Advances and Techniques in Medical Imaging and Minimally Invasive Interventions for Disorders of the Central Conducting and Mesenteric Lymphatic System
Abstract
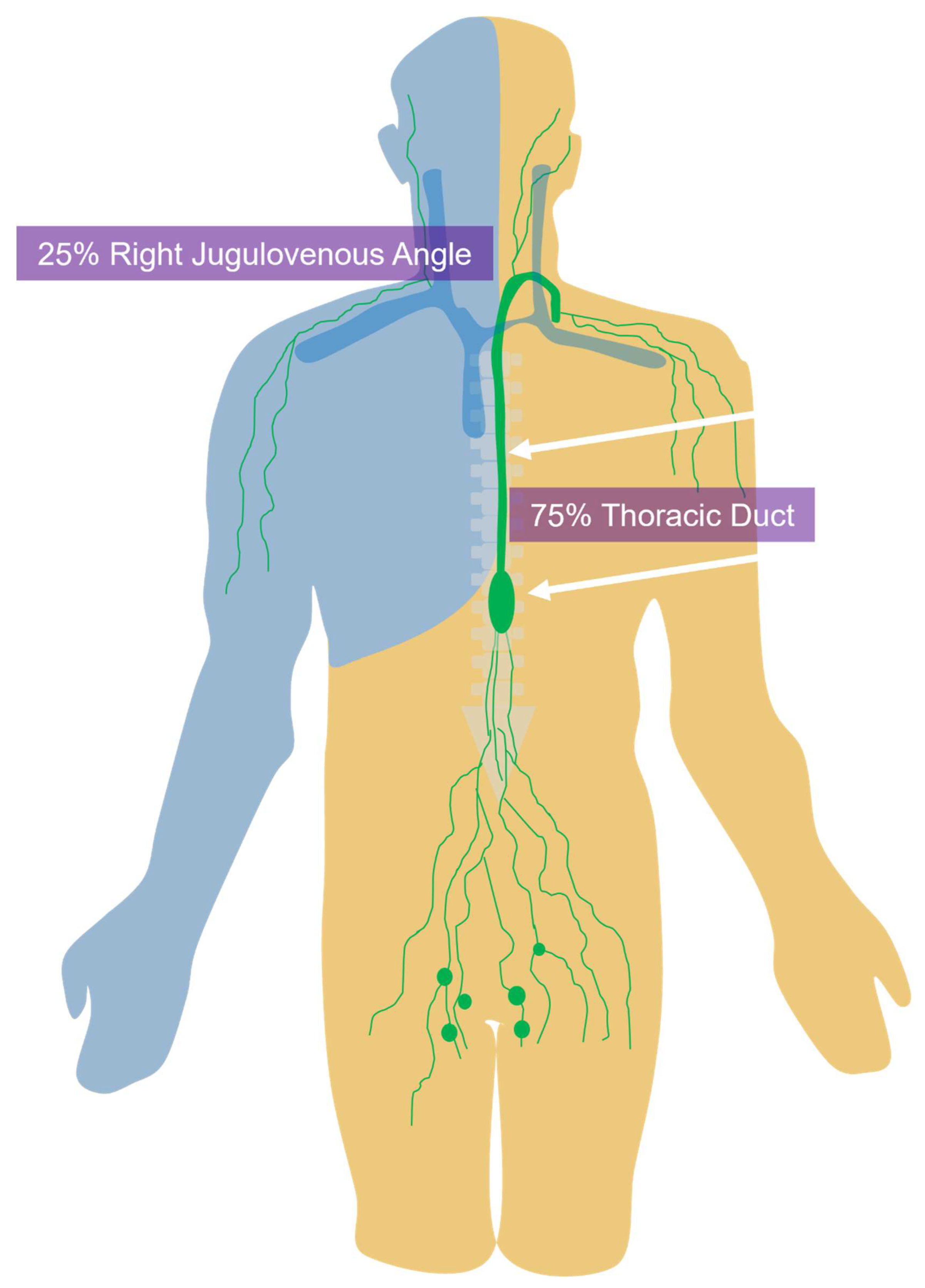
1. Background and Introduction
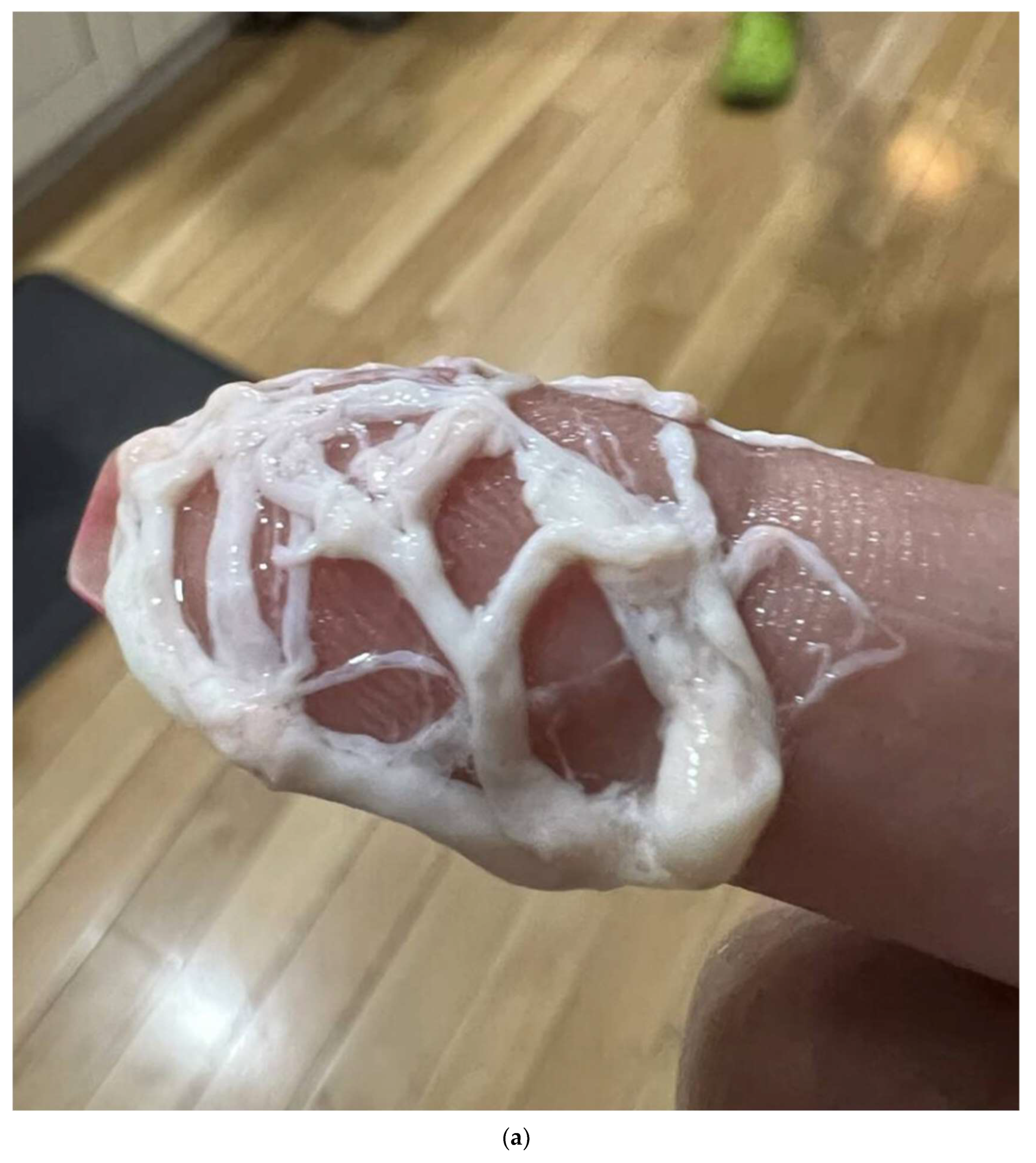
2. Imaging of the Central Conducting Lymphatics
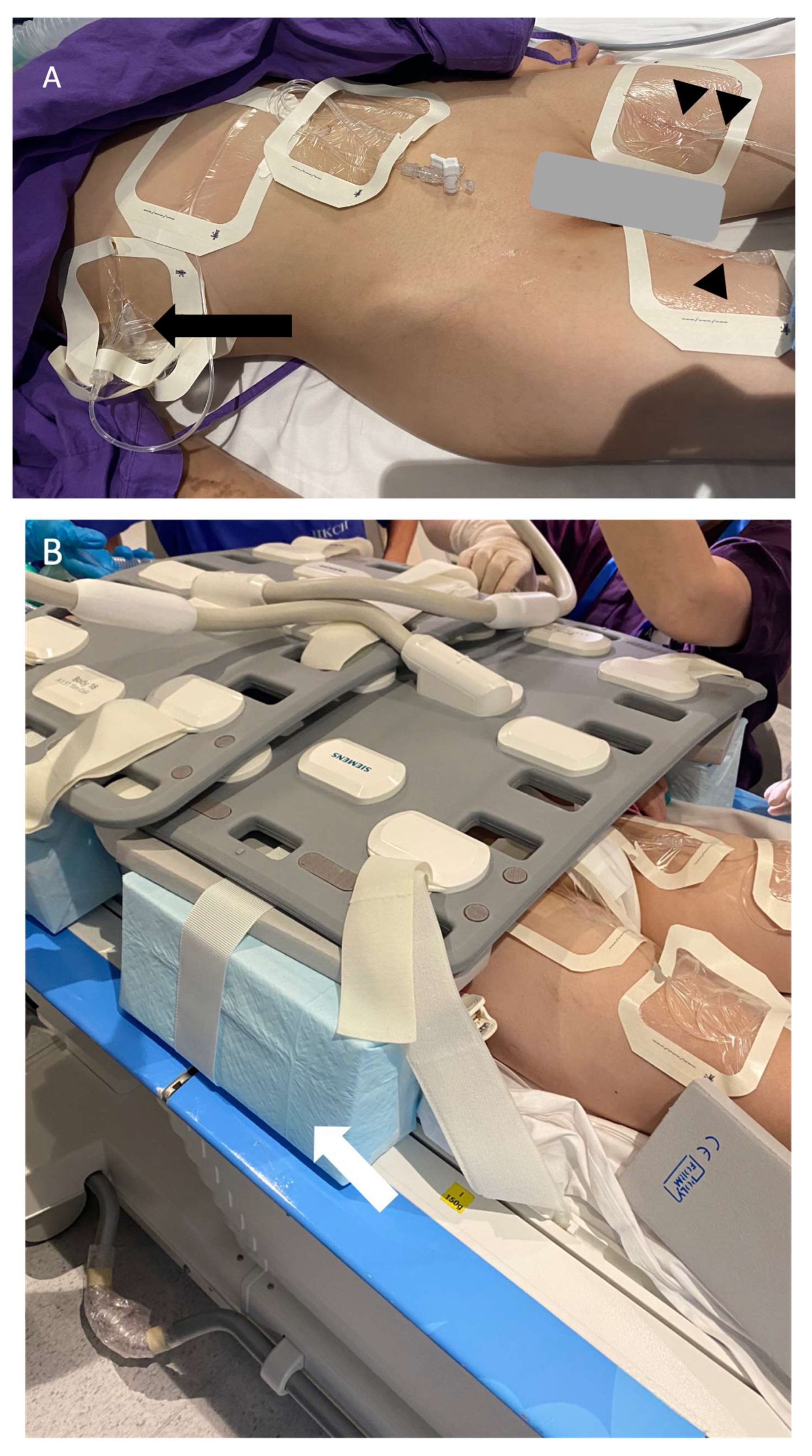
3. Magnetic Resonance Lymphangiography
General Technique
4. Computed Tomography Lymphangiography
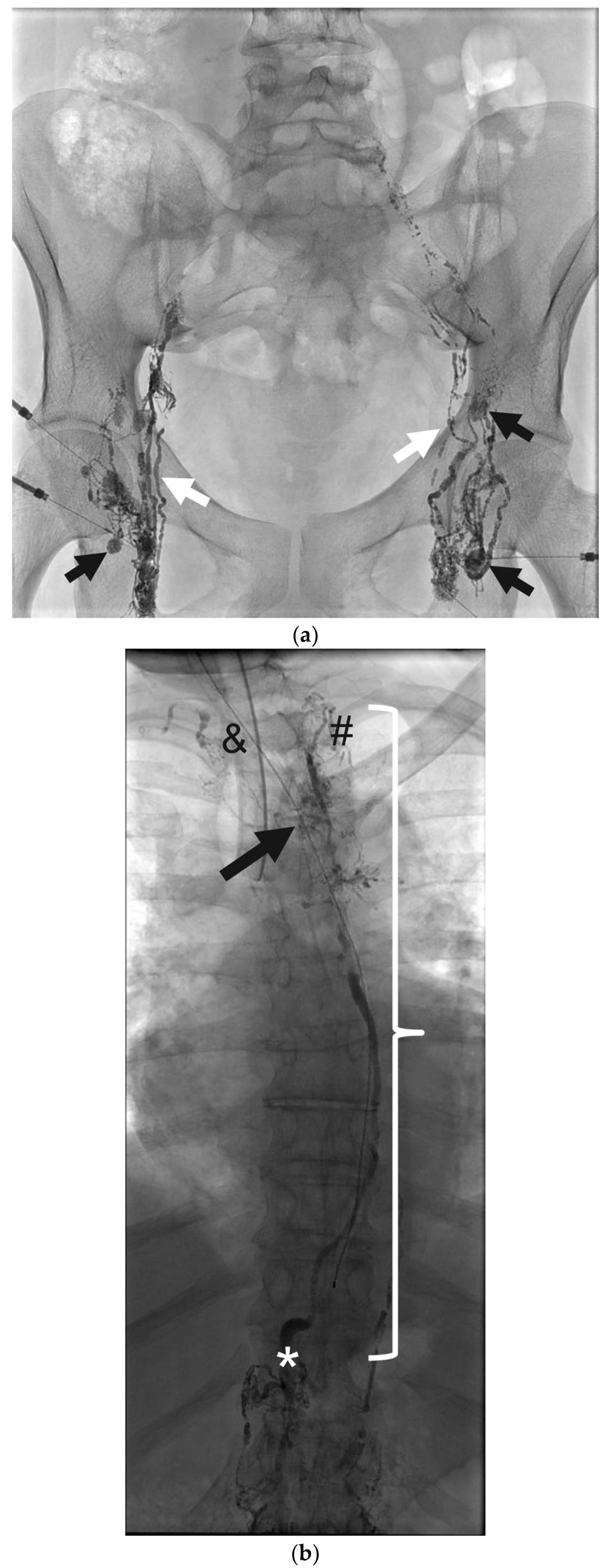
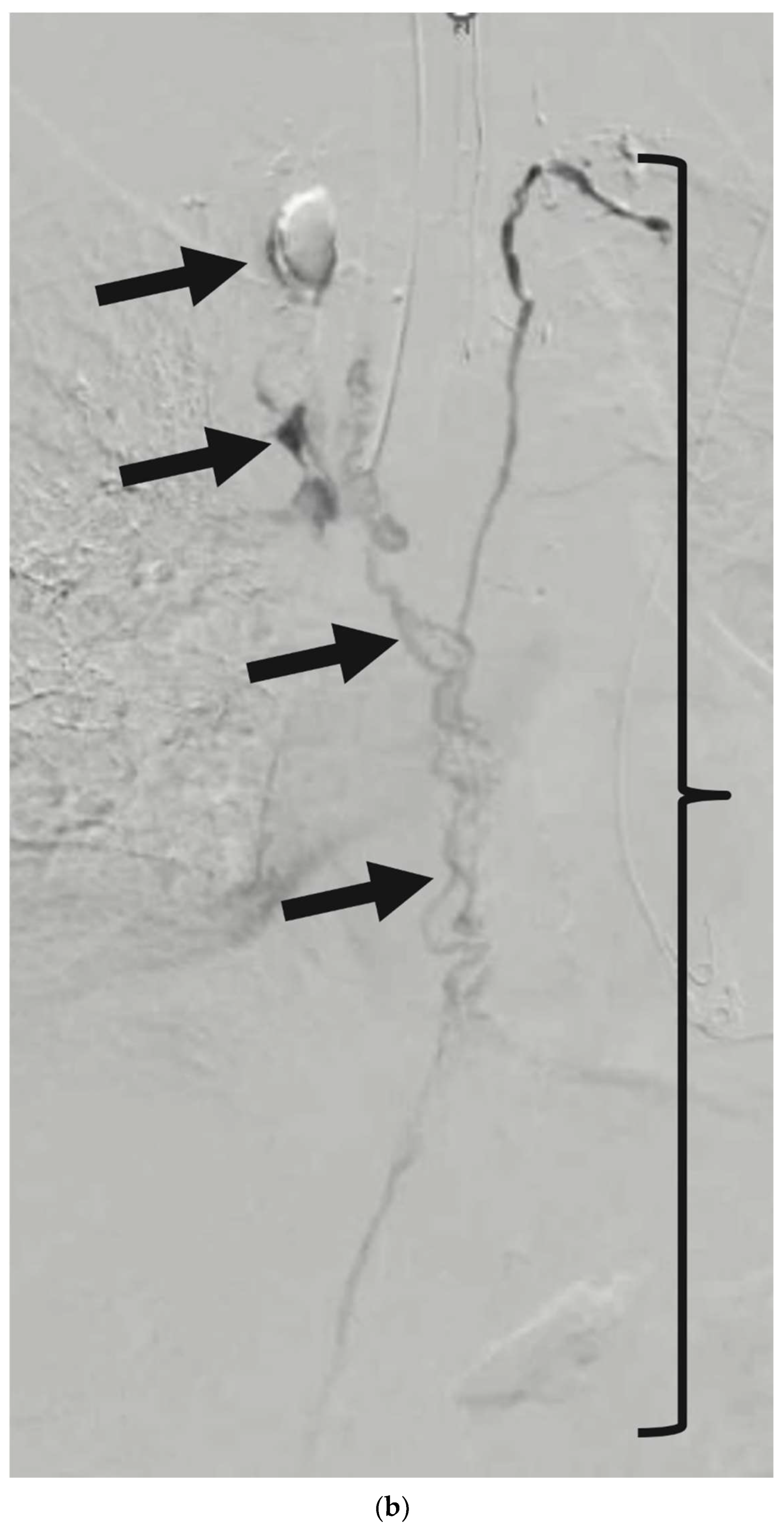
5. Fluoroscopic Lymphangiography
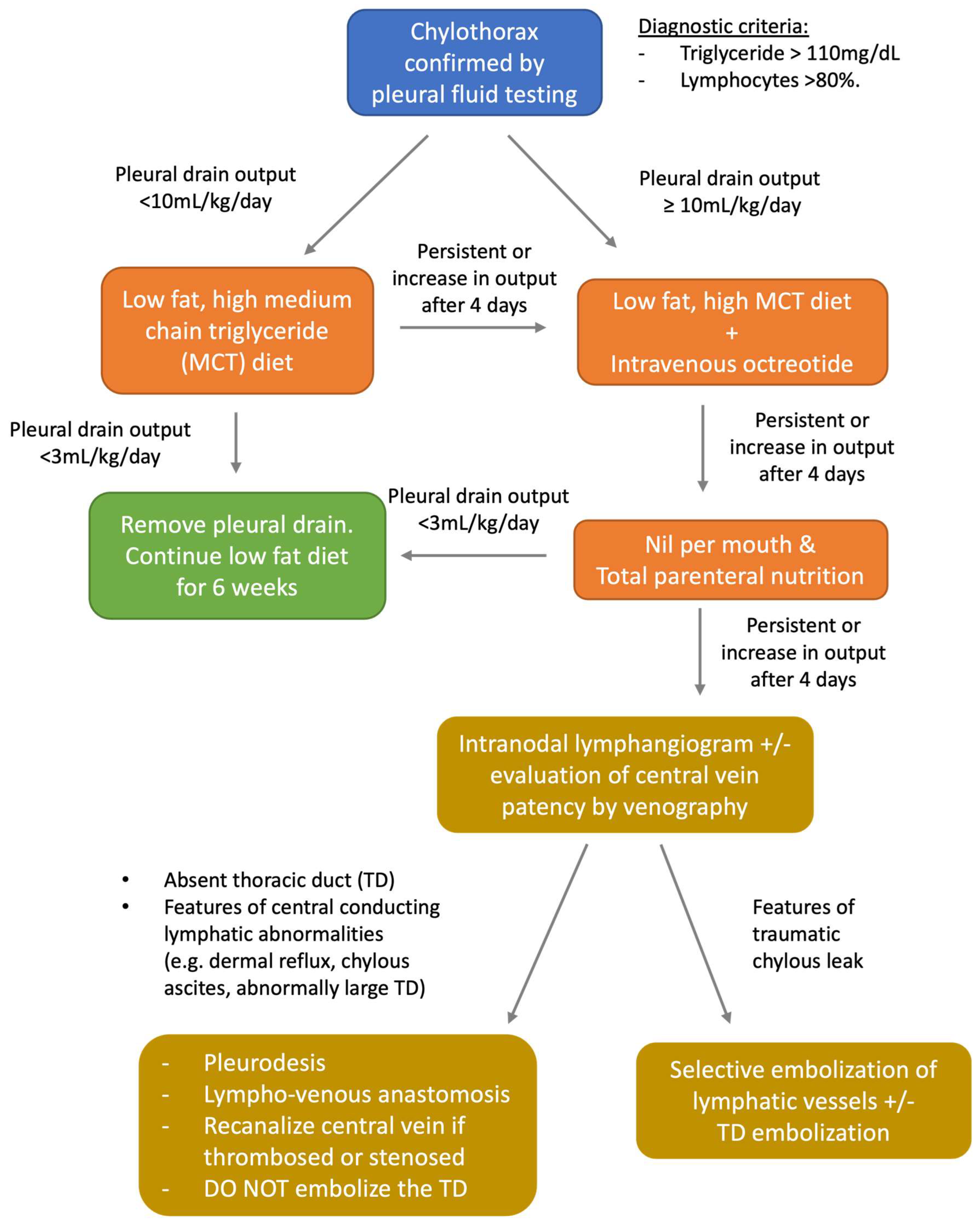
6. Chylothorax
7. Plastic Bronchitis
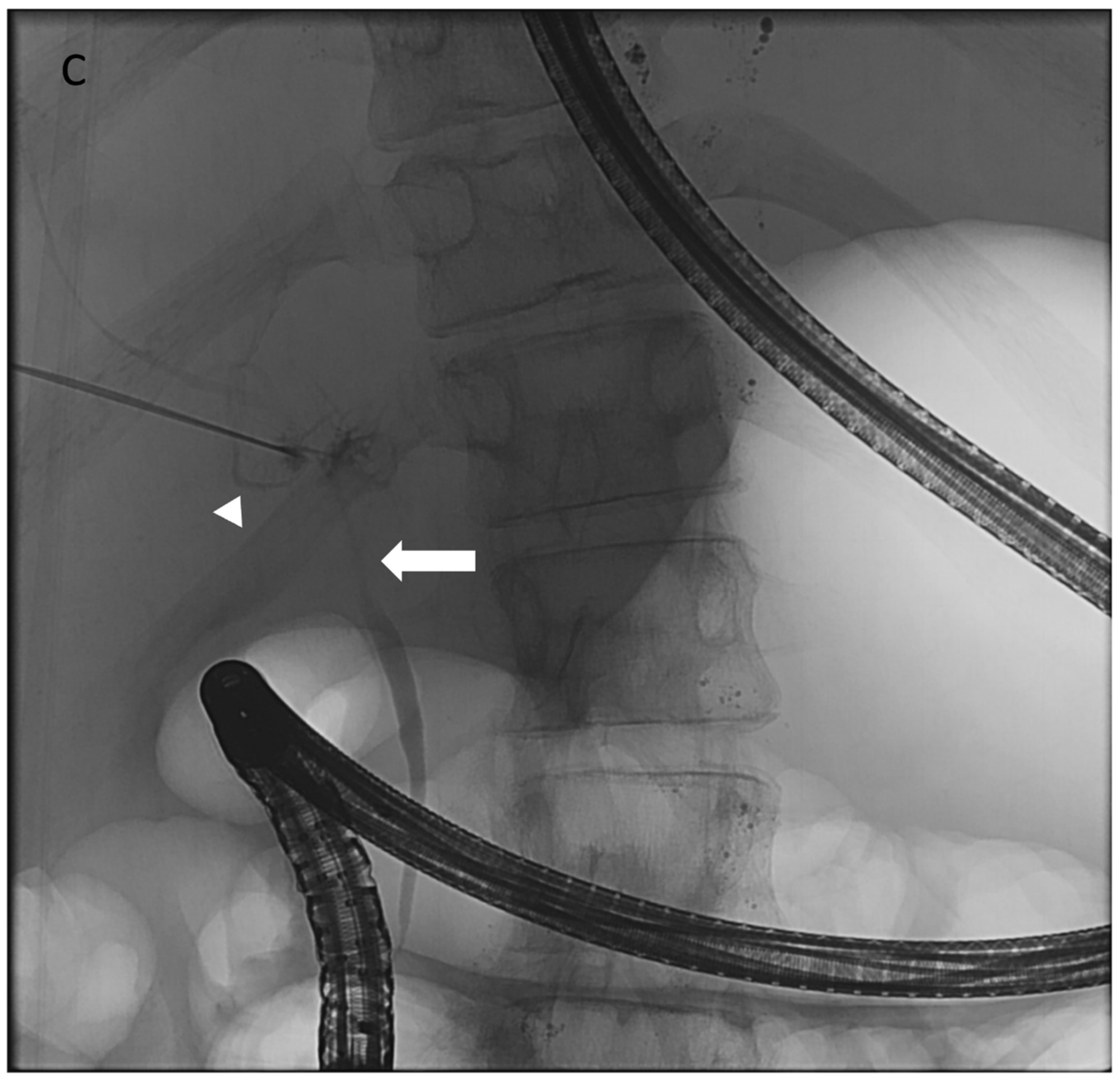
8. Thoracic Duct Embolization (TDE)
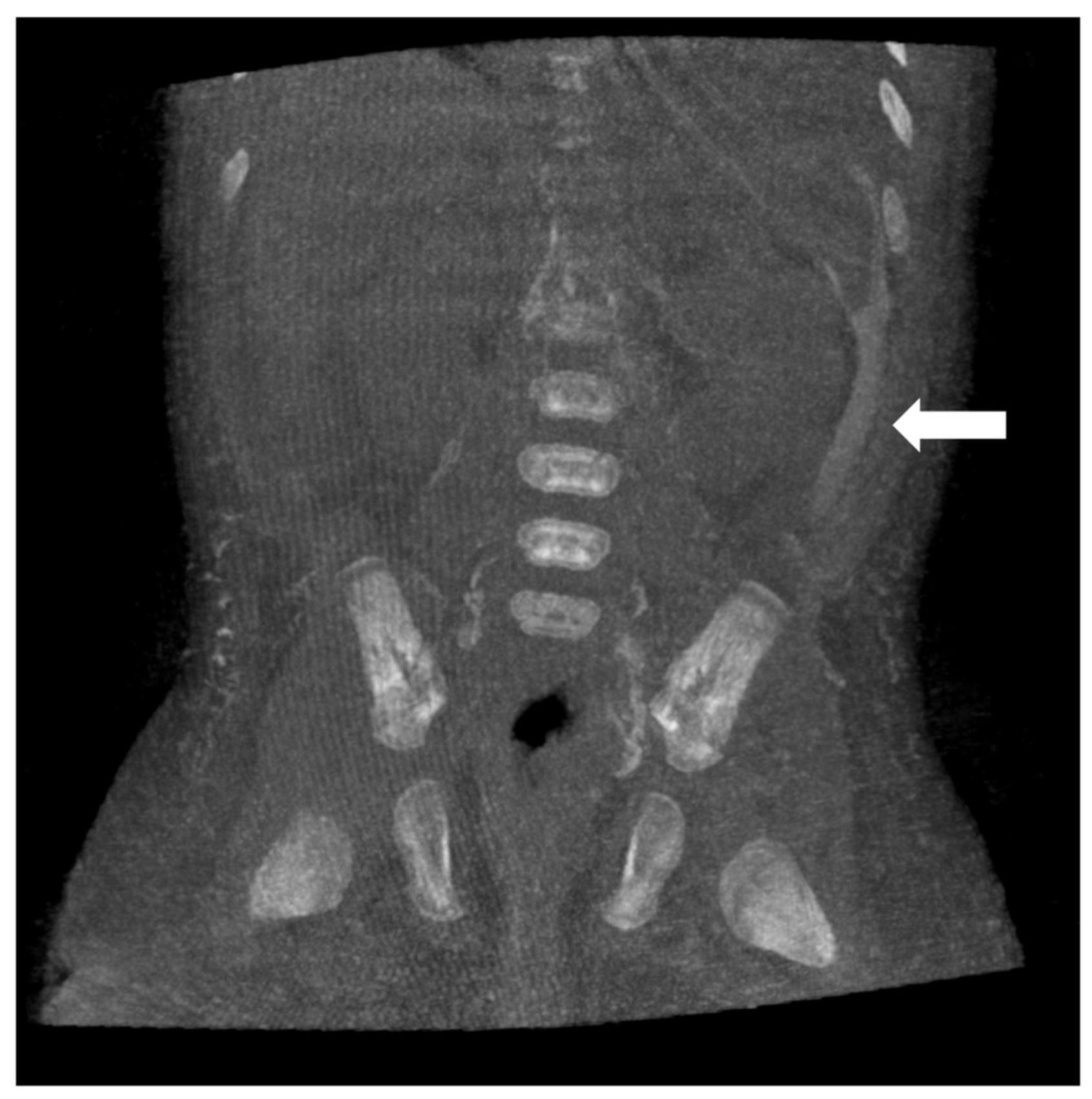
9. Post-Operative Lymphocele
10. Protein-Losing Enteropathy and Chylous Ascites
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goswami, A.K.; Khaja, M.S.; Downing, T.; Kokabi, N.; Saad, W.E.; Majdalany, B.S. Lymphatic Anatomy and Physiology. Semin. Interv. Radiol. 2020, 37, 227–236. [Google Scholar] [CrossRef]
- Jeong, J.; Tanaka, M.; Iwakiri, Y. Hepatic lymphatic vascular system in health and disease. J. Hepatol. 2022, 77, 206–218. [Google Scholar] [CrossRef]
- Tanaka, M.; Iwakiri, Y. The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 733–749. [Google Scholar] [CrossRef]
- Witte, M.H.; Jones, K.; Wilting, J.; Dictor, M.; Selg, M.; McHale, N.; Gershenwald, J.E.; Jackson, D.G. Structure function relationships in the lymphatic system and implications for cancer biology. Cancer Metastasis Rev. 2006, 25, 159–184. [Google Scholar] [CrossRef]
- van der Putte, S.C. The development of the lymphatic system in man. Adv. Anat. Embryol. Cell Biol. 1975, 51, 3–60. [Google Scholar]
- Rockson, S.G. Embryology of the Lymphatic System and Lymphangiogenesis. In Lymphedema: A Concise Compendium of Theory and Practice [Internet]; Lee, B.B., Rockson, S.G., Bergan, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 47–55. [Google Scholar] [CrossRef]
- Oliver, G. Lymphatic vasculature development. Nat. Rev. Immunol. 2004, 4, 35–45. [Google Scholar] [CrossRef]
- Hogan, B.M.; Bos, F.L.; Bussmann, J.; Witte, M.; Chi, N.C.; Duckers, H.J.; Schulte-Merker, S. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 2009, 41, 396–398. [Google Scholar] [CrossRef]
- Bos, F.L.; Caunt, M.; Peterson-Maduro, J.; Planas-Paz, L.; Kowalski, J.; Karpanen, T.; van Impel, A.; Tong, R.; Ernst, J.A.; Korving, J.; et al. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ. Res. 2011, 109, 486–491. [Google Scholar] [CrossRef]
- Wigle, J.T.; Chowdhury, K.; Gruss, P.; Oliver, G. Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 1999, 21, 318–322. [Google Scholar] [CrossRef]
- Yang, Y.; García-Verdugo, J.M.; Soriano-Navarro, M.; Srinivasan, R.S.; Scallan, J.P.; Singh, M.K.; Epstein, J.A.; Oliver, G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 2012, 120, 2340–2348. [Google Scholar] [CrossRef]
- Hägerling, R.; Pollmann, C.; Andreas, M.; Schmidt, C.; Nurmi, H.; Adams, R.H.; Alitalo, K.; Andresen, V.; Schulte-Merker, S.; Kiefer, F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. MBO J. 2013, 32, 629–644. [Google Scholar] [CrossRef]
- François, M.; Short, K.; Secker, G.A.; Combes, A.; Schwarz, Q.; Davidson, T.-L.; Smyth, I.; Hong, Y.-K.; Harvey, N.L.; Koopman, P. Segmental territories along the cardinal veins generate lymph sacs via a ballooning mechanism during embryonic lymphangiogenesis in mice. Dev. Biol. 2011, 364, 89–98. [Google Scholar] [CrossRef]
- Wigle, J.T.; Harvey, N.; Detmar, M.; Lagutina, I.; Grosveld, G.; Gunn, M.D.; Jackson, D.G.; Oliver, G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002, 21, 1505–1513. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, X.; Wu, Z.; Qu, B.; Yuan, M.; Xing, Y.; Song, Y.; Wang, Z. Lymphatic vessel: Origin, heterogeneity, biological functions and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 1–37. [Google Scholar] [CrossRef]
- Yaniv, K.; Isogai, S.; Castranova, D.; Dye, L.; Hitomi, J.; Weinstein, B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006, 12, 711–716. [Google Scholar] [CrossRef]
- Buttler, K.; Kreysing, A.; von Kaisenberg, C.S.; Schweigerer, L.; Gale, N.; Papoutsi, M.; Wilting, J. Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev. Dyn. 2006, 235, 1554–1562. [Google Scholar] [CrossRef]
- Suami, H.; Kato, S. Anatomy of the Lymphatic System and Its Structural Disorders in Lymphoedema. In Lymphedema: A Concise Compendium of Theory and Practice [Internet]; Lee, B.B., Rockson, S.G., Bergan, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 57–78. [Google Scholar] [CrossRef]
- Johnson, O.W.; Chick, J.F.B.; Chauhan, N.R.; Fairchild, A.H.; Fan, C.-M.; Stecker, M.S.; Killoran, T.P.; Suzuki-Han, A. The thoracic duct: Clinical importance, anatomic variation, imaging, and embolization. Eur. Radiol. 2015, 26, 2482–2493. [Google Scholar] [CrossRef]
- Mazzei, F.G.; Gentili, F.; Guerrini, S.; Squitieri, N.C.; Guerrieri, D.; Gennaro, P.; Scialpi, M.; Volterrani, L.; Mazzei, M.A. MR Lymphangiography: A Practical Guide to Perform It and a Brief Review of the Literature from a Technical Point of View. BioMed Res. Int. 2017, 2017, 2598358. [Google Scholar] [CrossRef]
- Calderwood, A.; Thompson, B.; Ho-Shon, K.; Suami, H. Overview of magnetic resonance lymphography for imaging lymphoedema. Plast. Aesthetic Res. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Pieper, C.C. Nodal and Pedal MR Lymphangiography of the Central Lymphatic System: Techniques and Applications. Semin. Interv. Radiol. 2020, 37, 250–262. [Google Scholar] [CrossRef]
- Lee, E.W.; Shin, J.H.; Ko, H.K.; Park, J.; Kim, S.H.; Sung, K.-B. Lymphangiography to Treat Postoperative Lymphatic Leakage: A Technical Review. Korean J. Radiol. 2014, 15, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.F.K.; Chen, H.-S.R.; Ng, W.K.C.; Che, K.Y.J.; Poon, M.Y.; Lun, K.S.; Kan, Y.L.E. Feasibility of contrast-enhanced ultrasound in confirming intranodal needle position for dynamic contrast-enhanced magnetic resonance lymphangiography in children. Pediatr. Radiol. 2023, 53, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Wagenpfeil, J.; Kupczyk, P.A.; Henkel, A.; Geiger, S.; Köster, T.; Luetkens, J.A.; Schild, H.H.; Attenberger, U.I.; Pieper, C.C. Ultrasound-guided needle positioning for nodal dynamic contrast-enhanced MR lymphangiography. Sci. Rep. 2022, 12, 3621. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Suarez, K.I.; Tierradentro-Garcia, L.O.; Stern, J.A.; Dori, Y.; Escobar, F.A.; Otero, H.J.; Rapp, J.B.; Smith, C.L.; Krishnamurthy, G.; Biko, D.M. State-of-the-art imaging for lymphatic evaluation in children. Pediatr. Radiol. 2022, 53, 1380–1390. [Google Scholar] [CrossRef]
- Matsumoto, S.; Mori, H.; Tada, I. Successful Demonstration of Post-Operative Lymphatic Fistula by Percutaneous Transhepatic Lymphography: Case Reports. Clin. Radiol. 2000, 55, 485–486. [Google Scholar] [CrossRef]
- Ramirez-Suarez, K.I.; Tierradentro-Garcia, L.O.; Smith, C.L.; Krishnamurthy, G.; Escobar, F.A.; Otero, H.J.; Rapp, J.B.; Dori, Y.; Biko, D.M. Dynamic contrast-enhanced magnetic resonance lymphangiography. Pediatr. Radiol. 2022, 52, 285–294. [Google Scholar] [CrossRef]
- Biko, D.M.; Smith, C.L.; Otero, H.J.; Saul, D.; White, A.M.; DeWitt, A.; Glatz, A.C.; Piccoli, D.A.; Mamula, P.; Rome, J.J.; et al. Intrahepatic dynamic contrast MR lymphangiography: Initial experience with a new technique for the assessment of liver lymphatics. Eur. Radiol. 2019, 29, 5190–5196. [Google Scholar] [CrossRef]
- Wong, D.; Fung, K.F.K.; Chen, H.-S.R.; Lun, K.S.; Kan, Y.L.E. Intranodal cone-beam computed tomographic lymphangiography with water-soluble iodinated contrast agent for evaluating chylothorax in infants—Preliminary experience at a single institution. Pediatr. Radiol. 2023, 53, 179–183. [Google Scholar] [CrossRef]
- Kirschen, M.P.; Dori, Y.; Itkin, M.; Licht, D.J.; Ichord, R.; Vossough, A. Cerebral Lipiodol Embolism after Lymphatic Embolization for Plastic Bronchitis. J. Pediatr. 2016, 176, 200–203. [Google Scholar] [CrossRef]
- Schwartz, F.R.; James, O.; Kuo, P.H.; Witte, M.H.; Koweek, L.M.; Pabon-Ramos, W.M. Lymphatic Imaging: Current Noninvasive and Invasive Techniques. Semin. Interv. Radiol. 2020, 37, 237–249. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Fisher, A.; Ramsaroop, S.; Carter, A.; Pippard, B. Chylothorax: Pathophysiology, diagnosis, and management—A comprehensive review. J. Thorac. Dis. 2024, 16, 1645–1661. [Google Scholar] [CrossRef] [PubMed]
- Bialkowski, A.; Poets, C.F.; Franz, A.R.; Erhebungseinheit für Seltene Pädiatrische Erkrankungen in Deutschland Study Group. Congenital chylothorax: A prospective nationwide epidemiological study in Germany. Arch. Dis. Child.-Fetal Neonatal Ed. 2015, 100, F169–F172. [Google Scholar] [CrossRef] [PubMed]
- Haines, C.; Walsh, B.; Fletcher, M.; Davis, P.J. Chylothorax development in infants and children in the UK. Arch. Dis. Child. 2014, 99, 724–730. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Chen, H. Risk factors of chylothorax after esophagectomy. J. Thorac. Dis. 2019, 11, 1749–1752. [Google Scholar] [CrossRef]
- Rudrappa, M.; Paul, M. Chylothorax. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK459206/ (accessed on 21 December 2023).
- Ur Rehman, K.; Sivakumar, P. Non-traumatic chylothorax: Diagnostic and therapeutic strategies. Breathe 2022, 18, 210163. [Google Scholar] [CrossRef]
- Dixon, J.B. Mechanisms of chylomicron uptake into lacteals. Ann. N. Y. Acad. Sci. 2010, 1207 (Suppl. 1), E52–E57. [Google Scholar] [CrossRef]
- Hussain, M.M. Intestinal Lipid Absorption and Lipoprotein Formation. Curr. Opin. Infect. Dis. 2014, 25, 200–206. [Google Scholar] [CrossRef]
- Huggins, J.T. Chylothorax and cholesterol pleural effusion. Semin. Respir. Crit. Care Med. 2010, 31, 743–750. [Google Scholar] [CrossRef]
- Prajapati, S.; Ramasamy, S.; Vats, M.; Neogi, S.; Kantamaneni, K.; Tudu, S.K. Effect of Octreotide on Lymphorrhea in Patients After Modified Radical Mastectomy for Carcinoma Breast: A Randomized Controlled Trial. Cureus 2021, 13, e19225. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, R.; Wang, J.; Yuan, L.; Hu, L.; Jiang, S.; Chen, C.; Cao, Y. Effects of somatostatin/octreotide treatment in neonates with congenital chylothorax. Medicine 2017, 96, e7594. [Google Scholar] [CrossRef]
- Ntiamoah, P.; Mukhopadhyay, S.; Ghosh, S.; Mehta, A.C. Recycling plastic: Diagnosis and management of plastic bronchitis among adults. Eur. Respir. Rev. 2021, 30, 210096. [Google Scholar] [CrossRef] [PubMed]
- Quysner, A.; Surani, S.; Roberts, D. Plastic Bronchitis. West. J. Emerg. Med. 2011, 12, 118–119. [Google Scholar] [PubMed]
- Nayar, S.; Parmar, R.; Kulkarni, S.; Cherian, K.M. Treatment of Plastic Bronchitis. Ann. Thorac. Surg. 2007, 83, 1884–1886. [Google Scholar] [CrossRef]
- Muller, A.F.; Mithal, A. A case of plastic bronchitis. J. R. Soc. Med. 1988, 81, 360. [Google Scholar] [CrossRef]
- Patel, N.; Patel, M.; Inja, R.; Krvavac, A.; Lechner, A.J. Plastic Bronchitis in Adult and Pediatric Patients: A Review of its Presentation, Diagnosis, and Treatment. Mo. Med. 2021, 118, 363–373. [Google Scholar]
- Dori, Y.; Keller, M.S.; Rome, J.J.; Gillespie, M.J.; Glatz, A.C.; Dodds, K.; Goldberg, D.J.; Goldfarb, S.; Rychik, J.; Itkin, M. Percutaneous Lymphatic Embolization of Abnormal Pulmonary Lymphatic Flow as Treatment of Plastic Bronchitis in Patients With Congenital Heart Disease. Circulation 2016, 133, 1160–1170. [Google Scholar] [CrossRef]
- Chen, E.; Itkin, M. Thoracic Duct Embolization for Chylous Leaks. Semin. Interv. Radiol. 2011, 28, 63–74. [Google Scholar] [CrossRef]
- Nadolski, G.J.; Itkin, M. Lymphangiography and thoracic duct embolization following unsuccessful thoracic duct ligation: Imaging findings and outcomes. J. Thorac. Cardiovasc. Surg. 2018, 156, 838–843. [Google Scholar] [CrossRef]
- Cope, C.; Salem, R.; Kaiser, L.R. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: Prospective trial. J. Vasc. Interv. Radiol. 1999, 10, 1248–1254. [Google Scholar] [CrossRef]
- Cope, C.; Kaiser, L.R. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J. Vasc. Interv. Radiol. 2002, 13, 1139–1148. [Google Scholar] [CrossRef]
- Itkin, M.; Kucharczuk, J.C.; Kwak, A.; Trerotola, S.O.; Kaiser, L.R. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: Experience in 109 patients. J. Thorac. Cardiovasc. Surg. 2010, 139, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Tsauo, J.; Shin, J.H. Lymphatic Interventions for Chylothorax: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2018, 29, 194–202.e4. [Google Scholar] [CrossRef] [PubMed]
- Majdalany, B.S.; Saad, W.A.; Chick, J.F.B.; Khaja, M.S.; Cooper, K.J.; Srinivasa, R.N. Pediatric lymphangiography, thoracic duct embolization and thoracic duct disruption: A single-institution experience in 11 children with chylothorax. Pediatr. Radiol. 2017, 48, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Savla, J.J.; Itkin, M.; Rossano, J.W.; Dori, Y. Post-Operative Chylothorax in Patients with Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 2410–2422. [Google Scholar] [CrossRef]
- Laslett, D.; Trerotola, S.O.; Itkin, M. Delayed complications following technically successful thoracic duct embolization. J. Vasc. Interv. Radiol. JVIR 2012, 23, 76–79. [Google Scholar] [CrossRef]
- Nerli, R.B.; Sushant, D.; Ghagane, S.C.; Nutalpati, S.; Mohan, S.; Dixit, N.S.; Neelagund, S.; Hiremath, M.B. Lymphocele Complications Following Renal Transplantation. Indian J. Transplant. 2019, 13, 273–276. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef]
- Scheer, J.K.; Haddad, A.F.; Chan, A.K.; Eichler, C.M.; Tay, B.; Burch, S.; Chou, D.; Ames, C.P.; Mummaneni, P.V. Lymphocele after anterior lumbar interbody fusion: A review of 1322 patients. J. Neurosurg. Spine 2021, 35, 722–728. [Google Scholar] [CrossRef]
- Guacheta-Bomba, P.L.; Guerrero, M.F.S.; Ramirez, G.; Garcia-Perdomo, H.A. Lymphocele Complication After Kidney Transplant: Current Literature Review and Management Algorithm. Exp. Clin. Transplant. 2023, 21, 855–859. [Google Scholar]
- Tsaur, I.; Thomas, C. Risk factors, complications and management of lymphocele formation after radical prostatectomy: A mini-review. Int. J. Urol. 2019, 26, 711–716. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.W.; Kim, S.H.; Kim, Y.T.; Kim, J.H. An Analysis of the Risk Factors and Management of Lymphocele after Pelvic Lymphadenectomy in Patients with Gynecologic Malignancies. Cancer Res. Treat. 2004, 36, 377–383. [Google Scholar] [CrossRef] [PubMed]
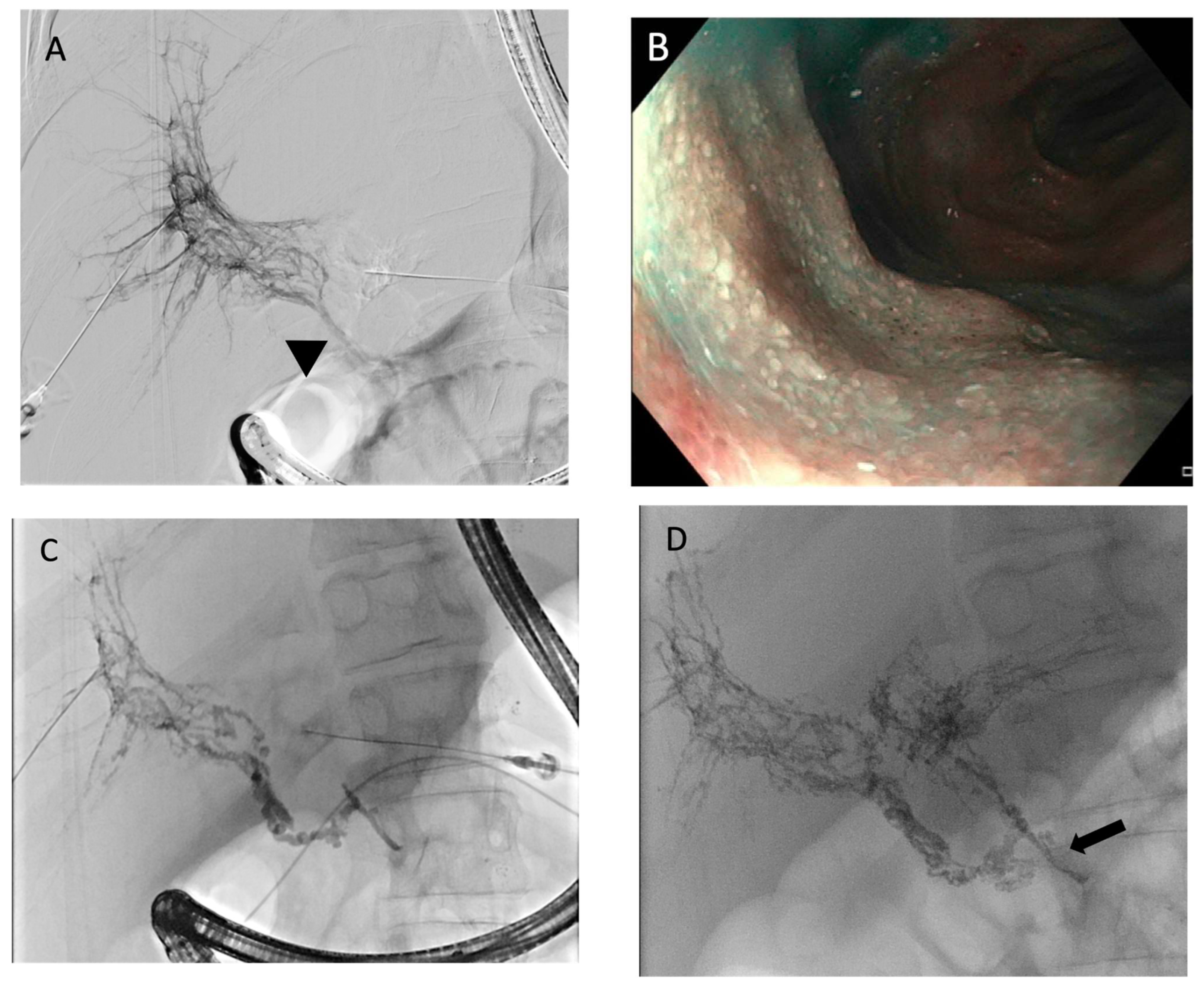
- Smolock, A.R.; Nadolski, G.; Itkin, M. Intranodal Glue Embolization for the Management of Postsurgical Groin Lymphocele and Lymphorrhea. J. Vasc. Interv. Radiol. 2018, 29, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Kwon, L.M.; Hur, S.; Jeong, C.W.; Jae, H.J.; Chung, J.W. Glue Embolization of Lymphopseudoaneurysm for Chylous Ascites after Retroperitoneal Surgery. Korean J. Radiol. 2021, 22, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Won, J.H.; Chang, S.-J.; Ryu, H.-S.; Song, S.-Y.; Yim, B.; Kim, J. Lymphatic Embolization for the Treatment of Pelvic Lymphoceles: Preliminary Experience in Five Patients. J. Vasc. Interv. Radiol. 2016, 27, 1170–1176. [Google Scholar] [CrossRef]
- Bertino, F.J.; Hawkins, C.M. Contemporary management of extracranial vascular malformations. Pediatr. Radiol. 2023, 53, 1600–1617. [Google Scholar] [CrossRef]
- Hawkins, C.M.; Chewning, R.H. Diagnosis and Management of Extracranial Vascular Malformations in Children: Arteriovenous Malformations, Venous Malformations, and Lymphatic Malformations. Semin Roentgenol [Internet]. 2019. Available online: http://www.sciencedirect.com/science/article/pii/S0037198X19300410 (accessed on 28 July 2019).
- Mahrer, A.; Ramchandani, P.; Trerotola, S.O.; Shlansky-Goldberg, R.D.; Itkin, M. Sclerotherapy in the management of postoperative lymphocele. J. Vasc. Interv. Radiol. 2010, 21, 1050–1053. [Google Scholar] [CrossRef]
- Kim, Y.; Jeon, G.S.; Choi, S.Y.; Kim, M.D.; Lee, S.J. Evaluation of sclerotherapy for the treatment of infected postoperative lymphocele. Taiwan J. Obstet. Gynecol. 2017, 56, 477–481. [Google Scholar] [CrossRef]
- Pieper, C.C.; Wagenpfeil, J.; Henkel, A.; Geiger, S.; Köster, T.; Hoss, K.; Luetkens, J.A.; Hart, C.; Attenberger, U.I.; Müller, A. MR lymphangiography of lymphatic abnormalities in children and adults with Noonan syndrome. Sci. Rep. 2022, 12, 11164. [Google Scholar] [CrossRef]
- Maleux, G.; Storme, E.; Cools, B.; Heying, R.; Boshoff, D.; Louw, J.J.; Frerich, S.; Malekzadeh-Milanii, S.; Hubrechts, J.; Brown, S.C.; et al. Percutaneous embolization of lymphatic fistulae as treatment for protein-losing enteropathy and plastic bronchitis in patients with failing Fontan circulation. Catheter. Cardiovasc. Interv. 2019, 94, 996–1002. [Google Scholar] [CrossRef]
- Mertens, L.; Hagler, D.J.; Sauer, U.; Somerville, J.; Gewillig, M. Protein-losing enteropathy after the Fontan operation: An international multicenter study. J. Thorac. Cardiovasc. Surg. 1998, 115, 1063–1073. [Google Scholar] [CrossRef]
- Alsaied, T.; Lubert, A.M.; Goldberg, D.J.; Schumacher, K.; Rathod, R.; Katz, D.A.; Opotowsky, A.R.; Jenkins, M.; Smith, C.; Rychik, J.; et al. Protein losing enteropathy after the Fontan operation. Int. J. Cardiol. Congenit. Hear. Dis. 2022, 7, 100338. [Google Scholar] [CrossRef] [PubMed]
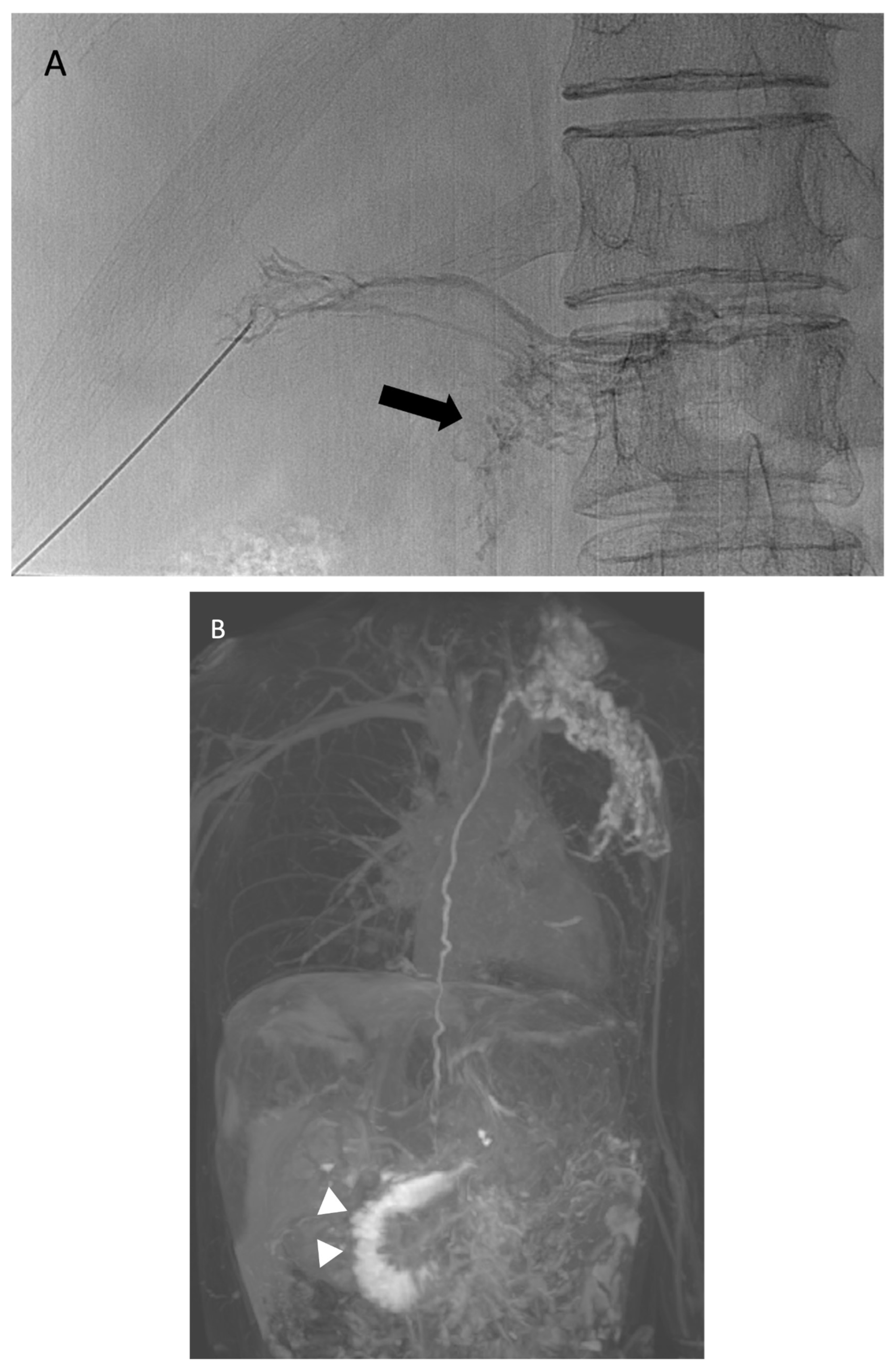
- Lemley, B.A.; Biko, D.M.; Dewitt, A.G.; Glatz, A.C.; Goldberg, D.J.; Saravanan, M.; O’byrne, M.L.; Pinto, E.; Ravishankar, C.; Rome, J.J.; et al. Intrahepatic Dynamic Contrast-Enhanced Magnetic Resonance Lymphangiography: Potential Imaging Signature for Protein-Losing Enteropathy in Congenital Heart Disease. J. Am. Heart Assoc. 2021, 10, e021542. [Google Scholar] [CrossRef] [PubMed]
- Itkin, M.; Piccoli, D.A.; Nadolski, G.; Rychik, J.; DeWitt, A.; Pinto, E.; Rome, J.; Dori, Y. Protein-Losing Enteropathy in Patients with Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Krishnamurthy, G.; Srinivasan, A.; Dori, Y. Lymphatic interventions in congenital heart disease. Semin. Pediatr. Surg. 2024, 33, 151419. [Google Scholar] [CrossRef]
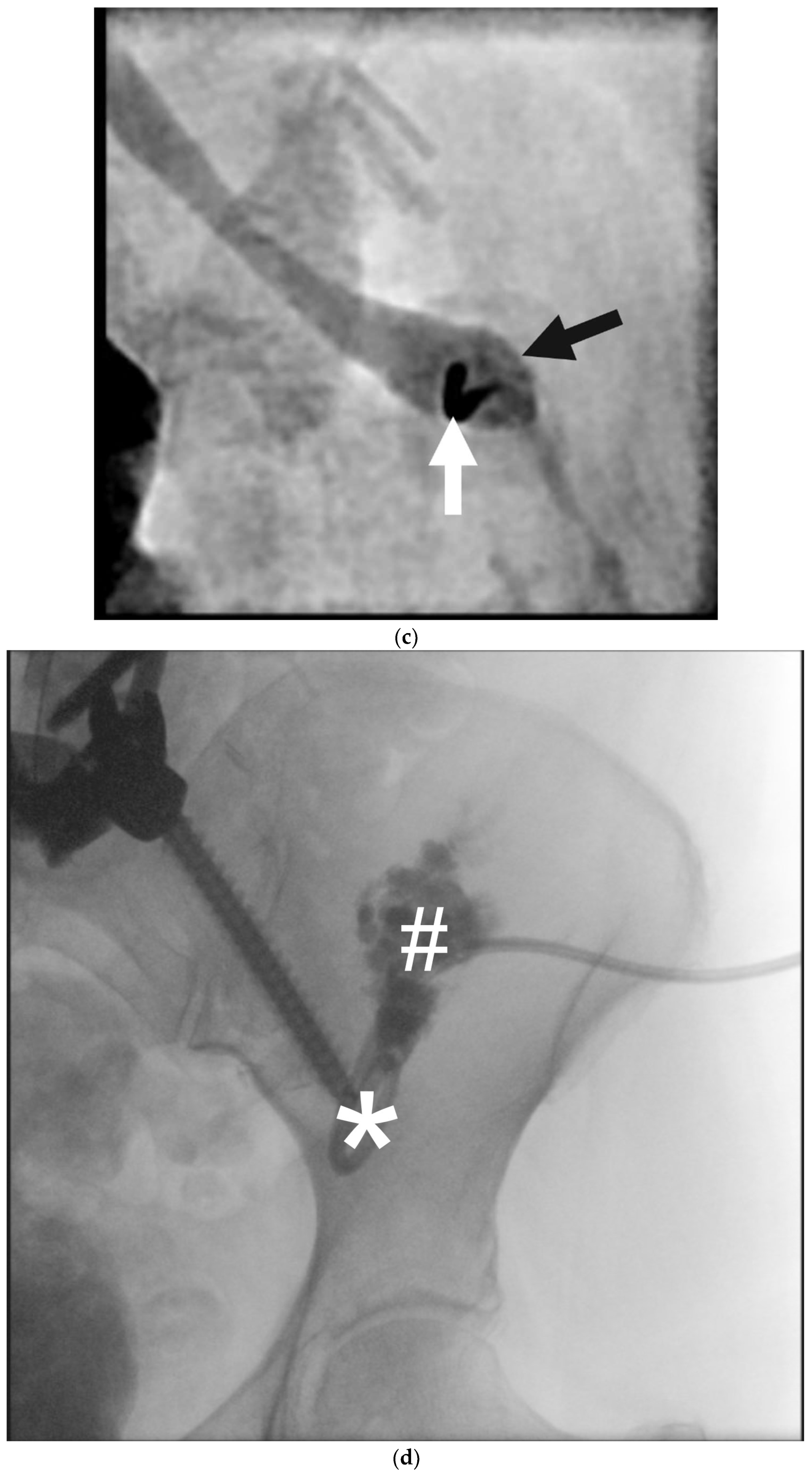
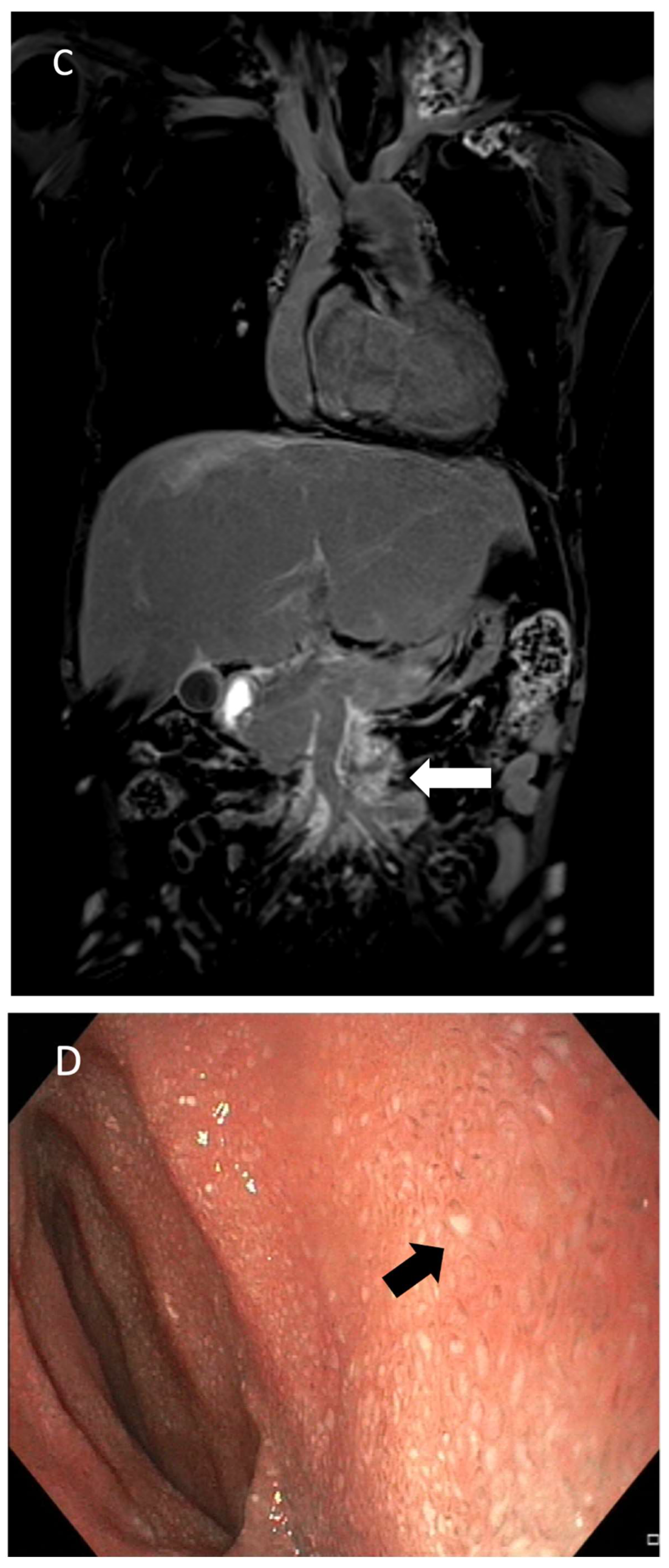
- Gartenberg, A.J.; Krishnamurthy, G.; Srinivasan, A.; Escobar, F.A.; Brownell, J.N.; Mamula, P.; O’byrne, M.L.; Dori, Y.; Smith, C.L. Intrahepatic and Periduodenal Embolization for Protein-Losing Enteropathy Patients with Congenital Heart Disease. J. Am. Coll. Cardiol. 2023, 81, 2476–2478. [Google Scholar] [CrossRef]
- Itkin, M.; Teeple, E.A.; Spurrier, E.L.; Srivastava, S.; Rabinowitz, D.A. Transendoscopic retrograde hepatoduodenal lymphatic embolization as a treatment for protein losing enteropathy in patients with congenital heart disease. J. Am. Coll. Cardiol. 2022, 79 (Suppl. 9), 1384. [Google Scholar] [CrossRef]
- Husnain, A.; Aadam, A.A.; Reiland, A.; Salem, R.; Baker, J.; Nemcek, A.A.; Green, J.; Ganger, D.; De Freitas, R.A.; Riaz, A. Combined Percutaneous Transhepatic Lymphatic Embolization and Peroral Duodenal Mucosal Radiofrequency Ablation to Manage Protein-Losing Enteropathy. J. Vasc. Interv. Radiol. 2024, 35, 1351–1356.e1. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertino, F.J.; Fung, K.F.K. Advances and Techniques in Medical Imaging and Minimally Invasive Interventions for Disorders of the Central Conducting and Mesenteric Lymphatic System. Lymphatics 2025, 3, 8. https://doi.org/10.3390/lymphatics3010008
Bertino FJ, Fung KFK. Advances and Techniques in Medical Imaging and Minimally Invasive Interventions for Disorders of the Central Conducting and Mesenteric Lymphatic System. Lymphatics. 2025; 3(1):8. https://doi.org/10.3390/lymphatics3010008
Chicago/Turabian StyleBertino, Frederic J., and Kin Fen Kevin Fung. 2025. "Advances and Techniques in Medical Imaging and Minimally Invasive Interventions for Disorders of the Central Conducting and Mesenteric Lymphatic System" Lymphatics 3, no. 1: 8. https://doi.org/10.3390/lymphatics3010008
APA StyleBertino, F. J., & Fung, K. F. K. (2025). Advances and Techniques in Medical Imaging and Minimally Invasive Interventions for Disorders of the Central Conducting and Mesenteric Lymphatic System. Lymphatics, 3(1), 8. https://doi.org/10.3390/lymphatics3010008





