016, 3, e163–e175. [Google Scholar] [CrossRef] [PubMed]
- Lin, O.; Frizzera, G. Angiomyoid and follicular dendritic cell proliferative lesions in Castleman disease of hyaline-vascular type: A study of 10 cases. Am. J. Surg. Pathol. 1997, 21, 1295–1306. [Google Scholar] [CrossRef]
- Izumi, M.; Mochizuki, M.; Kuroda, M.; Iwaya, K.; Mukai, K. Angiomyoid proliferative lesion: An unusual stroma-rich variant of Castleman disease of hyaline-vascular type. Virchow. Arch. 2002, 441, 400–405. [Google Scholar] [CrossRef]
- Walsh-Jahake, R.; Cui, W.; Zhang, D. Late recurrence of Castleman’s disease with mixed angiomyoid, histiocytic reticulum cell, follicular dendritic cell stroma-rich proliferations: A case report and review of the literature. J. Hematop. 2015, 8, 43–47. [Google Scholar] [CrossRef]
- Chan, J.K.C.; Tsang, W.Y.; Ng, C.S. Follicular dendritic cell tumor and vascular neoplasm complicating hyaline-vascular Castleman disease. Am. J. Surg. Pathol. 1994, 18, 517–525. [Google Scholar] [CrossRef]
- Chan, J.K.C.; Luk, S.C.; Ho, P.L. Stroma-rich Castleman’s disease with superimposed Kikuchi’s lymphadenitis-like changes. Int. J. Surg. Pathol. 1997, 4, 197–202. [Google Scholar] [CrossRef]
- Evans, R.S.; Takahashi, K.; Duane, R.T.; Payne, R.; Liu, C.K. Primary thrombocytopenic purpura and acquired hemolytic anemia. AMA Arch. Intern. Med. 1951, 87, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Miichel, M.; Chanet, V.; Dechartes, A.; Morin, A.-S.; Piette, J.-C.; Cirasino, L.; Emilia, G.; Zaja, F.; Ruggeri, M.; Andres, E.; et al. The spectrum of Evans syndrome in adults: New insight into the disease based on the analysis of 68 cases. Blood 2009, 114, 3167–3172. [Google Scholar] [CrossRef]
- Kumar, D.; Prince, C.; Bennett, C.M.; Briones, M.; Lucas, L.; Russell, A.; Patel, K.; Chonat, S.; Graciaa, S.; Edington, H.; et al. T-follicular helper cell expansion and chronic T-cell activation are characteristic immune anomalies in Evans Syndrome. Blood 2022, 139, 369–383. [Google Scholar] [CrossRef]
- Hadjadj, J.; Aladjidi, N.; Fermandes, H.; members of the French Reference Center for Pediatric Autoimmune Cytopenias (CEREVANCE). Pediatric Evans Syndrome is associated with a high frequency of potentially damaging variants in immune genes. Blood 2019, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M. Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytosis. Nippo N Ketsueki Gakki Zashi 1972, 35, 379–380. [Google Scholar]
- Fujimoto, Y.; Koyima, Y.; Yamaguchi, K. Cervical subacute necrotizing lymphadenitis. Naika 1972, 30, 920–927. [Google Scholar]
- Kuo, T.T. Kikuchi’s disease (Histiocytic necrotizing lymphadenitis). A clinicopathologic study of 79 cases with an analysis of histologic subtypes, immunohistology and DNA ploidy. Am. J. Surg. Pathol. 1995, 19, 798–809. [Google Scholar] [CrossRef]
- Pileri, S.; Kikuchi, M.; Helbron, D.; Lennert, K. Histiocytic necrotizing lymphadenitis without granulocytic infiltration. Virchow. Arch. A Pathol. Anat. 1982, 395, 257–271. [Google Scholar] [CrossRef]
- Papadimitriou, C.S.; Rapacharalampous, N.X. Histiocytic necrotizing lymphadenitis without granulocytic infiltration. Arch. Pathol. Lab. Med. 1985, 109, 107–108. [Google Scholar]
- Dorfman, R.F.; Berry, G.J. Kikuchi’s histiocytic necrotizing lymphadenitis: An analysis of 108 cases with emphasis on differential diagnosis. Sem. Diagn. Pathol. 1988, 5, 329–345. [Google Scholar]
- Tsang, W.Y.W.; Chan, J.K.C.; Ng, C.S. Kikuchi’s lymphadenitis. A morphologic analysis of 75 cases with special reference to unusual features. Am. J. Surg. Pathol. 1994, 18, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.Y.W.; Chan, J.K.C. Fine-needle aspiration cytologic diagnosis of Kikuchi’s lymphadenitis: A report of 27 cases. Am. J. Clin. Pathol. 1994, 102, 454–458. [Google Scholar] [CrossRef]
- Deaven, D.; Horna, P.; Cualing, H.; Soloi, L. Pathogenesis, diagnosis and management of Kikuchi-Fujimoto disease. Cancer Control. 2014, 21, 313–321. [Google Scholar] [CrossRef]
- Yu, F.; Ba, X.; Yang, H.; Huang, K.; Zhang, Y.; Zhang, H.; Xu, L.; Wang, J.; Wang, L.; Wang, Z.; et al. Kikuchi disease with an exuberant proliferation of large T-cells: A study of 25 cases that can mimic T-cell lymphoma. Histopathology 2023, 82, 340–353. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, H.; Ohara, M.; Suzuki, C.; Naishiro, Y.; Yamamoto, H.; Shinomura, Y.; Imai, K. A new conceptualization for Mikulicz’s diseases as an IgG4-related plasmacytic disease. Mod. Rheumatol. 2006, 16, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V.S.; Mattoo, H.; Deshpande, V.; Pillai, S.S.; Stone, J.H. IgG4-related disease. Ann. Rev. Pathol. 2014, 9, 315–347. [Google Scholar] [CrossRef]
- Hamano, H.; Kawa, S.; Horiuchi, A.; Unno, H.; Furuya, N.; Akamatsu, T.; Fukushima, M.; Nikaido, T.; Nakayama, K.; Usuda, N.; et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N. Eng. J. Med. 2001, 344, 732–738. [Google Scholar] [CrossRef]
- Kamisawa, T.; Funata, N.; Hayashi, Y.; Eishi, Y.; Koike, M.; Tsuruta, K.; Okamoto, A.; Egawa, N.; Nakajima, H. A new clinicopathological entity of IgG4-related autoimmune disease. J. Gastroenterol. 2003, 38, 982–984. [Google Scholar] [CrossRef]
- Cheuk, W.; Chan, J.K.C. IgG4-related sclerosing disease. A critical appraisal of an evolving clinicopathologic entity. Adv. Anat. Pathol. 2010, 17, 303–332. [Google Scholar] [CrossRef]
- Deshpande, V.; Zea, Y.; Chan, J.K.C.; Yi, E.E.; Sato, Y.; Yoshino, Y.; Kloppel, G.; Heathcote, J.G.; Khosroshahi, A.; Ferry, J.A.; et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 2012, 25, 1181–1192. [Google Scholar] [CrossRef]
- Umehara, H.; Okazaki, K.; Kawa, S.; Takahashi, H.; Goto, H.; Matsui, S.; Ishizaka, N.; Akamizu, T.; Sato, Y.; Kawano, M.; et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod. Rheumatol. 2021, 31, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Ng, C.S.; Yin, W. A comparative study of Kimura’s disease and IgG4-related disease: Similarities, differences and overlapping features. Histopathology 2021, 79, 801–809. [Google Scholar] [CrossRef]
- Nehring, P.; Przybytkowski, A. Think twice before operating on a pancreatic mass: Could it be IgG4-related disease? Lancet 2020, 395, 816. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Perez, D.; Vaquero, E.C.; Ayuso, J.R.; Fernandez-Cruz, L. Autoimmune pancreatitis: A surgical dilemma. Cir. Esp. 2014, 92, 645–653. [Google Scholar] [CrossRef]
- Shiokawa, M.; Kodama, Y.; Sekiguchi, K.; Kuwada, T.; Tomono, T.; Kuriyama, K.; Yamzaki, H.; Morita, T.; Marui, S.; Sogabe, Y.; et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci. Transl. Med. 2018, 10, eaaq0997. [Google Scholar] [CrossRef]
- Hubers, L.M.; Vos, H.; Schuurman, A.L.; Erken, R.; Elferink, R.P.O.; Burgering, B.; van de Graaf, S.F.J.; Beuers, U. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut 2018, 67, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Munemura, R.; Maehara, T.; Murakami, Y.; Koga, R.; Aoyagi, R.; Kaneko, N.; Doi, A.; Perugino, C.A.; Della-Torre, E.; Saeki, T.; et al. Distinct disease-specific Tfh cell populations in 2 different fibrotic diseases: IgG4-related disease and Kimura disease. J. Allergy Clin. Immunol. 2022, 150, 440–455. [Google Scholar] [CrossRef]
- Jarrell, J.; Baker, M.C.; Perugino, C.A.; Liu, H.; Bloom, M.S.; Maehar, T.; Wong, H.H.; Lanz, T.V.; Adamska, J.Z.; Kongpachith, S.; et al. Neutralizing anti-IL-1 receptor antagonist autoantibodies include inflammatory and fibrotic mediators in IgG4-related disease. J. Allergy Clin. Immunol. 2022, 149, 358–368. [Google Scholar] [CrossRef]
- Akiyama, M.; Alshehri, W.; Ishigai, S.; Saiti, K.; Kanebo, Y. The immunological pathogenesis of IgG4-related disease categorized by clinical characteristics. Immunol. Med. 2025, 48, 11–23. [Google Scholar] [CrossRef]
- Langerhans, P. Ueber die Nerven der menschlichen Ilaut. Arch. Anat. Physiol. Kin. Med. 1868, 44, 325–338. [Google Scholar] [CrossRef]
- Rowden, G.; Lewis, M.G.; Sullivan, A.K. Ia antigen expression on human epidermal Langerhans cells. Nature 1977, 268, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Kapur, P.; Reickson, C.; Rakheja, D.; Carden, K.R.; Hoang, M.P. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): Ten-year experience at Dallas Children’s Medical Center. J. Am. Acad. Derm. 2007, 56, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Matubayashi, T.; Koizumi, M. BRAF mutation analysis in two cases of congenital self-healing Langerhans cell histiocytosis. Cureus 2022, 14, e32497. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Wang, Y.; Greter, M.; See, P.; Teo, P.; Malleret, B.; Leboeuf, M.; Low, D.; Oller, G.; Almeida, F.; et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012, 209, 1167–1181. [Google Scholar] [CrossRef]
- Allen, C.E.; Li, L.; Peters, T.L.; Leung, H.C.E.; Yu, A.; Man, T.K.; Gurusiddappa, S.; Phillips, M.T.; Hicks, M.J.; Gaikwad, A.; et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveal a distinct profile compared with epidermal Langerhans cells. J. Immunol. 2010, 184, 4557–4567. [Google Scholar] [CrossRef]
- Kemps, P.G.; Hebeda, K.; Pals, S.T.; Verdijk, R.M.; Lam, K.H.; Bruggink, A.H.; de Lil, H.S.; Ruiterkamp, B.; de Heer, K.; van Laar, J.; et al. Spectrum of histiocytic neoplasms associated with diverse haematological malignancies bearing the same oncogenic mutation. J. Pathol. Clin. Res. 2021, 7, 10–26. [Google Scholar] [CrossRef]
- Badalian-Veoy, G.; Verogilo, J.-A.; Degar, B.A.; MacConaill, L.E.; Brandner, B.; Calicchio, M.L.; Kuo, F.; Ligon, A.H.; Stevenson, K.E.; Kehoe, S.M.; et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 2010, 116, 1919–1923. [Google Scholar] [CrossRef]
- Yousem, S.A.; Dacic, S.; Nikiforov, Y.E.; Nikiforov, M. pulmonary Langerhans cell histiocytosis: Profiling of multifocal tumors using next-generation sequencing identifies concordant occurrence of BRAFV600E mutations. Chest 2013, 143, 1679–1684. [Google Scholar] [CrossRef]
- Brown, N.A.; Furtado, L.V.; Betz, B.L.; Kiel, M.J.; Weigelin, H.C.; Lim, M.S.; Elenitoba-Johnson, K.C.J. High prevalence of somatic MAP2k1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood 2014, 124, 1655–1658. [Google Scholar] [CrossRef]
- Harmon, C.M.; Brown, N. Langerhans cell histiocytosis. A clinicopathologic review and molecular pathogenetic update. Arc. Pathol. Lab. Med. 2015, 139, 1211–1214. [Google Scholar] [CrossRef]
- Allen, C.E.; Ladisch, S.; McClain, K.L. How I treat Langerhans cell histiocytosis. Blood 2015, 126, 26–35. [Google Scholar] [CrossRef]
- Hutter, C.; Minkoy, M. Insights into the pathogenesis of Langerhans cell histiocytosis: The development of targeted therapies. Immunotargets Ther. 2016, 5, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.D.; Manerish-Mazor, M.; Shoenfield, Y. Erdheim-Chester disease: A comprehensive review of the literature. Orphanet. J. Rare. Ds. 2013, 8, 137. [Google Scholar] [CrossRef]
- Bruce-Brand, C.; Schneider, J.W.; Schubert, P. Rosai-Dorfman disease: An overview. J. Clin. Pathol. 2020, 73, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Garces, S.; Medeiros, L.J.; Patel, K.P.; Li, S.; Pina-Oviedo, S.; Li, J.; Garces, J.C.; Khoury, J.D.; Yin, C.C. Mutually exclusive recurrent KRAS and MAP2K1 mutations in Rosai-Dorfman disease. Mod. Pathol. 2017, 30, 1367–1377. [Google Scholar] [CrossRef]
- Maric, I.; Pittaluga, S.; Dale, J.K.; Niemela, J.E.; Delsol, G.; Diment, J.; Rosai, J.; Raffeld, M.; Puck, J.M.; Straus, S.E.; et al. Histologic features of sinus histiocytosis with massive lymphadenopathy in patients with autoimmune lymphoproliferative syndrome. Am. J. Surg. Pathol. 2005, 29, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Emile, J.-F.; Abla, O.; Fraitag, S.; Horne, A.; Haroche, J.; Donadieu, J.; Requena-Caballero, L.; Jordan, M.B.; Abdal-Wahab, O.; Allen, C.E.; et al. Revised classification of histiocytosis and neoplasms of the macrophage-dendritic cell lineages. Blood 2016, 127, 2672–2681. [Google Scholar] [CrossRef]
- Hodgkin, T. On some morbid appearance of the absorbent glands and spleen. Med. Chir. Trans. 1832, 17, 68–114. [Google Scholar] [CrossRef]
- Lakhtakia, R.; Burney, I. A historical tale of two lymphomas. Sultan. Quaboos. Uni. Med. J. 2015, 15, e202–e206. [Google Scholar]
- Kanzler, H.; Kuppers, R.; Hansmann, M.L.; Rajewshy, K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J. Exp. Med. 1996, 184, 1495–1505. [Google Scholar] [CrossRef]
- Kuppers, R.; Schwering, I.; Brauninger, A.; Rajewsky, K.; Hansmann, M.-L. Biology of Hodgkin’s lymphoma. Ann. Oncol. 2002, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Marafiot, T.; Hummel, M.; Foss, H.D.; Laumen, H.; Korbjuhn, P.; Anagnostopoulos, I.; Lammert, H.; Demel, G.; Thiel, J.; Wirth, T.; et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood 2000, 95, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Schwering, I.; Brauninger, A.; Klein, U.; Jungnickel, B.; Tinguely, M.; Diehl, V.; Hansmann, M.-L.; Dalla-Favera, R.; Rajewsky, K.; Kuppers, R.L. Loss of the B-lineage –specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood 2003, 101, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Marafiot, T.; Hummel, M.; Anagnostopoulos, I.; Foss, H.D.; Falini, B.; Delsol, G.; Isaacson, P.G.; Pileri, S.; Stein, H. Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal center B cells. N. Engl. J. Med. 1997, 33, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Braeuninger, A.; Kuppers, R.; Strickler, J.G.; Wacker, H.H.; Rajewsky, K.; Hansmann, M.-L. Hodgkin and Reed-Sternberg cells in predominant Hodgkin disease represent clonal populations of germinal center-derived tumor B cells. Proc. Natl. Acad. Sci. USA 1997, 94, 9337–9342. [Google Scholar] [CrossRef]
- Saarinen, S.; Aavikko, M.; Aittomaki, K.; Launonen, V.; Lehtonen, R.; Franssila, K.; Lehtonen, H.J.; Kassinen, E.; Broderick, P.; Tarkkanen, J.; et al. Exome sequencing reveals germline NPAT mutation as a candidate risk factor for Hodgkin lymphoma. Blood 2011, 118, 493–498. [Google Scholar] [CrossRef]
- Strobbe, L.; Valke, L.L.F.G.; Diets, I.J.; van den Brand, M.; Aben, K.; Raemaekers, J.M.M.; Hebeda, K.M.; van Krieken, H.J.M. A 20-year population-based study on the epidemiology, clinical features, treatment, and outcome of nodular lymphocyte predominant Hodgkin lymphoma. Ann. Hematol. 2016, 95, 417–423. [Google Scholar] [CrossRef]
- Van den Berg, A.; Maggio, E.; Diepstra, A.; de Jong, D.; van Krieken, J.; Poppema, S. Germline FAS gene mutation in a case of ALPS and NLP Hodgkin lymphoma. Blood 2002, 15, 1492–1494. [Google Scholar] [CrossRef]
- Elodarska, I.; Nooyen, P.; Maes, B.; Martin-Subero, J.I.; Siebert, R.; Pauwels, P.; DeWolf-Peters, C.; Hagemeijer, A. Frequent occurrence of BCL6 rearrangements in nodular lymphocyte predominant Hodgkin lymphoma but not in classical Hodgkin lymphoma. Blood 2003, 101, 706–710. [Google Scholar] [CrossRef]
- Thurner, L.; Hartmann, S.; Fadle, N.; Regitz, E.; Kemele, M.; Kim, Y.-J.; Bohle, R.M.; Nimmesgern, A.; von Muller, L.; Kempf, V.A.J.; et al. Lymphocyte predominant cells detect Moraxella catarrhalis-derived antigens in nodular lymphocyte predominant Hodgkin lymphoma. Nat. Commun. 2020, 11, 2465. [Google Scholar] [CrossRef]
- Al-Mansour, M.; Connors, J.M.; Gasciyne, R.D.; Skinner, B.; Savage, K.J. Transformation to aggressive lymphoma in nodular lymphocyte-predominant Hodgkin lymphoma. J. Clin. Oncol. 2010, 28, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Paschold, L.; Willscher, E.; Bein, J.; Vornanen, M.; Eichenauer, D.A.; Simnica, D.; Thiele, B.; Wickenhauser, C.; Rosenwald, A.; Bernd, H.W.; et al. Evolutionary clonal trajectories in nodular lymphcyte-predominant Hodglin lymphoma with high risk transformation. Haematologica 2021, 106, 2654–2666. [Google Scholar] [CrossRef]
- Burkitt, D. A sarcoma involving the jaws in African childen. Br. J. Surg. 1958, 46, 218–223. [Google Scholar] [CrossRef]
- Roschewski, M.; Dunleavy, K.; Abramson, J.S.; Powell, B.L.; Link, B.K.; Patel, P.; Bierman, P.J.; Jagadeesh, D.; Mitsuyasu, R.T.; Peace, D.; et al. Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with intreated Burkitt lymphoma. J. Clin. Oncol. 2020, 38, 2519–2529. [Google Scholar] [CrossRef]
- Salzburg, J.; Burkhardt, B.; Zimmermann, M.; Wachowski, O.; Woessmann, W.; Oschlies, I.; Klapper, W.; Wacker, H.-H.; Ludwig, W.-D.; Niggli, F.; et al. Prevalence, clinical pattern and outcome of CNS involvement in childhood and adolescent non-Hodgkin’s lymphoma differ by non-Hodgkin’s lymphoma subtype: A Berlin-Frankfurt-Munster Group Report. J. Clin. Oncol. 2007, 25, 3915–3922. [Google Scholar] [CrossRef]
- Fitzsimmons, L.; Boyce, A.J.; Wei, W.; Chang, C.; Croomo-Cartet, D.; Tierney, R.J.; Herold, M.J.; Bell, A.I.; Strasser, A.; Kelly, G.L.; et al. Coordinated repression of BIM and PUMA by Epstein-Barr virus latent genes maintains the survival of Burkitt lymphoma cells. Cell Death Differ. 2018, 25, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Bellan, C.; Lazzi, S.; Hummel, M.; Palwmmo, N.; de Santi, M.; Amato, T.; Nyagol, J.; Sabattini, E.; Lazure, T.; Pileri, S.A.; et al. Immunoglobulin gene analysis reveals 2 distinct cells of origin for EBV-positive and EBV-negative Burkitt lymphoma. Blood 2005, 106, 1031–1036. [Google Scholar] [CrossRef]
- Grande, B.M.; Gerhard, D.S.; Jiang, A.; Griner, N.B.; Abramson, J.S.; Alexander, T.B.; Allen, H.; Ayers, L.W.; Bathony, J.M.; Bhatia, K.; et al. Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood 2019, 133, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Robbiani, D.; Deroubaix, S.; Feldhahn, N.; Oliveira, T.Y.; Callen, E.; Wang, Q.; Jankovic, M.; Silva, I.T.; Rommel, P.C.; Bosque, D.; et al. Plasmodium infection promotes genomic instability and AID-dependent B cell lymphoma. Cell 2015, 162, 727–737. [Google Scholar] [CrossRef]
- Schmitz, R.; Young, R.M.; Ceribelli, M.; Jhavar, S.; Xiao, W.; Zhang, M.; Wright, G.; Shaffer, A.L.; Hodson, D.J.; Bursa, E.; et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 2012, 290, 116–120. [Google Scholar] [CrossRef]
- Saleh, K.; Michot, J.M.; Camara-Clayette, V.; Vassetsky, Y.; Ribrag, V. Burkitt and Burkitt-like lymphomas: A systematic review. Currr. Oncol. Rep. 2020, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- McBride, P. Case of rapid destruction of the Nose and Face. J. Laryngol. Otol. 1897, xii, 64. [Google Scholar]
- Stewart, J.P. The Hisopathology of Mastoiditis. J. Laryngol. Otol. 1928, 43, 689–712. [Google Scholar] [CrossRef]
- Ng, C.S.; Chan, J.K.C.; Cheng, P.N.M.; Szeto, S.C. Nasal T cell lymphoma associated with hemophagocytic sundrome. Cancer 1986, 58, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S. From the midfacial destructive drama to the unfolding EBV story: A short history of EBV-positive NK-cell and T-cell lymphoproliferative diseases. Pathology 2024, 56, 773–785. [Google Scholar] [CrossRef]
- Ng, C.S.; Chan, J.K.C.; Lo, S.T.H. Expression of natural killer cell markers in non-Hodgkin’s lymphoma. Hum. Pathol. 1987, 18, 1257–1262. [Google Scholar] [CrossRef]
- Tse, E.; Kwong, Y.L. The diagnosis and management of NK/T-cell lymphomas. J. Hematol. Oncol. 2017, 10, 85. [Google Scholar] [CrossRef]
- Harabuchi, Y.; Imai, S.; Wakashima, J.; Hirao, M.; Kataura, A.; Osato, T.; Kon, S. Nasal T-cell lymphoma causally associated with Epstein-Barr virus: Clinicopathologic, phenotypic and genotypic studies. Cancer 1996, 77, 2137–2149. [Google Scholar] [CrossRef]
- Yoon, T.Y.; Lee, H.T.; Chang, S.H. Nasal type T/natural killer cell angiocentric lymphoma. Epstein Barr virus associated and showing clonal T-cell receptor gamma gene rearrangement. Br. J. Dermatol. 1999, 140, 505–508. [Google Scholar]
- Nagata, H.; Konno, A.; Kimura, N.; Zhang, Y.; Kimura, M.; Demachi, A.; Sekine, T.; Yamaoto, K.; Shimizu, N. Characterization of novel natural killer (NK)—Cell and gammadelta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with Epstein-Barr Virus. Blood 2001, 97, 708–713. [Google Scholar] [CrossRef]
- Wang, H.; Fu, B.B.; Gale, R.P.; Liang, Y. NK/T-cell lymphomas. Leukemia 2021, 35, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-N.; Yang, Y.; Zhang, Y.-J.; Huang, H.-Q.; Wang, Y.; He, X.; Zhang, L.-N.; Wu, G.; Qu, B.-L.; Qian, L.-T.; et al. Risk-based, response-adapted therapy for early stage extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: A China Lymphoma Collaborative Group (CLCG) study. Am. J. Hematol. 2020, 95, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yang, D.H.; Kim, J.S.; Kwak, J.Y.; Eoni, H.S.; Hong, D.S.; Won, J.H.; Lee, J.H.; Yoon, D.H.; Cjo, J.; et al. Concurrent chemoraiotherapyfollowed by L-asparaginase-containing chemotherapy, VIDL, for localised nasal extranodal NK/T cell lymphoma, phase II study. Ann. Hematol. 2014, 93, 1895–1901. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.; Liu, P.; Zhou, S.; Gui, L.; He, X.; Qin, Y.; Zhang, C.; Yang, S.; Xing, P.; et al. Intensity-modulated radiation therapy followed by GDP chemotherapy for newly diagnosed Stage I/II extranodal natural killer/T cell lymphoma, naal type. Ann. Hematol. 2017, 96, 1477–1483. [Google Scholar] [CrossRef]
- Kim, H.J.; Ock, C.Y.; Kim, T.M.; Lee, S.H.; Lee, J.-Y.; Jung, S.H.; Cho, Y.S.; Kim, M.; Keam, B.; Kim, D.-W.; et al. Comparison of native Escheria Coli L-asparaginase versus pegylated asparaginase in combination with ifosfamide, methotrexate, ectoposide and prednisolone (IMEP), I extranodal NK/T cell lymphoma, nasal type (NTCL). Cancer Res. Treat. 2018, 50, 670–680. [Google Scholar] [CrossRef]
- Suzuki, R. Pathogenesis and treatment of extranodal natural killer/T-cell lymphoma. Semin. Hematol. 2014, 51, 42–51. [Google Scholar] [CrossRef]
- Kwong, Y.L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.-M.; Mow, B.; Khong, P.-L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing L-asparaginase. Blood 2017, 129, 2437–2442. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-G.; Kim, N.; Sohn, H.-J.; Lee, S.K.; Oh, S.T.; Lee, H.-J.; Cho, H.-I.; Yim, H.W.; Jung, S.E.; Park, G.; et al. Long-term outcome of extranodal NK/T cell lymphoma patients treated with postremission therapy using EBV LMP1 and LMP2a-specific CTLs. Mod. Ther. 2015, 23, 1501–1509. [Google Scholar] [CrossRef]
- Steffen, C. The man behind the eponym dermatology in historical perspective: Albert Sezary and the Sezary syndrome. Am. J. Dermatopathol. 2006, 28, 357–367. [Google Scholar] [CrossRef]
- Jones, L.; Degasperi, A.; Grandi, V.; Amarante, T.D.; Genomics England Research Consortium; Mitchell, T.J.; Nik-Zainal, S.; Whittaker, J.S. Spectrum of mutational signatures in T-cell lymphoma reveals a key role for UV radiation in cutaneous T-cell lymphoma. Sci. Rep. 2021, 11, 3962. [Google Scholar] [CrossRef]
- Park, J.; Daniels, J.; Wartewig, T.; Ringbloom, K.G.; Martinez-Escala, M.E.; Choi, S.; Thomas, J.J.; Doukas, P.G.; Yang, J.; Snowden, C.; et al. Integrated genomic analysis of cutaneous T-cell lymphomas reveals the molecular basis for disease heterogeneity. Blood 2021, 138, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Almeida, A.C.; Abate, F.; Khiabanian, H.; Martinez-Escala, E.; Guitart, J.; Jensen, C.P.; Vermeer, M.H.; Rabadan, R.; Fernando, A.; Palomero, T. The mutational landscape of cutaneous T cell lymphoma and sezart syndrome. Nat. Genet. 2015, 47, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Williamze, R.; Jaffe, E.S.; Burg, G.; Cerroni, G.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).