Effect of Pre- and Postharvest Chitosan and Calcium Applications on the Yield and Major Biochemical Qualities of Tomato (Lycopersicon esculentum Mill.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Layout in Field
2.3. Treatments Used During Field Trials
2.4. Preparation of Chitosan and Ca Solutions
2.5. Intercultural Operations and Data Collection at Harvest
2.6. Chitosan Treatments for Postharvest Storage
2.7. Quality Assessment During Postharvest Storage of Tomato Fruits
2.7.1. Weight Loss
2.7.2. Shrinkage Percentage
2.7.3. Visual Quality
2.7.4. Measurement of Titratable Acidity
2.7.5. Determination of Lycopene
2.7.6. Determination of Vitamin C
2.7.7. Determination of Total Sugar
2.8. Statistical Analysis
3. Results
3.1. Effect of Preharvest Application of Chitosan and Ca on Yield Contributing Parameters
3.1.1. Fruit Length
3.1.2. Fruit Diameter
3.1.3. Number of Fruits per Plant
3.1.4. Single-Fruit Weight
3.1.5. Yield of Tomato
3.2. Effect of Preharvest Application of Chitosan and Ca on Biochemical Qualities of Tomatoes
3.3. Effect of Postharvest Application of Chitosan on Physical Properties of Tomatoes
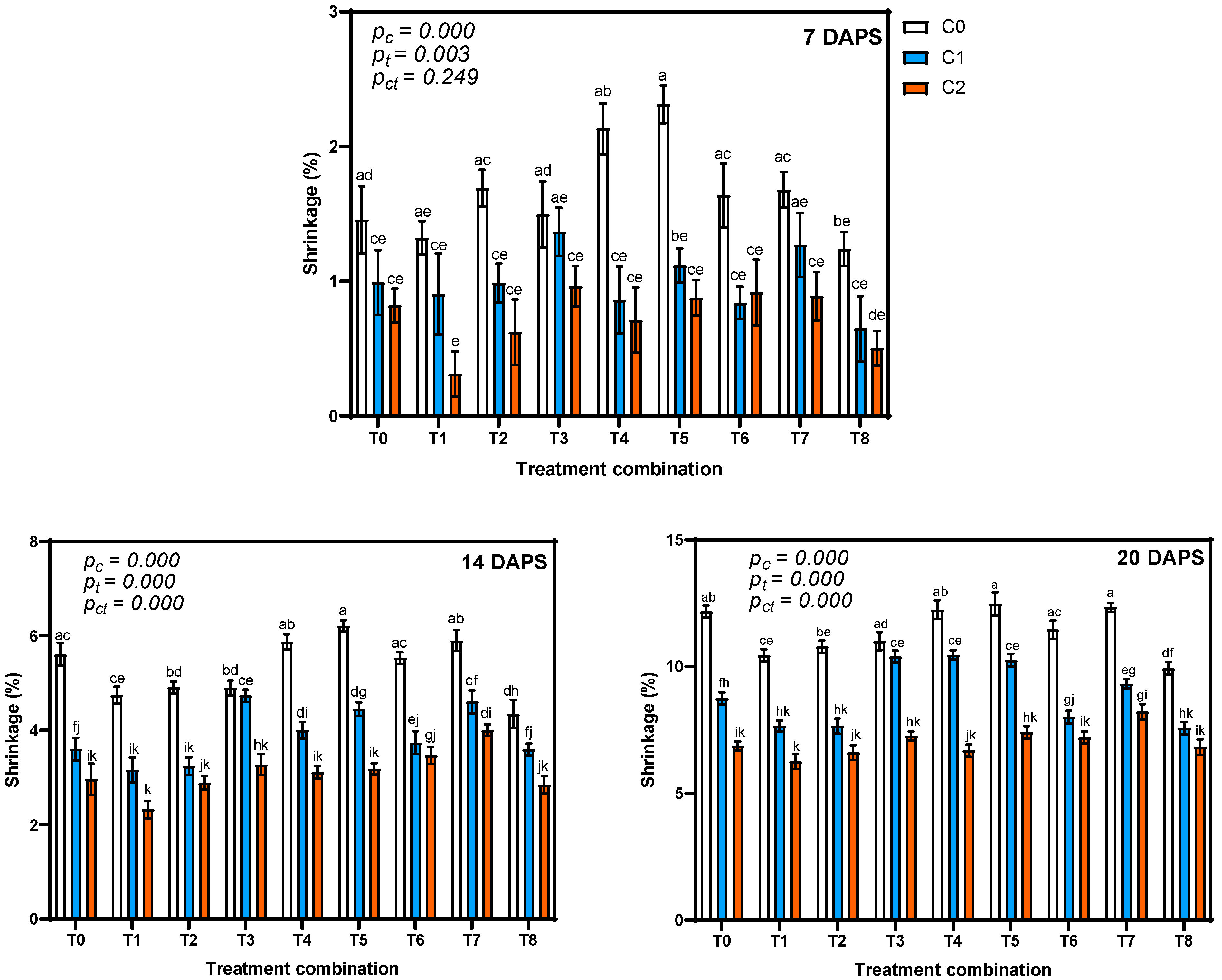
3.3.1. Effect on Shrinkage of Tomato Fruits
3.3.2. Effect on Visual Qualities of Tomato Fruits
3.4. Effect of Postharvest Application of Chitosan on Biochemical Qualities of Tomatoes
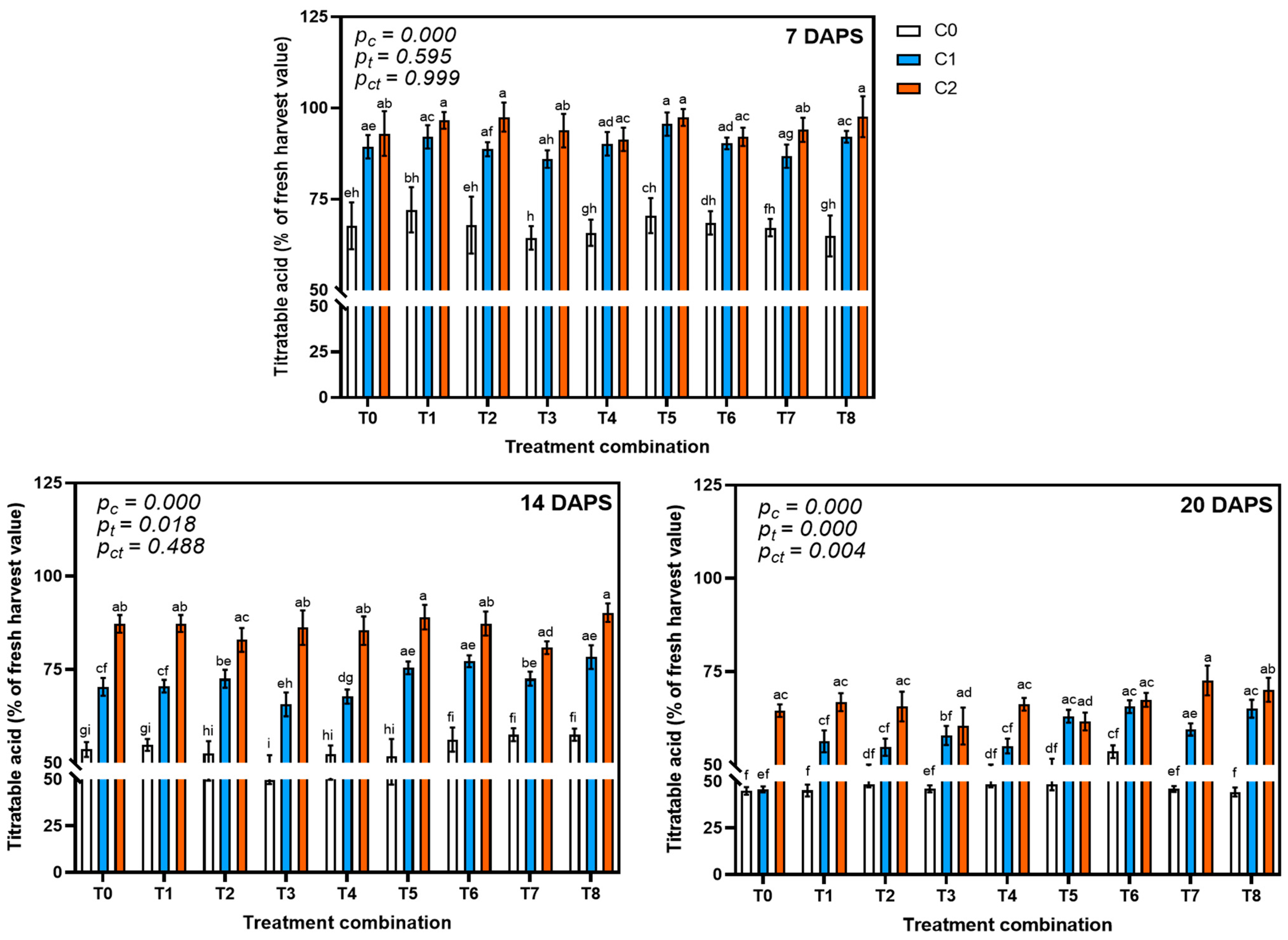
3.4.1. Effect on Titratable Acidity
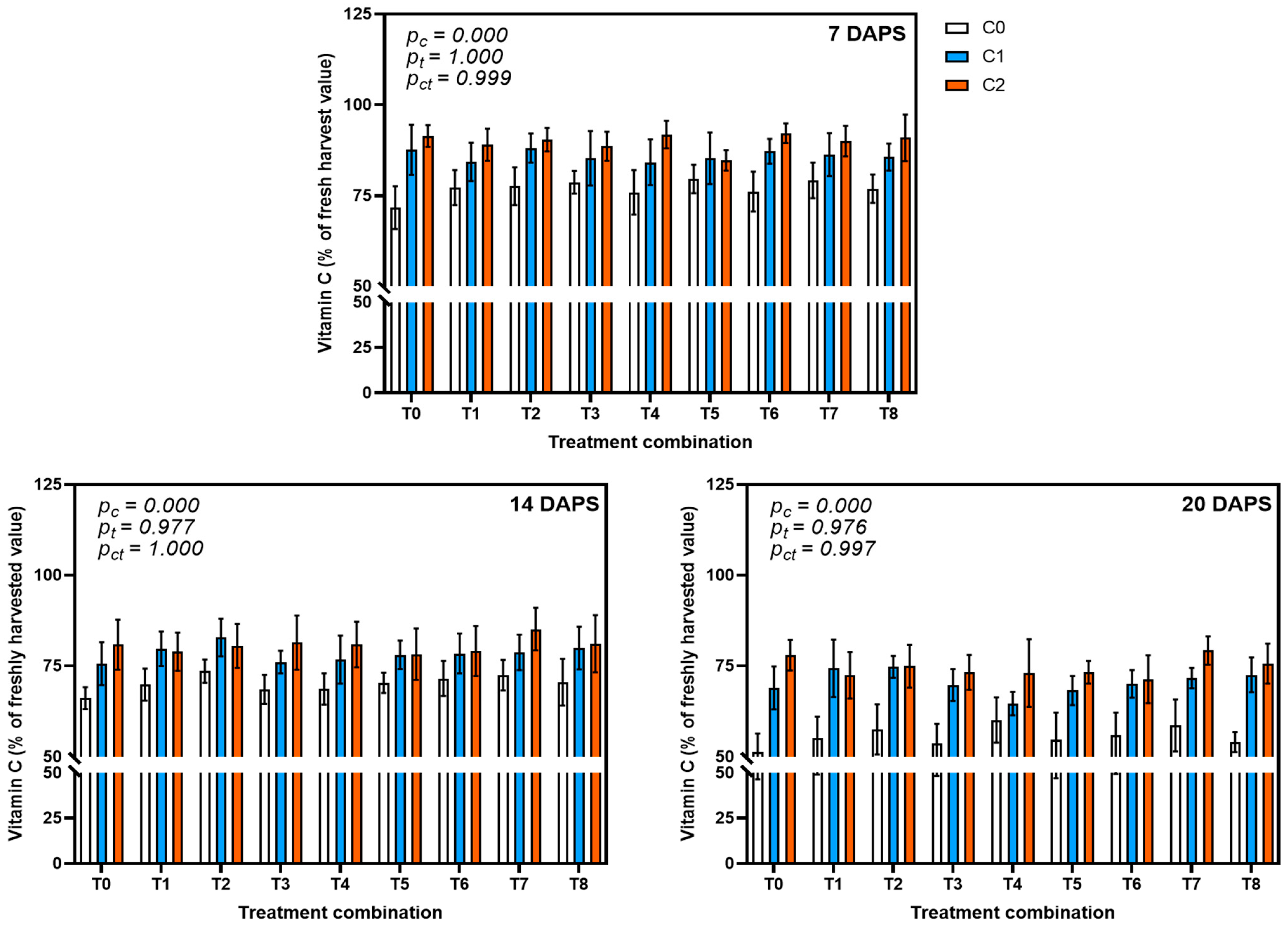
3.4.2. Effect on Vitamin C
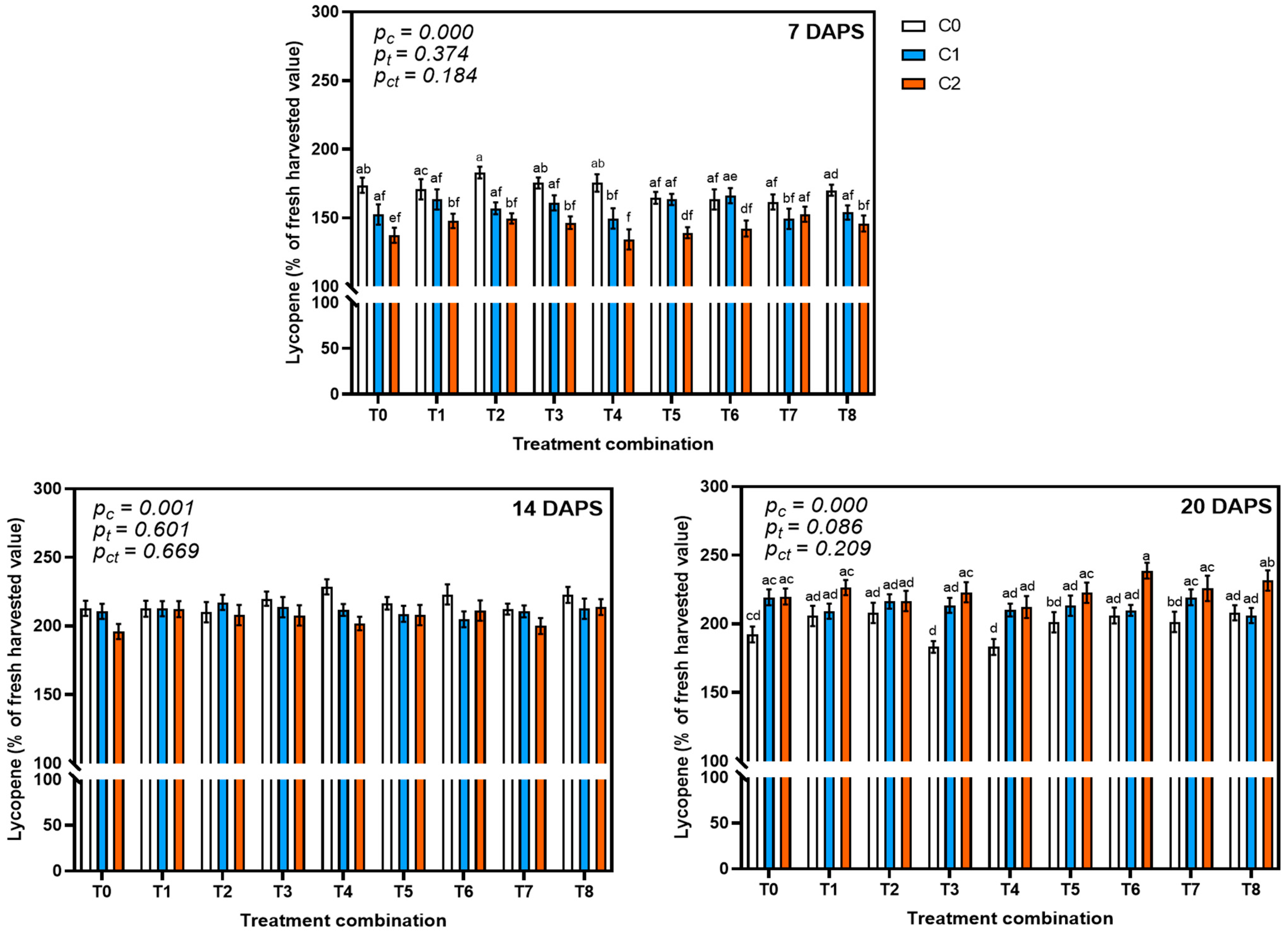
3.4.3. Effect on Lycopene
3.4.4. Effect on Total Sugar
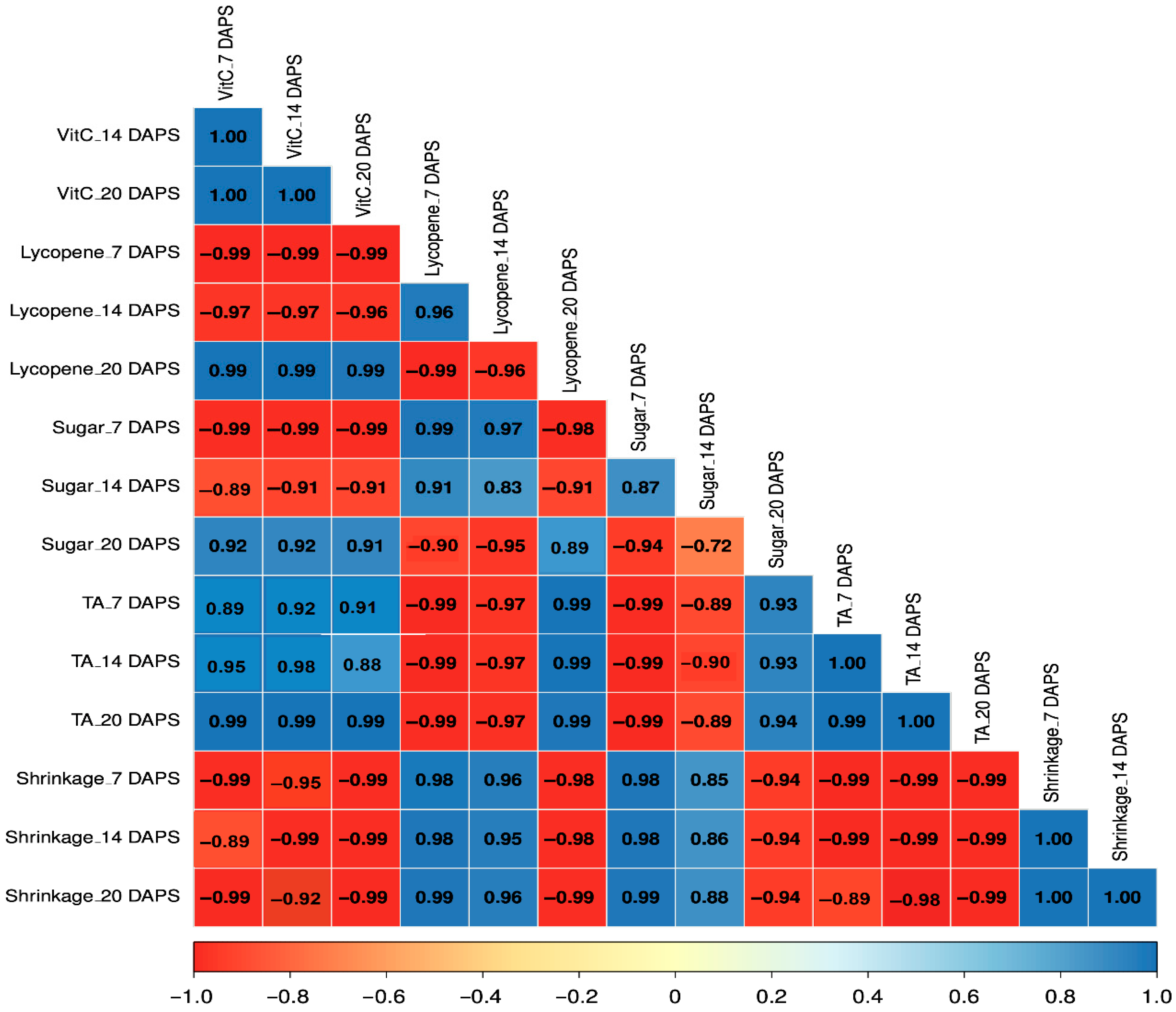
3.5. Correlations Among the Analyzed Parameters
4. Discussion
4.1. Preharvest Application of Chitosan and Ca on Tomato Yield
4.2. Preharvest Application of Chitosan and Ca on Biochemical Quality of Tomato
4.3. Postharvest Application of Chitosan on Physicochemical Qualities of Tomato
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAPS | Days after postharvest storage |
| BAU | Bangladesh Agricultural University |
| g | Gram |
| L | Liter |
| mL | Milliliter |
| mg | Milligram |
| µg | Microgram |
| CA | Citric acid |
| °C | Degree centigrade |
| ppm | Parts per million |
| TA | Titratable acidity |
| RCBD | Randomized Complete Block Design |
| N | Normality |
| CaCl2 | Calcium chloride |
| NaOH | Sodium hydroxide |
| H2SO4 | Sulphuric acid |
References
- FAOSTAT. Food and Agriculture Data; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 July 2025).
- BBS. Yearbook of Agricultural Statistics-2022; Bangladesh Bureau of Statistics, Statistics and Informatics Division (SID), Ministry of Planning, Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2023. Available online: https://www.bbs.gov.bd (accessed on 20 April 2025).
- Hassan, M.K.; Chowdhury, B.L.D.; Akhter, N. Post-Harvest Loss Assessment: A Study to Formulate Policy for Loss Reduction of Fruits and Vegetables and Socioeconomic Uplift of the Stakeholders. In Final Report of USAID and EO Funded Project (Jointly Implemented by FAO and FPMO of MoFDM); FAO: Dhaka, Bangladesh, 2010; p. 189. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=e19f18cf7099166eb73f9b16e52b4778&site=xueshu_se (accessed on 30 July 2025).
- Zhang, S.; Yu, Y.; Xiao, C.; Wang, X.; Tian, Y. Effect of carbon monoxide on browning of fresh-cut lotus root slice in relation to phenolic metabolism. LWT Food Sci. Technol. 2013, 53, 555–559. [Google Scholar] [CrossRef]
- Deepmala, K.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Betchem, G.; Johnson, N.A.N.; Wang, Y. The application of chitosan in the control of post-harvest diseases: A review. J. Plant Dis. Prot. 2019, 126, 495–507. [Google Scholar] [CrossRef]
- Ait Barka, E.; Eullaffroy, P.; Clement, C.; Vernet, G. Chitosan improves development and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Baños, S.; Hernandez-Lauzardo, A.N.; Hernandez-Lopez, M.; Barka, E.A.; Velazquez-del Valle, M.G.; Bosquez-Molina, E.; Wilson, C.L. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Sivakumar, D.; Bello-Pérez, A.; Villanueva-Arce, R.; Hernández-López, M. A review of the management alternatives for controlling fungi on papaya fruit during the postharvest supply chain. Crop Prot. 2013, 49, 8–20. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Canovas, G.V. Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Liu, W. Effects of chitin and its derivative chitosan on postharvest decay of fruits: A review. Int. J. Mol. Sci. 2011, 12, 917–934. [Google Scholar] [CrossRef]
- Ban, Z.; Yan, J.; Wang, Y.; Zhang, J.; Yuan, Q.; Li, L. Effects of postharvest application of chitosan-based layer-by-layer assemblies on regulation of ribosomal and defense proteins in strawberry fruit (Fragaria × ananassa). Sci. Hortic. 2018, 240, 293–302. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Lennox, C.L.; Meitz-Hopkins, J.C.; Caleb, O.J.; Sigge, G.O.; Opara, U.L. Investigating the effects of crab shell chitosan on fungal mycelial growth and postharvest quality attributes of pomegranate whole fruit and arils. Sci. Hortic. 2017, 220, 78–89. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Meng, X.; Yong, X. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Parvin, M.A.; Zakir, H.M.; Sultana, N.; Kafi, A.; Seal, H.P. Effects of different application methods of chitosan on growth, yield and quality of tomato (Lycopersicon esculentum Mill.). Arch. Agric. Environ. Sci. 2019, 4, 261–267. [Google Scholar] [CrossRef]
- Sultana, N.; Zakir, H.M.; Parvin, M.A.; Sharmin, S.; Seal, H.P. Effect of chitosan coating on physiological responses and nutritional qualities of tomato fruits during postharvest storage. Asian J. Adv. Agric. Res. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2015; p. 773. [Google Scholar]
- El-Badawy, H.E.M. Effect of chitosan and calcium chloride spraying on fruits quality of Florida Prince peach under cold storage. Res. J. Agric. Biol. Sci. 2012, 8, 272–281. [Google Scholar]
- Gayed, A.A.N.A.; Shaarawi, S.A.M.A.; Elkhishen, M.A.; Elsherbini, N.R.M. Pre-harvest application of calcium chloride and chitosan on fruit quality and storability of ‘Early Swelling’ peach during cold storage. Ciênc. Agrotec. 2017, 41, 220–231. [Google Scholar] [CrossRef]
- FRG. Fertilizer Recommendation Guide-2018; Bangladesh Agricultural Research Council (BARC): Dhaka, Bangladesh, 2018; p. 223. [Google Scholar]
- Sree, K.P.; Sree, M.S.; Supriya, P.; Samreen. Application of chitosan edible coating for preservation of tomato. Int. J. Chem. Stud. 2020, 8, 3281–3285. [Google Scholar] [CrossRef]
- Kibar, H.F.; Sabir, F.K. Chitosan coating for extending postharvest quality of tomatoes (Lycopersicon esculentum Mill.) maintained at different storage temperatures. AIMS Agric. Food 2018, 3, 97–108. [Google Scholar]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 2nd ed.; New Age International (P) Limited: New Delhi, India, 1996. [Google Scholar]
- Erba, D.; Casiraghi, M.C.; Ribas, A.; Cáceres, R.; Marfà, O.; Castellari, M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Comp. Anal. 2013, 31, 245–251. [Google Scholar] [CrossRef]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An Update on the health effects of tomato lycopene. Ann. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef]
- Su, L.; Diretto, G.; Purgatto, E.; Danoun, S.; Zouine, M.; Li, Z.; Roustan, J.-P.; Bouzayen, M.; Giuliano, G.; Chervin, C. Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol. 2015, 15, 114. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, H.; Wang, T.; Mustafa, G.; Liu, L.; Wang, Q.; Shao, Z. Quality improvement of tomato fruits by preharvest application of chitosan oligosaccharide. Horticulturae 2023, 9, 300. [Google Scholar] [CrossRef]
- Mondal, M.M.A.; Puteh, A.B.; Dafader, N.C. Foliar application of chitosan improved morpho-physiological attributes and yield in summer tomato (Solanum lycopersicum). Pak. J. Agric. Sci. 2016, 53, 339–344. [Google Scholar] [CrossRef]
- Mondal, M.M.; Malek, M.A.; Puteh, A.B.; Ismail, M.R.; Ashrafuzzaman, M.; Naher, L. Effect of foliar application of chitosan on growth and yield in okra. Aust. J. Crop Sci. 2012, 6, 918–921. [Google Scholar]
- El Amerany, F.; Rhazi, M.; Balcke, G.; Wahbi, S.; Meddich, A.; Taourirte, M.; Hause, B. The Effect of chitosan on plant physiology, wound response, and fruit quality of tomato. Polymers 2022, 14, 5006. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Islam, M.; Khatun, M.A.; Hassain, M.A.; Huque, R. Effect of foliar application of oligo-chitosan on growth, yield and quality of tomato and eggplant. Asian J. Agric. Res. 2017, 11, 36–42. [Google Scholar] [CrossRef][Green Version]
- Hassnain; Alam, M.; Ahmad, I.; Basit, A.; Ullah, I.; Alam, N.; Ullah, I.; Khalid, M.A.; Muhammad, B.; Shair, M. Efficacy of chitosan on performance of tomato (Lycopersicon esculentum L.) plant under water stress condition. Pak. J. Agric. Res. 2020, 33, 27–41. [Google Scholar] [CrossRef]
- Abdelaleem, H.M.; EL-Dkeshy, M.H.Z.; Abdalla, R.M. Preharvest spraying of calcium chloride, chitosan and their combination effects on tomato growth, yield, fruit characteristics, and quality. Assiut J. Agric. Sci. 2025, 56, 296–313. [Google Scholar] [CrossRef]
- Badawy, I.F.M.; Abou-Zaid, E.A.A.; Hussein, E.M.E. Cracking and fruit quality of “Manfalouty” pomegranate as affected by pre-harvest of chitosan, calcium chloride and gibberellic acid spraying. Middle East J. Agric. Res. 2019, 8, 873–882. [Google Scholar]
- Matteo, M.; Zoffoli, J.P.; Ayala, M. Calcium sprays and crop load reduction increase fruit quality and postharvest storage in sweet cherry (Prunus avium L). Agronomy 2022, 12, 829. [Google Scholar] [CrossRef]
- Vance, A.J.; Jones, P.; Strik, B.C. Foliar calcium applications do not improve quality or shelf life of strawberry, raspberry, blackberry, or blueberry fruit. Hort. Sci. 2017, 52, 382–387. [Google Scholar] [CrossRef]
- Mazumder, M.; Misran, A.; Ding, P.; Wahab, P. Preharvest foliar spray of calcium chloride on growth, yield, quality, and shelf life extension of different lowland tomato varieties in Malaysia. Horticulturae 2021, 7, 466. [Google Scholar] [CrossRef]
- Hernández, V.; Botella, M.Á.; Hellín, P.; Fenoll, J.; Flores, P. Dose-dependent potential of chitosan to increase yield or bioactive compound content in tomatoes. Horticulturae 2022, 8, 1152. [Google Scholar] [CrossRef]
- Tagele, A.; Woldetsadik, K.; Gedamu, F.; Rafi, M.M. Effects of preharvest applications of chemicals and storage conditions on the physico-chemical characteristics and shelf life of tomato (Solanum lycopersicum L.) fruit. Heliyon 2022, 8, e09494. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Montanha, G.S.; Agostinho, L.F.; Polezi, S.; Marques, J.P.R.; de Carvalho, H.W.P. Foliar calcium absorption by tomato plants: Comparing the effects of calcium sources and adjuvant usage. Plants 2023, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Chen, H.; Hu, S.; Liu, H.; Meng, F.; Li, S.; Wang, Q. Chitosan oligosaccharide treatment improves quality attributes of tomato fruit stored under room temperature. Postharvest Biol. Technol. 2022, 189, 111914. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Mohamed, A.H.; Odero, D.C.; Gomaa, A.M. Biochemical effects of chitosan coating and hot water dipping on green bean decay during cold storage. J. Appl. Sci. 2019, 19, 101–108. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Parmar, A.; Ali, M.R.; Abdel-Aziz, M.E.; Abdeldaym, E.A. Improving postharvest storage of fresh artichoke bottoms by an edible coating of Cordia myxa gum. Postharvest Biol. Technol. 2020, 163, 111143. [Google Scholar] [CrossRef]
- Saha, S.; Zakir, H.M.; Mallick, S.; Alam, M.S.; Quadir, Q.F. Impact of chitosan coatings on shelf-life, nutrient elements and biochemical qualities of country beans (Phaseolus lunatus L.) at postharvest storage. J. Hort. Postharvest Res. 2024, 7, 1–14. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Dai, L.; Wang, X.; Zhang, J.; Li, C. Application of chitosan and its derivatives in postharvest coating preservation of fruits. Foods 2025, 14, 1318. [Google Scholar] [CrossRef]
- Singh, H.; Bhasin, J.K.; Dash, K.K.; Shams, R.; Shaikh, A.K.; Béla, K. Effect of chitosan based edible coating in management of postharvest losses in Papaya: A comprehensive review. Appl. Food Res. 2024, 4, 100456. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Quantick, P.C. Antifungal effects of chitosan coating on fresh strawberries and raspberries during storage. J. Hort. Sci. Biotechnol. 1998, 73, 763–767. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Ponnampalam, R.; Boulet, M. Chitosan coating effect on storability and quality of fresh strawberries. J. Food Sci. 1991, 56, 1618–1621. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Pysiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Das, D.K.; Dutta, H.; Mahanta, C.L. Development of a rice starch-based coating with antioxidant and microbe-barrier properties and study of its effect on tomatoes stored at room temperature. LWT Food Sci. Technol. 2013, 50, 272–278. [Google Scholar] [CrossRef]
- Oz, A.T.; Ulukanli, Z. Application of edible starch-based coating including glycerol plus oleum nigella on arils from long-stored whole pomegranate fruits. J. Food Process. Preserv. 2012, 36, 81–95. [Google Scholar] [CrossRef]
- Bico, S.L.S.; Raposo, M.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh cut banana. Food Control 2009, 20, 508–514. [Google Scholar] [CrossRef]
- Khan, W.M.; Prithiviraj, B.; Smith, D.L. Effect of foliar application of chitin and chitosan oligosaccharides on photosynthesis of maize and soybean. Photosynthetica 2002, 40, 621–624. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.G.; Bondok, A.M. Response of tomato plants to salicylic acid and chitosan under infection with tomato mosaic virus. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 1520–1529. [Google Scholar]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Gottschalk, K. Effect of moisture content, storage temperature and storage period on colour, ascorbic acid, lycopene and total flavonoids of dried tomato halves. Int. J. Food Sci. Technol. 2009, 44, 1245–1253. [Google Scholar] [CrossRef]
- Javanmardi, J.; Kubota, C. Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during postharvest storage. Postharvest Biol. Technol. 2006, 41, 151–155. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Czékus, Z.; Iqbal, N.; Pollák, B.; Martics, A.; Ördög, A.; Poór, P. Role of ethylene and light in chitosan-induced local and systemic defence responses of tomato plants. J. Plant Physiol. 2021, 263, 153461. [Google Scholar] [CrossRef]


| Treatment | Fruit Length (cm) | Fruit Diameter (cm) | Number of Fruits/Plant | Single Fruit wt. (g) | Yield (tons/Hectare) | Titratable Acidity (%) | Vitamin C (mg/100 g Fresh wt.) | Lycopene (mg/100 g Fresh wt.) | Total Sugar (%) |
|---|---|---|---|---|---|---|---|---|---|
| Control (T0) | 4.1 ± 0.92 b | 3.3 ± 1.4 | 12.4 ± 2.1 c | 34.5 ± 3.1 c | 33.1 ± 2.2 d | 0.76 ± 0.12 | 29.1 ± 1.3 | 3.2 ± 0.55 | 1.3 ± 0.42 |
| 50 ppm chitosan (T1) | 4.6 ± 1.2 a | 3.6 ± 1.3 | 14.8 ± 2.4 ab | 36.7 ± 3.3 ab | 42.0 ± 1.8 c | 0.74 ± 0.11 | 27.5 ± 2.1 | 3.0 ± 0.24 | 1.2 ± 0.23 |
| 80 ppm chitosan (T2) | 4.6 ± 1.1 a | 3.6 ± 1.3 | 16.8 ± 2.2 a | 38.9 ± 2.4 a | 46.5 ± 2.0 a | 0.74 ± 0.13 | 28.1 ± 1.9 | 3.1 ± 0.93 | 1.2 ± 0.11 |
| 0.50% Ca solution (T3) | 4.3 ± 0.75 b | 3.6 ± 1.2 | 13.0 ± 1.9 bc | 33.4 ± 2.8 c | 33.3 ± 1.1 d | 0.76 ± 0.10 | 29.3 ± 0.52 | 3.6 ± 0.42 | 1.5 ± 0.15 |
| 1.0% Ca solution (T4) | 4.1 ± 0.72 b | 3.4 ± 1.1 | 12.3 ± 2.0 c | 34.8 ± 2.3 bc | 34.5 ± 1.2 d | 0.77 ± 0.10 | 29.2 ± 1.4 | 3.4 ± 0.88 | 1.3 ± 0.31 |
| Combined T1 and T3 (T5) | 4.4 ± 1.1 ab | 3.5 ± 1.3 | 15.0 ± 2.3 ab | 34.6 ± 3.1 bc | 41.7 ± 1.6 c | 0.77 ± 0.11 | 28.6 ± 2.5 | 3.4 ± 1.3 | 1.3 ± 0.22 |
| Combined T2 and T3 (T6) | 4.5 ± 0.78 a | 3.6 ± 1.2 | 15.3 ± 2.2 ab | 37.0 ± 2.9 ab | 44.6 ± 1.4 abc | 0.74 ± 0.13 | 27.1 ± 0.65 | 3.4 ± 0.65 | 1.2 ± 0.10 |
| Combined T1 and T4 (T7) | 4.3 ± 1.1 b | 3.5 ± 1.1 | 14.5 ± 2.0 ab | 37.3 ± 2.6 a | 43.3 ± 2.0 c | 0.76 ± 0.11 | 27.0 ± 0.44 | 3.2 ± 0.22 | 1.4 ± 0.32 |
| Combined T2 and T4 (T8) | 4.6 ± 0.94 a | 3.6 ± 1.3 | 16.4 ± 2.1 a | 38.6 ± 3.2 a | 45.0 ± 1.9 ab | 0.75 ± 0.11 | 26.9 ± 2.2 | 3.2 ± 0.85 | 1.4 ± 0.28 |
| CV (%) | 4.6 | 3.1 | 11.3 | 5.4 | 13.2 | 1.64 | 3.6 | 6.5 | 8.6 |
| Level of Significance | * | NS | * | * | * | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossen, M.Z.; Nayeem, S.M.M.R.; Quadir, Q.F.; Sharmin, S.; Das, P.; Moury, T.J.; Sathi, L.A.; Das, R.C.; Rashid, M.H.O. Effect of Pre- and Postharvest Chitosan and Calcium Applications on the Yield and Major Biochemical Qualities of Tomato (Lycopersicon esculentum Mill.). Agrochemicals 2025, 4, 13. https://doi.org/10.3390/agrochemicals4030013
Hossen MZ, Nayeem SMMR, Quadir QF, Sharmin S, Das P, Moury TJ, Sathi LA, Das RC, Rashid MHO. Effect of Pre- and Postharvest Chitosan and Calcium Applications on the Yield and Major Biochemical Qualities of Tomato (Lycopersicon esculentum Mill.). Agrochemicals. 2025; 4(3):13. https://doi.org/10.3390/agrochemicals4030013
Chicago/Turabian StyleHossen, Md. Zakir, S. M. Mashiur Rahman Nayeem, Quazi Forhad Quadir, Shaila Sharmin, Phalguni Das, Tasnuva Jahan Moury, Laila Arafat Sathi, Ronzon Chandra Das, and Md. Harun Or Rashid. 2025. "Effect of Pre- and Postharvest Chitosan and Calcium Applications on the Yield and Major Biochemical Qualities of Tomato (Lycopersicon esculentum Mill.)" Agrochemicals 4, no. 3: 13. https://doi.org/10.3390/agrochemicals4030013
APA StyleHossen, M. Z., Nayeem, S. M. M. R., Quadir, Q. F., Sharmin, S., Das, P., Moury, T. J., Sathi, L. A., Das, R. C., & Rashid, M. H. O. (2025). Effect of Pre- and Postharvest Chitosan and Calcium Applications on the Yield and Major Biochemical Qualities of Tomato (Lycopersicon esculentum Mill.). Agrochemicals, 4(3), 13. https://doi.org/10.3390/agrochemicals4030013







