T-Cells Rich Classical Hodgkin Lymphoma, a Pathology Diagnostic Pitfall for Nodular Lymphocyte-Predominant Hodgkin Lymphoma; Case Series and Review
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The international consensus classification of mature lymphoid neoplasms: A report from the clinical advisory committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef]
- Shankar, A.G.; Kirkwood, A.A.; Hall, G.W.; Hayward, J.; O’Hare, P.; Ramsay, A.D. Childhood and adolescent nodular lymphocyte predominant Hodgkin lymphoma—A review of clinical outcome based on the histological variants. Br. J. Haematol. 2015, 171, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Diehl, V.; Sextro, M.; Franklin, J.; Hansmann, M.L.; Harris, N.; Jaffe, E.; Poppema, S.; Harris, M.; Franssila, K.; Van Krieken, J.; et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyterich classical Hodgkin’s disease: Report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J. Clin. Oncol. 1999, 17, 776–783. [Google Scholar] [CrossRef]
- El Hussein, S.; Wang, X.; Fang, H.; Jelloul, F.Z.; Wang, W.; Loghavi, S.; Medeiros, L.J. Nodular lymphocyte-predominant Hodgkin lymphoma with nodular sclerosis: An underrecognized feature associated with pattern D. Am. J. Surg. Pathol. 2022, 46, 1291–1297. [Google Scholar] [CrossRef]
- Nam-Cha, S.H.; Montes-Moreno, S.; Salcedo, M.T.; Sanjuan, J.; Garcia, J.F.; Piris, M.A. Lymphocyte-rich classical Hodgkin’s lymphoma: Distinctive tumor and microenvironment markers. Mod. Pathol. 2009, 22, 1006–1015. [Google Scholar] [CrossRef]
- O’Malley, D.P.; Dogan, A.; Fedoriw, Y.; Medeiros, L.J.; Ok, C.Y.; Salama, M.E. American Registry of Pathology expert opinions: Immunohistochemical evaluation of classic Hodgkin lymphoma. Ann. Diagn. Pathol. 2019, 39, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, I.; Hansmann, M.L.; Franssila, K.; Harris, M.; Harris, N.L.; Jaffe, E.S.; Stein, H. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: Histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood 2000, 96, 1889–1899. [Google Scholar] [PubMed]
- Cirillo, M.; Reinke, S.; Klapper, W.; Borchmann, S. The translational science of Hodgkin lymphoma. Br. J. Haematol. 2019, 184, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Scharf, S.; Steiner, Y.; Loth, A.G.; Donnadieu, E.; Flinner, N.; Hansmann, M.L. Landscape of 4D cell interaction in Hodgkin and non-Hodgkin lymphomas. Cancers 2021, 13, 5208. [Google Scholar] [CrossRef] [PubMed]
- Brune, V.; Tiacci, E.; Pfeil, I.; Döring, C.; Eckerle, S.; Van Noesel, C.J.; Küppers, R. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J. Exp. Med. 2008, 205, 2251–2268. [Google Scholar] [CrossRef]
- Menke, J.R.; Spinner, M.A.; Natkunam, Y.; Warnke, R.A.; Advani, R.H.; Gratzinger, D.A. CD20-negative nodular lymphocyte-predominant Hodgkin lymphoma: A 20-year consecutive case series from a tertiary cancer center. Arch. Pathol. Lab. Med. 2021, 145, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Seliem, R.M.; Ferry, J.A.; Hasserjian, R.P.; Harris, N.L.; Zukerberg, L.R. Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) with CD30-positive lymphocyte- predominant (LP) cells. J. Hematop. 2011, 4, 175. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, G.; Raffeld, M.; Pittaluga, S.; Jaffe, E.S. CD15-expressing nodular lymphocyte-predominant Hodgkin lymphoma. Histopathology 2011, 58, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Wienand, K.; Chapuy, B.; Stewart, C.; Dunford, A.J.; Wu, D.; Kim, J.; Shipp, M.A. Genomic analyses of flow-sorted Hodgkin Reed-Sternberg cells reveal complementary mechanisms of immune evasion. Blood Adv. 2019, 3, 4065–4080. [Google Scholar] [CrossRef] [PubMed]
- Huppmann, A.R.; Nicolae, A.; Slack, G.W.; Pittaluga, S.; Davies-Hill, T.; Ferry, J.A.; Hasserjian, R.P. EBV may be expressed in the LP cells of nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) in both children and adults. Am. J. Surg. Pathol. 2014, 38, 316–324. [Google Scholar] [CrossRef] [PubMed]
- El Hussein, S.; Shaw, K.R.M.; Vega, F. Evolving insights into the genomic complexity and immune landscape of diffuse large B-cell lymphoma: Opportunities for novel biomarkers. Mod. Pathol. 2020, 33, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.S. Anaplastic large cell lymphoma: The shifting sands of diagnostic hematopathology. Mod. Pathol. 2001, 14, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Younes, S.; Rojansky, R.B.; Menke, J.R.; Gratzinger, D.; Natkunam, Y. Pitfalls in the diagnosis of nodular lymphocyte predominant Hodgkin lymphoma: Variant patterns, borderlines and mimics. Cancers 2021, 13, 3021. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.J.; Fergie, M.; Young, M.D.; Jones, C.; Sachdeva, A.; Blain, A.; Carey, C.D. Spatial and molecular profiling of the mononuclear phagocyte network in classic Hodgkin lymphoma. Blood J. Am. Soc. Hematol. 2023, 141, 2343–2358. [Google Scholar] [CrossRef] [PubMed]

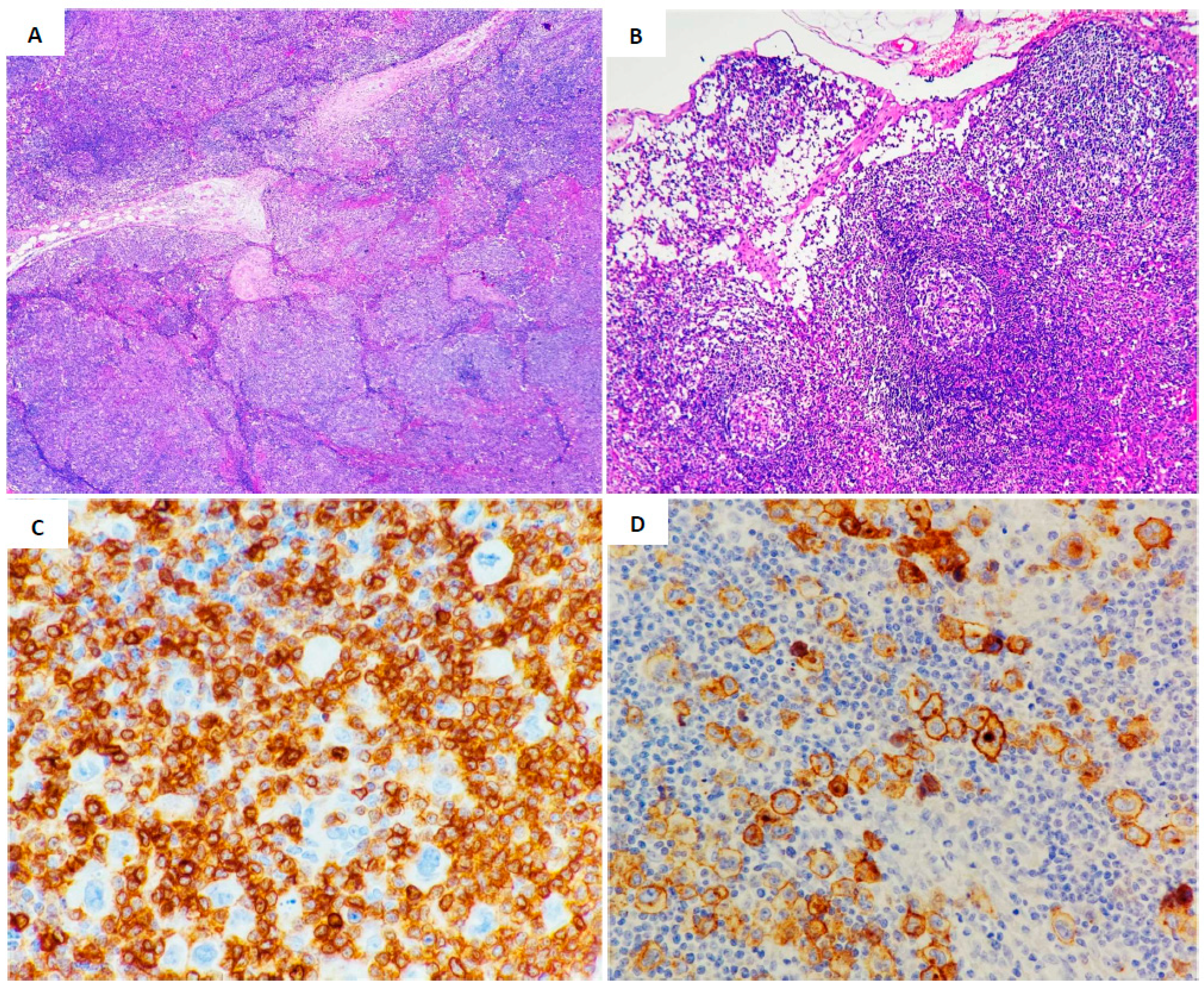
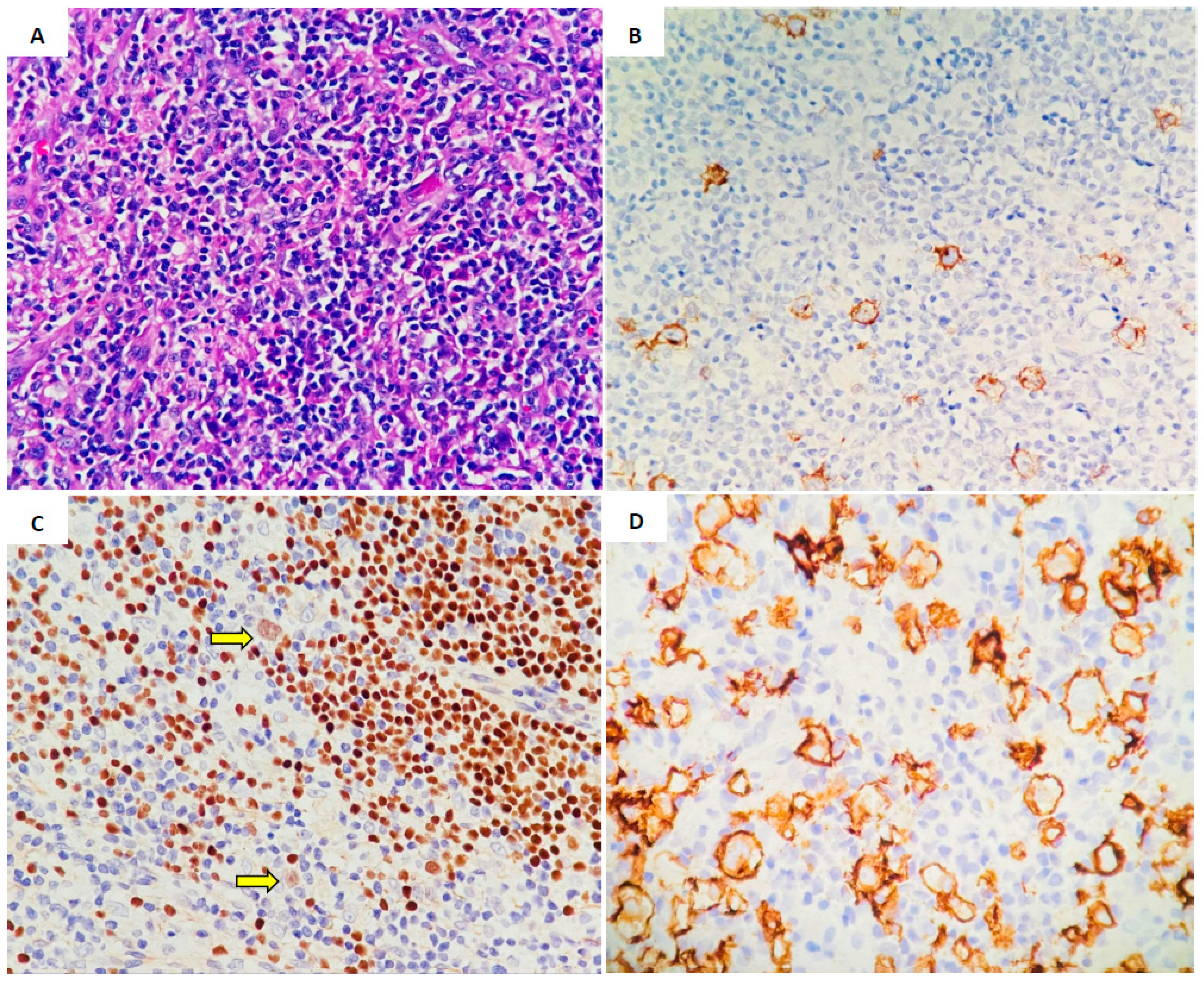
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age (year)/sex | 39 y/Female | 16 y/Female | 34 y/Male |
| Stage | IIA | IIA | I |
| B-symptoms | No | No | Fever, loss of weight |
| Location | Left neck lymph node | Left supraclavicular | Right iliac lymph node |
| LP-like cells | Present | Present | Present |
| T-cell Rosette | Present | Present | Present |
| Residual germinal center | No | Yes | No |
| CD3 positive T-cell rich background | Present | Present | Present |
| Inflammatory cells background (Eosinophils and plasma cells) | No | No | Present |
| CD45 | Negative | Negative | Partial positivity |
| CD20 | Negative | Negative | Partial positivity |
| CD79a | Partial positivity | Negative | Partial positivity |
| PAX5 | Dim positive | Dim positive | Strong positive/subset |
| CD30 | Positive | Positive | Positive/subset |
| CD15 | Positive | Positive | Positive |
| CD3 | Positive T-cells rich | Positive T-cells rich | Positive T-cells rich |
| BOB1 | Negative | Negative | Negative |
| OCT2 | Negative | Negative | Negative |
| EBER | Negative | Negative | Negative |
| IGH rearrangement by PCR testing | Scant material, no result (repeated twice) | Positive | Weak positive |
| TRG rearrangement by PCR testing | Negative | Negative | Negative |
| TRB rearrangement by PCR testing | Negative | Negative | Negative |
| Treatment | Surgical excision followed by 4 cycles ABVD | Surgical excision followed by 6 cycles ABVD | Surgical excision followed by 4 cycles ABVD + Rituximab |
| Radiation therapy | No | No | No |
| Clinical outcome and latest follow up | Complete remission after 1 year | Complete remission after 9 months | Complete remission after 11 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Maghrabi, H.; Mokhtar, G.; Noorsaeed, A. T-Cells Rich Classical Hodgkin Lymphoma, a Pathology Diagnostic Pitfall for Nodular Lymphocyte-Predominant Hodgkin Lymphoma; Case Series and Review. Lymphatics 2024, 2, 168-176. https://doi.org/10.3390/lymphatics2030014
Al-Maghrabi H, Mokhtar G, Noorsaeed A. T-Cells Rich Classical Hodgkin Lymphoma, a Pathology Diagnostic Pitfall for Nodular Lymphocyte-Predominant Hodgkin Lymphoma; Case Series and Review. Lymphatics. 2024; 2(3):168-176. https://doi.org/10.3390/lymphatics2030014
Chicago/Turabian StyleAl-Maghrabi, Haneen, Ghadeer Mokhtar, and Ahmed Noorsaeed. 2024. "T-Cells Rich Classical Hodgkin Lymphoma, a Pathology Diagnostic Pitfall for Nodular Lymphocyte-Predominant Hodgkin Lymphoma; Case Series and Review" Lymphatics 2, no. 3: 168-176. https://doi.org/10.3390/lymphatics2030014
APA StyleAl-Maghrabi, H., Mokhtar, G., & Noorsaeed, A. (2024). T-Cells Rich Classical Hodgkin Lymphoma, a Pathology Diagnostic Pitfall for Nodular Lymphocyte-Predominant Hodgkin Lymphoma; Case Series and Review. Lymphatics, 2(3), 168-176. https://doi.org/10.3390/lymphatics2030014






