Gata3 Insufficiency Accelerates Recanalization of Damaged Lymphatics via Adjusting Collagen Composition
Abstract
1. Introduction
2. Results
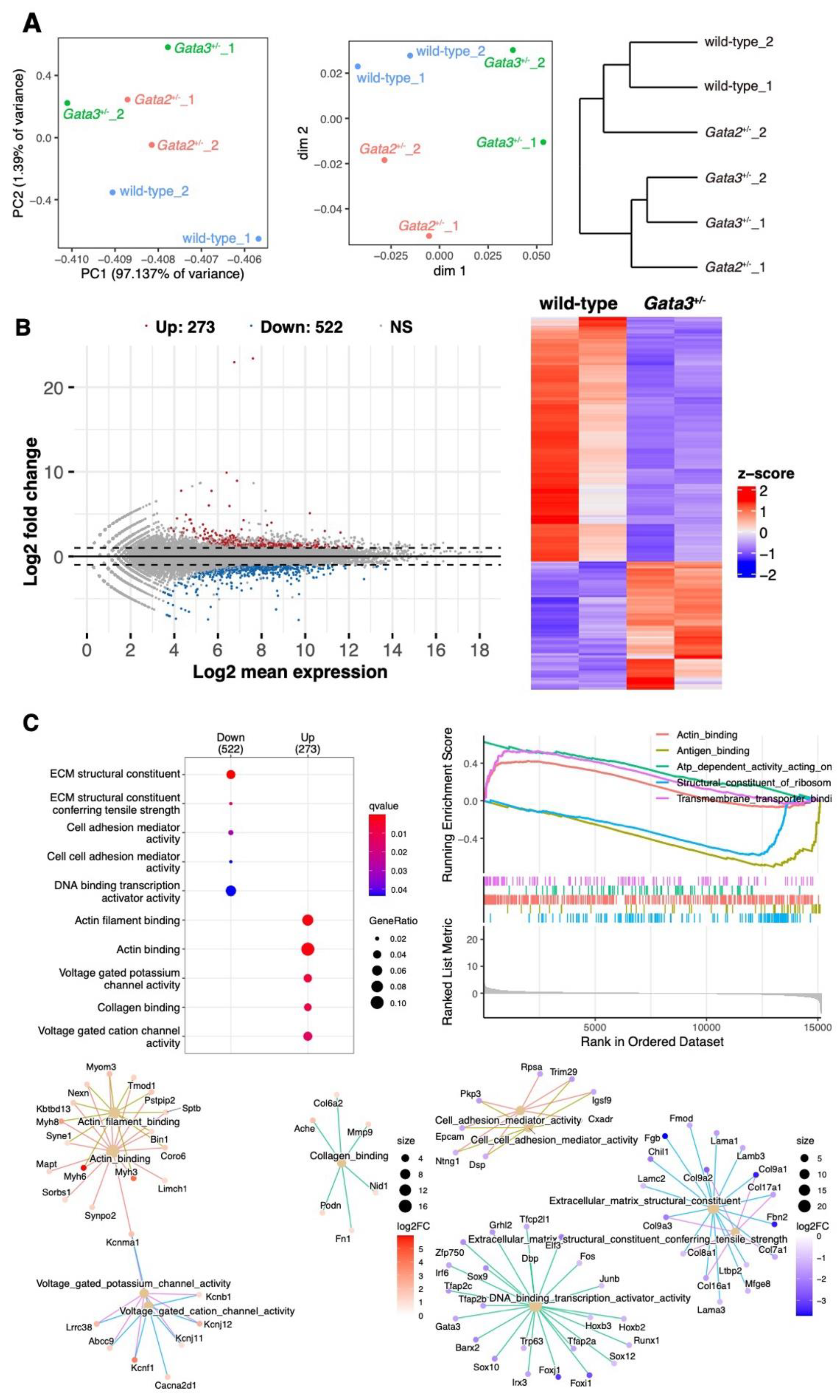
2.1. Different Gene Expression Patterns in the Subcutaneous Tissue of the Popliteal Region of Gata3+/− Mice
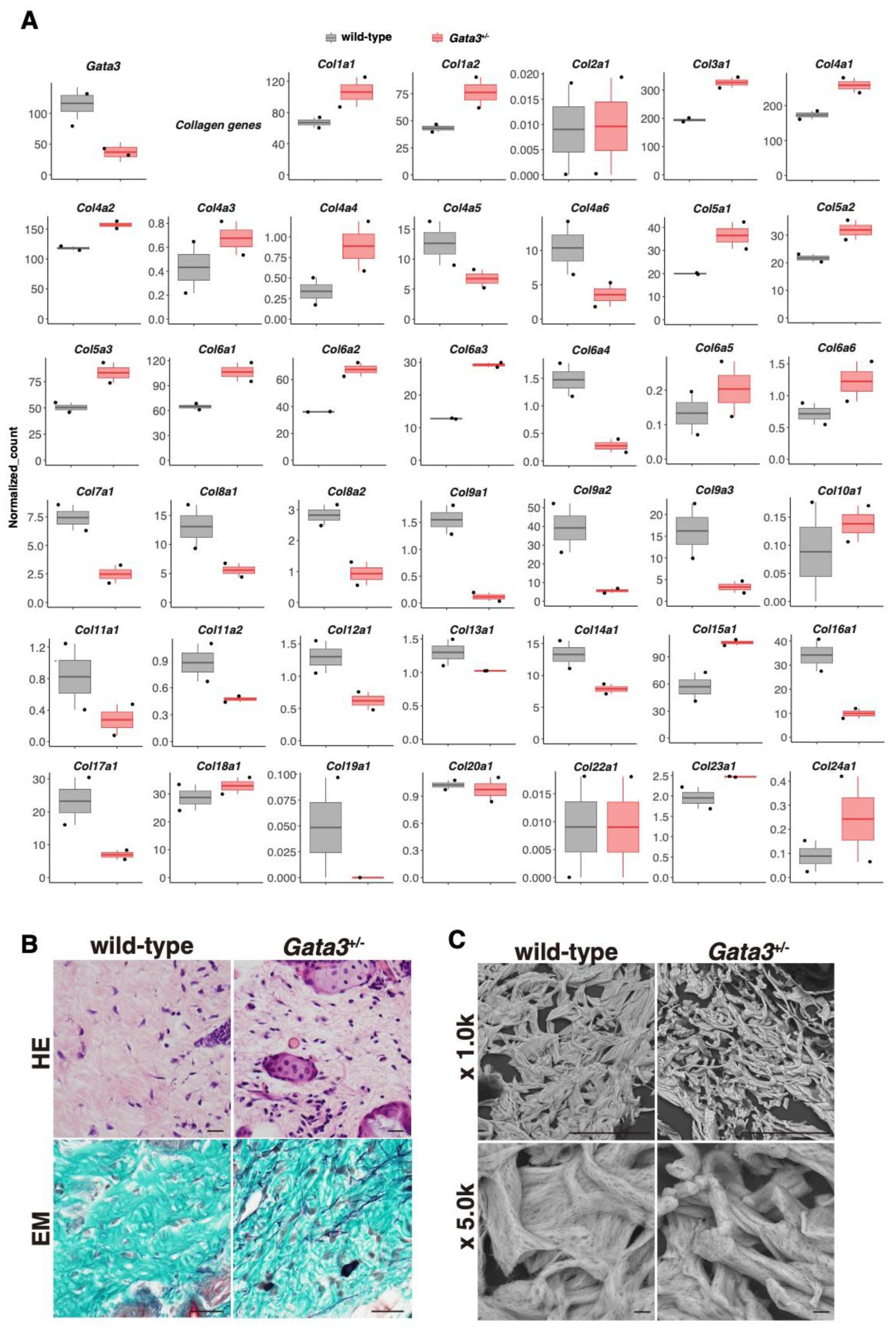
2.2. Altered Expression of the Collagen Gene Cluster and the Presence of Thin Dermal Collagen Fibers in Gata3+/− Skin
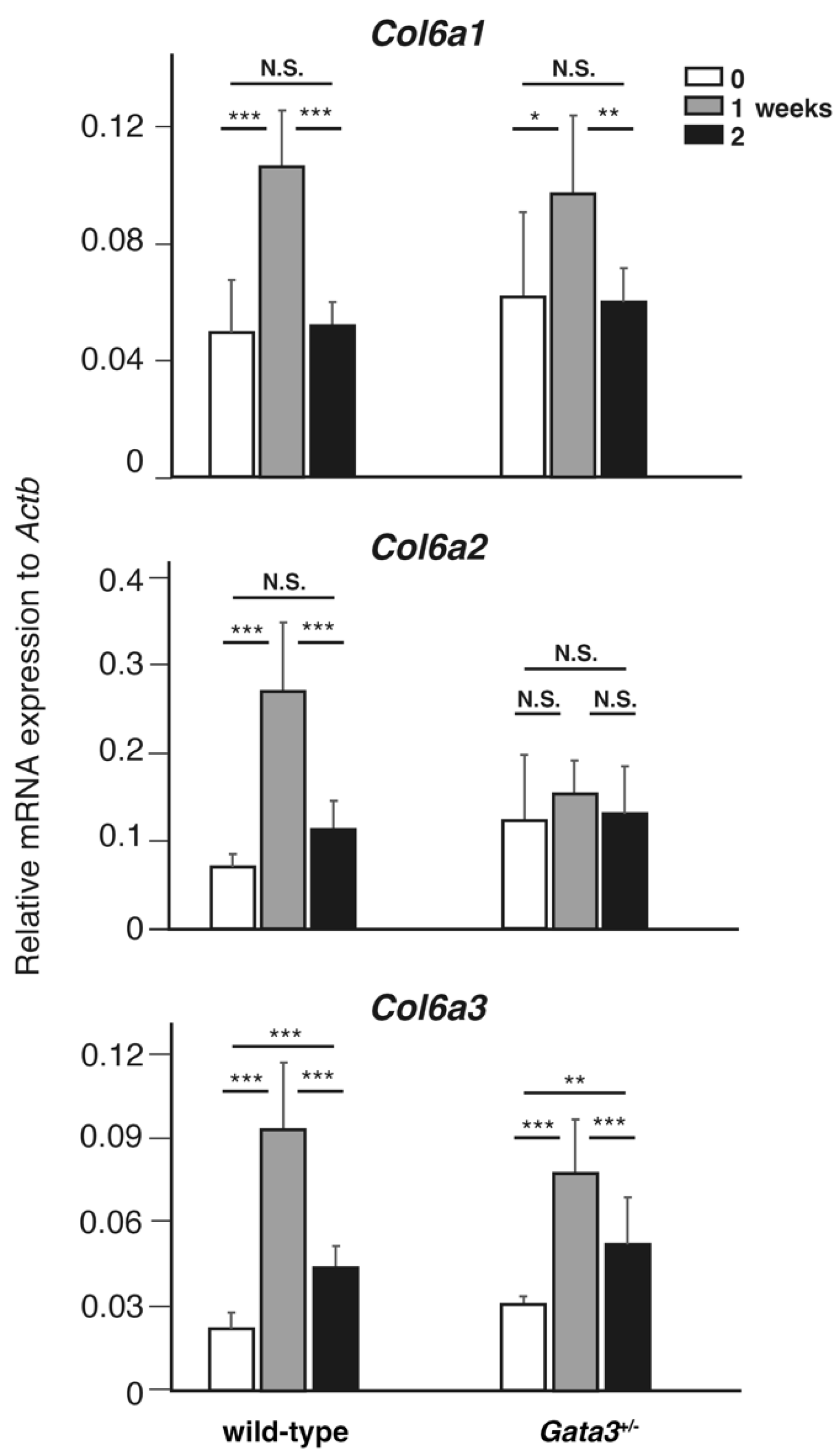
2.3. Transient Upregulation of Col6a Genes During Lymphatic Vessel Recanalization
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Popliteal Lymph Node Extirpation
4.3. RNA-Seq and Bioinformatic Analysis
4.4. Histology
4.5. Scanning Electron Microscopy (SEM)
4.6. cDNA Synthesis and RT-PCR Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G. Advances in Lymphedema. Circ. Res. 2021, 128, 2003–2016. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Wiser, I.; Baik, J.E.; Park, H.J.; Rehal, S.; Shin, J.Y.; Mehrara, B.J. Fibrosis and secondary lymphedema: Chicken or egg? Transl. Res. 2019, 209, 68–76. [Google Scholar] [CrossRef]
- Brown, S.; Dayan, J.H.; Kataru, R.P.; Mehrara, B.J. The Vicious Circle of Stasis, Inflammation, and Fibrosis in Lymphedema. Plast. Reconstr. Surg. 2023, 151, 330e–341e. [Google Scholar] [CrossRef] [PubMed]
- Kazenwadel, J.; Betterman, K.L.; Chong, C.E.; Stokes, P.H.; Lee, Y.K.; Secker, G.A.; Agalarov, Y.; Demir, C.S.; Lawrence, D.M.; Sutton, D.L.; et al. GATA2 is required for lymphatic vessel valve development and maintenance. J. Clin. Investig. 2015, 125, 2979–2994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostergaard, P.; Simpson, M.A.; Connell, F.C.; Steward, C.G.; Brice, G.; Woollard, W.J.; Dafou, D.; Kilo, T.; Smithson, S.; Lunt, P.; et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 2011, 43, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Polat, A.; Dinulescu, M.; Fraitag, S.; Nimubona, S.; Toutain, F.; Jouneau, S.; Poullot, E.; Droitcourt, C.; Dupuy, A. Skin manifestations among GATA2-deficient patients. Br. J. Dermatol. 2018, 178, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Asaka, T.; Hayashi, M.; Uemura, S.; Takai, J.; Suzuki, A.; Moriguchi, T.; Kawai, Y. GATA2 participates in the recanalization of lymphatic vessels after surgical lymph node extirpation. Genes Cells 2021, 26, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Asaka, T.; Hayashi, M.; Harada, T.; Uemura, S.; Takai, J.; Nakamura, Y.; Moriguchi, T.; Kawai, Y. Perturbed collagen metabolism underlies lymphatic recanalization failure in Gata2 heterozygous deficient mice. J. Biochem. 2024, 175, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.C.; Tai, T.S.; Pai, S.Y. GATA3 and the T-cell lineage: Essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 2009, 9, 125–135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yagi, R.; Zhu, J.; Paul, W.E. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 2011, 23, 415–420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bovay, E.; Sabine, A.; Prat-Luri, B.; Kim, S.; Son, K.; Willrodt, A.H.; Olsson, C.; Halin, C.; Kiefer, F.; Betsholtz, C.; et al. Multiple roles of lymphatic vessels in peripheral lymph node development. J. Exp. Med. 2018, 215, 2760–2777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Oliver, G. Development of the mammalian lymphatic vasculature. J. Clin. Investig. 2014, 124, 888–897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frye, M.; Taddei, A.; Dierkes, C.; Martinez-Corral, I.; Fielden, M.; Ortsäter, H.; Kazenwadel, J.; Calado, D.P.; Ostergaard, P.; Salminen, M.; et al. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat. Commun. 2018, 9, 1511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ross, R. The fibroblast and wound repair. Biol. Rev. Camb. Philos. Soc. 1968, 43, 51–96. [Google Scholar] [CrossRef] [PubMed]
- Knoedler, S.; Broichhausen, S.; Guo, R.; Dai, R.; Knoedler, L.; Kauke-Navarro, M.; Diatta, F.; Pomahac, B.; Machens, H.G.; Jiang, D.; et al. Fibroblasts—The cellular choreographers of wound healing. Front. Immunol. 2023, 14, 1233800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Rest, M.; Mayne, R.; Ninomiya, Y.; Seidah, N.G.; Chretien, M.; Olsen, B.R. The structure of type IX collagen. J. Biol. Chem. 1985, 260, 220–225. [Google Scholar] [CrossRef] [PubMed]
- van der Rest, M.; Dublet, B.; Champliaud, M.F. Fibril-associated collagens. Biomaterials 1990, 11, 28–31. [Google Scholar] [PubMed]
- Sun, B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep. Phys. Sci. 2021, 2, 100515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Y.; Sardar, S.; Bay-Jensen, A.C.; Port, H.; Karsdal, M.A. Chapter 9—Type IX collagen. In Biochemistry of Collagens, Laminins and Elastin, 3rd ed.; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 89–95. ISBN 9780443156175. [Google Scholar] [CrossRef]
- Port, H.; He, Y.; Karsdal, M.A.; Madsen, E.A.; Bay-Jensen, A.C.; Willumsen, N.; Holm Nielsen, S. Type IX Collagen Turnover Is Altered in Patients with Solid Tumors. Cancers 2024, 16, 2035. [Google Scholar] [CrossRef]
- Myllyharju, J.; Kivirikko, K.I. Collagens and collagen-related diseases. Ann. Med. 2001, 33, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations on Human Health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nielsen, S.H.; Sardar, S.; Karsdal, M.A.; Henriksen, K. Chapter 36—Collagen diseases. In Biochemistry of Collagens, Laminins and Elastin, 3rd ed.; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 371–398. ISBN 9780443156175. [Google Scholar] [CrossRef]
- Salles Rosa Neto, N.; Pereira, I.A.; Sztajnbok, F.R.; Azevedo, V.F. Unraveling the genetic collagen connection: Clinical and therapeutic insights on genetic connective tissue disorders. Adv. Rheumatol. 2024, 64, 32. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Castori, M.; Francomano, C.A.; Giunta, C.; Kosho, T.; Byers, P.H. The Ehlers-Danlos syndromes. Nat. Rev. Dis. Primers 2020, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Nixon, T.R.W.; Alexander, P.; Richards, A.; McNinch, A.; Bearcroft, P.W.P.; Cobben, J.; Snead, M.P. Homozygous Type IX collagen variants (COL9A1, COL9A2, and COL9A3) causing recessive Stickler syndrome-Expanding the phenotype. Am. J. Med. Genet. A 2019, 179, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Nixon, T.R.W.; Richards, A.J.; Martin, H.; Alexander, P.; Snead, M.P. Autosomal Recessive Stickler Syndrome. Genes 2022, 13, 1135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acke, F.R.E.; De Leenheer, E.M.R. Hearing Loss in Stickler Syndrome: An Update. Genes 2022, 13, 1571. [Google Scholar] [CrossRef]
- Bardhan, A.; Bruckner-Tuderman, L.; Chapple, I.L.C.; Fine, J.D.; Harper, N.; Has, C.; Magin, T.M.; Marinkovich, M.P.; Marshall, J.F.; McGrath, J.A.; et al. Epidermolysis bullosa. Nat. Rev. Dis. Primers 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- South, A.P.; Laimer, M.; Gueye, M.; Sui, J.Y.; Eichenfield, L.F.; Mellerio, J.E.; Nyström, A. Type VII Collagen Deficiency in the Oncogenesis of Cutaneous Squamous Cell Carcinoma in Dystrophic Epidermolysis Bullosa. J. Investig. Dermatol. 2023, 143, 2108–2119. [Google Scholar] [CrossRef] [PubMed]
- van der Wees, J.; van Looij, M.A.; de Ruiter, M.M.; Elias, H.; van der Burg, H.; Liem, S.S.; Kurek, D.; Engel, J.D.; Karis, A.; van Zanten, B.G.; et al. Hearing loss following Gata3 haploinsufficiency is caused by cochlear disorder. Neurobiol. Dis. 2004, 16, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.E.; Park, H.J.; Kataru, R.P.; Savetsky, I.L.; Ly, C.L.; Shin, J.; Encarnacion, E.M.; Cavali, M.R.; Klang, M.G.; Riedel, E.; et al. TGF-β1 mediates pathologic changes of secondary lymphedema by promoting fibrosis and inflammation. Clin. Transl. Med. 2022, 12, e758. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Hirakawa, S.; Sasaki, T.; Inuzuka, K.; Katahashi, K.; Kayama, T.; Yamanaka, Y.; Tsuyuki, H.; Endo, Y.; Naruse, E.; et al. Role of Subcutaneous Adipose Tissues in the Pathophysiology of Secondary Lymphedema. Lymphat. Res. Biol. 2022, 20, 593–599. [Google Scholar] [CrossRef]
- Duhon, B.H.; Phan, T.T.; Taylor, S.L.; Crescenzi, R.L.; Rutkowski, J.M. Current Mechanistic Understandings of Lymphedema and Lipedema: Tales of Fluid, Fat, and Fibrosis. Int. J. Mol. Sci. 2022, 23, 6621. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Yoshida, S.; Yoshimoto, H.; Fujioka, M.; Saijo, H.; Migita, K.; Kumaya, M.; Akita, S. Adipose-Derived Stem Cells and Vascularized Lymph Node Transfers Successfully Treat Mouse Hindlimb Secondary Lymphedema by Early Reconnection of the Lymphatic System and Lymphangiogenesis. Plast. Reconstr. Surg. 2017, 139, 639–651. [Google Scholar] [CrossRef]
- Will, P.A.; Kilian, K.; Bieback, K.; Fricke, F.; Berner, J.E.; Kneser, U.; Hirche, C. Lymphedema-Associated Fibroblasts Are Related to Fibrosis and Stage Progression in Patients and a Murine Microsurgical Model. Plast. Reconstr. Surg. 2024, 154, 688e–700e. [Google Scholar] [CrossRef]
- Son, H.; Lee, S.; Kim, K.; Koo, K.I.; Hwang, C.H. Deep learning-based quantitative estimation of lymphedema-induced fibrosis using three-dimensional computed tomography images. Sci. Rep. 2022, 12, 15371. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, P.P.; Roth, M.E.; Karis, A.; Leonard, M.W.; Dzierzak, E.; Grosveld, F.G.; Engel, J.D.; Lindenbaum, M.H. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat. Genet. 1995, 11, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Takako, N.; Hamada, M.; Maeda, A.; Fujioka, Y.; Kuroha, T.; Huber, R.E.; Hasegawa, S.L.; Rao, A.; Yamamoto, M.; et al. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development 2006, 133, 3871–3881. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Cock, P.J.; Fields, C.J.; Goto, N.; Heuer, M.L.; Rice, P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010, 38, 1767–1771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Etoh, K.; Nakao, M. A web-based integrative transcriptome analysis, RNAseqChef, uncovers the cell/tissue type-dependent action of sulforaphane. J. Biol. Chem. 2023, 299, 104810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yabe, Y.; Hagiwara, Y.; Ando, A.; Tsuchiya, M.; Minowa, T.; Takemura, T.; Honda, M.; Hatori, K.; Sonofuchi, K.; Kanazawa, K.; et al. Chondrogenic and fibrotic process in the ligamentum flavum of patients with lumbar spinal canal stenosis. Spine 2015, 40, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Yabe, Y.; Hagiwara, Y.; Tsuchiya, M.; Honda, M.; Hatori, K.; Sonofuchi, K.; Kanazawa, K.; Koide, M.; Sekiguchi, T.; Itaya, N.; et al. Decreased elastic fibers and increased proteoglycans in the ligamentum flavum of patients with lumbar spinal canal stenosis. J. Orthop. Res. 2016, 34, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sense Primer | Antisense Primer | Assay |
|---|---|---|---|
| Gata3LacZ | TTCGCCAGCTGGCGTAATAGCGAAGAGGC | TAGGTCACGTTGGTGTAGATGGGCGCATCG | genotyping |
| Col6a1 | AACAGGAATAGGAAATGTGACCC | ACACCACGGATAGGTTAGGGG | qPCR |
| Col6a2 | AAGGCCCCATTGGATTCCC | CTCCCTTCCGACCATCCGAT | qPCR |
| Col6a3 | GCTGCGGAATCACTTTGTGC | CACCTTGACACCTTTCTGGGT | qPCR |
| β-actin | AGATCAAGATCATTGCTCCTCCT | ACGCAGCTCAGTAACAGTCC | qPCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, M.; Harada, T.; Takai, J.; Uemura, S.; Moriguchi, T.; Watanabe-Asaka, T.; Kawai, Y. Gata3 Insufficiency Accelerates Recanalization of Damaged Lymphatics via Adjusting Collagen Composition. Lymphatics 2025, 3, 7. https://doi.org/10.3390/lymphatics3010007
Hayashi M, Harada T, Takai J, Uemura S, Moriguchi T, Watanabe-Asaka T, Kawai Y. Gata3 Insufficiency Accelerates Recanalization of Damaged Lymphatics via Adjusting Collagen Composition. Lymphatics. 2025; 3(1):7. https://doi.org/10.3390/lymphatics3010007
Chicago/Turabian StyleHayashi, Moyuru, Takuya Harada, Jun Takai, Satoshi Uemura, Takashi Moriguchi, Tomomi Watanabe-Asaka, and Yoshiko Kawai. 2025. "Gata3 Insufficiency Accelerates Recanalization of Damaged Lymphatics via Adjusting Collagen Composition" Lymphatics 3, no. 1: 7. https://doi.org/10.3390/lymphatics3010007
APA StyleHayashi, M., Harada, T., Takai, J., Uemura, S., Moriguchi, T., Watanabe-Asaka, T., & Kawai, Y. (2025). Gata3 Insufficiency Accelerates Recanalization of Damaged Lymphatics via Adjusting Collagen Composition. Lymphatics, 3(1), 7. https://doi.org/10.3390/lymphatics3010007






