Uptake, Distribution, and Activity of Pluronic F68 Adjuvant in Wheat and Its Endophytic Bacillus Isolate
Abstract
1. Introduction
2. Materials and Methods
2.1. F68 Properties
2.2. F68 Effect on Seed Water Uptake, Germination, Emergence, and Endophyte Colonization of Spermosphere
2.3. Assessment of Drought Stress Responses in F68, GB, and F68 + GB Wheat Seedlings
2.4. Fluorescent F68 (fF68) Labeling of Plant and Microbiome
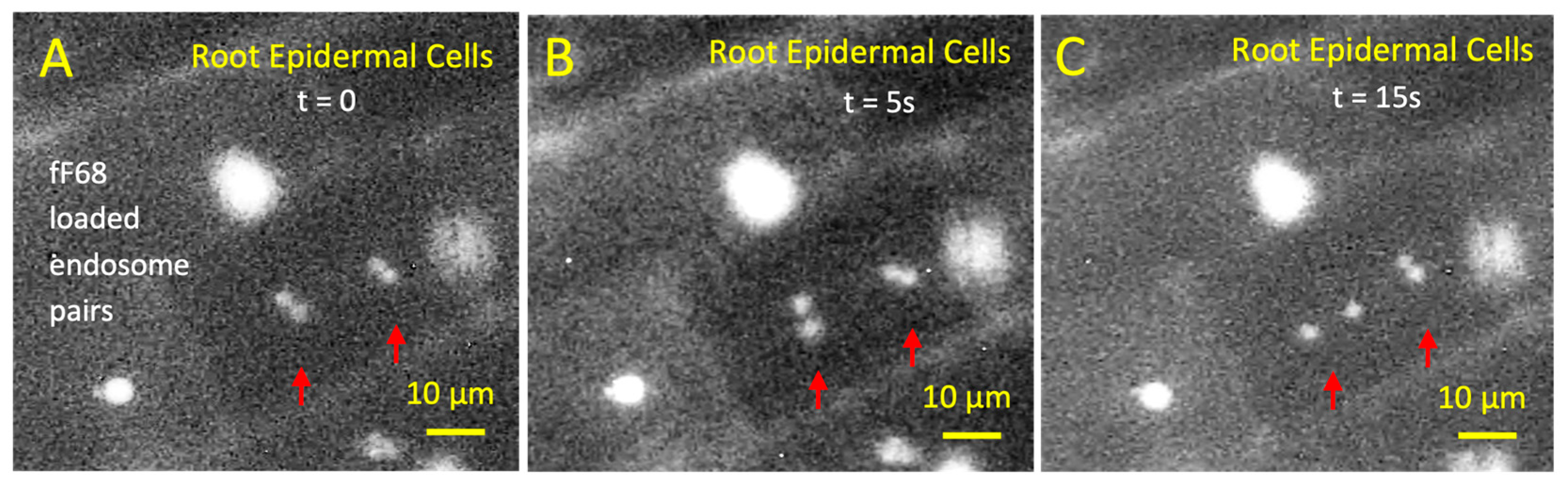
2.4.1. Interaction of fF68 with Root Epidermal Cells and Root-Epiphytic Bacteria
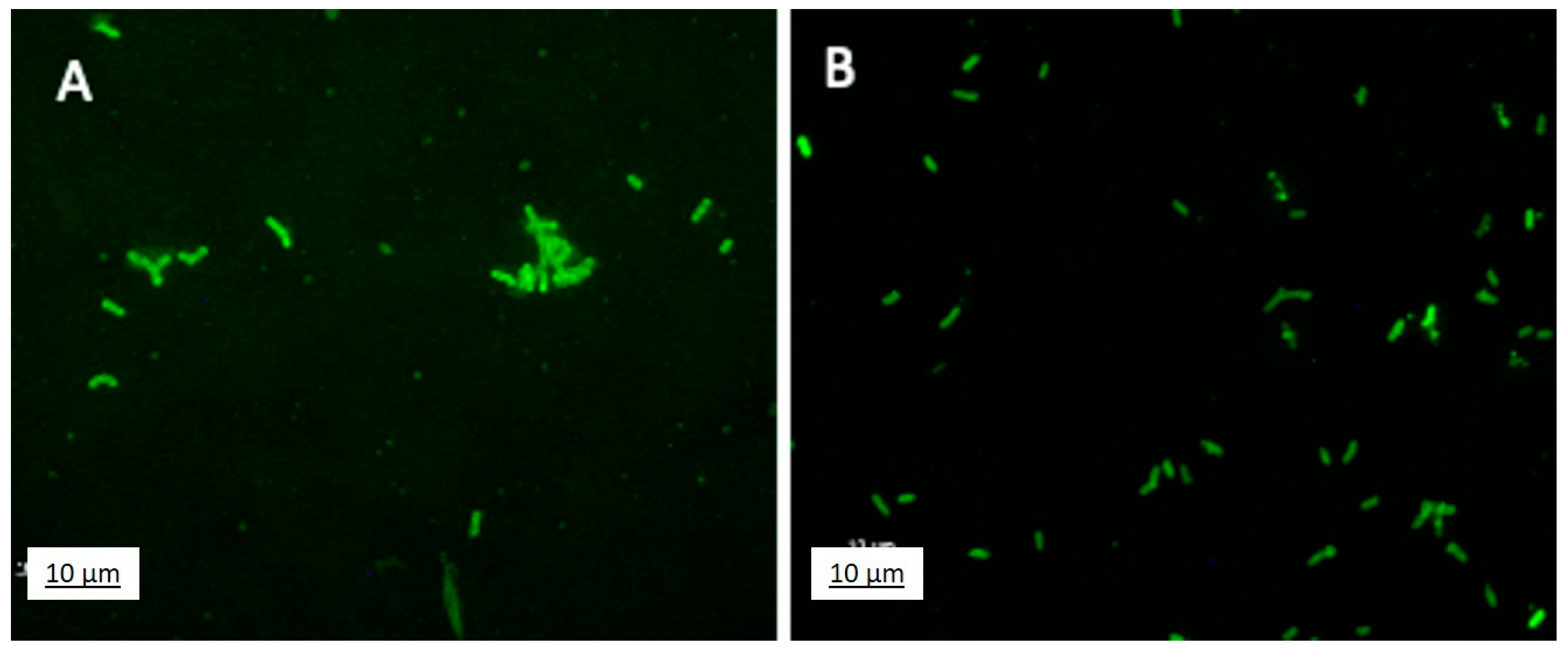
2.4.2. Effect of F68 on JunSE1L: Fluorescent Labeling and Planktonic Growth
2.5. Statistical Analyses
3. Results
3.1. F68 Characterization: Size and Surface Activity
3.2. F68 Effects on Seed Water Uptake, Germination, Emergence, and Endophyte Growth into the Spermosphere
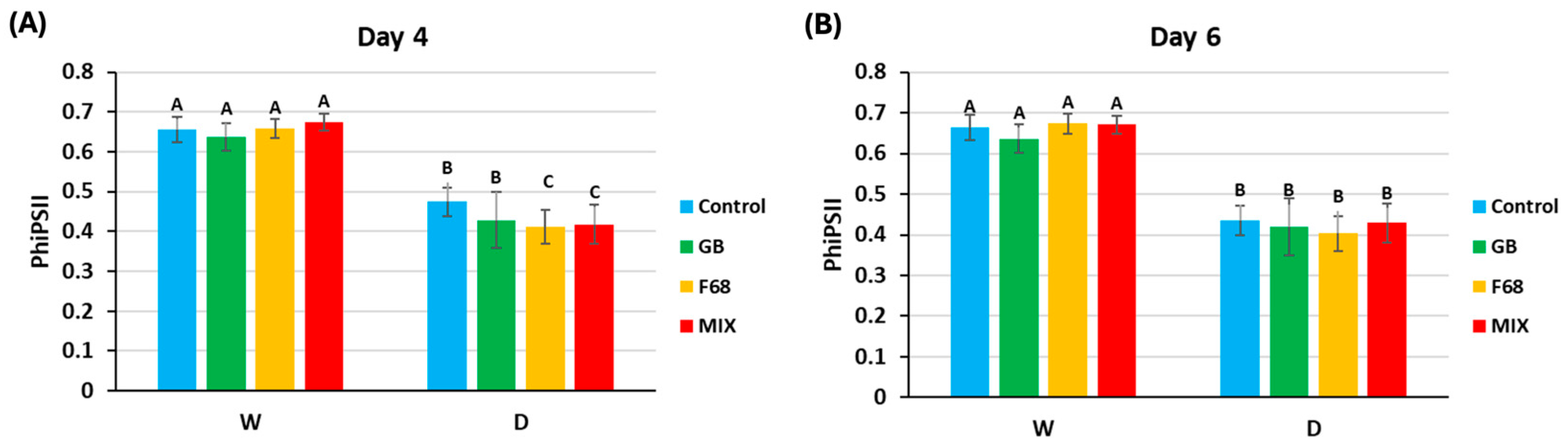
3.3. F68 Influence on Growth of PcO6—Inoculated Wheat Seedlings Under Drought Stress
3.4. Fluorescent F68 (fF68) Labeling of Plant and Microbiome
3.4.1. Interaction of fF68 with Root Epidermal Cells and Root-Epiphytic Bacteria
3.4.2. Effect of F68 on JunSE1L: Fluorescent Labeling and Planktonic Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hazen, J.L. Adjuvants—Terminology, classification, and chemistry. Weed Technol. 2000, 14, 773–784. [Google Scholar] [CrossRef]
- Kwiatkowski, T.A.; Rose, A.L.; Jung, R.; Capati, A.; Hallak, D.; Yan, R.; Weisleder, N. Multiple poloxamers increase plasma membrane repair capacity in muscle and nonmuscle cells. Am. J. Physiol.-Cell Physiol. 2020, 318, C253–C262. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; River, L.P.; Pan, F.S.; Ji, L.; Wollmann, R.L. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc. Natl. Acad. Sci. USA 1992, 89, 4524–4528. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Goliaei, A.; Tsereteli, L.; Berkowitz, M.L. Properties of poloxamer molecules and poloxamer micelles dissolved in water and next to lipid bilayers: Results from computer simulations. J. Phys. Chem. B 2016, 120, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Moloughney, J.; Weisleder, N. Poloxamer 188 (p188) as a membrane resealing reagent in biomedical applications. Recent Pat. Biotechnol. 2012, 6, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Casella, J.F.; Kronsberg, S.S.; Gorney, R.T. Poloxamer 188 vs. placebo for painful vaso-occlusive episodes in children and adults with sickle cell disease—Reply. JAMA 2021, 326, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Orringer, E.P.; Casella, J.F.; Ataga, K.I.; Koshy, M.; Adams-Graves, P.; Luchtman-Jones, L.; Wun, T.; Watanabe, M.; Shafer, F.; Kutlar, A.; et al. Purified poloxamer 188 for treatment of acute vaso-occlusive crisis of sickle cell disease: A randomized controlled trial. JAMA 2001, 286, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Kirova, E.; Pecheva, D.; Simova-Stoilova, L. Drought response in winter wheat: Protection from oxidative stress and mutagenesis effect. Acta Physiol. Plant. 2021, 43, 8. [Google Scholar] [CrossRef]
- Tyagi, M.; Pandey, G.C. Physiology of heat and drought tolerance in wheat: An overview. J. Cereal Res. 2022, 14, 13–25. [Google Scholar] [CrossRef]
- Impa, S.M.; Nadaradjan, S.; Jagadish, S.V.K. Drought stress induced reactive oxygen species and anti-oxidants in plants. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Springer: New York, NY, USA, 2012; pp. 131–147. [Google Scholar] [CrossRef]
- Kumar, M.; Lee, S.C.; Kim, J.Y.; Kim, S.J.; Aye, S.S.; Kim, S.R. Over-expression of dehydrin gene, OsDhn1, improves drought and salt stress tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). J. Plant Biol. 2014, 57, 383–393. [Google Scholar] [CrossRef]
- Hellung-Larsen, P.; Assaad, F.; Pankratova, S.; Saietz, B.L.; Skovgaard, L.T. Effects of Pluronic F-68 on Tetrahymena cells: Protection against chemical and physical stress and prolongation of survival under toxic conditions. J. Biotechnol. 2000, 76, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Camaille, M.; Fabre, N.; Clément, C.; Ait Barka, E. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Biocontrol of plant diseases by Bacillus spp. Physiol. Mol. Plant Pathol. 2023, 126, 102048. [Google Scholar] [CrossRef]
- Lastochkina, O.V. Adaptation and tolerance of wheat plants to drought mediated by natural growth regulators Bacillus spp.: Mechanisms and practical importance. Agric. Biol. 2021, 56, 843–867. [Google Scholar] [CrossRef]
- Streeter, A.R.; Cartwright, A.; Zargaran, M.; Wankhade, A.; Anderson, A.J.; Britt, D.W. Adjuvant Pluronic F68 is compatible with a plant root-colonizing probiotic, Pseudomonas chlororaphis O6. Agrochemicals 2023, 3, 1–11. [Google Scholar] [CrossRef]
- Cartwright, A. Surface-Functionalized Silica Nanocarriers for Mitigating Water Stress in Wheat and Benefiting the Root Microbiome. Master’s Thesis, Utah State University, Logan, UT, USA, 2023. Available online: https://digitalcommons.usu.edu/etd/8803 (accessed on 18 July 2025).
- Kok, A.D.X.; Wan Abdullah, W.M.A.N.; Tan, N.P.; Ong-Abdullah, J.; Sekeli, R.; Wee, C.Y.; Lai, K.S. Growth promoting effects of Pluronic F-68 on callus proliferation of recalcitrant rice cultivar. 3 Biotech 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.D.X.; Mohd Yusoff, N.F.; Sekeli, R.; Wee, C.Y.; Lamasudin, D.U.; Ong-Abdullah, J.; Lai, K.S. Pluronic F-68 improves callus proliferation of recalcitrant rice cultivar via enhanced carbon and nitrogen metabolism and nutrients uptake. Front. Plant Sci. 2021, 12, 667434. [Google Scholar] [CrossRef] [PubMed]
- Murungu, F.S. Effects of seed priming and water potential on seed germination and emergence of wheat (Triticum aestivum L.) varieties in laboratory assays and in the field. Afr. J. Biotechnol. 2011, 10, 4365–4371. [Google Scholar] [CrossRef]
- Wang, G.P.; Hui, Z.; Li, F.; Zhao, M.R.; Zhang, J.; Wang, W. Improvement of heat and drought photosynthetic tolerance in wheat by overaccumulation of glycine betaine. Plant Biotechnol. Rep. 2010, 4, 213–222. [Google Scholar] [CrossRef]
- Kjar, A.; Wadsworth, I.; Vargis, E.; Britt, D.W. Poloxamer 188–quercetin formulations amplify in vitro ganciclovir antiviral activity against cytomegalovirus. Antivir. Res. 2022, 204, 105362. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Nakashima, K.; Anzai, T.; Fujimoto, Y. Fluorescence studies on the properties of a Pluronic F68 micelle. Langmuir 1994, 10, 658–661. [Google Scholar] [CrossRef]
- Mamou, G.; Mohan, G.B.M.; Rouvinski, A.; Rosenberg, A.; Ben-Yehuda, S. Early developmental program shapes colony morphology in bacteria. Cell Rep. 2016, 14, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Chichkova, S.; Arellano, J.; Vance, C.P.; Hernández, G. Transgenic tobacco plants that overexpress alfalfa NADH-glutamate synthase have higher carbon and nitrogen content. J. Exp. Bot. 2001, 52, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Garshina, D.; Ivanov, S.; Yuldashev, R.; Khafizova, R.; Allagulova, C.; Fedorova, K.; Avalbaev, A.; Maslennikova, D.; Bosacchi, M. Seed priming with endophytic Bacillus subtilis modulates physiological responses of two different Triticum aestivum L. cultivars under drought stress. Plants 2020, 9, 1810. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Yuldashev, R.; Avalbaev, A.; Allagulova, C.; Veselova, S. The contribution of hormonal changes to the protective effect of endophytic bacterium Bacillus subtilis on two wheat genotypes with contrasting drought sensitivities under osmotic stress. Microorganisms 2023, 11, 2955. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.Y.; Lee, Y.H.; Cho, B.H.; Yang, K.Y.; Ryu, C.M.; Kim, Y.C. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Stebe, K.; Murphy, J.C.; Tung, L. Poloxamer 188 decreases susceptibility of artificial lipid membranes to electroporation. Biophys. J. 1996, 71, 3229–3241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, H.; McFaul, C.; Titushkin, I.; Cho, M.; Lee, R. Surfactant copolymer annealing of chemically permeabilized cell membranes. Regen. Eng. Transl. Med. 2018, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Houang, E.M.; Bates, F.S.; Sham, Y.Y.; Metzger, J.M. All-atom molecular dynamics-based analysis of membrane-stabilizing copolymer interactions with lipid bilayers probed under constant surface tensions. J. Phys. Chem. B 2017, 121, 10657–10664. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Hortin, J.M.; Jacobson, A.R.; Britt, D.W.; McLean, J.E. Changes in metal-chelating metabolites induced by drought and a root microbiome in wheat. Plants 2023, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Voigt, B.; Timmers, A.C.; Šamaj, J.; Hlavacka, A.; Ueda, T.; Preuss, M.; Nielsen, E.; Mathur, J.; Emans, N.; Stenmark, H.; et al. Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur. J. Cell Biol. 2005, 84, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Von Wangenheim, D.; Rosero, A.; Komis, G.; Šamajová, O.; Ovečka, M.; Voigt, B.; Šamaj, J. Endosomal interactions during root hair growth. Front. Plant Sci. 2016, 6, 1262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Z.; Chu, B. Light-scattering study on the association behavior of triblock polymers of ethylene oxide and propylene oxide in aqueous solution. J. Colloid Interface Sci. 1988, 126, 171–180. [Google Scholar] [CrossRef]



| Treatments 12 h F68 (g/L) | H2O Imbibed Per Seed (g) | % Germination @ 48 h on LB Agar | % Emergence @ 5 d Planted in Sand |
|---|---|---|---|
| 0 | 0.018 ± 0.002 | 80 ± 13 | 92 ± 6 |
| 0.1 | 0.018 ± 0.002 | 85 ± 9 | 92 ± 6 |
| 1 | 0.019 ± 0.002 | 80 ± 18 | 92 ± 6 |
| 10 | 0.019 ± 0.002 | 80 ± 9 | 96 ± 6 |
| 100 | 0.017 ± 0.001 | 47 ± 8 | 96 ± 6 |
| 250 | 0.015 ± 0.001 | 57 ± 3 | 96 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartwright, A.; Zargaran, M.; Wankhade, A.; Jacobson, A.; McLean, J.E.; Anderson, A.J.; Britt, D.W. Uptake, Distribution, and Activity of Pluronic F68 Adjuvant in Wheat and Its Endophytic Bacillus Isolate. Agrochemicals 2025, 4, 12. https://doi.org/10.3390/agrochemicals4030012
Cartwright A, Zargaran M, Wankhade A, Jacobson A, McLean JE, Anderson AJ, Britt DW. Uptake, Distribution, and Activity of Pluronic F68 Adjuvant in Wheat and Its Endophytic Bacillus Isolate. Agrochemicals. 2025; 4(3):12. https://doi.org/10.3390/agrochemicals4030012
Chicago/Turabian StyleCartwright, Anthony, Mohammad Zargaran, Anagha Wankhade, Astrid Jacobson, Joan E. McLean, Anne J. Anderson, and David W. Britt. 2025. "Uptake, Distribution, and Activity of Pluronic F68 Adjuvant in Wheat and Its Endophytic Bacillus Isolate" Agrochemicals 4, no. 3: 12. https://doi.org/10.3390/agrochemicals4030012
APA StyleCartwright, A., Zargaran, M., Wankhade, A., Jacobson, A., McLean, J. E., Anderson, A. J., & Britt, D. W. (2025). Uptake, Distribution, and Activity of Pluronic F68 Adjuvant in Wheat and Its Endophytic Bacillus Isolate. Agrochemicals, 4(3), 12. https://doi.org/10.3390/agrochemicals4030012







