1. Introduction
The lymphatic system has long been viewed as a passive component of the immune and circulatory systems. However, in the last 15 years more evidence has emerged to show that the lymphatic system plays an active role in wound healing, cancer, and fibrosis [
1] through regulation and dysregulation of the vasculature. In many of these healing and disease states, the tissue surrounding lymphatic vasculature undergoes dynamic biochemical (e.g., soluble factors) and biophysical (e.g., stiffness) changes that influence cell behavior [
2,
3,
4,
5]. One important biophysical change is the increased tissue stiffening that is often associated with chronic fibrosis—a pathological wound healing condition marked by excess tissue deposition and scarring [
6]. Increased tissue deposition and the subsequent tissue densification also alter tissue transport properties, which change the biochemical environment by altering how signaling molecules are produced and move through the tissue to reach cells. However, little is known about how tissue stiffening affects lymphatic vessel growth and function, particularly under disease conditions where tissue stiffness is much higher than in healthy tissue or during lymphatic development. Thus, it is important to leverage in vitro modeling approaches that use materials capable of exhibiting mechanical properties of healthy and fibrotic tissue environments to understand the impact of stiffness on lymphatic vasculature in fibrosis and wound healing.
Over the last several years, there has been more interest in investigating the relationship between tissue stiffness and lymphatic vasculature. To date, these studies primarily focus on lymphatic development and/or employ modeling strategies that approximate tissue stiffness levels observed across developmental and growth stages. Both Frye et al. [
7] and Alderfer et al. [
8] have demonstrated that Human Dermal Lymphatic Endothelial Cells (HDLECs) appear to be primed for lymphatic capillary formation on softer extracellular matrix (ECM) substrates of approximately 0.03–0.2 kilopascal (kPa). In these studies, HDLECs show increased expression for genes related to cell–matrix adhesion, cell migration (e.g., proteolytic enzymes), and new lymphatic vessel growth, while genes for cell proliferation were more likely to be downregulated. Collectively, they demonstrate the mechanosensing capabilities of HDLECs and highlight the importance of ECM stiffness in directing HDLEC behavior with stiffer substrates appearing to be more inhibitory to behaviors related to lymphatic capillary growth [
7,
8]. However, stiff matrices produced in vitro have the potential to represent stages of in vivo fibrosis where increased ECM stiffness plays a role in hindering lymphatic capillary growth (e.g., myocardial edema, secondary lymphedema) or supporting it (e.g., cancer, renal fibrosis).
As we consider materials for in vitro lymphatic studies, they should also reflect the ECM composition of tissues surrounding lymphatic vessels [
4,
7,
8,
9]. Substrate stiffness differences have been achieved using materials that support a range of stiffness levels but only for 2D monolayer cultures (i.e., Softwell™ and Softslip™ dishes [
7]). Tunable hydrogels that support 2D or 3D cultures and have ECM composition relevant to lymphatic tissue interactions [
10,
11] (e.g., hyaluronic acid (HA) composites [
8]) have also been used. Type I collagen is a viable ECM candidate for lymphatic studies due to its prominence in connective tissues and increased deposition and crosslinking in fibrotic tissues [
12]. Lymphatic capillaries are also directly attached to collagen-rich ECM through adhesion molecules like integrins that bind collagens and fibronectin, as well as elastin microfibril interfacer 1 [
13,
14,
15]. Therefore, fibrosis-mediated changes in type I collagen structure (e.g., crosslinking) and stiffness can be sensed by HDLECs. Methacrylated type I collagen can be used to generate matrices across a wide stiffness range with “on demand” photo-crosslinking (up to 8 kPa) [
16,
17], while maintaining the higher-order fibrillar microstructure found in in vivo tissue. However, despite methacrylated collagens being shown to have a robust mechanical response and support cell viability of multiple cell populations [
16,
17,
18,
19], they have not been leveraged for lymphatic endothelial cell (LEC) cultures.
In the current study, PhotoCol®, a methacrylated type I collagen formulation (Advanced BioMatrix, Inc., Carlsbad, CA, USA) was characterized on its “on demand” stiffening properties and permeability prior to serving as a substrate to assess the effects of ECM stiffness on HDLEC morphology. We hypothesized that PhotoCol®, crosslinked with one of its three commercially available photoinitiators, would increase stiffness to pathological levels while simultaneously decreasing its permeability. We also hypothesized that stiffer hydrogels would induce an LEC phenotype that was indicative of the fibrosis response (i.e., thicker, zipper-like cellular junctions). Of the three photoinitiators tested (Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), Irgacure 2959 (IRG), and Ruthenium/Sodium Persulfate (Ru/SPS)), Ru/SPS yielded photo-crosslinked PhotoCol® samples with the greatest dynamic stiffness range (0.5–6 kPa) with no stiffness difference upon burst light exposures. We then showed that photo-crosslinking also decreased permeability of 40 kilodalton (kDa) dextran but not 60–76 kDa dextran. Morphological assessment of HDLECs cultured on PhotoCol® (with Ru/SPS) at high stiffness (6 kPa) showed thicker cell–cell junctions of vascular endothelial (VE)-cadherin) and increased average cell area and shape irregularity compared to soft hydrogels, which is consistent with morphological changes in lymphatic capillaries within fibrotic tissues. Overall, these results demonstrate that we can leverage on-demand stiffening of PhotoCol®, along with its fibrillar microstructure, as a substrate for in vitro models of lymphatic capillaries focused on modeling fibrotic disease states.
3. Discussion
Recently, there has been an increased focus on the relationship between tissues stiffness and lymphatic vessel response, primarily with lymphatic capillaries and LECs [
6,
7,
8]. For our study, we focused on tissue stiffness levels that are found in fibrotic disease conditions, since tissue stiffening from excess ECM deposition and crosslinking are major features of fibrosis that alter the biophysical and biochemical microenvironments surrounding lymphatic vasculature. Methacrylated type I collagen, combined with photoinitiators (LAP, IRG, or Ru/SPS) and light exposure (356–405 nm), was utilized as an “on-demand” stiffened material for fibrotic tissue disease modeling. The three photoinitiators were evaluated on how they altered the stiffening profiles of PhotoCol
®, and Ru/SPS showed the highest average maximum stiffness, highest fold change after photo-crosslinking (i.e., dynamic range), and the quickest time to stiffness plateau. Importantly, the maximum stiffness of Ru/SPS PhotoCol
® reached an average of approximately 6 kPa, which is within the stiffness range of some diseased fibrotic tissue [
22,
23]. When Ru/SPS PhotoCol
® was tested for stiffness tunability using burst light exposures, a range of 5–9 kPa was achieved, but the fold change in the burst exposure was not statistically different despite an observable trend in increasing stiffness with light exposure. There was also a visual difference in how the PhotoCol
® fibrils formed under different stiffening conditions with longer, more densely packed fibrils with increased photo-crosslinking. The permeability of 40 kDa dextran was statistically different for photo-crosslinked versus uncrosslinked samples, but these effects were not significant for 60–76 kDa dextran. This result shows how molecular transport through methacrylated type I collagen is impacted by photo-crosslinking, which relates to how fibrosis-induced changes in tissues (i.e., crosslinking and stiffening) alters soluble factor signals that direct cell response. HDLEC morphology was also significantly affected by substrate stiffness with increased cell area and irregularity observed on stiffer PhotoCol©. These results were similar to other studies where soft substrates induced smaller cells, and stiff substrates induced flattened and spread-out morphologies [
7]. Notably, our study also quantified the thickness differences in VE-cadherin cell junctions, which are known to be related to healthy and diseased LEC states [
21]. Our approach extends the analysis of LEC response to include cell and nuclear shape metrics, along with VE-cadherin thickness. This morphological assessment is more robust and helpful in assessing LEC cultures, because morphology is less commonly used to describe changes in lymphatic vasculature outside of flow-based studies that focus on changes in elongation and alignment [
24,
25,
26].
The use of methacrylated type I collagen in our study offers an alternative to unmodified type I collagen hydrogels that are unable to achieve high stiffness values. It can support 2D monolayer cultures of LECs, as well as LEC sprouting or outgrowth cultures within 3D. Methacrylated type I collagen can also reach stiffness levels that go beyond that of developing tissues, which shifts the potential for performing in vitro lymphatic studies at stiffness levels observed in diseased, fibrotic tissues. For instance, in conditions like pancreatic cancer with intratumoral and peritumoral lymphatic capillaries, tissue stiffness has been measured at 5.46 ± 3.18 kPa compared to 1.06 ± 0.25 kPa for normal pancreatic tissue [
27]. The ability for methacrylated type I collagen to reach stiffness levels as high as 8 kPa positions us well to study lymphatics across a wide dynamic range of stiffnesses [
16,
17]. Methacrylated type I collagen has an additional advantage over other methacrylated materials, such as gelatin, HA, and various polysaccharides (e.g., dextran, alginate, chitosan) that are routinely used for cell and tissue culture. Although these materials can achieve high stiffness, they lack fibrillar structures on their own [
28,
29], which is important for recreating in vivo connective tissues and dictating ECM biophysical properties—mechanics, microstructure, and transport—that influence cell responses.
Photo-crosslinked methacrylated collagens from human, bovine, and rat tissue sources with LAP and IRG photoinitiators have high mechanical integrity and low cytotoxicity [
16,
17,
18,
19]. While stiffness and lack of toxicity are important to establish, there has been little work exploring other characteristics that impact cell behavior (e.g., permeability, and diffusivity). Our current work helps to fill that gap by evaluating permeability in PhotoCol
® with Ru/SPS. Prior studies have been performed to measure transport of soluble factors like dextran and bovine serum albumin through non-methacrylated type I collagens. Hsu et al. showed an inverse relationship between permeability speed and size of soluble factors but not within the context of changing collagen concentration or crosslinking [
30]. Chen et al. investigated the interplay between concentration and crosslinking to establish release characteristics of methacrylated collagen hydrogels photo-crosslinked with IRG [
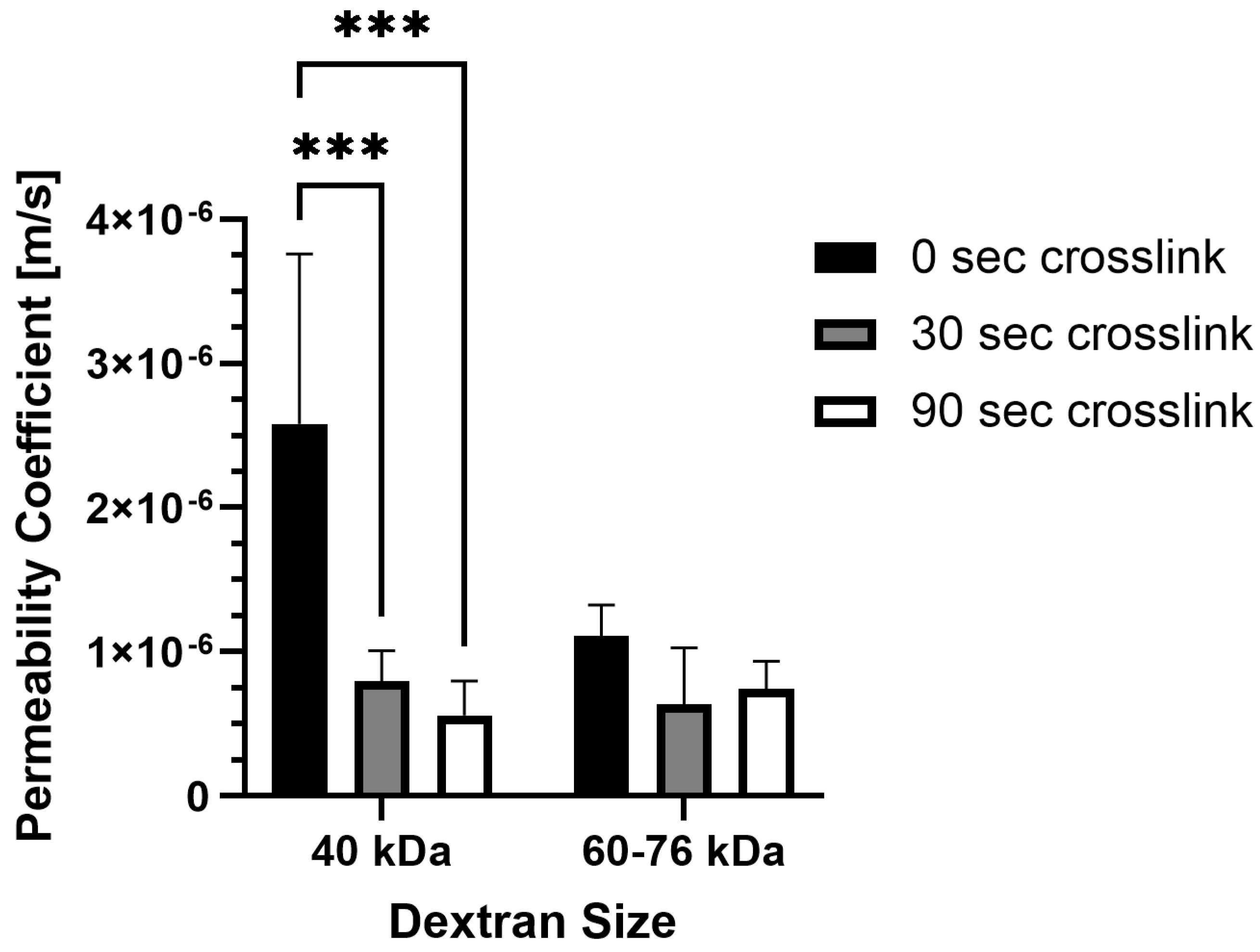
19]. Using 70 kDa FITC-labeled dextran, diffusion was observed to be more dependent on collagen concentration rather than photo-crosslinking, with the highest diffusion rate observed in uncrosslinked collagen at a low concentration. They cited increased fibril density from a higher collagen concentration as the driving factor in hindering diffusion rather than increased stiffness from photo-crosslinking (~40 Pa vs. 200 Pa). However, the effects of crosslinking on fibril density were not explicitly addressed. High fibril density in our samples, resulting from a high collagen concentration, also appeared to hinder transport of higher molecular weight dextran (>60 kDa) compared to 40 kDa (
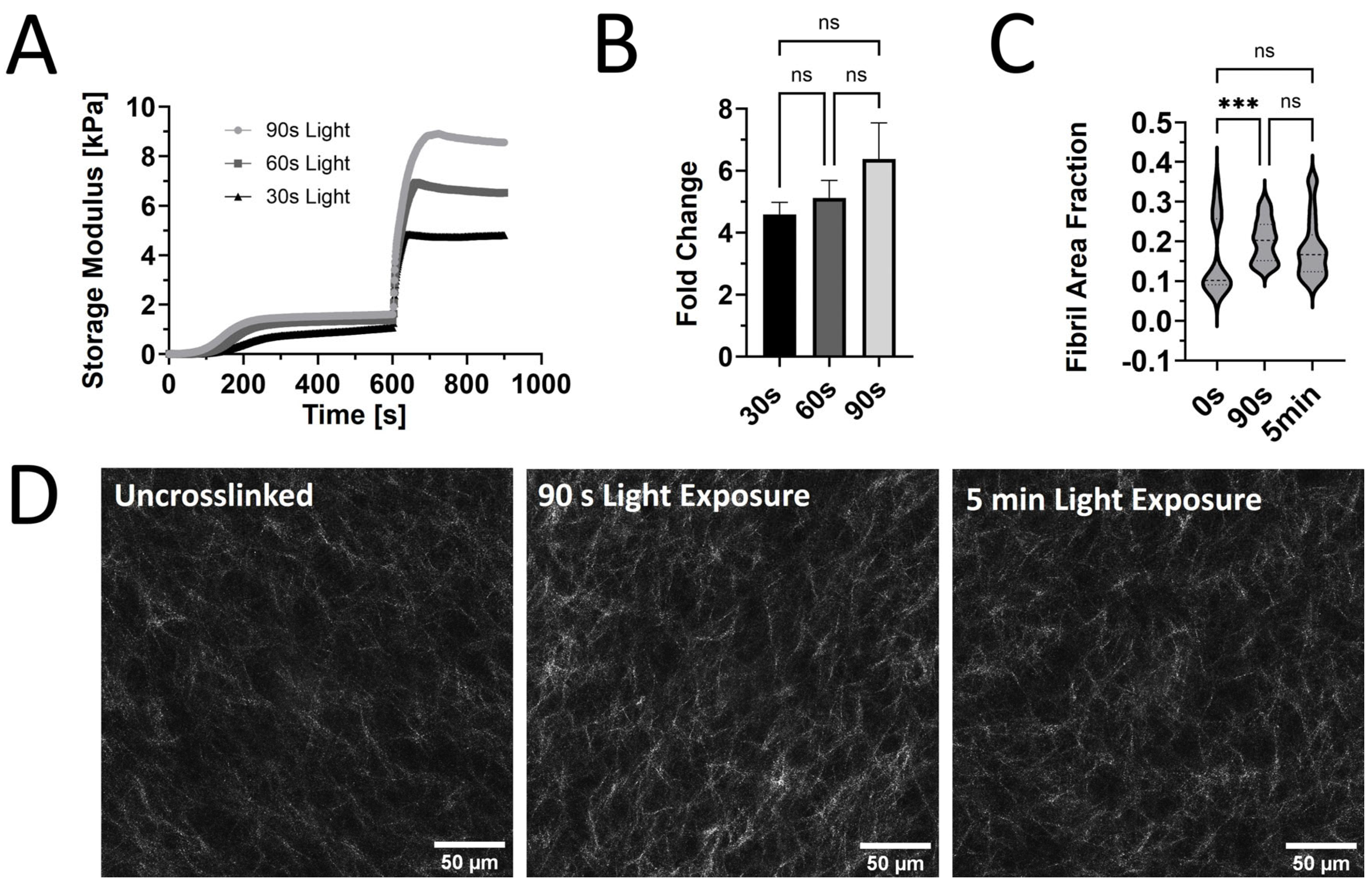
Figure 3) and smaller dextran molecules. Yet, our results also showed a significant decrease in transport with increased photo-crosslinking, which might relate, in part, to the microstructural differences we observed in the fibril area fraction for uncrosslinked PhotoCol
® compared to samples with 30 s light exposure (
Figure 2C). Although we did not observe significant differences in fibril area fraction between 30 s and 90 s light exposure samples or uncrosslinked and 90 s light exposure samples, the differences in permeability observed in the latter comparison supports the idea that the two samples have different microstructures. Collectively, these studies, including our study, provide insight into molecular transport through native and methacrylated collagen gels. We contribute to this area of study by widening the range of molecular sizes and matrix properties (e.g., stiffness and microstructure) to include more features that reflect fibrotic tissues.
Our results also align with other lymphatic studies showing show that HDLECs are responsive to differences in stiffness of the underlying substrate. Alderfer et al. [
8] used modified HA/polyethylene glycol diacrylate (PEG-DA) composite hydrogels to tune ECM stiffness from 30–900 Pa and observed upregulation of genes involved in cell migration and tube formation (i.e., metalloproteinases 2 and 14) on softer matrices. This study also focused on the role of ECM stiffness in activating vascular endothelial growth factor receptor (VEGFR)-3, the primary receptor for vascular endothelial growth factor (VEGF)-C. On softer matrices, VEGFR-3 activation increased, which enabled more VEGF-C binding and subsequent formation of more extensive cord-like structures when compared to stiffer matrices. Frye et al. [
7] cultured HDLECs on Softwell™ or Softslip™ dishes (Matrigen) to represent the tissue surrounding the cardinal vein (0.2 kPa,) and stiffer tissues throughout the body (4 kPa, embryonic cardinal vein; 8 and 12 kPa, muscle; 25 kPa, bone). Similar to Alderfer et al. [
8], they observed upregulation of genes for cell–matrix adhesion, cell migration, and new lymphatic vessel growth (e.g., metalloproteinases 1, 2, and 10), as well as valve formation (e.g., GATA binding protein 2), while genes for cell proliferation were downregulated. These results were consistent with cells preparing for vessel formation and sprouting. In a concurrent in vivo study, LECs that migrated outside of the cardinal vein into a softer ECM were more elongated and spindle-shaped as a precursor to network formation, whereas LECs within the stiffer cardinal vein were flatter and appeared to be tightly attached to the underlying basement membrane [
7]. Our quantitative assessment of LEC morphology was similarly aligned with HDLECs on soft, uncrosslinked PhotoCol
® being smaller (lower area) compared to HDLECs on stiff PhotoCol
® that had a higher cell area (flattened cells) with slightly more elongation (
Figure 5). This result provides more insight into how LECs change morphologically in response to stiffness changes. In general, LEC responsiveness to ECM stiffness is expected since LECs within lymphatic capillaries are directly attached to the underlying ECM [
13,
14,
15]. Interactions between LECs and collagen within connective tissues are uniquely tied to collagen structure. For instance, results from HDLEC studies in vitro and in vivo have shown stiffness-mediated changes in expression of matrix metalloproteinases that target a1(I) and a2(I) peptide chains within the type I collagen triple helix (e.g., metalloproteinases -1, -2, and -14) during cell migration and lymphatic sprouting [
7,
8,
9,
31]. These studies do not exclusively use type I collagen as a substrate for LECs, but the involvement of collagen-associated metalloproteinases shows the importance of leveraging collagen in lymphatic studies with and without stiffness changes. Moreover, we contribute to the field by using methacrylated type I collagen (PhotoCol
®) at higher stiffness levels that are more representative of tissues undergoing fibrosis rather than development.
As lymphatic studies, including our own work, continue to move toward more mechanistic studies in the future, we can leverage our quantitative morphological assessments of cell shape and VE-cadherin junctions to relate alterations in mechanosensing pathways to morphological changes for more robust characterization of model systems. For instance, the Yes-associated protein (YAP)/transcriptional co-activator with PDZ binding motif (TAZ) pathway is notable for its mechanosensing capability of LECs when exposed to oscillatory shear and varied ECM stiffness [
7,
8,
32]. Increased substrate stiffness has been shown to promote YAP/TAZ expression and translocation to the nucleus, which blocks Prox-1 and its downstream targets, VEFGR-3, and matrix metalloproteinase-14. Without robust activation of those two targets, lymphatic sprouting and subsequent formation of cord-like structures decreases. However, studies have not investigated whether this signaling pathway has any impact on cell morphology. Less is also known about relationships between VE-cadherin and either YAP/TAZ or ECM stiffness. Almost all lymphatic studies stain VE-cadherin, many assess VE-cadherin expression via western blot, but very few studies measure VE-cadherin morphology as we did in the current study [
33]. Moreover, the driving factors behind VE-cadherin expression are not typically investigated. In our study, we not only observed thicker VE-cadherin junctions formed in HDLECs on stiff photo-crosslinked samples, but we also noted a potential association between VE-cadherin junctions and F-actin formation. A similar observation was noted in HDLECs on viscoelastic substrates [
11]. Although neither study investigated the link between the two molecules within the context of HDLECs and stiffness, there are established connections between the VE-cadherin/catenin complex with actin [
34]. These molecules are integrated through the processes of junction formation and maturation and remain associated during junction remodeling and maintaining junction integrity. Moreover, additional molecules, such as the Actin Related Protein (ARP)2/3 complex, α-catenin, and p120
ctn, also help coordinate interactions. It will be important to investigate these mechanisms further within the context of lymphatic vasculature and ECM stiffness. Materials like methacrylated collagen will be helpful in these pursuits, because they retain collagen fibrillar microstructure that is important for cell attachment and appropriate force balance between cells and the surrounding ECM.
Through photo-crosslinking, we produced elastic hydrogels in the current studies that have covalent crosslinks that achieve static stiffness. However, there are temporal elements to the fibrotic process as excess ECM is deposited and crosslinked over time. Beyond stiffness, the biochemical environment also shifts with changes in ECM permeability that affect the movement of soluble factors and altered biodegradation that impacts tissue remodeling. Moreover, temporal changes in tissue stiffness and remodeling also affect ECM viscoelasticity. Fan et al. recently looked beyond static ECM stiffness and identified viscoelasticity as an important ECM property that impacts lymphatic morphogenesis and tube formation [
11]. They combined supramolecular and covalent crosslinking to create dynamic HA hydrogels with tunable viscoelasticity that is spatially controlled with UV light exposure. Although the hydrogels exhibit the same maximum shear storage modulus (~1500 Pa) before and after UV irradiation, they differ in stress-relaxation behavior from static stiffness hydrogels (fully covalently crosslinked and elastic). In standard and photo-patterned viscoelastic HA substrates, HDLECs showed evidence of a higher degree of cell spreading and migration with increased F-actin stress fiber formation and focal adhesion assembly. We also observed greater cell area and F-actin formation on our photo-crosslinked samples, even though our stiffness levels were ~4 times higher than Fan et al. HDLECs on viscoelastic HA hydrogels also formed a more extensive and branched lymphatic tube network compared to elastic (static) hydrogels with increased expression of characteristic lymphatic markers such as lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), Prospero homeobox protein 1 (Prox-1), podoplanin, and VEGFR-3. Matrix metalloproteinases 1, 2, and 14, which are involved in ECM degradation and remodeling events necessary for tube formation, also increased on viscoelastic hydrogels. Interestingly, these are some of the same markers that others observed as being expressed at higher levels on softer substrates (30–200 Pa) [
7,
8]. Collectively, these observations and study results provide rationale for why viscoelasticity and temporal stiffening are important factors to consider beyond static stiffness when modeling dynamic processes like fibrosis. These differences may also be a contributing factor to why in vitro lymphatic capillaries do not match the dense, non-functional capillary beds seen in vivo in disease.
Even though the current work focuses on a new material approach to investigate the lymphatic response to ECM stiffness, we recognize other work within the field that is making progress toward improved understanding of the lymphatic system. Beyond our work and other work with natural materials that were previously discussed, Hooks et al. recently used a synthetic poly(ethylene glycol) (PEG) hydrogel functionalized with four maleimide groups (PEG-4MAL) and binding arginylglycylaspartic acid (RGD) ligands to observe the relationship between matrix elasticity (stiffness), ligand binding density, and degradability on lymphatic sprouting [
35]. Their study was designed to target one of the drawbacks of collagen, being that ligand density and stiffness profiles of gels are not independent when using collagen concentration to alter collagen stiffness. They were able to control matrix elasticity via PEG weight percentage without altering the RGD ligand density and generate matrices at 680 Pa with comparable ligand density to 2 mg/mL collagen at 20 Pa. Moreover, they showed successful lymphatic sprouting in vitro and functional grafting into host vasculature in vivo. Although they were able maintain ligand density at increased stiffness levels, methacrylated collagen behaves similarly by achieving multiple stiffness levels via photo-crosslinking at a single collagen concentration [
16]. Methacrylated collagens are also capable of being integrated into lymphatic-on-a-chip models, which are popular models for studying lymphatic sprouting and growth [
25,
36,
37,
38,
39,
40]. Lymphatic capillaries sprout into ECM materials from LEC-lined channels, usually guided by a gradient of growth factors such as VEGF-C and sphingosine 1 phosphate [
24]. Disease is currently a part of lymphatic-on-a-chip modeling; however, the focus tends to be on using soluble factors and co-cultures to recreate a disease environment. As with most lymphatic capillary models, ECM-based features of fibrosis have not been included in lymphatic-on-a-chip models. Therefore, there is an opportunity to merge the two approaches to advance the field of lymphatic modeling to include more robust disease models with altered stiffness and transport properties that impact LEC behavior.
4. Materials and Methods
Table 1 includes all product information for reagents, consumables, major equipment, and software used in the study, including catalog number and company.
4.1. Preparation of Methacrylated Collagen Hydrogels (PhotoCol®)
Lyophilized methacrylated type I collagen (PhotoCol®, Advanced BioMatrix, Carlsbad, CA, USA) was solubilized on a rotator at 4 °C in sterile 20 mM acetic acid to a final concentration of 8 mg/mL. Three photoinitiators were prepared according to manufacturer (Advanced BioMatrix) protocols: Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP, 17 mg/mL stock volume), Irgacure 2959 (IRG, 10% stock), and Ruthenium (Ru)/Sodium Persulfate (SPS) (Ru at 37.4 mg/mL and SPS at 119 mg/mL stock volume). A neutralization solution (Advanced BioMatrix) at 8% (v/v) (7.407 mg/mL) and the photoinitiator at 2% (v/v) were mixed with PhotoCol® to make the final hydrogel mixture for each photoinitiator (LAP—7.262 mg/mL; IRG—7.334 mg/mL; and Ru/SPS—7.122 mg/mL). Neutralized PhotoCol® hydrogels with the photoinitiator were incubated at 37 °C for a minimum of 30 min for hydrogel self-assembly prior to photo-crosslinking in subsequent studies.
4.2. Rheological Assessment of PhotoCol® Viscoelasticity
Matrix viscoelastic properties (shear storage modulus, G′ and shear loss modulus, G″) were measured using a MCR 302e WESP rheometer (Anton Paar, Graaz, Austria) with a quartz stage to photo-crosslink PhotoCol® hydrogel samples (365–405 nm) during testing. Frequency and strain sweeps were conducted in oscillatory shear on fully crosslinked and un-crosslinked hydrogels to determine the linear viscoelastic region. Temporal changes in stiffness in response to photo-crosslinking were conducted in time sweeps (constant: 0.1% strain, 1 Hz). Hydrogel samples were allowed to self-assemble for 10 min at 37 °C before exposure to one of two light exposure conditions—constant and burst exposure—using 365 or 405 nm UV mounted LED (Thorlabs, Newton, NJ, USA). Constant light was applied for 20 min after self-assembly for complete photo-crosslinking and maximum stiffness (G’). Burst light exposures consisted of a single light pulse for 30, 60, or 90 s after self-assembly with continued measurement for 10 min after light exposure.
Using MATLAB (MathWorks, Natick, MA, USA), a locally weighted regression was used on each replicate to create a fitted curve that could be analyzed [
41]. The first derivate was taken of these regressions to determine rate of change in stiffness. A threshold of 3 Pa/s rate was used to determine the regions of plateau and regions of stiffening. Average stiffness values of the plateau regions before and after light exposure, within the regions of stiffening, were used to calculate fold change by the ratio of each replicate’s post-stiffening average to the same replicate’s pre-stiffening average. The post-stiffening averages of each replicate were then averaged to give each group’s average maximum stiffness. The average number of seconds that the rate of change in stiffening was above the 3 Pa/s threshold was used to calculate the amount of time to stiffness plateau.
4.3. Microstructural Assessment of PhotoCol® Hydrogels
Confocal laser scanning microscopy in reflectance mode was used to obtain images of fibrillar collagen microstructure for qualitative assessment. All samples were prepared in Nunc™ Lab-Tek™ II Chambered Coverglass #1.5 slides (ThermoFisher Scientific, Waltham, MA, USA). Briefly, PhotoCol® hydrogels with Ru/SPS were neutralized and allowed to self-assemble for 30 min at 37 °C as previously described. Photo-crosslinked samples were exposed to 405 nm light for 90 s (longest burst exposure) or 5 min (sufficient time to reach maximum G’) using a 405 nm UV mounted LED (Thorlabs). Images were collected in reflectance mode with a Leica TCS SP5 Spectral Confocal Microscope (Leica Microsystems, Wetzlar, Germany) using a 40× air objective (3D z-stack). Each z-stack began 20 μm above the cover glass and consisted of 15 slices with 2 μm spacing between slices (30 μm total thickness). Three stacks were taken per sample. Using CellProfiler™ (Broad Institute, Cambridge, MA, USA), images were enhanced and clarified with the “openlines” function. Threshold images were then created from these and the pixel area ratio was calculated to determine the fibril area ratio.
4.4. Permeability Assessment of PhotoCol® Hydrogels
A transwell membrane insert system protocol adapted from Hsu et al. [
30] was used to characterize the permeability of PhotoCol
® hydrogels with the Ru/SPS photoinitiator. Neutralized PhotoCol
® (60 μL; 7.122 mg/mL) was polymerized for 60 min at 37 °C on an 8.0 μm PET membrane 24-well transwell insert (Corning, New York, NY, USA) within a tissue-cultured treated 24-well plate (Corning). For photo-crosslinking, transwell inserts containing PhotoCol
® were removed and exposed to 405 nm near UV light for 30 or 90 s. Lyophilized human fibronectin (Advanced BioMatrix) was reconstituted in Milli-Q water and diluted in 1X PBS (Fisher Bioreagents, Waltham, MA, USA) to achieve 5 μg/cm
2 (0.03 mg/mL) on the PhotoCol
® surface. Samples were then incubated at 37 °C for 1 h to establish a fibronectin coating and submerged in 1X PBS (overnight at 37 °C) to remove any excess Ru/SPS from the PhotoCol
® gels. A 2 mg/mL solution of fluorescein isothiocyanate (FITC)-labeled dextran at 40 kDa or 60–76 kDa (Sigma Aldrich, St. Louis, MO, USA) was added on top of the fibronectin-coated PhotoCol
® samples within the inserts as the donor solution, while 1X PBS was pipetted into the outer well to serve as the acceptor solution. Solution volumes were controlled to be at equal heights to minimize flow due to hydrostatic pressure. PhotoCol
® with FITC-dextran was incubated at 37 °C, and 20 μL was removed from the acceptor solution at 1 h increments for a total of 3–5 h. The sampled solution was then diluted at 1:25 in 1X PBS and transferred to a black-walled polystyrene 96-well plate (Corning) to be read on a PerkinElmer VICTOR Nivo plate reader (Revvity, Waltham, MA, USA) (480 nm excitation/530 nm emission). A standard curve (0–0.0125 mg/mL of FITC-dextran) was used to calculate the concentration of measured samples. To calculate the permeability coefficient, Fick’s second law was used, using the dimensions of the PhotoCol
® sample (i.e., surface area) and the change in concentration of the acceptor solution in the linear region of permeability.
4.5. Lymphatic Endothelial Cell Culture
Human Dermal Lymphatic Endothelial Cells (HDLECs) isolated from adult skin (PromoCell, Heidelberg, Germany) were maintained according to manufacturer’s instructions in endothelial growth media with MV2 growth supplements (PromoCell) that contain the following: fetal calf serum (5% v/v), epidermal growth factor (recombinant human, 5 ng/mL), basis fibroblast growth factor (recombinant human, 10 ng/mL), insulin-like growth factor (Long R3 IGF, recombinant human, 20 ng/mL), vascular endothelial growth factor 165 (recombinant human, 0.5 ng/mL), ascorbic acid (1 μg/mL), and hydrocortisone (0.2 μg/mL) (PromoCell). All cell culture surfaces were coated with human fibronectin (3.5 μg/cm2; Advanced BioMatrix) prior to HDLEC seeding to promote cell attachment. Cells were passaged and maintained in Fisherbrand™ Vented Cap Surface Treated Sterile Tissue Culture Flasks (Fisher Scientific, Waltham) until ready for experimental use. Cells were maintained at 37 °C in a humidified incubator (5% CO2) and passaged at 70–90% confluency. All cells were used between passages 6 and 12 for experiments.
4.6. Morphological Assessment of Lymphatic Endothelial Cell Stiffness Response
HDLEC viability was confirmed via direct and indirect exposure to Ru/SPS followed with a Live/Dead™ stain (Invitrogen, Waltham, MA, USA) and quantified using FIJI (See
Supplementary Materials). HDLECs were characterized by their morphological response to hydrogels at different stiffness levels. Cells were seeded in a 15-well glass bottom μ-slide (ibidi GmbH, Gräfelfing, Germany) at 10,000 cells/cm
2 on top of fibronectin-coated PhotoCol
® prepared with Ru/SPS (photo-crosslinking, 90 s of light exposure, and high G’) and without photoinitiator (uncrosslinked, no light exposure, and low G’) for a total culture time of 3 days (37 °C, 5% CO
2). All groups were fixed with 4% paraformaldehyde (ThermoFisher Scientific) in 1X PBS for 15 min at 37 °C and permeabilized using 0.1% of Triton™ X-100 (Sigma Aldrich) diluted in 1X PBS. Samples were stained with Alexa Fluor™ 488 Phalloidin (1:400, ThermoFisher Scientific) to visualize F-actin and counterstained with 4′,6-diamidino-2-phenylindole (300 nM DAPI; ThermoFisher Scientific) to visualize the nuclei. For immunostaining of characteristic HDLEC markers, fixed samples were blocked with bovine albumin fraction V (7.5% solution; ThermoFisher Scientific) diluted in 1X PBS to 1% and incubated with primary antibodies for VE-cadherin (1:1000 ThermoFisher Scientific). Samples were then rinsed and incubated with Alexa Fluor™ Plus 647-conjugated secondary antibody donkey anti-rabbit IgG (H + L) (1:200, ThermoFisher Scientific). All samples were imaged with a Keyence BZX810 All-in-One Fluorescence Microscope (KEYENCE Corp. of America, Itasca, IL, USA). Images were collected at 20× magnification for cellular and nuclear area, cellular and nuclear eccentricity, cellular form factor, and VE-cadherin thickness, utilizing the Keyence BZ-X800 analysis software (Version 1.1.2.4) to create full focus images from z-stacks. The threshold of these images was obtained utilizing CellProfiler™, removing signal from cell nuclei and noise (including rounded dead cells with prominent staining) before obtaining the threshold image of just the VE-cadherin cellular outline (see “Original” and “Threshold” images in
Figure 5C to show signal removal). Primary (nuclei) and secondary objects (cell body) were obtained and quantified using CellProfiler™ as well. Threshold images were then quantified in FIJI, using distance map and Vessel Analysis plugins. Due to the visual similarity between threshold images of vasculature and threshold images of cell boundaries, the Vessel Analysis plugin was able to accurately obtain average vessel diameters of four random regions of interest in each image (n = 4, n = 3).
4.7. Statistical Analysis
Statistical analysis was carried out using GraphPad Prism 10.0 (GraphPad Software Inc., La Jolla, CA, USA). Stiffness data were expressed as mean storage modulus + standard deviation (SD) without removal of outliers. Permeability data were expressed as a mean + SD after outliers were removed using Grubbs’ test (α = 0.05). Morphological values were expressed as mean ± SD without removal of outliers. Normality was verified through a Shapiro–Wilk normality test using α = 0.05. Multiple comparisons were performed using one-way analysis of variance (ANOVA) (maximum stiffness, fold change, and fibril area fraction) or a two-way ANOVA (permeability), and parametric data were analyzed using Tukey’s post-hoc method (significance: * p < 0.05, ** p < 0.001, *** p < 0.0001, and **** p < 0.00001). Nonparametric data for morphological analysis were analyzed with the Mann–Whitney U test (significance: (** p < 0.005 and **** p < 0.0001).