Abstract
Hodgkin’s lymphoma (HL) is a monoclonal lymphoid neoplasm that is mainly characterized by multinucleated Reed–Sternberg cells on a background of non-neoplastic inflammatory cells. The incidence rate of Hodgkin’s lymphoma is 2.5 new cases per 100,000 people per year (1). Paraneoplastic syndromes are conditions that are related to malignancy; however, they are not a result of tumor invasion or compression of malignant tissues. These paraneoplastic syndromes can occur virtually at any point in the disease course, and paraneoplastic syndromes in HL and their various forms are not well studied. In this review article, we will be discussing paraneoplastic syndromes in general and then delve into specific syndromes seen in HL, followed by a brief discourse regarding their early recognition and timely management.
1. Introduction
Hodgkin’s lymphoma (HL) is a rare monoclonal lymphoid neoplasm with two main variants with distinct microscopic and clinical features: (1) classical and (2) nodular lymphocyte-predominant (NLP) HL. Classic HL has a characteristic microscopic finding of multinucleated Reed–Sternberg cells on a background of non-neoplastic inflammatory cells and accounts for approximately 95% of cases of HL. It is further characterized into subtypes including nodular sclerosis, mixed cellularity, lymphocyte rich, and lymphocyte depleted. Conversely, nodular lymphocyte-predominant HL, representing approximately 5% of HL cases, does not have Reed–Sternberg cells; rather, it has characteristic lymphocyte-predominant (LP) cells surrounded by either B or T lymphocytes on histology. HL typically presents with painless lymphadenopathy, profound fatigue, and B symptoms, including fever, night sweats, and unintentional weight loss [1]. Compared to classic HL, NLP-HL tends to have a more indolent course, which often leads to earlier diagnoses and typically spares mediastinal lymph nodes. HL can also present without the classical aforementioned findings, as paraneoplastic syndromes (PNSs). PNSs are uncommon in HL, making their diagnosis particularly challenging. However, it is important to recognize these entities early as they can lead to earlier diagnoses of malignancy in cases where the PNS manifests before the malignancy itself, having the potential to impact clinical outcomes significantly [2,3]. In the years 2016–2020, HL had an incidence rate of approximately 2.5 new cases per 100,000, and the death rate was 0.3 per 100,000 people per year. Approximately 220,000 people in the US had HL in 2022. Over time, with therapeutic advances, the death rate of HL has been gradually declining without much change in the incidence of cases, leading to increasing prevalence [4]. Neurological syndromes are by far the most common type of PNS associated with HL, constituting nearly half of cases (42%) [5].
A systematic review of classic HL showed that the simultaneous diagnosis of PNSs and HL occurred in 42.2% of cases, and in 33.6% of cases, the diagnosis of HL antedated the diagnosis of PNSs, while in 16.4% of cases, it was the diagnosis of PNSs that preceded that of HL [5]. Limited data also exist on the type of PNS in relation to the time of HL diagnosis. Paraneoplastic cerebellar degeneration is a type of PNS associated with HL, which tends to occur before the diagnosis of HL in approximately 80% of cases. Studies of limbic encephalitis (LE), another PNS associated with HL, showed that the delay between the onset of LE and HL ranged from 0 to 52 months, with a mean value of 8.5 months and a median of 6 months, amongst 11 cases [2]. It is difficult to determine how much these estimates are affected by the missed or delayed diagnosis of PNSs. Hence, our understanding of the temporality between HL and PNSs may change as we learn to better recognize PNSs associated with HL.
PNSs in HL are not well studied due to their low incidence, making their diagnosis and management challenging. This review aims to synthesize currently available data on PNSs related to HL to aid clinicians with their recognition and management.
2. General Overview of Paraneoplastic Syndromes and Pathophysiology
PNSs refer to a condition that is related to malignancy; however, it occurs neither by tumor invasion nor by compression of tissues by the tumor. They are also unrelated to infection, nutritional deficiencies, or treatment-related adverse effects. PNSs can occur at any point in the course of cancer and affect virtually any system. The diagnosis of PNSs requires the exclusion of alternative causes, which are usually more likely.
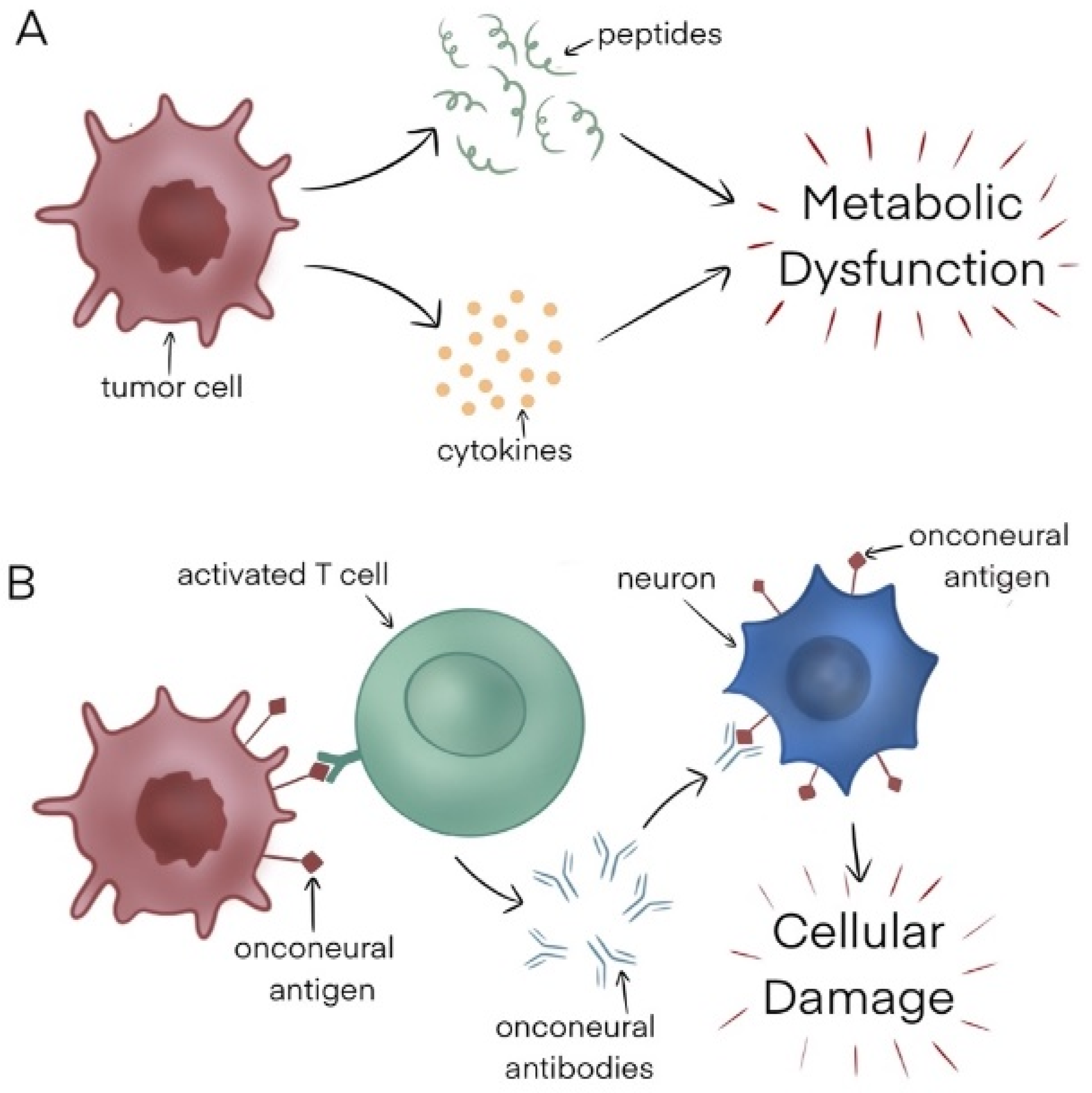
Although the pathophysiology underlying paraneoplastic syndromes is poorly understood, in general, there are two proposed mechanisms—nonimmune-mediated and immune-mediated (Figure 1).
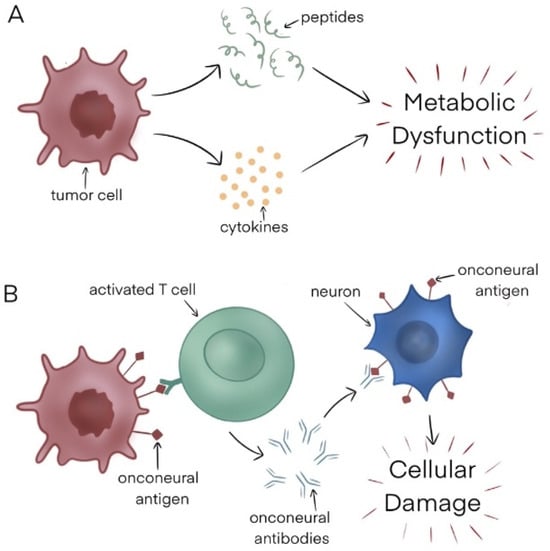
Figure 1.
(A) Nonimmune mediated mechanism. (B) Immune-mediated mechanism.
Nonimmune mediated: One nonimmune mediated mechanism is the tumor secretion of various chemical entities, including hormones, cytokines, and peptides, which in turn impacts host tissues, leading to metabolic abnormalities that manifest as a constellation of symptoms. An example of this is hyponatremia caused by ectopic production of the antidiuretic hormone by small-cell lung cancer cells.
Another way PNSs may occur is when there is competition between the tumor and a specific system for a particular substrate. An example of this is tryptophan depletion caused by neuroendocrine tumors, resulting in carcinoid syndrome [6].
Finally, immunoglobulins produced by certain malignancies like Waldenstrom macroglobulinemia can react with the peripheral nervous system, resulting in several neurological manifestations.
Immune-mediated: Tumor cells may be highly immunogenic, with the ability to elicit both cell-mediated and humoral immune responses. This leads to immune cell activation by antigens present on tumor cells, stimulating the production of antibodies that can attack host tissues when their host and tumor antigens share biochemical similarities. For instance, myasthenia gravis is caused by acetylcholine receptor-directed antibodies, which are made in response to thymoma tumor cells.
3. Paraneoplastic Syndromes in Hodgkin Lymphoma by System
PNSs in HL can affect multiple organs, including neurologic, dermatologic, endocrine, hepatic, renal, hematologic, and rheumatologic. They appear to be more common in males and present across all stages of HL [5].
3.1. Neurological Syndromes
Paraneoplastic neurological syndromes are by far the most common subtype of PNS associated with HL, accounting for approximately 42.2% of cases [5]. The incidence of neurological PNSs in HL reported in population-based European studies ranges from 0.21 to 1.22 per 100,000 person years [7,8]. They often result from specific autoantibodies directed either against the underlying tumor cells, normal nervous system cells, or both. A paraneoplastic neurological syndrome care score (PNS-Care), proposed by a panel of international experts, can be used to aid in diagnosing these conditions [9]. It considers three components: clinical phenotype, detection of neuronal antibodies, and the presence of cancer. By scoring the patient in these areas, the diagnostic certainty of a condition being a paraneoplastic neurological syndrome is categorized as ‘definite’, ‘probable’, ‘possible’, or ‘non-PNS’, in decreasing order of certainty. The term ‘high-risk phenotype’ is now used when referring to syndromes that are classically characterized as paraneoplastic neurological syndromes. High-risk neurological phenotypes include rapidly progressive neurological syndrome, limbic encephalitis, encephalomyelitis, opsoclonus–myoclonus syndrome, sensory neuropathy, Lambert–Eaton myasthenic syndrome, and enteric neuropathy. The detection of onconeural antibodies can support a diagnosis of paraneoplastic neurological syndrome but on their own cannot definitively confirm nor reject a diagnosis of PNS. An onconeural antibody can be considered to be a ‘high-risk antibody’ (i.e., associated with cancer in >70% of cases), an ‘intermediate-risk antibody’ (associated with cancer in 30–70% of cases), or a ‘low-risk antibody’ (i.e., associated with cancer in less than 30% of cases). Table 1 shows onconeural antibodies associated with HL and describes the risk of association with malignancies as per the PNS-Care panel criteria [9]. Generally speaking, treatment options for suspected paraneoplastic neurological syndromes include IVIG (intravenous immunoglobulin), plasmapheresis, corticosteroids, cyclophosphamide, and rituximab when the concomitant lymphoma has not been identified [10]. It is important to note that paraneoplastic neurological syndromes, like PNSs in general, are diagnoses of exclusion.

Table 1.
Onconeural antibodies associated with HL.
3.1.1. Cerebellar Degeneration
Paraneoplastic cerebellar degeneration (PCD) is amongst the most frequently cited PNSs associated with HL. The hallmark of PCD is severe loss of Purkinje cells of the cerebellum, causing subacute pancerebellar dysfunction. In 1976, Trotter et al. first detailed the discovery of antibodies targeting cerebellar Purkinje cells with the use of immunofluorescent staining in a patient with subacute cerebellar degeneration who was found to have HL. This suggested that Purkinje cells contain antigens that can cross-react with onconeural bodies produced as part of the host’s immune response [11]. The term ‘subacute cerebellar degeneration’ is synonymous with ‘rapidly progressive cerebellar syndrome’. Symptoms of this condition typically lead to severe disability within a period of 3 months [12].
The diagnosis of rapidly progressive cerebellar degeneration as a PNS requires the presence of truncal and limb ataxia [9]. Characteristic signs and symptoms of PCD include ataxia—both truncal and limb—dizziness, dysarthria, nystagmus, and diplopia, which are all cerebellar signs. This can be preceded by a prodromal syndrome with the occurrence of fever, nausea, vomiting, and headaches [13]. Marsili et al. describe anecdotal evidence of nuanced symptoms presenting months before the diagnosis of PCD including cognitive fluctuation during the same day, mild sensations of imbalance, dizziness, nausea, and vomiting [12]. Data supporting this diagnosis include classical imaging findings and the detection of high-risk antibodies. Magnetic resonance imaging (MRI) is the gold standard imaging modality to diagnose PCD, although it can be normal in the early course of disease. Hyperintensity of cerebellar hemispheres in T2 sequences appears to be the next chronological imaging feature, which is followed by the characteristic finding of cerebellar atrophy. Cerebrospinal fluid analysis may typically reveal pleocytosis or elevated protein levels in most patients, with a minority demonstrating oligoclonal bands [14]. While there are several different antibodies associated with PCD, the association is typically with antibodies to the Tr or delta/notch-like epidermal growth factor-related receptor (Tr/DNER) in HL [13,14,15]. These antibodies are usually detected in cerebrospinal fluid but can also be present in serum [16]. Another antibody that is associated with HL-related PCD is anti-mGluR1, which targets a glutamate receptor [17,18]. Autoantibodies against the intracellular Purkinje cell protein RGS8 have also been identified in one patient with HL and PCD, but further studies are needed to confirm this association [19]. The diagnosis of PCD typically precedes the diagnosis of HL by months to years, and treatment of the underlying lymphoma can lead to partial to complete remission of symptoms related to PCD, but this is infrequent [20,21,22,23]. There are reports of PCD presenting as a relapse of HL [16,18].
In general, the use of therapies beyond chemotherapy including corticosteroids, intravenous immunoglobulin (IVIG), cyclophosphamide, and tacrolimus does not seem to improve neurological outcomes in the majority of patients with PCD related to any malignancy [24]. With regard to HL specifically, management strategies have included either individual therapies or a combination of chemotherapy, steroids, IVIG, or plasma exchange, and even radiation chemotherapy alone with complete remission of neurological symptoms [18], partial remission [16], minimal improvement with severe disability, or no improvement [16,18,20,23].
While the dataset is very limited, outcomes appear to be better with improved response to treatment when PCD is diagnosed early. However, the treatment of HL takes precedence when concurrently diagnosed. In the urgent setting, IVIG +/− steroids and plasma exchange can be utilized. The Tr (or DNER) antibody titers usually disappear after HL treatment [21].
3.1.2. Limbic Encephalitis (LE)
LE is a neuropsychiatric disorder that classically presents with rapidly progressive symptoms including acute and subacute memory impairment, seizures, and psychiatric manifestations including anxiety, depression, and psychosis, which typically lead to severe disability within three months of symptom onset. There are numerous antibodies associated with LE and this condition can be paraneoplastic or nonparaneoplastic. Antibodies to mGluR5 are associated with HL-related PNSs [25]. The association of HL and memory impairment has been referred to as ‘Ophelia syndrome’ [26]. Characteristic MRI findings for LE include hyperintensity and swelling of mesial temporal lobes, which often exhibit an increase in a fludeoxyglucose-18 (FDG) positron emission tomography (PET) scan [27]. Supportive CSF findings include pleocytosis and oligoclonal bands [28]. In general, chemotherapy is an effective regimen, and ABVD (doxorubicin, vinblastine, bleomycin, and dacarbazine) is the most commonly used regimen and usually leads to partial neurological recovery at least and even complete recovery in some cases. Table 2 contains further details of cases of limbic encephalitis associated with HL.

Table 2.
Neurological paraneoplastic syndromes.
3.1.3. Granulomatous Angiitis of the Central Nervous System
This disorder is a necrotizing vasculitis that affects small vessels of both the venous and arterial systems of the leptomeninges, brain parenchyma, and spinal cord in the absence of systemic vasculitis. The pathophysiology behind this PNS in HL remains unclear. Most patients present with neurological dysfunction prior to a diagnosis of HL with symptoms of headache, weakness, ataxia, confusion, or altered consciousness [50].
Diagnosis can be challenging depending on the vascular territory involved. A brain biopsy can help exclude other etiology including malignancies like intravascular lymphoma, secondary CNS involvement of HL, infection, etc., and thus confirm a diagnosis.
A combination of cyclophosphamide, steroids, and HL-specific chemotherapy has been seen to be an effective treatment [2,50].
Other less frequently encountered neurological PNSs have been described further in Table 2.
3.2. Hepatic Syndromes
3.2.1. Vanishing Bile Duct Syndrome (VBDS)
VBDS is a syndrome consisting of paraneoplastic intrahepatic cholestasis with the hallmark histological findings of intrahepatic bile duct loss. The exact mechanism behind this PNS remains unknown; however, it has been theorized to be either immune-mediated or related to cytokine production by tumor cells promoting cholestasis. This is a diagnosis of exclusion and a thorough work-up is necessary to exclude hepatic infiltration by tumor, lymphomatous liver infiltration, external biliary tract compression by tumor or lymph nodes, hemolysis, infections such as hepatitis and cytomegalovirus, autoimmune hepatitis, and the effects of hepatotoxic drugs, amongst others. A liver biopsy is necessary to make the diagnosis. Recognition of HL-related VBDS is of paramount importance, as the hepatic dysfunction can quickly progress to liver failure with high mortality if definitive treatment of HL is not promptly initiated; it can sometimes progress despite this, potentially requiring liver transplantation. A literature review by Ballonoff et al. suggested that this occurs mostly in males in their 30s and may occur across different stages of HL. Radiotherapy was often utilized to treat early-stage HL, as the use of traditional chemotherapies was limited by the degree of hepatic dysfunction. Early (stage I or II HL) disease at diagnosis, use of radiotherapy, and complete remission of HL were all associated with improved liver-failure-free survival at one year since diagnosis of VBDS [53]. Another review consisting of a total of 46 patients with VBDS conducted by Scalabrini et al. compared the usage of full vs. reduced dose upfront chemotherapy with resolution of VBDS. A total of 18 patients had resolution of VBDS and, of these, only one received a reduced dose regimen, suggesting the benefit of using standard dosing of chemotherapy initially. All of these patients with VBDS resolution also received upfront steroids and had a resolution of HL as well. The role of high-dose ursodeoxycholic acid in treating liver disease was also noted [54]. Various chemotherapy regimens have been utilized with varying degrees of success, as summarized in Table 3.

Table 3.
Vanishing bile duct syndrome.
In summary, the use of radiotherapy to treat underlying early-stage HL is a reasonable choice when faced with VBDS, as traditional regimens are hepatotoxic and generally not well tolerated. When chemotherapy is given upfront, dose adjustments for hepatic function are necessary, often with the elimination of doxorubicin, which may be resumed once hepatotoxicity has resolved.
3.2.2. Paraneoplastic Intrahepatic Cholestasis
This syndrome is very similar to VBDS, with the absence of ductopenia, which is theorized to be a precursor condition to VBDS. This is also a diagnosis of exclusion and a work-up similar to the one described for VBDS is crucial to arriving at this diagnosis. Dose-reduced chemotherapy can be used initially to minimize hepatotoxicity, with ursodeoxycholic acid and steroids as an adjunct. Standard chemotherapy regimens can be used once liver function tests have normalized [64].
3.3. Renal Syndromes
Nephrotic syndrome is one of the PNSs that is mainly demonstrated as intrinsic renal lesions associated with glomerular injury. Although the association between HL and renal PNS is difficult to prove, it may be supported by the temporal relationship between the two manifested by improvement or resolution of symptoms with treatment of underlying malignancy.
The link between HL and nephrotic syndrome has been described before and is most often due to minimal change disease [65,66]; it is seen with classical HL in particular [67]. This association was first described in 1922 by Gagliano et al. and was the first recorded instance of paraneoplastic glomerulopathy [68]. The incidence of this entity is reported in the range of 0.6% to 1% of HL cases. The poor response of minimal change disease-type nephrotic syndrome to steroids and cyclosporine has been suggestive of underlying HL [69]. While minimal change disease is the most commonly seen glomerulonephropathy associated with HL, other forms have also been seen in association with HL. This includes focal segmental glomerulosclerosis (FSGS) [66], AA amyloidosis, crescentic glomerulonephritis [70], and immunoactoid glomerulopathy, which is characterized by Congo red-negative deposits in renal microtubules on biopsy [71].
The pathogenesis has been linked to the overexpression of certain paraneoplastics that result in both lymphomagenesis and renal manifestations [72]. In addition, renal manifestations are associated with the expression of VEGF and TGF-β1, which are reportedly increased in Reed–Sternberg cells in HL [73,74]. In the various case reports described, this entity was treated with chemotherapy using various regimens including OEPA (araneoplasticone, vincristine, doxorubicin, and etoposide) and ABVD [65]. There was resolution of renal manifestations in both scenarios.
3.4. Dermatological Syndromes
3.4.1. Eczematous Eruptions
Paraneoplastic skin conditions like diffuse hyperpigmentation, erythema nodosum, and acquired ichthyosis have been described in the literature [75,76,77]. The main pathophysiology is hypothesized to be T cell dysregulation and increased Th2 cytokine profiles conducive to atopy. There are reports of biopsy-proven chronic eczema diagnosed in a young woman preceding a diagnosis of HL [78]. The patient was later treated with ABVD chemotherapy. Her eczema resolved after the second cycle, suggestive of a paraneoplastic phenomenon.
3.4.2. Paraneoplastic Pemphigus (PNP)
PNP is characterized by diffuse mucocutaneous involvement resulting in painful blisters, skin erosions, and lichen planus-like eruptions. Non-Hodgkin’s lymphoma and chronic lymphocytic lymphoma are classically associated with paraneoplastic pemphigus [79]. PNP has rarely been reported in association with HL, comprising approximately 0.6% of cases of PNP [77]. Steroids, along with typical standard HL therapy, have been seen to be effective treatment [80].
3.5. Other Hematological Syndromes
Autoimmune Cytopenias (AICs)
Various autoimmune cytopenias including autoimmune hemolytic anemia (AIHA), autoimmune neutropenia, and immune thrombocytopenia purpura (ITP) have been associated with HL, although the incidence is quite rare at <1% of patients [81]. They may occur prior to, concurrent with, or at the time of recurrence of HL or in complete remission after treatment. However, they most commonly appear to precede a diagnosis of HL [82]. Autoimmune hemolytic anemias in this context tend to involve warm autoantibodies and occur more often in men. Standard chemotherapy for AIC rather than steroids has been seen to be effective in all these cases. Adjuncts seen to be effective for AIHA include combinations of steroids, IVIG, and splenectomy [82]. Autoimmune neutropenia and thrombocytopenia have been seen to respond well to steroids [82,83].
3.6. Miscellaneous Paraneoplastic Arthritis
Paraneoplastic arthritis in general is hypothesized to be related to immune complex deposition in joints. Rarely, this can be associated with HL. A case report by Erlij et al. describes a patient presenting with symmetric, seronegative polyarthritis and acute renal failure about three months prior to a diagnosis of HL. Although his arthritis responded to steroids initially, he had a recurrence of renal failure which resolved only with ABVD chemotherapy [84]. Although polyarthritis did not recur like kidney dysfunction, its timing and lack of recurrence are certainly suggestive of paraneoplastic pathology related to HL. A combination of migratory polyarthritis and acute renal failure occurring prior to a diagnosis of HL was also demonstrated in a case report by Aruch et al. Knee joint arthrocentesis revealed an inflammatory, noninfectious arthritis which was refractory to steroid treatment. However, it resolved with ABVD chemotherapy [85]. Although paraneoplastic arthritis has been associated with antinuclear antibodies and rheumatoid factor in other malignancies [84], both these cases were seronegative.
Other miscellaneous PNSs associated with HL have been described in Table 4. Of note, twenty-three patients in this study had ‘paraneoplastic cerebellar degeneration’, one had ‘subacute cerebellar syndrome’, one had ‘chronic mild, cerebellar ataxia’, and one had Tr antibodies and symptoms suggestive of limbic encephalitis but normal MRI. The results were not split up among the aforementioned categories.

Table 4.
Miscellaneous paraneoplastic syndromes.
4. Challenges and Future Directions
With the increasing use of immune checkpoint inhibitors, we are seeing an increase in the incidence of paraneoplastic phenotypes of autoimmune neurological disorders, particularly amongst malignancies that are not typically associated with PNSs. Improved detection techniques undoubtedly also play a role in this. Theoretically, it does make sense that immunotherapy could amplify the immunological process leading to the development of PNSs, just as the antitumor effect is augmented, with uninhibited T cell activation [96]. Neurological adverse effects related to immunotherapy are particularly important as they are often complicated by long-term sequelae and can even be fatal [97]. A review by Farina et al. investigates the occurrence of neurological immune-related adverse effects (n-irAE) in 147 patients with malignancies receiving immune checkpoint inhibitor therapy. Out of this cohort, 20.4% of patients were determined to have paraneoplastic-like syndromes. Conditions that are deemed high risk to represent PNS, including rapidly progressive cerebellar syndrome, limbic encephalitis, Lambert–Eaton myasthenic syndrome (LEMS), and sensory neuropathy, were characterized as ‘paraneoplastic-like syndromes’. Older age and the diagnosis of PNSs were both associated with poor neurological outcomes, including disability and death, in this study. The majority of patients were treated with immune-active therapies, which only consisted of steroids in 95.8% of cases. Other therapies included the use of intravenous immunoglobulin, plasmapheresis, immunosuppressants, and biotherapies [98]. Interestingly, immunotherapy can be used as a treatment modality for patients with paraneoplastic neurological syndromes who do not respond to first-line therapy, but data on this are limited [99].
Recognizing stereotypical clinical syndromes, testing for onconeural antibodies, obtaining imaging to look for specific findings associated with neurological paraneoplastic syndromes, and conducting a thorough work-up to exclude alternate diagnoses including direct tumor-mediated pathology are all important steps in arriving at the diagnosis of immunotherapy-triggered PNSs [97]. This is an emerging area of medicine and we will likely see more studies in the next few years, which will hopefully allow us to better understand and manage these conditions.
5. Discussion
A recent systematic review by El Fakih et al. with a composite of 128 patients best summarizes PNSs in classical HL. Neurological manifestations appear to be the most frequent PNS, and within this category, central nervous system (CNS)-related pathology was most common. This was followed by hepatic and then renal pathology, in order of decreasing frequency. Most cases did not have antibodies associated with them, which highlights the challenge these diagnoses present. In 54.7% of cases, the PNSs resolved with treatment of HL, and another 26.6% of patients showed improvement of the PNSs with residual symptoms remaining [5].
Paraneoplastic syndromes in Hodgkin lymphoma represent a rare manifestation, which can occur prior to or concurrently with the malignancy. Due to this rarity, there is a paucity of data pertaining to these conditions, which makes diagnosing as well as treating them a difficult task.
There is a paucity of data on the role of routine surveillance for cancer when PNSs are diagnosed in the absence of malignancy. Although some authors have advocated for routine surveillance every 4–6 months for 2 years following the diagnosis of a condition that is suspicious for a PNS [13], we recommend having a high degree of clinical suspicion and a low threshold to pursue imaging when they develop symptoms.
6. Conclusions
In conclusion, PNSs are conditions related to malignancy that are uncommon and, especially in HL, are a rare entity. It is imperative to gain knowledge of different clinical entities or presenting symptoms for established paraneoplastic syndromes. In patients with no history of HL, recognition of PNSs may help with early diagnosis and management of HL. In patients with HL, treatment with chemotherapy and syndrome-specific preferred therapy will significantly impact clinical outcomes.
Author Contributions
Y.J.—writing original draft and editing. G.P.—writing original draft, C.L.—review and editing, P.V.B.—conceptualization, writing (review and editing), supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
PB has received honoraria and stock options with Doximity.
References
- Kaseb, H.; Babiker, H.M. Hodgkin Lymphoma; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Graus, F.; Ariño, H.; Dalmau, J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood 2014, 123, 3230–3238. [Google Scholar] [CrossRef]
- Pelosof, L.C.; Gerber, D.E. Paraneoplastic Syndromes: An approach to diagnosis and treatment. Mayo Clin. Proc. 2010, 85, 838–854. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program Populations. Available online: https://seer.cancer.gov/statfacts/html/hodg.html (accessed on 9 October 2023).
- El Fakih, R.; Bajuaifer, Y.S.; Shah, A.Y.; Sulaiman, R.; Almohamady, R.; Elgohary, G.; Alothaimeen, H.S.; Aljurf, M. Paraneoplastic syndromes associated with classic Hodgkin lymphoma, a systematic literature review. Ann. Hematol. 2023, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- de Celis Ferrari, A.C.R.; Glasberg, J.; Riechelmann, R.P. Carcinoid syndrome: Update on the pathophysiology and treatment. Clinics 2018, 73 (Suppl. S1), e490s. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Gigli, G.L.; Segatti, S.; Corazza, E.; Marini, A.; Bernardini, A.; Valent, F.; Fabris, M.; Curcio, F.; Brigo, F.; et al. Epidemiology of paraneoplastic neurological syndromes: A population-based study. J. Neurol. 2020, 267, 26–35. [Google Scholar] [CrossRef]
- Hébert, J.; Riche, B.; Vogrig, A.; Muñiz-Castrillo, S.; Joubert, B.; Picard, G.; Rogemond, V.; Psimaras, D.; Alentorn, A.; Berzero, G.; et al. Epidemiology of paraneoplastic neurologic syndromes and autoimmune encephalitides in France. Neurol.-Neuroimmunol. Neuroinflammation 2020, 7, e883. [Google Scholar] [CrossRef]
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.-C.G.; Desestret, V.; Dubey, D.; Giometto, B.; Irani, S.R.; Joubert, B.; Leypoldt, F.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol.-Neuroimmunol. Neuroinflammation 2021, 8, e1014. [Google Scholar] [CrossRef]
- Nayak, L.; Batchelor, T.T. How I treat neurologic complications in patients with lymphoid cancer. Blood 2022, 139, 1469–1478. [Google Scholar] [CrossRef]
- Trotter, J.L.; Hendin, B.A.; Osterland, C.K. Cerebellar degeneration with hodgkin disease: An immunological study. Arch. Neurol. 1976, 33, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Marcucci, S.; LaPorta, J.; Chirra, M.; Espay, A.J.; Colosimo, C. Paraneoplastic Neurological Syndromes of the Central Nervous System: Pathophysiology, Diagnosis, and Treatment. Biomedicines 2023, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, P.A.; Zieger, L.; Simon, O.J. Update on Paraneoplastic Cerebellar Degeneration. Brain Sci. 2021, 11, 1414. [Google Scholar] [CrossRef]
- Campana, I.G.; Silva, G.D. Anti-Tr/DNER Antibody–Associated Cerebellar Ataxia: A Systematic Review. Cerebellum 2022, 21, 1085–1091. [Google Scholar] [CrossRef]
- Greene, M.; Lai, Y.; Baella, N.; Dalmau, J.; Lancaster, E. Antibodies to Delta/notch-like epidermal growth factor-related receptor in patients with anti-Tr, paraneoplastic cerebellar degeneration, and Hodgkin lymphoma. JAMA Neurol. 2014, 71, 1003–1008. [Google Scholar] [CrossRef]
- Christensen, P.B.; Gregersen, H.; Almasi, C. Anti-Tr/DNER antibody paraneoplastic cerebellar degeneration preceding a very late relapse of Hodgkin Lymphoma after 12 years. Cerebellum Ataxias 2021, 8, 14. [Google Scholar] [CrossRef]
- Galli, J.; Greenlee, J. Paraneoplastic Diseases of the Central Nervous System. F1000Research 2020, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Smitt, P.S.; Kinoshita, A.; De Leeuw, B.; Moll, W.; Coesmans, M.; Jaarsma, D.; Henzen-Logmans, S.; Vecht, C.; De Zeeuw, C.; Sekiyama, N.; et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N. Engl. J. Med. 2000, 342, 21–27. [Google Scholar] [CrossRef]
- Miske, R.; Scharf, M.; Stark, P.; Dietzel, H.; Bien, C.I.; Borchers, C.; Kermer, P.; Ott, A.; Denno, Y.; Rochow, N.; et al. Autoantibodies Against the Purkinje Cell Protein RGS8 in Paraneoplastic Cerebellar Syndrome. Neurol.-Neuroimmunol. Neuroinflammation 2021, 8, e987. [Google Scholar] [CrossRef] [PubMed]
- Arratibel, N.; Sobejano, E.; Moran, J.C.; Diaz, L.G.; Blázquez, A.; Baile, M.; Veiga, Á.; Caballero, M.D.; García-Sanz, R. A Case of Paraneoplastic Cerebellar Degeneration that Preceded the Diagnosis of Classical Hodgkin’s Lymphoma by 16 Months. Am. J. Case Rep. 2020, 21, e922342-1–e922342-5. [Google Scholar] [CrossRef] [PubMed]
- Bernal, F.; Shams’Ili, S.; Rojas-Marcos, I.; Sanchez-Valle, R.; Saiz, A.; Dalmau, J.; Honnorat, J.; Sillevis Smitt, P.S.; Graus, F. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology 2003, 60, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Hammack, J.; Kotanides, H.; Rosenblum, M.K.; Posner, J.B. Paraneoplastic cerebellar degeneration. II. Clinical and immunologic findings in 21 patients with Hodgkin’s disease. Neurology 1992, 42, 1938–1943. [Google Scholar] [PubMed]
- Chepovetsky, J.; Duffield, A.S.; Pu, J.J. Paraneoplastic cerebellar degeneration as an early sign of classical Hodgkin lymphoma. Ann. Hematol. 2016, 95, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Rosenfeld, M.R. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008, 7, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Lin, M.-I.; Weng, W.-C.; Lee, W.-T. Neuropsychiatric Disorders Due to Limbic Encephalitis: Immunologic Aspect. Int. J. Mol. Sci. 2020, 22, 389. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rincón, C.A.; Castillo-Torres, S.A.; Cantú-García, D.A.; Estrada-Bellmann, I.; Chávez-Luévanos, B.; Marfil, A. The poor insane Ophelia: Reconsidering Ophelia syndrome. Arq. Neuro-Psiquiatria 2019, 77, 828–831. [Google Scholar] [CrossRef]
- Madhavan, A.A.; Carr, C.M.; Morris, P.P.; Flanagan, E.P.; Kotsenas, A.L.; Hunt, C.H.; Eckel, L.J.; Lindell, E.P.; Diehn, F.E. Imaging Review of Paraneoplastic Neurologic Syndromes. Am. J. Neuroradiol. 2020, 41, 2176–2187. [Google Scholar] [CrossRef]
- Zrzavy, T.; Höftberger, R.; Wimmer, I.; Berger, T.; Rommer, P.; Macher, S. Longitudinal CSF Findings in Autoimmune Encephalitis—A Monocentric Cohort Study. Front. Immunol. 2021, 12, 646940. [Google Scholar] [CrossRef]
- Briani, C.; Vitaliani, R.; Grisold, W.; Honnorat, J.; Graus, F.; Antoine, J.; Bertolini, G.; Giometto, B.; Euronetwork, F.T.P. Spectrum of paraneoplastic disease associated with lymphoma. Neurology 2011, 76, 705–710. [Google Scholar] [CrossRef]
- Suri, V.; Jadhao, N.; Khan, N.; Gupta, R. Paraneoplastic cerebellar degeneration in Hodgkin′s lymphoma. Ann. Indian Acad. Neurol. 2012, 15, 205–207. [Google Scholar] [CrossRef]
- Ypma, P.F.; Wijermans, P.W.; Koppen, H.; Smitt, P.A.E.S. Paraneoplastic cerebellar degeneration preceding the diagnosis of Hodgkin’s lymphoma. Neth. J. Med. 2006, 64, 243–247. [Google Scholar]
- Shams’ili, S.; Grefkens, J.; de Leeuw, B.; van den Bent, M.; Hooijkaas, H.; van der Holt, B.; Vecht, C.; Sillevis Smitt, P. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: Analysis of 50 patients. Brain 2003, 126, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Spyridonidis, A.; Fischer, K.-G.; Glocker, F.X.; Fetscher, S.; Klisch, J.; Behringer, D. Paraneoplastic cerebellar degeneration and nephrotic syndrome preceding Hodgkin’s disease: Case report and review of the literature. Eur. J. Haematol. 2002, 68, 318–321. [Google Scholar] [CrossRef]
- Lancaster, E.; Martinez-Hernandez, E.; Titulaer, M.; Boulos, M.; Weaver, S.; Antoine, J.-C.; Liebers, E.; Kornblum, C.; Bien, C.; Honnorat, J.; et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology 2011, 77, 1698–1701. [Google Scholar] [CrossRef]
- Zandi, M.S.; Irani, S.R.; Follows, G.; Moody, A.M.; Molyneux, P.; Vincent, A. Limbic encephalitis associated with antibodies to the NMDA receptor in Hodgkin lymphoma. Neurology 2009, 73, 2039–2040. [Google Scholar] [CrossRef] [PubMed]
- Kung, S.; Mueller, P.S.; Geda, Y.E.; Krahn, L.E. Delirium Resulting from Paraneoplastic Limbic Encephalitis Caused by Hodgkin’s Disease. Psychosomatics 2002, 43, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Hentschke, S.; Malzfeldt, E.; Salwender, H.J.; Braumann, D.; Stang, A.; Hentschke, M. Hu-antibody positive limbic encephalitis in a patient with Hodgkin lymphoma. Leuk. Lymphoma 2008, 49, 2374–2376. [Google Scholar] [CrossRef] [PubMed]
- Bernard, P.; Vinzio, S.; Talarmin, F.; Kadouri, A.; Flocard, F. Hodgkin’s disease manifesting as paraneoplastic limbic encephalitis. Rev. Med. Interne 2003, 24, 257–260. [Google Scholar] [CrossRef]
- Deodhare, S.; O’connor, P.; Ghazarian, D.; Bilbao, J.M. Paraneoplastic Limbic Encephalitis in Hodgkin’s Disease. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 1996, 23, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Gärtner, J.; Körholz, D.; Janßen, G.; Schneider, D.; Engelbrecht, V.; Göbel, U.; Lenard, H.-G. Paraneoplastic limbic encephalitis in two teenage girls. Neuropediatrics 1998, 29, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Derouesne, C.; Signoret, J.L.; Gray, F.; Escourolle, R.; Castaigne, P. Bilateral and limited amygdalohippocampal lesions causing a pure amnesic syndrome. Ann. Neurol. 1985, 18, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Pfliegler, G.; Pósán, E.; Glaub, D.; Telek, B.; Rak, K. Hodgkin’s disease and memory loss: Another case of the ophelia syndrome. Br. J. Haematol. 1990, 74, 232. [Google Scholar] [CrossRef]
- Borellini, L.; Lanfranconi, S.; Bonato, S.; Trezzi, I.; Franco, G.; Torretta, L.; Bresolin, N.; Di Fonzo, A.B. Progressive Encephalomyelitis with Rigidity and Myoclonus Associated with Anti-GlyR Antibodies and Hodgkin’s Lymphoma: A Case Report. Front. Neurol. 2017, 8, 401. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Aroor, S.; Mundkur, S.C.; Shashidhara, S.; Reddy, K.V. Alopecia Areata and Demyelination as Paraneoplastic Manifestation in Paediatric Hodgkin’s Lymphoma. Int. J. Hematol. Oncol. Stem Cell Res. 2018, 12, 98–102. [Google Scholar]
- Al, I.O.; Koç, B.; Bayram, C.; Paslı, E.U.; Yıldız, E.P.; Ayçiçek, A.; Çalışkan, M.; Özdemir, G.N. Variant Guillain-Barré syndrome in a patient with Hodgkin lymphoma: AMSAN. Turk. Arch. Pediatr. 2018, 53, 263–266. [Google Scholar] [CrossRef]
- Anderson, D.; Beecher, G.; Steve, T.A.; Jen, H.; Camicioli, R.; Zochodne, D.W. Neurological Nuance: Hodgkin lymphoma presenting with Guillain-BarrÉ syndrome. Muscle Nerve 2017, 55, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Milanesio, M.; Vera, S.; Sturich, A.G.; Guanchiale, L.A.; Basquiera, A.L. Hodgkin’s lymphoma: Sensitive and autonomic neuropathy as a paraneoplastic manifestation. Medicina 2023, 83, 484–488. [Google Scholar]
- Flanagan, E.P.; Sandroni, P.; Pittock, S.J.; Inwards, D.J.; Jones, L.K. Paraneoplastic lower motor neuronopathy associated with Hodgkin lymphoma. Muscle Nerve 2012, 46, 823–827. [Google Scholar] [CrossRef]
- Johnson, M.; Maciunas, R.; Dutt, P.; Clinton, M.E.; Collins, R. Granulomatous angiitis masquerading as a mass lesion. Magnetic resonance imaging and stereotactic biopsy findings in a patient with occult Hodgkin’s disease. Surg. Neurol. 1989, 31, 49–53. [Google Scholar] [CrossRef]
- Lopez-Chiriboga, A.S.; Yoon, J.W.; Siegel, J.L.; Harriott, A.M.; Pirris, S.; Eidelman, B.H.; Freeman, W.D. Granulomatous Angiitis of the Central Nervous System Associated with Hodgkin’s Lymphoma: Case Report and Literature Review. J. Stroke Cerebrovasc. Dis. 2018, 27, e5–e8. [Google Scholar] [CrossRef]
- Delobel, P.; Brassat, D.; Danjoux, M.; Lotterie, J.-A.; Irsutti-Fjørtoft, M.; Clanet, M.; Laurent, G. Granulomatous angiitis of the central nervous system revealing Hodgkin’s disease. J. Neurol. 2004, 251, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Valappil, A.; Nair, R.R.; Madhusoodhanan, S.; Narendran, A. Rhombencephalomyelitis due to possible paraneoplastic syndrome associated with Hodgkin’s lymphoma. BMJ Case Rep. 2022, 15, e249089. [Google Scholar] [CrossRef]
- Ballonoff, A.; Kavanagh, B.; Nash, R.; Drabkin, H.; Trotter, J.; Costa, L.; Rabinovitch, R. Hodgkin lymphoma-related vanishing bile duct syndrome and idiopathic cholestasis: Statistical analysis of all published cases and literature review. Acta Oncol. 2008, 47, 962–970. [Google Scholar] [CrossRef]
- Scalabrini, D.R.; Caravelli, D.; Schianca, F.C.; D’ambrosio, L.; Tolomeo, F.; Boccone, P.; Manca, A.; De Rosa, G.; Nuzzo, A.; Aglietta, M.; et al. Complete remission of paraneoplastic vanishing bile duct syndrome after the successful treatment of Hodgkin’s lymphoma: A case report and review of the literature. BMC Res. Notes 2014, 7, 529. [Google Scholar] [CrossRef]
- Iturriagagoitia, A.C.; Bastarrica, M.I.; de Equiza, E.P.; Urmeneta, J.M.Z.; Martínez-Peñuela, J.M.; Pérez, R.B. Ductal regeneration in vanishing bile duct syndrome in Hodgkin’s lymphoma. Gastroenterol. Hepatol. 2005, 28, 275–278. [Google Scholar]
- Crosbie, O.M.; Crown, J.P.; Nolan, N.P.; Murray, R.; Hegarty, J.E. Resolution of paraneoplastic bile duct paucity following successful treatment of Hodgkin’s disease. Hepatology 1997, 26, 5–8. [Google Scholar] [CrossRef]
- Yalçın, S.; Kars, A.; Sökmensüer, C.; Atahan, L. Extrahepatic Hodgkin’s Disease with Intrahepatic Cholestasis: Report of Two Cases. Oncology 1999, 57, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Carretero, L.; Sabin, P.; Alvarez, E.; Marrupe, D.; Banares, R. Idiopathic cholestasis associated with progressive ductopenia in two patients with hodgkin’s disease. Gastroenterol. Hepatol. 2002, 25, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Barta, S.K.; Yahalom, J.; Shia, J.; Hamlin, P.A. Idiopathic cholestasis as a paraneoplastic phenomenon in Hodgkin’s lymphoma. Clin. Lymphoma Myeloma Leuk. 2006, 7, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, I.; Lugtenburg, E.P.; van Buuren, H.R.; Zondervan, P.E.; de Man, R.A. Severe jaundice, due to vanishing bile duct syndrome, as presenting symptom of Hodgkin’s lymphoma, fully reversible after chemotherapy. Eur. J. Gastroenterol. Hepatol. 2008, 20, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Pass, A.K.; McLin, V.A.; Rushton, J.R.; Kearney, D.L.; Hastings, C.A.; Margolin, J.F. Vanishing bile duct syndrome and hodgkin disease: A case series and review of the literature. J. Pediatr. Hematol. Oncol. 2008, 30, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-M.; Chang, C.-S.; Wu, C.-C.; Yin, H.-L. Hodgkin’s lymphoma-related vanishing bile duct syndrome: A case report and literature review. Kaohsiung J. Med. Sci. 2013, 29, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Anugwom, C.; Goetz, G.; Hassan, M. Vanishing Bile Duct Syndrome Preceding the Diagnosis of Hodgkin Lymphoma. ACG Case Rep. J. 2020, 7, e00336. [Google Scholar] [CrossRef]
- Papakonstantinou, I.; Kosmidou, M.; Papathanasiou, K.; Koumpis, E.; Kapsali, E.; Milionis, H.; Vassilakopoulos, T.P.; Papoudou-Bai, A.; Hatzimichael, E. Paraneoplastic Intrahepatic Cholestasis in Supradiaphragmatic Classical Hodgkin Lymphoma Successfully Treated with Brentuximab Vedotin: A Case Report and Review of the Literature. In Vivo 2021, 35, 1951–1957. [Google Scholar] [CrossRef]
- Sfrijan, D.; Tieranu, I.; Necula, I.; Popa, L.; Balgradean, M. Nephrotic Syndrome, Paraneoplastic Syndrome Associated to Hodgkin Lymphoma. Maedica 2016, 11, 64–67. [Google Scholar]
- Mallouk, A.; Pham, P.-C.T. Concurrent FSGS and Hodgkin’s lymphoma: Case report and literature review on the link between nephrotic glomerulopathies and hematological malignancies. Clin. Exp. Nephrol. 2006, 10, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Audard, V.; Larousserie, F.; Grimbert, P.; Abtahi, M.; Sotto, J.-J.; Delmer, A.; Boue, F.; Nochy, D.; Brousse, N.; Delarue, R.; et al. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: Report of 21 cases and review of the literature. Kidney Int. 2006, 69, 2251–2260. [Google Scholar] [CrossRef]
- Gagliano, R.G.; Costanzi, J.J.; Beathard, G.A.; Sarles, H.E.; Bell, J.D. The nephrotic syndrome associated with neoplasia: An unusual paraneoplastic syndrome. Report of a case and review of the literature. Am. J. Med. 1976, 60, 1026–1031. [Google Scholar] [CrossRef]
- Lien, Y.-H.H.; Lai, L.-W. Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat. Rev. Nephrol. 2011, 7, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.P.; Krzesinski, J.-M.; Launay-Vacher, V.; Sprangers, B. Onco-nephrology: Core Curriculum 2015. Am. J. kidney Dis. Off. J. Natl. Kidney Found. 2015, 66, 869–883. [Google Scholar] [CrossRef]
- Nagaharu, K.; Sugimoto, Y.; Kawakami, K. A Rare Case of Immunotactoid Glomerulopathy Associated with Hodgkin Lymphoma. Case Rep. Nephrol. 2021, 2021, 5527966. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, J.; Weening, J.J.; van der Valk, M.; Bobeldijk, R.; Berns, A. Overexpression of Frat1 in transgenic mice leads to glomerulosclerosis and nephrotic syndrome, and provides direct evidence for the involvement of Frat1 in lymphoma progression. Oncogene 1999, 18, 5982–5990. [Google Scholar] [CrossRef][Green Version]
- Doussis-Anagnostopoulou, I.A.; Talks, K.L.; Turley, H.; Debnam, P.; Tan, D.C.; Mariatos, G.; Gorgoulis, V.; Kittas, C.; Gatter, K.C. Vascular endothelial growth factor (VEGF) is expressed by neoplastic Hodgkin–Reed–Sternberg cells in Hodgkin’s disease. J. Pathol. 2002, 197, 677–683. [Google Scholar] [CrossRef]
- Newcom, S.R.; Gu, L. Transforming growth factor beta 1 messenger RNA in Reed-Sternberg cells in nodular sclerosing Hodgkin’s disease. J. Clin. Pathol. 1995, 48, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Riesco Martínez, M.C.; Muñoz Martín, A.J.; Zamberk Majlis, P.; Adeva Alfonso, J.; Sabin Domínguez, P.; García Alfonso, P.; Pérez Fernández, R. Acquired ichthyosis as a paraneoplastic syndrome in Hodgkin’s disease. Clin. Transl. Oncol. 2009, 11, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Didona, D.; Fania, L.; Didona, B.; Eming, R.; Hertl, M.; Di Zenzo, G. Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis. Int. J. Mol. Sci. 2020, 21, 2178. [Google Scholar] [CrossRef]
- de Souza, P.K.; Amorim, R.O.; Sousa, L.S.; Batista, M.D. Dermatological manifestations of hematologic neoplasms. Part II: Nonspecific skin lesions/paraneoplastic diseases. An. Bras. Dermatol. 2023, 98, 141–158. [Google Scholar] [CrossRef]
- Asad, U.; Austin, B.; Sturgeon, A.; Stetson, C. Paraneoplastic eczematous eruption associated with Hodgkin’s lymphoma. Bayl. Univ. Med. Cent. Proc. 2019, 32, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Kappius, R.H.; Ufkes, N.A.T.B. Paraneoplastic Pemphigus; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Marjon, P.; Kim, B.; Lazic, T.; Cernik, C.; Rathore, R. Paraneoplastic Pemphigus (PNP) As a Presenting Finding in a Patient with Hodgkin’s Lymphoma. Blood 2011, 118, 4864. [Google Scholar] [CrossRef]
- Váróczy, L.; Gergely, L.; Zeher, M.; Szegedi, G.; Illés, A. Malignant lymphoma-associated autoimmune diseases—A descriptive epidemiological study. Rheumatol. Int. 2002, 22, 233–237. [Google Scholar] [CrossRef]
- Lechner, K.; Chen, Y.-A. Paraneoplastic autoimmune cytopenias in Hodgkin lymphoma. Leuk. Lymphoma 2010, 51, 469–474. [Google Scholar] [CrossRef]
- Poponea, N.; Suede, M.; Muhsin Chisti, M. Idiopathic Thrombocytopenia Purpura Masking Hodgkin Disease: A Paraneoplastic Syndrome or Simply a Mere Association. Case Rep. Oncol. 2017, 10, 1116–1120. [Google Scholar] [CrossRef]
- Erlij, D.; Calderón, B.; Rivera, A.; Mella, C.; Valladares, X.; Roessler, E.; Rivera, M.T.; Méndez, G. Polyarthritis and membranoproliferative glomerulonephritis as paraneoplastic manifestation of hodgkin’s lymphoma: A Case Report and Literature Review. Reumatol. Clin. 2016, 12, 282–284. [Google Scholar] [CrossRef]
- Aruch, D.B.; Mims, M.P. Paraneoplastic nephrotic syndrome and inflammatory arthritis at diagnosis in hodgkin lymphoma. Clin. Lymphoma Myeloma Leuk. 2013, 13, 77–79. [Google Scholar] [CrossRef]
- Jurkovic, J.; Cirone, M. Mucocutaneous Paraneoplastic Syndrome Secondary to Classical Hodgkin’s Lymphoma. Clin. Prac. Cases Emerg. Med. 2019, 3, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Lim, S.W. Alopecia areata as a paraneoplastic syndrome of Hodgkin’s lymphoma: A case report. Mol. Clin. Oncol. 2014, 2, 596–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villafranca, J.J.A.; Siles, M.G.; Casanova, M.; Goitia, B.T.; Domínguez, A.R. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: A case report. J. Med. Case Rep. 2014, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Hinshaw, M.A. Palisaded Neutrophilic Granulomatous Dermatitis Leading to Diagnosis of Hodgkin Lymphoma: Report of Rare Case and Literature Review of Paraneoplastic Granulomatous Dermatitides. Am. J. Dermatopathol. 2019, 41, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.M.; Novoa, R.A.; Martires, K.J. Paraneoplastic granulomatous dermatitis in a patient with Hodgkin’s disease: A diagnostic pitfall. BMJ Case Rep. 2018, 2018, bcr2018224961. [Google Scholar] [CrossRef] [PubMed]
- Farruggia, P.; Trizzino, A.; Maringhini, S.; Grigoli, A.; Sapia, C.; D’alessandro, M.; Tropia, S.; D’angelo, P. Hodgkin lymphoma and nephrotic syndrome in childhood. Indian J. Pediatr. 2010, 77, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Deacon, A.J.; Goetz, N.N.; Weber, N.; Clouston, A.; Gonsalkorala, E.; Baskerville, C.; Leggett, B. Relapsed nodular lymphocyte-predominant Hodgkin lymphoma presenting as severe paraneoplastic hepatitis: A case report. J. Med. Case Rep. 2023, 17, 269. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, B.; Crivellaro, C.; Mitterer, M.; Zingerle, H.; Egarter-Vigl, E.; Wiedermann, C.J. Paraneoplastic stiff-person syndrome, heterotopic soft tissue ossification and gonarthritis in a HLA B27-positive woman preceding the diagnosis of Hodgkin’s lymphoma. Haematologica 2006, 91 (Suppl. S12), ECR59. [Google Scholar]
- Villano, F.; Peixoto, A.; Riva, E.; Di Matteo, C.; Díaz, L. Digital Ischemia as an Unusual Manifestation of Hodgkin’s Lymphoma. Case Rep. Hematol. 2018, 2018, 1980749. [Google Scholar] [CrossRef]
- AlRasbi, S.; Al-Badi, A.H.; Al Alawi, A.M. Paraneoplastic acral vascular syndrome: Case presentation and literature review. BMJ Case Rep. 2021, 14, e237258. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Sanchez, C.; Zekeridou, A. Paraneoplastic Neurological Syndromes and Beyond Emerging with the Introduction of Immune Checkpoint Inhibitor Cancer Immunotherapy. Front. Neurol. 2021, 12, 642800. [Google Scholar] [CrossRef] [PubMed]
- Duong, S.L.; Prüss, H. Paraneoplastic Autoimmune Neurological Syndromes and the Role of Immune Checkpoint Inhibitors. Neurotherapeutics 2022, 19, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Birzu, C.; Elsensohn, M.-H.; Picca, A.; Muñiz-Castrillo, S.; Vogrig, A.; Villagrán-García, M.; Ciano-Petersen, N.L.; Massacesi, L.; Hervier, B.; et al. Neurological outcomes in immune checkpoint inhibitor-related neurotoxicity. Brain Commun. 2023, 5, fcad169. [Google Scholar] [CrossRef]
- Binks, S.; Uy, C.; Honnorat, J.; Irani, S.R. Paraneoplastic neurological syndromes: A practical approach to diagnosis and management. Prac. Neurol. 2021, 22, 19–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).