Pseudobulbar Affect in Patients with Multiple Sclerosis: A Systematic Review
Abstract
1. Introduction
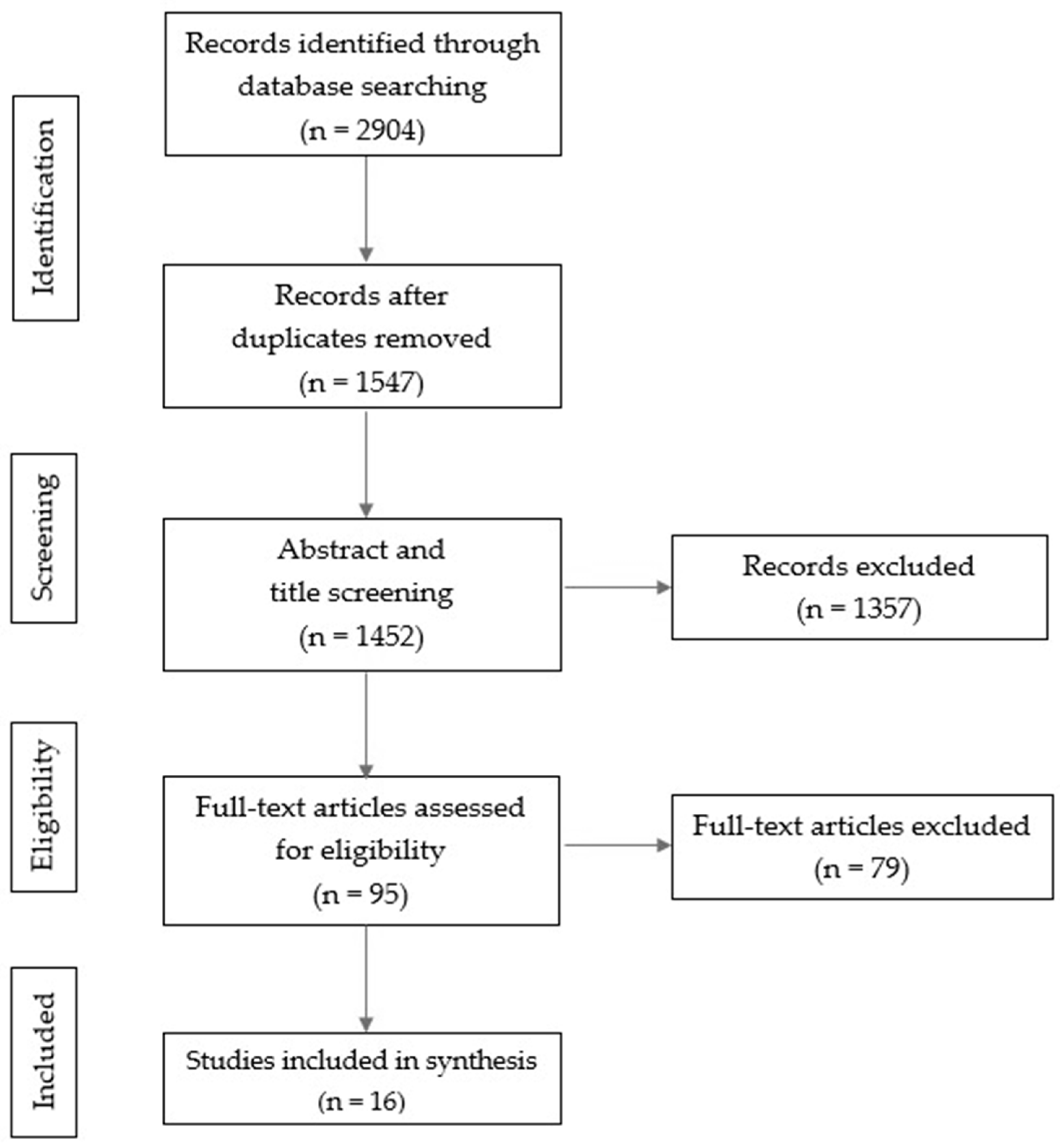
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Article Selection Process
2.4. Assessment of Methodological Quality and Risk of Bias
3. Results
3.1. Characteristics of the Reviewed Studies
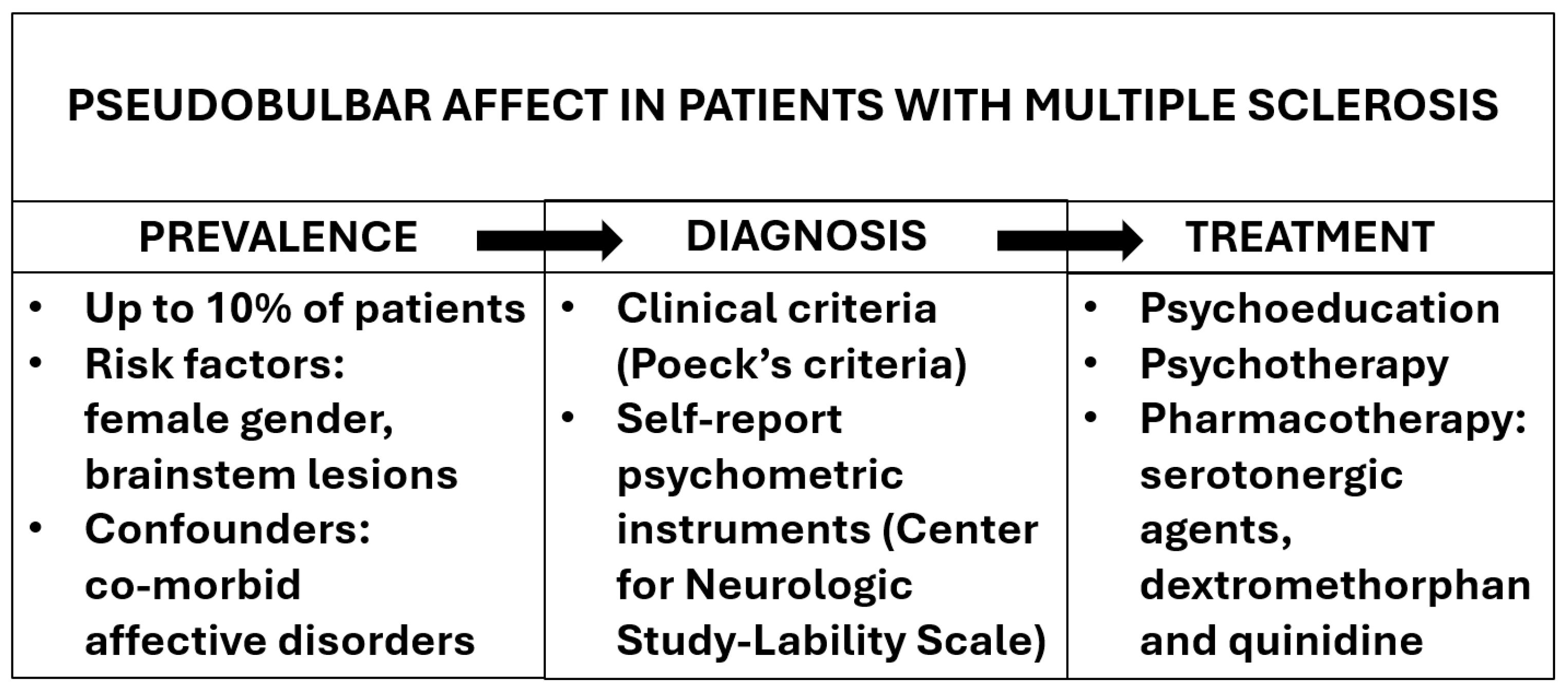
3.2. Prevalence of Pseudobulbar Affect
3.3. Clinical Correlates of Pseudobulbar Affect
3.3.1. Cognitive and Affective Symptoms
3.3.2. Quality of Life and Disability
3.4. Pathophysiology of Pseudobulbar Affect
3.4.1. Clinical Course
3.4.2. Structural Neuroimaging
3.4.3. Functional Neuroimaging
3.5. Treatment of Pseudobulbar Affect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poser, C.M.; Paty, D.W.; Scheinberg, L.; McDonald, W.I.; Davis, F.A.; Ebers, G.C.; Johnson, K.P.; Sibley, W.A.; Silberberg, D.H.; Tourtellotte, W.W. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann. Neurol. 1983, 13, 227–231. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Paparrigopoulos, T.; Ferentinos, P.; Kouzoupis, A.; Koutsis, G.; Papadimitriou, G.N. The neuropsychiatry of multiple sclerosis: Focus on disorders of mood, affect and behaviour. Int. Rev. Psychiatry 2010, 22, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Minden, S.L.; Feinstein, A.; Kalb, R.C.; Miller, D.; Mohr, D.C.; Patten, S.B.; Bever, C.; Schiffer, R.B., Jr.; Gronseth, G.S.; Narayanaswami, P. Guideline Development Subcommittee of the American Academy of Neurology. Evidence-based guideline: Assessment and management of psychiatric disorders in individuals with MS: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 82, 174–181. [Google Scholar] [PubMed]
- Raimo, S.; Santangelo, G.; Trojano, L. The emotional disorders associated with multiple sclerosis. Handb. Clin. Neurol. 2021, 183, 197–220. [Google Scholar] [PubMed]
- Murphy, R.; O’Donoghue, S.; Counihan, T.; McDonald, C.; Calabresi, P.A.; Ahmed, M.A.; Kaplin, A.; Hallahan, B. Neuropsychiatric syndromes of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Filser, M.; Buchner, A.; Fink, G.R.; Gold, S.M.; Penner, I.K. The manifestation of affective symptoms in multiple sclerosis and discussion of the currently available diagnostic assessment tools. J. Neurol. 2023, 270, 171–207. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Arciniegas, D.B.; Brooks, B.R.; Herndon, R.M.; Lauterbach, E.C.; Pioro, E.P.; Robinson, R.G.; Scharre, D.W.; Schiffer, R.B.; Weintraub, D. Defining and diagnosing involuntary emotional expression disorder. CNS Spectr. 2006, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wortzel, H.S.; Oster, T.J.; Anderson, C.A.; Arciniegas, D.B. Pathological laughing and crying: Epidemiology, pathophysiology and treatment. CNS Drugs 2008, 22, 531–545. [Google Scholar] [CrossRef]
- Parvizi, J.; Coburn, K.L.; Shillcutt, S.D.; Coffey, C.E.; Lauterbach, E.C.; Mendez, M.F. Neuroanatomy of pathological laughing and crying: A report of the American Neuropsychiatric Association Committee on Research. J. Neuropsychiatry Clin. Neurosci. 2009, 21, 75–87. [Google Scholar] [CrossRef]
- Ahmed, A.; Simmons, Z. Pseudobulbar affect: Prevalence and management. Ther. Clin. Risk Manag. 2013, 9, 483–489. [Google Scholar] [PubMed]
- Iacovides, A.; Andreoulakis, E. Bipolar disorder and resembling special psychopathological manifestations in multiple sclerosis: A review. Curr. Opin. Psychiatry 2011, 24, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.; Gracey, F.; Trigg, E.; Broomfield, N. Predictors and correlates of emotionalism across acquired and progressive neurological conditions: A systematic review. Neuropsychol. Rehabil. 2023, 33, 945–987. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Ali, F.; Leckman, J.F.; Robertson, M.M. Pathological laughter in Gilles de la Tourette syndrome: An unusual phonic tic. Mov. Disord. 2010, 25, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Poeck, K. Handbook of Clinical Neurology; Academic Press: Cambridge, MA, USA, 1969. [Google Scholar]
- Moore, S.R.; Gresham, L.S.; Bromberg, M.B.; Kasarkis, E.J.; Smith, R.A. A self report measure of affective lability. J. Neurol. Neurosurg. Psychiatry 1997, 63, 89–93. [Google Scholar] [CrossRef]
- Robinson, R.G.; Parikh, R.M.; Lipsey, J.R.; Starkstein, S.E.; Price, T.R. Pathological laughing and crying following stroke: Validation of a measurement scale and a double-blind treatment study. Am. J. Psychiatry 1993, 150, 286–293. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Rogers, F.B. Medical subject headings. Bull. Med. Libr. Assoc. 1963, 51, 114–116. [Google Scholar]
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare; University of York: York, UK, 2008. [Google Scholar]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews; ESRC Methods Programme: Swindon, UK, 2006. [Google Scholar]
- National Heart, Lung, and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies; Department of Health and Human Services, National Institutes of Health: Bethesda, MD, USA, 2014.
- Miller, J. The Scottish intercollegiate guidelines network (SIGN). Br. J. Diabetes Vasc. Dis. 2002, 2, 47–49. [Google Scholar] [CrossRef]
- Haiman, G.; Pratt, H.; Miller, A. Brain responses to verbal stimuli among multiple sclerosis patients with pseudobulbar affect. J. Neurol. Sci. 2008, 271, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Work, S.S.; Colamonico, J.A.; Bradley, W.G.; Kaye, R.E. Pseudobulbar affect: An under-recognized and under-treated neurological disorder. Adv. Ther. 2011, 28, 586–601. [Google Scholar] [CrossRef]
- Colamonico, J.; Formella, A.; Bradley, W. Pseudobulbar affect: Burden of illness in the USA. Adv. Ther. 2012, 29, 775–798. [Google Scholar] [CrossRef]
- Brooks, B.R.; Crumpacker, D.; Fellus, J.; Kantor, D.; Kaye, R.E. PRISM: A novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS ONE 2013, 8, 8. [Google Scholar] [CrossRef]
- Vidović, V.; Rovazdi, M.Č.; Kraml, O.; Kes, V.B. Pseudobulbar affect in multiple sclerosis patients. Acta Clin Croat. 2015, 54, 159–163. [Google Scholar]
- Fitzgerald, K.C.; Salter, A.; Tyry, T.; Fox, R.J.; Cutter, G.; Marrie, R.A. Pseudobulbar affect: Prevalence and association with symptoms in multiple sclerosis. Neurol. Clin. Pract. 2018, 8, 472–481. [Google Scholar] [CrossRef]
- Özer, D.; Ata, E.E.; Dikeç, G.; Demir, S. The relationship between stress, anxiety, and depression levels and pseudobulbar affect in patients with multiple sclerosis. Contemp. Nurse 2022, 58, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, A.; Feinstein, K.; Gray, T.; O’Connor, P. Prevalence and neurobehavioral correlates of pathological laughing and crying in multiple sclerosis. Arch. Neurol. 1997, 54, 1116–1121. [Google Scholar] [CrossRef]
- Feinstein, A.; O’Connor, P.; Gray, T.; Feinstein, K. Pathological laughing and crying in multiple sclerosis: A preliminary report suggesting a role for the prefrontal cortex. Mult. Scler. 1999, 5, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, O.; Chamelian, L.; Feinstein, A. Neuroanatomy of pseudobulbar affect: A quantitative MRI study in multiple sclerosis. J. Neurol. 2008, 255, 406–412. [Google Scholar] [CrossRef]
- Haiman, G.; Pratt, H.; Miller, A. Effects of dextromethorphan/quinidine on auditory event-related potentials in multiple sclerosis patients with pseudobulbar affect. J. Clin. Psychopharmacol. 2009, 29, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Feinstein, A.; Morrow, S.A. The association of pathological laughing and crying and cognitive impairment in multiple sclerosis. J. Neurol. Sci. 2016, 361, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Luhoway, J.A.; Sharma, M.; Menon, S.; Rosehart, H.; Morrow, S.A. Posterior fossa lesion load and pathological laughing and crying in multiple sclerosis. Int. J. MS Care 2019, 21, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.N.; Johri, S.; Gorthi, S.P.; Dubey, A.K.; Sharma, J.R.; Ramdas, G.V.; Yadav, K.K. Pathological laughter, multiple sclerosis, behavioural abnormality. Med. J. Armed Forces India 2006, 62, 383–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, B.; Nichols, S. Crying and suicidal, but not depressed. Pseudobulbar affect in multiple sclerosis successfully treated with valproic acid: Case report and literature review. Palliat. Support. Care 2015, 13, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Panitch, H.S.; Thisted, R.A.; Smith, R.A.; Wynn, D.R.; Wymer, J.P.; Achiron, A.; Vollmer, T.L.; Mandler, R.N.; Dietrich, D.W.; Fletcher, M.; et al. Pseudobulbar Affect in Multiple Sclerosis Study Group. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann. Neurol. 2006, 59, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, K.A.; Morrow, S.A.; Sellers, E.M. Evaluating the safety and efficacy of dextromethorphan/quinidine in the treatment of pseudobulbar affect. Neuropsychiatr. Dis. Treat. 2014, 10, 1161–1174. [Google Scholar] [CrossRef][Green Version]
- Yang, L.P.H.; Deeks, E.D. Dextromethorphan/quinidine: A review of its use in adults with pseudobulbar affect. Drugs 2015, 75, 83–90. [Google Scholar] [CrossRef]
| Study | Country | Setting | Study Design | MS Sample (F, %) | MS Age (Mean, Range) | MS Diagnosis | PBA Measure | QATOCCS Rating | SIGN Score |
|---|---|---|---|---|---|---|---|---|---|
| Feinstein et al., 1997 [32] | Canada | Specialist clinic | Case–control | 152 (NA) | 45 (NA) | Poser criteria | Poeck’s criteria; PLACS | Fair | 2+ |
| Feinstein et al., 1999 [33] | Canada | Specialist clinic | Case–control | 152 (NA) | 45 (NA) | Poser criteria | Poeck’s criteria; PLACS | Good | 2++ |
| Swamy et al., 2006 [38] | India | Specialist clinic | Case report | 1 male | 38 | NA | Clinical assessment | Poor | 3 |
| Panitch et al., 2006 [40] | America | Specialist clinics | Double-blind, placebo-controlled trial | 150 (124 F, 83%) | 45 (NA) | McDonald criteria | CNS-LS > 13 | Good | 1+ |
| Ghaffar et al., 2007 [34] | Canada | Specialist clinic | Case–control | 28 (19 F, 68%) | 47 (NA) | McDonald criteria | Poeck’s criteria | Fair | 2+ |
| Haiman et al., 2008 [25] | Israel | Specialist clinic | Cross-sectional | 22 (14 F, 64%) | 46 (23–63) | Poser criteria | CNS-LS > 15 | Good | 2+ |
| Haiman et al., 2009 [35] | Israel | Specialist clinic | Case–control | 6 (5 F, 80%) | 49 (32–60) | Poser criteria | CNS-LS > 17 | Good | 2++ |
| Work et al., 2011 [26] | USA | Registry | Cross-sectional | 504 (NA) | NA | NA | PLACS > 13 CNS-LS > 13 CNS-LS > 21 | Fair | 2+ |
| Colamonico et al., 2012 [27] | USA | Online survey | Cross-sectional | 173 (145 F, 84%) | 49 (NA) | NA | CNS-LS > 13 | Fair | 2++ |
| Brooks et al., 2013 [28] | USA | Registry | Cross-sectional | 1215 (979 F, 81%) | 49 (NA) | NA | CNS-LS > 13 CNS-LS > 21 | Fair | 1+ |
| Johnson and Nichols, 2015 [39] | USA | Specialist clinic | Case report | 1 female | 60 | NA | Poeck’s criteria | Poor | 3 |
| Vidović et al., 2015 [29] | Croatia | Specialist clinic | Cross-sectional | 79 (48 F, 61%) | 49 (21–71) | McDonald criteria | CNS-LS > 17 | Fair | 2- |
| Hanna et al., 2016 [36] | Canada | Specialist clinic | Retrospective chart review | 153 (119 F, 78%) | 46 (21–58) | McDonald criteria | CNS-LS > 17 | Fair | 2- |
| Fitzgerald et al., 2018 [30] | USA | Registry | Cross-sectional | 8136 (6312 F, 78%) | 57 (NA) | NARCOMS registry | CNS-LS > 13 CNS-LS > 17 CNS-LS > 21 | Good | 1++ |
| Luhoway et al., 2019 [37] | Canada | Specialist clinic | Retrospective chart review | 77 (51 F, 66%) | 39 (18–65) | NA | CNS-LS > 17 | Fair | 2- |
| Özer et al., 2022 [31] | Turkey | Online survey | Cross sectional | 442 (336 F, 76%) | 34 (16–65) | McDonald criteria | CNS-LS > 15 | Fair | 2- |
| Study | PBA Prevalence (F, %) | PBA Age (Mean, Range) | PBA Features | PBA Correlates | Psychiatric Co-Morbidities |
|---|---|---|---|---|---|
| Feinstein et al., 1997 [32] | 11 (10%) (7 F, 64%) | 44 (NA) | Laughing and crying | Lower IQ; higher EDSS | Anxiety and affective symptoms |
| Feinstein et al., 1999 [33] | 11 (10%) (7 F, 64%) | 44 (NA) | Laughing and crying | Lower IQ; chronic progressive MS | Depression |
| Swamy et al., 2006 [38] | 1 male | 38 | Laughing | NA | NA |
| Panitch et al., 2006 [40] | 150 (124 F, 83%) | 45 (NA) | Laughing or crying | NA | Excluded |
| Ghaffar et al., 2007 [34] | 14 (9 F, 64%) | 47 (NA) | Laughing or crying | Higher number of hyperintense lesions | NA |
| Haiman et al., 2008 [25] | 11 (7 F, 64%) | 47 (30–60) | Laughing or crying | Lower IQ; greater activation in response to neutral stimuli in somatosensory and motor areas | NA |
| Haiman et al., 2009 [35] | 6 (5 F, 80%) | 49 (32–60) | Laughing or crying | Early and late event-related potential effects in response to subjectively significant stimuli | Excluded |
| Work et al., 2011 [26] | PLACS > 13: 49 (10%) (NA) CNS-LS > 13: 231 (47%) (NA) CNS-LS > 21: 49 (10%) (NA) | NA | Laughing or crying | NA | NA |
| Colamonico et al., 2012 [27] | 73 (42%) (60 F, 82%) | 49 (NA) | Laughing or crying | Lower QoL; increased rate of depression; increased burden for caregivers | Depression |
| Brooks et al., 2013 [28] | CNS-LS > 13: 556 (46%) (NA) CNS-LS > 21: 145 (12%) (NA) | 49 (NA) | Laughing and crying | Lower QoL; increased use of antidepressant and antipsychotic pharmacotherapy | NA |
| Johnson and Nichols, 2015 [39] | 1 female | 60 | Crying | NA | NA |
| Vidović et al., 2015 [29] | 33 (42%) (24 F, 73%) | 49 (NA) | Laughing or crying | NA | NA |
| Hanna et al., 2016 [36] | 58 (38%) (NA) | NA | Laughing or crying | Lower IQ; lower number of years of education; higher EDSS; relapsing-remitting MS | Anxiety and depression |
| Fitzgerald et al., 2018 [30] | CNS-LS > 13: 1740 (21%) (1427 F, 82%) CNS-LS > 17: 574 (7%) (476 F, 83%) CNS-LS > 21: 174 (2%) (154 F, 89%) | CNS-LS > 13: 56 (NA) CNS-LS > 17: 54 (NA) CNS-LS > 21: 53 (NA) | Laughing and crying | Lower IQ; lower number of years of education; higher rates of depression | Depression |
| Luhoway et al., 2019 [37] | 22 (NA) | NA | Laughing and crying | Lower number of years of education; fewer lesions in the posterior fossa | Depression |
| Özer et al., 2022 [31] | 280 (NA) | NA | Laughing or crying | Lower number of years of education; higher rates of anxiety and depression | Anxiety and depression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiereghin, S.; Purpura, G.; Riva, A.; Nacinovich, R.; Cavanna, A.E. Pseudobulbar Affect in Patients with Multiple Sclerosis: A Systematic Review. Sclerosis 2024, 2, 186-198. https://doi.org/10.3390/sclerosis2030013
Chiereghin S, Purpura G, Riva A, Nacinovich R, Cavanna AE. Pseudobulbar Affect in Patients with Multiple Sclerosis: A Systematic Review. Sclerosis. 2024; 2(3):186-198. https://doi.org/10.3390/sclerosis2030013
Chicago/Turabian StyleChiereghin, Silvia, Giulia Purpura, Anna Riva, Renata Nacinovich, and Andrea Eugenio Cavanna. 2024. "Pseudobulbar Affect in Patients with Multiple Sclerosis: A Systematic Review" Sclerosis 2, no. 3: 186-198. https://doi.org/10.3390/sclerosis2030013
APA StyleChiereghin, S., Purpura, G., Riva, A., Nacinovich, R., & Cavanna, A. E. (2024). Pseudobulbar Affect in Patients with Multiple Sclerosis: A Systematic Review. Sclerosis, 2(3), 186-198. https://doi.org/10.3390/sclerosis2030013






