Lipoperoxides as Prognostic Markers in Pediatric B-Acute Lymphocytic Leukemia Patients Undergoing Induction Chemotherapy
Abstract
1. Introduction
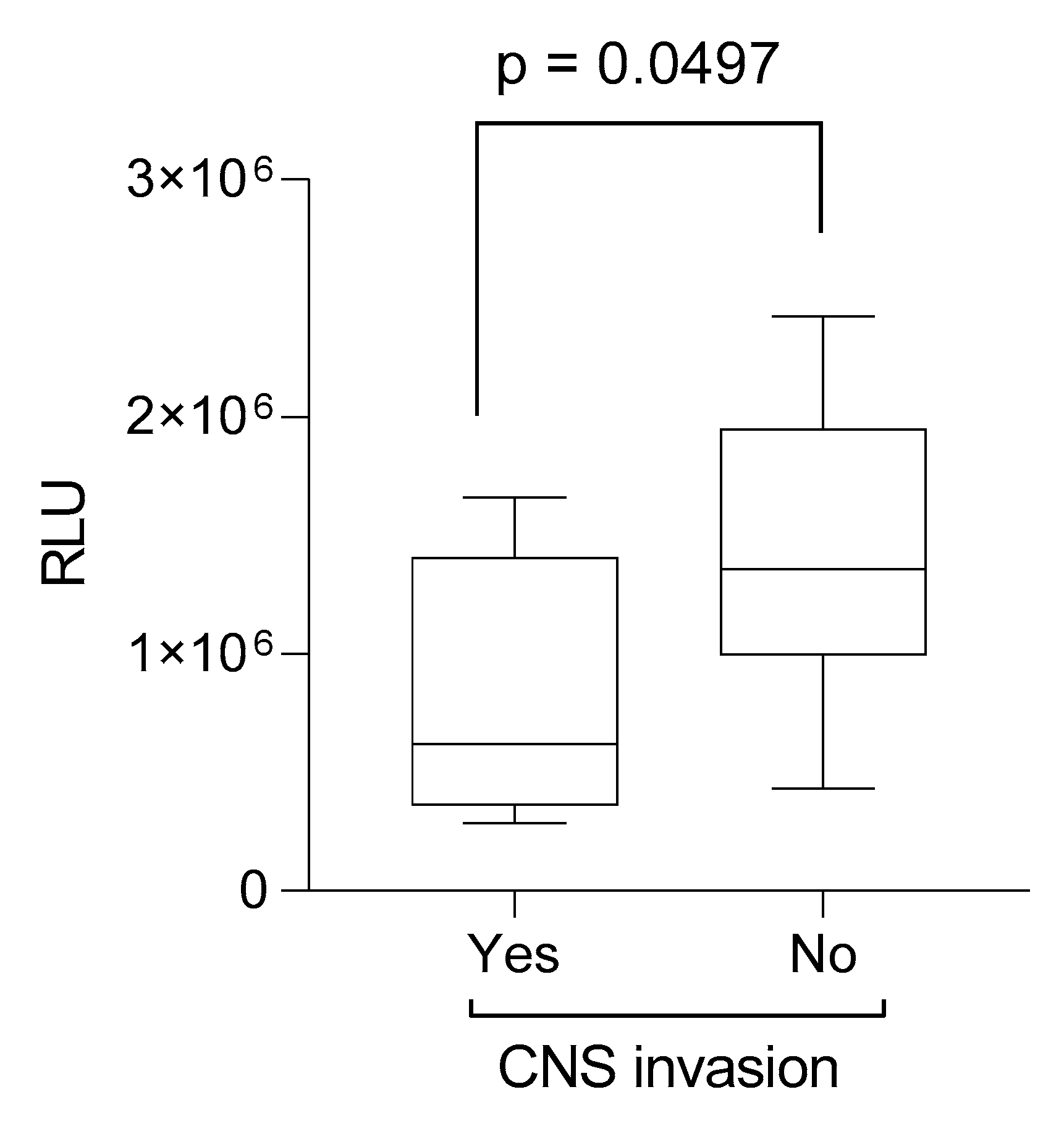
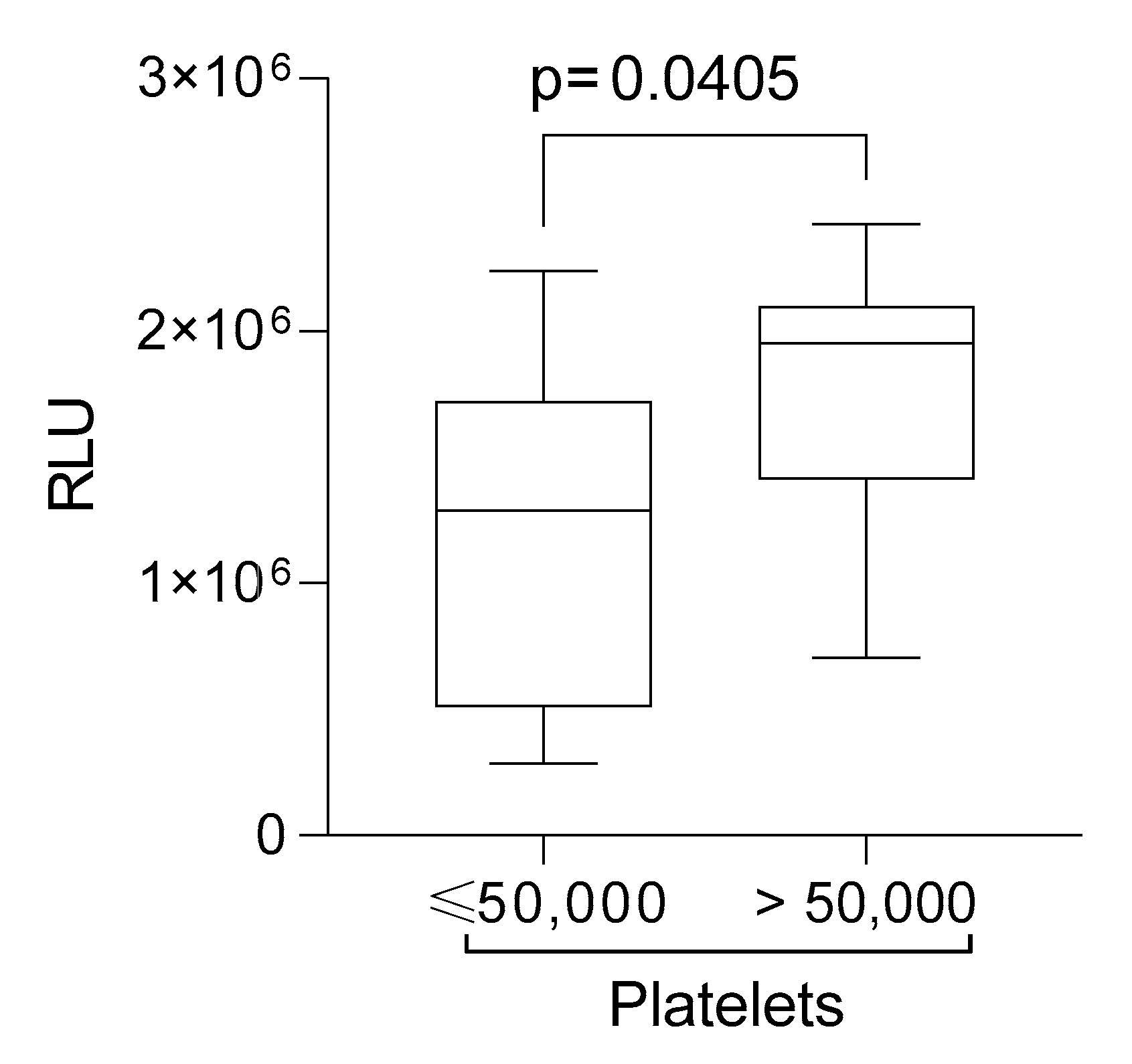
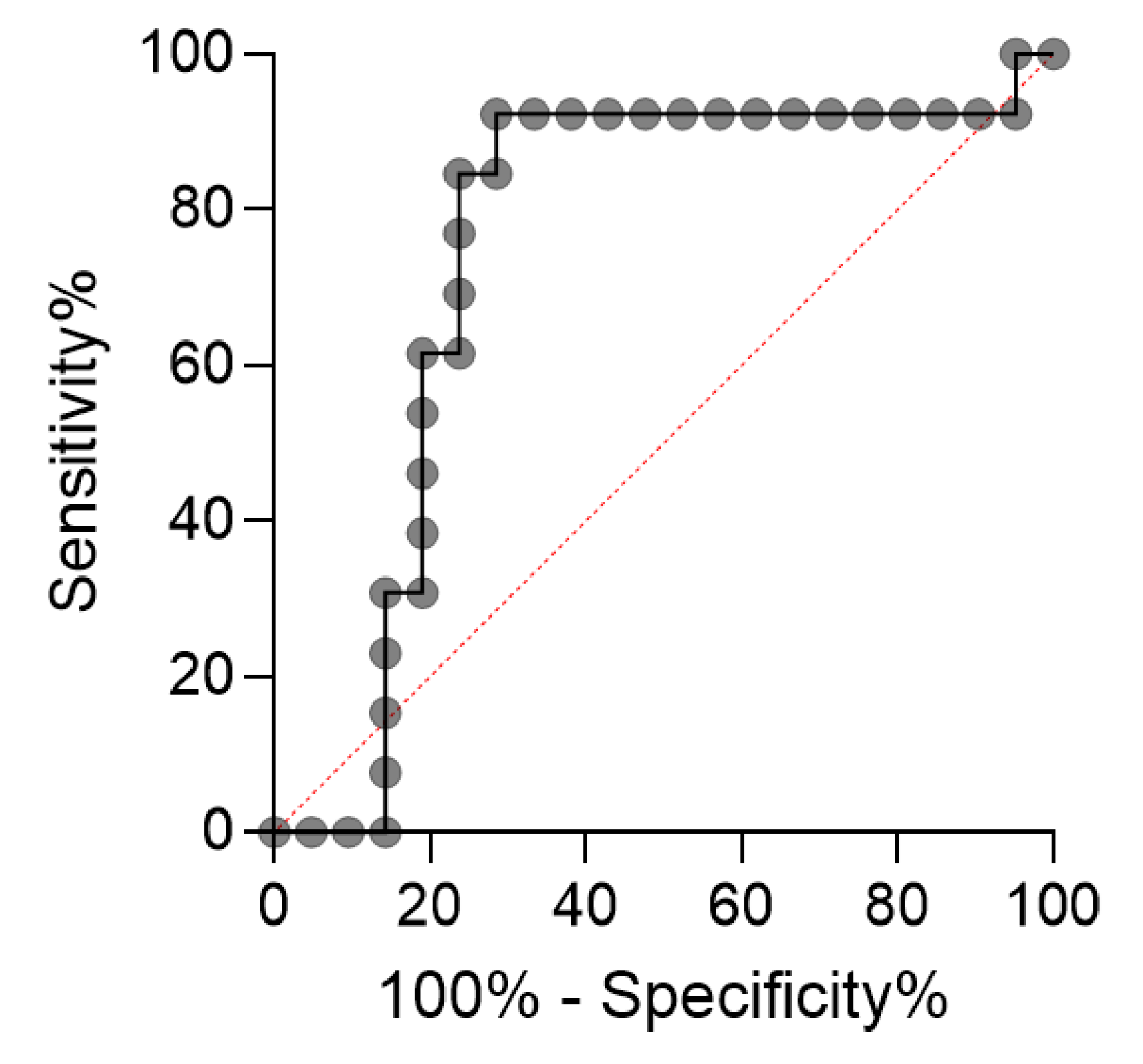
2. Results
3. Discussion
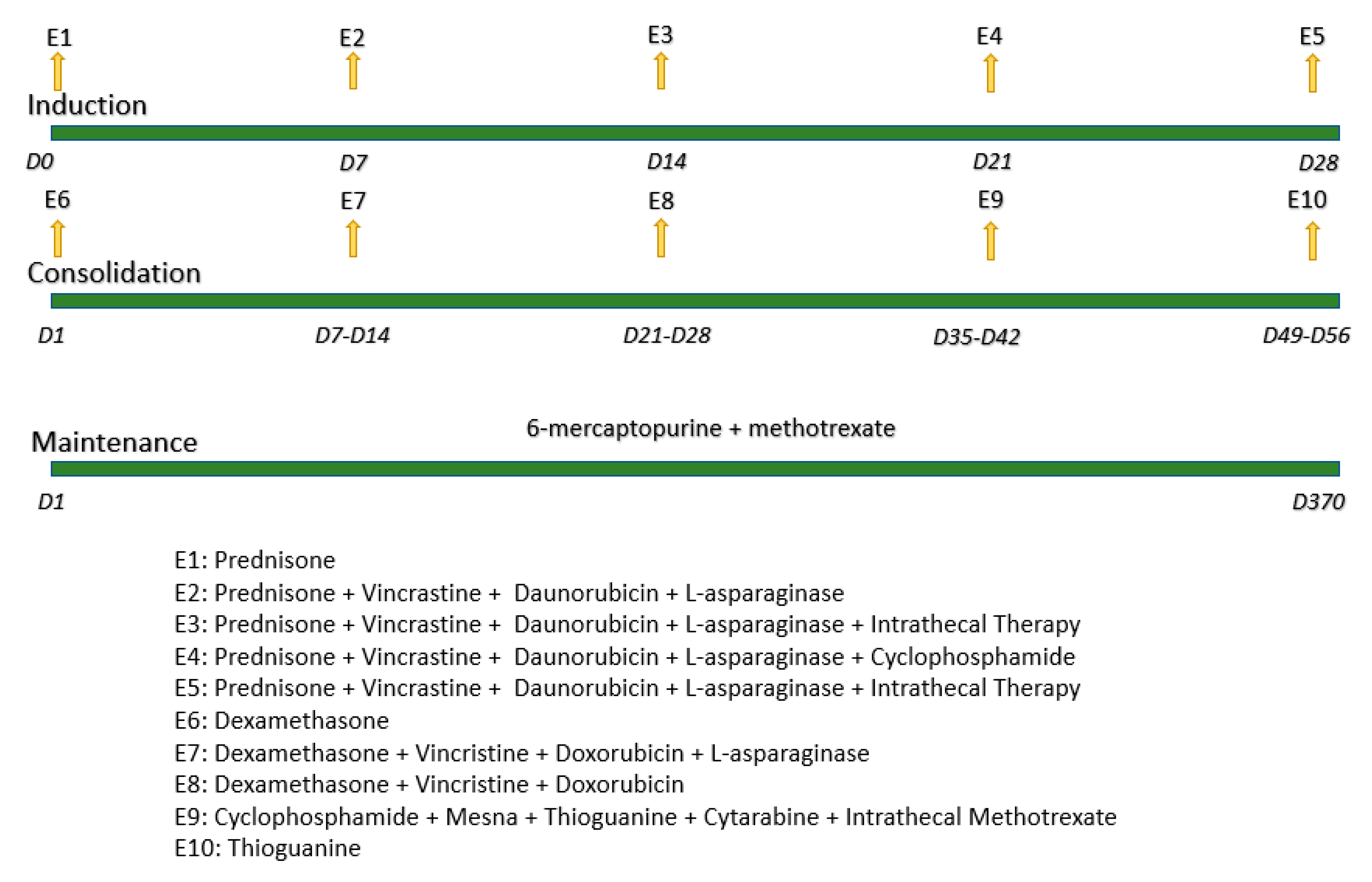
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| B-ALL | B-type acute lymphoblastic leukemia |
| N | number of patients |
| CNS | central nervous system |
| ROC | receiver operating characteristic |
| ALL | acute lymphoblastic leukemia |
| RLU | relative light units |
| CSF | cerebrospinal fluid |
| ROS | reactive oxygen species |
| EFS | event-free survival |
References
- National Cancer Institute. Available online: https://www.gov.br/inca/pt-br/assuntos/cancer/numeros/estimativa/estado-capital/brasil/cancer-infantojuvenil (accessed on 16 April 2024).
- Brazilian Society of Pediatrics. Available online: https://www.sbp.com.br/fileadmin/user_upload/publicacoes/C-Doc-Cientifico-Oncologia-Epidemiol-30-mar-17.pdf (accessed on 16 April 2024).
- Pedrosa, F.; Lins, M. Acute lymphocytic leukemia: A curable disease. Rev. Bras. Saúde Materno Infantil. 2002, 2, 63–68. [Google Scholar] [CrossRef]
- Canto, M.E.B.S.; Ferreira, J.A.; Rosa, F.C.; Gomes, A.C.D.B.; Zveibil, G.S. Perfil epidemiológico da leucemia linfoide no Brasil. Hematology. Transfus. Cell Ther. 2023, 45 (Suppl. 4), S163. [Google Scholar] [CrossRef]
- Wang, L.; Yao, X.; Yang, L. Acute Lymphoblastic Leukemia Collaborators. (2025). Global, regional, and national burden of children and adolescents with acute lymphoblastic leukemia from 1990 to 2021: A systematic analysis for the Global Burden of Disease Study 2021. Front. Public Health 2021, 13, 1525751. [Google Scholar] [CrossRef]
- Associação Brasileira de Linfoma e Leucemia. Leucemia Linfoide Aguda. Available online: https://www.abrale.org.br/doencas/leucemia/lla/o-que-e/ (accessed on 16 April 2024).
- Zago, M.A.; Falcão, R.P.; Pasquini, R. Leucemia Linfoide da Criança e do Adolescente. In Tratado de Hematologia, 1st ed.; Atheneu: São Paulo, Brasil, 2013; pp. 391–401. [Google Scholar]
- Hong, Z.; Wei, Z.; Xie, T.; Fu, L.; Sun, J.; Zhou, F.; Jamal, M.; Zhang, Q.; Shao, L. Targeting chemokines for acute lymphoblastic leukemia therapy. J. Hemat. Oncol. 2021, 14, 48. [Google Scholar] [CrossRef]
- Cole, P.D.; Finkelstein, Y.; Stevenson, K.E.; Blonquist, T.M.; Vijayanathan, V.; Silverman, L.B.; Neuberg, D.S.; Sallan, S.E.; Robaey, P.; Waber, D.P. Polymorphisms in genes related to oxidative stress are associated with inferior cognitive function after therapy for childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2015, 33, 2205–2211. [Google Scholar] [CrossRef]
- Clemente, S.M.; Martínez-Costa, O.H.; Monsalve, M.; Samhan-Arias, A.K. Targeting lipid peroxidation for cancer treatment. Molecules 2020, 25, 5144. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Ferreira, A.L.A.; Matsubara, L.S. Free radicals: Concepts, related diseases, defense system and oxidative stress. J. Braz. Med. Assoc. 1997, 43, 61–68. [Google Scholar] [CrossRef]
- Panis, C.; Victorino, V.J.; Herrera, A.C.; Cecchini, A.L.; Simão, A.N.; Tomita, L.Y.; Cecchini, R. Can breast tumors affect the oxidative status of the surrounding environment? A comparative analysis among cancerous breast, mammary Adjacent tissue, and plasma. Oxidative Med. Cell. Longev. 2015, 2015, 6429812. [Google Scholar] [CrossRef]
- Al-Tonbary, Y.; Al-Hasan, S.A.; Zaki, M.; Hammad, A.; Kandil, S.; Fouda, A. Impact of anti-oxidant status and apoptosis on the induction phase of chemotherapy in childhood acute lymphoblastic leukemia. Hematology 2011, 16, 14–19. [Google Scholar] [CrossRef]
- Babiak, R.M.V.; Campello, A.P.; Garnieri, E.G.S.; Oliveira, M.B.M. Metotrexate: Pentose cycle and oxidative stress. Cell Biochem. Funct. 1998, 16, 283–293. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.K.; Bagatini, M.D.; Santos, K.F.; Spanevello, R.M.; Maldonado, P.A.; Brulé, A.O.; de Araújo, M.C.; Schetinger, M.A.; Morsch, V.M. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin. Biochem. 2008, 41, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Broto, G.E.; Corrêa, S.; Trigo, F.C.; Santos, E.C.; Tomiotto-Pelissier, F.; Pavanelli, W.R.; Silveira, G.F.; Abdelhay, E.; Panis, C. Comparative analysis of systemic and tumor microenvironment proteomes from children with B-cell acute lymphocytic leukemia at diagnosis and after induction treatment. Front. Oncol. 2020, 10, 550213. [Google Scholar] [CrossRef] [PubMed]
- De Cavanagh, E.M.V.; Honegger, A.E.; Hofer, E.; Bordenave, R.H.; Bullorsky, E.O.; Chasseing, N.A.; Fraga, C. Higher oxidation and lower antioxidant levels in peripheral blood plasma and bone marrow plasma from advanced cancer patients. Cancer 2002, 94, 3247–3251. [Google Scholar] [CrossRef]
- Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S.; et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 2017, 23, 120–127. [Google Scholar] [CrossRef]
- Devi, G.S.; Prasad, M.H.; Saraswathi, I.; Raghu, D.; Rao, D.N.; Reddy, P.P. Free radicals, antioxidant enzymes, and lipid peroxidation in different types of leukemias. Clin. Chim. Acta 2000, 293, 53–62. [Google Scholar] [CrossRef]
- El-Sabagh, M.E.; Ramadan, K.S.; El-Slam, I.M.A.; Ibrahim, A.M. Antioxidants status in acute lymphoblastic leukemic patients. Am. J. Med. Med. Sci. 2011, 1, 1–6. Available online: http://article.sapub.org/10.5923.j.ajmms.20110101.01.html (accessed on 25 March 2025). [CrossRef]
- Er, T.-K.; Tsai, S.-M.; Wu, S.-H.; Chiang, W.; Lin, H.-C.; Lin, S.-F.; Wu, S.-H.; Tsai, L.-Y.; Liu, T.-Z. Antioxidant status and superoxide anion radical generation in acute myeloid leukemia. Clin. Biochem. 2007, 40, 1015–1019. [Google Scholar] [CrossRef]
- Oliveira, G.E.B. Impact of the Induction Phase of Chemotherapy on the Profile of Systemic Inflammatory Mediators and the Tumor Microenvironment in Childhood Acute Lymphocytic Leukemia. Ph.D. Thesis, State University of Londrina, Londrina, Brazil, 2021. [Google Scholar]
- Caron, J.E.; Krull, K.R.; Hockenberry, M.; Jain, N.; Kaemingk, K.; Moore, I.M. Oxidative stress and executive function in children receiving chemotherapy for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2009, 53, 551–556. [Google Scholar] [CrossRef]
- Kien, C.L.; Camitta, B.M. Close association of accelerated rates of whole body protein turnover (synthesis and breakdown) and energy expenditure in children with newly diagnosed acute lymphocytic leukemia. J. Parenter. Enter. Nutr. 1987, 11, 129–134. [Google Scholar] [CrossRef]
- Chaudhary, P.; Kumari, S.; Dewan, P.; Gomber, S.; Ahmed, R.S.; Kotru, M. Chemotherapy-Induced Oxidative Stress in Pediatric Acute Lymphoblastic Leukemia. Cureus 2023, 15, e35968. [Google Scholar] [CrossRef] [PubMed]
- Udensi, U.K.; Tchounwou, P.B. Dual effect of oxidative stress in the induction and treatment of leukemia cancer. J. Exp. Clin. Cancer Res. 2014, 106, 3594–3601. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathi, A.K.; Tripathi, P.; Singh, R.; Singh, S.; Singh, R.K. Oxidative stress and antioxidant status in patients with chronic myeloid leukemia. Indian. J. Clin. Bioch. 2008, 23, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Zhao, Z. Redox control in acute lymphoblastic leukemia: From physiology to pathology and therapeutic opportunities. Cells 2021, 10, 1218. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Moore, K.I.; Gundy, P.; Pasvogel, A.; Montgomery, D.W.; Taylor, O.A.; Koerner, K.M.; McCarthy, K.; Hockenberry, M.J. Increase in oxidative stress as measured by cerebrospinal fluid lipid peroxidation during treatment for childhood acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2015, 37, 86–93. [Google Scholar] [CrossRef]
- Tang, X.; Mai, H.; Wang, L.; Chen, S.; Chen, F.; Li, T.; Liu, Y.; Zhou, G.; Liu, S.; Wang, Y.; et al. Diagnostic significance of cerebrospinal fluid flow cytometry in Chinese children with B lineage acute lymphoblastic leukemia. BMC Pediatr. 2024, 24, 204. [Google Scholar] [CrossRef]
- Xu, L.H.; Geng, X.; Liao, N.; Yang, L.H.; Mai, H.R.; Wan, W.Q.; Huang, L.B.; Zheng, M.C.; Tian, C.; Chen, H.Q.; et al. Prognostic significance of CNSL at diagnosis of childhood B-cell acute lymphoblastic leukemia: A report from the South China Children’s Leukemia Group. Front. Oncol. 2022, 12, 943761. [Google Scholar] [CrossRef]
- Zeidler, L.; Zimmermann, M.; Möricke, A.; Meissner, B.; Bartels, D.; Tschan, C.; Schrauder, A.; Cario, G.; Goudeva, L.; Jäger, S.; et al. Low platelet counts after induction therapy for childhood acute lymphoblastic leukemia are strongly associated with poor early response to treatment as measured by minimal residual disease and are prognostic for treatment outcome. Haematologica 2012, 97, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Calzada, C.; Vericel, E.; Lagarde, M. Low concentrations of lipid hydroperoxides prime human platelet aggregation specifically via cyclo-oxygenase activation. Biochem. J. 1997, 325, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.P.S.S.; Magalhães-Gama, F.; Loiola, B.P.; Neves, J.C.F.; Araújo, N.D.; Silva, F.S.; Catão, C.L.S.; Alves, E.B.; Pimentel, J.P.D.; Barbosa, M.N.S.; et al. Systemic immunological profile of children with B-cell acute lymphoblastic leukemia: Performance of cell populations and soluble mediators as serum biomarkers. Front. Oncol. 2023, 13, 1290505. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.W.A.; Magalhães-Gama, F.; Ibiapina, H.N.S.; Hanna, F.S.A.; Xabregas, L.A.; Alves, E.B.; Pimentel, J.P.D.; Carvalho, M.P.S.S.; Tarragô, A.M.; Teixeira-Carvalho, A.; et al. Bone marrow soluble immunological mediators as clinical prognosis biomarkers in B-cell acute lymphoblastic leukemia patients undergoing induction therapy. Front. Oncol. 2021, 11, 696032. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Sun, M.; Yang, L.; Long, F.; Qi, S.; Luo, L.; Lv, X.; Wang, C.; Wu, X.; et al. Molecular characterization and biomarker identification in paediatric B-cell acute lymphoblastic leukaemia. J. Cell. Mol. Med. 2024, 28, e70126. [Google Scholar] [CrossRef]
- Moorman, A.V. New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia. Haematologica 2016, 101, 407–416. [Google Scholar] [CrossRef]
- Cavalcante, M.S.; Torres-Romero, J.C.; Lobo, M.D.; Moreno, F.B.; Bezerra, L.P.; Lima, D.S.; Matos, J.C.; Moreira, R.A.; Monteiro-Moreira, A.C. A panel of glycoproteins as candidate biomarkers for early diagnosis and treatment evaluation of B-cell acute lymphoblastic leukemia. Biomark. Res. 2016, 4, 1. [Google Scholar] [CrossRef]
- Hristozov, D.; Gadjeva, V.; Vlaykova, T.; Dimitrov, G. Evaluation of oxidative stress in patients with cancer. Arch. Physiol. Biochem. 2001, 109, 331–336. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Kim, R.; Hashimoto, A.; Markosyan, N.; Tyurin, V.A.; Tyurina, Y.Y.; Kar, G.; Fu, S.; Sehgal, M.; Garcia-Gerique, L.; Kossenkov, A.; et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 2022, 612, 338–346. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, A.; García-Vicente, R.; Morales, M.L.; Ortiz-Ruiz, A.; Martínez-López, J.; Linares, M. Protein carbonylation and lipid peroxidation in hematological malignancies. Antioxidants 2020, 9, 1212. [Google Scholar] [CrossRef] [PubMed]
- Garbim, M.R.; Broto, G.E.; Trigo, F.C.; Victorino, V.J.; Oliveira, S.T.; Barbosa, D.S.; Panis, C. Chemotherapy induces plasmatic antioxidant changes in pediatric patients with acute lymphoid leukemia B that correlate to disease prognosis. Curr. Res. Immunol. 2022, 3, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Sociedade Brasileira de Oncologia Pediátrica. GBTLI-LLA. In Protocolo de Tratamento da Leucemia Linfoblástica Aguda na Infância; Sociedade Brasileira de Oncologia Pediátrica: São Paulo, Brazil, 2009; pp. 1–6. [Google Scholar]
- Gonzales-Flecha, B.; Lleswy, S.; Boveris, A. Hydroperoxide-initiated chemiluminescence: An assay for oxidative stress in biopsies of heart, liver and muscle. Free Radic. Biol. Med. 1991, 10, 93–100. [Google Scholar] [CrossRef]
- Martínez-Camblor, P.; Pardo-Fernández, J. The Youden Index in the Generalized Receiver Operating Characteristic Curve Context. Int. J. Biostat. 2019, 15, 20180060. [Google Scholar] [CrossRef]
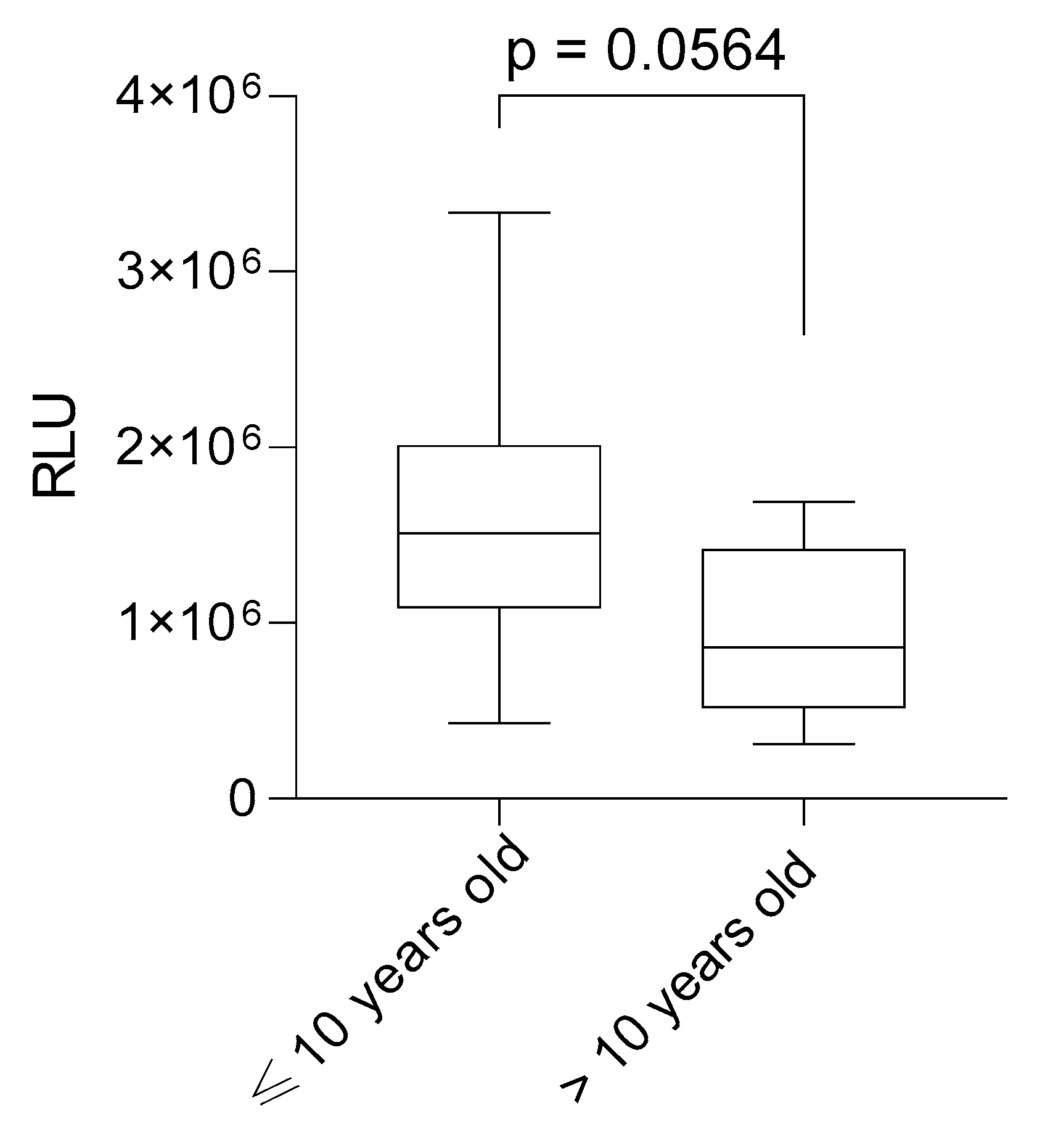
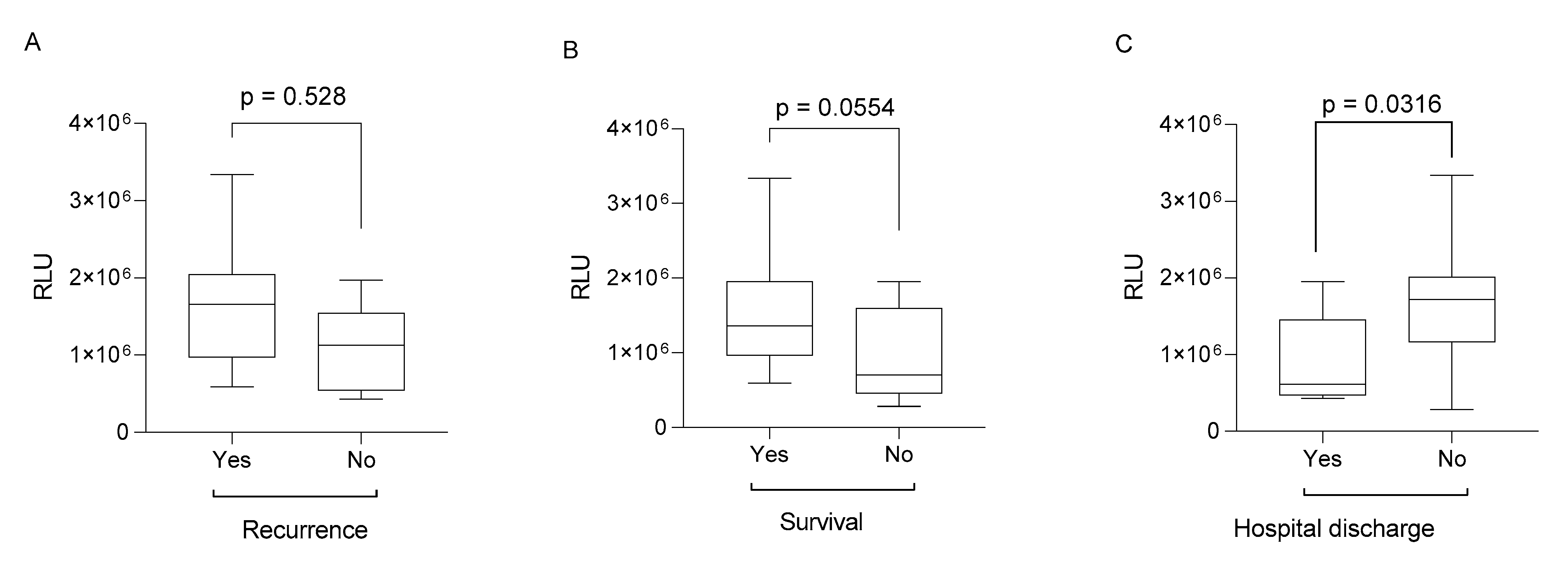
| Parameter | Number of Patients |
|---|---|
| Total number of patients | n = 40 |
| Molecular profile | |
| B cell type | n = 40 (100%) |
| Gender | |
| Female | n = 19 (47.5%) |
| Male | n = 21 (52.5%) |
| Age at diagnosis | |
| Age at diagnosis ≤ 10 | n = 24 (60%) |
| Age at diagnosis > 10 | n = 7 (17.5%) |
| No data | n = 9 (22.5%) |
| Recurrence | n = 10 (25%) |
| Death | n = 17 (42.5%) |
| Hospital discharge | n = 8 (20%) |
| Leukometry, mm3 | |
| Leukometry > 10,000 | n = 10 (25%) |
| Leukometry ≤ 10,000 | n = 17 (42.5%) |
| No data | n = 13 (32.5%) |
| Hemoglobin, g/dL | |
| Hemoglobin > 8 | n = 10 (25%) |
| Hemoglobin ≤ 8 | n = 16 (40%) |
| No data | n = 14 (35%) |
| Platelets, mm3 | |
| Platelet > 50,000 | n = 9 (22.5%) |
| Platelet ≤ 50,000 | n = 18 (45%) |
| No data | n = 13 (32.5%) |
| CNS involvement | n = 5 (12.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koizumi, B.Y.; Sotomayor, M.R.; Coradi, C.; Starck, A.L.G.; Ribeiro, A.W.; Leite, M.B.; Simonato, M.E.P.; Paz, R.G.; Tizzo, V.d.M.; Longo, S.T.; et al. Lipoperoxides as Prognostic Markers in Pediatric B-Acute Lymphocytic Leukemia Patients Undergoing Induction Chemotherapy. Lymphatics 2025, 3, 11. https://doi.org/10.3390/lymphatics3020011
Koizumi BY, Sotomayor MR, Coradi C, Starck ALG, Ribeiro AW, Leite MB, Simonato MEP, Paz RG, Tizzo VdM, Longo ST, et al. Lipoperoxides as Prognostic Markers in Pediatric B-Acute Lymphocytic Leukemia Patients Undergoing Induction Chemotherapy. Lymphatics. 2025; 3(2):11. https://doi.org/10.3390/lymphatics3020011
Chicago/Turabian StyleKoizumi, Bruna Yukie, Marina Rayciki Sotomayor, Carolina Coradi, Ana Luiza Goulart Starck, Anna Will Ribeiro, Maikely Bruna Leite, Maria Eduarda Pardal Simonato, Rafael Gomes Paz, Vinicius de Melo Tizzo, Stefania Tagliari Longo, and et al. 2025. "Lipoperoxides as Prognostic Markers in Pediatric B-Acute Lymphocytic Leukemia Patients Undergoing Induction Chemotherapy" Lymphatics 3, no. 2: 11. https://doi.org/10.3390/lymphatics3020011
APA StyleKoizumi, B. Y., Sotomayor, M. R., Coradi, C., Starck, A. L. G., Ribeiro, A. W., Leite, M. B., Simonato, M. E. P., Paz, R. G., Tizzo, V. d. M., Longo, S. T., Broto, G. E., Trigo, F. C., & Panis, C. (2025). Lipoperoxides as Prognostic Markers in Pediatric B-Acute Lymphocytic Leukemia Patients Undergoing Induction Chemotherapy. Lymphatics, 3(2), 11. https://doi.org/10.3390/lymphatics3020011






