A Review of Anti-CD20 Antibodies in the Management of B-Cell Lymphomas
Abstract
1. Introduction
2. Resistance to RTX and the Bioengineering of Newer Anti-CD20 Antibodies
3. Follicular Lymphoma (FL) and Other Indolent Non-Hodgkin’s lymphomas (NHLs)
4. Diffuse Large B-Cell Lymphoma (DLBCL)
5. Chronic Lymphocytic Leukemia (CLL)
6. Mantle Cell Lymphoma (MCL)
7. Other Investigational Anti CD-20 Antibodies
8. Rituximab Biosimilars
9. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cerny, T.; Borisch, B.; Introna, M.; Johnson, P.; Rose, A.L. Mechanism of action of rituximab. Anticancer. Drugs 2002, 13 (Suppl. S2), S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, T.M.; Limper, C.B.; Richards, K.L. Past, Present, and Future of Rituximab-The World’s First Oncology Monoclonal Antibody Therapy. Front. Oncol. 2018, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.M.; Tawbi, H.A.; Oddis, C.V.; Kulkarni, H.S. Clinical review: Serious adverse events associated with the use of rituximab—A critical care perspective. Crit. Care 2012, 16, 231. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; Davies, A.; Ando, K.; Klapper, W.; Opat, S.; Owen, C.; Phillips, E.; Sangha, R.; Schlag, R.; Seymour, J.F.; et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N. Engl. J. Med. 2017, 377, 1331–1344. [Google Scholar] [CrossRef]
- Teeling, J.L.; Mackus, W.J.M.; Wiegman, L.J.J.M.; van den Brakel, J.H.N.; Beers, S.A.; French, R.R.; van Meerten, T.; Ebeling, S.; Vink, T.; Slootstra, J.W.; et al. The Biological Activity of Human CD20 Monoclonal Antibodies Is Linked to Unique Epitopes on CD201. J. Immunol. 2006, 177, 362–371. [Google Scholar] [CrossRef]
- Teeling, J.L.; French, R.R.; Cragg, M.S.; van den Brakel, J.; Pluyter, M.; Huang, H.; Chan, C.; Parren, P.W.; Hack, C.E.; Dechant, M.; et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 2004, 104, 1793–1800. [Google Scholar] [CrossRef]
- Mössner, E.; Brünker, P.; Moser, S.; Püntener, U.; Schmidt, C.; Herter, S.; Grau, R.; Gerdes, C.; Nopora, A.; van Puijenbroek, E.; et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010, 115, 4393–4402. [Google Scholar] [CrossRef]
- Herter, S.; Herting, F.; Mundigl, O.; Waldhauer, I.; Weinzierl, T.; Fauti, T.; Muth, G.; Ziegler-Landesberger, D.; Van Puijenbroek, E.; Lang, S.; et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol. Cancer Ther. 2013, 12, 2031–2042. [Google Scholar] [CrossRef]
- Maloney, D.G.; Ogura, M.; Fukuhara, N.; Davis, J.; Lasher, J.; Izquierdo, M.; Banerjee, H.; Tobinai, K. A phase 3 randomized study (HOMER) of ofatumumab vs rituximab in iNHL relapsed after rituximab-containing therapy. Blood Adv. 2020, 4, 3886–3893. [Google Scholar] [CrossRef]
- Armitage, J.O.; Longo, D.L. Which Anti-CD20 Antibody Is Better in Follicular Lymphoma? N. Engl. J. Med. 2017, 377, 1389–1390. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, G.W.v.; McMillan, A.; Matasar, M.J.; Radford, J.; Ardeshna, K.M.; Kuliczkowski, K.; Kim, W.; Hong, X.; Goerloev, J.S.; Davies, A.; et al. Ofatumumab Versus Rituximab Salvage Chemoimmunotherapy in Relapsed or Refractory Diffuse Large B-Cell Lymphoma: The ORCHARRD Study. J. Clin. Oncol. 2017, 35, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Martelli, M.; Trněný, M.; Liu, W.; Bolen, C.R.; Knapp, A.; Sahin, D.; Sellam, G.; Vitolo, U. A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: Final analysis of GOYA. J. Hematol. Oncol. 2020, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Mulligan, S.P. Ofatumumab and its role as immunotherapy in chronic lymphocytic leukemia. Haematologica 2015, 100, 411–414. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef]
- Freeman, C.L.; Sehn, L.H. A tale of two antibodies: Obinutuzumab versus rituximab. Br. J. Haematol. 2018, 182, 29–45. [Google Scholar] [CrossRef]
- Torka, P.; Akhtar, O.S.; Reddy, N.M.; Baysal, B.E.; Kader, A.; Groman, A.; Nichols, J.; Mavis, C.; Tario, J.D.; Block, A.W.; et al. Ofatumumab plus HyperCVAD/HD-MA induction leads to high rates of minimal residual disease negativity in patients with newly diagnosed mantle cell lymphoma: Results of a phase 2 study. Cancer 2022, 128, 1595–1604. [Google Scholar] [CrossRef]
- Rezvani, A.R.; Maloney, D.G. Rituximab resistance. Best Pract. Res. Clin. Haematol. 2011, 24, 203–216. [Google Scholar] [CrossRef]
- Bonavida, B. Postulated mechanisms of resistance of B-cell non-Hodgkin lymphoma to rituximab treatment regimens: Strategies to overcome resistance. Semin. Oncol. 2014, 41, 667–677. [Google Scholar] [CrossRef]
- Wang, S.Y.; Veeramani, S.; Racila, E.; Cagley, J.; Fritzinger, D.C.; Vogel, C.W.; St John, W.; Weiner, G.J. Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood 2009, 114, 5322–5330. [Google Scholar] [CrossRef]
- Seyfizadeh, N.; Seyfizadeh, N.; Hasenkamp, J.; Huerta-Yepez, S. A molecular perspective on rituximab: A monoclonal antibody for B cell non Hodgkin lymphoma and other affections. Crit. Rev. Oncol. Hematol. 2016, 97, 275–290. [Google Scholar] [CrossRef]
- Cheson, B.D. Ofatumumab, a Novel Anti-CD20 Monoclonal Antibody for the Treatment of B-Cell Malignancies. J. Clin. Oncol. 2010, 28, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Bologna, L.; Gotti, E.; Manganini, M.; Rambaldi, A.; Intermesoli, T.; Introna, M.; Golay, J. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J. Immunol. 2011, 186, 3762–3769. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.; Grillo-López, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Colombat, P.; Brousse, N.; Salles, G.; Morschhauser, F.; Brice, P.; Soubeyran, P.; Delwail, V.; Deconinck, E.; Haioun, C.; Foussard, C.; et al. Rituximab induction immunotherapy for first-line low-tumor-burden follicular lymphoma: Survival analyses with 7-year follow-up. Ann. Oncol. 2012, 23, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Taverna, C.; Martinelli, G.; Hitz, F.; Mingrone, W.; Pabst, T.; Cevreska, L.; Del Giglio, A.; Vanazzi, A.; Laszlo, D.; Raats, J.; et al. Rituximab Maintenance for a Maximum of 5 Years after Single-Agent Rituximab Induction in Follicular Lymphoma: Results of the Randomized Controlled Phase III Trial SAKK 35/03. J. Clin. Oncol. 2016, 34, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Czuczman, M.S.; Weaver, R.; Alkuzweny, B.; Berlfein, J.; Grillo-López, A.J. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J. Clin. Oncol. 2004, 22, 4711–4716. [Google Scholar] [CrossRef] [PubMed]
- Hiddemann, W.; Kneba, M.; Dreyling, M.; Schmitz, N.; Lengfelder, E.; Schmits, R.; Reiser, M.; Metzner, B.; Harder, H.; Hegewisch-Becker, S.; et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005, 106, 3725–3732. [Google Scholar] [CrossRef]
- Hochster, H.; Weller, E.; Gascoyne, R.D.; Habermann, T.M.; Gordon, L.I.; Ryan, T.; Zhang, L.; Colocci, N.; Frankel, S.; Horning, S.J. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG1496 Study. J. Clin. Oncol. 2009, 27, 1607–1614. [Google Scholar] [CrossRef]
- Salles, G.; Seymour, J.F.; Offner, F.; López-Guillermo, A.; Belada, D.; Xerri, L.; Feugier, P.; Bouabdallah, R.; Catalano, J.V.; Brice, P.; et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 2011, 377, 42–51. [Google Scholar] [CrossRef]
- Salles, G.A.; Seymour, J.F.; Feugier, P.; Offner, F.; Lopez-Guillermo, A.; Belada, D.; Xerri, L.; Bouabdallah, R.; Catalano, J.; Pauline, B. Updated 6 year follow-up of the PRIMA study confirms the benefit of 2-year rituximab maintenance in follicular lymphoma patients responding to frontline immunochemotherapy. Blood 2013, 122, 509. [Google Scholar] [CrossRef]
- Forstpointner, R.; Dreyling, M.; Repp, R.; Hermann, S.; Hänel, A.; Metzner, B.; Pott, C.; Hartmann, F.; Rothmann, F.; Rohrberg, R.; et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2004, 104, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Forstpointner, R.; Unterhalt, M.; Dreyling, M.; Böck, H.P.; Repp, R.; Wandt, H.; Pott, C.; Seymour, J.F.; Metzner, B.; Hänel, A.; et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006, 108, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- van Oers, M.H.; Van Glabbeke, M.; Giurgea, L.; Klasa, R.; Marcus, R.E.; Wolf, M.; Kimby, E.; van t Veer, M.; Vranovsky, A.; Holte, H.; et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: Long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol 2010, 28, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.; Gafter-Gvili, A.; Salles, G.; Bousseta, S.; Oberman, B.; Rubin, C.; van Oers, M.H.; Fortpied, C.; Ghielmini, M.; Pettengell, R.; et al. Rituximab maintenance improves overall survival of patients with follicular lymphoma-Individual patient data meta-analysis. Eur. J. Cancer 2017, 76, 216–225. [Google Scholar] [CrossRef]
- Armitage, J.O.; Longo, D.L. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood 2016, 127, 2804–2808. [Google Scholar] [CrossRef]
- Hagenbeek, A.; Gadeberg, O.; Johnson, P.; Pedersen, L.M.; Walewski, J.; Hellmann, A.; Link, B.K.; Robak, T.; Wojtukiewicz, M.; Pfreundschuh, M.; et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: Results of a phase 1/2 trial. Blood 2008, 111, 5486–5495. [Google Scholar] [CrossRef]
- Pan, J.; Ghimire, S.; Alpdogan, S.O.; Chapman, A.; Carabasi, M.; DiMeglio, M.; Gong, J.; Martinez-Outschoorn, U.; Rose, L.; Ramirez, M.; et al. Phase I/II study of bendamustine in combination with ofatumumab, carboplatin, etoposide (BOCE) for relapsed or refractory aggressive B-cell non-Hodgkin lymphoma. Leuk. Lymphoma 2021, 62, 590–597. [Google Scholar] [CrossRef]
- Salles, G.; Morschhauser, F.; Lamy, T.; Milpied, N.; Thieblemont, C.; Tilly, H.; Bieska, G.; Asikanius, E.; Carlile, D.; Birkett, J.; et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood 2012, 119, 5126–5132. [Google Scholar] [CrossRef]
- Sehn, L.H.; Goy, A.; Offner, F.C.; Martinelli, G.; Caballero, M.D.; Gadeberg, O.; Baetz, T.; Zelenetz, A.D.; Gaidano, G.; Fayad, L.E.; et al. Randomized Phase II Trial Comparing Obinutuzumab (GA101) with Rituximab in Patients with Relapsed CD20+ Indolent B-Cell Non-Hodgkin Lymphoma: Final Analysis of the GAUSS Study. J. Clin. Oncol. 2015, 33, 3467–3474. [Google Scholar] [CrossRef]
- Salles, G.A.; Morschhauser, F.; Solal-Céligny, P.; Thieblemont, C.; Lamy, T.; Tilly, H.; Gyan, E.; Lei, G.; Wenger, M.; Wassner-Fritsch, E.; et al. Obinutuzumab (GA101) in Patients with Relapsed/Refractory Indolent Non-Hodgkin Lymphoma: Results from the Phase II GAUGUIN Study. J. Clin. Oncol. 2013, 31, 2920–2926. [Google Scholar] [CrossRef] [PubMed]
- Radford, J.; Davies, A.; Cartron, G.; Morschhauser, F.; Salles, G.; Marcus, R.; Wenger, M.; Lei, G.; Wassner-Fritsch, E.; Vitolo, U. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: Results of the GAUDI study (BO21000). Blood 2013, 122, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Chua, N.; Mayer, J.; Dueck, G.; Trněný, M.; Bouabdallah, K.; Fowler, N.; Delwail, V.; Press, O.; Salles, G.; et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): A randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016, 17, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Townsend, W.; Hiddemann, W.; Buske, C.; Cartron, G.; Cunningham, D.; Dyer, M.J.S.; Gribben, J.G.; Phillips, E.H.; Dreyling, M.; Seymour, J.F.; et al. Obinutuzumab Versus Rituximab Immunochemotherapy in Previously Untreated iNHL: Final Results from the GALLIUM Study. Hemasphere 2023, 7, e919. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Schmitz, R. Molecular Subgroups of Diffuse Large B Cell Lymphoma: Biology and Implications for Clinical Practice. Curr. Oncol. Rep. 2022, 24, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.D.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.G.; Thomopoulos, T.P.; Katagas, I.; Bouchla, A.; Pappa, V. Prognostic molecular biomarkers in diffuse large B-cell lymphoma in the rituximab era and their therapeutic implications. Ther. Adv. Hematol. 2021, 12, 20406207211013987. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.M.; Link, B.K.; Grossbard, M.L.; Czuczman, M.; Grillo-Lopez, A.; Gilman, P.; Lowe, A.; Kunkel, L.A.; Fisher, R.I. Phase II study of rituximab in combination with chop chemotherapy in patients with previously untreated, aggressive non-Hodgkin’s lymphoma. J. Clin. Oncol. 2001, 19, 389–397. [Google Scholar] [CrossRef]
- Coiffier, B.; Lepage, E.; Briere, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Trümper, L.; Osterborg, A.; Pettengell, R.; Trneny, M.; Imrie, K.; Ma, D.; Gill, D.; Walewski, J.; Zinzani, P.L.; et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006, 7, 379–391. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Kuhnt, E.; Trümper, L.; Osterborg, A.; Trneny, M.; Shepherd, L.; Gill, D.S.; Walewski, J.; Pettengell, R.; Jaeger, U.; et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011, 12, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Feugier, P.; Van Hoof, A.; Sebban, C.; Solal-Celigny, P.; Bouabdallah, R.; Fermé, C.; Christian, B.; Lepage, E.; Tilly, H.; Morschhauser, F.; et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 2005, 23, 4117–4126. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Thieblemont, C.; Van Den Neste, E.; Lepeu, G.; Plantier, I.; Castaigne, S.; Lefort, S.; Marit, G.; Macro, M.; Sebban, C.; et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010, 116, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Haioun, C.; Ketterer, N.; Engert, A.; Tilly, H.; Ma, D.; Johnson, P.; Lister, A.; Feuring-Buske, M.; Radford, J.A.; et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood 1998, 92, 1927–1932. [Google Scholar] [PubMed]
- Coiffier, B.; Radford, J.; Bosly, A.; Martinelli, G.; Verhoef, G.; Barca, G.; Davies, A.; Decaudin, D.; Gallop-Evans, E.; Padmanabhan-Iyer, S.; et al. A multicentre, phase II trial of ofatumumab monotherapy in relapsed/progressive diffuse large B-cell lymphoma. Br. J. Haematol. 2013, 163, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Matasar, M.J.; Czuczman, M.S.; Rodriguez, M.A.; Fennessy, M.; Shea, T.C.; Spitzer, G.; Lossos, I.S.; Kharfan-Dabaja, M.A.; Joyce, R.; Fayad, L.; et al. Ofatumumab in combination with ICE or DHAP chemotherapy in relapsed or refractory intermediate grade B-cell lymphoma. Blood 2013, 122, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Forero-Torres, A.; Costa, L.J.; Flinn, I.W.; Inhorn, L.; Kelly, K.; Bessudo, A.; Fayad, L.E.; Kaminski, M.S.; Evens, A.M.; et al. Obinutuzumab plus CHOP is effective and has a tolerable safety profile in previously untreated, advanced diffuse large B-cell lymphoma: The phase II GATHER study. Leuk. Lymphoma 2019, 60, 894–903. [Google Scholar] [CrossRef]
- Fischer, K.; Bahlo, J.; Fink, A.M.; Goede, V.; Herling, C.D.; Cramer, P.; Langerbeins, P.; von Tresckow, J.; Engelke, A.; Maurer, C.; et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: Updated results of the CLL8 trial. Blood 2016, 127, 208–215. [Google Scholar] [CrossRef]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grünhagen, U.; et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Robak, T.; Dmoszynska, A.; Solal-Céligny, P.; Warzocha, K.; Loscertales, J.; Catalano, J.; Afanasiev, B.V.; Larratt, L.; Geisler, C.H.; Montillo, M.; et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J. Clin. Oncol. 2010, 28, 1756–1765. [Google Scholar] [CrossRef]
- Eichhorst, B.; Fink, A.M.; Bahlo, J.; Busch, R.; Kovacs, G.; Maurer, C.; Lange, E.; Köppler, H.; Kiehl, M.; Sökler, M.; et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016, 17, 928–942. [Google Scholar] [CrossRef]
- Hillmen, P.; Gribben, J.G.; Follows, G.A.; Milligan, D.; Sayala, H.A.; Moreton, P.; Oscier, D.G.; Dearden, C.E.; Kennedy, D.B.; Pettitt, A.R.; et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: Final analysis of an open-label phase II study. J. Clin. Oncol. 2014, 32, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Dartigeas, C.; Van Den Neste, E.; Maisonneuve, H.; Berthou, C.; Dilhuydy, M.-S.; De Guibert, S.; Lepretre, S.; Rodon, P.; Aurran, T.; Vilque, J.-P. Rituximab maintenance after induction with abbreviated FCR in previously untreated elderly (≥65 years) CLL patients: Results of the randomized CLL 2007 SA trial from the French FILO Group (NCT00645606). J. Clin. Oncol. 2016, 34, 7505. [Google Scholar] [CrossRef]
- Hillmen, P.; Pitchford, A.; Bloor, A.; Broom, A.; Young, M.; Kennedy, B.; Walewska, R.; Furtado, M.; Preston, G.; Neilson, J.R.; et al. Ibrutinib Plus Rituximab Is Superior to FCR in Previously Untreated CLL: Results of the Phase III NCRI FLAIR Trial. Blood 2021, 138, 642. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: Updated results of the E1912 trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final Results of a Randomized, Phase III Study of Rituximab with or without Idelalisib Followed by Open-Label Idelalisib in Patients with Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.; Mikhael, J.; Gleeson, M.; Danese, M.; Dreyling, M. Addition of rituximab to chemotherapy alone as first-line therapy improves overall survival in elderly patients with mantle cell lymphoma. Blood 2011, 118, 4808–4816. [Google Scholar] [CrossRef]
- Kluin-Nelemans, H.C.; Hoster, E.; Hermine, O.; Walewski, J.; Trneny, M.; Geisler, C.H.; Stilgenbauer, S.; Thieblemont, C.; Vehling-Kaiser, U.; Doorduijn, J.K.; et al. Treatment of older patients with mantle-cell lymphoma. N. Engl. J. Med. 2012, 367, 520–531. [Google Scholar] [CrossRef]
- Le Gouill, S.; Thieblemont, C.; Oberic, L.; Moreau, A.; Bouabdallah, K.; Dartigeas, C.; Damaj, G.; Gastinne, T.; Ribrag, V.; Feugier, P.; et al. Rituximab after Autologous Stem-Cell Transplantation in Mantle-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 1250–1260. [Google Scholar] [CrossRef]
- Jain, P.; Zhao, S.; Lee, H.J.; Hill, H.A.; Ok, C.Y.; Kanagal-Shamanna, R.; Hagemeister, F.B.; Fowler, N.; Fayad, L.; Yao, Y.; et al. Ibrutinib with Rituximab in First-Line Treatment of Older Patients with Mantle Cell Lymphoma. J. Clin. Oncol. 2022, 40, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Jain, P.; Zhao, S.; Lee, H.J.; Nastoupil, L.; Fayad, L.; Ok, C.Y.; Kanagal-Shamanna, R.; Hill, H.A.; Yao, Y.; et al. Ibrutinib-rituximab followed by R-HCVAD as frontline treatment for young patients (≤65 years) with mantle cell lymphoma (WINDOW-1): A single-arm, phase 2 trial. Lancet Oncol. 2022, 23, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Casulo, C.; Joffe, E.; Moskowitz, C.; Gerecitano, J.; Moskowitz, A.; Younes, A.; Drullinsky, P.; Drill, E.; Choma, M.; et al. Bendamustine in combination with ofatumumab as first line treatment for elderly patients with mantle cell lymphoma: A phase II risk-adapted design. Leuk. Lymphoma 2022, 63, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Leonard, J.P.; Fayad, L.; Coiffier, B.; Petillon, M.-O.; Coleman, M.; Schuster, S.J.; Dyer, M.J.S.; Horne, H.; Teoh, N.; et al. Humanized Anti-CD20 Antibody, Veltuzumab, in Refractory/Recurrent Non-Hodgkin’s Lymphoma: Phase I/II Results. J. Clin. Oncol. 2009, 27, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Marlton, P.; Vitolo, U.; Lindén, O.; Seymour, J.F.; Crump, M.; Coiffier, B.; Foà, R.; Wassner, E.; Burger, H.U.; et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann. Oncol. 2010, 21, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Ganjoo, K.N.; de Vos, S.; Pohlman, B.L.; Flinn, I.W.; Forero-Torres, A.; Enas, N.H.; Cronier, D.M.; Dang, N.H.; Foon, K.A.; Carpenter, S.P.; et al. Phase 1/2 study of ocaratuzumab, an Fc-engineered humanized anti-CD20 monoclonal antibody, in low-affinity FcγRIIIa patients with previously treated follicular lymphoma. Leuk. Lymphoma 2015, 56, 42–48. [Google Scholar] [CrossRef]
- Sawas, A.; Farber, C.M.; Schreeder, M.T.; Khalil, M.Y.; Mahadevan, D.; Deng, C.; Amengual, J.E.; Nikolinakos, P.G.; Kolesar, J.M.; Kuhn, J.G.; et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br. J. Haematol. 2017, 177, 243–253. [Google Scholar] [CrossRef]
- Nastoupil, L.J.; Lunning, M.A.; Vose, J.M.; Schreeder, M.T.; Siddiqi, T.; Flowers, C.R.; Cohen, J.B.; Burger, J.A.; Wierda, W.G.; O’Brien, S.; et al. Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: A phase 1 dose escalation and expansion trial. Lancet Haematol. 2019, 6, e100–e109. [Google Scholar] [CrossRef]
- de Romeuf, C.; Dutertre, C.A.; Le Garff-Tavernier, M.; Fournier, N.; Gaucher, C.; Glacet, A.; Jorieux, S.; Bihoreau, N.; Behrens, C.K.; Béliard, R.; et al. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcgammaRIIIA/CD16. Br. J. Haematol. 2008, 140, 635–643. [Google Scholar] [CrossRef]
- Kim, W.S.; Buske, C.; Ogura, M.; Jurczak, W.; Sancho, J.M.; Zhavrid, E.; Kim, J.S.; Hernández-Rivas, J.; Prokharau, A.; Vasilica, M.; et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 compared with rituximab in patients with previously untreated advanced-stage follicular lymphoma: A randomised, double-blind, parallel-group, non-inferiority phase 3 trial. Lancet Haematol. 2017, 4, e362–e373. [Google Scholar] [CrossRef]
- Ogura, M.; Sancho, J.M.; Cho, S.G.; Nakazawa, H.; Suzumiya, J.; Tumyan, G.; Kim, J.S.; Lennard, A.; Mariz, J.; Ilyin, N.; et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 in comparison with rituximab in patients with previously untreated low-tumour-burden follicular lymphoma: A randomised, double-blind, parallel-group, phase 3 trial. Lancet Haematol. 2018, 5, e543–e553. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Liberati, A.M.; Ishizawa, K.; Khan, T.; Robbins, J.; Alcasid, A.; Rosenberg, J.A.; Aurer, I. A Randomized, Double-Blind, Efficacy and Safety Study of PF-05280586 (a Rituximab Biosimilar) Compared with Rituximab Reference Product (MabThera®) in Subjects with Previously Untreated CD20-Positive, Low-Tumor-Burden Follicular Lymphoma (LTB-FL). BioDrugs 2020, 34, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Niederwieser, D.; Hamm, C.; Cobb, P.; Mo, M.; Forsyth, C.; Tucci, A.; Hanes, V.; Delwail, V.; Hajek, R.; Chien, D. Efficacy and Safety of ABP 798: Results from the JASMINE Trial in Patients with Follicular Lymphoma in Comparison with Rituximab Reference Product. Target. Oncol. 2020, 15, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Lexicomp. Available online: https://www.uptodate.com/contents/rituximab-intravenous-including-biosimilars-drug-information?search=rituximab&selectedTitle=1~148&usage_type=panel&display_rank=1&kp_tab=drug_general&source=panel_search_result#F6133643 (accessed on 1 November 2023).
- Lexicomp. Available online: https://www.uptodate.com/contents/obinutuzumab-drug-information?search=obinutuzumab&source=panel_search_result&selectedTitle=1~52&usage_type=panel&kp_tab=drug_general&display_rank=1#F23613368 (accessed on 1 November 2023).

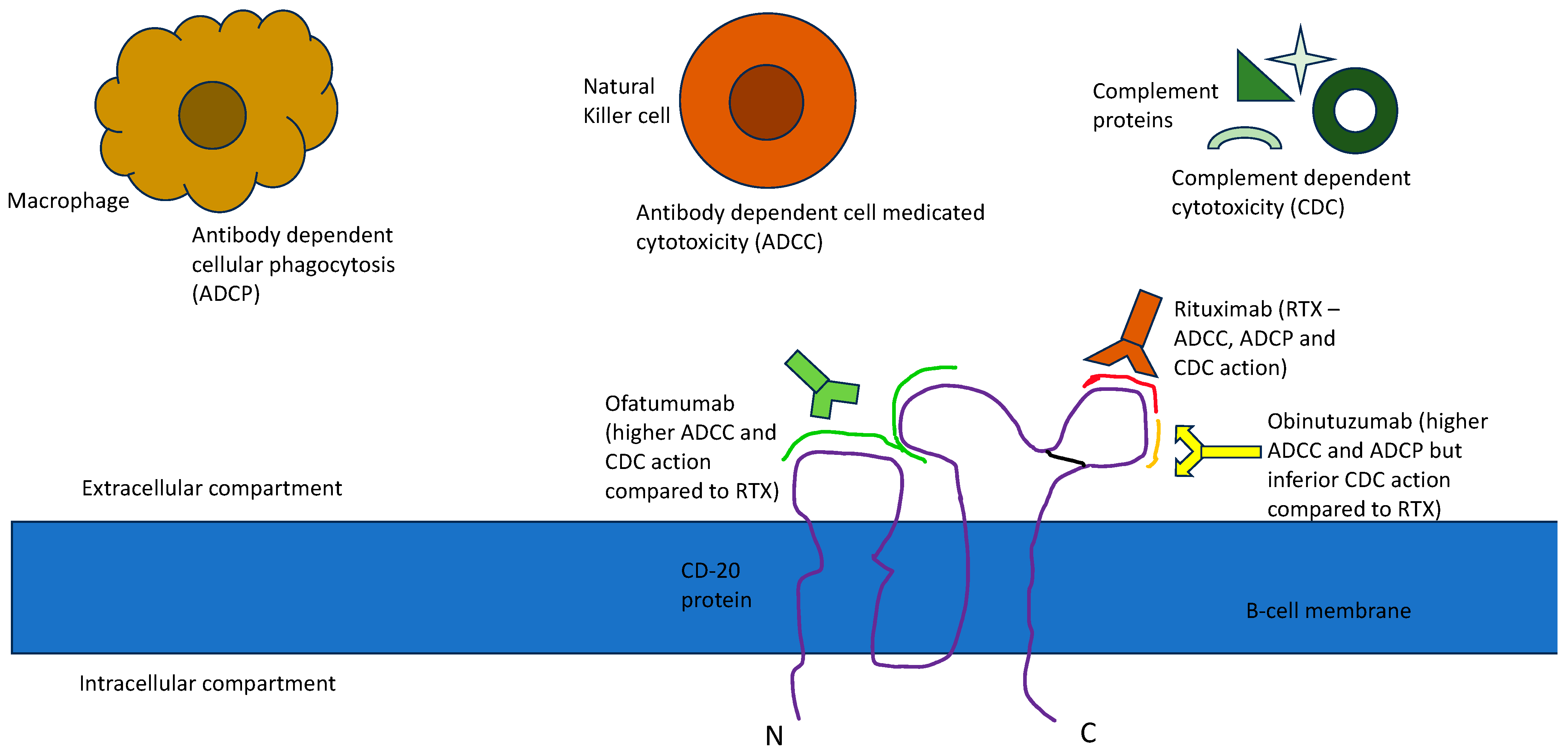
| Comparison Factors | Rituximab (RTX) | Ofatumumab | Obinutuzumab |
|---|---|---|---|
| Bioengineering-related mechanistic change |
| ||
| Proposed difference in therapeutic action |
|
| |
| Efficacy—Follicular lymphoma | Reference mAb—RTX | Comparable outcomes to RTX—PFS (16.33 vs. 21.29 months; not significant) and ORR (50% vs. 66%) compared to RTX [10]. |
|
| Efficacy—Diffuse large B-cell lymphoma | Reference mAb—RTX |
| |
| Efficacy—Chronic lymphocytic leukemia | Reference mAb—RTX |
|
|
| Efficacy—Mantle cell lymphoma | Reference mAb—RTX |
|
|
| Adverse effect profile | Reference mAb—RTX |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahadevia, H.; Ananthamurugan, M.; Shah, K.; Desai, A.; Shrestha, A. A Review of Anti-CD20 Antibodies in the Management of B-Cell Lymphomas. Lymphatics 2024, 2, 10-24. https://doi.org/10.3390/lymphatics2010002
Mahadevia H, Ananthamurugan M, Shah K, Desai A, Shrestha A. A Review of Anti-CD20 Antibodies in the Management of B-Cell Lymphomas. Lymphatics. 2024; 2(1):10-24. https://doi.org/10.3390/lymphatics2010002
Chicago/Turabian StyleMahadevia, Himil, Mirdhula Ananthamurugan, Kashish Shah, Atharva Desai, and Anuj Shrestha. 2024. "A Review of Anti-CD20 Antibodies in the Management of B-Cell Lymphomas" Lymphatics 2, no. 1: 10-24. https://doi.org/10.3390/lymphatics2010002
APA StyleMahadevia, H., Ananthamurugan, M., Shah, K., Desai, A., & Shrestha, A. (2024). A Review of Anti-CD20 Antibodies in the Management of B-Cell Lymphomas. Lymphatics, 2(1), 10-24. https://doi.org/10.3390/lymphatics2010002







