From Mechanisms to Treatment: A Comprehensive View of Lymphatic Metastasis in Cancer
Abstract
1. Introduction
2. Mechanisms of Lymphatic Metastasis
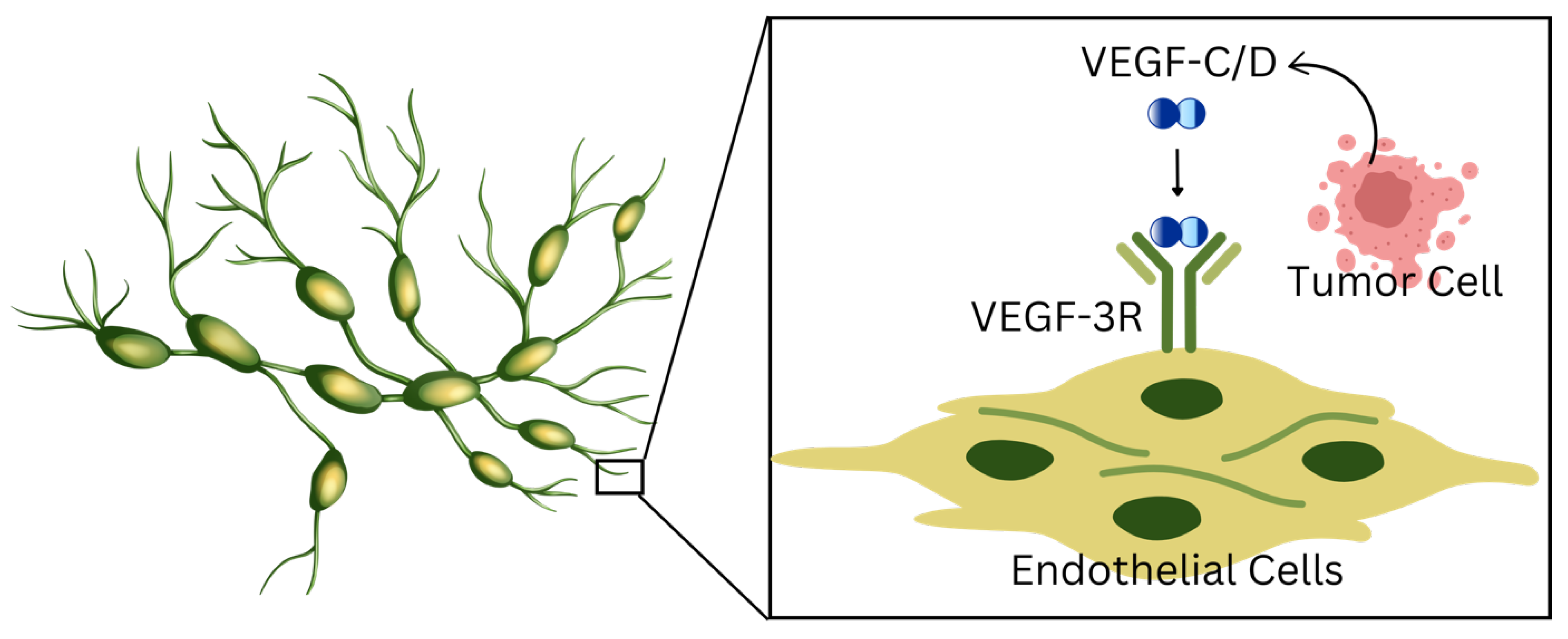
2.1. Role of Lymphangiogenesis
2.2. Tumor Cell Migration and Intravasation into Lymphatics
2.3. Tumor Cell Survival and Colonization in Lymph Nodes
2.4. Tumor Types and the Role of Lymphatic Metastasis
3. Clinical Implications of Lymphatic Metastasis
3.1. Prognostic Value of Lymphatic Involvement in Cancer
3.2. Diagnostic Approaches
3.3. Biomarkers for Lymphatic Spread
4. Therapeutic Strategies Targeting Lymphatic Metastasis
4.1. Surgical Approaches
4.2. Pharmacological Inhibition of Lymphangiogenesis
4.3. Immunotherapy and Targeted Therapies
4.4. Emerging Therapies and Clinical Trials
5. Challenges and Future Directions in Research
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Witte, T.; Dadras, M.; Heck, F.-C.; Heck, M.; Habermalz, B.; Welss, S.; Lehnhardt, M.; Behr, B. Water-jet-assisted liposuction for the treatment of lipedema: Standardized treatment protocol and results of 63 patients. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2020, 73, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhao, X.; Wu, Z.; Qu, B.; Yuan, M.; Xing, Y.; Song, Y.; Wang, Z. Lymphatic vessel: Origin, heterogeneity, biological functions and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Hu, C.; Yang, X.; Liu, Y.; Ji, G.; Ge, S.; Wang, X.; Wang, M. Lymph node metastasis in cancer progression: Molecular mechanisms, clinical significance and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 367. [Google Scholar] [CrossRef]
- Carr, I.; Pettigrew, N.; Weinerman, B. Lymphatic metastasis and its treatment. Cancer Treat. Rev. 1987, 14, 53–64. [Google Scholar] [CrossRef]
- Sleeman, J.P.; Thiele, W. Tumor metastasis and the lymphatic vasculature. Int. J. Cancer 2009, 125, 2747–2756. [Google Scholar] [CrossRef]
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef]
- Duff, S.E.; Li, C.; Jeziorska, M.; Kumar, S.; Saunders, M.P.; Sherlock, D.; O’Dwyer, S.T.; Jayson, G.C. Vascular endothelial growth factors C and D and lymphangiogenesis in gastrointestinal tract malignancy. Br. J. Cancer 2003, 89, 426–430. [Google Scholar] [CrossRef]
- Mohammed, R.A.A.; Green, A.; El-Shikh, S.; Paish, E.C.; Ellis, I.O.; Martin, S.G. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br. J. Cancer 2007, 96, 1092–1100. [Google Scholar] [CrossRef]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- He, Y.; Rajantie, I.; Pajusola, K.; Jeltsch, M.; Holopainen, T.; Yla-Herttuala, S.; Harding, T.; Jooss, K.; Takahashi, T.; Alitalo, K. Vascular Endothelial Cell Growth Factor Receptor 3–Mediated Activation of Lymphatic Endothelium Is Crucial for Tumor Cell Entry and Spread via Lymphatic Vessels. Cancer Res. 2005, 65, 4739–4746. [Google Scholar] [CrossRef]
- TNM Classification of Malignant Tumours|UICC. Available online: https://www.uicc.org/what-we-do/sharing-knowledge/tnm (accessed on 1 December 2024).
- Davydova, N.; Harris, N.C.; Roufail, S.; Paquet-Fifield, S.; Ishaq, M.; Streltsov, V.A.; Williams, S.P.; Karnezis, T.; Stacker, S.A.; Achen, M.G. Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D*. J. Biol. Chem. 2016, 291, 27265–27278. [Google Scholar] [CrossRef] [PubMed]
- Veikkola, T.; Jussila, L.; Makinen, T.; Karpanen, T.; Jeltsch, M.; Petrova, T.V.; Kubo, H.; Thurston, G.; McDonald, D.M.; Achen, M.G.; et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001, 20, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, S.; Brown, L.F.; Kodama, S.; Paavonen, K.; Alitalo, K.; Detmar, M. VEGF-C–induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007, 109, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, T.; Shayan, R.; Caesar, C.; Roufail, S.; Harris, N.C.; Ardipradja, K.; Zhang, Y.F.; Williams, S.P.; Farnsworth, R.H.; Chai, M.G.; et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 2012, 21, 181–195. [Google Scholar] [CrossRef]
- Karpanen, T.; Heckman, C.A.; Keskitalo, S.; Jeltsch, M.; Ollila, H.; Neufeld, G.; Tamagnone, L.; Alitalo, K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 2006, 20, 1462–1472. [Google Scholar] [CrossRef]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Chiang, S.P.H.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef]
- Shields, J.D.; Fleury, M.E.; Yong, C.; Tomei, A.A.; Randolph, G.J.; Swartz, M.A. Autologous Chemotaxis as a Mechanism of Tumor Cell Homing to Lymphatics via Interstitial Flow and Autocrine CCR7 Signaling. Cancer Cell 2007, 11, 526–538. [Google Scholar] [CrossRef]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Leone, P.; Malerba, E.; Susca, N.; Favoino, E.; Perosa, F.; Brunori, G.; Prete, M.; Racanelli, V. Frontiers|Endothelial cells in tumor microenvironment: Insights and perspectives. Front. Immunol. 2024, 15, 1367875. [Google Scholar] [CrossRef] [PubMed]
- He, M.; He, Q.; Cai, X.; Chen, Z.; Lao, S.; Deng, H.; Liu, X.; Zheng, Y.; Liu, X.; Liu, J.; et al. Role of lymphatic endothelial cells in the tumor microenvironment—A narrative review of recent advances. Transl. Lung Cancer Res. 2021, 10, 2252–2277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Li, G.; Wu, H.; Sun, K.; Chen, J.; Feng, Y.; Chen, C.; Cai, S.; Xu, J.; et al. CXCL1 from tumor-associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin β1/FAK/AKT signaling. Cancer Lett. 2017, 385, 28–38. [Google Scholar] [CrossRef]
- Issa, A.; Le, T.X.; Shoushtari, A.N.; Shields, J.D.; Swartz, M.A. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009, 69, 349–357. [Google Scholar] [CrossRef]
- Pereira, E.R.; Jones, D.; Jung, K.; Padera, T.P. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin. Cell Dev. Biol. 2015, 38, 98–105. [Google Scholar] [CrossRef]
- Regis, S.; Dondero, A.; Caliendo, F.; Bottino, C.; Castriconi, R. NK Cell Function Regulation by TGF-β-Induced Epigenetic Mechanisms. Front. Immunol. 2020, 11, 311. [Google Scholar] [CrossRef]
- Portale, F.; Di Mitri, D. NK Cells in Cancer: Mechanisms of Dysfunction and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 9521. [Google Scholar] [CrossRef]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. Frontiers|The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, B.; Liu, S.; Shi, Y.; Tao, Y.; Xiao, D.; Wang, W. What Happens to the Immune Microenvironment After PD-1 Inhibitor Therapy? Front. Immunol. 2021, 12, 773168. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Huang, R.; Kang, T.; Chen, S. The role of tumor-associated macrophages in tumor immune evasion. J. Cancer Res. Clin. Oncol. 2024, 150, 238. [Google Scholar] [CrossRef] [PubMed]
- Svanberg, R.; Janum, S.; Patten, P.E.M.; Ramsay, A.G.; Niemann, C.U. Targeting the tumor microenvironment in chronic lymphocytic leukemia. Haematologica 2021, 106, 2312–2324. [Google Scholar] [CrossRef]
- Roy, S.; Kumaravel, S.; Sharma, A.; Duran, C.L.; Bayless, K.J.; Chakraborty, S. Hypoxic tumor microenvironment: Implications for cancer therapy. Exp. Biol. Med. 2020, 245, 1073–1086. [Google Scholar] [CrossRef]
- Ubellacker, J.M.; Tasdogan, A.; Ramesh, V.; Shen, B.; Mitchell, E.C.; Martin-Sandoval, M.S.; Gu, Z.; McCormick, M.L.; Durham, A.B.; Spitz, D.R.; et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 2020, 585, 113–118. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Yao, W.; Shi, D.; Shao, X.; Lu, Z.; Chai, Y.; Song, J.; Tang, W.; Wang, X. Mechanism insights and therapeutic intervention of tumor metastasis: Latest developments and perspectives. Signal Transduct. Target. Ther. 2024, 9, 192. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- Zahoor, S.; Haji, A.; Battoo, A.; Qurieshi, M.; Mir, W.; Shah, M. Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update. J. Breast Cancer 2017, 20, 217–227. [Google Scholar] [CrossRef]
- Shankland, K.R.; Armitage, J.O.; Hancock, B.W. Non-Hodgkin lymphoma. Lancet 2012, 380, 848–857. [Google Scholar] [CrossRef]
- Küppers, R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer 2005, 5, 251–262. [Google Scholar] [CrossRef]
- Rosen, R.D.; Sapra, A. TNM Classification. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Singletary, S.E.; Allred, C.; Ashley, P.; Bassett, L.W.; Berry, D.; Bland, K.I.; Borgen, P.I.; Clark, G.; Edge, S.B.; Hayes, D.F.; et al. Revision of the American Joint Committee on Cancer Staging System for Breast Cancer. J. Clin. Oncol. 2002, 20, 3628–3636. [Google Scholar] [CrossRef] [PubMed]
- Wilczak, W.; Wittmer, C.; Clauditz, T.; Minner, S.; Steurer, S.; Büscheck, F.; Krech, T.; Lennartz, M.; Harms, L.; Leleu, D.; et al. Marked Prognostic Impact of Minimal Lymphatic Tumor Spread in Prostate Cancer. Eur. Urol. 2018, 74, 376–386. [Google Scholar] [CrossRef]
- Moncayo, V.M.; Alazraki, A.L.; Alazraki, N.P.; Aarsvold, J.N. Sentinel Lymph Node Biopsy Procedures. Semin. Nucl. Med. 2017, 47, 595–617. [Google Scholar] [CrossRef]
- Vinh-Hung, V.; Verkooijen, H.M.; Fioretta, G.; Neyroud-Caspar, I.; Rapiti, E.; Vlastos, G.; Deglise, C.; Usel, M.; Lutz, J.-M.; Bouchardy, C. Lymph Node Ratio as an Alternative to pN Staging in Node-Positive Breast Cancer. J. Clin. Oncol. 2009, 27, 1062–1068. [Google Scholar] [CrossRef]
- Siewert, J.R.; Stein, H.J. Classification of adenocarcinoma of the oesophagogastric junction. Br. J. Surg. 1998, 85, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.; Van Nieuwenhove, Y.; Pattyn, P. Prognostic Value of the Lymph Node Ratio in Stage III Colorectal Cancer: A Systematic Review. Ann. Surg. Oncol. 2010, 17, 2847–2855. [Google Scholar] [CrossRef]
- Kyzas, P.A.; Geleff, S.; Batistatou, A.; Agnantis, N.J.; Stefanou, D. Evidence for lymphangiogenesis and its prognostic implications in head and neck squamous cell carcinoma. J. Pathol. 2005, 206, 170–177. [Google Scholar] [CrossRef]
- Lauria, R.; Perrone, F.; Carlomagno, C.; De Laurentiis, M.; Morabito, A.; Gallo, C.; Varriale, E.; Pettinato, G.; Panico, L.; Petrella, G.; et al. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer 1995, 76, 1772–1778. [Google Scholar] [CrossRef]
- Schoppmann, S.F.; Bayer, G.; Aumayr, K.; Taucher, S.; Geleff, S.; Rudas, M.; Kubista, E.; Hausmaninger, H.; Samonigg, H.; Gnant, M.; et al. Prognostic Value of Lymphangiogenesis and Lymphovascular Invasion in Invasive Breast Cancer. Ann. Surg. 2004, 240, 306. [Google Scholar] [CrossRef]
- Rakha, E.A.; Martin, S.; Lee, A.H.S.; Morgan, D.; Pharoah, P.D.P.; Hodi, Z.; MacMillan, D.; Ellis, I.O. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 2012, 118, 3670–3680. [Google Scholar] [CrossRef]
- Chang, W.-C.; Chang, C.-F.; Li, Y.-H.; Yang, C.-Y.; Su, R.-Y.; Lin, C.-K.; Chen, Y.-W. A histopathological evaluation and potential prognostic implications of oral squamous cell carcinoma with adverse features. Oral Oncol. 2019, 95, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Bizzoca, M.E.; Contaldo, M.; Serpico, R.; Lo Muzio, L.; Santarelli, A. Lymphovascular invasion as a prognostic tool for oral squamous cell carcinoma: A comprehensive review. Int. J. Oral Maxillofac. Surg. 2022, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, B.; Zhao, W.; Guo, Y.; Chen, H.; Chu, H.; Liang, X.; Bi, J. Clinical Significance and Role of Lymphatic Vessel Invasion as a Major Prognostic Implication in Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE 2012, 7, e52704. [Google Scholar] [CrossRef] [PubMed]
- Ranzenberger, L.R.; Pai, R.B. Lymphoscintigraphy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mariani, G.; Moresco, L.; Viale, G.; Villa, G.; Bagnasco, M.; Canavese, G.; Buscombe, J.; Strauss, H.W.; Paganelli, G. Radioguided sentinel lymph node biopsy in breast cancer surgery. J. Nucl. Med. Off. Public Soc. Nucl. Med. 2001, 42, 1198–1215. [Google Scholar]
- Yoo, J.-N.; Cheong, Y.-S.; Min, Y.-S.; Lee, S.-W.; Park, H.Y.; Jung, T.-D. Validity of Quantitative Lymphoscintigraphy as a Lymphedema Assessment Tool for Patients with Breast Cancer. Ann. Rehabil. Med. 2015, 39, 931–940. [Google Scholar] [CrossRef]
- Polomska, A.K.; Proulx, S.T. Imaging technology of the lymphatic system. Adv. Drug Deliv. Rev. 2021, 170, 294–311. [Google Scholar] [CrossRef]
- Lasso, J.M. Lympho-SPECT/CT as a tool to evaluate postoperative outcomes after LVA for lymphedema repair. Plast. Aesthet. Res. 2020, 7, 30. [Google Scholar] [CrossRef]
- Dumitru, D.; Ghanakumar, S.; Provenzano, E.; Benson, J.R. A Prospective Study Evaluating the Accuracy of Indocyanine Green (ICG) Fluorescence Compared with Radioisotope for Sentinel Lymph Node (SLN) Detection in Early Breast Cancer. Ann. Surg. Oncol. 2022, 29, 3014–3020. [Google Scholar] [CrossRef]
- Luke, G.P.; Myers, J.N.; Emelianov, S.Y.; Sokolov, K.V. Sentinel Lymph Node Biopsy Revisited: Ultrasound-Guided Photoacoustic Detection of Micrometastases Using Molecularly Targeted Plasmonic Nanosensors. Cancer Res. 2014, 74, 5397–5408. [Google Scholar] [CrossRef]
- Liu, P.; Tan, J.; Song, Y.; Huang, K.; Zhang, Q.; Xie, H. The Application of Magnetic Nanoparticles for Sentinel Lymph Node Detection in Clinically Node-Negative Breast Cancer Patients: A Systemic Review and Meta-Analysis. Cancers 2022, 14, 5034. [Google Scholar] [CrossRef]
- Chen, S.L.; Iddings, D.M.; Scheri, R.P.; Bilchik, A.J. Lymphatic Mapping and Sentinel Node Analysis: Current Concepts and Applications. CA Cancer J. Clin. 2006, 56, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Tsopelas, C.; Sutton, R.; Bs, M. Why Certain Dyes Are Useful for Localizing the Sentinel Lymph Node. J. Nucl. Med. 2002, 43, 1377–1382. [Google Scholar] [PubMed]
- Scoggins, C.R.; Martin, R.C.G.; Ross, M.I.; Edwards, M.J.; Reintgen, D.S.; Urist, M.M.; Gershenwald, J.E.; Sussman, J.J.; Dirk Noyes, R.; Goydos, J.S.; et al. Factors Associated with False-Negative Sentinel Lymph Node Biopsy in Melanoma Patients. Ann. Surg. Oncol. 2010, 17, 709–717. [Google Scholar] [CrossRef]
- Pesek, S.; Ashikaga, T.; Krag, L.E.; Krag, D. The False-Negative Rate of Sentinel Node Biopsy in Patients with Breast Cancer: A Meta-Analysis. World J. Surg. 2012, 36, 2239–2251. [Google Scholar] [CrossRef]
- Rossin, G.; Zorzi, F.; De Pablos-Rodríguez, P.; Biasatti, A.; Marenco, J.; Ongaro, L.; Perotti, A.; Tulone, G.; Traunero, F.; Piasentin, A.; et al. Sentinel Lymph Node Biopsy in Prostate Cancer: An Overview of Diagnostic Performance, Oncological Outcomes, Safety, and Feasibility. Diagnostics 2023, 13, 2543. [Google Scholar] [CrossRef]
- De Britto, N.; Neeraja, R.; Anbarasi, L.J.; Ravi, V.; SP, S.I.; Jawahar, M.; Al Mazroa, A. Diagnosis of Lymphatic Metastasis in Breast Cancer Using Nanoparticle Technology—Diagnosis, Therapy, Imaging, Treatment. Open Neuroimaging J. 2024, 17, E18744400287726. [Google Scholar] [CrossRef]
- Chambers, A.F.; Matrisian, L.M. Changing Views of the Role of Matrix Metalloproteinases in Metastasis. JNCI J. Natl. Cancer Inst. 1997, 89, 1260–1270. [Google Scholar] [CrossRef]
- Jacob, A.; Jing, J.; Lee, J.; Schedin, P.; Gilbert, S.M.; Peden, A.A.; Junutula, J.R.; Prekeris, R. Rab40b regulates trafficking of MMP2 and MMP9 during invadopodia formation and invasion of breast cancer cells. J. Cell Sci. 2013, 126, 4647–4658. [Google Scholar] [CrossRef]
- Veikkola, T.; Karkkainen, M.; Claesson-Welsh, L.; Alitalo, K. Regulation of Angiogenesis via Vascular Endothelial Growth Factor Receptors1. Cancer Res. 2000, 60, 203–212. [Google Scholar]
- Brown, J.L.; Cao, Z.A.; Pinzon-Ortiz, M.; Kendrew, J.; Reimer, C.; Wen, S.; Zhou, J.Q.; Tabrizi, M.; Emery, S.; McDermott, B.; et al. A Human Monoclonal Anti-ANG2 Antibody Leads to Broad Antitumor Activity in Combination with VEGF Inhibitors and Chemotherapy Agents in Preclinical Models. Mol. Cancer Ther. 2010, 9, 145–156. [Google Scholar] [CrossRef]
- Rautiola, J.; Lampinen, A.; Mirtti, T.; Ristimäki, A.; Joensuu, H.; Bono, P.; Saharinen, P. Association of Angiopoietin-2 and Ki-67 Expression with Vascular Density and Sunitinib Response in Metastatic Renal Cell Carcinoma. PLoS ONE 2016, 11, e0153745. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhang, H.; Hui, R. Single chain Fv antibody against angiopoietin-2 inhibits VEGF-induced endothelial cell proliferation and migration in vitro. Biochem. Biophys. Res. Commun. 2003, 309, 946–951. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, G.; Huang, Y.; Zheng, W.; Hua, J.; Yang, S.; Zhuang, J.; Ye, J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol. Rep. 2016, 35, 1787–1795. [Google Scholar] [CrossRef]
- Huang, Q.; Duan, L.; Qian, X.; Fan, J.; Lv, Z.; Zhang, X.; Han, J.; Wu, F.; Guo, M.; Hu, G.; et al. IL-17 Promotes Angiogenic Factors IL-6, IL-8, and Vegf Production via Stat1 in Lung Adenocarcinoma. Sci. Rep. 2016, 6, 36551. [Google Scholar] [CrossRef]
- Fang, X.; Hong, Y.; Dai, L.; Qian, Y.; Zhu, C.; Wu, B.; Li, S. CRH promotes human colon cancer cell proliferation via IL-6/JAK2/STAT3 signaling pathway and VEGF-induced tumor angiogenesis. Mol. Carcinog. 2017, 56, 2434–2445. [Google Scholar] [CrossRef]
- Watanabe, S.; Mu, W.; Kahn, A.; Jing, N.; Li, J.H.; Lan, H.Y.; Nakagawa, T.; Ohashi, R.; Johnson, R.J. Role of JAK/STAT Pathway in IL-6-Induced Activation of Vascular Smooth Muscle Cells. Am. J. Nephrol. 2004, 24, 387–392. [Google Scholar] [CrossRef]
- Al-Rawi, M.a.A.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Interleukin 7 upregulates vascular endothelial growth factor D in breast cancer cells and induces lymphangiogenesis in vivo. Br. J. Surg. 2005, 92, 305–310. [Google Scholar] [CrossRef]
- Jian, M.; Qingfu, Z.; Yanduo, J.; Guocheng, J.; Xueshan, Q. Anti-lymphangiogenesis effects of a specific anti-interleukin 7 receptor antibody in lung cancer model in vivo. Mol. Carcinog. 2015, 54, 148–155. [Google Scholar] [CrossRef]
- Ming, J.; Zhang, Q.; Qiu, X.; Wang, E. Interleukin 7/interleukin 7 receptor induce c-Fos/c-Jun-dependent vascular endothelial growth factor-D up-regulation: A mechanism of lymphangiogenesis in lung cancer. Eur. J. Cancer 2009, 45, 866–873. [Google Scholar] [CrossRef]
- Krishnan, H.; Rayes, J.; Miyashita, T.; Ishii, G.; Retzbach, E.P.; Sheehan, S.A.; Takemoto, A.; Chang, Y.; Yoneda, K.; Asai, J.; et al. Podoplanin: An emerging cancer biomarker and therapeutic target. Cancer Sci. 2018, 109, 1292–1299. [Google Scholar] [CrossRef]
- Rizzetto, G.; Lucarini, G.; De Simoni, E.; Molinelli, E.; Mattioli-Belmonte, M.; Offidani, A.; Simonetti, O. Tissue Biomarkers Predicting Lymph Node Status in Cutaneous Melanoma. Int. J. Mol. Sci. 2022, 24, 144. [Google Scholar] [CrossRef]
- Chen, T.; Lin, Y.; Tan, Q. Risk factors for lower extremity lymphedema after inguinal lymphadenectomy in melanoma patients: A retrospective cohort study. Surg. Open Sci. 2022, 8, 33–39. [Google Scholar] [CrossRef]
- Chatterjee, A.; Serniak, N.; Czerniecki, B.J. Sentinel Lymph Node Biopsy in Breast Cancer: A Work in Progress. Cancer J. Sudbury Mass 2015, 21, 7–10. [Google Scholar] [CrossRef]
- Wang, C.; Chu, M. Advances in Drugs Targeting Lymphangiogenesis for Preventing Tumor Progression and Metastasis. Front. Oncol. 2022, 11, 783309. [Google Scholar] [CrossRef]
- Stacker, S.A.; Caesar, C.; Baldwin, M.E.; Thornton, G.E.; Williams, R.A.; Prevo, R.; Jackson, D.G.; Nishikawa, S.; Kubo, H.; Achen, M.G. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 2001, 7, 186–191. [Google Scholar] [CrossRef]
- Bock, F.; Maruyama, K.; Regenfuss, B.; Hos, D.; Steven, P.; Heindl, L.M.; Cursiefen, C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog. Retin. Eye Res. 2013, 34, 89–124. [Google Scholar] [CrossRef]
- Kodera, Y.; Katanasaka, Y.; Kitamura, Y.; Tsuda, H.; Nishio, K.; Tamura, T.; Koizumi, F. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res. 2011, 13, R66. [Google Scholar] [CrossRef]
- McDonald, D.M. New antibody to stop tumor angiogenesis and lymphatic spread by blocking receptor partnering. Cancer Cell 2010, 18, 541–543. [Google Scholar] [CrossRef]
- Gotink, K.J.; Verheul, H.M.W. Anti-angiogenic tyrosine kinase inhibitors: What is their mechanism of action? Angiogenesis 2010, 13, 1–14. [Google Scholar] [CrossRef]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef]
- Tomuleasa, C.; Tigu, A.-B.; Munteanu, R.; Moldovan, C.-S.; Kegyes, D.; Onaciu, A.; Gulei, D.; Ghiaur, G.; Einsele, H.; Croce, C.M. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Signal Transduct. Target. Ther. 2024, 9, 201. [Google Scholar] [PubMed]
- Peng, M.; Deng, J.; Li, X. Clinical advances and challenges in targeting FGF/FGFR signaling in lung cancer. Mol. Cancer 2024, 23, 256. [Google Scholar] [CrossRef] [PubMed]
- Kirthiga Devi, S.S.; Singh, S.; Joga, R.; Patil, S.Y.; Meghana Devi, V.; Chetan Dushantrao, S.; Dwivedi, F.; Kumar, G.; Kumar Jindal, D.; Singh, C.; et al. Enhancing cancer immunotherapy: Exploring strategies to target the PD-1/PD-L1 axis and analyzing the associated patent, regulatory, and clinical trial landscape. Eur. J. Pharm. Biopharm. 2024, 200, 114323. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining Immunotherapy and Targeted Therapies in Cancer Treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ni, Y.; Liang, X.; Lin, Y.; An, B.; He, X.; Zhao, X. Mechanisms of tumor resistance to immune checkpoint blockade and combination strategies to overcome resistance. Front. Immunol. 2022, 13, 915094. [Google Scholar] [CrossRef]
- Eulberg, D.; Frömming, A.; Lapid, K.; Mangasarian, A.; Barak, A. The prospect of tumor microenvironment-modulating therapeutical strategies. Front. Oncol. 2022, 12, 1070243. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- He, P.; Tang, H.; Zheng, Y.; Xiong, Y.; Cheng, H.; Li, J.; Zhang, Y.; Liu, G. Advances in nanomedicines for lymphatic imaging and therapy. J. Nanobiotechnol. 2023, 21, 292. [Google Scholar] [CrossRef]
- Zwaans, B.M.M.; Bielenberg, D.R. Potential therapeutic strategies for lymphatic metastasis. Microvasc. Res. 2007, 74, 145–158. [Google Scholar] [CrossRef]
- Rebaudi, F.; De Franco, F.; Goda, R.; Obino, V.; Vita, G.; Baronti, C.; Iannone, E.; Pitto, F.; Massa, B.; Fenoglio, D.; et al. The landscape of combining immune checkpoint inhibitors with novel Therapies: Secret alliances against breast cancer. Cancer Treat. Rev. 2024, 130, 102831. [Google Scholar] [CrossRef]
- Lu, C.; Tan, Y. Promising immunotherapy targets: TIM3, LAG3, and TIGIT joined the party. Mol. Ther. Oncol. 2024, 32, 200773. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, X.; Fu, J.; Wang, H. Progress and Challenges in Precise Treatment of Tumors With PD-1/PD-L1 Blockade. Front. Immunol. 2020, 11, 339. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Matsumura, N.; Abiko, K.; Baba, T.; Konishi, I. PD-1/PD-L1 blockade in cancer treatment: Perspectives and issues. Int. J. Clin. Oncol. 2016, 21, 462–473. [Google Scholar] [CrossRef]
- Bai, J.; Gao, Z.; Li, X.; Dong, L.; Han, W.; Nie, J. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget 2017, 8, 110693–110707. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Freeman, G.J.; McDermott, D.F. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin. Ther. 2015, 37, 764–782. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Wang, Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist 2019, 24, S31–S41. [Google Scholar] [CrossRef]
- Wu, M.; Huang, Q.; Xie, Y.; Wu, X.; Ma, H.; Zhang, Y.; Xia, Y. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J. Hematol. Oncol. 2022, 15, 24. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, H.; Zhao, J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther. Adv. Med. Oncol. 2020, 12, 1758835920937612. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Fang, K.K.-L.; Lee, J.B.; Zhang, L. Adoptive Cell Therapy for T-Cell Malignancies. Cancers 2022, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Liu, Z.; Wang, J.; Xia, J.; Liu, S.; Jia, Y.; Liu, H.; Li, K. Anlotinib suppresses lymphangiogenesis and lymphatic metastasis in lung adenocarcinoma through a process potentially involving VEGFR-3 signaling. Cancer Biol. Med. 2020, 17, 753–767. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Y.; Weng, S.; Xu, H.; Li, L.; Han, X. A New Trend in Cancer Treatment: The Combination of Epigenetics and Immunotherapy. Front. Immunol. 2022, 13, 809761. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. US National Library of Medicine. Available online: https://clinicaltrials.gov (accessed on 13 May 2025).
- Ottaviano, M.; De Placido, S.; Ascierto, P.A. Recent success and limitations of immune checkpoint inhibitors for cancer: A lesson from melanoma. Virchows Arch. 2019, 474, 421–432. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. Ecancermedicalscience 2012, 6, ed16. [Google Scholar] [CrossRef]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef]
- Shiga, T.; Hiraide, M. Cardiotoxicities of 5-Fluorouracil and Other Fluoropyrimidines. Curr. Treat. Options Oncol. 2020, 21, 27. [Google Scholar] [CrossRef]
- Polk, A.; Vistisen, K.; Vaage-Nilsen, M.; Nielsen, D.L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol. Toxicol. 2014, 15, 47. [Google Scholar] [CrossRef]
- Li, K.; Chen, W.; Ma, L.; Yan, L.; Wang, B. Approaches for reducing chemo/radiation-induced cardiotoxicity by nanoparticles. Environ. Res. 2024, 244, 117264. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Al-malky, H.S.; Al Harthi, S.E.; Osman, A.-M.M. Major obstacles to doxorubicin therapy: Cardiotoxicity and drug resistance. J. Oncol. Pharm. Pract. 2020, 26, 434–444. [Google Scholar] [CrossRef]
- Delaunay, M.; Prévot, G.; Collot, S.; Guilleminault, L.; Didier, A.; Mazières, J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur. Respir. Rev. 2019, 28, 190012. [Google Scholar] [CrossRef]
- Sunder, S.S.; Sharma, U.C.; Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: Pathophysiology, mechanisms and clinical management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef]
- Peerzada, M.M.; Spiro, T.P.; Daw, H.A. Pulmonary Toxicities of Tyrosine Kinase Inhibitors. Clin. Adv. Hematol. Oncol. 2011, 9, 824–836. [Google Scholar]
- Shippee, B.M.; Bates, J.S.; Richards, K.L. The role of screening and monitoring for bleomycin pulmonary toxicity. J. Oncol. Pharm. Pract. 2016, 22, 308–312. [Google Scholar] [CrossRef]
- Martin, W.G.; Ristow, K.M.; Habermann, T.M.; Colgan, J.P.; Witzig, T.E.; Ansell, S.M. Bleomycin Pulmonary Toxicity Has a Negative Impact on the Outcome of Patients with Hodgkin’s Lymphoma. J. Clin. Oncol. 2005, 23, 7614–7620. [Google Scholar] [CrossRef]
- Peterson, L.L.; Hurria, A.; Feng, T.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Glezerman, I.; et al. Association between renal function and chemotherapy-related toxicity in older adults with cancer. J. Geriatr. Oncol. 2017, 8, 96–101. [Google Scholar] [CrossRef]
- Ruggiero, A.; Ferrara, P.; Attinà, G.; Rizzo, D.; Riccardi, R. Renal toxicity and chemotherapy in children with cancer. Br. J. Clin. Pharmacol. 2017, 83, 2605–2614. [Google Scholar] [CrossRef]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Duan, Z.; Cai, G.; Li, J.; Chen, X. Cisplatin-induced renal toxicity in elderly people. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923430. [Google Scholar] [CrossRef] [PubMed]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Akbarali, H.I.; Muchhala, K.H.; Jessup, D.K.; Cheatham, S. Chapter Four—Chemotherapy induced gastrointestinal toxicities. In Advances in Cancer Research; Gewirtz, D.A., Fisher, P.B., Eds.; Strategies to Mitigate the Toxicity of Cancer Therapeutics; Academic Press: Cambridge, MA, USA, 2022; Volume 155, pp. 131–166. [Google Scholar]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. WJG 2014, 20, 3751–3761. [Google Scholar] [CrossRef]
- Di Fiore, F.; Van Cutsem, E. Acute and long-term gastrointestinal consequences of chemotherapy. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 113–124. [Google Scholar] [CrossRef]
- Yazbeck, V.; Alesi, E.; Myers, J.; Hackney, M.H.; Cuttino, L.; Gewirtz, D.A. Chapter One—An overview of chemotoxicity and radiation toxicity in cancer therapy. In Advances in Cancer Research; Gewirtz, D.A., Fisher, P.B., Eds.; Strategies to Mitigate the Toxicity of Cancer Therapeutics; Academic Press: Cambridge, MA, USA, 2022; Volume 155, pp. 1–27. [Google Scholar]
- Alotayk, L.I.; Aldubayan, M.A.; Alenezi, S.K.; Anwar, M.J.; Alhowail, A.H. Comparative evaluation of doxorubicin, cyclophosphamide, 5-fluorouracil, and cisplatin on cognitive dysfunction in rats: Delineating the role of inflammation of hippocampal neurons and hypothyroidism. Biomed. Pharmacother. 2023, 165, 115245. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Frey, N.V.; Porter, D.L. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology 2016, 2016, 567–572. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef]
- Charehjoo, A.; Majidpoor, J.; Mortezaee, K. Indoleamine 2,3-dioxygenase 1 in circumventing checkpoint inhibitor responses: Updated. Int. Immunopharmacol. 2023, 118, 110032. [Google Scholar] [CrossRef]
- Diab, A.; Gogas, H.; Sandhu, S.; Long, G.V.; Ascierto, P.A.; Larkin, J.; Sznol, M.; Franke, F.; Ciuleanu, T.E.; Pereira, C.; et al. Bempegaldesleukin Plus Nivolumab in Untreated Advanced Melanoma: The Open-Label, Phase III PIVOT IO 001 Trial Results. J. Clin. Oncol. 2023, 41, 4756–4767. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. Recent advances in mRNA cancer vaccines: Meeting challenges and embracing opportunities. Front. Immunol. 2023, 14, 1246682. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, V.; Tarazona, N.; Cejalvo, J.M.; Lombardi, P.; Huerta, M.; Roselló, S.; Fleitas, T.; Roda, D.; Cervantes, A. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers 2020, 12, 1009. [Google Scholar] [CrossRef]
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted cancer models: Revolutionizing personalized cancer therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef]

| Mechanisms of Lymphatic Metastasis | |||
|---|---|---|---|
| Section | Key Mechanisms/Processes | Molecules/Pathways Involved | Role/Impact |
| 2.1 Role of Lymphangiogenesis | Formation of new lymphatic vessels | VEGF-C, VEGF-D, VEGFR-3, Neuropilin-2 | -Promotes lymphatic vessel sprouting, expansion, and remodeling. -Creates favorable conditions for tumor cell intravasation. -VEGF-C/D overexpression increases lymphatic vessel density and metastatic spread. |
| Downstream signaling pathways | PI3K/Akt, MAPK | -Regulate lymphatic endothelial cell (LEC) proliferation, migration, and survival. -Support tumor dissemination through lymphatics. | |
| 2.2 Tumor Cell Migration and Intravasation Into Lymphatics | Tumor invasion into lymphatics through ECM remodeling and signaling | Chemokines (CCL21-CCR7), MMPs (MMP14, MMP16), adhesion molecules (VCAM-1), CXCL1-integrin β1 | -Chemokine gradients (e.g., CCL21-CCR7) guide tumor cells to lymphatic vessels. -MMPs degrade ECM barriers, facilitating migration. -VCAM-1 and integrin signaling enhance permeability and adhesion to lymphatic vessels. |
| Synergistic VEGF-C and chemokine signaling | VEGF-C-induced CCL21 secretion, CCR7 | -VEGF-C and CCL21 synergize to promote lymphatic vessel dilation and CCR7-dependent tumor invasion. -Enhances metastatic spread to lymph nodes. | |
| 2.3 Tumor Cell Survival and Colonization in Lymph Nodes | Immune evasion strategies | Tregs, TGF-β, PD-L1/PD-1 pathway | -Tregs suppress cytotoxic T cells, creating an immunosuppressive microenvironment. -TGF-β inhibits NK and cytotoxic T cell functions. -PD-L1 overexpression blocks T cell activation. |
| Adaptation to lymph node microenvironment | Tumor-associated macrophages (TAMs), CAFs, hypoxia, ECM remodeling | -TAMs (M2 phenotype) and CAFs remodel ECM and secrete chemokines, recruiting immunosuppressive cells. -Hypoxia induces genetic changes that enhance survival and resistance to treatment. -Creates a robust niche for tumor persistence. | |
| Tumor Type | Category | Role of Lymphatic Metastasis |
|---|---|---|
| Breast Cancer | Solid | Strong predictor of prognosis; lymph node status critical for staging and therapeutic decisions. |
| Melanoma | Solid | Early lymphatic spread to sentinel nodes; SLN biopsy is standard for staging and management. |
| Colorectal Cancer | Solid | Lymph node involvement defines stage III disease; lymphatic invasion predicts worse survival. |
| Non-Small Cell Lung Cancer (NSCLC) | Solid | Lymphatic spread associated with relapse risk; mediastinal node metastasis guides resection and adjuvant therapy. |
| Gastric Cancer | Solid | Lymphatic dissemination to perigastric and para-aortic nodes affects surgical planning and prognosis. |
| Cervical Cancer | Solid | Lymphatic invasion correlates with recurrence risk; guides need for radiotherapy and chemotherapy. |
| Lymphoma | Hematologic | Primary malignancy of lymphatic tissues; dissemination is systemic but disrupts lymphatic architecture. |
| Leukemia | Hematologic | Involves blood and lymphatic system; lymphadenopathy common but “metastasis” concept differs from solid tumors. |
| Biomarkers for Lymphangiogenesis | ||
|---|---|---|
| Name | Function | Role in Metastasis |
| Matrix metalloproteinases | Endopeptidases | Promote angiogenesis Degrade extracellular matrix |
| Vascular endothelial growth factor | Signal protein | Stimulates the formation of blood vessels |
| Fibroblast growth factor | Signal protein | Cell proliferation Stimulates growth of new blood vessels |
| Platelet-derived growth factor | Signal protein | Mitogen Activates endothelial cells during angiogenesis |
| Lymphatic vessel endothelial hyaluronan receptor 1 | Protein | Binds to hyaluronic acid Found on the surface of lymphatic endothelial cells |
| Podoplanin | Transmembrane glycoprotein | Associated with tumor mobility and metastasis |
| PROX1 | Transmembrane protein | Transforms blood vessels into lymphatic vessels |
| Clinical Trial | ID | Types of Cancer | Treatment Type | Status | Phase |
|---|---|---|---|---|---|
| “Radiotherapy With or Without Concurrent Chemotherapy for Limited Lymphatic Metastasis of Esophageal Cancer—3JECROG P-02” | NCT03308552 | Esophageal | Radiation therapy, Paclitaxel, platinum-based drugs | Unknown | 3 |
| “Radiotherapy With or Without Concurrent Chemotherapy for Extensive Lymphatic Metastasis of Esophageal Cancer—3JECROG P-03” | NCT03328234 | Esophageal | Radiation therapy, Paclitaxel, platinum-based drugs, involved field irradiation | Unknown | 3 |
| “Study of Neo-adjuvant Use of Vemurafenib Plus Cobimetinib for BRAF Mutant Melanoma with Palpable Lymph Node Metastases” | NCT02036086 | Melanoma | Vemurafenib, Cobimetinib | Unknown | 2 |
| “RP1 in Primary Melanoma to Reduce the Risk of Sentinel Lymph Node Metastasis” | NCT06216938 | Melanoma | Vusolimogene oderparepvec (RP1) | Recruiting | 1 |
| “Interferon Alfa-2b in Treating Patients with Melanoma and Early Lymph Node Metastasis” | NCT00004196 | Melanoma | Recombinant interferon alfa | Completed | 3 |
| “Clinical Trial Comparing Carnoy’s and GEWF Solutions” | NCT02704988 | Colorectal | Carnou procedure, GEWF procedure | Completed | N/A |
| “Targeted Resection of Axillary Metastatic Lymph Nodes After Breast Cancer Neoadjuvant Chemotherapy” | NCT04744506 | Breast | Carbon Nanoparticle Suspension Injection | Recruiting | N/A |
| “Trial of Xeloda and Oxaliplatin (XELOX) as Neo-adjuvant Chemotherapy Followed by Surgery in Advanced Gastric Cancer Patients with Para-aortic Lymph Node Metastasis” | NCT02071043 | Gastric | Capecitabine, Oxaliplatin | Completed | 2 |
| “Relation Between Tumor-draining Lymph Nodes Metastasis Pattern and Non-small Cell Lung Cancer Neoadjuvant Immunotherapy Effectiveness” | NCT06292052 | Lung | Immunotherapy | Completed | N/A |
| “Chemo-radio-immunotherapy With Nivolumab and Ipilimumab Treatment in Locally Advanced Cervical Cancer Patients (CERAD-IMMUNE)” | NCT05504642 | Cervical | Nivolumab/Ipilimumab | Withdrawn | 2 |
| “Antiandrogen Therapy with or Without Axitinib Before Surgery in Treating Patients With Previously Untreated Prostate Cancer With Known or Suspected Lymph Node Metastasis” | NCT01409200 | Prostate | Antiandrogen Therapy, Axitinib | Active | 2 |
| “Safety and Efficacy of Sintilimab in Combination with Chemoradiothrapy Followed by D2 Surgical Resection in Patients With Advanced Gastric Cancer With Retroperitoneal Lymph Node Metastasis” | NCT05002686 | Gastric | Sintilimab, Albumin–Paclitaxel, Capecitabine, Oxaliplatin | Unknown | 2/3 |
| “Oral Iohexol in the Management of Chylous Ascites After After Retroperitoneal or Extended Lymphadenectomy” | NCT06820320 | Abdominal or pelvic tumors | Oral Iohexol | Not yet recruiting | 2 |
| “A Prospective, Multicenter Randomized Controlled Study of the Application of Preoperative FOLFOXIRI Chemotherapy Combined with Lateral Lymph Node Dissection in Low- and Medium-lying Rectal Cancer With Lateral Lymph Node Metastasis” | NCT06048146 | Rectal | FOLFOXIRI (Irinotecan, Oxaliplatin), lymph node dissection | Enrolling by invitation | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devisetti, N.; Shah, P.; Liu, F.C. From Mechanisms to Treatment: A Comprehensive View of Lymphatic Metastasis in Cancer. Lymphatics 2025, 3, 12. https://doi.org/10.3390/lymphatics3020012
Devisetti N, Shah P, Liu FC. From Mechanisms to Treatment: A Comprehensive View of Lymphatic Metastasis in Cancer. Lymphatics. 2025; 3(2):12. https://doi.org/10.3390/lymphatics3020012
Chicago/Turabian StyleDevisetti, Nitya, Pushti Shah, and Farrah C. Liu. 2025. "From Mechanisms to Treatment: A Comprehensive View of Lymphatic Metastasis in Cancer" Lymphatics 3, no. 2: 12. https://doi.org/10.3390/lymphatics3020012
APA StyleDevisetti, N., Shah, P., & Liu, F. C. (2025). From Mechanisms to Treatment: A Comprehensive View of Lymphatic Metastasis in Cancer. Lymphatics, 3(2), 12. https://doi.org/10.3390/lymphatics3020012






