Pathophysiology in Systemic Sclerosis: Current Insights and Future Perspectives
Abstract
1. Introduction
2. Etiology and Risk Factors
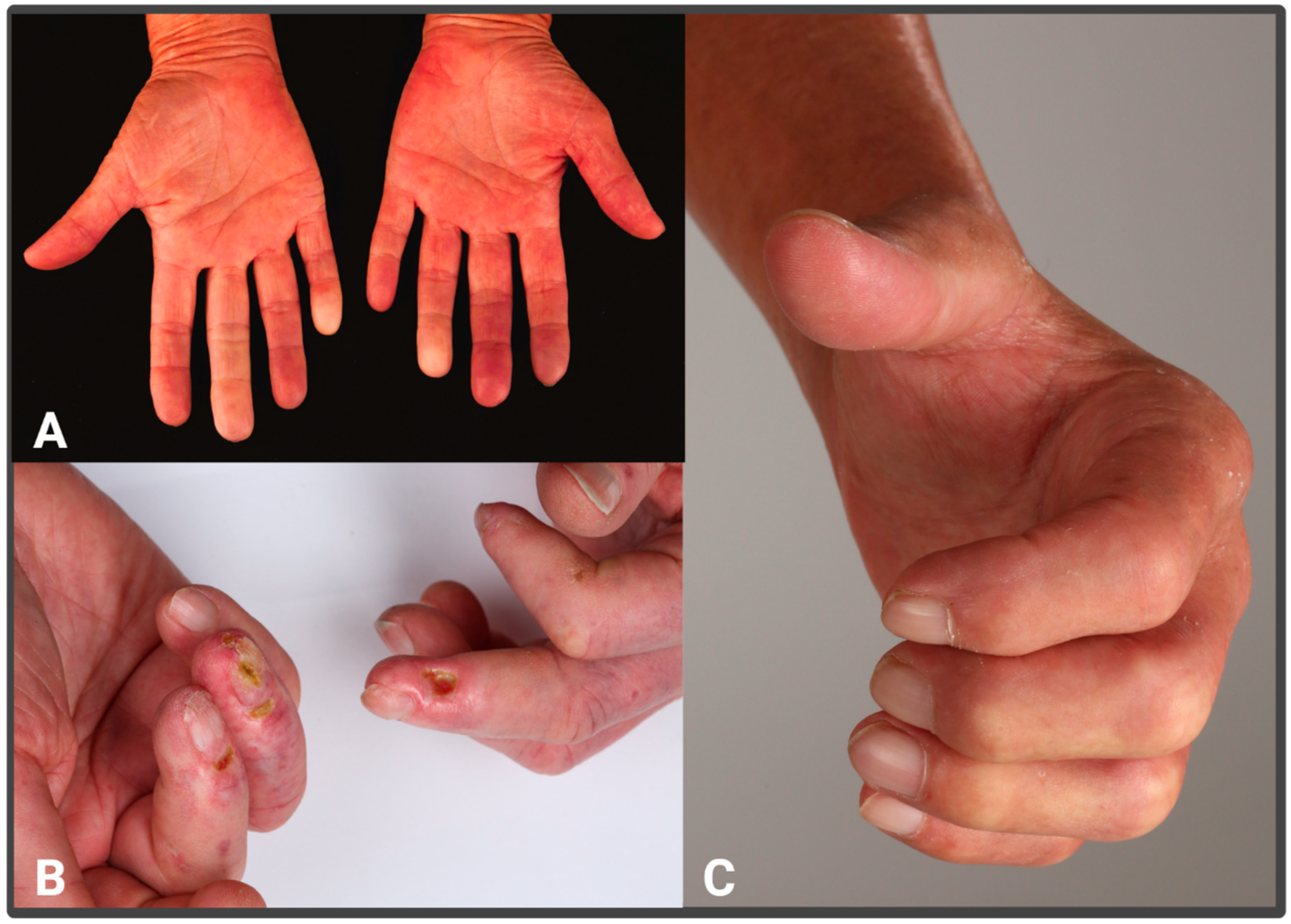
3. Vascular Alterations and Endothelial Damage in SSc
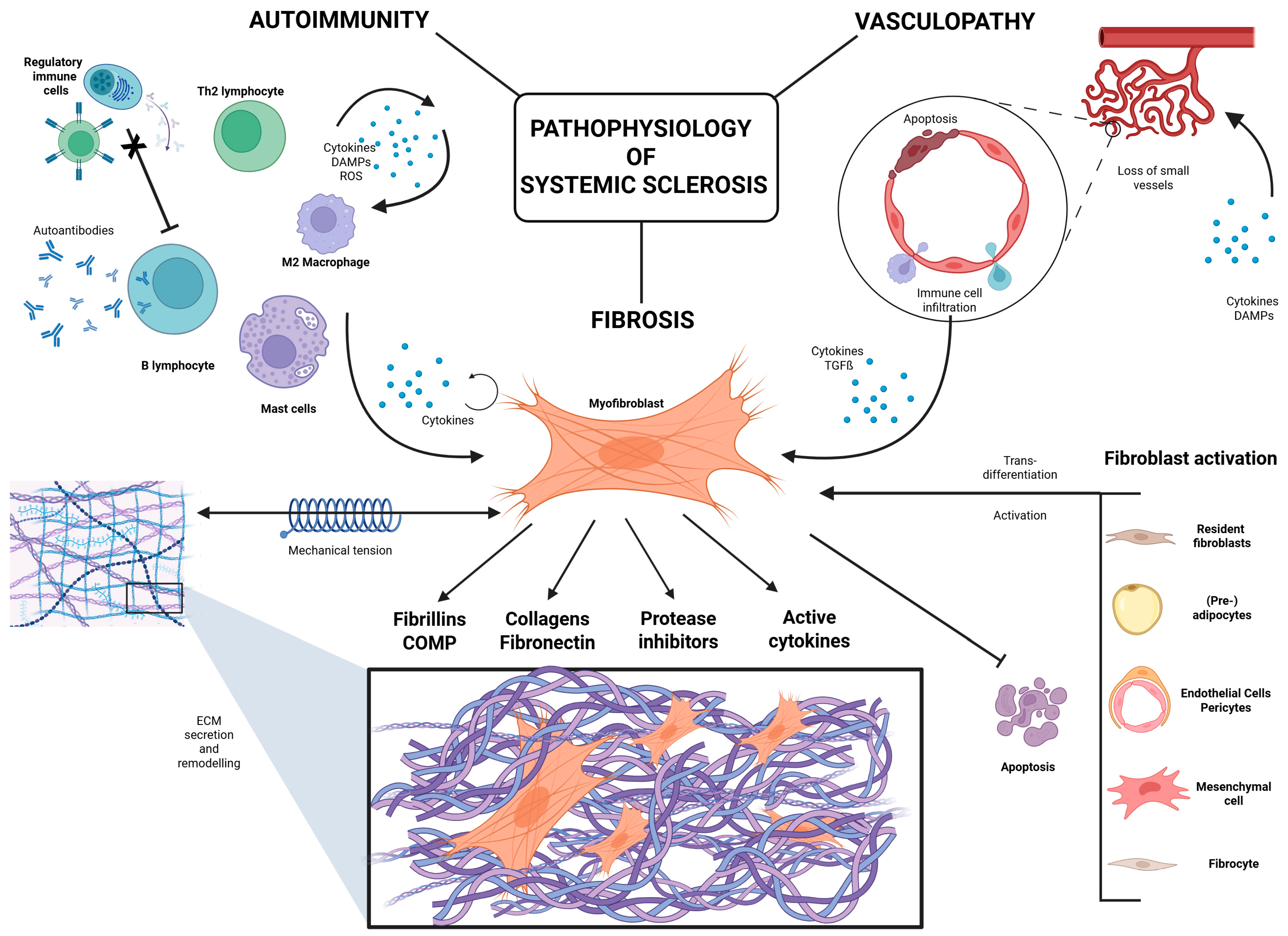
4. Autoimmune Dysregulation in Systemic Sclerosis
| Cytokines/chemokines/growth factors involved in vasculopathy | ||
|---|---|---|
| Endothelin-1 (ET-1) | Potent vasoconstrictor; promotes vascular dysfunction, fibroblast activation, and is elevated in SSc patients. Involved in PAH and DU development. | [17,28] |
| Nitric Oxide (NO) | Vasodilator; its impaired production leads to vascular tone dysregulation, platelet aggregation, and oxidative injury. | [18,19,29] |
| Vascular Endothelial Growth Factor (VEGF) | Key factor for angiogenesis; elevated in SSc but ineffective, leading to defective capillary repair and progressive ischemia. | [30,31] |
| Platelet-Derived Growth Factor (PDGF) | Induces fibroblast proliferation, contributing to vascular remodeling and fibrosis; linked to vascular dysfunction in SSc. | [26,48] |
| CXCL4 | Chemokine produced by plasmacytoid dendritic cells; amplifies immune activation, vascular injury, and fibrosis. | [47] |
| Interleukin-6 (IL-6) | Elevated early; drives endothelial activation, Th2 polarization, and chronic inflammation, and contributes to vascular damage. | [35,41] |
| Cytokines/chemokines/growth factors involved in fibrosis | ||
| Transforming Growth Factor-β (TGF-β) | Master regulator of fibrosis; promotes fibroblast activation, ECM production, myofibroblast differentiation, and suppresses ECM degradation. | [37,54,55] |
| Interleukin-4 (IL-4) | Th2 cytokine; enhances fibroblast proliferation and collagen production, suppresses ECM degradation, and promotes fibrotic progression. | [36,51] |
| Interleukin-13 (IL-13) | Works alongside IL-4; boosts collagen synthesis and fibroblast proliferation; and sustains fibrotic cycles. | [36,51] |
| Interleukin-6 (IL-6) | In addition to vascular roles, promotes M2 macrophage polarization and enhances fibrotic signaling. | [41,43] |
| Interferon-α (IFN-α) | Produced by plasmacytoid dendritic cells; promotes immune activation and maintains fibrotic and inflammatory environments. | [47] |
| Oncostatin M | Produced by mononuclear cells; acts synergistically with IL-6 to stimulate fibroblast activation and fibrosis. | [41] |
| Connective Tissue Growth Factor (CTGF) | Acts downstream of TGF-β; critical in fibroblast activation and persistent ECM accumulation. | [56] |
| Osteopontin (OPN) | Pro-inflammatory glycoprotein promoting fibroblast activation, myofibroblast differentiation, ECM deposition, and chronic inflammation; linked to disease severity in SSc. | [57] |
| Interleukin-17 (IL-17) | Pro-inflammatory cytokine from Th17 cells; enhances fibroblast proliferation, collagen expression, and synergizes with TGF-β in fibrotic pathways. | [58,59] |
| Interleukin-11 (IL-11) | Promotes fibroblast activation, ECM production, and collagen deposition; implicated in lung and skin fibrosis. Revelant for cardiac and renal fibrosis. | [60,61] |
| Interleukin-31 (IL-31) | Associated with pruritus in SSc; emerging evidence suggests profibrotic roles via immune–fibroblast crosstalk. | [62,63] |
5. Fibrosis and ECM Deposition in SSc
5.1. Activation and Origin of Fibroblasts in SSc
5.2. Fibroblast Survival and Resistance to Apoptosis
5.3. The ECM in SSc
6. Therapeutic Approaches for SSc Based on the Understanding of Its Pathophysiology
7. Conclusions
Funding
Conflicts of Interest
References
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef]
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Mechanisms of Disease Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Györfi, A.-H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.L.; Caballero-Ruiz, B.; Clarke, E.L.; Kakkar, V.; Wasson, C.W.; Mulipa, P.; De Lorenzis, E.; Merchant, W.; Di Donato, S.; Rindone, A.; et al. Biological hallmarks of systemic sclerosis are present in the skin and serum of patients with Very Early Diagnosis of Systemic Sclerosis (VEDOSS). Rheumatology 2024, keae698, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investigation. 2007, 117, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Avouac, J.; Riemekasten, G.; Meune, C.; Ruiz, B.; Kahan, A.; Allanore, Y. Autoantibodies against endothelin 1 Type A receptor are strong predictors of digital ulcers in systemic sclerosis. J. Rheumatol. 2015, 42, 1801–1807. [Google Scholar] [CrossRef]
- Lescoat, A.; Varga, J.; Matucci-Cerinic, M.; Khanna, D. New promising drugs for the treatment of systemic sclerosis: Pathogenic considerations, enhanced classifications, and personalized medicine. Expert Opin. Investig. Drugs 2021, 30, 635–652. [Google Scholar] [CrossRef]
- Del Galdo, F.; Lescoat, A.; Conaghan, P.G.; Bertoldo, E.; Čolić, J.; Santiago, T.; Suliman, Y.A.; Matucci-Cerinic, M.; Gabrielli, A.; Distler, O.; et al. EULAR recommendations for the treatment of systemic sclerosis: 2023 update. Ann. Rheum. Dis. 2024, 84, 29–40. [Google Scholar] [CrossRef]
- Broen, J.C.A.; Radstake, T.R.D.J.; Rossato, M. The role of genetics and epigenetics in the pathogenesis of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 671–681. [Google Scholar] [CrossRef]
- López-Isac, E.; Acosta-Herrera, M.; Kerick, M.; Assassi, S.; Satpathy, A.T.; Granja, J.; Mumbach, M.R.; Beretta, L.; Simeón, C.P.; Carreira, P.; et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Ciccarelli, F.; Sirufo, M.M.; Ginaldi, L. An overview of environmental risk factors in systemic sclerosis. Expert Rev. Clin. Immunol. 2016, 12, 465–478. [Google Scholar] [CrossRef]
- Moroncini, G.; Svegliati, S.; Grieco, A.; Cuccioloni, M.; Mozzicafreddo, M.; Paolini, C.; Agarbati, S.; Spadoni, T.; Amoresano, A.; Pinto, G.; et al. Adeno-Associated Virus Type 5 Infection via PDGFRα Is Associated With Interstitial Lung Disease in Systemic Sclerosis and Generates Composite Peptides and Epitopes Recognized by the Agonistic Immunoglobulins Present in Patients With Systemic Sclerosis. Arthritis Rheumatol. 2024, 76, 620–630. [Google Scholar] [CrossRef]
- Jung, P.; Trautinger, F. Kapillarmikroskopie. JDDG-J. Ger. Soc. Dermatol. 2013, 11, 731–736. [Google Scholar] [CrossRef]
- Fieischmajer, R.; Perlish, J.S. Capillary Alterations in Scleroderma. J. Am. Acad. Dermatol. 1980, 2, 161–170. [Google Scholar] [CrossRef]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef] [PubMed]
- Rokni, M.; Shaker, M.S.; Kavosi, H.; Shokoofi, S.; Mahmoudi, M.; Farhadi, E. The role of endothelin and RAS/ERK signaling in immunopathogenesis-related fibrosis in patients with systemic sclerosis: An updated review with therapeutic implications. Arthritis Res. Ther. 2022, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Borderie, D.; Hilliquin, P.; Hernvann, A.; Levacher, M.; Lemaréchal, H.; Ekindjian, O.G.; Kahan, A. Low Levels of Nitric Oxide (NO) in Systemic Sclerosis: Inducible NO Synthase Production Is Decreased in Cultured Peripheral Blood Monocyteumacrophage Cells. Rheumatology 2001, 40, 1089–1096. [Google Scholar] [CrossRef]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Binda, M.; Moccaldi, B.; Civieri, G.; Cuberli, A.; Doria, A.; Tona, F.; Zanatta, E. Autoantibodies Targeting G-Protein-Coupled Receptors: Pathogenetic, Clinical and Therapeutic Implications in Systemic Sclerosis. Int. J. Mol. Sci. 2024, 25, 2299. [Google Scholar] [CrossRef]
- Rosendahl, A.; Schönborn, K.; Krieg, T. Pathophysiology of systemic sclerosis (scleroderma). Kaohsiung J. Med. Sci. 2022, 38, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; An, X.-R.; Li, Q.; Li, X.-X.; Cong, X.-D.; Xu, M. Improvement of vascular dysfunction by argirein through inhibiting endothelial cell apoptosis associated with ET-1/Nox4 signal pathway in diabetic rats. Sci. Rep. 2018, 8, 12620. [Google Scholar] [CrossRef] [PubMed]
- Bruni, C.; Frech, T.; Manetti, M.; Rossi, F.W.; Furst, D.E.; De Paulis, A.; Rivellese, F.; Guiducci, S.; Matucci-Cerinic, M.; Bellando-Randone, S. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: Should the door be closed? Front. Immunol. 2018, 9, 2045. [Google Scholar] [CrossRef] [PubMed]
- De Ciuceis, C.; Amiri, F.; Brassard, P.; Endemann, D.H.; Touyz, R.M.; Schiffrin, E.L. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: Evidence for a role in inflammation in angiotensin-induced vascular injury. Arter. Thromb. Vasc. Biol. 2005, 25, 2106–2113. [Google Scholar] [CrossRef]
- Günther, J.; Rademacher, J.; van Laar, J.M.; Siegert, E.; Riemekasten, G. Functional autoantibodies in systemic sclerosis. Semin. Immunopathol. 2015, 37, 529–542. [Google Scholar] [CrossRef]
- Sartori-Valinotti, J.C.; Tollefson, M.M.; Reed, A.M. Updates on morphea: Role of vascular injury and advances in treatment. Autoimmune Dis. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Talotta, R.; Atzeni, F.; Ditto, M.C.; Gerardi, M.C.; Batticciotto, A.; Bongiovanni, S.; Puttini, P.S. Certainties and uncertainties concerning the contribution of pericytes to the pathogenesis of systemic sclerosis. J. Scleroderma Relat. Disord. 2018, 3, 14–20. [Google Scholar] [CrossRef]
- Cozzani, E.; Javor, S.; Laborai, E.; Drosera, M.; Parodi, A. Endothelin-1 Levels in Scleroderma Patients: A Pilot Study. ISRN Dermatol. 2013, 2013, 125632. [Google Scholar] [CrossRef]
- Takagi, K.; Kawaguchi, Y.; Hara, M.; Sugiura, T.; Harigai, M.; Kamatani, N. Serum nitric oxide (NO) levels in systemic sclerosis patients: Correlation between NO levels and clinical features. Clin. Exp. Immunol. 2003, 134, 538–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantatore, F.P.; Maruotti, N.; Corrado, A.; Ribatti, D. Angiogenesis Dysregulation in the Pathogenesis of Systemic Sclerosis. Biomed Res. Int. 2017, 2017, 5345673. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonte, F.; Desmouliere, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [PubMed]
- Hunzelmann, N.; Krieg, T. Sklerodermie. In Braun-Falco’s Dermatologie, Venerologie Und Allergologie, 7th ed.; Plewig, G., Ruzicka, T., Kaufmann, R., Hertl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 919–936. [Google Scholar] [CrossRef]
- Pattanaik, D.; Brown, M.; Postlethwaite, A.E. Vascular involvement in systemic sclerosis (scleroderma). J. Inflamm. Res. 2011, 4, 105–125. [Google Scholar] [CrossRef]
- Roumm, A.D.; Whiteside, T.L.; Medsger, T.A.; Rodnan, G.P. Lymphocytes in the skin of patients with progressive systemic sclerosis. Arthritis Rheum. 1984, 27, 645–653. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sato, S.; Ihn, H.; Takehara, K. Enhanced Production of Interleukin-6 (IL-6), Oncostatin M and Soluble IL-6 Receptor by Cultured Peripheral Blood Mononuclear Cells from Patients with Systemic Sclerosis. Rheumatology 1999, 38, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Shaw, T.J.; Martin, P. Molecular mechanisms linking wound inflammation and fibrosis: Knockdown of osteopontin leads to rapid repair and reduced scarring. J. Investig. Dermatol. 2008, 128, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Agarbati, S.; Benfaremo, D.; Viola, N.; Paolini, C.; Baroni, S.S.; Funaro, A.; Moroncini, G.; Malavasi, F.; Gabrielli, A. Increased expression of the ectoenzyme CD38 in peripheral blood plasmablasts and plasma cells of patients with systemic sclerosis. Front. Immunol. 2022, 13, 1072462. [Google Scholar] [CrossRef]
- Yoshizaki, A.; Fukasawa, T.; Ebata, S.; Yoshizaki-Ogawa, A.; Sato, S. Involvement of B cells in the development of systemic sclerosis. Front. Immunol. 2022, 13, 938785. [Google Scholar] [CrossRef]
- Grassegger, A.; Pohla-Gubo, G.; Frauscher, M.; Hintner, H. Autoantibodies in systemic sclerosis (scleroderma): Clues for clinical evaluation, prognosis and pathogenesis. Wien. Med. Wochenschr. 2008, 158, 19–28. [Google Scholar] [CrossRef]
- Higashi-Kuwata, N.; Jinnin, M.; Makino, T.; Fukushima, S.; Inoue, Y.; Muchemwa, F.C.; Yonemura, Y.; Komohara, Y.; Takeya, M.; Mitsuya, H.; et al. Characterization of Monocyte/Macrophage Subsets in the Skin and Peripheral Blood Derived from Patients with Systemic Sclerosis. Arthritis Res. Ther. 2010, 12, R128. [Google Scholar] [CrossRef]
- Knipper, J.A.; Willenborg, S.; Brinckmann, J.; Bloch, W.; Maaß, T.; Wagener, R.; Krieg, T.; Sutherland, T.; Munitz, A.; Rothenberg, M.E.; et al. Interleukin-4 Receptor α Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity 2015, 43, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Bereznayj, I.O.; Sporn, M.; Greenberg, A.H. Macrophage Production of Transforming Growth Factor r and Fibroblast Collagen Synthesis in Chronic Pulmonary Inflammation. J. Exp. Med. 1989, 170, 727–737. [Google Scholar] [CrossRef]
- Vona, R.; Giovannetti, A.; Gambardella, L.; Malorni, W.; Pietraforte, D.; Straface, E. Oxidative stress in the pathogenesis of systemic scleroderma: An overview. J. Cell Mol. Med. 2018, 22, 3308–3314. [Google Scholar] [CrossRef]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lande, R.; Lee, E.Y.; Palazzo, R.; Marinari, B.; Pietraforte, I.; Santos, G.S.; Mattenberger, Y.; Spadaro, F.; Stefanantoni, K.; Iannace, N.; et al. CXCL4 assembles DNA into liquid crystalline complexes to amplify TLR9-mediated interferon-α production in systemic sclerosis. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, N.; Capobianco, A.; Rovere-Querini, P.; Ramirez, G.A.; Tombetti, E.; Valle, P.D.; Monno, A.; D’Alberti, V.; Gasparri, A.M.; Franchini, S.; et al. Platelet Microparticles Sustain Autophagy-Associated Activation of Neutrophils in Systemic Sclerosis. Sci. Transl. Med. 2018, 10, 3089. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Caraffa, A.; Mastrangelo, F.; Tettamanti, L.; Ronconi, G.; Frydas, I.; Kritas, S.K.; Theoharides, T.C. Critical role of inflammatory mast cell in fibrosis: Potential therapeutic effect of IL-37. Cell Prolif. 2018, 51, e12475. [Google Scholar] [CrossRef]
- Overed-Sayer, C.; Rapley, L.; Mustelin, T.; Clarke, D.L. Are mast cells instrumental for fibrotic diseases? Front. Pharmacol. Front. Pharmacol. 2014, 4, 174. [Google Scholar] [CrossRef]
- Gasparini, G.; Cozzani, E.; Parodi, A. Interleukin-4 and interleukin-13 as possible therapeutic targets in systemic sclerosis. Cytokine 2020, 125, 154799. [Google Scholar] [CrossRef]
- McGaha, T.; Saito, S.; Phelps, R.G.; Gordon, R.; Noben-Trauth, N.; Paul, W.E.; Bona, C. Lack of Skin Fibrosis in Tight Skin (TSK) Mice with Targeted Mutation in the Interleukin-4Ra and Transforming Growth Factor-b Genes. J. Investig. Dermatol. 2001, 116, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.E.; Mariotti, J.; Ryan, K.; Eckhaus, M.; Fowler, D.H. Th2 Cell Therapy of Established Acute Graft-Versus-Host Disease Requires IL-4 and IL-10 and Is Abrogated by IL-2 or Host-Type Antigen-Presenting Cells. Biol. Blood Marrow Transplant. 2008, 14, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Kaliterna, D.M.; Petrić, M. Biomarkers of skin and lung fibrosis in systemic sclerosis. Expert Rev. Clin. Immunol. 2019, 15, 1215–1223. [Google Scholar] [CrossRef]
- Lafyatis, R. Transforming growth factor β—At the centre of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef]
- Yamakage, A.; Kikuchi, K.; Smith, E.A.; Leroy, E.C.; Trojanowska, M. Selective Upregulation of Platelet-Derived Growth Factor C~Receptors by Transforming Growth Factor/~ in Scleroder Fibroblasts. Available online: http://rupress.org/jem/article-pdf/175/5/1227/1673399/1227.pdf (accessed on 18 May 2025).
- Lang, F.; Li, Y.; Yao, R.; Jiang, M. Osteopontin in Chronic Inflammatory Diseases: Mechanisms, Biomarker Potential, and Therapeutic Strategies. Biology 2025, 14, 428. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Misra, D.P.; Agarwal, V. Interleukin-17 pathways in systemic sclerosis-associated fibrosis. Rheumatol. Int. 2019, 39, 1135–1143. [Google Scholar] [CrossRef]
- Chizzolini, C.; Dufour, A.M.; Brembilla, N.C. Is there a role for IL-17 in the pathogenesis of systemic sclerosis? Immunol. Lett. 2018, 195, 61–67. [Google Scholar] [CrossRef]
- Tan, Y.; Mosallanejad, K.; Zhang, Q.; O’brien, S.; Clements, M.; Perper, S.; Wilson, S.; Chaulagain, S.; Wang, J.; Abdalla, M.; et al. IL11-mediated stromal cell activation may not be the master regulator of pro-fibrotic signaling downstream of TGFβ. Front. Immunol. 2024, 15, 1293883. [Google Scholar] [CrossRef]
- O’Reilly, S. Interleukin-11 and its eminent role in tissue fibrosis: A possible therapeutic target. Clin. Exp. Immunol. 2023, 214, 154–161. [Google Scholar] [CrossRef]
- Kuzumi, A.; Yoshizaki, A.; Matsuda, K.M.; Kotani, H.; Norimatsu, Y.; Fukayama, M.; Ebata, S.; Fukasawa, T.; Yoshizaki-Ogawa, A.; Asano, Y.; et al. Interleukin-31 promotes fibrosis and T helper 2 polarization in systemic sclerosis. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Yaseen, B.; Lopez, H.; Taki, Z.; Zafar, S.; Rosario, H.; Abdi, B.A.; Vigneswaran, S.; Xing, F.; Arumalla, N.; Black, S.; et al. Interleukin-31 promotes pathogenic mechanisms underlying skin and lung fibrosis in scleroderma. Rheumatology 2020, 59, 2625–2636. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, G.; Ryan, G.B.; Majno, G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, G. 50 Years of Myofibroblasts: How the Myofibroblast Concept Evolved. In Myofibroblasts: Methods and Protocols; Hinz, B., Lagares, D., Eds.; Springer: New York, NY, USA, 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.-L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Schuster, R.; Younesi, F.; Ezzo, M.; Hinz, B. The Role of Myofibroblasts in Physiological and Pathological Tissue Repair. Cold Spring Harb. Perspect. Biol. 2022, 15, a041231. [Google Scholar] [CrossRef]
- van Caam, A.; Vonk, M.; Hoogen, F.v.D.; van Lent, P.; van der Kraan, P. Unraveling SSc pathophysiology; The myofibroblast. Front. Immunol. 2018, 9, 2452. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H. Scleroderma, Fibroblasts, Signaling, and Excessive Extracellular Matrix. Curr. Rheumatol. Rep. 2005, 7, 156–162. [Google Scholar] [CrossRef]
- Chadli, L.; Sotthewes, B.; Li, K.; Andersen, S.N.; Cahir-McFarland, E.; Cheung, M.; Cullen, P.; Dorjée, A.; de Vries-Bouwstra, J.K.; Huizinga, T.W.J.; et al. Identification of regulators of the myofibroblast phenotype of primary dermal fibroblasts from early diffuse systemic sclerosis patients. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Santiago, B.; Galindo, M.; Rivero, M.; Pablos, J.L. Decreased susceptibility to Fas-induced apoptosis of systemic sclerosis dermal fibroblasts. Arthritis Rheum. 2001, 44, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Jelaska, A.; Korn, J.H. Role of apoptosis and transforming growth factor beta1 in fibroblast selection and activation in systemic sclerosis. Arthritis Rheum. 2000, 43, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Lagares, D.; Santos, A.; Grasberger, P.E.; Liu, F.; Probst, C.K.; Rahimi, R.A.; Sakai, N.; Kuehl, T.; Ryan, J.; Bhola, P.; et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl. Med. 2017, 9, eaal3765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samuel, G.H.; Lenna, S.; Bujor, A.M.; Lafyatis, R.; Trojanowska, M. Acid sphingomyelinase deficiency contributes to resistance of scleroderma fibroblasts to Fas-mediated apoptosis. J. Dermatol. Sci. 2012, 67, 166–172. [Google Scholar] [CrossRef]
- Jafarinejad-Farsangi, S.; Farazmand, A.; Gharibdoost, F.; Karimizadeh, E.; Noorbakhsh, F.; Faridani, H.; Mahmoudi, M.; Jamshidi, A.R. Inhibition of MicroRNA-21 induces apoptosis in dermal fibroblasts of patients with systemic sclerosis. Int. J. Dermatol. 2016, 55, 1259–1267. [Google Scholar] [CrossRef]
- Rajkumar, V.S.; Sundberg, C.; Abraham, D.J.; Rubin, K.; Black, C.M. Activation of microvascular pericytes in autoimmune Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum. 1999, 42, 930–941. [Google Scholar] [CrossRef]
- Sonnylal, S.; Shi-Wen, X.; Leoni, P.; Naff, K.; Van Pelt, C.S.; Nakamura, H.; Leask, A.; Abraham, D.; Bou-Gharios, G.; de Crombrugghe, B. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010, 62, 1523–1532. [Google Scholar] [CrossRef]
- Nikitorowicz-Buniak, J.; Denton, C.P.; Abraham, D.; Stratton, R. Partially evoked epithelial-mesenchymal transition (EMT) is associated with increased TGFβ signaling within lesional scleroderma skin. PLoS ONE 2015, 10, e0134092. [Google Scholar] [CrossRef] [PubMed]
- Blumbach, K.; Zweers, M.C.; Brunner, G.; Peters, A.S.; Schmitz, M.; Schulz, J.-N.; Schild, A.; Denton, C.P.; Sakai, T.; Fässler, R.; et al. Defective granulation tissue formation in mice with specific ablation of integrin-linked kinase in fibroblasts—Role of TGFβ1 levels and RhoA activity. J. Cell Sci. 2010, 123, 3872–3883. [Google Scholar] [CrossRef]
- Schulz, J.-N.; Zeltz, C.; Sørensen, I.W.; Barczyk, M.; Carracedo, S.; Hallinger, R.; Niehoff, A.; Eckes, B.; Gullberg, D. Reduced granulation tissue and wound strength in the absence of α11β1 integrin. J. Investig. Dermatol. 2015, 135, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.-N.; Plomann, M.; Sengle, G.; Gullberg, D.; Krieg, T.; Eckes, B. New developments on skin fibrosis—Essential signals emanating from the extracellular matrix for the control of myofibroblasts. Matrix Biol. 2018, 68–69, 522–532. [Google Scholar] [CrossRef]
- Sawant, M.; Hinz, B.; Schönborn, K.; Zeinert, I.; Eckes, B.; Krieg, T.; Schuster, R. A story of fibers and stress: Matrix-embedded signals for fibroblast activation in the skin. Wound Repair Regen. 2021, 29, 515–530. [Google Scholar] [CrossRef]
- Lagares, D.; Busnadiego, O.; García-Fernández, R.A.; Kapoor, M.; Liu, S.; Carter, D.E.; Abraham, D.; Shi-Wen, X.; Carreira, P.; Fontaine, B.A.; et al. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 2012, 64, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, H.; Skaug, B.; Tabib, T.; Li, Y.-N.; Tao, Y.; Matei, A.-E.; Lyons, M.A.; Schett, G.; Lafyatis, R.; et al. Fibroblast Subpopulations in Systemic Sclerosis: Functional Implications of Individual Subpopulations and Correlations with Clinical Features. J. Investig. Dermatol. 2024, 144, 1251–1261.e13. [Google Scholar] [CrossRef] [PubMed]
- Schönborn, K.; Willenborg, S.; Schulz, J.-N.; Imhof, T.; Eming, S.A.; Quondamatteo, F.; Brinckmann, J.; Niehoff, A.; Paulsson, M.; Koch, M.; et al. Role of collagen XII in skin homeostasis and repair. Matrix Biol. 2020, 94, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.-N.; Nüchel, J.; Niehoff, A.; Bloch, W.; Schönborn, K.; Hayashi, S.; Kamper, M.; Brinckmann, J.; Plomann, M.; Paulsson, M.; et al. COMP-assisted collagen secretion—A novel intracellular function required for fibrosis. J. Cell Sci. 2016, 129, 706–716. [Google Scholar] [CrossRef]
- Pope, J.E.; Denton, C.P.; Johnson, S.R.; Fernandez-Codina, A.; Hudson, M.; Nevskaya, T. State-of-the-art evidence in the treatment of systemic sclerosis. Nat. Rev. Rheumatol. 2023, 19, 212–226. [Google Scholar] [CrossRef]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis–Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef]
- Stratton, R.J.; Wilson, H.; Black, C.M. Pilot Study of Anti-Thymocyte Globulin plus Mycophenolate Mofetil in Recent-Onset Diffuse Scleroderma. Rheumatology 2001, 40, 84–88. [Google Scholar] [CrossRef]
- Franco-Fuquen, P.; Figueroa-Aguirre, J.; Martínez, D.A.; Moreno-Cortes, E.F.; Garcia-Robledo, J.E.; Vargas-Cely, F.; Castro-Martínez, D.A.; Almaini, M.; Castro, J.E. Cellular therapies in rheumatic and musculoskeletal diseases. J. Transl. Autoimmun. 2025, 10, 100264. [Google Scholar] [CrossRef]
- Kuzumi, A.; Ebata, S.; Baron, M.; Sato, S.; Yoshizaki, A. Usefulness of rituximab for the treatment of systemic sclerosis-associated interstitial lung disease: Further analysis of the DESIRES trial. J. Am. Acad. Dermatol. 2025, 92, 1402–1404. [Google Scholar] [CrossRef]
- Davis, J.S.; Ferreira, D.; Paige, E.; Gedye, C.; Boyle, M. Infectious complications of biological and small molecule targeted immunomodulatory therapies. Clin. Microbiol. Rev. 2020, 33, 1–117. [Google Scholar] [CrossRef]
- Colussi, L.; Dagri, A.; Pastore, S.; Tommasini, A.; Pin, A.; Taddio, A. Effect of the Janus kinase inhibitor tofacitinib in the treatment of juvenile scleroderma: A single-center experience. Int. J. Rheum. Dis. 2024, 27, 15295. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Liu, C.; Wu, Y.; Liu, S.; Zheng, Y.; Hao, W.; Wang, D.; Sun, L. Efficacy and safety of mesenchymal stromal cell transplantation in the treatment of autoimmune and rheumatic immune diseases: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2025, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, C.; De Luca, G.; Lazzaroni, M.-G.; Armentaro, G.; Spinella, A.; Vigone, B.; Ruaro, B.; Stanziola, A.; Benfaremo, D.; De Lorenzis, E.; et al. Real-life efficacy and safety of nintedanib in systemic sclerosis-interstitial lung disease: Data from an Italian multicentre study. RMD Open 2023, 9, e002850. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Subias, P.E.; Feldman, J.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef]
- Matucci-Cerinic, M.; Kahaleh, B.; Wigley, F.M. Review: Evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 2013, 65, 1953–1962. [Google Scholar] [CrossRef]
- Rigau, A.R.; Li, Y.-N.; Matei, A.-E.; Györfi, A.-H.; Bruch, P.-M.; Koziel, S.; Devakumar, V.; Gabrielli, A.; Kreuter, A.; Wang, J.; et al. Characterization of Vascular Niche in Systemic Sclerosis by Spatial Proteomics. Circ. Res. 2024, 134, 875–891. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Smith, V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat. Rev. Rheumatol. 2010, 6, 578–587. [Google Scholar] [CrossRef]
- Lemmers, J.; Velauthapillai, A.; van Herwaarden, N.; Vonk, M. Change of the microvascularization in systemic sclerosis, a matter of air. Best Pr. Res. Clin. Rheumatol. 2021, 35, 101683. [Google Scholar] [CrossRef]
- Allanore, Y.; Wung, P.; Soubrane, C.; Esperet, C.; Marrache, F.; Bejuit, R.; Lahmar, A.; Khanna, D.; Denton, C.P. A randomised, double-blind, placebo-controlled, 24-week, phase II, proof-of-concept study of romilkimab (SAR156597) in early diffuse cutaneous systemic sclerosis. Ann. Rheum. Dis. 2020, 79, 1600–1607. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Gburi, S.; Moinzadeh, P.; Krieg, T. Pathophysiology in Systemic Sclerosis: Current Insights and Future Perspectives. Sclerosis 2025, 3, 17. https://doi.org/10.3390/sclerosis3020017
Al-Gburi S, Moinzadeh P, Krieg T. Pathophysiology in Systemic Sclerosis: Current Insights and Future Perspectives. Sclerosis. 2025; 3(2):17. https://doi.org/10.3390/sclerosis3020017
Chicago/Turabian StyleAl-Gburi, Suzan, Pia Moinzadeh, and Thomas Krieg. 2025. "Pathophysiology in Systemic Sclerosis: Current Insights and Future Perspectives" Sclerosis 3, no. 2: 17. https://doi.org/10.3390/sclerosis3020017
APA StyleAl-Gburi, S., Moinzadeh, P., & Krieg, T. (2025). Pathophysiology in Systemic Sclerosis: Current Insights and Future Perspectives. Sclerosis, 3(2), 17. https://doi.org/10.3390/sclerosis3020017






