Abstract
The conventional agricultural industry largely relies on pesticides to maintain healthy and viable crops. Application of fungicides, both pre- and post-harvest of crops, is the go-to method for avoiding and eliminating Botrytis cinerea, the fungal pathogen responsible for gray mold. However, conventional fungicides and their residues have purported negative environmental and health impacts. Natural products, such as essential oils, are viewed as a promising alternative to conventional fungicides. The current research is an in vitro study on the antifungal activity of a natural water-based fungicide (N.F.), which uses a blend of essential oils (ajowan, cassia, clove, eucalyptus, lemongrass, oregano) as the active ingredients against B. cinerea. Compared to conventional fungicides tested at the same concentration (50 μL/mL), those with active ingredients of myclobutanil or propiconazole; the N.F. demonstrated significant (F(3,16) = 54, p = <0.001) and complete fungal growth inhibition. While previous research has largely focused on the antifungal properties of single essential oils and/or isolated compounds from essential oils, this research focuses on the efficacy of using a blend of essential oils in a proprietary delivery system. This research is of importance to the fields of agronomy, ecology, and health sciences.
1. Introduction
The term ‘pesticide’ is used as a general description for any chemical treatment in agricultural practices to control or eliminate unwanted pests. While many specific forms of pesticides exist, the most common pesticides target, either specifically or non-specifically, weeds (herbicides), insects (insecticides), and fungi (fungicides) [1]. The agricultural industry is largely dependent on the use of pesticides in order to maintain high crop yields and presentable crops and food goods [1,2].
Gray mold, which is caused by Botrytis cinerea, is a fungal disease that attacks many different plants, including apples, grapes, peaches, strawberries, and tomatoes [2,3,4,5,6]. Fungicide treatments are the conventional means for fighting gray mold, either pre- or post-harvest of crops. However, there are many concerns with using conventional pesticides. Studies have shown that pesticides and pesticide residues can contaminate soil and water sources [7] and can persist in the environment for multiple growing seasons [8]. Some of these pesticide residues have been suspected to be detrimental to human and other animal health [7]. High use of fungicides also raises concerns about fungal pathogen resistance, and the perpetual need for new and stronger treatments [1].
Much research has been conducted to investigate novel alternatives to conventional fungicides. Essential oils (often called volatile oils) are seen as a viable active ingredient in fungicides that are both a natural and potentially safer alternative to those currently in use. Essential oils with high concentrations of phenolic compounds such as eugenol, carvacrol, and thymol have been shown to display antifungal properties against B. cinerea, Ascomycota (the phylum of pathogens causing powdery mildew and apple scab), and Plasmopara viticola (the cause of downy mildew) [2,4,6,9,10,11,12,13,14,15,16,17,18,19]. Additionally, research supports the use of essential oils that are aldehyde-rich (such as cinnamaldehyde, neral, geranial, etc.) and those high in 1,8-cineole as antifungal agents against the same pathogens and diseases [2,5,6,12,14,16,19,20,21]. Studies have also been conducted on isolated compounds that comprise essential oils, demonstrating compound-specific antifungal activity [4].
Naturally occurring compounds in essential oils are unlikely to contaminate soil and water [19], and their use as a natural fungicide lends itself as an environmentally responsible alternative to conventional fungicides. A study conducted on treating powdery mildew with a foliar spray of essential oils demonstrated that less than 10% of residues were detected seven days post-application [11]. Another study found that while essential oils were 92% effective in treating apple scab in dry conditions, rainfall after application reduced effectiveness to 65% [16]. While using biodegradable active ingredients in fungicides, such as essential oils, is ideal for maintaining healthy environments, this may also be viewed as a negative aspect from the agricultural industry, given that essential oil-based fungicides may be deemed less effective in treating pathogens over prolonged periods of time and require repeated applications based on weather conditions.
The current study demonstrates the antifungal properties of a plant-based fungicide that uses a blend of essential oils as active ingredients (hereafter referred to as ‘natural fungicide’ or ‘N.F.’). While previously referenced studies focused primarily on individual essential oils or isolated compounds from essential oils and their antifungal activity, this in vitro study focuses on a patent pending formula [22] comprised of a blend of essential oils (ajowan, cassia, clove, eucalyptus, lemongrass, oregano), demonstrating growth inhibition of gray mold. GC/MS and GC/FID analyses were conducted on each essential oil to establish both the chemotype and quality of said essential oils. This study is the first study to investigate the effectiveness of said N.F. against agricultural fungal pathogens.
2. Materials and Methods
2.1. Essential Oil Analysis
Essential oils of ajowan (Trachyspermum ammi), cassia (Cinnamomum cassia), clove leaf (Syzygium aromaticum), eucalyptus (Eucalyptus globulus), lemongrass (Cymbopogon flexuosus), and oregano (Origanum vulgare) were provided by Young Living Essential Oils (Lehi, UT, USA). Essential oils were analyzed by GC/MS and GC/FID to establish plant chemotype and essential oil quality.
Essential oil samples were analyzed, and volatile compounds identified by GC/MS using an Agilent 7890B GC/5977B MSD (Agilent Technologies, Santa Clara, CA, USA) and Agilent J&W DB-5 (Agilent Technologies, Santa Clara, CA, USA), 0.25 mm × 60 m, 0.25 μm film thickness, fused silica capillary column. Samples were made up at 20% soln. in HPLC-grade methylene chloride (MilliporeSigma, Sigma Aldrich, St. Louis, MS, USA). Operating conditions: 0.1 μL of sample, 100:1 split ratio, initial oven temp. of 40 °C with an initial hold time of 5 min, oven ramp rate of 4.5 °C per min to 310 °C with a hold time of 5 min. The electron ionization energy was 70 eV, scan range 35–650 amu, scan rate 2.4 scans per sec., source temp. 230 °C, and quadrupole temp. 150 °C. Volatile compounds were identified using the Adams volatile oil library [23] through Chemstation library search in conjunction with retention indices. Two volatile compounds detected in this study, styrene and 4-nonanone, were not found in the Adams volatile oil library. Their identification was made using the 2020 NIST mass spectral library and retention indices were manually calculated using alkane standards. Note that limonene/1,8-cineole eluted as a single peak in some samples. Their relative amounts were determined by the ratio of masses 41, 68, 79 (limonene) and 43, 71, 81 (1,8-cineole). Volatile compounds were quantified and are reported as a relative area percent by GC/FID using an Agilent 7890B GC and Agilent J&W DB-5, 0.25 mm × 60 m, 0.25 μm film thickness, fused silica capillary column. Essential oil samples were made up at 20% soln. and reference compounds at 1% soln. in HPLC-grade methylene chloride (MilliporeSigma, Sigma Aldrich, St. Louis, MS, USA). Operating conditions: 0.1 μL of sample, 25:1 split ratio, initial oven temp. of 40 °C with an initial hold time of 2 min, oven ramp rate of 3.0 °C per min to 250 °C with a hold time of 3 min. Essential oil samples were analyzed in triplicate by GC/FID to ensure repeatability of relative area % values. Compounds were identified using retention indices coupled with retention time data of reference compounds (MilliporeSigma, Sigma Aldrich, St. Louis, MS, USA).
2.2. Fungicide Preparation
The natural fungicide (N.F.), which is a water-based formula with embedded essential oils, is a patent-pending formula [22]. Essential oils (hereafter referred to as ‘essential oil blend’) were mixed in the following combination for the N.F.: lemongrass (8.3%), cassia (8.3%), eucalyptus (16.7%), ajowan (16.7%), clove leaf (25.0%), and oregano (25.0%). The N.F. was formulated by combining the following ingredients using a stir plate (500 RPM for 15 min): 92.4 g deionized water, 0.1 g bio saponin (Bio-Botanica Inc., Hauppauge, NY, USA), 1.0 g Pinna Leaf Extract (Biofos Biotechnology, Chicago, IL, USA), 2.5 g glycerin (Bulk Apothecary, Aurora, OH, USA), 3.0 g Sodium Sunflowerseedate (Eden Botanicals, Petaluma, CA, USA), and 1 g essential oil blend. To demonstrate the antifungal properties of the N.F., a second solution was made up of identical ingredients, minus the essential oil blend (93.4 g deionized water to compensate for the 1 g removal of essential oil blend). Two other conventional fungicides were made up at manufacturer-recommended concentrations with deionized water, with active ingredients of myclobutanil (9.8 mL/L) or propiconazole (4.0 mL/L). For simplicity, these four solutions will be referred to as the natural fungicide (N.F.), the natural fungicide control without essential oils (N.F.C), the myclobutanil-based fungicide (M.F.), and propiconazole-based fungicide (P.F.).
2.3. Fungal Pathogen Isolation, Inoculation, & Identification
Potato dextrose agar (PDA) medium is a viable nutrient medium for cultivating Botrytis cinerea [5,6,24]. PDA was made by mixing 7.5 g malt agar and 10.0 g potato dextrose agar (MilliporeSigma, Sigma Aldrich, St. Louis, MS, USA) with 500 mL deionized water. Once mixed, contents were sterilized by heating for 15 min at 15 psi. Under sterile conditions, 15 mL of the liquid solution were added to sterile petri dishes and allowed to cool and solidify. When evaluating antifungal properties of fungicides, fungicides were homogenously embedded at various concentrations into PDA medium prior to agar mixture cooling and solidifying. Prior to inoculation, all petri dishes were exposed to UV light for 5 min using UV Clave UV Chamber (Benchmark Scientific Inc., Sayreville, NJ, USA).
Gray mold, which is caused by Botrytis cinerea, was observed on and isolated from post-harvest strawberries. Symptomatic strawberries were washed three times with deionized water, soaked in 70% isopropyl alcohol, plant tissue was placed on potato dextrose agar (PDA) medium, and incubated at 25 °C (±2 °C) for 7 days. After the 7-day incubation period, fungal colony growth that displayed morphological characteristics of gray mold was selected and transferred to additional PDA medium for genetic identification.
To verify the identity of the fungal isolate, DNA was extracted from mycelial scrapings of freshly grown petri plates using the Plant Isolate DNA Extraction Kit (IBI Scientific, Dubuque, IA, USA) according to the manufacturer’s recommendations. The DNA was then used as a template for ITS PCR amplification using Apex Taq RED Master Mix (Apex Bioresearch, Houston, TX, USA) and the primer set ITS1F and TW13 [25]. The PCR amplicon was then purified using AMPure XP beads (Beckman Coulter Life Sciences, Indianapolis, IN, USA) according to the manufacturer’s recommendations. This was then subjected to Sanger Sequencing (Psomagen, Inc., Rockville, MD, USA) to a 3× coverage with both primer sets and assembled into a contig using CAP3 [26]. The contig was then subject to BLASTN [27] to identify the nearest relative, sharing 100% nucleotide identity to accession # OR544950.1. The contig was deposited into GenBank (accession # PQ460570).
2.4. Fungicidal Activity and Statistical Analysis
The fungal growth rate method [28] was used to evaluate antifungal activity. The four solutions previously mentioned (N.F., N.F.C., M.F., P.F.) were added to the PDA media at various concentrations (100 μL/mL, 50 μL/mL, 10 μL/mL, 5 μL/mL, 1 μL/mL, check plate). To best understand optimal growth inhibition, the N.F. was investigated at additional concentrations (40 μL/mL, 30 μL/mL, 20 μL/mL, check plate). Once homogeneously embedded, a B. cinerea colony (diameter of 5 mm) was placed in the center of the PDA medium plates and cultured at 25 °C (±2 °C) for 7 days. The colony diameter (mm) was measured using a ruler at the widest points. Culture inoculations and incubations were conducted in quintuplicate, and measured diameters are an average of those five. The fungicide antifungal property dataset was evaluated by an Analysis of Variance (one-way ANOVA).
3. Results
The patent-pending fungicide (N.F.) investigated in this study contains a blend of six essential oils (ajowan, cassia, clove, eucalyptus, lemongrass, oregano) that have either previously demonstrated antifungal activity or are composed of volatile compounds that have previously demonstrated antifungal activity [2,4,6,9,10,11,12,13,14,15,16,17,18,19,20,21]. Essential oil samples were analyzed by GC/MS and GC/FID to establish essential oil chemotype and quality (Table 1).

Table 1.
Summarized essential oil profiles of ajowan (Trachyspermum ammi), cassia (Cinnamomum cassia), clove (Syzygium aromaticum), eucalyptus (Eucalyptus globulus), lemongrass (Cymbopogon flexuosus), and oregano (Origanum vulgare) samples. Compounds detected in any essential oil at ≥1.0% are reported; the complete profiles can be found in Supplementary Table S1. Reported values represent averages from samples analyzed in triplicate, which was done to ensure repeatability of values (standard deviation ≤ 0.8 for all compounds). Values less than 0.1% are denoted as trace (tr), and those not detected as nd. KI is the Kováts Index value and was previously calculated by Robert Adams using a linear calculation on a DB-5 column [23]. Relative area percent was determined by GC/FID.
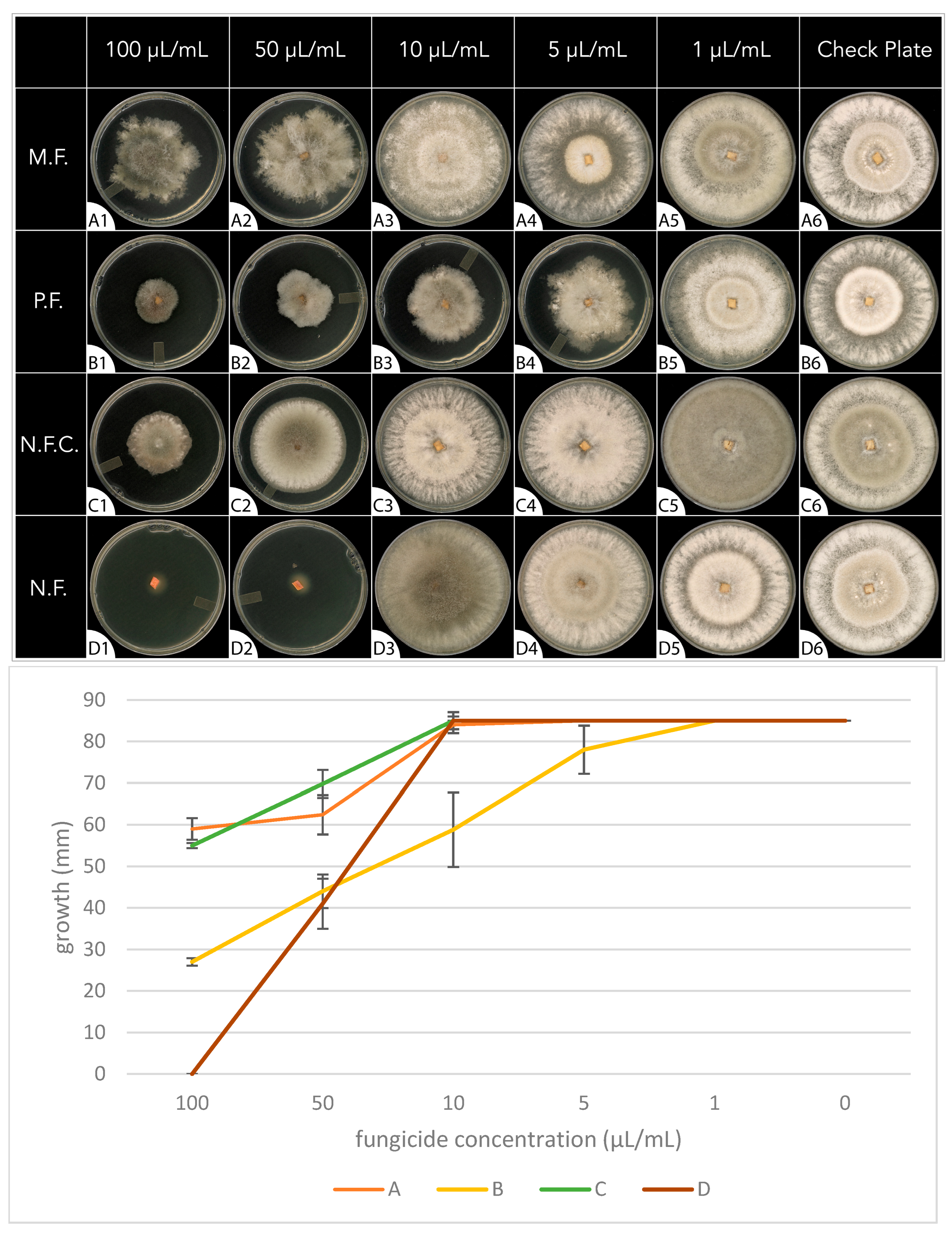
The fungal growth rate was used to evaluate the antifungal activity of the different fungicides. Referring to Figure 1, none of the fungicides demonstrated antifungal activity at the lowest concentration, 1 μL/mL (column 5). The conventional fungicide containing myclobutanil (M.F.) in row ‘A’ demonstrated the weakest antifungal activity, showing mild antifungal activity at the highest concentrations only (50 μL/mL, 100 μL/mL). The conventional fungicide containing propiconazole (P.F.) in row ‘B’ demonstrated increasing antifungal activity from low to high concentrations (5 μL/mL, 10 μL/mL, 50 μL/mL, 100 μL/mL). The P.F., compared to the other three solutions, displayed significantly higher fungal inhibition (F(3,16) = 29, p = <0.001) at a concentration of 10 μL/mL. The focus of this study, the natural fungicide (N.F.) in row ‘D’ was the only fungicide to demonstrate complete antifungal activity, which occurred at the highest concentration (100 μL/mL). The N.F. displayed significant fungal inhibition (F(3,16) = 54, p = <0.001) compared to the other fungicides, beginning at a concentration of 50 μL/mL. The control group (N.F.C.) in row ‘C’ demonstrated mild antifungal activity, which was similar to that of the M.F. at the highest concentrations (50 μL/mL, 100 μL/mL). The complete dataset can be found in Supplementary Table S2.
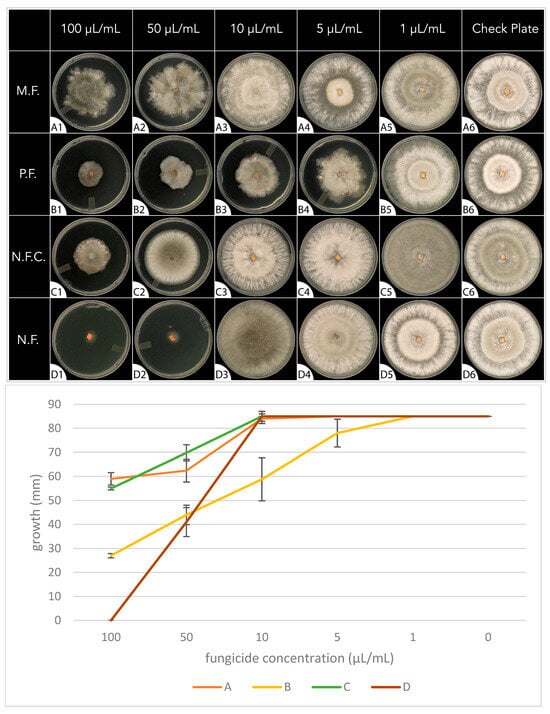
Figure 1.
The fungal growth rate of Botrytis cinerea on PDA media plates for 7 days in the absence (check plate, column 6) or presence of different concentrations of fungicides (columns 1–5). Row ‘A’ and row ‘B’ PDA plates were embedded with conventional fungicides containing myclobutanil (M.F.) and propiconazole (P.F.), respectively. Row ‘D’ PDA plates were embedded with the fungicide containing the essential oil blend. Row ‘C’ PDA plates acted as the control group and were embedded with the same fungicide as in row ‘D’ but with the essential oil blend removed. Concentrations of fungicides were 100 μL/mL (column 1), 50 μL/mL (column 2), 10 μL/mL (column 3), 5 μL/mL (column 4), 1 μL/mL (column 5), and check plate (column 6).
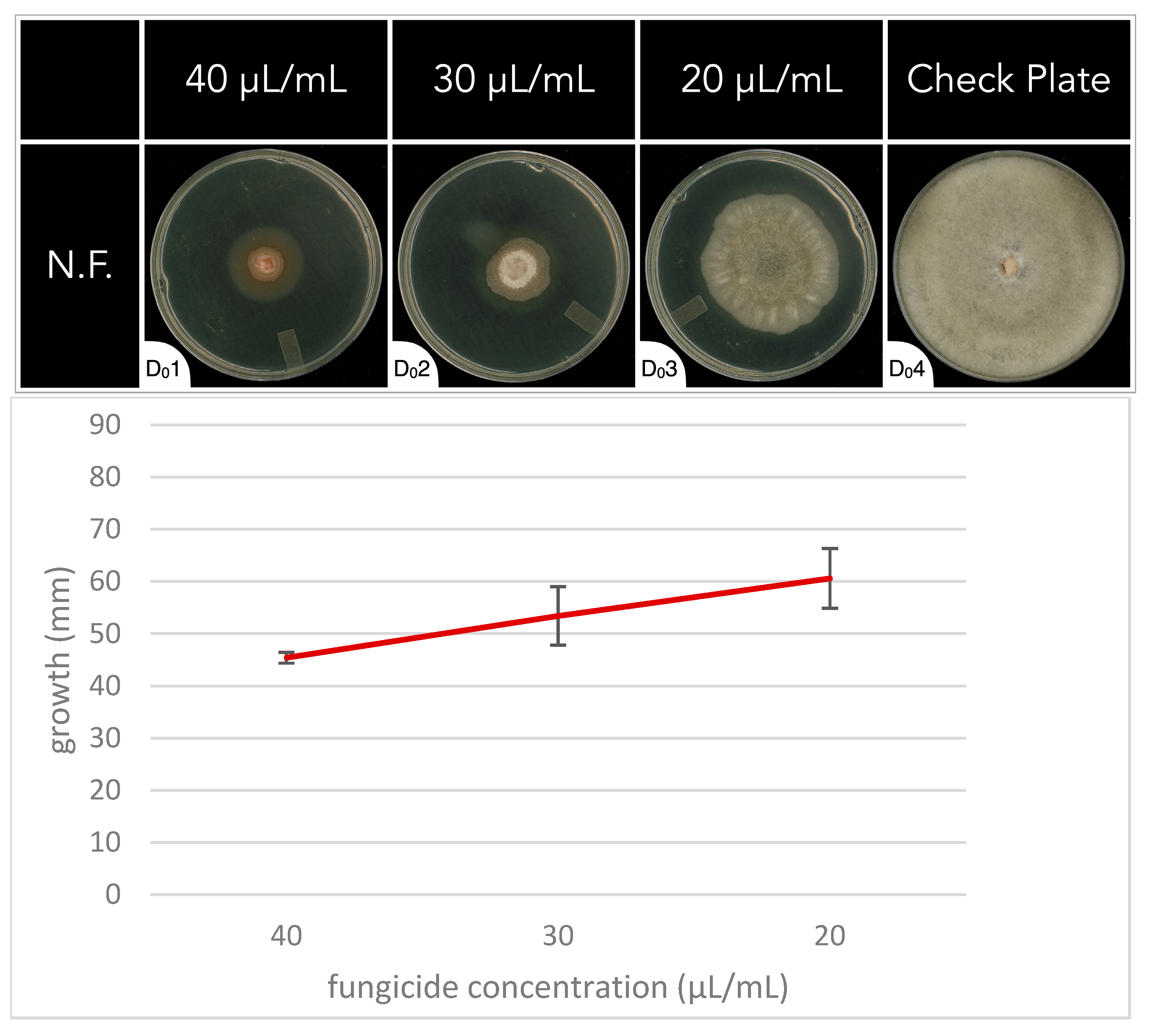
In the N.F., a sharp increase in antifungal activity was observed between concentrations of 10 μL/mL and 50 μL/mL in the initial fungal growth rate experiment. To better understand why, and what the optimal concentration of application is, additional concentrations were investigated at 40 μL/mL, 30 μL/mL, and 20 μL/mL (Figure 2). These increasing concentrations of N.F. correlated with a linear trend in antifungal activity (Figure 2). The complete dataset can be found in Supplementary Table S3.
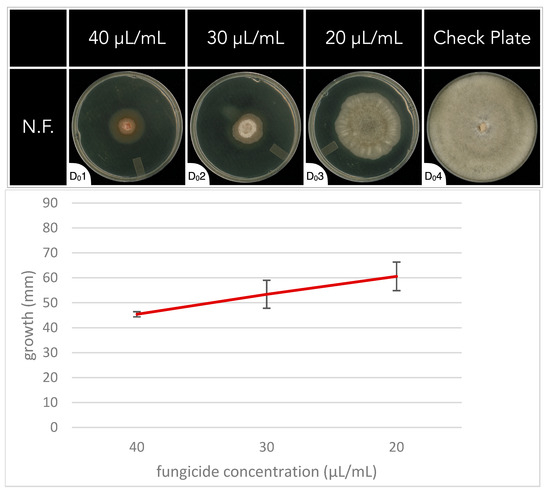
Figure 2.
The fungal growth rate of Botrytis cinerea on PDA media plates for 7 days in the absence (check plate, D04) or presence of different concentrations of the natural fungicide (N.F.) (D01 through D03). Concentrations of fungicides were 40 μL/mL (D01), 30 μL/mL (D02), and 20 μL/mL (D03).
4. Discussion
Within the same species of aromatic plants, there are often chemotypes (subspecies) that exist. These chemotypes are defined by prominent volatile compounds in the essential oil. Ajowan (Trachyspermum ammi) essential oil is largely composed of p-cymene (26.2%), γ-terpinene (43.2%), and thymol (21.0%). Cassia (Cinnamomum cassia) essential oil is largely composed of (E)-cinnamaldehyde (80.8%) and (E)-o-methoxy cinnamaldehyde (8.3%). Clove (Syzygium aromaticum) leaf essential oil is largely composed of eugenol (87.1%) and (E)-caryophyllene (7.4%). Eucalyptus (Eucalyptus globulus) essential oil is largely composed of p-cymene (5.9%) and 1,8-cineole (80.1%). Lemongrass (Cymbopogon flexuosus) essential oil is largely composed of neral (30.5%) and geranial (42.2%). Oregano (Origanum vulgare) essential oil is largely composed of p-cymene (7.6%) and carvacrol (69.6%). The patent-pending natural fungicide (N.F.) was prepared so that optimal amounts of these volatile compounds were present in the formulation. Most of the key volatile compounds mentioned are supplied either exclusively or primarily by a single essential oil, with two exceptions: p-Cymene, which is supplied by ajowan (26.2%), eucalyptus (5.9%), oregano (7.6%), and γ-terpinene, which is supplied by ajowan (43.2%) and oregano (3.5%). The antifungal activity of the N.F. is largely attributed to the presence of these prominent volatile compounds, which is supported by previously published research [2,4,5,6,9,10,11,12,13,14,15,16,17,18,19,20,21]. Using a blend of essential oils may be a suitable approach for fungicidal activity against multiple fungal pathogens and may help avoid the development of antifungal resistance by pathogens, however, this cannot be determined by a single study and should be the focus of future research.
Sources of targeted volatile compounds may be produced by multiple plant species. While most studies focusing on thymol, (E)-cinnamaldehyde, and eugenol are from essential oils extracted from thyme leaf (Thymus vulgaris), cinnamon bark (Cinnamomum zeylanicum), and clove bud (Syzygium aromaticum) respectively, these compounds are also found in other plant species and/or plant parts [6,9,12,13,14,15,16,17,19,29,30,31]. In the N.F. formula, these three compounds (thymol, (E)-cinnamaldehyde, and eugenol) were supplied by ajowan, cassia, and clove leaf, respectively, since these plants are more economical and sustainable sources of said compounds.
The natural fungicide control (N.F.C.) demonstrated some antifungal activity at the two highest concentrations (50 μL/mL and 100 μL/mL). While this control provides ample evidence that the essential oils in the N.F. act as the primary active ingredients in the formula, there are other ingredients at play here that provide antifungal activity. The small amounts of other plant-based ingredients used in the formation of the N.F., which act as surfactants, stabilizers, and preservatives, also have known antimicrobial properties. Their description and exact antifungal activity are beyond the scope of this article. While the focus of the current study is on the use of a natural fungicide that contains essential oils as active ingredients, it is seemingly impossible to apply essential oils directly to diseased plants in a safe, efficient, and sustainable manner. As such, let it be sufficient to state that findings from N.F.C. and N.F. provide evidence that the essential oil blend provides promising antifungal activity and that the formula of N.F. is a novel and reliable delivery system for said essential oil blend.
The conventional fungicide containing myclobutanil (M.F.) as well as the control (N.F.C.) performed similarly at all concentrations and demonstrated limited antifungal activity at the highest tested concentrations. The conventional fungicide containing propiconazole (P.F.) demonstrated the best antifungal activity at lower concentrations (5 μL/mL, 10 μL/mL). However, the N.F., displayed significantly higher antifungal activity (F(3,16) = 54, p = <0.001) at 50 μL/mL, and demonstrated complete inhibition of fungal growth at 100 μL/mL. Given findings from this study, the N.F. may provide sufficient antifungal activity against Botrytis cinerea at a concentration between 30–40 μL/mL. Fungal pathogens, including B. cinerea, grow and spread prolifically, and complete inhibition, as observed with the N.F., is promising. These findings show promise for future studies, both conducted in vitro and in vivo, against B. cinerea and other fungal pathogens.
5. Conclusions
The current study investigates the antifungal property of a patent-pending fungicide formula that uses a blend of six essential oils (ajowan, cassia, clove, eucalyptus, lemongrass, oregano) as the main active ingredients. Many previous studies investigated the fungicidal properties of each of these essential oils and/or isolated compounds from these oils. The current study entails the logical subsequent steps: (1) formulating a fungicide with previously investigated essential oils and (2) investigating the fungicidal properties of said fungicide against conventional fungicides.
In this study, the conventional fungicide containing propiconazole (P.F.) demonstrated the best antifungal activity at lower concentrations (5 μL/mL, 10 μL/mL). However, the natural fungicide (N.F.) displayed high antifungal activity at concentrations between 30–40 μL/mL, significantly higher antifungal activity (F(3,16) = 54, p = <0.001) at 50 μL/mL and demonstrated complete inhibition of fungal growth at 100 μL/mL. The N.F. is a water-based formula that delivers essential oils as antifungal active ingredients and shows promise as a natural crop application against Botrytis cinerea, the cause of gray mold.
Future studies will investigate other aspects of antifungal activity of the N.F., including in vivo efficacy on multiple plant species (both pre- and post-harvest), long-term environmental impact, and safety.
6. Patents
United States patent Application Publication Number: US 2025/0176562 A1. Publication Date: 5 June 2025. Publication Title: FUNGICIDAL COMPOSITIONS COMPRISING ESSENTIAL OILS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agrochemicals4030011/s1, Table S1: GC Dataset; Table S2: Fungal Growth Rate A-D; Table S3: Fungal Growth Rate D0.
Author Contributions
Conceptualization, T.M.W. and R.E.C.; methodology, T.M.W., A.L., and R.E.C.; software, T.M.W.; validation, R.E.C.; investigation and formal analysis, T.M.W., A.L., and Z.R.; resources, R.E.C.; data curation, T.M.W. and A.L.; writing original draft, T.M.W.; review and editing draft, A.L., Z.R., and R.E.C.; funding acquisition, R.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Young Living Essential Oils.
Institutional Review Board Statement
Not applicable to this study.
Informed Consent Statement
Not applicable to this study.
Data Availability Statement
Complete datasets can be found in Supplementary Tables S1–S3. Genetic information was deposited into GenBank (accession # PQ460570).
Acknowledgments
The authors would like to acknowledge the following individuals for their support and assistance with this research: Adrian Abad, Chris Packer, Emma A. Ziebarth, Michael Carter, and Riley Jackson.
Conflicts of Interest
Young Living is the entity that both funded this research and filed the patent for the natural fungicide discussed in this manuscript. The funders had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. The authors declare no conflict of interest.
References
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Ahmad, Z.; Abbas, H.; Murtaza, T.; Khan, A.U.R.; Ali, A.; Zahid, K.; Tahir, Z.; Mahmood, T.; Habib, A. Assessment of responses of peach cultivars to postharvest pathogen Botrytis cinerea and its mitigation using plant essential oils. Plant Prot. 2023, 7, 2. [Google Scholar] [CrossRef]
- Antuhu, Y.L.; Muanpuii, C.V.; Maisnam, R.; Kumari, A.; López-Menchero, J.R.; Coloma, A.G.; Andrés, M.F.; Kaushik, N. Assessing the efficacy of essential oil fumigation in mitigating Botrytis cinerea infection in cherry tomato. BIO Web Conf. 2024, 110, 02008. [Google Scholar] [CrossRef]
- Karakus, S.; Atıcı, O.; Turan, M.; Azizi, S.; Hajizadeh, H.S.; Kaya, O. Volatile organic compounds produced by some synthetic essential oils as biological fumigants against Botrytis cinerea on apples. Chem. Biol. Technol. Agric. 2023, 10, 136. [Google Scholar] [CrossRef]
- Nofia, N.; Martosudiro, M.; Muhibuddin, A. Growth Inhibition of Botrytis cinerea Fungus on Strawberry (Fragaria sp.) Using Kaffir Lime (Citrus hystrix) Leaf Essential Oil Emulsion. Agro. Bali. Agric. J. 2024, 7, 1. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, J.; Liu, H.; Lian, H.; Liu, J. In vitro antifungal activity of essential oils against Botrytis cinerea of postharvest grapes. IOP Conf. Ser. Earth Environ. Sci. 2022, 1035, 012008. [Google Scholar] [CrossRef]
- Gomes, H.D.O.; Menezes, J.M.C.; da Costa, J.G.M.; Coutinho, H.D.M.; Teixeira, R.N.P.; do Nascimento, R.F. A socio-environmental perspective on pesticide use and food production. Ecotoxicol. Environ. Saf. 2020, 197, 110627. [Google Scholar] [CrossRef]
- Neuwirthová, N.; Trojan, M.; Svobodová, M.; Vašíčková, J.; Šimek, Z.; Hofman, J.; Bielská, L. Pesticide residues remaining in soils from previous growing season (s)-Can they accumulate in non-target organisms and contaminate the food web? Sci. Total Environ. 2019, 646, 1056–1062. [Google Scholar] [CrossRef]
- Bi, Y.; Jiang, H.; Hausbeck, M.K.; Hao, J.J. Inhibitory effects of essential oils for controlling Phytophthora capsica. Plant Dis. 2012, 96, 6. [Google Scholar] [CrossRef]
- Christova, P.K.; Dobreva, A.M.; Dzhurmanski, A.G.; Dincheva, I.N.; Dimkova, S.D.; Zapryanova, N.G. The Impact of Plant Essential Oils on the Growth of the Pathogens Botrytis cinerea, Fusarium solani, and Phytophthora pseudocryptogea. Life 2024, 14, 817. [Google Scholar] [CrossRef]
- Donnarumma, L.; Milano, F.; Trotta, S.; Annesi, T. Use of essential oils in control strategies against zucchini powdery mildew. J. Phytopathol. 2015, 163, 877–885. [Google Scholar] [CrossRef]
- Hegazi, M.A.; El-Kot, G.A.N. Efficacy of Some Essential Oils on Controlling Powdery Mildew on Zinnia (Zinnia elegans, L.). J. Agric. Sci. 2010, 2, 4. [Google Scholar] [CrossRef]
- La Torre, A.; Mandalà, C.; Pezza, L.; Caradonia, F.; Battaglia, V. Evaluation of essential plant oils for the control of Plasmopara viticola. J. Essent. Oil Res. 2014, 26, 4. [Google Scholar] [CrossRef]
- Lu, M.; Han, Z.; Yao, L. In vitro and in vivo antimicrobial efficacy of essential oils and individual compounds against Phytophthora parasitica var. nicotianae. J. Appl. Microbiol. 2013, 115, 1. [Google Scholar] [CrossRef]
- Muchembled, J.; Deweer, C.; Sahmer, K.; Halama, P. Antifungal activity of essential oils on two Venturia inaequalis strains with different sensitivities to tebuconazole. Environ. Sci. Pollut. Res. 2018, 25, 29921–29928. [Google Scholar] [CrossRef]
- Nagy, G.; Hochbaum, T.; Sárosi, S.; Ladányi, M. In Vitro and in Planta Activity of Some Essential Oils against Venturia inaequalis (Cooke) G. Winter. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 1. [Google Scholar] [CrossRef]
- Nana, W.L.; Eke, P.; Fokom, R.; Bakanrga-Via, I.; Begoude, D.; Tchana, T.; Tchameni, N.S.; Kuate, J.; Menut, C.; Fekam Boyom, F. Antimicrobial activity of Syzygium aromaticum and Zanthoxylum xanthoxyloides essential oils against Phytophthora megakarya. J. Phytopathol. 2015, 163, 7–8. [Google Scholar] [CrossRef]
- Rienth, M.; Crovadore, J.; Ghaffari, S.; Lefort, F. Oregano essential oil vapour prevents Plasmopara viticola infection in grapevine (Vitis vinifera) and primes plant immunity mechanisms. PLoS ONE 2019, 14, 9. [Google Scholar] [CrossRef]
- Sturchio, E.; Donnarumma, L.; Annesi, T.; Milano, F.; Casorri, L.; Masciarelli, E.; Zanellato, M.; Meconi, C.; Boccia, P. Essential oils: An alternative approach to management of powdery mildew diseases. Phytopathol. Mediterr. 2014, 53, 3. [Google Scholar] [CrossRef]
- Maia, A.J.; Oliveira, J.S.B.; Schwan-Estrada, K.R.F.; Faria, C.M.R.; Batista, A.F.; Costa, W.F.; Batista, B.N. The control of isariopsis leaf spot and downy mildew in grapevine cv. Isabel with the essential oil of lemon grass and the activity of defensive enzymes in response to the essential oil. Crop Prot. 2014, 63, 57–67. [Google Scholar] [CrossRef]
- Sameza, M.L.; Bedine Boat, M.A.; Tchameni Nguemezi, S.; Nguemnang Mabou, L.C.; Jazet Dongmo, P.M.; Boyom, F.F.; Menut, C. Potential use of Eucalyptus globulus essential oil against Phytophthora colocasiae the causal agent of taro leaf blight. Eur. J. Plant Pathol. 2014, 140, 243–250. [Google Scholar] [CrossRef]
- Carlson, R.E.; Wilson, T.M. Fungicidal compositions comprising essential oils. US Patent 2025/0176562 A1, 5 June 2025. [Google Scholar]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Leyronas, C.; Duffaud, M.; Nicot, P.C. Compared efficiency of the isolation methods for Botrytis cinerea. Mycology 2012, 3, 4. [Google Scholar] [CrossRef]
- Asemaninejad, A.; Weerasuriya, N.; Gloor, G.B.; Lindo, Z.; Thorn, R.G. New Primers for Discovering Fungal Diversity Using Nuclear Large Ribosomal DNA. PLoS ONE 2016, 11, e0159043. [Google Scholar] [CrossRef]
- Huang, X.; Madan, A. CAP3: A DNA sequence assembly program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Tang, X.; Yangjing, G.; Zhuoma, G.; Guo, X.; Cao, P.; Yi, B.; Wang, W.; Ji, D.; Pasquali, M.; Baccelli, I.; et al. Biological characterization and in vitro fungicide screenings of a new causal agent of wheat Fusarium head blight in Tibet, China. Front. Microbiol. 2022, 13, 941734. [Google Scholar] [CrossRef]
- Howyzeh, M.S.; Noori, S.A.S.; Shariati, V. Essential oil profiling of Ajowan (Trachyspermum ammi) industrial medicinal plant. Ind. Crop. Prod. 2018, 119, 255–259. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Huang, R.S.; Liang, H.L.; Xu, C.G.; Wu, H. Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind. Crop. Prod. 2013, 47, 92–101. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).