Weed Control Increases the Growth and Above-Ground Biomass Production of Pinus taeda Plantations in Southern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Experimental Design and Treatments
2.3. Floristic Composition and Biomass of Weed Species
2.4. Tree Growth Assessment
2.5. Assessment of Tree Above-Ground Biomass
2.6. Statistical Analysis
3. Results
3.1. Tree Growth
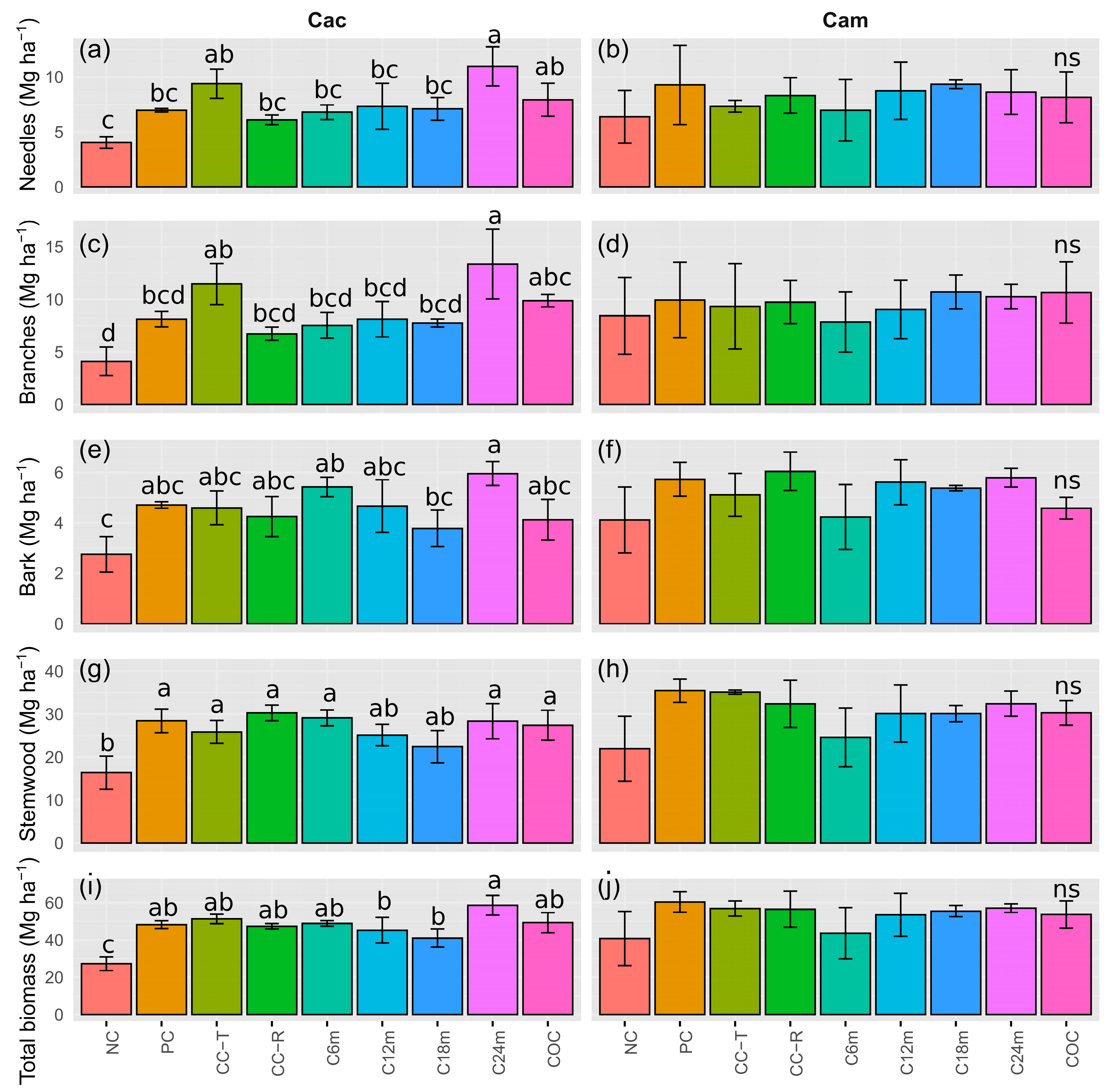
3.2. Tree Above-Ground Biomass
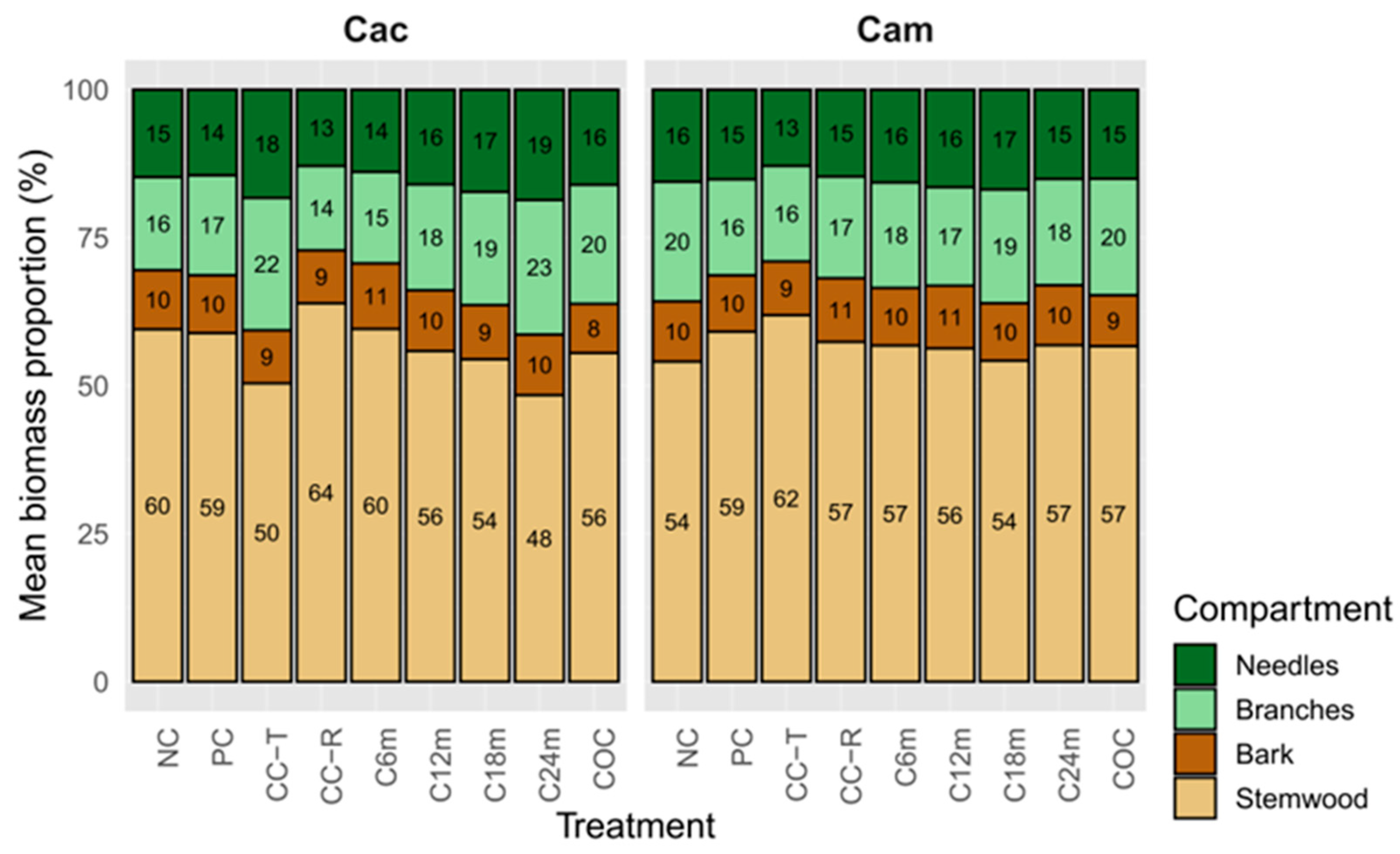
3.3. Proportion of Tree Above-Ground Biomass
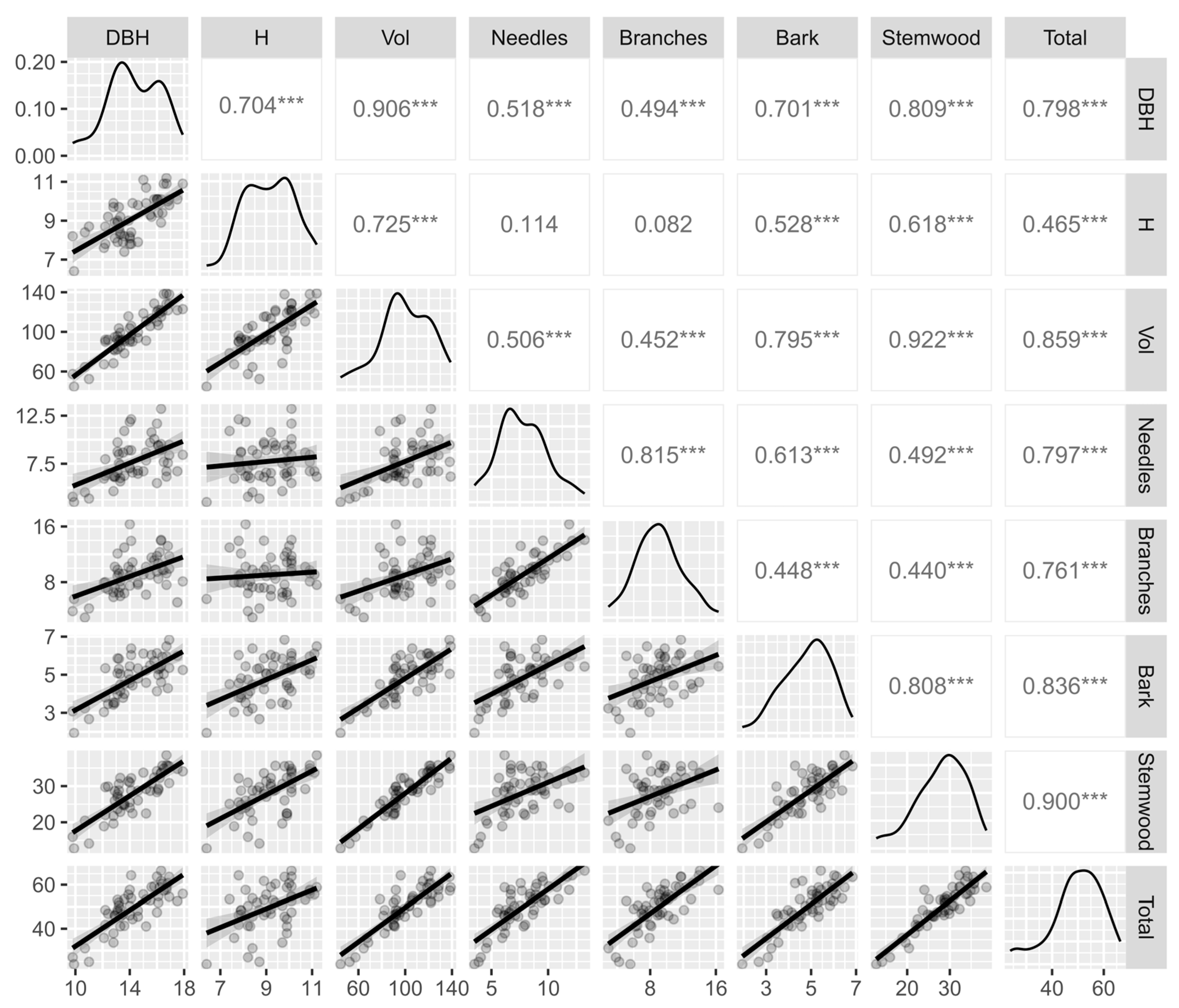
3.4. Correlation Between Growth and Above–Ground Biomass of Trees
3.5. Indirect Estimation of Above–Ground Biomass of Trees
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. Global Forest Resources Assessment; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Indústria Brasileira de Árvores. Relatório Anual 2022; IBÁ: São Paulo, Brazil, 2023; Available online: https://iba.org/datafiles/publicacoes/relatorios/relatorio-anual-iba2022-compactado.pdf (accessed on 7 December 2022).
- Dobner, M.; Campoe, O.C. Meteorological effects on 30-years-grown Pinus taeda under a gradient of crown thinning intensities in southern Brazil. For. Ecol. Manag. 2019, 453, 117624. [Google Scholar] [CrossRef]
- Schulte, M.L.; Cook, R.L.; Albaugh, T.J.; Allen, H.L.; Rubilar, R.A.; Pezzutti, R.; Caldato, S.L.; Campoe, O.; Carter, D.R. Mid-rotation response of Pinus taeda to early silvicultural treatments in subtropical Argentina. For. Ecol. Manag. 2020, 473, 118317. [Google Scholar] [CrossRef]
- Scherf, A.N.; Corley, J.C.; Gioia, C.D.; Eskiviski, E.R.; Carazzo, C.; Patzer, H.R.; Dimarco, R.D. Impact of a leaf-cutting ant (Atta sexdens L.) on a Pinus taeda plantation: A 6 year-long study. J. Appl. Entomol. 2022, 146, 1178–1184. [Google Scholar] [CrossRef]
- Consalter, R.; Motta, A.C.V.; Barbosa, J.Z.; Vezzani, F.M.; Rubilar, R.A.; Prior, S.A.; Nisgoski, S.; Bassaco, M.V.M. Fertilization of Pinus taeda L. on an acidic oxisol in southern Brazil: Growth, litter accumulation, and root exploration. Eur. J. For. Res. 2021, 140, 1095–1112. [Google Scholar] [CrossRef]
- Cardoso, D.J.; Lacerda, A.E.B.; Rosot, M.A.D.; Garrastazú, M.C.; Lima, R.T. Influence of spacing regimes on the development of loblolly pine (Pinus taeda L.) in Southern Brazil. For. Ecol. Manag. 2013, 310, 761–769. [Google Scholar] [CrossRef]
- Rodriguez, D.R.O.; de Guilherme, C.A.; Bellote, A.F.J.; Tomazello-Filho, M. Effect of pulp and paper mill sludge on the development of 17-year-old loblolly pine (Pinus taeda L.) trees in Southern Brazil. For. Ecol. Manag. 2018, 422, 179–189. [Google Scholar] [CrossRef]
- Munhoz, J.S.B.; Alvares, C.A.; Carneiro, R.L.; Kulmann, M.S.S.; Stape, J.L. Production patterns of loblolly pine plantations along a geographic gradient in southern Brazil. For. Ecol. Manag. 2024, 562, 121956. [Google Scholar] [CrossRef]
- Pinto, C.B.; Marques, R.; Dalmaso, C.A.; Kulmann, M.S.S.; Deliberali, I.; Schumacher, M.V.; de Oliveira Junior, J.C. Relationship between edaphoclimatic attributes and productivity of loblolly pine (Pinus taeda L.) in southern Brazil. For. Ecol. Manag. 2023, 544, 121162. [Google Scholar] [CrossRef]
- Campoe, O.C.; Stape, J.L.; Albaugh, T.J.; Allen, H.L.; Fox, T.R.; Rubilar, R.; Binkley, D. Fertilization and irrigation effects on tree level aboveground net primary production, light interception and light use efficiency in a loblolly pine plantation. For. Ecol. Manag. 2013, 288, 43–48. [Google Scholar] [CrossRef]
- Kulmann, M.S.S.; Aguilar, M.V.M.; Tassinari, A.; Schwalbert, R.; Tabaldi, L.A.; Araujo, M.M.; Antoniolli, Z.I.; Nicoloso, F.T.; Brunetto, G.; Schumacher, M.V. Effects of increasing soil phosphorus and association with ectomycorrhizal fungi (Pisolithus microcarpus) on morphological, nutritional, biochemical, and physiological parameters of Pinus taeda L. For. Ecol. Manag. 2023, 544, 121207. [Google Scholar] [CrossRef]
- Kulmann, M.S.S.; Deliberali, I.; Schumacher, M.V.; Stahl, J.; Figura, M.A.; Ludvichak, A.A.; Stape, J.L. Can fertilization and stand uniformity affect the growth and biomass production in a Pinus taeda plantation in southern Brazil. For. Ecol. Manag. 2023, 541, 121075. [Google Scholar] [CrossRef]
- Pellens, G.C.; Lessa, P.R.; Schorn, L.A.; Fenilli, T.A.B. Influência da Matocompetição em Povoamentos Jovens de Pinus taeda L. Ciênc. Florest. 2018, 28, 495–504. [Google Scholar] [CrossRef]
- Albaugh, T.J.; Maier, C.A.; Campoe, O.C.; Laviner, M.A.; Shively, T.J.; Aguiar, V.; Peer, K.R.; Carter, D.R.; Cook, R.L.; Rubilar, R.A.; et al. Quantifying above- and belowground biomass improved our understanding of site differences and demonstrated the importance of management decisions in sequestering carbon in Pinus taeda. For. Ecol. Manag. 2025, 593, 122901. [Google Scholar] [CrossRef]
- Amishev, D.Y.; Fox, T.R. The effect of weed control and fertilization on survival and growth of four pine species in the Virginia Piedmont. For. Ecol. Manag. 2006, 236, 93–101. [Google Scholar] [CrossRef]
- Rubilar, R.; Bozo, D.; Albaugh, T.; Cook, R.; Campoe, O.; Carter, D.; Allen, H.L.; Álvarez, J.; Pincheira, M.; Zapata, Á. Rotation-age effects of subsoiling, fertilization, and weed control on radiata pine growth at sites with contrasting soil physical, nutrient, and water limitations. For. Ecol. Manag. 2023, 544, 121213. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- INMET. Clima e Tempo—Português (Brasil). Available online: https://www.gov.br/agricultura/pt-br/assuntos/inmet (accessed on 1 August 2022).
- Staff, S.S. Keys to Soil Taxonomy, 11th ed.; United States Department of Agriculture, Natural Resource Conservation Service: Washington, DC, USA, 2010; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=CuWEKWc1wIgC&oi=fnd&pg=PP7&dq=Keys+to+soil+taxonomy&ots=Mg7-wECwaM&sig=QJQgLzt8CfHUB-SfR9tAXXKrkBk#v=onepage&q=Keys%20to%20soil%20taxonomy&f=false (accessed on 25 July 2021).
- de Moraes Gonçalves, J.L.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; de Barros Ferraz, S.F.; de Paula Lima, W.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Moro, L.; Gatiboni, L.C.; Simonete, M.A.; Cassol, P.C.; Chaves, D.M. Resposta de Pinus taeda com diferentes idades à adubação NPK no Planalto Sul Catarinense. Rev. Bras. Cienc. Solo 2014, 38, 1181–1189. [Google Scholar] [CrossRef]
- Gatiboni, L.C.; da Silva, W.C.; Mumbach, G.L.; Schmitt, D.E.; Iochims, D.A.; Stahl, J.; Vargas, C.O. Use of exchangeable and nonexchangeable forms of calcium, magnesium, and potassium in soils without fertilization after successive cultivations with Pinus taeda in southern Brazil. J. Soils Sediments 2020, 20, 665–674. [Google Scholar] [CrossRef]
- Forest Stewardship Council. Política de Pesticidas do FSC; FSC: Bonn, Germany, 2019; Available online: https://br.fsc.org/sites/default/files/assets/FSC_newsentry_1567173471_file.pdf (accessed on 15 June 2025).
- Schumacher, F.X.; Hall, F.S. Logarithmic expression of timber-tree volume. J. Agric. Res. 1933, 47, 719–734. [Google Scholar]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R Package for ANOVA and Experimental Designs. Appl. Math. 2014, 5, 2952–2958. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package “corrplot” Title Visualization of a Correlation Matrix. CRAN 2017. Available online: https://www.maths.bris.ac.uk/R/web/packages/corrplot/corrplot.pdf (accessed on 2 July 2025).
- Kassambara, A.; Mundt, F. Package ‘Factoextra’. Extract and Visualize the Results of Multivariate Data Analyses. CRAN R Package 2020. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 6 December 2022).
- The R Core Team. R: A Language and Environment for Statistical Computing; The R Core Team: Vienna, Austria, 2019; Available online: https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf (accessed on 2 July 2025).
- Subedi, P.; Jokela, E.J.; Vogel, J.G.; Martin, T.A. Sustained productivity of intensively managed loblolly pine plantations: Persistence of fertilization and weed control effects across rotations. For. Ecol. Manag. 2019, 446, 38–53. [Google Scholar] [CrossRef]
- Albaugh, T.J.; Allen, H.L.; Dougherty, P.M.; Johnsen, K.H. Long term growth responses of loblolly pine to optimal nutrient and water resource availability. For. Ecol. Manag. 2004, 192, 3–19. [Google Scholar] [CrossRef]
- Fox, T.R.; Allen, H.L.; Albaugh, T.J.; Rubilar, R.; Carlson, C.A. Tree nutrition and forest fertilization of pine plantations in the southern United States. South. J. Appl. For. 2007, 31, 5–11. [Google Scholar] [CrossRef]
- Carlson, C.A.; Fox, T.R.; Allen, H.L.; Albaugh, T.J.; Rubilar, R.A.; Stape, J.L. Growth responses of loblolly pine in the southeast United States to midrotation applications of nitrogen, phosphorus, potassium, and micronutrients. For. Sci. 2014, 60, 157–169. [Google Scholar] [CrossRef]
- Samuelson, L.J.; Butnor, J.; Maier, C.; Stokes, T.A.; Johnsen, K.; Kane, M. Growth and physiology of loblolly pine in response to long-term resource management: Defining growth potential in the southern United States. Can. J. For. Res. 2008, 38, 721–732. [Google Scholar] [CrossRef]
- Pezzutti, R.; Schumacher, M.V. Efect of Chemical Root Pruning on Eucalyptus globulus subsp. maidenii Seedlings Growth. Rev. For. Yvyrareta 2000, 1–6. [Google Scholar]
- Albaugh, T.J.; Fox, T.R.; Maier, C.A.; Campoe, O.C.; Rubilar, R.A.; Cook, R.L.; Raymond, J.E.; Alvares, C.A.; Stape, J.L. A common garden experiment examining light use efficiency and heat sum to explain growth differences in native and exotic Pinus taeda. For. Ecol. Manag. 2018, 425, 35–44. [Google Scholar] [CrossRef]
- Campoe, O.C.; Stape, J.L.; Nouvellon, Y.; Laclau, J.P.; Bauerle, W.L.; Binkley, D.; Le Maire, G. Stem production, light absorption and light use efficiency between dominant and non-dominant trees of Eucalyptus grandis across a productivity gradient in Brazil. For. Ecol. Manag. 2013, 288, 14–20. [Google Scholar] [CrossRef]




| Site | Depth | Clay | Silt | Sand | SOM | pH | P | K | Ca | Mg | H + Al | BS | CEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | g kg−1 | – | mg dm−3 | cmolc dm−3 | |||||||||
| Cac | 0–20 | 653 | 241 | 106 | 45.0 | 4.9 | 3.3 | 73.7 | 1.4 | 0.3 | 13.3 | 2.0 | 15.3 |
| 20–40 | 698 | 202 | 100 | 35.7 | 4.8 | 2.6 | 54.3 | 1.3 | 0.4 | 12.1 | 2.0 | 14.1 | |
| Cam | 0–20 | 605 | 284 | 111 | 51.7 | 4.6 | 2.4 | 50.0 | 1.0 | 0.5 | 16.5 | 1.7 | 18.2 |
| 20–40 | 606 | 251 | 143 | 38.0 | 4.5 | 1.9 | 34.7 | 0.5 | 0.3 | 16.0 | 1.0 | 17.0 | |
| Treatment Abbreviation | Operation Description |
|---|---|
| NC | No control—with weeds present |
| PC | Physical weed control |
| CC-T | Chemical weed control in total area |
| CC-R | Chemical weed control in rows |
| C6m | Weed control up to 6 months after planting |
| C12m | Weed control up to 12 months after planting |
| C18m | Weed control up to 18 months after planting |
| C24m | Weed control up to 24 months after planting |
| COC | Company operational weed control |
| Site | Weed Structure | Weed Biomass | Main Species |
|---|---|---|---|
| (Mg ha−1) | |||
| Cac | Abundant | 1.98 | Melinis minutiflora; Baccharis dracunculifolia; Baccharis uncinella; Cyperus esculentus |
| Dominant | 7.76 | Brachiaria mutica; Cyperus esculentus; Austroeupatorium inulaefolium | |
| Other weeds | 1.17 | ||
| Total | 10.91 | ||
| Cam | Abundant | 6.21 | Baccharis spp.; Melinis minutiflora; Ageratum conyzoides |
| Dominant | 9.67 | Melinis minutiflora; Baccharis uncinella; Callisia repens; Miconia theaezans | |
| Other weeds | 2.34 | ||
| Total | 18.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulmann, M.S.d.S.; Pereira, M.G.; Witschoreck, R.; Schumacher, M.V. Weed Control Increases the Growth and Above-Ground Biomass Production of Pinus taeda Plantations in Southern Brazil. Agrochemicals 2025, 4, 14. https://doi.org/10.3390/agrochemicals4030014
Kulmann MSdS, Pereira MG, Witschoreck R, Schumacher MV. Weed Control Increases the Growth and Above-Ground Biomass Production of Pinus taeda Plantations in Southern Brazil. Agrochemicals. 2025; 4(3):14. https://doi.org/10.3390/agrochemicals4030014
Chicago/Turabian StyleKulmann, Matheus Severo de Souza, Marcos Gervasio Pereira, Rudi Witschoreck, and Mauro Valdir Schumacher. 2025. "Weed Control Increases the Growth and Above-Ground Biomass Production of Pinus taeda Plantations in Southern Brazil" Agrochemicals 4, no. 3: 14. https://doi.org/10.3390/agrochemicals4030014
APA StyleKulmann, M. S. d. S., Pereira, M. G., Witschoreck, R., & Schumacher, M. V. (2025). Weed Control Increases the Growth and Above-Ground Biomass Production of Pinus taeda Plantations in Southern Brazil. Agrochemicals, 4(3), 14. https://doi.org/10.3390/agrochemicals4030014






