The Cognitive Reserve May Influence Fatigue after Rehabilitation in Progressive Multiple Sclerosis: A Secondary Analysis of the RAGTIME Trial
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
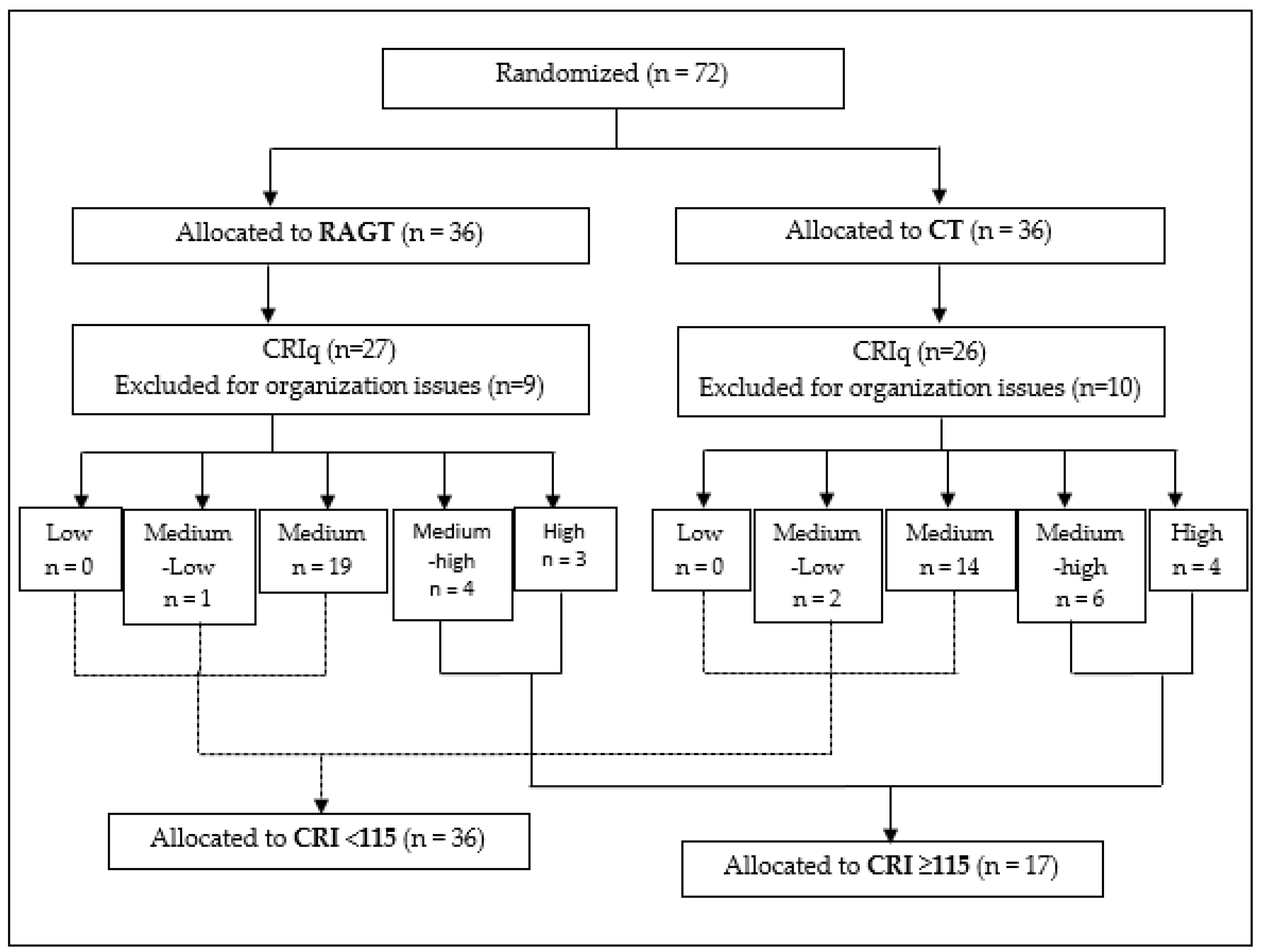
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ben-Zacharia, A.B. Therapeutics for Multiple Sclerosis Symptoms. Mt. Sinai J. Med. 2011, 78, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S. Natural History of Multiple Sclerosis: A Unifying Concept. Brain 2006, 129, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Wiles, C.M.; Newcombe, R.G.; Fuller, K.J.; Shaw, S.; Furnival-Doran, J.; Pickersgill, T.P.; Morgan, A. Controlled Randomised Crossover Trial of the Effects of Physiotherapy on Mobility in Chronic Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2001, 70, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Benedetti, M.G.; Venturini, E.; Manca, M.; Foti, C.; Basaglia, N. Does Robot-Assisted Gait Training Ameliorate Gait Abnormalities in Multiple Sclerosis? A Pilot Randomized-Control Trial. NeuroRehabilitation 2013, 33, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Fanciullacci, C.; Martinuzzi, C.; Pavarelli, C.; Rossi, B.; Chisari, C.; Basaglia, N. The Effects of Robot-Assisted Gait Training in Progressive Multiple Sclerosis: A Randomized Controlled Trial. Mult. Scler. 2016, 22, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Manfredini, F.; Lamberti, N.; Martinuzzi, C.; Maietti, E.; Basaglia, N. Robot-Assisted Gait Training Is Not Superior to Intensive Overground Walking in Multiple Sclerosis with Severe Disability (the RAGTIME Study): A Randomized Controlled Trial. Mult. Scler. 2020, 26, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Mondini, S.; Madella, I.; Zangrossi, A.; Bigolin, A.; Tomasi, C.; Michieletto, M.; Villani, D.; Di Giovanni, G.; Mapelli, D. Cognitive Reserve in Dementia: Implications for Cognitive Training. Front. Aging Neurosci. 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Sumowski, J.F.; Leavitt, V.M. Cognitive Reserve in Multiple Sclerosis. Mult. Scler. 2013, 19, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Altieri, M.; Enzinger, C.; Gallo, A.; Trojano, L. Cognitive Reserve and Neuropsychological Performance in Multiple Sclerosis: A Meta-Analysis. Neuropsychology 2019, 33, 379–390. [Google Scholar] [CrossRef]
- Imbimbo, I.; Coraci, D.; Santilli, C.; Loreti, C.; Piccinini, G.; Ricciardi, D.; Castelli, L.; Fusco, A.; Bentivoglio, A.R.; Padua, L. Parkinson’s Disease and Virtual Reality Rehabilitation: Cognitive Reserve Influences the Walking and Balance Outcome. Neurol. Sci. 2021, 42, 4615–4621. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive Reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Kartschmit, N.; Mikolajczyk, R.; Schubert, T.; Lacruz, M.E. Measuring Cognitive Reserve (CR)—A Systematic Review of Measurement Properties of CR Questionnaires for the Adult Population. PLoS ONE 2019, 14, e0219851. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. What Is Cognitive Reserve? Theory and Research Application of the Reserve Concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index Questionnaire (CRIq): A New Instrument for Measuring Cognitive Reserve. Aging Clin. Exp. Res. 2012, 24, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Artemiadis, A.; Bakirtzis, C.; Ifantopoulou, P.; Zis, P.; Bargiotas, P.; Grigoriadis, N.; Hadjigeorgiou, G. The Role of Cognitive Reserve in Multiple Sclerosis: A Cross-Sectional Study in 526 Patients. Mult. Scler. Relat. Disord. 2020, 41, 102047. [Google Scholar] [CrossRef] [PubMed]
- Chillemi, G.; Scalera, C.; Terranova, C.; Calamuneri, A.; Buccafusca, M.; Dattola, V.; Rizzo, V.; Bruschetta, D.; Girlanda, P.; Quartarone, A. Cognitive Processess and Cognitive Reserve in Multiple Sclerosis. Arch. Ital. Biol. 2015, 153, 19–24. [Google Scholar]
- Fuchs, T.A.; Benedict, R.H.B.; Bartnik, A.; Choudhery, S.; Li, X.; Mallory, M.; Oship, D.; Yasin, F.; Ashton, K.; Jakimovski, D.; et al. Preserved Network Functional Connectivity Underlies Cognitive Reserve in Multiple Sclerosis. Hum. Brain Mapp. 2019, 40, 5231–5241. [Google Scholar] [CrossRef] [PubMed]
- Ifantopoulou, P.; Artemiadis, A.K.; Bakirtzis, C.; Zekiou, K.; Papadopoulos, T.-S.; Diakogiannis, I.; Hadjigeorgiou, G.; Grigoriadis, N.; Orologas, A. Cognitive and Brain Reserve in Multiple Sclerosis—A Cross-Sectional Study. Mult. Scler. Relat. Disord. 2019, 35, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Cadden, M.H.; Guty, E.T.; Arnett, P.A. Cognitive Reserve Attenuates the Effect of Disability on Depression in Multiple Sclerosis. Arch. Clin. Neuropsychol. 2019, 34, 495–502. [Google Scholar] [CrossRef]
- Guzzetti, S.; Mancini, F.; Caporali, A.; Manfredi, L.; Daini, R. The Association of Cognitive Reserve with Motor and Cognitive Functions for Different Stages of Parkinson’s Disease. Exp. Gerontol. 2019, 115, 79–87. [Google Scholar] [CrossRef]
- Padua, L.; Imbimbo, I.; Aprile, I.; Loreti, C.; Germanotta, M.; Coraci, D.; Piccinini, G.; Pazzaglia, C.; Santilli, C.; Cruciani, A.; et al. Cognitive Reserve as a Useful Variable to Address Robotic or Conventional Upper Limb Rehabilitation Treatment after Stroke: A Multicentre Study of the Fondazione Don Carlo Gnocchi. Eur. J. Neurol. 2020, 27, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, G.; Imbimbo, I.; Ricciardi, D.; Coraci, D.; Santilli, C.; Lo Monaco, M.R.; Loreti, C.; Vulpiani, M.C.; Silveri, M.C.; Padua, L. The Impact of Cognitive Reserve on the Effectiveness of Balance Rehabilitation in Parkinson’s Disease. Eur. J. Phys. Rehabil. Med. 2018, 54, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Castelli, L.; De Giglio, L.; Haggiag, S.; Traini, A.; De Luca, F.; Ruggieri, S.; Prosperini, L. Premorbid Functional Reserve Modulates the Effect of Rehabilitation in Multiple Sclerosis. Neurol. Sci. 2020, 41, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-W.; Lin, L.-F.; Tam, K.-W.; Tsai, C.-P.; Hong, C.-H.; Kuan, Y.-C. Efficacy of Robot-Assisted Gait Training in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2020, 41, 102034. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Manfredini, F.; Lamberti, N.; Zamboni, P.; Bernardi, F.; Marchetti, G.; Pinton, P.; Bonora, M.; Secchiero, P.; Tisato, V.; et al. The Effectiveness of Robot-Assisted Gait Training versus Conventional Therapy on Mobility in Severely Disabled progressIve MultiplE Sclerosis Patients (RAGTIME): Study Protocol for a Randomized Controlled Trial. Trials 2017, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Young, C.A. A Medical Definition of Fatigue in Multiple Sclerosis. QJM 2008, 101, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.; Hogan, N.; Larkin, A.; Saunders, J.; Jakeman, P.; Coote, S. Exercise in the Community for People with Minimal Gait Impairment Due to MS: An Assessor-Blind Randomized Controlled Trial. Mult. Scler. 2013, 19, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Heine, M.; van de Port, I.; Rietberg, M.B.; van Wegen, E.E.H.; Kwakkel, G. Exercise Therapy for Fatigue in Multiple Sclerosis. Cochrane Database Syst. Rev. 2015, 9, CD009956. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, N.; Manfredini, F.; Baroni, A.; Crepaldi, A.; Lavezzi, S.; Basaglia, N.; Straudi, S. Motor Cortical Activation Assessment in Progressive Multiple Sclerosis Patients Enrolled in Gait Rehabilitation: A Secondary Analysis of the RAGTIME Trial Assisted by Functional Near-Infrared Spectroscopy. Diagnostics 2021, 11, 1068. [Google Scholar] [CrossRef]
- Manjaly, Z.-M.; Harrison, N.A.; Critchley, H.D.; Do, C.T.; Stefanics, G.; Wenderoth, N.; Lutterotti, A.; Müller, A.; Stephan, K.E. Pathophysiological and Cognitive Mechanisms of Fatigue in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 642–651. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Galea, M. Management of Fatigue in Persons with Multiple Sclerosis. Front. Neurol. 2014, 5, 177. [Google Scholar] [CrossRef] [PubMed]
- Tur, C. Fatigue Management in Multiple Sclerosis. Curr. Treat. Options Neurol. 2016, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Sepulcre, J.; Masdeu, J.C.; Goñi, J.; Arrondo, G.; Vélez de Mendizábal, N.; Bejarano, B.; Villoslada, P. Fatigue in Multiple Sclerosis Is Associated with the Disruption of Frontal and Parietal Pathways. Mult. Scler. 2009, 15, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Pardini, M.; Bonzano, L.; Mancardi, G.L.; Roccatagliata, L. Frontal Networks Play a Role in Fatigue Perception in Multiple Sclerosis. Behav. Neurosci. 2010, 124, 329–336. [Google Scholar] [CrossRef]
- Penner, I.-K. Evaluation of Cognition and Fatigue in Multiple Sclerosis: Daily Practice and Future Directions. Acta Neurol. Scand. 2016, 134 (Suppl. S200), 19–23. [Google Scholar] [CrossRef] [PubMed]
- Zackowski, K.M.; Freeman, J.; Brichetto, G.; Centonze, D.; Dalgas, U.; DeLuca, J.; Ehde, D.; Elgott, S.; Fanning, V.; Feys, P.; et al. Prioritizing Progressive MS Rehabilitation Research: A Call from the International Progressive MS Alliance. Mult. Scler. 2021, 27, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Kos, D.; Kerckhofs, E.; Nagels, G.; D’hooghe, M.B.; Ilsbroukx, S. Origin of Fatigue in Multiple Sclerosis: Review of the Literature. Neurorehabilit. Neural Repair 2008, 22, 91–100. [Google Scholar] [CrossRef]
- Buss, S.S.; Fried, P.J.; Macone, J.; Zeng, V.; Zingg, E.; Santarnecchi, E.; Pascual-Leone, A.; Bartrés-Faz, D. Greater Cognitive Reserve Is Related to Lower Cortical Excitability in Healthy Cognitive Aging, but Not in Early Clinical Alzheimer’s Disease. Front. Hum. Neurosci. 2023, 17, 1193407. [Google Scholar] [CrossRef] [PubMed]
- Hechenberger, S.; Helmlinger, B.; Penner, I.-K.; Pirpamer, L.; Fruhwirth, V.; Heschl, B.; Ropele, S.; Wurth, S.; Damulina, A.; Eppinger, S.; et al. Psychological Factors and Brain Magnetic Resonance Imaging Metrics Associated with Fatigue in Persons with Multiple Sclerosis. J. Neurol. Sci. 2023, 454, 120833. [Google Scholar] [CrossRef]
- Rottoli, M.; La Gioia, S.; Frigeni, B.; Barcella, V. Pathophysiology, Assessment and Management of Multiple Sclerosis Fatigue: An Update. Expert Rev. Neurother. 2017, 17, 373–379. [Google Scholar] [CrossRef]
- Téllez, N.; Río, J.; Tintoré, M.; Nos, C.; Galán, I.; Montalban, X. Does the Modified Fatigue Impact Scale Offer a More Comprehensive Assessment of Fatigue in MS? Mult. Scler. 2005, 11, 198–202. [Google Scholar] [CrossRef] [PubMed]

| CRI < 115 (n = 36) | CRI ≥ 115 (n = 17) | Total (n = 53) | p-Value | |
|---|---|---|---|---|
| sex (M/F) (a) | 10/26 | 8/9 | 18/35 | 0.16 |
| MS type (PP/SP) (a) | 19/17 | 7/10 | 26/27 | 0.43 |
| treatment (CT/RAGT) (b) | 16/20 | 10/7 | 26/27 | 0.33 |
| age (years) (b) | 54.0 [46–60.3] | 60 [56–63] | 56 [48–63] | 0.02 |
| MS years (b) | 12 [7.50–20.5] | 19 [12–25] | 13.5 [8–21.3] | 0.13 |
| EDSS (b) | 6.50 [6–6.50] | 6.50 [6–6.50] | 6.50 [6–6.5] | 0.21 |
| CRI < 115 (n = 36) | CRI ≥ 115 (n = 17) | Total(n = 53) | p-Value | |
|---|---|---|---|---|
| 6MWT T0 (m) | 147 [68.3–260] | 106 [32–157] | 130 [60–237] | 0.05 |
| Speed25FW T0 (speed, m s−1) | 0.558 [0.328–0.748] | 0.427 [0.121–0.566] | 0.495 [0.288–0.731] | 0.06 |
| Berg T0 | 40.0 [26.8–49.5] | 30 [18–49] | 39 [25–49] | 0.24 |
| PHQ9 T0 | 9.00 [5–13] | 5 [3–11] | 8 [5–12] | 0.09 |
| FSS T0 | 6.28 [5.52–6.66] | 5.77 [4.77–6.66] | 6.11 [5.33–6.66] | 0.74 |
| MSWS12 T0 | 47.5 [42.5–55] | 49 [43–55] | 49 [43–55] | 0.98 |
| MSIS29mot T0 | 62.5 [50.5–75] | 56 [53–75] | 61 [51–75] | 0.82 |
| MSIS29psyc T0 | 23 [16.8–31] | 19 [14–25] | 23 [16–27] | 0.06 |
| T0 | T1 | p-Value ANCOVA ∆T1-T0 | |||
|---|---|---|---|---|---|
| CRI < 115 (n = 36) | CRI ≥ 115 (n = 17) | CRI < 115 (n = 36) | CRI ≥ 115 (n = 17) | ||
| Speed25FW (speed, m s−1) | 0.558 [0.328–0.748] | 0.427 [0.121–0.566] | 0.552 [0.344–0.967] | 0.420 [0.192–0.661] | 0.42 |
| 6MWT (m) | 147 [68.3–260] | 106 [32–157] | 160 [78.0–288] | 128 [41.2–202] | 0.83 |
| Berg | 40.0 [26.8–49.5] | 30 [18–49] | 43.0 [30.3–52.0] | 33 [26.0–46.0] | 0.74 |
| PHQ9 | 9.00 [5–13] | 5 [3–11] | 6.00 [3.75–10.00] | 5 [2.00–6.00] | 0.89 |
| FSS | 6.28 [5.52-6.66] | 5.77 [4.77–6.66] | 6.00 [5.30–6.36] | 4.77 [3.33–6.11] | 0.01 |
| MSWS12 | 47.5 [42.5-55] | 49 [43–55] | 42.5 [31.8–51.0] | 42 [33.0–51.0] | 0.28 |
| MSIS29mot | 62.5 [50.5–75] | 56 [53–75] | 59.5 [45.0–67.5] | 55 [38.0–66.0] | 0.24 |
| MSIS29psyc | 23 [16.8-31] | 19 [14–25] | 19.0 [14.0–27.0] | 17 [9.00–23.0] | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzeri, A.; Lamberti, N.; Baroni, A.; Basaglia, N.; Bergonzoni, A.; Stablum, F.; Manfredini, F.; Straudi, S. The Cognitive Reserve May Influence Fatigue after Rehabilitation in Progressive Multiple Sclerosis: A Secondary Analysis of the RAGTIME Trial. Sclerosis 2024, 2, 108-116. https://doi.org/10.3390/sclerosis2020008
Balzeri A, Lamberti N, Baroni A, Basaglia N, Bergonzoni A, Stablum F, Manfredini F, Straudi S. The Cognitive Reserve May Influence Fatigue after Rehabilitation in Progressive Multiple Sclerosis: A Secondary Analysis of the RAGTIME Trial. Sclerosis. 2024; 2(2):108-116. https://doi.org/10.3390/sclerosis2020008
Chicago/Turabian StyleBalzeri, Ambra, Nicola Lamberti, Andrea Baroni, Nino Basaglia, Antonella Bergonzoni, Franca Stablum, Fabio Manfredini, and Sofia Straudi. 2024. "The Cognitive Reserve May Influence Fatigue after Rehabilitation in Progressive Multiple Sclerosis: A Secondary Analysis of the RAGTIME Trial" Sclerosis 2, no. 2: 108-116. https://doi.org/10.3390/sclerosis2020008
APA StyleBalzeri, A., Lamberti, N., Baroni, A., Basaglia, N., Bergonzoni, A., Stablum, F., Manfredini, F., & Straudi, S. (2024). The Cognitive Reserve May Influence Fatigue after Rehabilitation in Progressive Multiple Sclerosis: A Secondary Analysis of the RAGTIME Trial. Sclerosis, 2(2), 108-116. https://doi.org/10.3390/sclerosis2020008








