Abstract
Background/Objectives: Since the emergence of the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the discovery of compounds with antiviral potential from medicinal plants has been extensively researched. This study aimed to investigate plant metabolites with in vitro inhibitory potential against SARS-CoV-2 targets, including 3CLpro, PLpro, Spike protein, and RdRp. Methods: A systematic review was conducted following PRISMA guidelines, with literature searches performed in six electronic databases (Scielo, ScienceDirect, Scopus, Springer, Web of Science, and PubMed) from January 2020 to February 2024. Computational analyses using SwissADME, pkCSM, ADMETlab, ProTox3, Toxtree, and DataWarrior were performed to predict the absorption, distribution, metabolism, excretion, and toxicity (ADMET) profiles as well as other medicinal chemistry parameters of these compounds. Results: A total of 330 plant-derived compounds with inhibitory activities against the proposed targets were identified, with compounds showing IC50 values as low as 0.01 μM. Our findings suggest that several plant metabolites exhibit significant in vitro inhibition of SARS-CoV-2 targets; however, few molecules exhibit drug development viability without further adjustments. Additionally, after these evaluations, two phenolic acids, salvianic acid A and protocatechuic acid methyl ester, stood out for their potential as candidates for developing antiviral therapies, with IC50 values of 2.15 μM against 3CLpro and 3.76 μM against PLpro; respectively; and satisfactory in silico drug-likeness and ADMET profiles. Conclusions: These results reinforce the importance of plant metabolites as potential targets for antiviral drug discovery.
1. Introduction
As COVID-19 cases persist into 2025, the World Health Organization (WHO) continues to monitor cases and emerging variants of SARS-CoV-2, the virus responsible for the disease. Since the outbreak began in China, over 777 million cases and 7.0 million deaths have been reported worldwide [1,2]. In March 2023, the WHO updated its tracking system and variant classification to better reflect the evolving landscape. SARS-CoV-2 variants are now categorized as Variants of Concern (VOCs), Variants of Interest (VOIs), and Variants Under Monitoring (VUMs) [3]. The Centers for Disease Control and Prevention (CDC) has been tracking SARS-CoV-2 evolution through national genomic surveillance since December 2020, revealing that the Omicron lineages, characterized by increased transmissibility and immune escape, continue to evolve [4].
Emerging and re-emerging infectious diseases are appearing with increasing frequency, posing a growing threat to global health [5,6]. The risk of future pandemics, potentially more severe than COVID-19, is both real and imminent [7]. Research has consistently shown that the cost of preventing infectious diseases is significantly lower than that of managing them, particularly on a global scale [8,9,10].
The COVID-19 pandemic exemplifies this ongoing challenge. Despite worldwide vaccination programs, infections continue to rise due to emerging variants, some of which may reduce vaccine efficacy [11,12]. This highlights the urgent need for effective antiviral drugs alongside vaccines to control and reduce cases [13,14].
Given the rapid evolution and mutagenicity of these viruses, proactive measures are essential. Expanding efforts to understand the origin, transmission, and molecular targets of infectious diseases are crucial for developing effective prevention and treatment strategies. Strengthening research in this field will ultimately help mitigate the impact of future outbreaks and enhance global preparedness [15,16].
Research on SARS-CoV-2 has progressed rapidly, particularly regarding its molecular mechanism and pathogenesis. Highly exploited drug targets for COVID-19 include structural proteins such as Spike protein (S protein) and non-structural proteins (NSPs), including papain-like protease (PLpro), RNA-dependent RNA polymerase (RdRp), and main protease (Mpro), known as 3-chymotrypsin-like protease (3CLpro) [13,17]. By understanding the proteins involved in SARS-CoV-2 infection, researchers can use this information to identify appropriate drugs for COVID-19 management [18].
According to the WHO, 70–90% of the world’s population currently depends on traditional medicine as their primary healthcare means [19]. A considerable number of antiviral agents extracted from herbal species have been used in several studies. In addition, several complex herbal medicines have been used in clinical research to treat coronavirus-related symptoms [20,21].
Natural products serve as excellent sources for discovering antiviral agents due to their diversity and complexity and can offer remarkable efficacy and specificity to target viral infections [22,23]. Plant metabolites are a good source for anti-COVID-19 drug research since these compounds have demonstrated the capacity to inhibit viral invasion and replication and modulate the immune-inflammatory response [12,24,25].
Several computational methods have been used for the discovery and development of various drugs. In recent decades, modeling of in silico ADMET (absorption, distribution, metabolism, excretion, and toxicity) has garnered significant attention as a rational drug design tool [26,27]. The cost-effectiveness and high-throughput nature of these models facilitate a streamlined drug development process, enabling the prediction of compound accessibility, guided hit identification, and structural optimization [28].
Several reviews have emphasized the in silico anti-SARS-CoV-2 potential of plant metabolites [29,30,31,32,33]. However, this is the first systematic review focusing on the molecular targets of plant metabolites that act as anti-SARS-CoV-2 agents. Furthermore, the ADMET properties of these compounds were predicted to identify potential drug candidates.
2. Results and Discussion
2.1. Search Results
PubMed, Scielo, Science Direct, Scopus, Springer, and Web of Science were used to perform the search from January 2020 to February 2024 (Figure 1). A total of 2760 papers were identified during the identification stage of the search. Science Direct presented the larger number of studies, representing over 43.1% of the total, followed by Springer (37.6%), Scopus (11.5%), PubMed (4.4%), Web of Science (2.5%) and Scielo (0.9%). Of the 2760 records, 1373 were duplicates. After removal, 1387 papers were used in the next step.
The first screening was performed with 1387 papers by analyzing their titles, abstracts, and keywords. Based on the inclusion criteria for selecting studies that provided data on the plant-derived compounds that inhibited the RBD:ACE2 interaction and the 3CLpro/Mpro, PLpro, and RdRp activities, 820 studies followed the next screening, and 567 were removed. For the second screening, after evaluating the complete texts of the articles, 764 records were removed, and 56 papers were considered valid; thus, they were suitable for inclusion and data extraction.
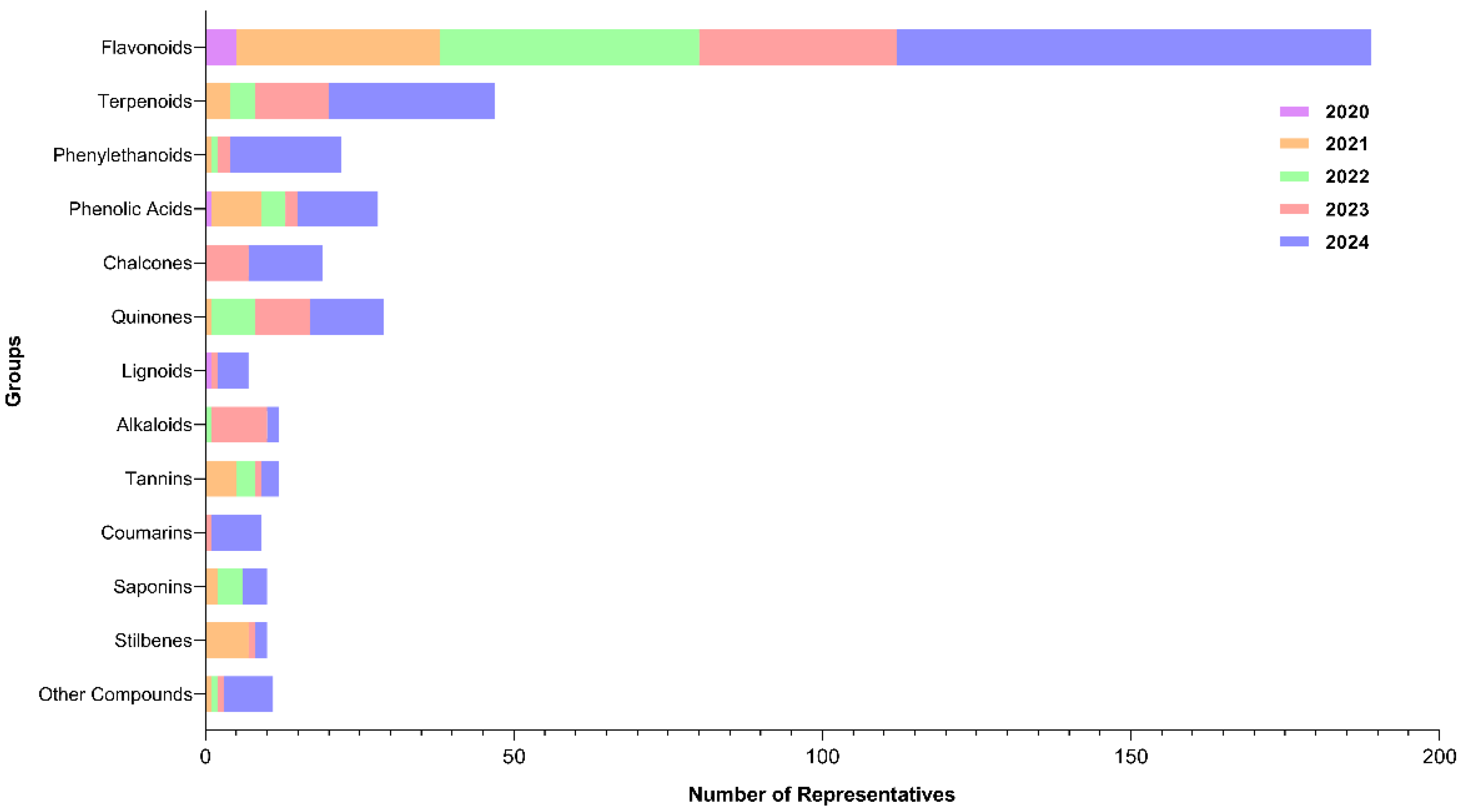
A total of 405 bibliographic citations, distributed across 13 groups, were used. There was a successive increase from 2020 to 2024, starting with 1.7% and reaching 47.2% of citations that addressed the inhibitory activity of metabolites against SARS-CoV-2 Flavonoids and terpenoids were the most representative groups with higher percentages, accounting for 46.7% and 10.6% of the total citations, respectively (Supplementary Table S1; Figure 2).
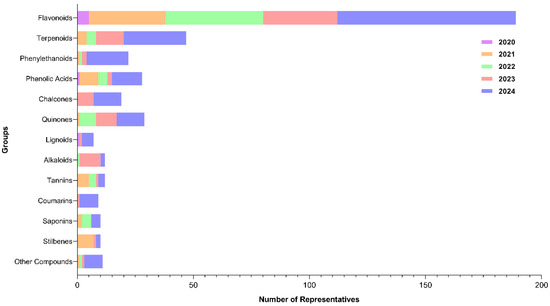
Figure 2.
Graphical representation of the number of citations between 2020 and 2024 for each chemical class with inhibitory potential against different SARS-CoV-2 targets.
2.2. Study Characteristics
2.2.1. Plant Compounds Evaluated as Anti-SARS-CoV-2
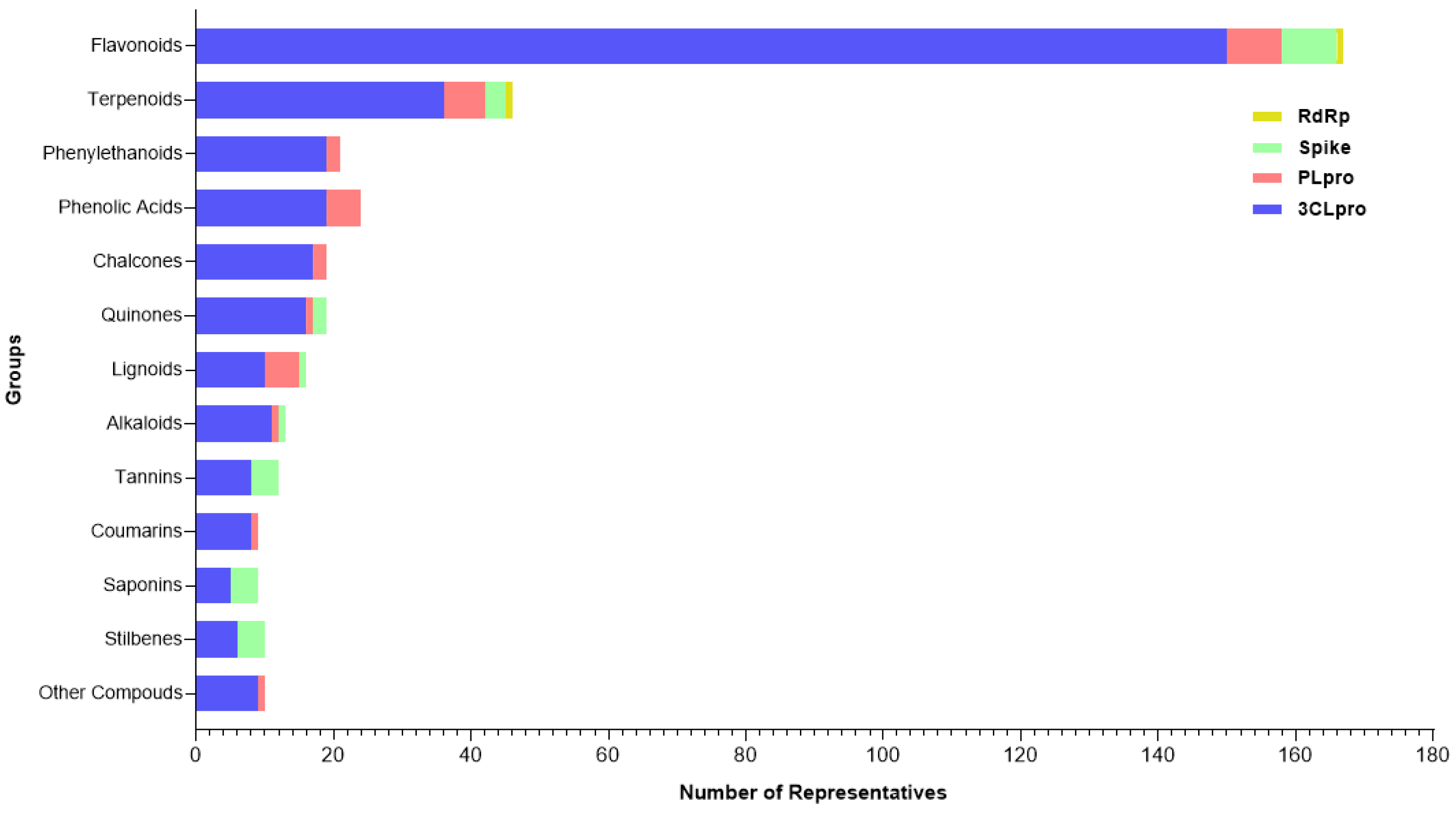
Following data extraction from the reviewed valid papers, 334 plant-derived compounds were identified to act on the proposed SARS-CoV-2 targets. These molecules could be classified into four categories, some of which included subclasses. Regarding to the major classes, the search revealed 20 different groups (Figure 3), among flavonoids (n = 145), terpenoids (n = 41), phenylethanoids (n = 20), phenolic acids (n = 19), chalcones (n = 19), quinones (n = 18), lignoids (n = 12), alkaloids (n = 13), tannins (n = 10), coumarins (n = 9), saponins (n = 8), stilbenes (n = 7), and other compounds: steroids (n = 2), curcuminoids (n = 2), capsaicinoids (n = 2), organic acids (n = 1), benzaldehyde (n = 1), aurones (n = 1), coumestans (n = 1) and benzophenones (n = 1).
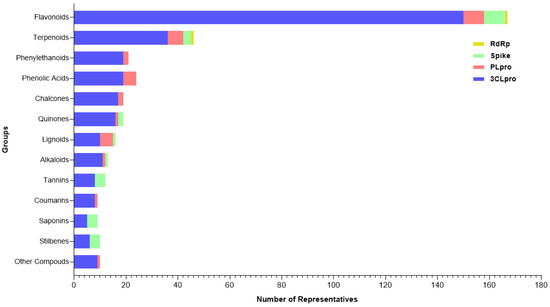
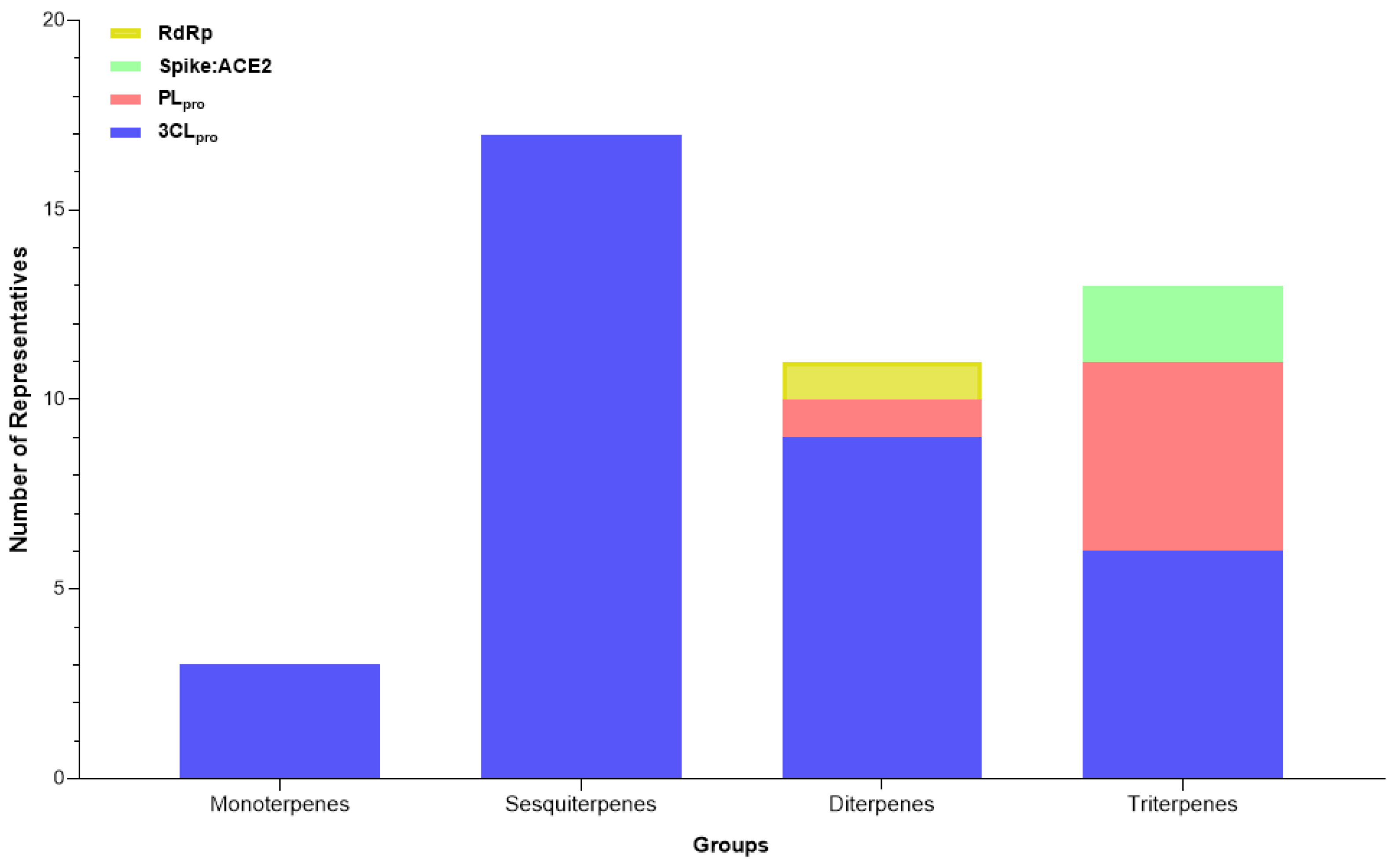
Figure 3.
Categories of compounds and their number of representatives tested against selected targets of SARS-CoV-2 (3CLpro, PLpro, Spike:ACE2, and RdRp), tracked by the review.
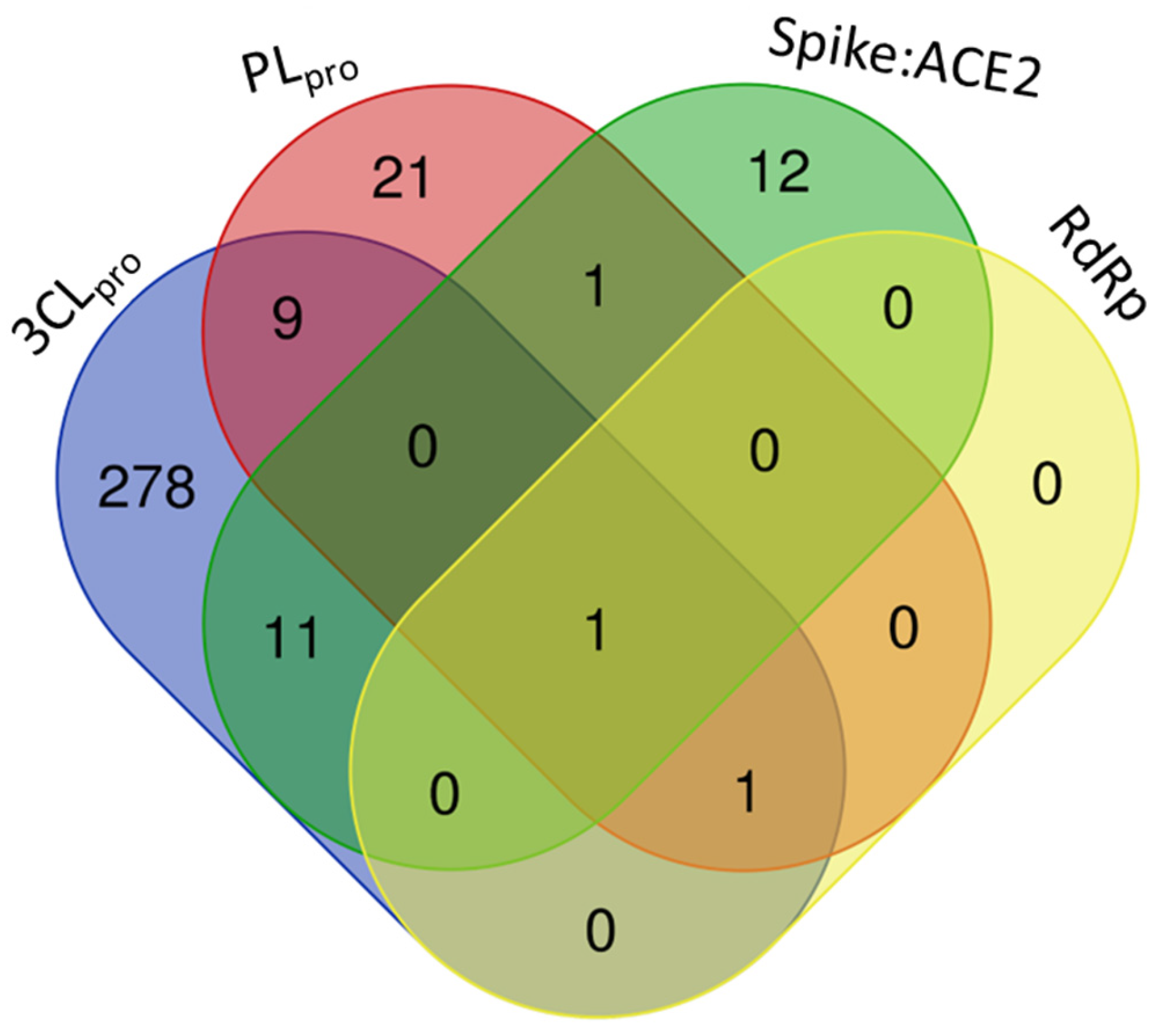
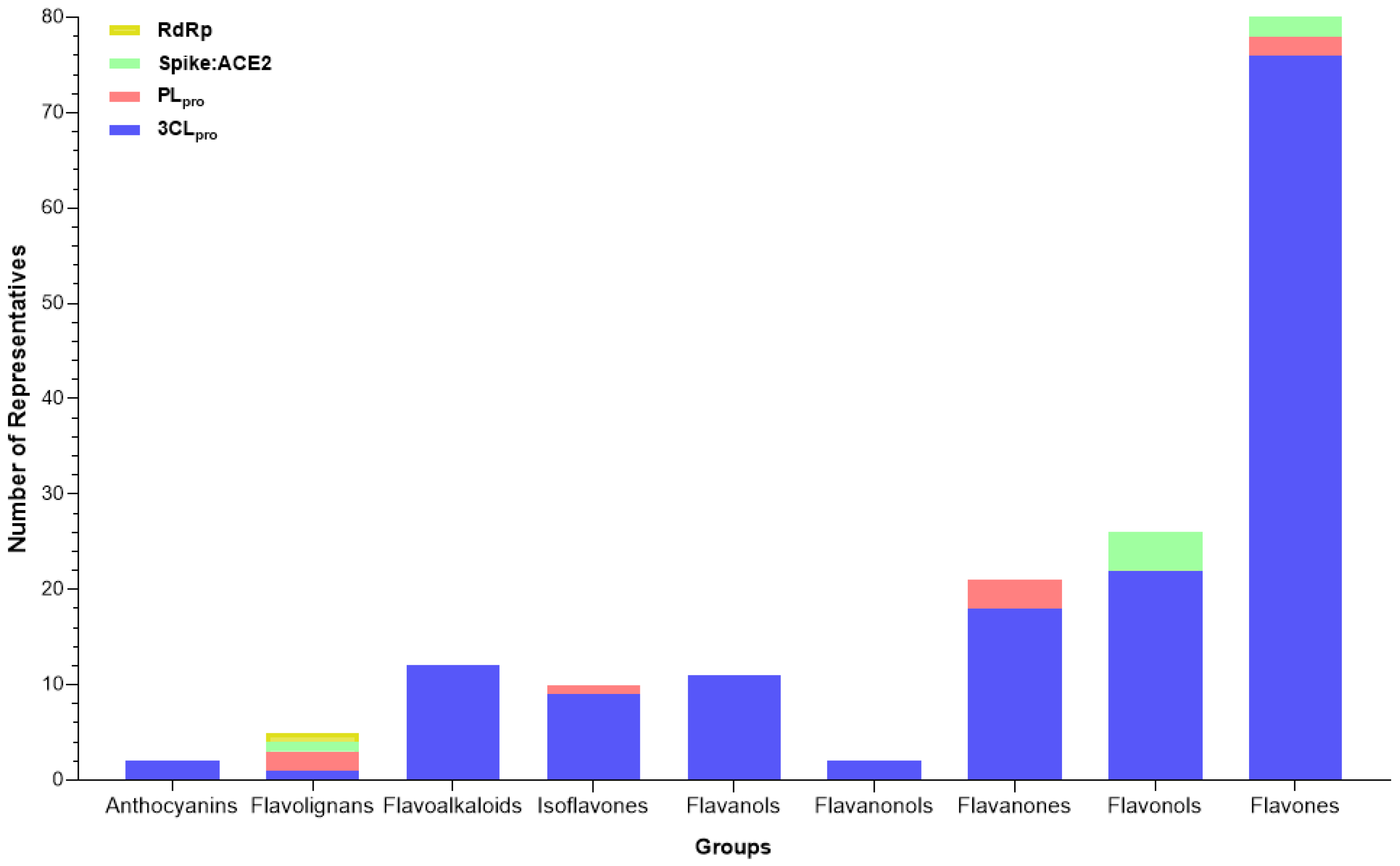
Among the target molecules analyzed, there was a clear concentration of studies focused on 3CLpro, which accounted for 84.4% of the citations (Supplementary Table S2). In contrast, lower percentages were observed for PLpro (8.1%), Spike protein (6.9%), and RNA-dependent RNA polymerase (RdRp), which represented only 0.5%, indicating a significant knowledge gap in the investigation of enzymes involved in viral RNA replication. This trend was also reflected in the experimental assays reported, with 301 compounds tested for 3CLpro inhibition, followed by 33 for PLpro, 25 targeting the Spike-ACE2 interaction, and only two compounds evaluated for RdRp inhibition (Figure 4).
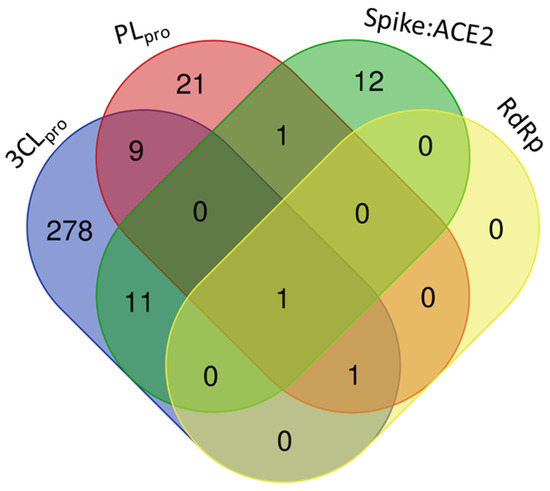
Figure 4.
Number of compounds tested against each selected target of SARS-CoV-2 (3CLpro, PLpro, Spike:ACE2, and RdRp), tracked by the review.
Overall, more than half (n = 172) of the compounds were based on their IC50 values. Across all targets, 21 molecules (6%) exhibited significant anti-SARS-CoV-2 effects, as indicated by IC50 values that were lower than those of the reference compounds. The lowest IC50 found was sennoside B (0.01 μM), against 3CLpro, followed by 11-keto-β-boswellic acid (1.1 μM), vaticanol B (0.07 μM) and silibilin (20.3 μM), for PLpro, Spike:ACE2 interaction and RdRp, respectively.
Among the multitarget molecules—those exhibiting inhibitory activity at more than one stage of the SARS-CoV-2 replicative cycle, notable examples include kaempferitrin, kuwanon A, luteolin, myricetin, quercetin, and glycyrrhizin, which possess both anti-spike and anti-3CLpro activities. In contrast, honokiol displays multitarget activity against Spike and PLpro. Furthermore, silibinin demonstrated inhibitory effects on all four targets studied.
2.2.2. Controls and References Evaluated as Anti-SARS-CoV-2
More than 65% (n = 39) of the selected papers included internal controls for the assays. These molecules were divided into the following categories: protein inhibitors (n = 19), anti-inflammatory drugs (n = 7), antiviral drugs (n = 5), natural products (n = 4), synthetic products (n = 4), recombinant antibodies (n = 3), and dyes (n = 1). Some records employed more than one category; however, most papers had one reference for each target.
Out of the ten articles examining the inhibition of the Spike:ACE2 interaction, seven employed some form of reference. The majority (>50%) used antibodies that neutralize the spike protein, thereby preventing the interaction, which is the most employed form of reference for these assays. For the records aiming at RdRp inhibition, only one used a specific control, remdesivir, which belongs to the antiviral category. The use of this reference is justified by its mechanism of action, aimed directly at the action of SARS-CoV-2 polymerase.
The highest prevalence of references was found in the category of protein inhibitors (>48%), which can be attributed to the critical role of viral proteases in the replication cycle of SARS-CoV-2 and the greater number of records of 3CLpro inhibition. Notably, the 3CLpro (the main protease) of SARS-CoV-2 is essential for processing viral polyproteins into functional units, making it an indispensable target for antiviral intervention. Consequently, many studies have focused on screening natural products against 3CLpro to identify potent inhibitors that can disrupt the viral life cycle.
2.2.3. SARS-CoV-2 Targets
SARS-CoV-2 is a positive-strand RNA virus (+ssRNA) with a genome of approximately 30 kb and is one of the largest pathogenic viruses [34]. Its genome primarily consists of two Open Reading Frames (ORFs), ORF1a and ORF1ab, which encode pp1a and pp1ab polyproteins. These polyproteins are proteolytically processed by viral proteases to generate non-structural proteins (nsps) that play essential roles in viral replication and transcription [35,36]. Additionally, the genome produces subgenomic RNAs that encode structural and accessory proteins [37]. Among structural proteins, spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins are crucial for virion assembly, release, and host cell interactions [38]. Given their key functions in viral infection and replication, both non-structural and structural proteins are promising targets for therapeutic strategies against SARS-CoV-2 and COVID-19 [36]. After data extraction from the valid records, four targets were analyzed: the spike (RBD):ACE2 interaction, 3CLpro and PLpro proteases, and RNA polymerase (RdRp).
The primary focus of most reviewed articles was the enzymatic inhibition of 3CLpro and PLpro proteases. Upon entry into the host cell, the viral genome is translated into two polyproteins: pp1a (~450 kDa, nsp1-11) and pp1ab (~750 kDa, nsp1-16). These polyproteins are cleaved by the viral proteases nsp3 (PLpro) and nsp5 (3CLpro), generating 16 non-structural proteins (nsps) that play essential roles in viral replication and transcription [39,40]. These proteases undergo self-cleavage to cleave other nsps [41].
3CLpro of SARS-CoV-2 is a cysteine protease that shares 97% sequence similarity with the 3CLpro of SARS-CoV [42]. This enzyme plays a crucial role in viral replication by mediating the co-translational and post-translational cleavage of the pp1a/pp1ab polyproteins at 11 sites on the conserved amino acid sequences Leu-Gln↓Ser-AlaGly playing [40,43,44]. Due to its crucial role in the viral life cycle, 3CLpro is a promising target for developing and identifying new drugs, either as viral inhibitors or through drug repositioning.
Moreover, the absence of human proteins homologous to 3CLpro is an advantage in the development of inhibitors, as it reduces the risk of adverse side effects on human proteases [45]. Various approaches have been utilized to inhibit 3CLpro, including drug repurposing, high-throughput virtual screening (HTVS), high-throughput screening (HTS), and structure- and ligand-based drug design (SBDD and LBBD) [46,47,48,49,50,51,52]. Notably, research focusing on natural products for the development of 3CLpro inhibitors has gained significant attention [53,54].
3CLpro is a homodimer comprising three domains: I (residues 10–99), II (residues 100–182), and III (residues 198–303). Domains I and II feature six barrel structures arranged in antiparallel β-strands, while domain III consists of five α-helices, which are involved in the dimerization of 3CLpro [55,56]. This 33.79 kDa protein consists of 306 amino acid residues per monomer, with the homodimer being the catalytically active form of the protease [57].
The substrate-binding site of 3CLpro comprises four subsites (S1, S1′, S2, and S4), forming a “butterfly-shaped” pocket. Established 3CLpro inhibitors occupy all these subsites. Promising compounds identified via docking were predicted to form hydrogen bonds with Leu141, Asn142, and His163 in S1 and exhibit lipophilic interactions in S2. They were also expected to form hydrogen bonds with Thr26 and Glu166 in S1′ and S4, respectively. These interactions are essential for ligand binding [58].
The catalytic dyad of 3CLpro is composed of Cys145 and His41, with additional conserved amino acid residues interacting with the substrate at the catalytic site (Thr24, Thr25, Cys44, Met49, Tyr54, Phe140, Asn142, Gly143, His163, His164, Met165, Glu166, Leu167, Pro168, Asp187, Arg188, Gln189, and Thr190). This broadens the application of inhibitory compounds to other coronaviruses [59,60,61,62,63].
PLpro is one of the eight domains of nsp3, a large (212 kDa) multidomain protein that is essential for the formation of the replication/transcription complex [64,65]. PLpro is located on residues 746–1060 of nsp3, consists of 315 amino acids (36 kDa), and is highly conserved among coronaviruses, sharing 83% identity with SARS-CoV PLpro [66,67]. SARS-CoV-2 PLpro is a cysteine protease with four domains, and the catalytic triad is located between the thumb (62–178) and palm (241–315) domains and consists of Cys111, His272, and Asp286 [68,69]. The recognition site is a consensus sequence of Leu-X-Gly Gly ↓XX between nsp1-4 [70,71]. Like 3CLpro, PLpro functions as a homodimer, with the substrate-binding sites of the protomers positioned close to each other [72].
PLpro, in addition to its proteolytic function, plays a crucial role in immune evasion and the modulation of inflammatory responses by de-ubiquitination and de-ISGylation, processes that suppress the IFN-I signaling pathway [42,73,74,75,76,77,78,79,80,81,82,83]. This action of PLpro is a key factor in the suppression of the host immune system by SARS-CoV-2, as highlighted by Mahmoudvand and Shokri [84]. Specifically, PLpro removes post-translational modifications such as ubiquitination and ISG-ylation, which are essential for initiating the antiviral immune response [66,67].
Due to its critical role in the viral replication cycle, SARS-CoV-2 PLpro has emerged as a promising target for antiviral therapies. Several inhibitors of SARS-CoV-2 PLpro were initially identified as inhibitors of the SARS-CoV PLpro. One notable example is GRL0617, discovered by Ratia et al. [85], which has since become one of the most widely studied inhibitors of SARS-CoV-2 PLpro, acting as a reversible competitive inhibitor [85,86,87].
The interaction between the Spike protein and its receptor ACE2 was the focus of 17% (n = 10) of the articles covered in this review. Similar to other enveloped viruses, as with many human pathogenic viruses, coronaviruses have unique proteins responsible for recognition and binding to the host cell receptor and initiation of cell entry [88,89]. In the case of SARS-CoV-2 infection, this process is mediated by the interaction between the spike protein and angiotensin-converting enzyme 2 (ACE2) on the cell surface, which is widely spread in different types of tissues, including the lungs, heart, kidneys, and intestines [38,90]. There are two possible routes for SARS-CoV-2 to enter permissive cells: one mediated by endosomes and the other mediated by proteases on the cell surface. Both begin with the recognition and binding of the spike protein to ACE2 via the receptor-binding domain (RBD) [36,38,90].
Spike protein is a structural glycoprotein with a molecular weight of 180–200 kDa that is common to all coronaviruses and is formed by 1273 amino acids [90,91]. It is found in the homotrimeric form and creates a crown-like ornamentation around the viral particle, which has been named the coronavirus group [90]. Structurally, each monomer of the Spike protein is composed of two functional subunits, S1 and S2, which are bound together and have different roles during the viral entry process [36]. The S1 subunit contains the RBD, which is crucial for the ability of the virus to recognize and attach to the host cell. Between SARS-CoV and SARS-CoV-2 RBDs, there is more than 70% similarity, and although mutations in this region are listed for the variants that emerged over time, its affinity with ACE2 is considerably preserved [36,38,90,92]. The RBD is the primary target for neutralizing antibodies [36,38,90], which is composed of a core structure formed by a five-stranded antiparallel β-sheet and an extended loop known as the receptor-binding motif (RBM), which directly interacts with ACE2, facilitating viral entry into the host cell [38,93].
As the Spike protein plays a crucial role in viral infection, initiating the process of cell entry, the interaction between the RBD and ACE2 has been a major focus of therapeutic strategies, including the development of neutralizing antibodies, small-molecule inhibitors, and vaccines. Since 2020, extensive research, including investigations into natural products, has focused on identifying molecules that inhibit Spike protein binding to ACE2, emphasizing the importance of this interaction as a critical target to block viral entry and thus reduce disease progression [21,94,95,96,97,98].
The nsp12 of SARS-CoV-2, also known as RNA-dependent RNA polymerase (RdRp), is the most conserved enzyme among various species of viruses, including influenza, hepatitis C virus (HCV), Zika virus (ZIKV), and coronaviruses (CoV). The RdRp of SARS-CoV-2 shares 96% similarity with that of SARS-CoV, with primary structural differences located in the catalytically inactive region [99]. RdRp is the main enzyme responsible for viral replication, and in coronaviruses, it catalyzes the synthesis of the RNA genome using the positive RNA strand as a template to produce a complementary negative RNA strand from the 3′ poly-A tail. Because RdRp is indispensable in the life cycle of RNA viruses, it has become a crucial target for combating various viral infections [99,100]. Considering COVID-19, Remdesivir, an adenosine triphosphate analog, acts as an RdRp inhibitor that directly interferes with viral replication. Initially developed for other viral infections, its mechanism of action enabled its successful repurposing as the first drug approved for the treatment of COVID-19 [101,102,103].
Structurally, RdRp features a highly conserved polymerase domain resembling a cupped right hand, with three interconnected subdomains: fingers (residues L366 to A581 and K621 to G679), palm (residues T582 to P620 and T680 to Q815), and thumb (residues H816 to E920) [100,104]. The active site of the SARS-CoV-2 RdRp domain is composed of conserved polymerase motifs A–G within the palm domain, following a structural arrangement similar to that of other RNA polymerases [104]. In addition, RdRp does not function alone, as it requires interaction with nsp7 and nsp8 to enhance enzymatic activity and processivity. The nsp7-nsp8 complex acts as a cofactor, forming a processivity clamp that stabilizes RdRp on the RNA template, ensuring efficient and continuous RNA synthesis [100].
The critical roles of these four targets in the SARS-CoV-2 lifecycle make them prime candidates for therapeutic interventions, as highlighted by multiple studies in this review. Many of these components are highly conserved across coronaviruses, underscoring their relevance as potential therapeutic targets. Natural products have long provided bioactive compounds with diverse mechanisms of action, and their potential to inhibit key viral functions in SARS-CoV-2 emphasizes the need for further exploration, particularly for isolated compounds targeting multiple viral components. This review reinforces the growing evidence supporting natural products as valuable sources for discovering inhibitors that may effectively reduce viral replication and spread, offering accessible and sustainable treatment options.
2.2.4. Evaluation Methods for SARS-CoV-2 Targets
Among the selected articles, the evaluation of the inhibitory activity of substances against proteases, particularly 3CLpro, stands out the most. Therefore, the most frequently cited methodology is the Fluorescent Resonance Energy Transfer (FRET) assay, comprising around 90% of the selected articles. The prevalence of interaction assays between the spike protein and its receptor ACE2 using commercial kits was observed, representing 17% (n = 10) of the total, and finally, primer-dependent assays for RNA-dependent RNA polymerase with 4% (n = 2).
Spike:ACE2 binding assays are widely used for screening samples regarding their antibody titers and nature and for discovering inhibitors, especially due to spike protein size and complexity. They offer results obtained quickly and in a miniaturized manner, using both 96- and 384-well plates, allowing a vast range of dilutions and making these approaches suitable for HTS-like platforms [105,106,107].
Typically, these kits use the receptor-binding domain (RBD) of the Spike protein, which is around 26 kDa, instead of trimeric and whole Spike proteins. Briefly, one of the reagents, Spike RBD or ACE2, and the samples to be investigated are incubated together. A second reagent is then added to the reaction. Detection is performed after incubation (30 min to 2 h) based on the assay principle. The kits rely on fluorescent, luminescent, or colorimetric principles, which is a straightforward method of reading the response in a microplate reader [96,105,107,108].
FRET assays are commonly employed for viral protease studies, covering a spectrum of human immunodeficiency virus type I (HIV), enterovirus (EV), hepatitis C virus (HCV), and various coronaviruses [109,110]. These assays are particularly valuable for screening enzymatic inhibitors targeting proteases from SARS-CoV-2, SARS-CoV, and MERS-CoV due to their effective indirect assessment of proteolytic activity. They detect enzyme-substrate interactions with high sensitivity, allowing for the real-time monitoring of enzymatic activity and detailed tracking of inhibitor-enzyme interactions. The adaptability of FRET assays supports automation, adjustment to diverse pH ranges, minimal reaction volumes, and flexible modulation of the enzyme and substrate concentrations. This versatility enables the rapid screening of multiple inhibitors across varying concentrations, such as in 96-well plates, facilitating detailed studies of enzyme kinetics and optimization of experimental conditions to evaluate inhibitor efficacy and mechanisms of action. Moreover, the assay specificity toward targeted enzymes is enhanced through the use of substrates containing enzyme-specific cleavage sequences, which is critical for validating and complementing data obtained from molecular docking and rational drug design strategies. This integrated approach combines experimental and computational methodologies to advance the development of viral protease inhibitors.
FRET assay is a quantitative method based on the quenching phenomenon between two fluorophores. Its principle relies on a distance-dependent physical phenomenon that transfers energy from a donor fluorophore to an acceptor fluorophore [111]. In FRET assays, when two fluorophores (donor and acceptor) are nearby, energy transfer from the donor to the acceptor results in quenching, reducing the fluorescence of the donor and increasing the fluorescence of the acceptor. This process is highly efficient when the distance between the particles is less than 10 nm. When the peptide connecting the two fluorophores is cleaved, they separate, increasing the distance between the donor and acceptor. This results in a decrease in FRET efficiency and, consequently, an increase in donor fluorescence, as less energy is transferred to the acceptor and a reduction in acceptor fluorescence due to reduced energy reception. FRET assays are commonly used for viral protease assays, including those for human immunodeficiency virus type I (HIV), enterovirus (EV), hepatitis C virus (HCV), and other coronaviruses [109,110].
In these studies, the donor and acceptor fluorophores were connected by a short peptide containing the protease cleavage site, and the emission wavelength of the donor fluorophore must overlap with the absorption wavelength of the acceptor fluorophore. Thus, peptide cleavage results in the separation of donor and acceptor fluorophores by diffusion, a decrease in FRET efficiency (quenching), and an increase in the donor’s fluorescence emission over time, which can be measured and related to enzymatic activity. This can be quantified by comparing the enzymatic reaction product formation under control conditions and in the presence of an inhibitor [112].
Although RdRps are the main target for the development of antivirals due to their indispensable role in the replicative cycles of RNA viruses, there are some difficulties related to their expression, purification, and catalytic activity in vitro, which may be reflected in the very few articles tracked by the review. One strategy used for SARS-CoV involves using accessory factors (nsp7 and nsp8) complexed with nsp12, which increases the template-binding activity and RdRp processivity [100].
2.2.5. Anti-SARS-CoV-2 Effects
Overall, more than half (n = 172) of the compounds were based on their IC50 values. Across all targets, 21 molecules (6%) exhibited significant anti-SARS-CoV-2 effects, as indicated by IC50 values that were lower than those of the reference compounds. The lowest IC50 found was sennoside B (0.01 μM), against 3CLpro, followed by 11-keto-β-boswellic acid (1.1 μM), vaticanol B (0.07 μM) and silibilin (20.3 μM), for PLpro, Spike:ACE2 and RdRp interaction, respectively.
Among the multitarget molecules—those exhibiting inhibitory activity at more than one stage of the SARS-CoV-2 replicative cycle, notable examples include kaempferitrin, kuwanon A, luteolin, myricetin, quercetin, and glycyrrhizin, which possess both anti-spike and anti-3CLpro activities. In contrast, Honokiol displays multitarget activity against Spike and PLpro. Furthermore, the potential of silibinin is particularly noteworthy, as it demonstrates inhibitory effects on all four targets studied: Spike, 3CLpro, PLpro, and RdRp, indicating its promise as a broad-spectrum antiviral agent.
2.3. Plant Metabolites Against SARS-CoV-2
Potential binding inhibitors developed using natural compounds represent safe and powerful treatment options for coronavirus. Consequently, these natural medicines may be used to treat various signs and symptoms of SARS-CoV-2 infection and other coronaviruses as alternative therapies when no specific antiviral medicine is currently available. Furthermore, natural products are essential for infection prevention, particularly in high-risk patients with coronavirus transmission [20]. In the following section, the current research results on compounds against SARS-CoV-2 are discussed, and their specific molecular targets are summarized (Supplementary Table S3). Structures with undefined stereochemistry are represented as such due to the lack of relative or absolute stereochemical information in the original sources.
2.3.1. Phenolic Acids
Phenolic acids are widely distributed in the plant kingdom. They can be categorized into two groups: benzoic acid derivatives (hydroxybenzoic acids) (C6-C1) and cinnamic acid derivatives (hydroxycinnamic acids) (C6-C3), also known as phenylpropanoids [113]. Studies have shown that the carbonyl and hydroxyl groups of phenolic acids play important roles in forming strong binding interactions with SARS-CoV-2 proteins [114].
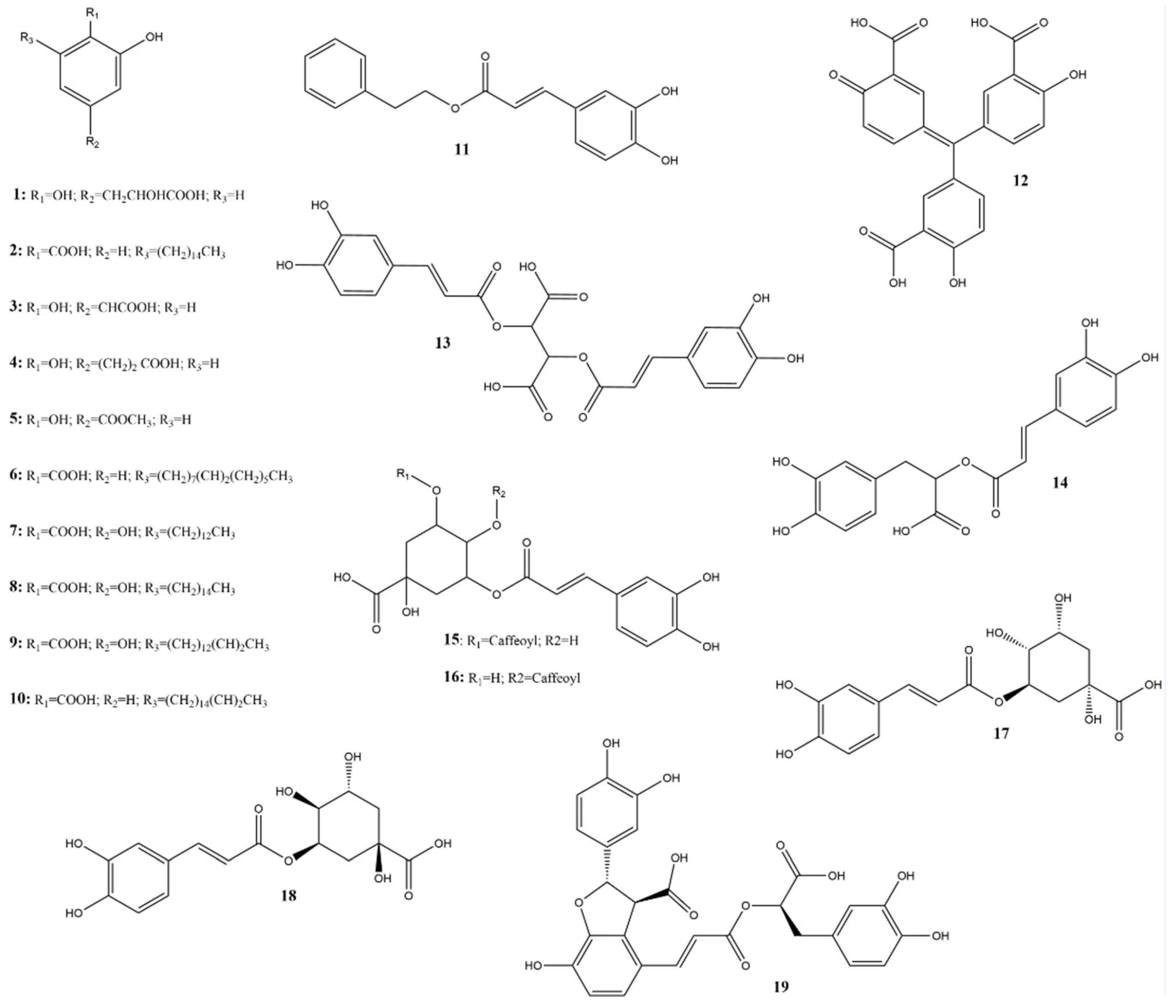
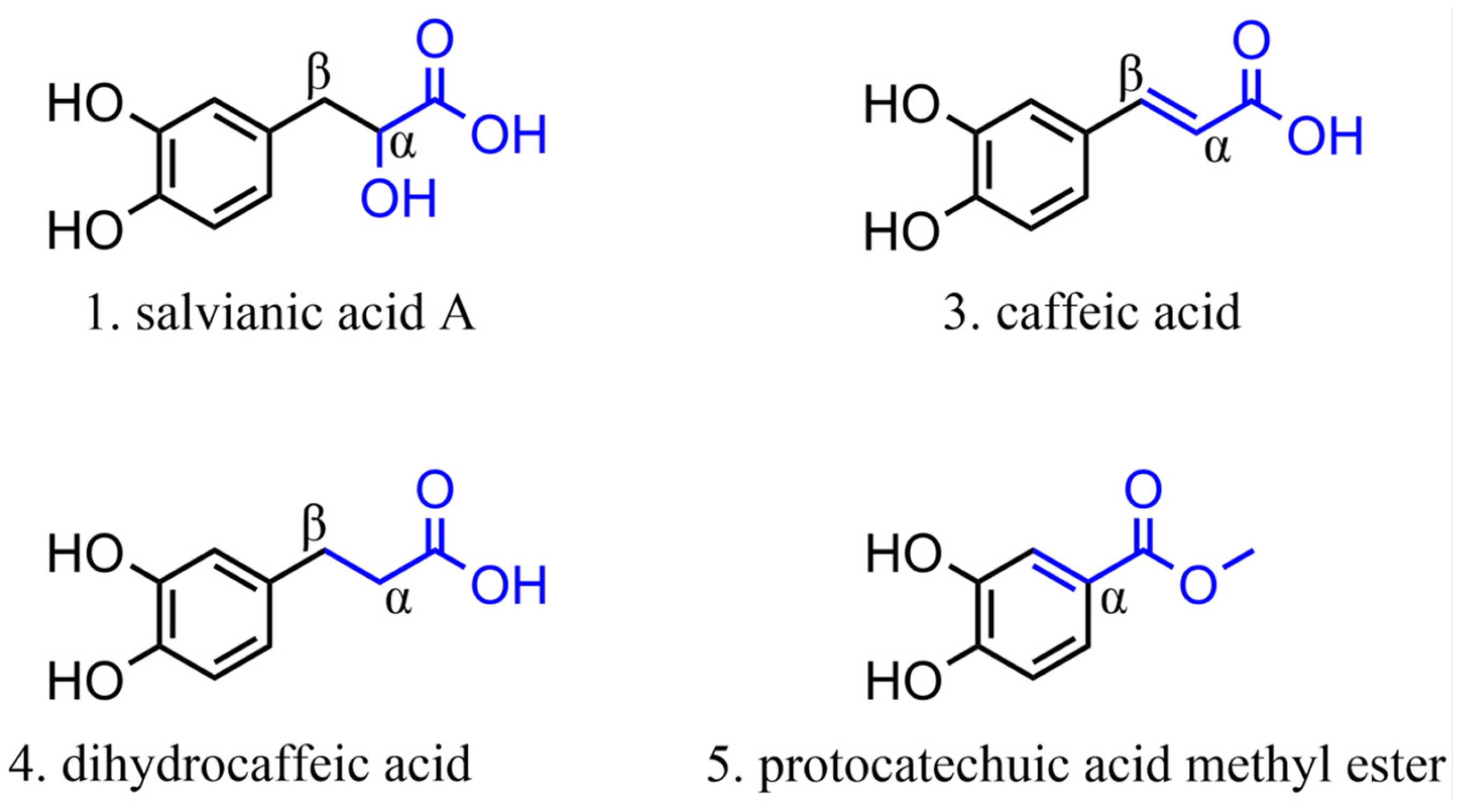
Among the phenolic acids analyzed, 19 compounds (1–19) (Figure 5) were selected and examined, with a focus on the molecular targets of these plant metabolites as anti-SARS-CoV-2 agents.
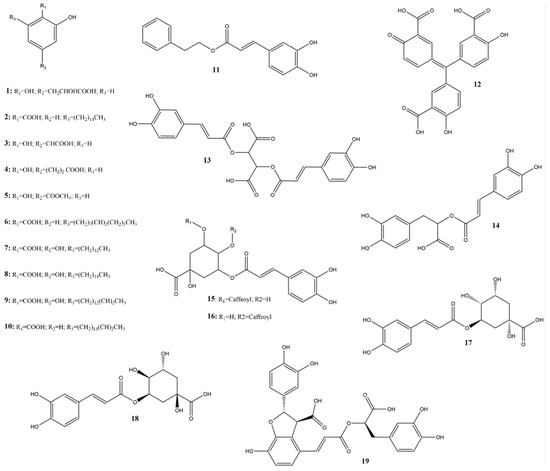
Figure 5.
Chemical structures of phenolic acids tested against SARS-CoV-2 targets.
Twelve molecules were examined for 3CLpro activity, showing inhibition rates between 0% and 50% at a concentration of 100 µM and IC50 values ranging from 0.7 to 394 µM. The compound with the highest inhibition value was caffeic acid (3), which showed 98.07% inhibition at a concentration of 100 μM. This compound, containing a catechol structure, was identified by FRET-based screening [115].
The rosmarinic acid (13), mentioned in two articles [115,116], has an IC50 value of 6.84 µM for inhibiting 3CLpro. Krüger et al. [117] suggest that the inhibition occurs through covalent modification between Cys145 and the Michael acceptor donor, contrary to the initial theory that orthoquinone is formed by oxidation of the phenolic hydroxyl groups of compound 13.
Chlorogenic acid (16) showed the lowest inhibition rate of 22.81% at a concentration of 100 µM (3CLpro) [115]. Compound 16, for instance, in the study by Shafiq et al. [118], performed through molecular docking, mainly interacted with the RBD via hydrogen bonds. Interestingly, it has been reported that hydrogen bond formation is important for selectivity and optimization of compound structures, maximizing interactions with target proteins [119,120]. This suggested that it binds strongly and stably to the protein.
Salvianic acid A (18) and lithospermic acid (19) have shown antiviral potential against SARS-CoV-2 by inhibiting its main protease (3CLpro). For compound 19, one study reported an IC50 of 4.92 μM with 50% inhibition [121], while another found 88.74% inhibition at 100 μM [115]. This variation suggests that the efficacy of compound 19 may depend on specific experimental conditions or variations in assay methodologies. Despite discrepancies in the adequate concentrations, both studies indicate its therapeutic potential. For compound 18, the observed IC50 was 2.33 µM, demonstrating significant activity of this molecule in inhibiting 3CLpro, suggesting great potential as an anti-SARS-CoV-2 agent, possibly acting on viral replication. However, there is no further information in the literature regarding the in silico potential of these molecules during in vitro (viral cell model) or in vivo assays.
Anacardic acid (2) was tested by Hicks et al. [122] and Chen et al. [123]. Hicks et al. [122] reported 96.10% inhibition at a concentration of 100 μM. However, additional dose-response curve analyses revealed an IC50 value of 6.8 μM, indicating that its potential toxicity may hinder its development as an antiviral agent.
In the study by Chen et al. [123], compound 2 was tested for both proteases, showing only 50% inhibition of 3CLpro and PLpro. The cytotoxicity assay on Vero-E6 cells had an IC50 of 25.48 ± 0.69 µM, and no apparent toxicity (>90% cell viability) was observed at 20 µM. In a plaque reduction assay with authentic SARS-CoV-2, compound 2 significantly reduced viral plaque formation at a concentration of 15 µM, suggesting that this inhibitor can block SARS-CoV-2 at non-toxic concentrations, with 13% inhibition at 7.5 µM and an estimated EC50 value of 9.0 ± 2.5 µM.
Therefore, compound 2 inhibits SARS-CoV-2 3CLpro and PLpro by acting as a potent cysteine protease inhibitor [124]. Although this typically implies that compounds may be toxic to host cells by targeting cellular cysteine proteases, this study demonstrated that compound 2 exhibits antiviral activities against authentic SARS-CoV-2 at non-toxic concentrations. This suggests that this compound deserves further exploration as a potential new therapy for COVID-19.
Phenolic acids have revealed their potential as anti-SARS-CoV-2 agents, highlighting the important interactions between the carbonyl and hydroxyl groups of these molecules and viral proteins. Among the compounds analyzed, caffeic acid (3) showed the highest inhibition rate of 3CLpro, followed by compound 2, although the latter has limitations due to its toxicity [123]. Docking analysis showed that the formation of hydrogen bonds is crucial for optimizing interactions with target proteins. Despite the promising antiviral activities observed in vitro, further investigations are required to confirm the efficacy and safety of these compounds in cell models and in vivo assays.
2.3.2. Phenylethanoids
Phenylethanoids belong to a class of organic compounds derived from phenylacetic acid. They have a basic structure comprising Ae3 attached to a two-carbon chain (C2). These compounds are found in various plants and possess diverse biological properties, including antioxidant, anti-inflammatory, and antimicrobial activities [125].
Among this class, 20 compounds (20–39) (Figure 6) were evaluated, and nineteen molecules were examined for 3CLpro activity, showing average inhibition rates between 0% and 50% at a concentration of 100 µM and IC50 values ranging from 0.04 573.9 µM. Compound 4-(4-hydroxyphenyl) butan-2-one (36) exhibited 0% inhibition based on FRET assay and an IC50 of 100 µM [115].
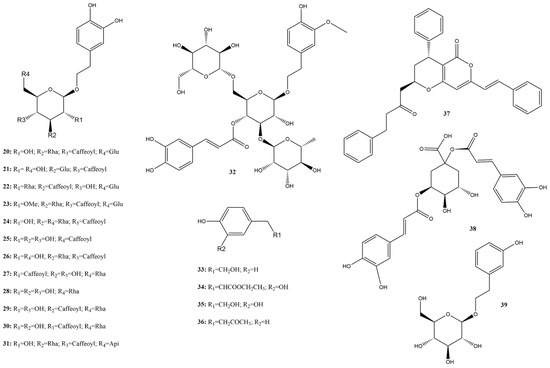
Figure 6.
Chemical structures of phenylethanoids tested against SARS-CoV-2 targets.
The phenylethanoid glycoside verbascoside (26) exhibited computational affinity toward the viral protease (3CLpro) with a binding energy of −10.13 kcal/mol. The in vitro activity of this compound was assessed using the FRET assay (IC50 = 0.043 μM), and it showed potency similar to that of the positive control GC376 (IC50 = 0.044 μM). Furthermore, 75.76% inhibition of enzyme activity was achieved at 100 µM. Docking experiments on acteoside using the PDB structure 6Y2F (3CLpro) showed covalent inhibitors of SARS-CoV-2 (−8.98 kcal/mol) [126].
Ethyl caffeate (34) showed the highest potential among the inventoried molecules with 99.24% inhibition against 3CLpro at a concentration of 100 μM using screening based on the FRET assay. This compound is an ester of the hydroxycinnamic acid group found in various plants [115].
The phenylethanoid 4-(2-hydroxyethyl)phenol (33) binds to PLpro at the ISG15/Ub-S2 allosteric binding site in a hydrophobic cavity with a predicted binding energy of −7.17 kcal/mol (calculated using Prodigy42). The in vitro activity was assessed using the FRET assay, revealing activity at a concentration of 3.76 ± 1.20 μM. The known PLpro inhibitor GRL0617 was used as a control (0.82 μM). Compound 33 showed no cytotoxicity in cellular cytotoxicity assays, suggesting its potential as a promising lead compound for developing specific coronaviral PLpro inhibitors [127].
Compound 33 stood out for its ability to bind to the allosteric site of PLpro, which is a less conserved but critical region for enzymatic activity. The predicted binding energy of −7.17 kcal/mol suggests a high affinity, and the absence of cytotoxicity makes this compound a viable candidate for therapeutic development, minimizing side effects. Compound 34 showed 99.24% inhibition of 3CLpro, demonstrating the importance of additional functional groups that enhance biological activity. The specific structure of this compound, belonging to the hydroxycinnamic acid group, facilitates more effective interaction with the target enzyme, resulting in nearly complete inhibition of enzymatic activity at a concentration of 100 μM.
Phenylethanoids are promising compounds due to their intrinsic characteristics that contribute to their biological efficacy. Firstly, the basic structure of phenylethanoids, which is composed of a phenyl ring attached to a two-carbon chain, provides a favorable combination of stability and chemical reactivity. This structure allows for effective interactions with various active sites of viral enzymes, potentially inhibiting their function [115].
2.3.3. Chalcones
Chalcones are natural compounds consisting of an aromatic ketone and enone, forming a flat structure with two aromatic rings connected by a propanone bridge [128]. They have various pharmacological benefits and comprise the backbone of open-chain flavonoids, wherein the three-carbon aliphatic system serves as a linker between aromatic rings A and B [129].
Chalcone pharmacophores represent an attractive structure for discovering and targeting anti-SARS-CoV-2 agents. This review identified 19 chalcones (40–58) (Figure 7) that were tested in vitro using a FRET assay against the targets of SARS-CoV-2. Seventeen compounds were examined for 3CLpro activity, displaying inhibition rates between 0 and 50% at a concentration ranging from 20 to 100 µM in an initial screening and IC50 values from 1.5 µM to 57.42 µM. For PLpro suppression, only xanthohumol (42) (IC50 162 µM) and sophotonin I (57) (IC50 16.74 µM) were assessed [58,115,130,131,132]. Furthermore, the best candidates were subjected to antiviral activity and cytotoxicity evaluations in Caco-2 and Calu-3 cells, along with in silico tests.
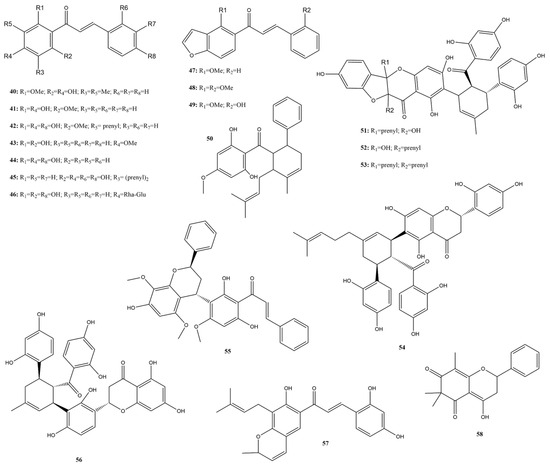
Figure 7.
Chemical structures of chalcones tested against SARS-CoV-2 targets.
Khamto et al. [132] conducted a study on the in vitro 3CLpro proteolytic activity across various compounds belonging to the chalcone class, with baicalein (IC50 86.57 μM) and nirmatrelvir (IC50 < 1 μM) as positive controls. All compounds underwent initial screening at a concentration of 100 μM, along with cytotoxicity testing in Vero cells. These chalcones exhibited robust antiproteolytic activity during the initial screening, with inhibition percentages ranging from 43.96% for pinostrobin chalcone (43) to 94.54% for panduratin A (50), a Diels-Alder chalcone hybrid. The main candidate, compound 50, demonstrated an IC50 value of 13.28 μM but displayed considerable cytotoxicity with a CC50 value of 1.81 μM.
In studies conducted by Kanjanasirirat et al. [133], the compound 50 hindered SARS-CoV-2 infectivity in Vero E6 cells post-viral infection (IC50 0.81 μΜ, CC50 14.71 µM), and pre-entry treatment inhibited infection (IC50 5.30 µM, CC50 43.47 µM). This research underscores the inhibitory effects of compound 50 on SARS-CoV-2 infection during the pre-and post-infection stages, not only in Vero E6 cells but also in human airway epithelial cells, highlighting its potential as an anti-SARS-CoV-2 agent.
Wasilewicz et al. [58] found that compounds known as Mulberry Diels-Alder-type adducts (MDAAs): sanggenon C, D, O, and G (51–54), and kuwanon L (56), from Morus alba L., exhibited pronounced anti-SARS-CoV-2 activities. They are biosynthesized from the [4+2] cycloaddition of chalcones (dienophiles) and prenylated phenols (dienes) and display a range of bioactivities, including antioxidant, cytotoxic, neuroprotective, anti-inflammatory, and antiviral properties [58,134]. The MDAAs also act as ligands of 3CLpro and inhibit its activity at low micromolar concentrations available by FRET assay (IC50 ranged from 4.8 μM for compound 51 to 13.9 μM for compound 52) [58]. According to the authors’ findings, the antiviral activity and cytotoxicity in Calu-3 cells showed IC50 values ranging from 4.6 μM for compound 51 to >20 μM for compound 56. Despite their slight cytotoxicity, these compounds exhibited reasonable SI values of >5.
The docking poses of MDAAs in SARS-CoV-2 3CLpro revealed a comparable occupancy of four subsites by all these compounds, except for compound 54, maintaining the conserved benzoyl and cyclohexene moieties in the S1 and S2 pockets, respectively. Except for compound 52, the others had a chalcone phenyl ring in S1′ and a flavonoid part in S3, while compound 52 showed an inverted binding orientation in S1′ and S4 due to its (R)-configuration at C-3″. Except for compound 52, these compounds were also predicted to form hydrogen bonds with Thr26 and Glu166 in S1′ and S4, respectively, which are crucial for ligand binding. In addition, the results from the CPMG-RD experiments further supported the assumed competitive 3CLpro inhibition of MDAAs by binding to the substrate-binding site. The two resorcyl residues flank the catalytic Cys134 in the S1 and S1′ pockets, while the cyclohexene ring with methyl (51 and 53) or prenyl (54) groups is accommodated in the lipophilic S2 pocket. The benzopyran moiety loosely covers the solvent-accessible S4 pocket. Since these compounds share a similar docking pose, the authors proposed the chalcone moiety as a promising starting point for improved potent 3CLpro inhibitors [58].
The compound 42, a prenylated chalcone derived from Humulus lupulus L. (Cannabaceae), has been demonstrated to be a potent inhibitor of 3CLpro with IC50 1.5 μM, as tested using a FRET assay [131,135]. Based on these promising results, in silico docking analysis and in vitro inhibition against PLpro using a FRET assay were also performed by Herzog et al. [131]. The findings indicate that compound 42 binds to PLpro at the catalytic site using hydrophobic interactions and multiple hydrogen bonds to block substrate access to the active site cysteine; however, it had an IC50 of 162 ± 46 μM.
The efficacy of compound 42 in inhibiting SARS-CoV-2 replication in Caco-2 cells was also evaluated, showing inhibitory activity with an IC50 of 3.3 μM despite its cytotoxicity with a CC50 of 12.3 μM (IS: 3.73). This issue is circumvented by compound 42 occurring in hop-related beverages, presenting advantages such as widespread availability and tolerability. The authors concluded that PLpro inhibition is needed, suggesting its potential impact on multiple stages of the viral infection cycle. This highlights the potential of chemical modifications for the development of potent compounds [131].
In summary, these compounds exhibited significant activity against SARS-CoV-2 proteases, although they were cytotoxic. Particularly noteworthy are those containing prenyl groups, which facilitate hydrophobic interactions, and hydroxyl groups, which enable hydrophilic interactions at the catalytic sites.
2.3.4. Flavonoids
Flavonoids are a diverse group of polyphenolic compounds characterized by their common flavone backbone, which consists of a 15-carbon skeleton arranged in a C6-C3-C6 configuration. They were subdivided based on the oxidation and substitution pattern levels of the central pyran ring. Flavonoids include several subclasses, such as flavones, flavonols, flavanones, flavanonols, flavonols, isoflavones, anthocyanins, and derivatives (Figure 8).
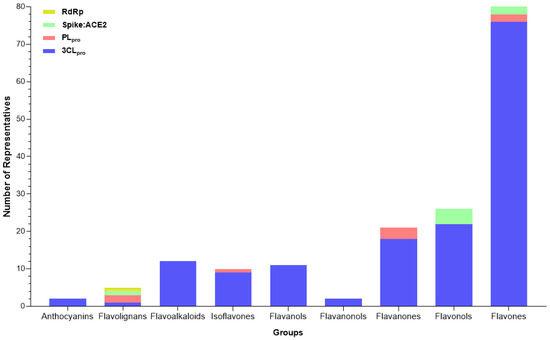
Figure 8.
Subclasses of flavonoids and their number of representatives tested against selected targets of SARS-CoV-2 (3CLpro, PLpro, Spike:ACE2, and RdRp), tracked by the review.
Flavones are a subclass of flavonoids characterized by the presence of saturation between carbons 2 and 3, the absence of a hydroxyl group in carbon 3, and the B ring linked in carbon 2. Many compounds of this class have antiviral activities, such as baicalein against the Japanese Encephalitis Virus (JEP) and Chikungunya Virus and Apigenin against the Hepatitis C virus [136].
Among the flavonoids identified in this review, flavones are the most representative subclass, with sixty-five compounds (59–124) (Figure 9) described for their in silico and in vitro activities.
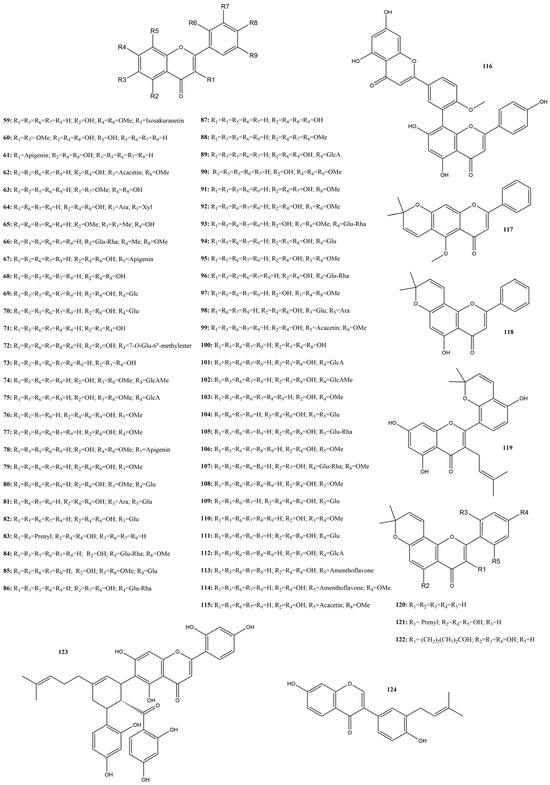
Figure 9.
Chemical structures of flavones tested against SARS-CoV-2 targets.
Several studies have evaluated compound activities against 3CLpro by FRET assay, demonstrating inhibitions that varied from 0 to 87.85% and IC50 from 0.074 to 230 μM. Luteolin tetramethyl ether (88) and diosmin (107) showed inhibition rates as low as 0% [115]. However, various compounds showed prominent activity in the 3CLpro assay. Based on FRET results, the ten most active compounds were selected, considering an IC50 below 7.0 μM. Compounds that satisfy this value are divided into three groups: aglycones: 7-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-6-methoxychromen-4-one) (63), baicalein (71), ganhuangenin (76), and glycosilated; flavones: apigetrin (70), schaftoside (98), vicenin-2 (104), and baicalin (112); and isoflavones: ginkgetin (78), sciadopitysin (99), and isoginkgetin (115) [1,126,137,138,139,140,141,142,143].
Two aglycones showed IC50 below 1 μM: compound 71 and 76 (0.94 μM and 0.84 μM). They were assessed in the work of Zhu et al. [142], who described the screening of compounds in six species used in Traditional Chinese Medicine (TCM) using an affinity-selection method. Both compounds were identified and screened in the extract of Scutellaria baicalensis Georgi. The screening method indicates the degree of activity by selection based on affinity with the target. After selection, the inhibition of 3CLpro by the compounds was tested by FRET assay and evaluated for their binding affinities using Native MS. The binding results showed a positive correlation with FRET results, indicating stronger binding to more potent compounds.
Compound 71 3CLpro activity was previously reported by other authors, and this compound was used as a positive control for the tests [142]. In the work of Liu et al. [138], compound 71 showed an IC50 of 0.39 μM, even lower than demonstrated in the studies based on this work. Notably, the activity of compound 71 varied in the studies, ranging from strong to moderate. This can be attributed to the catechol moiety, which is a Pan-Assay Interference Compound (PAINS) motif that forms nonspecific covalent bonds with amino acids, such as cysteine in the 3CLpro active site [58].
The third aglycone, compound 62, is a dimethoxylated aglycone present in Saussureae involucratea Matsum and Koidz, both in silico and in vitro against SARS-CoV-2 [143]. The authors performed a structure-based virtual screening of 18.263 traditional Chinese medicinal compounds, pointing to nineteen compounds with never-reported antiviral activity. The compounds were then tested in vitro, leading to compound 62 as the best result, with an IC50 of 4.64 ± 0.11 μM and EC50 12.25 ± 1.68 μM. The compound was also assessed by in silico assays for its expected activity and toxicity, with both results indicating promising activity and low toxicity.
The best flavone result observed in this review was an IC50 value of 0.074 μM for the glycosylated flavone compound 70, as reported by Abdallah et al. [126]. In this study, authors screened 8739 natural products by docking and selecting eighteen promising compounds for further in vitro analysis. Of these, compound 70 emerged as the second most active against 3CLpro, with a value of inhibition near the IC50 of 0.044 μM, achieved by the control compound GC376. According to Abdallah et al. [126], the in vitro activity of the compound could be explained by its interactions with the protein binding site, similar to that of the co-crystallized compound. In an extra-precision docking study of the 3CLpro active site, compound 70 formed a network of hydrogen bonds with several residues of the active site, as well as π-π stacking and van der Waals interactions, and came second as the best-docked molecule. These interactions could explain the blocking of substrate access to the active site and, consequently, inhibition.
Although not as potent as apigetrin, three other glycosylated flavones showed low values of IC50, 98, 104, and 112. Compounds 98 and 104 showed IC50 values of 1.73 and 1.43 μM against SARS-CoV-2 3CLpro in FRET assays [141], while 112 presented an IC50 of 6.41 μM [137]. Even though 104 showed higher potency in FRET, it only showed moderate to weak activity when tested against the virus in vitro, which made 97 the best candidate, as much as in vitro activity due to low cell toxicity, with a CC50 of more than 200 μM.
Three bisflavones, 78, 99, and 115, were assessed by Xiong et al. [139], who aimed to discover candidates against SARS-CoV-2 3CLpro in Ginkgo biloba leaves. Compound 99 was the second-best candidate, with an IC50 of 1.09 μM, and inhibition kinetics analysis demonstrated that it strongly inhibited the protein in a reversible and mixed-inhibition manner and could be docked in two druggable pockets. This could lead to the development of novel inhibitors of this target. Xiong et al. [139] also demonstrated that compounds 78 and 115 are potent inhibitors with IC50 of 2.33 and 2.98 μM, respectively.
Lastly, two studies, conducted by Lopes et al. [1] and Kim et al. [140], tested the capacity of three compounds: amentoflavone (67), kuwanon C (83), and luteolin (87) to inhibit SARS-CoV-2 spike protein interaction with host receptors, using a RBD:ACE2 kit. These compounds yielded inhibitions varying from 0 to 50% and IC50 values of 0 to 91.4 μM. The study by Kim et al. [140] demonstrated that kuwanon C was capable of inhibiting the Spike S1 RBD interaction with the ACE2 receptor with an IC50 of 91.4 µM, which motivated other tests with this compound, demonstrating that it was capable of preventing a clinical isolate of SARS-CoV-2 from infecting Vero cells. In contrast, compounds 67 and 87 showed 0% inhibition in the spike assay [1].
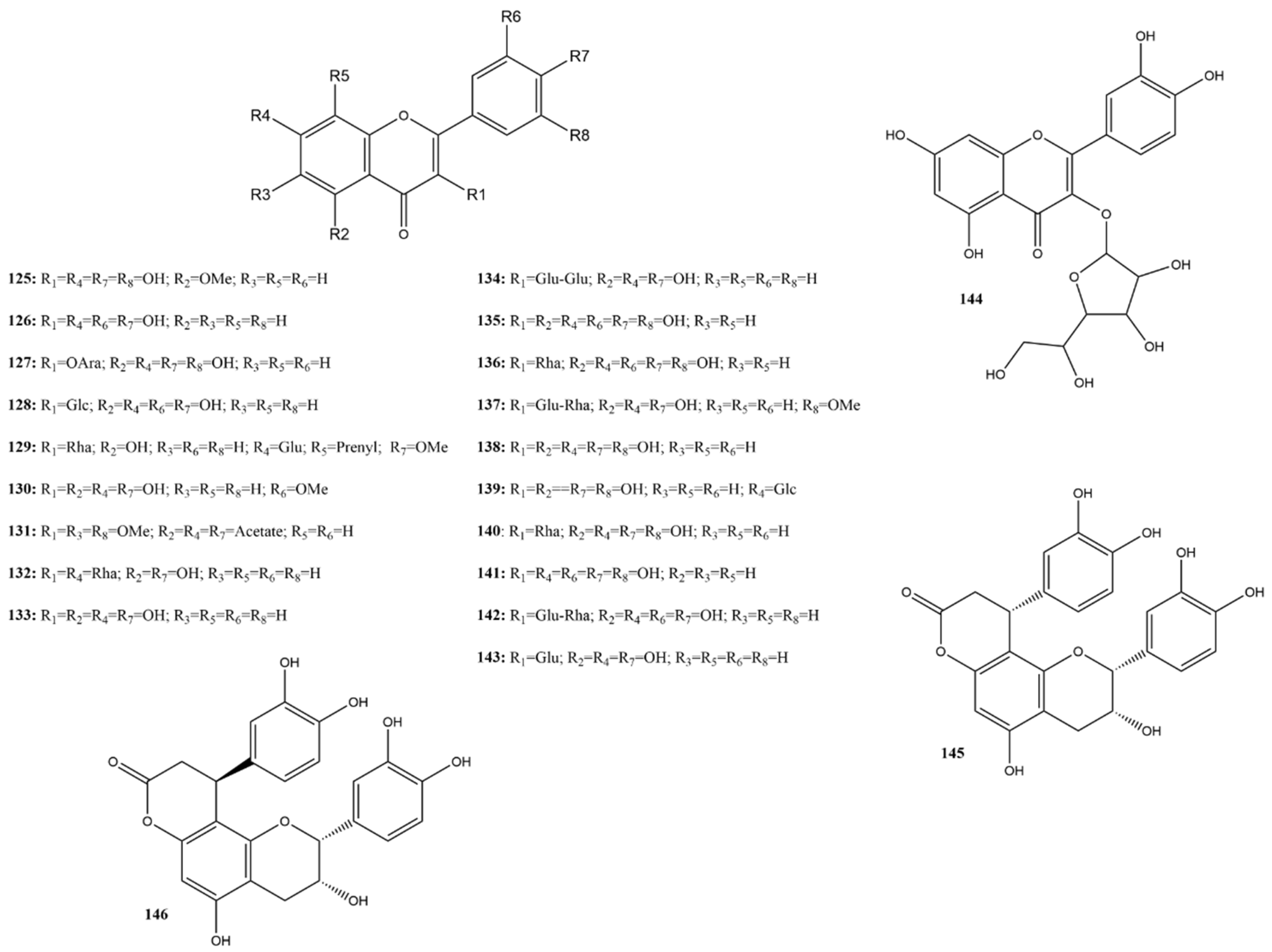
Flavonols are a widely distributed subclass of flavonoids in the plant kingdom. They are characterized by a 3-hydroxyflavone backbone. This review predominantly reported the inhibition of 24 reported flavonols (125–146) (Figure 10). In studies of 3CLpro inhibition, only fisetin (126) showed 0% inhibition at 100 μM [115]. For this subclass, the substances considered most active were those with an inhibition greater than 80% or an IC50 below 10 μM. Therefore, the selected compounds were the aglycones isorhamnetin (130), myricetin (135), and robinetin (141), and the glycosides kaempferitrin (132) and astragalin (143) [115,116,143,144].
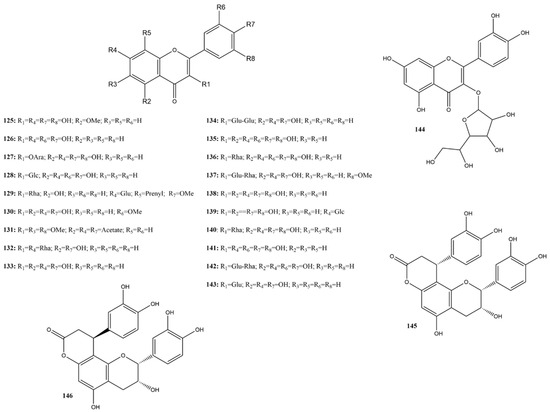
Figure 10.
Chemical structures of flavonols tested against SARS-CoV-2 targets.
Wang et al. [115] utilized compound 135 as a positive inhibitor against 3CLpro, presenting an IC50 of 0.43 μM. Previous studies corroborate this activity both in FRET assays (IC50 of 0.63 μM) and Vero E6 cells (EC50 of 8.00 μM, SI > 25). Additionally, in silico assays demonstrated the formation of a covalent bond between cysteine in the catalytic site of SARS-CoV-2 3CLpro and the pyrogallol group of compound 135. Structure-based design studies of compound 135, using both in silico and in vitro analyses, reinforce the importance of the pyrogallol group [145]. Interestingly, Lin et al. [146] reported an IC50 of 44 μM for compound 135 in another study included in this review. The most notable difference between these studies is the protease concentration used in the assays.
Compound 141 also contains the pyrogallol group-like compound 135. In a study by Jin et al. [143], this flavonol showed an IC50 of 11.16 μM against 3CLpro, which was higher than that of the control dipyridamole (IC50 of 0.51 μM). In assays using Vero E6 cells, an EC50 of 29.32 μM was found. Another study demonstrated inhibition against 3CLpro with an IC50 of 0.96 μM and an EC50 of 1.3 nM in Calu-3 cells [117]. The evaluation time used in each assay was a distinguishing factor between the studies. This is an important variable, as evaluated in the study by Li et al. [147], where compound 141 showed IC50 values of 3.91 μM and 0.96 μM at 3 and 30 min, respectively. However, all authors agree that the pyrogallol group is important for protease binding.
Two studies included in this review reported on the activity of compound 130 against 3CLpro. Shahhamzehei et al. [116] showed an IC50 of 8.42 μM using a commercial assay kit (SensoLyte SARS-CoV-2 3CL Protease Activity Assay Kit). Additionally, in silico analyses indicated the formation of hydrogen bonds or hydrophobic interactions with at least one of the catalytic residues (Cys145 and His41), comparable to that of the control GC-376. An assay with MRC-5 lung fibroblasts presented a CC50 of 36.80 μM with a therapeutic index of 4.37. Xiong et al. [139] observed an IC50 of 31.59 μM using recombinant 3CLpro expressed in Escherichia coli. The different methods of evaluating protease activity may have influenced the discrepancy in the observed values. It is worth noting that studies indicate the antiviral activity of compound 130 against SARS-CoV and influenza, reinforcing the antiviral potential of this compound [116,148].
The compound 143, exhibited an IC50 of 0.13 μM, with in silico analyses indicating the formation of hydrogen bonds and hydrophobic interactions with 3CLpro residues [144]. Alhadrami et al. [149] investigated the potential of this flavonol through in silico assays against PLpro and 3CLpro. However, in a study with Vero-E6 cells, astragalin showed minimal inhibitory activity with an IC50 > 100 µM. Another study in the literature highlights the anti-inflammatory potential of this compound [150].
Interestingly, in all studies included in this review reporting the activity of kaempferol (compound 133), the IC50 values ranged from 45.42 to >100 μM, with 4.51% inhibition observed at a concentration of 100 μM against 3CLpro [115,139,144,151]. The main differences between these assays lie in the reading time of the experiment or the incubation period of the protein with the evaluated material before the addition of the fluorescent substrate. Compound 132 differs from kaempferol in having a rhamnose unit attached to rings A and C. Zhang et al. [144] demonstrated that at a concentration of 10 μM, compound 132 inhibited 96.81% of the 3CLpro. Studies have indicated the potential antiviral activity of this flavonol against H1N1 and H3N2, as well as its anti-inflammatory properties [152,153].
Kaempferol-3-O-gentiobioside (134), which structurally differs by having two glucose units-one directly attached to ring C and the other attached to the first glucose- was reported in one study to have an IC50 of 35.89 μM against 3CLpro, with in silico analyses indicating a high binding affinity, forming numerous hydrogen bonds at the catalytic site of the protease [154]. Previous studies have indicated the potential of kaempferol and its glycosides as inhibitors of different coronavirus pathways, with glycosylated structures considered more potent inhibitors, in addition to having higher water solubility [155].
The four flavonols evaluated for inhibition of the Spike:ACE2 interaction showed 0% activity: kaempferitrin (132), myricetin (135), quercetin (138), and quercimeritrin (139) at a concentration of approximately 45 μM [1].
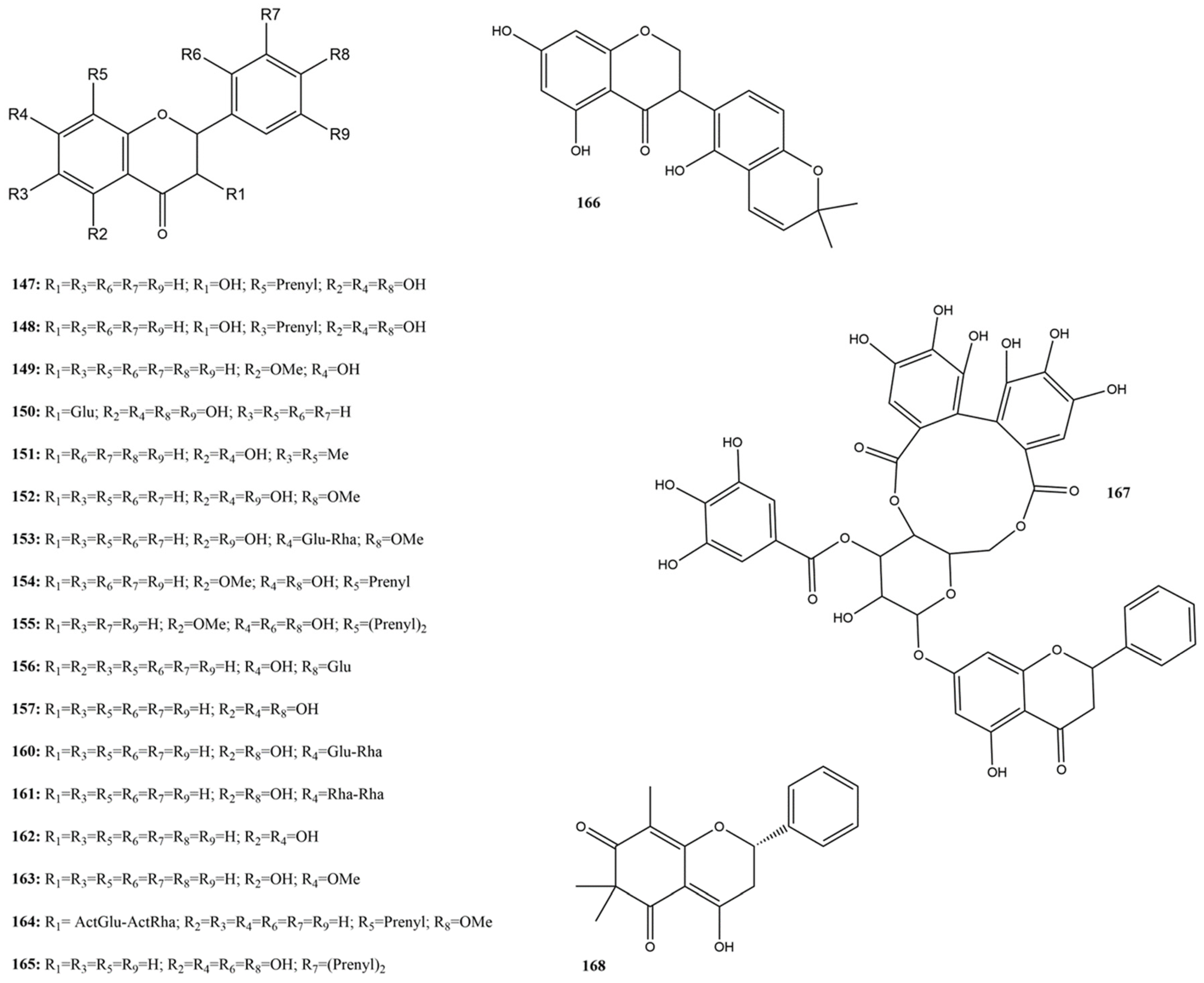
Flavanones are a subclass of flavonoids widely distributed in various plants, mainly in the Citrus genus [156]. Known for their anti-inflammatory and antiviral properties [156,157], flavanones have been the subject of numerous studies due to their therapeutic potential. This study discusses the inhibitory actions of 21 flavanones (147–168) (Figure 11) concerning their efficacy as inhibitors of the SARS-CoV-2 protease 3CLpro.
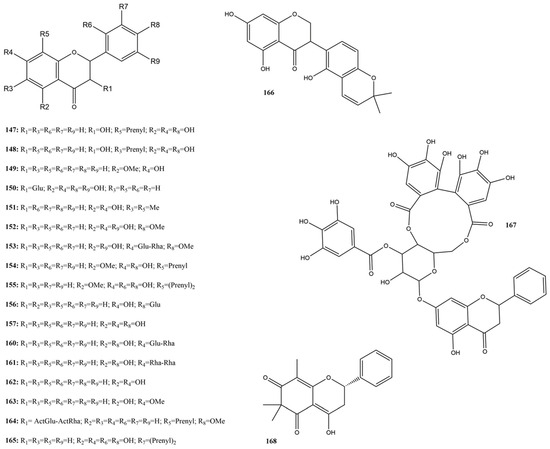
Figure 11.
Chemical structures of flavanone tested against SARS-CoV-2 targets.
Among these compounds, some demonstrated 3CLpro inhibition, with rates ranging from 11 to 82% at a concentration of 100 µM and IC50 values between 0.09 and 100 µM in the FRET assay. Naringenin (157) showed the lowest inhibition of 11.86% [115]. In contrast, hesperidin (153) and kurarinone (155) exhibited the highest inhibition of 82.14% and 72.14%, respectively [115].
The compound 157, highlighted in Abdallah et al. [126], presented an IC50 value of 0.09 µM, showing promise as a SARS-CoV-2 3CLpro inhibitor. The ability of compound 157 to form hydrogen interactions with key residues in 3CLpro, such as Tyr54 and Thr190, significantly contributed to its efficacy. Additionally, this compound exhibits low cytotoxicity in host cells, reinforcing its potential as an effective therapeutic agent [126].
Wasilewicz et al. [58] verified the 3CLpro inhibition values of compound 155 and sanggenol A (163) at concentrations of 20 μM and 100 μM. Compound 155 showed moderate inhibition, with an IC50 value of 30.2 µM, while 163 showed an IC50 value of 31.9 at 20 μM. Both compounds showed reduced efficacy at higher concentrations, with IC50 values greater than 100 μM, suggesting that their efficacy could be improved through structural modifications or combinations with other antiviral compounds.
Khamto et al. [158] evaluated alpinetin (149), demethoxymatteucinol (151), pinocembrin (160), and pinostrobin (161) against SARS-CoV-2 3CLpro. Compound 149 showed moderate inhibitory activity with an IC50 of 53.10 μM, while the other compounds exhibited weak activity (IC50 > 100 μM), indicating the need for structural optimization. Licoisoflavanone (166), a compound found in licorice, has emerged as a promising 3CLpro inhibitor with a half-maximal inhibitory concentration (IC50 of 1.52 μM, standing out among the tested flavonoids [141]. The ability of flavanone 166 to form stable interactions with the key residues in 3CLpro contributes to its efficacy and low cytotoxicity. Zhang et al. [144] found that astilbin (150), a flavanone extracted from cotton flowers, demonstrated significant inhibition of SARS-CoV-2 3CLpro, with an IC50 of 7.92 µM, indicating high efficacy in inhibiting the viral protease.
Among the flavanones studied, naringenin (157) stands out for its efficacy in inhibiting SARS-CoV-2 3CLpro protease, presenting a low IC50 value and low cytotoxicity in host cells. The results of this study suggest that compound 157 could be a promising basis for developing new treatments against SARS-CoV-2, encouraging new research and structural optimization [126].
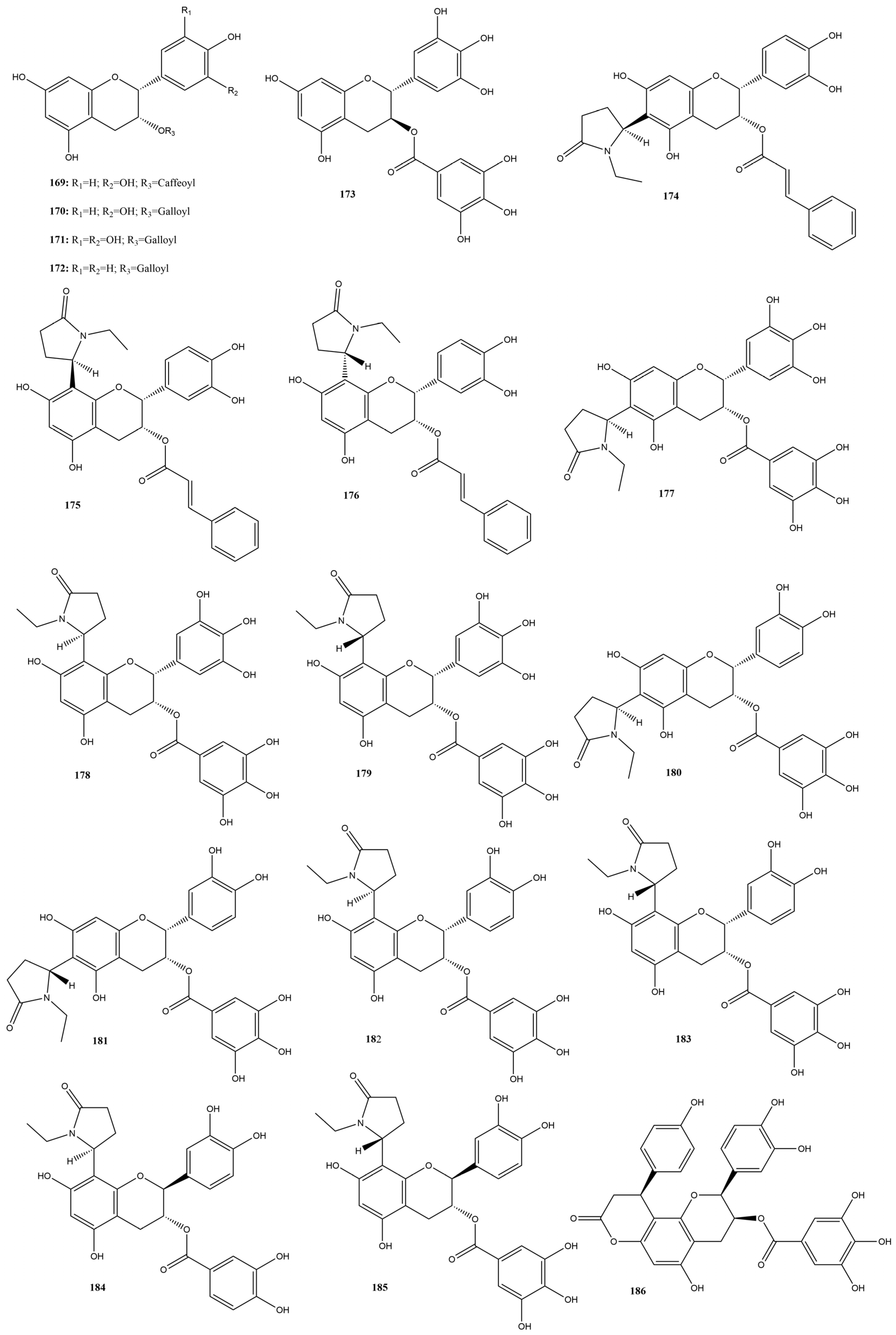
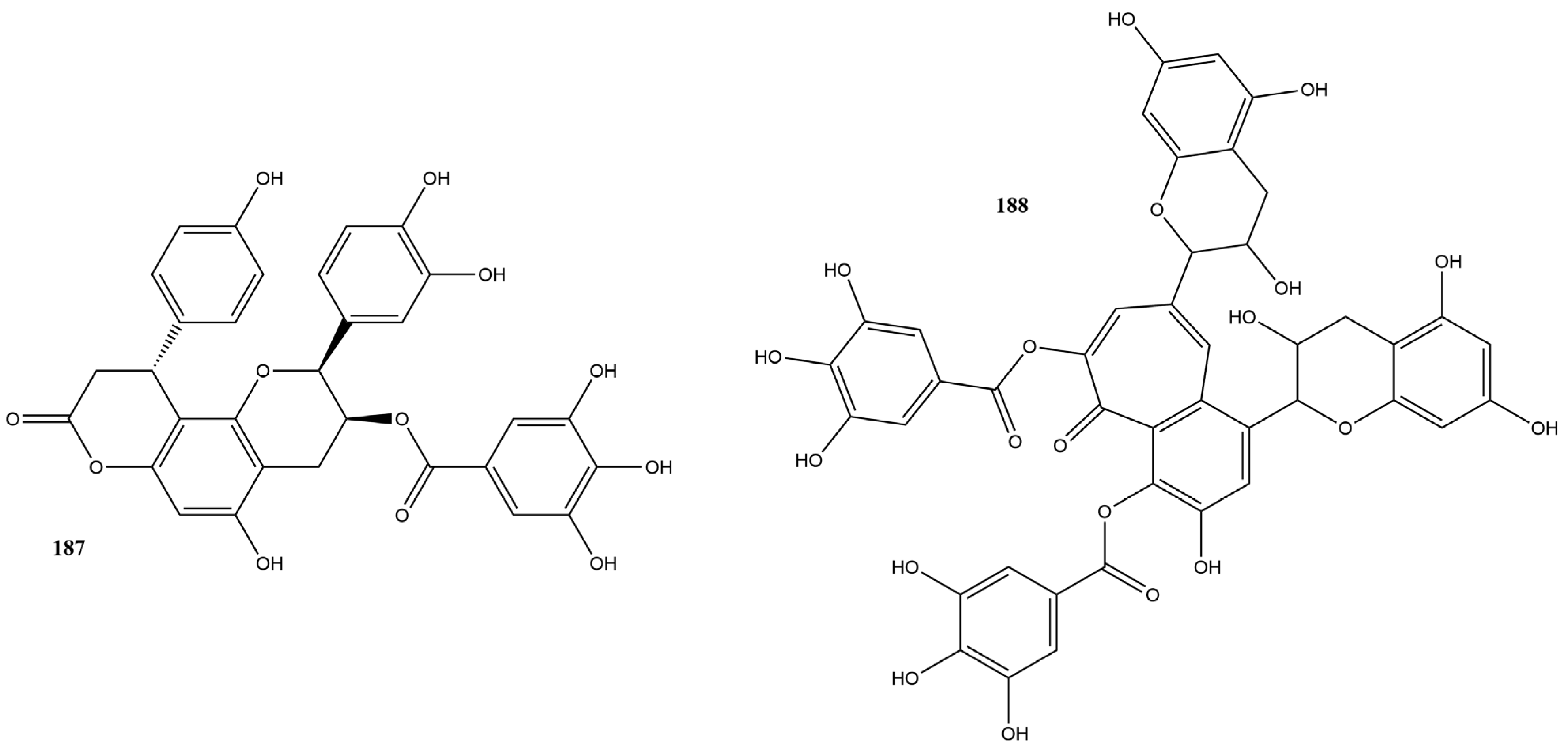
Flavanols are a distinct subclass of flavonoids characterized by the absence of a double bond between the C2 and C3 carbons of the C ring and the absence of a carbonyl group on the C4 carbon of the C ring. These compounds have hydroxyl groups on the C3 or C4 carbons, contributing to their biological properties [159]. They are widely distributed in foods, such as green tea, dark chocolate, grapes, and red wine. The most common flavanols include catechin and epicatechin, which are known for their antioxidant, anti-inflammatory, and antiviral properties [159,160].
In this review, 20 flavanols (169–188) (Figure 12) were identified in the literature with possible actions regarding their efficacy as inhibitors of SARS-CoV-2 3CLpro. Five of these compounds had IC50 values ranging from 0.85 to 3.38 µM in inhibition assays based on the FRET assay, showing inhibition rates of 50% at a concentration of 100 µM.
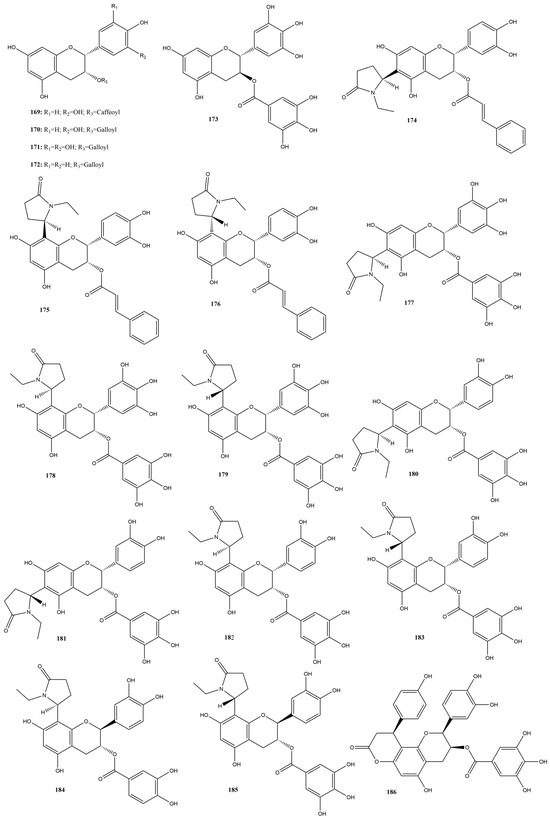
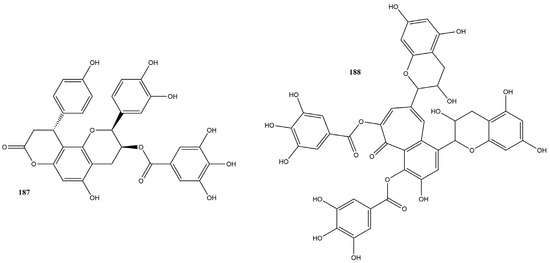
Figure 12.
Chemical structures of flavanols tested against SARS-CoV-2 targets.
Epigallocatechin gallate (171) had the lowest IC50 value, 0.85 µM, indicating high efficacy in inhibiting the activity of the protease essential for viral replication [151]. Surface Plasmon Resonance (SPR) tests conducted by Du et al. [151] demonstrated that compound 171 has a high binding affinity with 3CLpro. Additionally, the dissociation constant was calculated, showing a value of 6.17 μM. Molecular modeling indicated that compound 171 formed hydrogen bonds with critical residues of 3CLpro, reinforcing its therapeutic potential against SARS-CoV-2.
Other research groups have studied compound 171. In a study by Rubio-Martínez et al. [161], virtual screening of natural compounds was conducted to identify SARS-CoV-2 3CLpro protease inhibitors. Compound 171 was recognized as one of the five compounds with an antiviral profile, inhibiting SARS-CoV-2 replication in vitro, with an IC50 of 22 μM. Tun et al. [162] verified the antiviral activity of Japanese green tea packaged in polyethylene terephthalate (PET) bottles and tea compounds, including 171. It exhibited activity against SARS-CoV-2, with an IC50 value of 56 µM for inhibiting 3CLpro activity. Furthermore, the molecule significantly inhibited the entry and post-entry of the virus, as well as SARS-CoV-2 protease activity. The results of the three studies confirmed that compound 171 is a possible inhibitor of the main protease of SARS-CoV-2, with the ability to inhibit viral replication and the activity of the 3CLpro protease, which is essential for the virus’s life cycle.
Liu et al. [163] verified the possible interactions between the main protease 3CLpro of SARS-CoV-2 and the catechins present in Camellia sinensis (L.) Kuntze tea. The IC50 values of the catechins epicatechin 3-O-caffeoate (169), epicatechin gallate (170), gallocatechin gallate (173), etc-pyrrolidinone C and D (178–179), etc-pyrrolidinone F (181), zijuanin C (186) were 1.58, 71.78, 3.38, 0.90, 0.90, 46.71 and 41.2 µM, respectively. The results showed that catechins 169, 173, 178, and 179 have potent inhibitory activities against the main protease 3CLpro of SARS-CoV-2, with low IC50 values, indicating high efficacy. These compounds can serve as the basis for developing new antivirals, and structural modifications can further increase their effectiveness.
Isoflavones are a subclass of flavonoids characterized by the attachment of A and C rings (chromane ring) to the B ring at the C3 position, unlike the more common C2 linkage found in other flavonoids [164]. They can be found as aglycones or, more commonly, as glycosides. Isoflavones have a more limited distribution than other flavonoid groups and are primarily found in Fabaceae plants. Best known for their estrogenic effects, isoflavones are also reported to have other activities, including cardiovascular benefits [165].
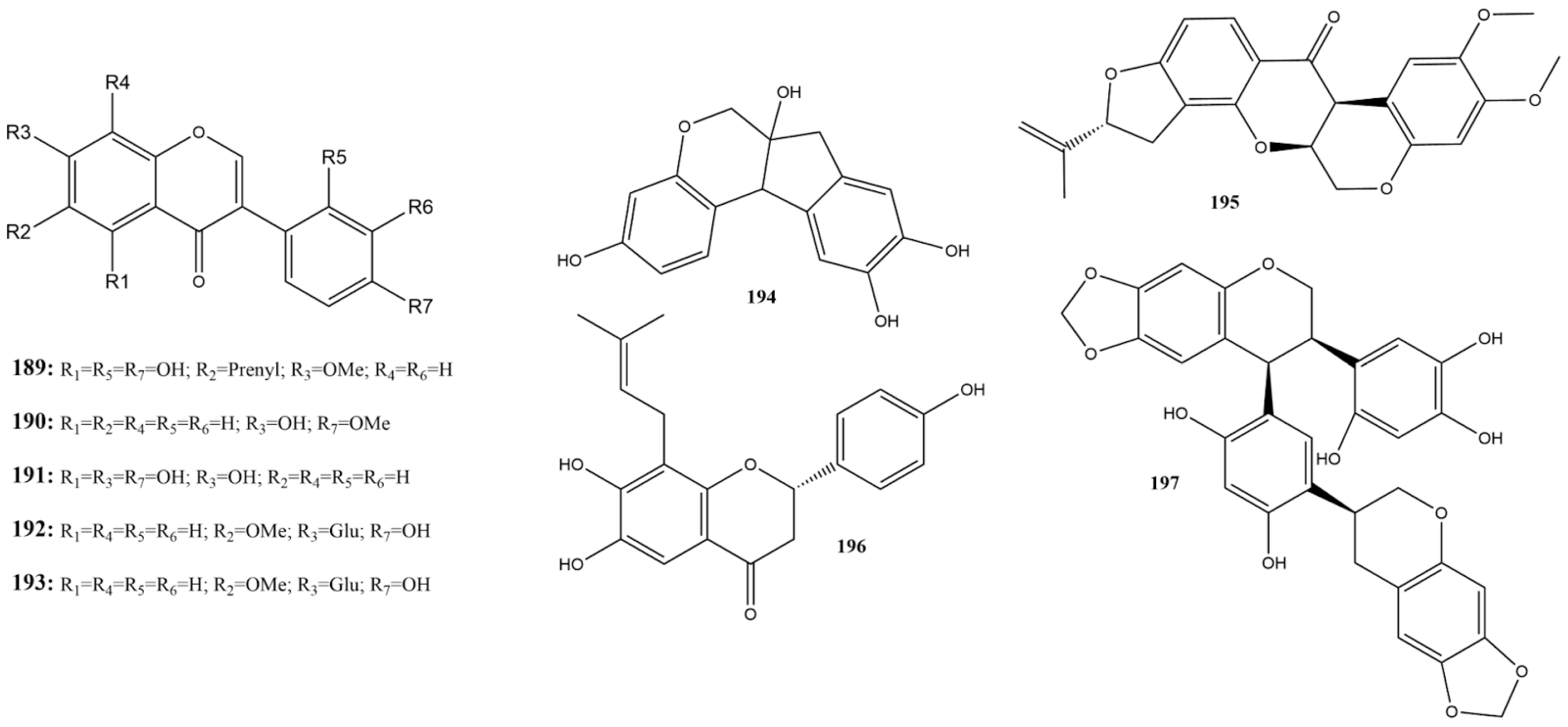
Three studies reported the activity of nine isoflavones (189–197) against 3CLpro (Figure 13) [115,130,158]. Among these, glycitin (192) and rotenone (195) showed no activity against 3CLpro at 100 μM [115]. Among the substances evaluated against 3CLpro, those with an IC50 below 20 μM were considered more active, namely sophotokin (196) and brazilin (194) [115,130].
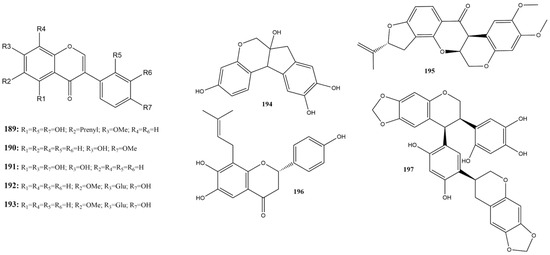
Figure 13.
Chemical structures of isoflavones tested against SARS-CoV-2 targets.
Compound 196 was identified as the second most active compound in this subclass, with an IC50 of 19.88 μM against 3CLpro, which was lower than the value found using the control PF-07321332 (IC50 of 27.36 μM) reported by Li et al. [130]. This isoflavonoid is also characterized as a pterocarpan, a member of a group of rare substances typically composed of benzo-pyrano-furano-benzene units [166]. It has been found in the species Sophora tonkinensis Gagnep. (ST) (Fabaceae) is used in TCM for treating respiratory tract infections. No additional studies have reported the antiviral activities of this substance against SARS-CoV-2 or other viruses. However, studies have demonstrated an anti-neuroinflammatory effect and inhibition of LPS-stimulated NO production [167]. Studies have shown antiviral effects against MERS-CoV ranging from 96% to 88% and inhibition of SARS-CoV-2 from 100% to 98.75% at concentrations of 12.50 to 3.13 µg/mL [168].
In the same species as a compound 196, the only isoflavone tested against PLpro was 7-O-methylluteone (189), which demonstrated superior activity compared to the control GRL0618, with an IC50 of 79.38 μM and 1.77 μM, respectively [130]. However, further studies are required to understand its activity against PLpro and to evaluate its efficacy against other SARS-CoV-2 targets.
Compound 194 is also a homoisoflavonoid found in the genus Caesalpinia L. and Haematixylum L. (Fabaceae) [166]. Present in the Brazilwood, or redwood tree, is one of the main coloring constituents [169]. In addition to its use as a dye, brazilin has the potential to chelate zinc and is capable of disaggregating amyloid fibers into non-toxic amyloid aggregates [170,171].
Wang et al. [115] demonstrate that compound 194 exhibits significant values of strong reversible and irreversible binding affinity during in vitro assays for 3CLpro, with a dose-response effect with an IC50 of 1.18 μM. Molecular dynamics simulations indicated hydrogen bonds with Cys145 and Cys44, as well as π-π interactions with His41. Additionally, studies in cells infected by wild-type SARS-CoV-2 showed potent antiviral activity with an EC50 of 7.85 ± 0.20 μM during full-time treatment and an EC50 of 5.24 ± 0.21 μM in post-infection treatment. Research on the binding of the SARS-CoV-2 RBD-spike protein to the human ACE2 receptor demonstrates that compound 194 can inhibit approximately 60% of this interaction at 10 μM, with inhibition persisting even when pseudovirus particles are used [95]. These findings indicate the potential of this flavonoid as a multitarget agent against SARS-CoV-2.
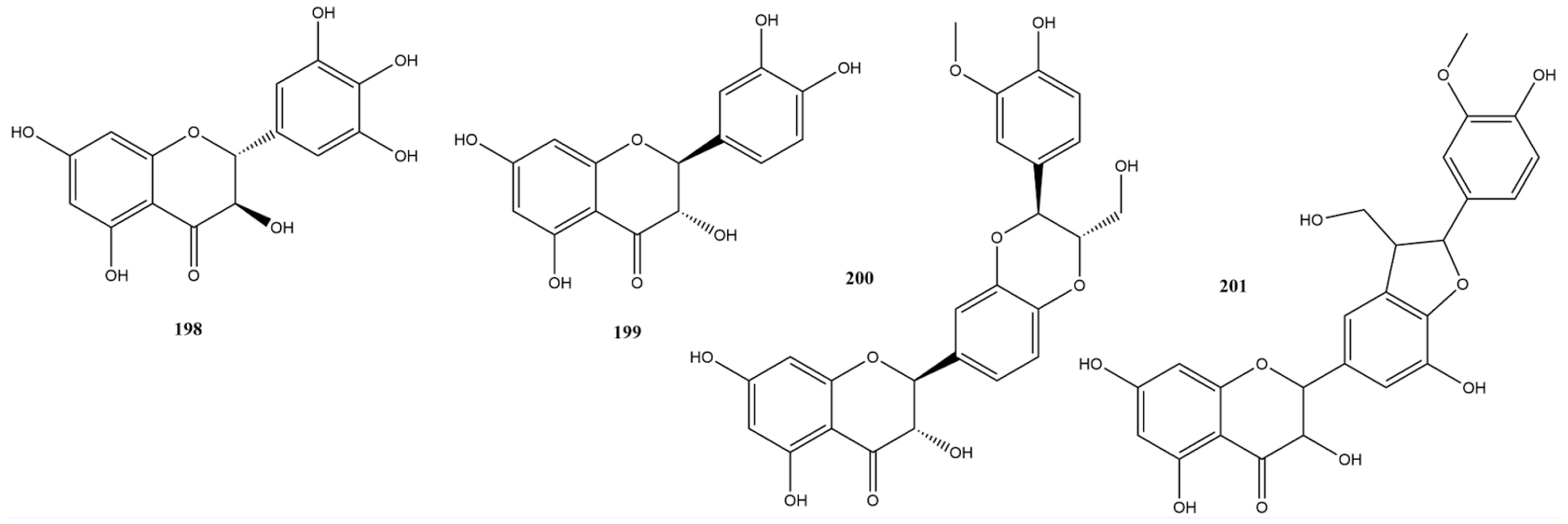
Flavanonols structurally resemble flavonols, the only difference being the saturated bond between positions 2 and 3 [172]. This subgroup is highly diversified and multisubstituted, presenting variations such as flavolignans found in various fruits and vegetables [173]. In this study, four research papers identified four flavanonols: dihydromyricetin (198), taxifolin (199), silibinin (200), and silicristin (201) (Figure 14).
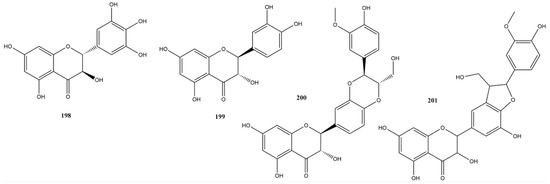
Figure 14.
Chemical structures of flavanonols tested against SARS-CoV-2 targets.
Compound 198 showed the best result among the substances tested against 3CLpro, with an IC50 value of 1.72 μM, similar to that of the control, ebselen, which had an IC50 of 1.63 μM [174]. Flavanonol has demonstrated antioxidative, anti-inflammatory, anticancer, antimicrobial, and various other activities [175]. According to Xiao et al. [174], molecular docking analysis revealed that dihydromyricetin occupies the active center of 3CLpro. It binds to the protease through hydrogen bonds at Leu141, Glu166, Gln189, and Thr190 and forms a π-π stacking interaction with His163. In vivo assays using dihydromyricetin in models of pulmonary inflammation and fibrosis indicated its potential to inhibit inflammatory factors and preserve affected lung cells, thereby preventing or delaying the progression of pulmonary fibrosis. Furthermore, other studies in the literature have underscored the potential of compound 198 for the treatment of inflammation-related diseases [176]. This highlights the potential of this flavonoid as a therapeutic target for COVID-19, given the inflammatory response triggered by the disease.
Only one study has reported testing compound 199 against 3CLpro, indicating an inhibitory activity close to 70% at a concentration of 100 μM [115]. In silico studies indicated a strong interaction of this flavonoid with RdRp [177], strong interaction, and stability of binding with 3CLpro and PLpro in molecular dynamics studies [178].
Compound 201 showed low inhibitory activity against PLpro, with only 3.4% inhibition at a concentration of 20 μM. This was significantly lower than the inhibition observed with the control GRL-0617, which had an IC50 of 0.8 μM [58].
Compound 200 was reported in three studies using assays against Spike, RdRp, 3CLpro, and PLpro [58,115,179]. Noteworthy results include assays against Spike with an IC50 of 14 μM and against 3CLpro with an IC50 of 9.93 μM, with remdesivir as the positive control showing an IC50 of 4.01 μM in both targets [179]. Hamdy et al. [179] also demonstrated that compound 200 exhibits various interactions with amino acid residues of the S protein, RdRp, and 3CLpro. Molecular dynamics studies have demonstrated the stability of these interactions. ADME studies have indicated acceptable pharmacokinetic parameters. However, Wang et al. [115] reported 31.94% inhibition at a concentration of 100 μM. mL−1. There is no detailed description of the expression and purification processes in the work by Hamdy et al. [179]. However, it should be noted that there are some differences in the final concentration values of the protease and substrate between these studies, which may influence the variability in the observed responses.
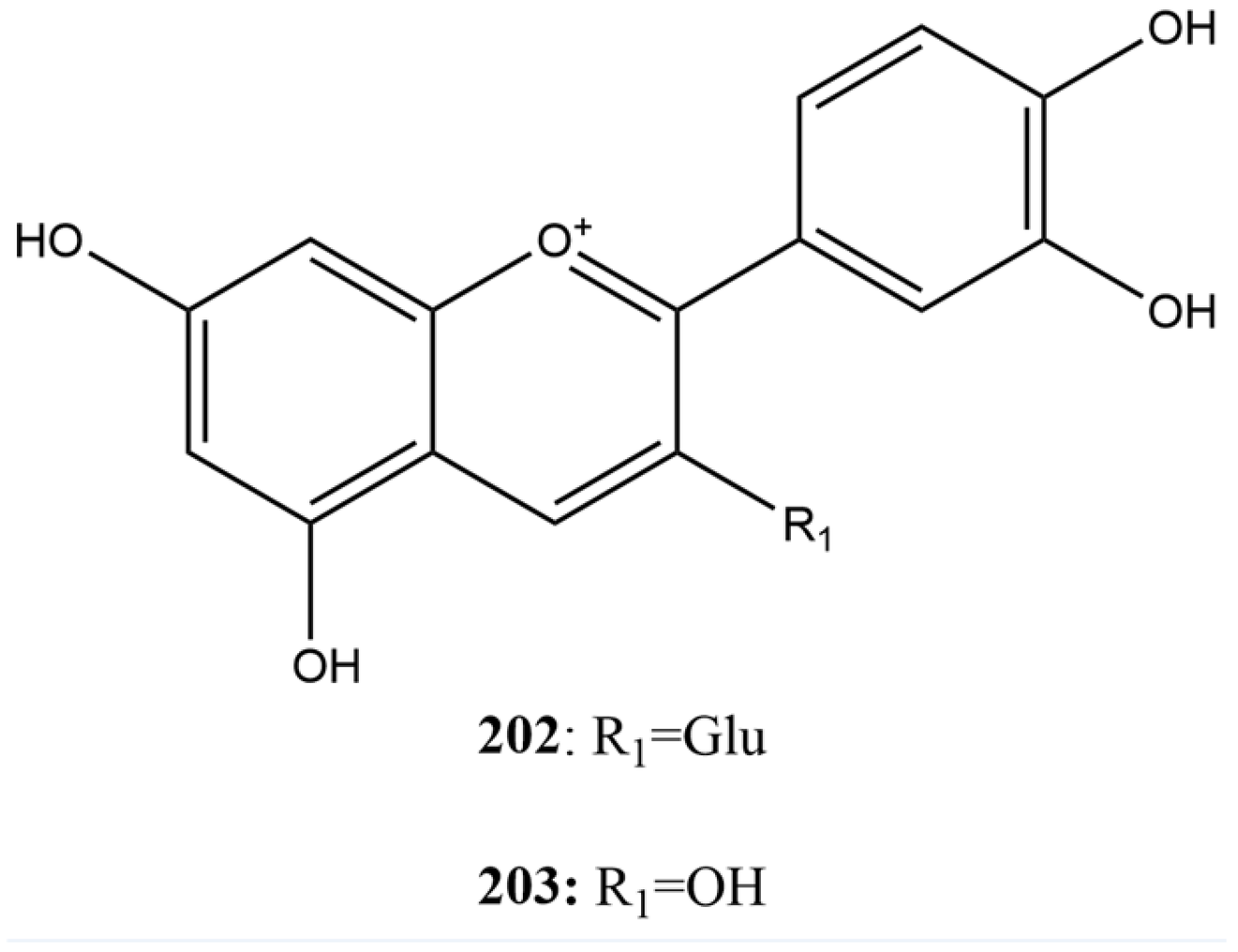
Anthocyanins are flavonoid glycosides with an anthocyanidin skeleton of C6-C3-C6, also known as the flavylium ion [156,160]. To identify potential inhibitors of SARS-CoV-2 3CLpro, Wang et al. [115] tested the compounds asterin (202) and cyanidin (203) (Figure 15) at a concentration of 100 µM using FRET assay. The IC50 values of the anthocyanins were 81.69 µM for compound 203 and 30.57 µM for 202. In this case, compound 202 was found to be more effective at inhibiting SARS-CoV-2 3CLpro. This difference may be attributed to the molecular structure of compound 202, which facilitates better interaction and binding with the active site of the protease, resulting in more effective inhibition.
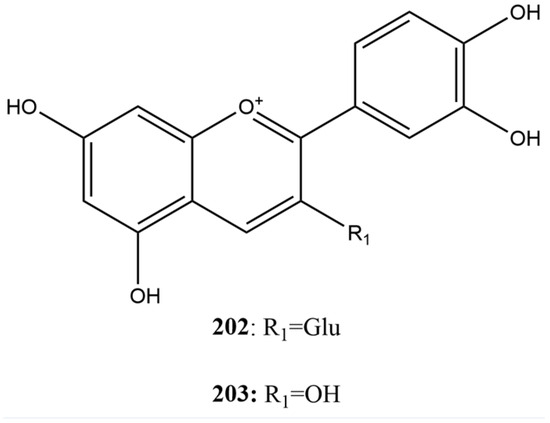
Figure 15.
Chemical structures of anthocyanins tested against SARS-CoV-2.
Another study conducted by Pillai et al. [180] investigated the antiviral potential of phytochemicals derived from cranberry, with a special focus on compound 202, to inhibit the main protease 3CLpro of SARS-CoV-2. Using molecular docking techniques, 202 was identified as one of the principal phytochemical compounds, exhibiting the lowest binding energy (−10.85 kcal/mol), indicating a high binding affinity to the protease’s active site. The inhibitory efficacy of 202 was confirmed through in vitro assays using the FRET technique, where an IC50 of 9.98 µM was determined. This value was the lowest among the compounds studied, highlighting compound 202 as the most potent 3CLpro inhibitor among the phytochemicals tested.
The studies conducted by Wang et al. [115] and Pillai et al. [180] highlighted compound 202 as an inhibitor of the SARS-CoV-2 3CLpro protease, with significantly lower IC50 values than other tested compounds. These findings suggest that compound 202 is a promising molecule for developing antiviral therapies against SARS-CoV-2 due to its ability to bind to the active site of the protease and inhibit its essential function in viral replication.
In summary, flavonoids demonstrated a wide range of inhibitory activities against SARS-CoV-2 targets, particularly 3CLpro. Flavones, flavonols, and flavanones showed the most promising results, with compounds like baicalein (71), myricetin (135), and naringenin (157) with an IC50 ranging from 0.09 to 0.94 μM standing out for their high efficacy. Despite belonging to different subclasses, the three most active compounds share a degree of structural similarity with the carbonyl in position 4 and possess hydroxyl groups at positions 5 and 7. Since many authors [115,126,143] have pointed out the importance of intermolecular interactions between compound groups and protein active site residues to demonstrate activity, the presence of the cited groups in these positions can lead to specific interactions with residues in a way that allows for better inhibition of the observed protein. However, to confirm this proposition, further studies focusing on these interactions are necessary.
2.3.5. Tannins
Tannins are polyphenol compounds that are widely found in plants and can be divided into two main groups: hydrolyzable and condensed tannins (or proanthocyanidins). Hydrolyzable tannins encompass polyesters of gallic acid and hexahydroxydiphenic acid (gallotannins and ellagitannins, respectively), with a sugar core of mainly glucose. Condensed tannins include oligomers and polymers of flavan-3-ols [181]. These secondary metabolites offer numerous health benefits, including anti-inflammatory, anthelmintic, antimicrobial, and antiviral properties, as documented in the literature [182].
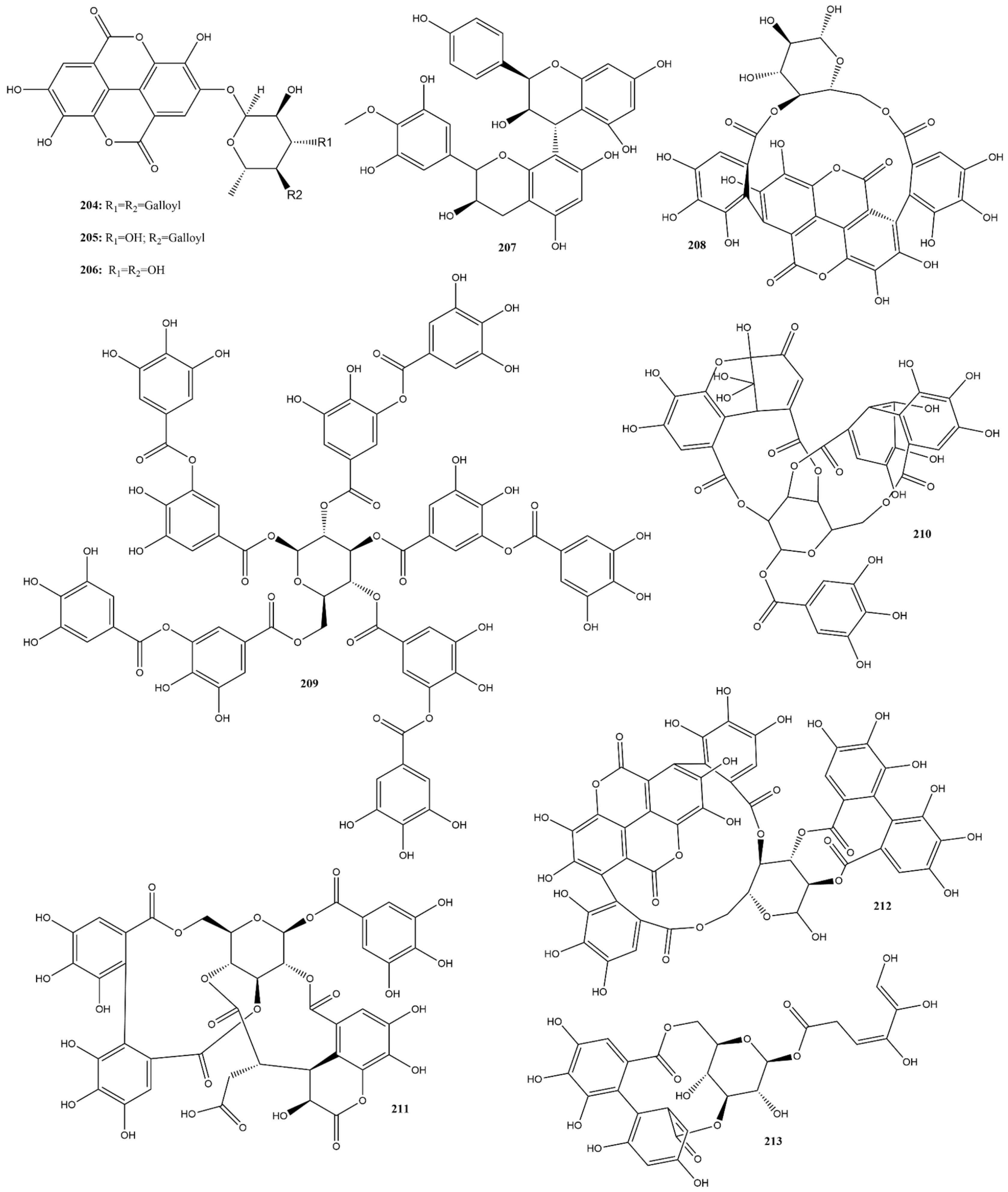
Among the compounds analyzed in this study, nine (204–206 and 208–213) were hydrolyzable tannins (Figure 16) with IC50 values ranging from 1.2−125 μM in the FRET assay for evaluating 3CLpro inhibition [108,144,151,161]. Regarding condensed tannins, only compound 207 was reported and had an IC50 value exceeding 125 μM for the same target analyzed [161]. Additionally, compounds punicalin (208), punicalagin (212), and tannic acid (209), all hydrolyzable tannins, were tested for the potential inhibition of RBD:ACE2 interaction, evaluated in vitro by different kits, presenting IC50 values of 9.0, 29.0 and 49.71 μM, respectively [96,105,108].
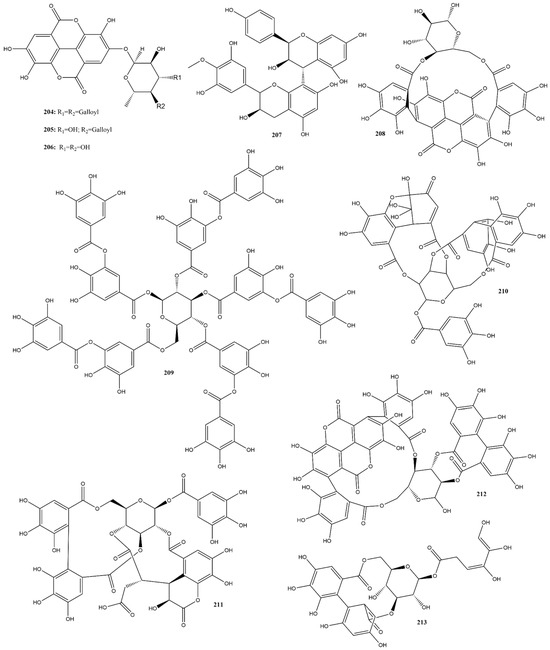
Figure 16.
Chemical structures of tannins tested against SARS-CoV-2 targets.
In a study conducted by Alharbi et al. [183], several hydrolyzable tannins isolated from alcoholic extracts of Terminalia brownii Fresen exhibited significant 3CLpro inhibitory activity. The most potent compound (4-O-(4″-O-galloyl-α-L-rhamnopyranosyl)-ellagic acid (205)) presented an IC50 value of 1.2 μM, while the other two representatives of this class, eschweilenol C (206) and 4-O-(3″,4″-di-O-galloyl-α-L-rhamnopyranosyl) ellagic acid (204), exhibited IC50 values of 10.0 and 20.1 μM respectively, using GC376 as a positive control (IC50 0.42 µM). Furthermore, cytotoxicity analysis indicated that none of these compounds demonstrated any toxic effects at concentrations corresponding to their antiviral activity, with CC50 values greater than 100 μM in A549 and HUVEC cells [183]. These findings confirm the potential of these compounds as safe antiviral agents.
Molecular docking studies performed with these compounds using AutoDock 4.2, showed binding energies of −9.3, −8.1, and −8.5 kcal.mol−1 for compounds 205, 206, and 204, respectively. The overlay confirmed that they occupied a similar binding site on 3CLpro as employed by ritonavir as a positive control. In addition, a structural activity relationship (SAR) analysis concluded that the presence of a sugar moiety notably influenced the activity [183]. Compound 205, with a single galloyl moiety acylation at OH-4, showed higher activity than those with double acylation at OH-3, suggesting that increased acylation diminished activity due to steric hindrance. These results complement the in vitro finding that compound 205 is a promising agent against 3CLpro of SARS-CoV-2 [183].
Two other hydrolyzable tannins, chebulagic acid (211) and punicalagin (212) exhibited moderated antiviral activity in vitro against 3CLpro, as observed by Du et al. [151], with IC50 values of 9.09 ± 0.87 μM and 4.62 ± 0.27 μM, respectively, also evaluated using a FRET assay. Additionally, the CC50 values of 211 and 212 were around 100 μM, conferring SI values above 10 and 13, respectively [151]. Molecular docking studies have revealed that both can form stable interactions within the cleft between domains II and III of 3CLpro. This binding site is positioned slightly apart from the substrate-binding pocket, where the catalytic dyads His41 and Cys145 are formed. This study demonstrated that compounds 211 and 212 are a novel class of SARS-CoV-2 inhibitors that allosterically regulate 3CLpro activity.
In conclusion, tannins, particularly hydrolyzable tannins, have shown promising antiviral activity against SARS-CoV-2. Several studies have highlighted the potential of specific tannin compounds to inhibit the 3CLpro enzyme, providing insights into their potential as therapeutic agents. However, condensed tannins have limited commercially available analytical standards, and their isolation is a time-consuming endeavor due to their diverse range of structures, which may explain the scarcity of studies on their effectiveness against SARS-CoV-2.
2.3.6. Coumarin
Coumarins (2H-1-benzopyran-2-ones) are naturally occurring heterocyclic compounds consisting of fused benzene and α-pyrone rings [184]. Natural coumarins are classified as simple coumarins (1,2-benzopyrone), furanocoumarins (psoralen and angelicin types), pyranocoumarins (xanthyletin and seseline types), phenylcoumarins and dicoumarins [185]. The computational analyses identified coumarin derivatives as potential agents against SARS-CoV-2 [186,187,188].
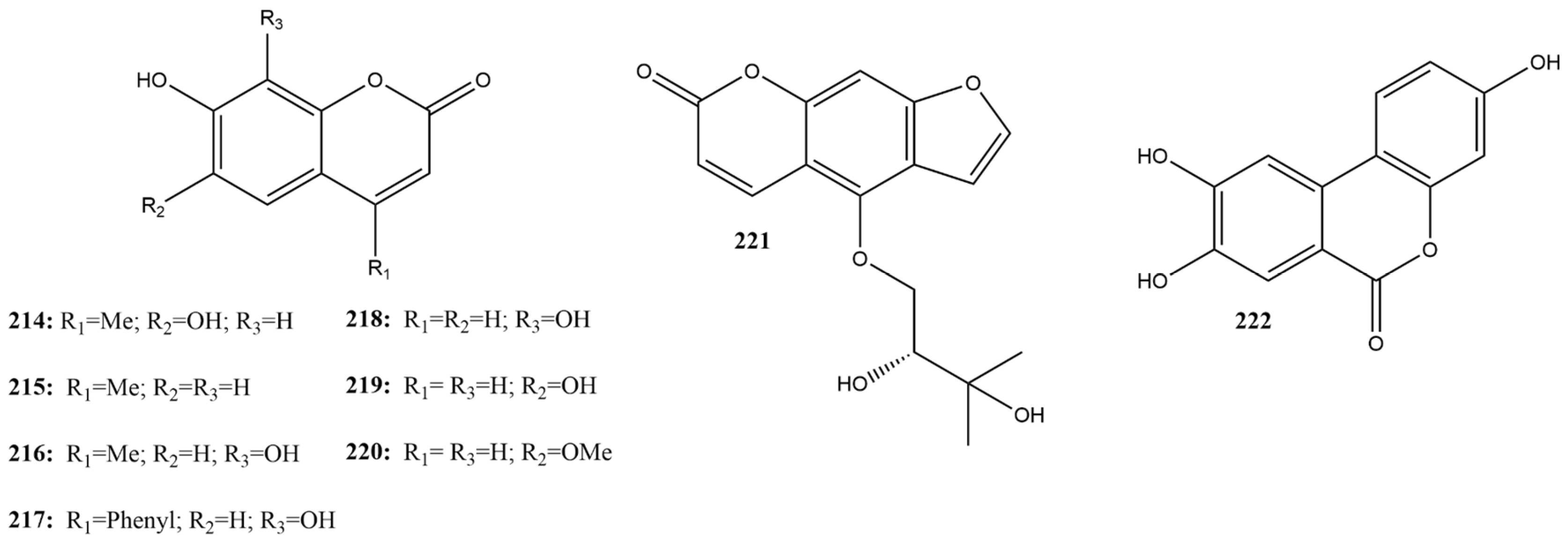
In this review, nine coumarins (214–222) were identified in the literature (Figure 17), of which four were effective 3CLpro inhibitors of SARS-CoV-2, with compounds containing hydroxyl groups at positions 7 and 8 showing greater potential.
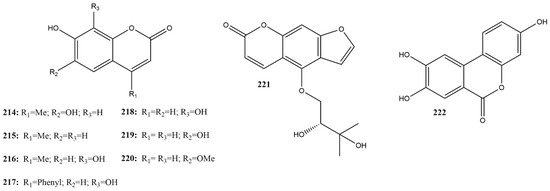
Figure 17.
Chemical structures of coumarins tested against SARS-CoV-2 targets.
In studies that screened catechol derivatives against SARS-CoV-2 3CLpro, myricetin (135) was used as a positive control (inhibition rates of 100% at a concentration of 100 µM). Simple coumarins with a 7,8-dihydroxy-2H-chromen-2-one structure, such as 7,8-dihydroxy-4-methylcoumarin (216), 7,8-dihydroxy-4-phenylcoumarin (217), 7,8-dihydroxycoumarin (218), and urolithin C (222), showed inhibition percentages at 100 μM of 94.91, 83.33, 66.11, and 9.59%, respectively [115].
The isomeric form of coumarins, chromans (1,4-benzopyrone), presents important results in the inhibition of SARS-CoV-2 3CLpro. The chromones 7-O-methylaloeresin A (237), aloeresin D (239), and aloesin (240) are present in the plant of the genus Aloe, including the species A. perryi Baker and A. barbadensis Miller [122,189], and show activity against 3CLpro inhibitors with percentages of inhibition 60.5 ± 1.7%, 72.6 ± 1.1% (IC50 125.3 ± 24.5 μM), and 80.1 ± 2.9% (IC50 38.9 ± 8.6 μM), respectively [122].
In an in silico study, the compounds 239 and 240, anchored in the active site of 3CLpro, showed interaction with compound 240 binding with the lowest free energy −7.5 kcal/mol and then with 239 at −6.8 kcal/mol. Compound 240 was bound in an extended conformation and completely occupied the active sites. In contrast, 239 presented a more sterically restricted conformation with possible interference from the vinylphenol ring. This may explain the difference in IC50 values between compounds 239 and 240 [122].
In evaluating the inhibitory activities of SARS-CoV-2 PLpro, furanocoumarin oxypeucedanine hydrate (221) showed an enzymatic inhibition at 100 μM of 15.7 ± 3.2% and at 20 μM and 10.9 ± 9.0% [58].
Wang et al. [115] evaluated the influence of vicinal hydroxyls in coumarins; the simple coumarins that have the structure 6,7-dihydroxy-2H-chromen-2-one did not show inhibitory potential at a concentration of 100 μM carried out in 3CLpro, for the compounds 4-methylesculetin (214), esculetin (219) and scopoletin (220). Similar inactivity was observed for the monohydroxylated compound 4-methylumbelliferone (215).
In this review, for inhibitory activity on SARS-CoV-2 proteins, molecules with the structure 7,8-dihydroxy-2H-chromen-2-one showed greater potential to be used as precursor molecules in the development of medicines and studies on a possible effect of transannular resonance of these structures [138]. In studies of the structure-activity relationship of coumarins in antiviral activity against influenza viruses, the presence of the aglycone 6,7,8-trihydroxy coumarin proved to be indispensable [190].
2.3.7. Stilbenes
Stilbenes are a class of plant secondary metabolites widely found in food and medicinal plants. They can exist as monomers or polymers, increasing their structural diversity through polymerization [191]. Structurally, they have a C6–C2–C6 skeleton, usually in two isomeric forms. Pharmacological research has shown that stilbenes exhibit various biological activities, including antitumor, anti-inflammatory, antioxidant, antibacterial, and antiviral effects [192]. Due to their structural properties and diverse biological activities, stilbenes have gained increasing attention from scientists.
Regarding anti-SARS-CoV activity, this review identified seven compounds (223–229) (Figure 18) from this class that were tested for their potential to inhibit 3CLpro. In the in vitro FRET assay, all compounds had IC50 values greater than 42.5 μM. Although the compounds vatalbinoside A (227), hopeaphenol (228), and vaticanol B (229) were described by Tietjen et al. [107] as potent and selective inhibitors of RBD:ACE2 binding and viral entry with IC50 values of 0.24, 0.11, and 0.07 μM, respectively, using RBD:ACE2 binding assay. Conversely, the resveratrol monomer exhibited almost no activity in this assay (IC50 > 100 μM), suggesting that inhibition requires a multimeric structure.
Figure 18.
Chemical structures of stilbenes tested against SARS-CoV-2 targets.
In this work, the authors screened a natural product library using the AlphaScreen-based RBD–ACE2 interaction in an in vitro assay. The top hits from this screen, which included the plant stilbenoids described above [107], demonstrated significant inhibition of in vitro replication of infectious SARS-CoV-2. Their IC50 values ranged from 10.2 to 37.0 μM, with no observed cytotoxicity. These inhibitory effects were similar to those of the control antiviral remdesivir (IC50 1.0 μM). Furthermore, these compounds effectively inhibited the B.1.1.7 (alpha) and B.1.351 (beta) variants in both viral and spike-containing pseudovirus assays, showing comparable or superior activity to the USA-WA1/2020 variant. This suggests that compound 228 and its related stilbenoid analogs possess potent and selective inhibitory properties against viral entry across various SARS-CoV-2 variants [107].
Therefore, the findings described herein provide valuable insights that underscore the potential of stilbenes and their derivatives as promising candidates for the development of antiviral therapies targeting SARS-CoV-2. In summary, this class of compounds had considerable activity in inhibiting the RBD:ACE2 interaction but not so prominent in inhibiting proteases. The most active compounds have a multimeric structure, which appears to be important for the observed inhibition. This effect, coupled with their low cytotoxicity, highlights the importance of further exploration of stilbenes as potential sources of antiviral agents.
2.3.8. Quinones
Quinones are natural compounds that are present in plants, bacteria, fungi, and animals. Structurally, they have a ring formed by a system of double bonds and ketonic groups (benzoquinones), with the possibility of structural variations due to the inclusion of the benzene ring, differentiating them from other classes such as naphthoquinones, anthraquinones, and phenanthraquinone [193,194]. Several studies have demonstrated that these compounds are potential alternatives to SARS-CoV-2 [32,195,196].
In this review, seventeen compounds (230–246) (Figure 19) were selected due to their structural similarities. One group is related to compounds containing 1,4-benzoquinone or 1,2-benzoquinone, as observed in quinones, and the other group is related to compounds containing the pyran-4-one ring, as observed in chromones. Of these compounds, twelve showed a percentage of enzyme inhibition above 50%.
Figure 19.
Chemical structures of quinones tested against SARS-CoV-2 targets.
Wang et al. [115] developed a study that indicated the influence of catechol group in inhibiting activity and covalent binding with 3CLpro protease—included in this study the quinones and their derivatives, such as the benzoquinone ubidecarenone (246), which presented low inhibition 4.15% in 100 μM, and the anthraquinones, alizarin (230), anthracenic (231), and purpurin (235), with a percentage of 18.5, 1.18, and 37.53% in 100 μM, respectively.
The physcione (234), anthraquinone present in Rhei Radix et Rhizoma showed an antiviral effect in the reduction test of plates to determine the activity anti-SARS-CoV-2 with EC50 of 15.58 ± 0.77 μM and inhibiting effect in the activity 3CLpro of SARS-CoV-2 with IC50 7.56 ± 0.78 μM. In computational analyses, the interaction between the inhibitor and SARS-CoV-2 3CLpro occurred at amino acids Gln 19, Thr 26, Leu 27, Asn 28, Tyr 118, Asn 119, Gly 143, and Cys 145 [143].
Enzymatic inhibition tests, using GC376 as a positive control, revealed that hypericin (242) exhibits inhibitory activity against the 3CLpro of SARS-CoV-1 (19.43 ± 3.11 μM), SARS-CoV-2 (23.30 ± 1.21 μM), and MERS-CoV (49.65 ± 5.41 μM). This indicates that naphthodianthrone can potentially be a pan-anti-coronavirus agent, as it interacts with and inhibits the 3CLpro of different human coronavirus pathogens [116].
Other dimers of quinones, the dianthrones sennoside A (244) and sennoside B (245), had inhibitory action on SARS-CoV-2 3CLpro, with 67.41% and 78.46% inhibition of enzymatic activity at 100 µM, respectively [115]. For the compound 245 was calculated IC50 0.10 μM, and the positive control (GC376) IC50 0.044 μM [126].
In an in silico study to evaluate the interaction of inhibitors with the SARS-CoV-2 3CLpro active site residue, compound 245 formed hydrogen bonds with sites Thr190, Glu166, Asn142, and Cys44 being two connections with carbonyl, another two with the lateral chain of carboxylic acid of Thr190, and connections with the lateral chain of carbonyl of Asn142 and thiol group of Cys44. Other existing interactions were van der Waal with sites Pro168, Gln189, and Met49 [126].
It is possible to include other oxygen compounds with structural similarities to the catechol group, such as xanthones dibenzo-ỵ-pyrone and xanthene. Phenylfluorone (243) and gallein (241) inhibited 3CLpro by 92.51% and 100% at 100 μM, respectively. The Mangiferin xanthone (236), on the other hand, had an inhibition rate of 28.40% in the same concentration [115].
Naphthoquinone acetylshikonin (238) demonstrated enzymatic inhibition against SARS-CoV-2 PLpro, and it achieved 84.3 ± 4.6% at 100 μM and 41.7 ± 3.8% at 20 μM. However, it was considered a weak inhibitor of PLpro due to its IC50 value of 24.3 μM [58].
Anthraquinone (232) (anthracene-9,10-dione) obtained an EC50 of 55.9 μM and could effectively block the entry of pseudovirus in cells with superexpression of ACE2, but overall, compound 232 did not present significant inhibitory effects [96].
The natural compound emodin (233) was used as a positive control (EC50 126.1 μM), as it was previously identified to be capable of blocking the binding of the S protein of coronavirus to the ACE2 protein. Another important fact is that compound 233 was a less effective inhibitor of binding the S protein to ACE2 than the synthetic inhibitor nafamostat mesylate as a positive control (EC50 11.34 μM) [96].
The best results were observed for quinones with a hydroxyl vicinal to the ketone group or vicinal hydroxyls in the benzene ring. It is noteworthy that the size of the molecules did not prove to be a determining factor, as seen in the quinone dimers 244 and 245.
2.3.9. Lignoids
Lignoids are a large class of natural compounds comprising two phenylpropane units with diverse chemical structures and biological activities [197,198] (The monomers that form lignoids include cinnamic acid, cinnamyl alcohol, propenyl benzene, and allyl benzene. When the molecular bonding of monomers occurs between the β-β′ positions (also referred to as 8-8′), these compounds are designated as “classical lignans”. In contrast, compounds are grouped as “neolignans” if the main structural units are coupled in any other manner (not β-β′ bonding). Neolignans exhibit more varied structures than classical lignans [199]. Plant-derived lignans and other lignoids have been investigated in the fight against COVID-19 [200,201,202].
In this review, twelve lignoids (247–258) were cataloged (Figure 20), seven of which were effective as SARS-CoV-2 inhibitors, three of which inhibited 3CLpro and PLpro.
Figure 20.
Chemical structures of lignoids tested against SARS-CoV-2 targets.
The two epimeric 7,8-secolignans-(1S,2R)-1-(3,4-dimethoxyphenyl) -2-methyl-3-oxobutyl-3,4-dimethoxybenzoate (257) and 7,8-secolignans-(1S,2S)-1-(3,4-dimethoxyphenyl)-2-methyl-3-oxobutyl-3,4-dimethoxybenzoate (258) was shown to inhibit 3CLpro with IC50 values of 4.88 ± 0.60 μM and 4.75 ± 0.34 μM, respectively. These secolignans also exerted an action in regulating the inflammatory response and preventing lung injury, and are associated with SARS-CoV-2 inhibition via 3CLpro. During in silico studies, secolignans showed a strong binding affinity with energies of −29.75 and −28.48 kcal/mol, respectively. These binding sites are similar, emphasizing Thr26, Cys145, Met49, and Asp187 [203].
Honokiol (252) demonstrated inhibitory activity against the entry of the SARS-CoV-2 spike pseudovirus into HEK-293 T-ACE2h cells at maximum non-toxic concentrations (CC50 and IC50 of 50μM). In assays of in vitro α-glucosidase inhibitory activity, compound 252 showed an inhibition rate of 17.1 ± 1.7% [198], and in a study evaluating PLpro inhibition using an assay fluorescence analysis based on the cleavage of a Z-RLRGG-AMC substrate showed an enzymatic inhibition of 5.3 ± 3.2% at 100 μM and 4.9 ± 4.6% at 20 μM. In a similar assay evaluating PLpro inhibition, magnolol (251) showed a percentage of enzyme inhibition of 23.5 ± 5.7 at 100 μM and 4.3 ± 6.6 at 20 μM [58].
The neolignans of the diarylbutane type, mesodihydroguaiaretic acid (247), nordihydroguaiaretic acid (248), and pregomisin (249), in in vitro studies showed promising results as inhibitors and promising anti-inflammatories of SARS-CoV-2 3CLpro with values of 44.97% (IC50 4.12 ± 0.38 μM), 48.43% (IC50 6.06 ± 0.62 μM), and 58.02% (IC50 3.07 ± 0.38 μM), respectively, and positive control GC376 (80.98%), and for PLpro with 56.40% (IC50 4.24 ± 0.46 μM), 30.69% (IC50 16.28 ± 0.54 μM), and 51.39% (IC50 5.23 ± 0.33 μM), respectively, and positive control GRL0617 (83.45%) [202].
The studies of compounds 247, 248, and 249 by molecular docking confirmed their binding affinities to the SARS-CoV-2 protein target in relation to 3CLpro of −31.26, −34.50, and −21.33 kcal/mol, respectively, and binding affinities with PLpro of −26.64, −33.00, and −21.32 kcal/mol, respectively. Compound 249, which has the highest number of methoxy groups, showed a higher percentage of inhibition and binding energy [202].
Lignan phillyrin (254) did not show inhibitory activity against 3CLpro and PLpro at concentrations of 10 and 100 μM [138]. Likewise, other lignans have not been shown to inhibit SARS-CoV-2 3CLpro using a FRET assay, such as 4′-demethylepipodophyllotoxin (255), podophyllotoxin (256) and secoisolariciresinol diglucoside (250) or the allolignan denudatin B (253), which showed a low rate of inhibition (9.66%) [115].
Examples described in this review include lignoids among the metabolic classes that show potential as future inhibitors of 3CLpro and PLpro. However, more in-depth studies of some factors need to be carried out, such as the conformation and size of the molecule and the influence of ligands, highlighting the presence of vicinal methoxy groups replacing vicinal hydroxyls, hindering a decrease in this activity, as observed in compounds 253 and 255.
2.3.10. Alkaloids
Alkaloids are a diverse group of metabolites found in higher plants that are categorized by the presence of a heterocyclic nitrogen ring system. Many alkaloids exhibit significant biological activities and have been utilized in medicinal and pharmacological research [204].
Regarding SARS-CoV-2 activity, in a recent study, bisbenzylisoquinolines (259–270) (Figure 21) were tested against SARS-CoV-2 3CLpro using the covalent inhibitor GC-373 as a positive control (97.24% inhibition) with a FRET assay at a concentration of 100 µM [132]. From the root of Tiliacora triandra (Colebr.) Diels isolated dinklacorine (259), 2′-nortiliacorinine (260), tiliacorinine (261), and yanangcorinine (262), while liensinine (263), isoliensinine (264), and neferine (265), were obtained from an embryo of Nelumbo nucifera Gaertn. The compound 264 displayed the highest inhibition with 63.89% (IC50 29.93 µM), followed by 265 with 55.23% (IC50 76.98 µM), 263 with 53.57% (IC50 93.75 µM) and 259 with 50.72% (IC50 < 100 µM).
Figure 21.
Chemical structures of alkaloids tested against SARS-CoV-2 targets.
Compounds 260, 261, and 262 displayed lower activity (<35%). Their molecular docking and molecular dynamics simulations exhibited strong affinity with binding energies ranging from −8.1 to −9.3 kcal/mol due to van der Waals and π-interactions of the aromatic system. They showed RMSD values ranging from 0.5 to 2.5 Å, with high negative energies for the catalytic site Cys145, except 260, interacting with aromatic and piperidine rings through π-cation and alkyl interactions. At the same time, the residues Leu167, Pro168, and Gln192 also contribute to π-sigma, alkyl, and van der Waals interactions [132].
Two quinolizidine alkaloids, matrine N-oxide (266) and matrine (267), were tested against 3CLpro at concentrations of 10 and 100 µM. Nevertheless, only 267 were active, exhibiting 0.82% inhibition at 100 µM [115].
The naphthylisoquinoline alkaloid korupensamine A (269A) was tested against SARS-CoV-2 3CLpro using an FRET-based enzymatic assay to evaluate protease inhibition, compared to the reference covalent inhibitor GC-376 (IC50 of 0.88 ± 0.15 μM). It showed an IC50 value of 2.53 ± 0.14 μM, while its atropisomer korupensamine B (269B) was inactive up to 50 μM, with an IC50 of 71.89 ± 0.49 μM. However, both alkaloids exhibited similar molecular dynamics simulations with a docking score of −7.90 kcal/mol and comparable ΔGbinding (by free energy perturbation method) of −8.57 and −7.04 kcal/mol, respectively. Also, their binding stability in the catalytic site was similar with RMSDs of 2.43 Å and 3.72 Å for 269A and 269B. The simulation data only exhibited differences for 269A, forming stable hydrogen bonds with Ser144, His163, and Arg188, and hydrophobic interactions with Met165 and Cys145 [205].
Moreover, the piperidine alkaloid lobeline (270) was tested for inhibitory activity against SARS-CoV-2 PLpro using a FRET-based assay, but it showed a 3.7 ± 2.6% inhibition at a 20 µM concentration and 14.6 ± 7.7 at 100 µM [58].
In another study, an indole alkaloid, 9-methoxycanthin-6-one (268), was tested against the spike protein with the RBD:ACE2 kit, displaying an EC50 of 36.21 μM, using emodin (EC50 126.1 μM) and nafamostat mesilate (EC50 11.34 μM) as positive controls. Molecular docking simulation indicated that 268 inhibits the binding of the spike protein to ACE2, blocking any interaction between the spike protein and ACE2 complex. In addition, 268 has mainly polar interactions with the catalytic site, including the residues Arg403, Asp405, Tyr453, and Tyr505 in the spike protein, and Asp30, Asn33, His34, Glu37, Lys353, Ala387, Gln388, Pro389, and Phe390 in ACE2 [96].
The cited examples demonstrate that alkaloids have received limited attention during in vitro assays of SARS-CoV-2 activity. The vast and diverse nature of alkaloids makes it challenging to thoroughly explore all possible antiviral compounds. Additionally, the complex mechanisms of SARS-CoV-2 infection require the targeting of specific areas within the virus, making it difficult to predict which alkaloids might be effective. Furthermore, the focus on other compounds or drugs with established antiviral activity may have overshadowed research on alkaloids. These combined factors have contributed to the limited exploration of alkaloids during in vitro assays of SARS-CoV-2 activity.
2.3.11. Terpenes
Terpenoids, also known as isoprenoids, are a significant family of natural metabolites consisting mainly of isoprene units (Figure 22). They are primarily of plant origin and comprise hydrocarbons as well as oxygen-based compounds, such as alcohols, aldehydes, and ketones, which enhance their chemical diversity. Several terpenoids have potential pharmacological applications, including managing type 2 diabetes, hyperlipidemia, insulin resistance, cardiovascular disease, and metabolic syndrome [206].
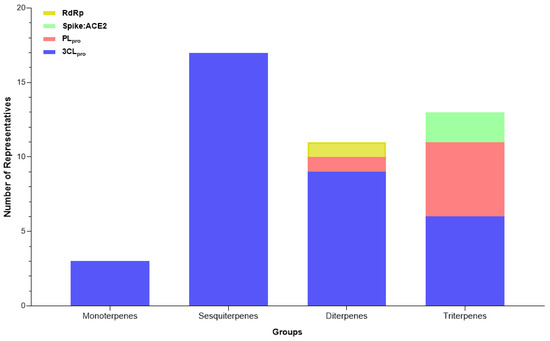
Figure 22.
Subclasses of terpenoids and their number of representatives, tested against selected targets of SARS-CoV-2 (3CLpro, PLpro, Spike:ACE2, and RdRp), tracked by the review.
Many terpenoids (271–311) (Figure 23) have been screened against 3CLpro and have shown a wide range of inhibitory activities against SARS-CoV-2. Most studies have used the FRET assay, with ebselen, GC376, or baicalein as a positive control.
Figure 23.
Chemical structures of terpenes tested against SARS-CoV-2 targets.
Secoiridoid-derived oleuropein (304) showed excellent inhibitory activity against 3CLpro with an IC50 of 4.18 ± 0.19 μM. Other monoterpenoid glycosides tested (compounds 306 and 310) exhibited low activity (IC50 ≥ 100) [115]. Isolated sesquiterpenes from Artemisia argyi H. Lév. Vaniot showed promising inhibitory activity against the 3CLpro, displaying interesting IC50 values, such as 8.74 µM for argyin A (280), 21.46 µM for chrysanthemulide I (286), 23.17 µM for achillinin C (279), 24.64 µM for tanaphilin (307), 26.34 µM for argyin C (281), 33.45 µM for 3,3-dimethyl-γ-methylene-2-(3-oxobutyl)-cyclobutanoic acid (278), 35.27 µM for ilicic alcohol (303), and 35.58 µM for epi-baynol A (294) [207].
Compounds 279, 294, and 303 also showed reduced in vitro anti-inflammatory effects on nitric oxide secretion. Furthermore, molecular docking simulations indicated that unsaturated ketones in these metabolites could form hydrogen bonds with Thr24 and Thr26 residues, thereby achieving stability [207].
In guaiacane-type sesquiterpene lactones, such as 280 and 281, the diol hydroxyl groups at C3 and C4 of their five-membered rings form a stable hydrogen bond with residue Ser46. Compound 286, another guaiacane-type sesquiterpene lactone, also exhibited stable interaction with Asn119, indicating that these interactions can improve the inhibition of 3CLpro [207]. Furthermore, other isolated sesquiterpenes from Carpesium abrotanoides L. were also tested against 3CLpro, with 1β-hydroxy-8-epi-inuviscolide displaying the better IC50 of 16.58 μM followed by (4α,5α,8β,10β)-4,10-dihydroxy-1,11(13)-guaidien-12,8-olide with an IC50 of 36.81 μM. More compounds (288–290) were screened, but they lacked noticeable activity with an IC50 > 50 μM [208].
Additionally, diterpenes from dried roots of Salvia miltiorrhiza Bunge were also screened for 3CLpro, with tanshinone IIA (309) showed better results with an IC50 of 6.47 ± 0.92 μM, followed by dihydrotanshinone I (293) with an IC50 of 9.00 ± 3.87 μM, cryptotanshinone (291) with an IC50 of 9.70 ± 0.86 μM, compound 293 with an IC50 of 11.36 ± 0.52 μM and tanshinone I (308) with an IC50 of 14.20 ± 2.63 μM [121]. However, in another study, compound 309 exhibited only 53.9 ± 3.3% inhibition against 3CLpro at 100 μM [122].
From the Ginkgo biloba L. leaves, the terpene trilactones bilobalide (284) (sesquiterpene), ginkgolide A, B, and C (297–299) (diterpenes) were screened for 3CLpro and displayed extremely weak inhibition (IC50 > 100 µM) [139].
Triterpenoids from the resin of Boswellia sacra Flück were extracted and tested for PLpro. All the compounds showed more than 50% inhibition during the screening process with 3-O-acetyl-11-hydroxy-β-boswellic acid (273) exhibited the most significant inhibition with an IC50 of 5.95 ± 0.38 μM, followed by 3α,11α-dihydroxyurs-12-en-24-oic acid (271) with IC50 of 6.25 ± 0.41μM, α-acetyl 11-keto-β-boswellic acid (302) with an IC50 of 9.85 ± 0.59 μM, and 11-keto-β-boswellic acid with an IC50 of 11.10 ± 0.75 μM. Molecular docking simulations showed a docking score ranging from −8.18 to −8.69 kcal/mol, indicating interactions with the PLpro active site residues. Additionally, compound 271 formed multiple hydrogen bonds with Asp164, Arg166, and Tyr268 and displayed ionic interactions with Arg166. The carbonyl oxygen of compound 271 formed hydrogen bonds with Arg166, and its hydroxyl group interacted with Gly266 within the binding site. The hydroxyl group of compound 273 participated in hydrogen bonding with Tyr268 and Gln269, while carbonyl oxygen formed a bond with Lys157, which also provided an ionic interaction with the ligand. The compound 302 hydroxyl group and carbonyl oxygen also established hydrogen bonds with Glu167 and Tyr268, respectively [209].
Other triterpenoids were also screened but displayed weak interactions, such as friedelin (296), with an IC50 of 100.5 µM [210], celastrol (285) exhibited 57.7 ± 1.3% inhibition [122], xanthophyll (311) showed 10.33% inhibition, betulin (282) only inhibited 6.63%, while 18β-glycyrrhetinic acid (272) and sesquiterpene farnesol (295) were inactive [115].
Triterpenes from Glycyrrhiza uralensis Fisch., betulinic acid (283), and ursolic acid (301) displayed an EC50 of 15.1 and 9.0 μM, respectively, against the spike protein (ELISA). However, in an in vitro assay against SARS-CoV-2 pseudovirus, although they showed noticeable effects, they exhibited high cytotoxicity and were not evaluated further. Other triterpenes, such as betulin (282), were also tested at 10 μM and showed <50% inhibition of the spike protein [141]. Another study tested the triterpenoids ursolic acid (301) and glycyrrhetinic acid (300) against SARS-CoV-2 spike protein at 50 μM. The results showed that compound 301 produced 47% inhibition (CC50 49.16 ± 5.53 μM), while compound 300 had an 11.1% inhibition (CC50 > 100 μM). Docking simulations revealed that the steric effect of the carboxyl group at C17 in compound 301 could form a hydrogen bond that influenced its activity. In contrast, compound 300 possesses an oxo group at C13, which is unfavorable for exerting a biological effect [98].
Compound 283, isolated from Eurya chinensis R.Br, also inhibited PLpro, with an IC50 value of 2.03 μM. This was achieved by forming a direct hydrogen bond between Met208 and its carbonyl oxygen. Van der Waals interactions were also observed for Pro248, Thr301, Thr268, Gly163, Leu162, and Lys157 in the bottomless and broad S4 pocket of PLpro [211]. Compound 283 was also tested for 3CLpro. However, it showed weak inhibition, with an EC50 > 20 μM and 72.2% inhibition at 100 μM [212]. The diterpenoid oridonin (305) displayed an EC50 13.46 μM for 3CLpro, with over 90% inhibition. However, it exhibited a weak inhibitory effect against PLpro with an EC50 611.8 μM [213].
Research on terpenoids as potential agents against SARS-CoV-2 has identified several promising compounds. Notably, compounds 304 (seco-iridoid) and 309 (diterpene) showed inhibitory activity against 3CLpro, highlighting the therapeutic potential of terpenoids in combating viral infections. Further exploration of other subclasses, such as sesquiterpenes and triterpenes, is warranted to investigate their potential as antiviral agents. However, there have been no studies on monoterpenes in essential oils, possibly due to the challenges in screening these compounds due to their high volatility. Fluctuations in temperature and other reaction conditions can affect stability, making it difficult to obtain accurate data [214].
2.3.12. Saponins
Saponins are plant glycosides classified as steroids or triterpene compounds. Their oligosaccharides can be either linear or branched, and, based on the number of attachment sites, they are categorized as monodesmosides (one attachment site), bisdesmosides (two attachment sites), or tridesmosides (three attachment sites) [215].
Saponins exhibit various biological and pharmacological activities, including immunomodulatory and antitumor properties [216]. However, only a few have been examined for interactions with SARS-CoV-2 proteins (312–319) (Figure 24), including 3CLpro activity, using the FRET assay or spike protein with the RBD:ACE2 kit. In this review’s bibliographical summary listed in Supplementary Material, the inhibition rates ranged from 0% to 50% at a concentration of 8.3 µM up to >125 µM.
Figure 24.
Chemical structures of saponins tested against SARS-CoV-2 targets.
Glycyrrhizin (314), a saponin isolated from licorice roots (Glycyrrhiza uralensis Fisch.) reported contradictory results with respect to 3CLpro inhibition activity in different studies. In one, it showed complete inhibition of 3CLpro at 2000 µM, similar to the positive control GC376 at 100 µM, and reduced 3CLpro activity by 70.3% at 30 µM [217]. However, another report stated that 314 did not inhibit 3CLpro at concentrations of 10 and 100 µM [115]. Even so, a case report detailed the compassionate use of 314, specifically diammonium glycyrrhizinate, combined with ascorbic acid, for treating COVID-19. The treatment was administered orally three times daily for 8 days, with 150 mg of diammonium glycyrrhizinate and 200 mg of ascorbic acid. The patient’s condition improved after 12 h of treatment and eventually recovered from the disease. Nevertheless, further controlled studies are needed to substantiate the therapeutic effects of 314 in COVID-19 [218]. In addition, ziyu-glycoside I (317) and ginsenosides Rb1 and Rb2 (318 and 319) were tested for 3CLpro; however, none displayed inhibitory activity at the concentrations analyzed [161,219].
In a recent study with Glycyrrhiza uralensis Fisch., two isolated saponins, licorice-saponin A3 (312) and glycyrrhizin (314), showed an IC50 of 8.3 ± 1.6 µM and 10.9 ± 1.3 µM, respectively, for the spike protein. At 10 µM, compound 314 exhibited an inhibition rate of 51.9%, while compound 312 featured 45.1%. The binding affinity was determined by surface plasmon resonance (SPR) analysis, with KD values of 314 and 312 with spike protein 2.33 µM and 33.6 µM, respectively. Molecular docking indicated that the 314 hydroxyl group in C3, carbonyl group in C11, and carboxyl group in C30 formed hydrogen bonds with Y453, N501, and G502 residues, respectively. Meanwhile, 312 carboxyl groups of 2′-O-glucuronyl and 3-O-glucuronyl interact with Y453 and N501 residues, specifically, while the hydroxyl group of the C30 glucosyl residue interacts with the G446 residue [219].
Recent studies have shown that plant saponins have potential antiviral activities against SARS-CoV-2. Because of their amphiphilic structure, saponins have natural surfactant properties, allowing them to interact with the viral envelope and disrupt its integrity [220]. Additionally, saponins have been found to modulate the host immune response, potentially reducing the severity of viral infections [94]. However, they can increase the foamability of aqueous solutions, even at low concentrations, or aggregate to form micelles at critical micelle concentrations [221]. These properties may pose a challenge for in vitro testing of saponins with SARS-CoV-2 proteins, indicating that only a few have been tested as natural antiviral agents.
2.3.13. Other Compounds
Other chemical compounds with in vitro activity against various SARS-CoV-2 targets have been reported in the literature. There are two representative classes of curcuminoids (curcumin (322) and tetrahydrocurcumin (323)), steroids (estriol (324) and estrone (325)), and capsaicinoids (cis-capsaicin (326) and trans-capsaicin (327)), as well as a representative class of organic acids (myristic acid (320)), benzaldehyde (4-hydroxybenzaldehyde (321)), benzophenone (2,3′,4,4′,6-pentahydroxybenzophenone (328)), coumestan (wedelolactone (329)), and aurone (6-hydroxy-4-methoxy-5,7-dimethylaurone (330)) (Figure 25).
Figure 25.
Chemical structures of other compounds tested against SARS-CoV-2 targets.
Curcuminoids are natural polyphenol compounds derived from turmeric (Curcuma longa L.), which have garnered significant attention for their potential in treating conditions characterized by immune system perturbations and inflammatory responses, including COVID-19 [222]. Among these compounds, 322 and 323 have received considerable attention due to their anti-inflammatory, anticarcinogenic, antiviral, and antitumor activities [175,223,224].
Wang et al. [115], in their screening for SARS-CoV-2 3CLpro inhibitors, observed a significant difference in the activity profiles of 322 and 323 at a concentration of 100 μM using a FRET substrate. Inhibition percentages were 79.14% and 0%, respectively. On the other hand, molecular docking studies revealed that these compounds exhibited low binding energies and high affinity for 3CLpro, with binding energy values of −5.7 and −7.1 kcal/mol for compounds 322 and 323, respectively [225]. This demonstrates the importance of in vitro assays for confirming computational analyses.
Steroids are compounds characterized by a skeleton composed of isoprenoid precursors and a fused tetracyclic system, such as androstane and related structures, estrane, gonane, cholestane, and protostane [226]. Despite the structural differences in plant steroids, they affect viral transcription/replication alteration, and their inhibitory, antioxidant, and immunomodulatory activities support host defense mechanisms and cell survival during viral infections [227,228].
Wang et al. [115], in their in vitro screening of 101 natural products using the FRET assay for 3CLpro, tested two steroids: 324 and 325. However, no inhibition was observed at the tested concentration (100 μM). This does not rule out the possibility of the activity of these compounds on other SARS-CoV-2 targets, considering that various studies have highlighted the potential of this class and its derivatives for combating this virus [228,229].
Capsaicinoids are amides from the plant genus Capsicum, which are secondary metabolites that impart a hot taste to fruits and vegetables, also known as pungency. They are used for flavoring and preserving food as well as for medicinal purposes [230]. Capsaicin (326–327), an active component of red chili peppers, is chemically known as 8-methyl-N-vanillyl-6-nonenamide. It is the most abundant compound in Capsicum species, accounting for 69% of total capsaicinoids [231,232].
An integrated silico–in vitro approach was used to identify natural products with activity against SARS-CoV-2 [58]. Initial experimental validation of selected virtual hits was conducted using a commercial PLpro kit, with GRL-0617 (20 μM) as the positive control, achieving an enzyme inhibition of 96.6% ± 10.3, with an IC50 of 0.8 μM. In this screening, the compound 327 exhibited low enzyme inhibition, with 5.3 ± 0.1% at 100 μM and 5.6 ± 9.7% at 20 μM. Another screening conducted with compound 326 by Wang et al. [115] also showed no inhibition of 3CLpro at 100 μM.
These results do not corroborate previous in silico studies that suggested the potential of compound 327 against various targets (S protein, 3CLpro, PLpro, TMPRSS2, and RdRp) with binding energies ranging from −5.7 to −7.8 kcal/mol [233,234,235]. This highlights the importance of in vitro analyses to validate computational assays and underscores the interest in exploring other potential targets of SARS-CoV-2.
Carboxylic and organic acids contain a carboxyl functional group attached to a hydrocarbon radical. These acids are widely distributed in nature and serve as intermediates in the degradation pathways of amino acids, fats, and carbohydrates [236]. Recent studies have investigated the possible mechanisms of action of this class against SARS-CoV-2 targets, primarily by evaluating its role in proteases [237,238].
Wang et al. [115] conducted in vitro screenings of a high-content protease inhibitor library against 3CLpro, using FRET assay-based screening to identify structurally diverse compounds with potential as antiviral leads. Compound 319, at a concentration of 100 μM, showed 71.64% inhibition of 3CLpro and was characterized as a substance with irreversible covalent inhibition.
Benzaldehyde, C6H5CHO) is the simplest and most industrially useful member of the family of aromatic aldehydes. This aromatic aldehyde exists in nature, primarily in combined forms such as glycosides in almonds, apricots, cherries, and peach seeds [239]. Srinivasan et al. [127], using the FRET assay for PLpro, tested 321 and found it exhibited 73% inhibition of PLpro replication with an IC50 of 3.99 μM. In this case, the compound GRL-0617 was utilized as a positive control, showing 100% inhibition of PLpro at a concentration of 0.82 μM.
These results corroborate those of in silico studies that indicate the potential of compounds 320 and 321 against SARS-CoV-2 targets. Molecular docking analysis showed that the compound 320 has high interactions with the receptors, exhibiting binding affinities of −4.3, −5.3, −4.3, and −3.7 kcal/mol for 3CLpro, Spike protein, PLpro, and RdRp, respectively [240]. Compound 321 also showed high binding potential with target proteins, exhibiting binding energy of −4.93 and −6.97 kcal/mol for 3CLpro and PLpro, respectively. Additionally, compound 321 was predicted to have high absorption and solubility levels and non-hepatotoxicity by ADMET prediction [127,241].
Benzophenones constitute a group of compounds characterized by a common phenol-carbonyl-phenol skeleton, exhibiting excellent structural diversity, with various substituents such as -OMe, -OH, geranyl, or prenyl groups [242,243]. Naturally occurring derivatives of benzophenones can be found in flowering plants such as mango (Mangifera indica L.) and muscat grape (Vitis vinifera L.) [244]. This class demonstrates a diverse range of biological activities [245], and recent studies have investigated its activity against SARS-CoV-2 [246,247,248].
Compound 328 exhibited the highest computational affinity toward the viral protease (3CLpro) with a binding energy of −8.34 kcal/mol. The in vitro activity of the compound was assessed using FRET assay. The results demonstrated that compound 328 achieved a remarkable 87.9% inhibition of 3CLpro at a concentration of 100 µM with IC50 0.1 µM, with the GC376 (positive control) with IC50 0.44 µM. Additionally, it showed no violations of drug-like parameters, indicating favorable predicted oral bioavailability. Furthermore, it exhibited a satisfactory Topological Polar Surface Area (TPSA) value of <140 Å, suggesting potential intestinal permeability, and demonstrated high absorption (50%) [126].
Coumestans are oxidative derivatives of pterocarpans similar to coumarins. The first coumestan compound, named wedelolactone (329), was isolated from Wedelia calendulacea (L.) Less. (Asteraceae) in 1956. Since then, many efforts have been made in the extraction and isolation, structural elucidation, biological evaluation, pharmacokinetics, and chemical synthesis of coumestans, suggesting that coumestans can serve as an important chemical scaffold for the development of novel drug leads [249,250].
The compound 329 showed one of the best inhibitory activities in the FRET assay against 3CLpro of SARS-CoV-2 with 95.04% inhibition at 10 μM and IC50 value of 1.35 ± 0.16 μM [115]. These data corroborate a study conducted by Chen et al. [251], in which compound 329 at a concentration of 80 μM inhibited 100% of 3CLpro with an IC50 value of 1.00 ± 0.24 μM.
In silico studies also indicated the anti-SARS-CoV-2 potential of compound 329 in the RBD:ACE2 interaction (−7.4 kcal/mol), suggesting that it could be utilized to develop a potent drug candidate against COVID-19 by blocking viral entry into the host cell [97,252]. This has sparked interest in in vitro studies that target this interaction.
Aurones are a part of a wide family of polyphenols. As has been observed in detail, the basic structure of an aurone consists of a main 6:5 benzofuran-one core instead of the 6:6 chromane ring of flavones, but it shares a 2-aryl decoration with higher homologs [253]. This class has shown various biological activities, and recent studies have demonstrated its anti-SARS-CoV-2 potential [254].
Khamto et al. [158] analyzed in vitro 3CLpro inhibition by FRET assay of the semisynthetic compound 330, which showed moderate protease inhibitory activity at a concentration of 100 μM, which showed 93.44 ± 0.28% inhibition, with an IC50 value of 46.18 μM. Nirmatrelvir (positive control), on the other hand, inhibited 100% of the protease at the same concentration. This is the first reported study of this compound for SARS-CoV-2, encouraging further studies to investigate other possible mechanisms of action.
2.4. ADMET and Drug-likeness Computational Analysis
To calculate and predict the ADMET profiles, drug-likeness, and medicinal chemistry characteristics of the 330 plant-derived molecules analyzed against different SARS-CoV-2 targets, all the compounds were categorized into their respective chemical classes. Table 1 provides a structured summary of the number of molecules retained or eliminated at each filtering stage, ultimately identifying those with the most promising pharmacokinetic and pharmacological potential.

Table 1.
Screening drug-likeness, ADMET, and other medicinal chemistry parameters and biological activities of plant compounds with inhibitory potential against different SARS-CoV-2 targets.
The selection process was based on a sequential screening strategy in which each step refined the dataset by eliminating compounds that did not meet the well-established pharmacokinetic and medicinal chemistry criteria [255,256,257]. The evaluation encompassed all the parameters, each of which was assessed using at least two computational platforms. To ensure the robustness and reliability of the predictions, only parameters with converging results between different tools were considered in the filtering process. The complete dataset containing the ADMET analysis results for all molecules, generated using multiple computational platforms, is publicly available on Zenodo and can be accessed via doi.org/10.5281/zenodo.14984521.
The table visually condenses a large volume of information into a concise format, ensuring clarity and facilitating interpretation. Each filtering stage is thoroughly detailed in the text to provide a comprehensive understanding of the selection process. This discussion highlights the key parameters that influence compound retention at each step, allowing the reader to follow the rationale behind the final selection.
Initially, drug-likeness evaluations were conducted using the SwissADME, DataWarrior, and ADMETlab tools. This step assesses the molecules against the Lipinski, Veber, Ghose, Egan, and Muegge rules. Molecules that did not meet the drug-likeness criteria and violated more than one (Veber and Egan) or two (Lipinski, Ghose, and Muegge) of the rules were excluded.
From the initial 330 molecules belonging to 13 different groups (chemical classes) established in this study, 149 were approved. The coumarin (100%) and lignoid (83%) classes had the highest number of molecules that passed this stage, while the tannin (0%) and saponin (0%) classes had the highest number of retained molecules.
The approval of these chemical classes (coumarin and lignoids) can be attributed to their stable and relatively small chemical structures, which meet other pharmaceutical viability criteria, contributing to their higher bioavailability and distribution in the body [258,259]. Additionally, the chemical diversity within these classes allows structural modifications that can optimize their pharmacokinetic properties [259].
Saponins and tannins have large and complex structures, often exceeding 500 Da, and exhibit high polar surface area (TPSA) values due to the presence of multiple hydrophilic groups [260,261]. Additionally, saponins often have many hydrogen bond donors and acceptors, which further reduces their permeability [259,262]. On the other hand, tannins have a high affinity for proteins, which can lead to low bioavailability due to the formation of insoluble complexes in the body [259,263].
The results of this first stage are of significant relevance, as molecules that do not meet the drug-likeness criteria typically have a lower probability of being viable drug candidates and can thus be excluded early in the process.
For the remaining stages (absorption, distribution, metabolism, and excretion), the same tools (SwissADME, pkCSM, and ADMETlab) were used to modify only the evaluated factors to align them with the focus of each stage. In these steps, any infraction against any criteria evaluated in each stage eliminated the molecule.
In absorption screening, the following critical stages of drug efficacy were evaluated: oral bioavailability, human colon epithelial carcinoma cell line (Caco-2) permeability, water solubility, and human intestinal absorption (HIA). Approximately 29 compounds were retained, with an emphasis on the chalcones, stilbenes, and alkaloid classes (average 35% retention).
Chalcones have significant and complex structures, resulting in low water solubility and limited oral bioavailability [264,265]. Stilbenes typically have low oral bioavailability due to first-pass metabolism as well as low aqueous solubility and rigid structures, hindering passive diffusion and intestinal absorption [266]. Alkaloids have complex structures and high polarity, which reduce permeability in Caco-2 cells and low bioavailability [267].
Parameters such as volume of distribution (VDss), plasma protein binding (PPB), unbound fraction in plasma (Fu), and blood−brain barrier permeability (BBB) were used to assess the distribution criteria. After screening, 38 molecules showed a viable distribution and had great potential to reach the site of action.
Molecules with higher distributions typically have moderate values for LogP, molecular weight, and water solubility. These characteristics indicate that the molecule is sufficiently lipophilic to cross biological membranes but not so lipophilic that it accumulates in adipose tissues. Furthermore, these values tend to improve cellular permeability and tissue distribution, ensuring that the molecule can cross membranes and reach the target tissues [268,269].
Quinones generally have high logP values, making them excessively lipophilic, which can result in their accumulation in adipose tissues and compromise their uniform distribution throughout the body [270,271]. Chalcones, although with variable logP values, also face similar challenges. Both classes of molecules tend to exhibit high plasma protein binding (PPB) rates, reducing the free fraction in the plasma available to cross cell membranes and reach target tissues [264,265]. Additionally, they may have problems with water solubility and cellular permeability, negatively affecting their ability to distribute efficiently across body tissues [264,265,271].
The metabolic stage involved analyzing the interactions with cytochrome P450 (CYP450) enzymes. Molecules that do not significantly inhibit or induce major CYP isoforms (CYP1A2, CYP3A4, CYP2C9, CYP2C19, and CYP2D6) are preferable. However, significant inhibition or induction can lead to undesirable drug interactions. Additionally, molecules that are rapidly metabolized may not be effective, while slowly metabolized molecules can induce bioaccumulation and trigger toxic processes in the body [272].
At this stage of analyzing interactions with cytochrome P450 enzymes, compounds with significant interactions with three or more CYPs were excluded. Consequently, the total number of compounds in the screen was reduced to 35, eliminating all representatives of the lignoid and stilbene classes.
Lignoids can significantly inhibit CYP450 isoforms like CYP3A4 and CYP2C9, which can lead to adverse drug interactions by increasing the plasma levels of other medications and the risk of toxicity [273,274]. Stilbenes exhibit similar behavior, being capable of inhibiting or inducing CYP1A2, thus altering the metabolism of other drugs and potentially reducing their therapeutic efficacy [266,275]. Additionally, the inhibition of CYPs by these compounds can result in reduced metabolic clearance, leading to bioaccumulation and potential toxicity, especially for compounds with long half-lives [272,276].
Subsequently, excretion screening was applied, focusing on the elimination of half-life and total clearance. In the final ADME stage, compounds with a short to moderate half-life (1–12 h) and moderate total clearance (2 to 5 mL/min/kg) were considered approved, as this offers a balance between convenient administration and adequate control of plasma levels. This balance improves treatment adherence and reduces the risk of adverse effects between doses [277].
Only 15 molecules passed this screening stage, and the flavonoid class was eliminated. This is because molecules with excessively long or short half-lives present a significant challenge. Those with long half-lives can accumulate in the body, increasing the risk of toxicity, such as nephrotoxicity or hepatotoxicity, because they are not efficiently eliminated [278,279]. Conversely, molecules with very short half-lives are rapidly eliminated, often requiring frequent dosing to maintain therapeutic levels, which can lead to poor patient adherence [279]. Additionally, the total clearance rate, which integrates all elimination pathways, must be balanced; a high clearance rate can result in subtherapeutic drug levels, while a low clearance rate can cause drug accumulation and potential toxicity [279]. Understanding excretion mechanisms is essential for predicting and managing drug interactions and ensuring patient safety, as drugs that are not adequately excreted can accumulate and cause serious adverse effects [279,280]. Therefore, finding an optimal range of half-life and total clearance is crucial for the successful excretion of drugs, ensuring that they are safe and effective for patient use.
Finally, the molecules were subjected to toxicity screening using ProTox3, ToxTree, and DataWarrior. This step is essential to ensure the safety of the molecules before investing time in additional analyses. Twelve potential toxic effects of the investigated molecules were considered, including hepatotoxic, neurotoxic, nephrotoxic, cardiotoxic, and respiratory toxic, as well as potentially carcinogenic, immunotoxic, mutagenic, cytotoxic, hematotoxic, and acute oral toxicity in rats, and clinical toxicity. The potential toxic effects of four or more of these factors led to the elimination of the compound. After applying these criteria, the pool was reduced to eight molecules, and the alkaloid class was eliminated.
Natural products often perform poorly during the toxicity stage of ADMET due to the complexity and structural diversity of their chemical components, which can lead to a wide range of adverse effects. Among natural compounds, alkaloids stand out due to their high biological activity and potential toxic effects [281]. These compounds can be metabolized into toxic metabolites and interfere with hepatic enzymes and renal transport systems, causing tissue damage in these organs [282]. Another risk associated with this chemical class is its severe neurotoxic and cardiotoxic effects, resulting from the inhibition of sodium and potassium channels, which are essential for neuromuscular and cardiac function [281,282]. Therefore, despite their potential therapeutic benefits, alkaloids require rigorous toxicity evaluations to ensure their safety in clinical use [267].
In addition to these screenings, other medicinal chemistry parameter filters were applied, which included criteria such as potential pan-assay interference compounds or invalid metabolic panaceas (PAINS/IMPS) alerts (cut-off two or more infractions), Brenk alerts (cut-off three or more infractions), and violations of lead-likeness (cut-off two or more infractions). A synthetic accessibility score (1 = very easy to 10 = very difficult) was also evaluated, in which molecules with values greater than or equal to 5 were eliminated. Analyses were performed using SwissADME, DataWarrior, and ADMETlab tools. Any break in any of these four criteria would eliminate the compound.
At this stage, only one compound (belonging to the coumarin class) was eliminated. Some alerts indicated a high potential for false positives in biological assays. Additionally, there may be inadequacy of coumarin as an efficient lead molecule and other additional development challenges [187,270]. Evaluating medicinal chemistry parameters, such as PAINS, Brenk alerts, lead-likeness, and synthetic accessibility, is crucial in drug development due to their importance in identifying compounds with the potential for undesirable effects and development challenges [256]. Rigorously assessing these parameters in the initial screening helps minimize risks and costs, ensuring that only compounds with truly viable aspects advance to the later stages of drug development [256].
Furthermore, in the end, compounds with a concentration greater than or equal to 10 μM, with inhibition values against at least one SARS-CoV-2 target greater than or equal to 50%, were manually selected.
Only two molecules were approved: compound 1 (salvianic acid A) and compound 5 (protocatechuic acid methyl ester), both belonging to the class of phenolic acids. These molecules showed great potential for biological activity, with IC50 of 2.15 μM inhibiting 3CLpro activity and 3.76 μM inhibiting PLpro activity.
Phenolic acids are bioactive compounds with significant antioxidant, anti-inflammatory, and antiviral properties. In silico and in vitro studies have shown that these compounds can inhibit viral replication and modulate immune responses, primarily due to their ability to interfere with essential viral proteins and critical cellular processes for infection. This includes their efficacy against a variety of viruses, such as arboviruses, influenza viruses, and coronaviruses [283,284].
Phenolic acids, despite their structural diversity, share important functional groups such as hydroxyl (-OH) and carboxyl (-COOH) groups. They are capable of interacting with several proteins mainly through non-covalent interactions such as hydrogen bonds, electrostatic interactions, hydrophobic interactions, van der Waals interactions, and π bonding/π-π stacking [285].
The relevance of salvianic acid A (alpha-hydroxy acid) (1) can be demonstrated by comparing compounds with structural similarities, such as caffeic acid (3), dihydrocaffeic acid (4), and protocatechuic acid methyl ester (5). Compound 1 is also related to the structural scans of 2-hydroxybenzoic acid (beta-hydroxy acid) seen in compounds 2, 6, 7, 8, and 9 (Figure 26 and Figure 27).
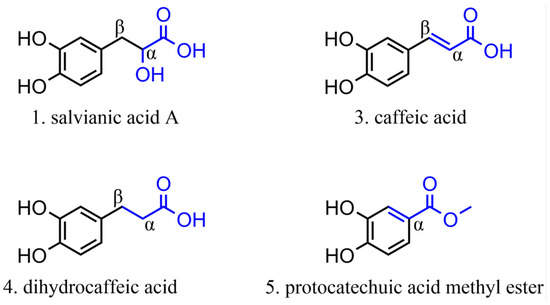
Figure 26.
Chemical structures used in the specification of alpha-hydroxy acid structures.
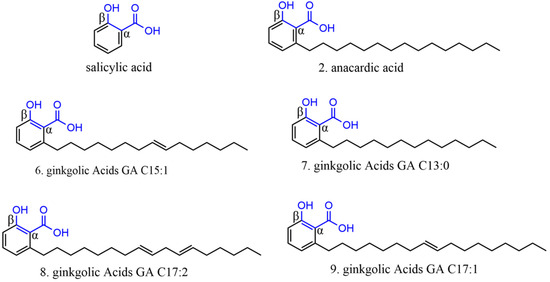
Figure 27.
Chemical structures used in the specification of the beta-hydroxy acid structures.
Structurally related compounds, such as compound 3 (IC50 98.07% inhibition at 100 µM) and compound 4 (IC50 7.15 µM, 3CLpro inhibitor), exhibited weaker activity. Initially, the presence of the hydroxyl in the alpha position in relation to the carboxyl can be highlighted as being responsible for an increase in the IC50 from 2.15 µM of compound 1 to 7.15 µM of compound 4.
Chlorogenic acid (IC50 39.48 µM, 3CLpro inhibitor), which has an esterified region between the two units of compound 3, demonstrated lower potency. In silico analyses demonstrated the action of the catecholic group but with greater emphasis on the carboxylic group because this region has a high electron density [286].
In contrast, compound 5 is a simpler dihydroxybenzoic acid, but it has a catecholic region (-OH at C3 and C4), which is a key pharmacophoric group responsible for covalent bonds with the spike protein and 3CLpro of SARS-CoV-2 [115]. Another significant factor of the molecule is the presence of an ortho-oriented electron-donating substituent, such as the methoxy group, which is responsible for maintaining the bond with the 3CLpro protein [287].
These cases of hydroxyl suppression demonstrate that it is essential to evaluate the occurrence of intermolecular hydrogen bonding and the influence of intramolecular hydrogen bonding on the structural properties of these molecules.
In a study of ligand-receptor interactions, compound 1, when evaluated by saturation transfer difference nuclear magnetic resonance (STD NMR), demonstrated that all its protons present efficient STD effects. However, the H-2 proton, which was closer to the carboxylic group, showed a stronger effect with an STD amplification factor (STDAMP) value of 100%. This fact allows studies to be initially directed toward the alpha-hydroxy acid molecule instead of compound 4 [288] (Papaemmanouil et al., 2020).
Ginkgolic acid and anacardic acid, both long-chain alkylphenols, showed inhibitory effects against 3CLpro and PLpro; however, their IC50 values were significantly higher, suggesting a less favorable interaction profile (Figure 27). Their hydrophobic alkyl chains, although potentially increasing lipophilicity and membrane permeability, may limit direct enzyme-binding affinity and aqueous solubility, which may negatively impact bioavailability. A molecular docking study showed that the aliphatic chain interferes with the accessibility of ACE2 to the spike protein RBD [289].
Another important factor for discussion is the structural scans of 2-hydroxybenzoic acid (salicylic acid) since it was responsible for reducing the expression of SARS-CoV-2 viral RNA in 24 h, with viral replication quantified by determining the number of copies of the viral genome by quantitative reverse transcription polymerase chain reaction (RTqPCR [290]).
The structural properties of the compounds containing 2-hydroxybenzoic acid were recorded by the ortho arrangement of the carboxyl and hydroxyl ligands. Another relevant aspect is that this compound undergoes the angular group-induced bond alternation effect (AGIBA) due to the influence of asymmetric functional groups on the structure of the phenyl ring and the electronic behavior of the aromatic ring [291].
Intramolecular hydrogen bonds are more stable than intermolecular hydrogen bonds because they are influenced by resonance-assisted hydrogen bonding, RAHB [291]. This may explain the stronger electrostatic interactions between these compounds and their target proteins.
Studies on compound 2 and ginkgoic acids (6–9) are needed to clarify the possible forms of inter-and intramolecular hydrogen bond interactions as well as the influence of the alkyl chain on the molecular properties of these compounds and their interactions with the proteins present in the SARS-CoV-2 virus.
Considering the potential enzymatic action and favorable molecular characteristics described in this article, compounds 1 and 5 were reinforced as the main candidates for additional preclinical studies. With the research and selection of active molecules described, it is suggested that these molecules be subjected to future optimization strategies. Thus, focusing on increasing the specificity of the molecules to the target, reducing metabolic liability, improving bioavailability, docking studies, and molecular dynamics simulations are valuable tools to refine structure-activity relationships (SARs).
In the studies by Wang et al. [292], compound 1 demonstrated potent antiviral activity in vitro against SARS-CoV-2 and, in in vivo assays, reduced pulmonary inflammation, tissue damage, and levels of inflammatory cytokines in infected mice. The compound also inhibited the activation of the TLR4 pathway and hyperphosphorylation of NF-κB p65.
Compound 5, a methylated derivative of protocatechuic acid, exhibits increased lipophilicity, enhanced bioavailability and metabolic stability, and potentiated antioxidant and neuroprotective activities [293,294,295]. Studies have indicated its efficacy in scavenging reactive oxygen species, inhibiting lipid oxidation, and preventing glutamate-induced toxicity in cortical cells, suggesting its potential therapeutic applications in neurodegenerative diseases [295]. Additionally, its antimicrobial activity against Gram-positive and Gram-negative bacteria is enhanced by increased cell membrane permeability conferred by methylation [294]. In the oncological context, this compound has been shown to modulate oxidative stress, induce apoptosis, and inhibit tumor cell proliferation, possibly through improved interactions with intracellular targets [293,295]. Although specific studies on protocatechuic acid methyl ester remain limited, the broad range of pharmacological activities reported for protocatechuic acid, including neuroprotective, antibacterial, antiviral, anticancer, antiosteoporotic, analgesic, anti-aging, and metabolic syndrome-preventive effects, suggests that its methylated derivative may share similar or even enhanced mechanisms due to its greater stability and cellular permeability [295,296]. Notably, compound 5 has demonstrated antiviral activity, inhibiting viruses such as HIV, influenza, hepatitis, and avian influenza by promoting apoptosis and reducing viral replication [297]. Furthermore, while evidence for its efficacy against SARS-CoV-2 remains scarce, protocatechuic acid has shown promising results against H1N1 during in vivo rat models, preventing weight loss and death, reducing the viral load in the lungs, and suppressing immune cell infiltration and inflammatory cytokine production. These effects are associated with inhibition of the TLR4/NF-κB pathway, highlighting its potential as an anti-influenza therapeutic [298]. Additionally, protocatechuic acid has been shown to inhibit HBV replication by activating the extracellular signal-regulated kinase 1/2 pathway and reducing HNF4α and HNF1α expression in HepG2.2.15 cells, as well as exhibiting in vitro anti-HSV-2 activity [299].
In addition to their antiviral properties, phenolic acids stand out for their favorable ADMET profile. Studies have indicated that many phenolic acids have high oral bioavailability and are capable of crossing biological barriers effectively, which is crucial for their therapeutic use [259,260,300]. These characteristics make phenolic acids promising candidates not only for the treatment of viral infections but also for other pathological conditions where immune modulation and antioxidant activity are beneficial. Additionally, the low toxicity associated with these compounds at therapeutic doses reinforces their potential as safe and effective antiviral agents [300]. The combination of a favorable ADMET profile and a broad range of biological activities makes phenolic acids a class of compounds of great interest for the development of new therapies. Additionally, all the evaluated results for compounds 1 and 5, along with their findings reported in the literature, indicate their great potential for further research and development as promising anti-SARS-CoV-2 drugs.
These results demonstrate that, even among the various molecules tested and with relevant inhibition potential against some SARS-CoV-2 targets found in this review, few showed complete potential viability to become drugs without additional structural adjustments. However, considering the great bioactive potential of some molecules, it is important to consider the cost-benefit relationship, as some adaptations in their core structure can be made to adjust these new molecules to meet any criteria (Drug-likeness, ADMET, and other medicinal chemistry parameters) that may have caused them to be filtered out.
These analyses allowed the refinement of the selection of compounds, ensuring that the approved molecules not only have favorable ADMET properties but also possess high potential for successful drug development. This step is crucial for focusing on compounds that are not only biologically active but also chemically viable and cost-effective for large-scale synthesis and development.
The filtering workflow allowed for the exclusion of molecules with less favorable profiles, thereby focusing on compounds with the potential to become pharmaceuticals. Specifically, it is the only approved molecule that demonstrates significant inhibitory activity against SARS-CoV-2 targets and exhibits highly desirable pharmacological properties.
3. Materials and Methods
3.1. Study Design
This systematic review followed the PRISMA—Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [301]. The question of this study was formulated following the PICO (problem, intervention, control, and outcome) framework as recommended in the PRISMA method: “In in vitro studies (I) using SARS-CoV-2 target models, what is the potential of plant-derived molecules (P) in inhibiting the Spike:ACE2 interaction and the activity of the 3CLpro, PLpro and RdRp enzymes (O) compared to control groups and reference treatments (C)?” Due to the scope of the studies evaluated in this systematic review, which focused on in vitro research on plant metabolites targeting the main SARS-CoV-2 targets, this study was not registered in an electronic database of systematic reviews.
3.2. Search Strategy
Three reviewers independently conducted the systematic review based on searches in six electronic databases: PubMed, Scielo,
Science Direct, Scopus, Springer, and Web of Science. Ultimately, the references in each database list were cross-checked for potential discrepancies by each reviewer. When inconsistencies were identified, they were adjusted according to patterns identified by most authors. In case of disagreement, the final decision was made by another external reviewer. These searches encompassed the period from January 2020 to February 2024. The following keywords were used as search terms: [“Natural Product” OR “Natural Compound” OR “Plant Metabolite” OR “Plant Compound” OR Phytocompound] AND [“In Vitro”] AND [Spike OR Spike:ACE2 OR RBD:ACE2 OR 3CLpro OR Mpro OR “Main protease” OR 3-chymotrypsin-like OR 3-chymotrypsin? OR PLpro OR Papain-like OR Papain? OR RdRp OR “RNA-dependent RNA polymerase” OR “RNA dependent RNA polymerase”] AND [SARS-CoV-2]. Searches were carried out using the titles, abstracts, and keywords of each article. Complete scientific articles written in English without geographic limitations were included. All other document types, such as reviews, books, case studies/reports, theses, and dissertations, were excluded. Nevertheless, we conducted manual searches of the references of these studies to identify any articles that might not have been initially detected in the databases. These articles were then subjected to a critical evaluation for potential inclusion in the comprehensive collection of gathered materials.
3.3. Eligibility Criteria
Two validations of the identified articles during the search phase were conducted. In the first selection, all titles, abstracts, and keywords were independently evaluated by three reviewers. Disagreements in this inclusion/exclusion stage were resolved through consensus, and when necessary, the decision was adjudicated by a third reviewer. In the second phase of selection, which involved data extraction, the complete text of the articles was reviewed by three evaluators. During this phase of inclusion/exclusion, any disagreements were resolved through consensus; in cases where consensus could not be reached, a third reviewer made the final decision.
3.3.1. Inclusion Criteria
Articles that provided data on the effectiveness of plant-derived compounds in inhibiting the interaction between spike (RBD)ACE2 and the 3CLpro/Mpro, PLpro, and RdRp of SARS-CoV-2 during in vitro assays were included.
3.3.2. Exclusion Criteria
Articles that exclusively featured compounds from non-plant sources were excluded. Additionally, articles that presented data on the effectiveness of raw extracts, fractions, or mixtures were excluded. Furthermore, articles that reported solely on in vivo or in silico experiments or outcomes in different SARS-CoV-2 target models were also excluded. Articles on trials involving cellular models were excluded. Articles that included only qualitative assay results and/or lacked the essential information necessary to complete the standardized spreadsheet were also excluded.
3.4. Processing and Extraction of Data
To compile the material, we organized all articles in a table, including the discovery database, search term combination employed, title, publication year, and DOI. Subsequently, duplicates were eliminated from the retrieved documents, and a new file containing all material and consolidated information was generated. Both tasks were performed using a conventional free spreadsheet editor. Three reviewers extracted the information from a standardized spreadsheet, gathering the following details: name of the plant-derived compound, its chemical class and subclass, name, and type of controls or references (when used), the target model under investigation, the type of in vitro assay employed, concentrations of plant compounds, quantitative (numeric) results of inhibitory activity, units of measurement for concentration and assay results, publication year, publication authors, and DOI. The extracted information underwent a cross-verification process for completeness and accuracy between the reviewers, with any inconsistencies addressed through discussion and resolved through consensus or, if necessary, arbitrated by a third reviewer.
3.5. Risk of Bias
The potential sources of bias in this study included exclusion/inclusion criteria, chosen database, language, differences between studies, and selected articles. The quality of the studies was assessed based on the peer-reviewed journal publications. Protocols such as PICO and PRISMA were meticulously followed to identify and mitigate bias. These biases encompass inclusion/exclusion criteria, missing data, selected database, publication date, language, impact of missing primary results, and type of articles.
3.6. Methodology Summary
All numerical data were calculated using a data collection process. This included determining the number of articles discovered in each database using each term combination and the total count of articles in the concatenated file, those removed as duplicates, and those either included or excluded during independent assessments (Figure 1).
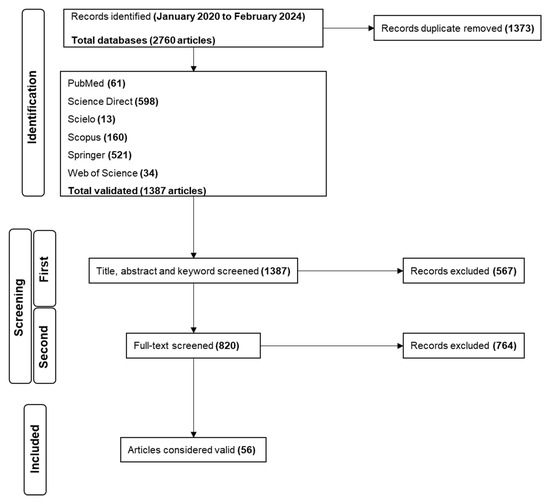
Figure 1.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flowchart of the screening process.
Figure 1.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flowchart of the screening process.
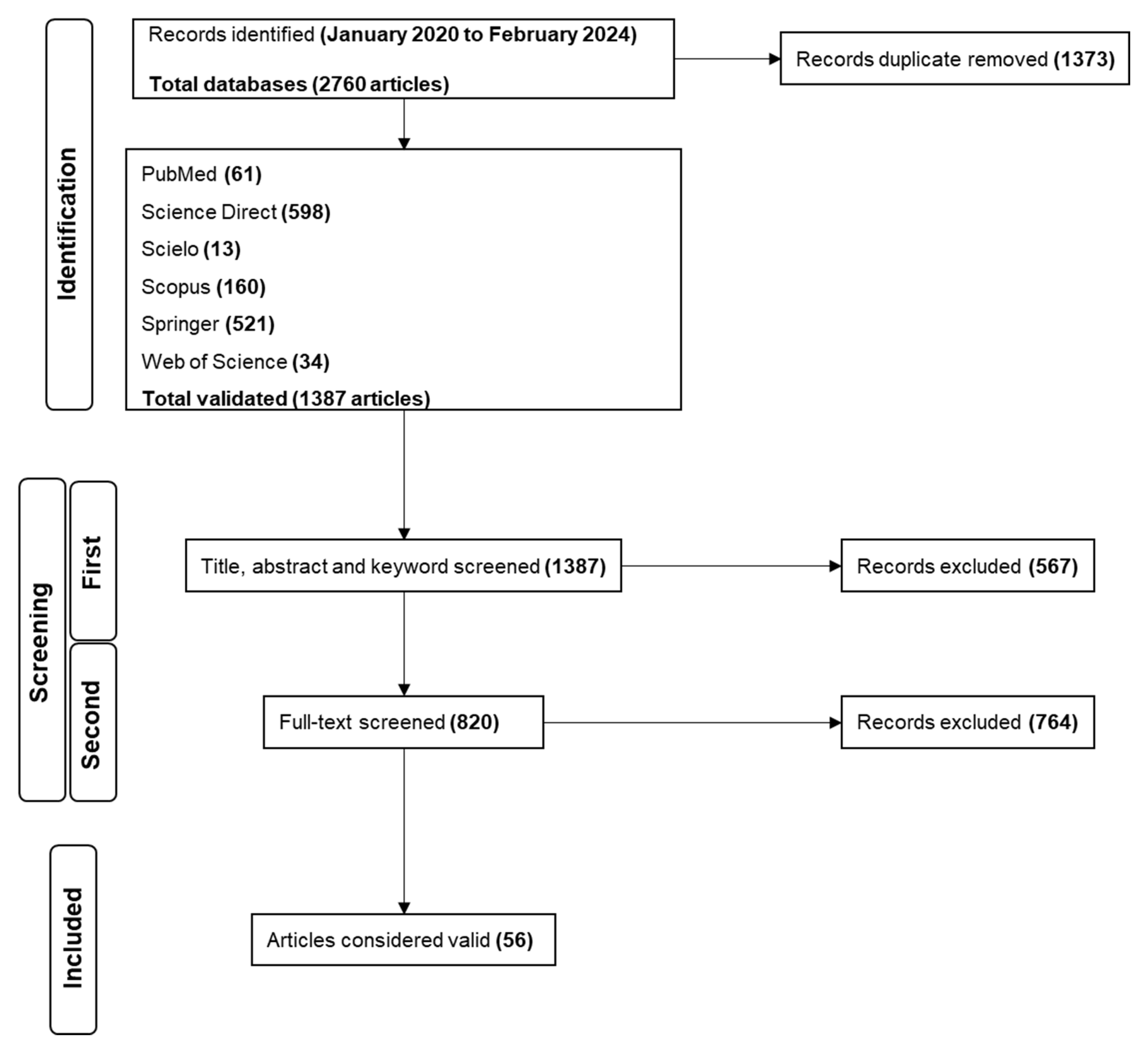
3.7. Computational Analysis
For ADMET, drug-likeness, and other medicinal chemistry analyses, the tools SwissADME (http://www.swissadme.ch/ (accessed on 13 June 2024)), pkCSM (https://biosig.lab.uq.edu.au/pkcsm/ (accessed on 15 June 2024)), ADMETlab (http://admet.scbdd.com/ (accessed on 13 June 2024)), ProTox3 (https://tox-new.charite.de/protox_III/ (accessed on 28 June 2024)), Toxtree (https://toxtree.sourceforge.net/ (accessed on 28 June 2024)), and DataWarrior (http://www.openmolecules.org/datawarrior/ (accessed on 02 July 2024)) were used in combination.
To ensure the accuracy of the SMILES notation used in this study, a multi-step validation approach was employed. Initially, SMILES was retrieved from reliable chemical databases, such as PubChem and ChEMBL. These structures were used to generate molecular representations in ChemDraw v.20 (https://revvitysignals.com/chemdraw (accessed on 25 May 2024)) and their integrity was verified.
For compounds not available in these databases, their molecular structures were manually drawn using ChemDraw based on the chemical representations provided in the original reference articles. The built-in function of the software was then used to convert these manually drawn structures into SMILES notation. Given the sensitivity of the SMILES notation in computational and ADMET analyses, all generated SMILES underwent a final check against publicly available data to ensure consistency and accuracy.
The factors evaluated in each category (drug-likeness (DL), absorption (A), distribution (D), metabolism (M), excretion (E), toxicity (T), and medicinal chemistry (MC)) were calculated using at least two platforms and software. The data obtained from the tools used in each step were compared, and only the factors with coinciding results were used in the filtering process, ensuring the greater reliability and robustness of the predictions. The molecules were subjected to a sequential screening and filter process, where each step reduced the number of compounds based on well-established cut-off criteria [255,256,257]. This refinement process selected the molecules with the highest pharmacokinetic and pharmacological potential.
4. Methodological Limitations of the Studies
This review aimed to analyze the literature on the potential of plant-derived compounds against SARS-CoV-2; however, it has some limitations. Due to the methodological choice of a systematic review based on the PRISMA method, only scientific articles that included the specified descriptors in their titles, abstracts, and keywords were included. Consequently, some articles may have been missed if they did not contain all mandatory terms, potentially leading to incomplete coverage of the literature.
The study focused on in vitro research; however, even after the COVID-19 pandemic period, most of the studies found were still in silico. While valuable for virtual drug screening, in silico data must be validated through in vitro and in vivo approaches to accurately assess the antiviral potential of phytocompounds. This resulted in a reduced number of studies being included. Most studies compared treatments with positive controls (references), but some did not detail or use any controls, limiting their ability to make proper inferences about the assessed activity. Inadequate data reporting, particularly regarding the IC50 values of both active and inactive molecules, further complicates these analyses. Some articles explicitly provided activity values, while others only reported values for molecules that they considered active.
There is an uneven distribution of studies targeting various SARS-CoV-2 proteins. Significant research has focused on 3CLpro and Spike, while PLpro and RdRp have received less attention despite their importance in investigating potential anti-SARS-CoV-2 molecules. Other targets were excluded due to a lack of in vitro assay support. The number of molecules evaluated in each chemical class was unbalanced, with a strong preference for phenolics such as flavonoids. This imbalance limits broader inferences and discussions about the potential of other important chemical classes in phytochemistry.
Another important limitation is the lack of stereochemical information for some plant-derived compounds, as illustrated in the structural figures. An accurate representation of chiral centers is essential, especially for bioactive molecules with pharmacological applications. However, several structures extracted from the primary studies did not report their absolute or relative stereochemistry, nor did they provide sufficient experimental details to infer it. In these cases, we opted to depict the molecules in their achiral or undefined form to avoid speculative assumptions and to preserve the scientific integrity of the data. When stereochemical information is available, it is properly represented. This disparity in data availability highlights a common limitation in the phytochemical literature and represents a challenge for rational drug development.
The use of ADMET analysis, which is a theoretical method, is a limitation of this study. Although this computational approach serves as an important filtering tool, particularly when dealing with a large number of compounds, it lacks the robustness of in vitro and in vivo experimental validations. The predictions generated by ADMET modeling provide valuable preliminary insights but do not replace empirical data regarding pharmacokinetics, metabolic stability, and toxicity.
Additionally, while ADMET analysis may highlight potential drawbacks in a compound’s profile, a negative prediction does not necessarily imply that the molecule should be definitively discarded from further investigation. Many compounds with initially unfavorable computational predictions can still demonstrate viable pharmacological properties when tested in biological systems. To enhance the reliability of our findings, multiple computational platforms were used, and only the converging results were considered in the selection process. However, experimental validation is essential to confirm the therapeutic potential of these plant-derived metabolites.
Despite these limitations, this study compiled extensive information on plant-derived molecules from various chemical classes against different SARS-CoV-2 targets, supplemented by computational pharmacological data. This study contributes valuable insights to the scientific literature on the potential of natural products to combat COVID-19. Future studies can build on this information, guiding idea generation, project development, and discussions in other works, thereby improving the reliability and comparability of findings regarding the antiviral potential of plant-derived compounds against SARS-CoV-2.
5. Conclusions and Perspectives
This systematic review highlighted a diverse array of plant-derived metabolites with potential inhibitory effects on key SARS-CoV-2 targets, such as 3CLpro, PLpro, Spike protein, and RdRp. Most experimental assays have focused on inhibiting 3CLpro, followed by investigations on PLpro, Spike-ACE2 interaction, and RNA polymerase. Most studies were in silico ones with respect to in vitro assays, which explains the limited experimental data. Notable in vitro methodologies include FRET-based assays, RBD:ACE2 kits, and methods specific for RdRp.
The most active classes of compounds were phenolic acids and flavonoids (subdivided into flavones, flavonols, and flavanones)—these compounds showed prominent inhibition with IC50 values below 10 μM in several cases. These results indicate that natural compounds, especially flavonoids and phenolic acids, have a promising therapeutic potential against SARS-CoV-2.
Other promising compounds identified as 3CLpro, PLpro, and Spike:ACE2 inhibitors were sennoside B (IC50 0.01 μM), 11-keto-β-boswellic acid (IC50 1.1 μM), and vaticanol B (IC50 0.07 μM), respectively. However, no compound with significant activity against RdRp with favorable IC50 values was identified, with silibinin being the best (20.3 μM). Despite the higher inhibitory activity, the predicted in silico ADMET profiles of these compounds were unfavorable in this study.
Out of an initial pool of 330 compounds, only two phenolic acids, salvianic acid A (IC50 2.15 μM), active against 3CLpro, and protocatechuic acid methyl ester (IC50 3.76 μM), active against PLpro, met the stringent criteria for drug-likeness, ADMET properties, and other medicinal chemistry and biological activity parameters, which further supports their potential as drug candidates. Despite the known antiviral properties of other classes, such as flavonoids and alkaloids, these were excluded due to inadequate ADMET profiles obtained in the evaluations and screening of this study or insufficient inhibitory activity in the assays evaluated and available in the literature found by this review. Flavonoids were eliminated in the excretion screening due to challenges with half-life and clearance rate, while alkaloids were excluded in the toxicity screening because of their high potential for toxic effects, such as neurotoxicity and cardiotoxicity.
In a study like this review, where ADMET profiles are used as a filter, it is common for many molecules to be excluded due to unfavorable characteristics. However, rigorous screening should not be considered a definitive limitation. Active molecules with poor ADMET profiles can still be made viable using various strategies. Therapeutic targets can be adjusted, chemical structure modifications can be implemented to improve pharmacokinetic properties, and monitoring methods or precautions can be developed to mitigate the potential adverse effects. Additionally, advanced formulation techniques, such as controlled-release systems and nanocarriers, can be employed to enhance the bioavailability and distribution of compounds. These strategies are widely used, even for the molecules described in this study, which are already extensively used as medications. Thus, molecules initially considered unsuitable can be optimized for effective therapeutic use, ensuring that their bioactive potential is fully realized. The filter used in this review aims to identify naturally promising molecules with inherent biological potential, drug-likeness, ADMET, and medicinal chemistry properties. This approach facilitates the search for new natural products with pharmaceutical potential, reducing the financial and time expenditure on research and development to bring a new drug to the market.
This research provides an in-depth evaluation of the pharmacokinetic and toxicological properties of these compounds, emphasizing their drug-likeness and development potential. These findings highlight the significance of natural products as sources of antiviral agents and offer promising leads for new anti-SARS-CoV-2 therapies. This study also highlights the importance of continued exploration of plant-derived compounds, integrating in silico, in vitro, and in vivo studies to validate and optimize their efficacy.
Overall, this research provides a robust foundation for future studies aimed at discovering and developing effective antiviral agents from natural sources. The detailed literature review and rigorous screening of plant metabolites presented here significantly contribute to the field, offering insights into their potential therapeutic applications against COVID-19.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ddc4020027/s1, Table S1: Survey of citations between 2020 and 2024 of substances with inhibitory potential against different SARS-CoV-2 targets, organized according to their chemical classes; Table S2: Survey of citations between 2020 and 2024 of substances with inhibitory potential against different SARS-CoV-2 targets, organized according to the target protein; Table S3: Plant compounds and metadata from the systematic review (PRISMA) of natural products evaluated in vitro against different SARS-CoV-2 targets (3CLpro, PLpro, Spike, RdRp) in the literature from January 2020 to February 2024.
Author Contributions
Conceptualization and methodology: B.A.G. and D.A.F.; Software: B.A.G. Investigation, data curation, formal analysis, and writing—original draft preparation: B.A.G., D.A.F., T.S.d.F., M.F.C., P.A.J., M.V.T.e.S., L.E.C.C., A.A.S.d.V., B.R.F., E.S.M., H.B.M.P., B.G.M.d.M., B.A.C.d.O. and S.d.S.C. Visualization, supervision, writing—review, and editing: F.M.M.d.A., D.R.d.O., I.C.R.L., G.R.M., G.G.L., D.A., S.C.M., M.T.S. and S.G.L. Project administration and funding acquisition: S.G.L. All authors have read and agreed to the published version of this manuscript.
Funding
This work was supported by Brazilian agencies, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Data Availability Statement
Data will be made available upon request.
Acknowledgments
We would like to thank the Brazilian funding agencies and teams from the laboratories Fitoquímica e Farmacognosia (FitoFar), Fitoquímica e Cromatografia Contracorrente (FitoCCC), and Biotecnologia e Bioengenharia Estrutural (LABGENEST).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3CLpro | 3-Chymotrypsin-like Protease |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| ADMET | Absorption, Distribution, Metabolism, Excretion, and Toxicity |
| BBB | Blood-brain barrier permeability |
| CDC | Centers for Disease Control and Prevention |
| CC50 | 50% Cytotoxic Concentration |
| COVID-19 | Coronavirus Disease 2019 |
| CoV | Coronaviruses |
| DL | Drug-likeness |
| EC50 | 50% Effective Concentration |
| E | Envelope protein |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EV | Enterovirus |
| FRET | Fluorescence Resonance Energy Transfer |
| FU | Fraction unbound in plasma |
| H1N1 | Hemagglutinin type 1/Neuraminidase type 1 |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C virus |
| HIA | Human intestinal absorption |
| HIV | Human immunodeficiency virus type I |
| HTS | High-Throughput Screening |
| HSV | Herpes Simplex Virus |
| HTVS | High-Throughput Virtual Screening |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| IC50 | 50% Inhibitory Concentration |
| IFN-I | Type I Interferon |
| JEP | Japanese Encephalitis Virus |
| LBBD | Ligand-Based Drug Design |
| M | Membrane protein |
| MC | Medicinal Chemistry |
| MERS-CoV | Middle East Respiratory Syndrome Coronavirus |
| N | Nucleocapsid protein |
| Nsps | Non-structural Proteins |
| ORF | Open Reading Frame |
| PAINS | Pan-Assay Interference Compounds |
| PICO | Population, Intervention, Comparator, Outcome |
| PLpro | Papain-like Protease |
| PPB | Plasma protein binding |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| pH | Potential of Hydrogen |
| ProTox3 | Plataforma de predição de toxicidade |
| RBD | Receptor-Binding Domain |
| RBM | Receptor-Binding motif |
| RMSD | Root Mean Square Deviation |
| RNA | Ribonucleic Acid |
| RdRp | RNA-dependent RNA Polymerase |
| RTqPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| S protein | Spike protein |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SBDD | Structure-Based Drug Design |
| SI | Selectivity Index |
| Scielo | Scientific Electronic Library Online |
| SMILES | Simplified Molecular Input Line Entry System |
| STD NMR | Saturation Transfer Difference Nuclear Magnetic Resonance |
| SwissADME | Plataforma de predição de propriedades ADME |
| TCM | Traditional Chinese Medicine |
| TMPRSS2 | Transmembrane Protease, Serine 2 |
| TPSA | Topological Polar Surface Area |
| Toxtree | Plataforma de predição toxicológica |
| WHO | World Health Organization |
| VDss | Volume of distribution |
| VOIs | Variants of Interest |
| VOCs | Variants of Concern |
| VUMs | Variants Under Monitoring |
| ZIKV | Zika virus |
References
- Lopes, A.J.O.; Calado, G.P.; Fróes, Y.N.; Araújo, S.A.D.; França, L.M.; Paes, A.M.D.A.; Morais, S.V.D.; Rocha, C.Q.D.; Vasconcelos, C.C. Plant Metabolites as SARS-CoV-2 Inhibitors Candidates: In Silico and In Vitro Studies. Pharmaceuticals 2022, 15, 1045. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Coronavirus (COVID-19) Dashboard. Available online: http://covid19.who.int (accessed on 2 May 2024).
- World Health Organization (WHO). Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 6 March 2025).
- Ma, K.C.; Castro, J.; Lambrou, A.S.; Rose, E.B.; Cook, P.W.; Batra, D.; Cubenas, C.; Hughes, L.J.; MacCannell, D.R.; Mandal, P.; et al. Genomic Surveillance for SARS-CoV-2 Variants: Circulation of Omicron XBB and JN.1 Lineages—United States, May 2023–September 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Lefrançois, T.; Malvy, D.; Atlani-Duault, L.; Benamouzig, D.; Druais, P.-L.; Yazdanpanah, Y.; Delfraissy, J.-F.; Lina, B. After 2 Years of the COVID-19 Pandemic, Translating One Health into Action Is Urgent. Lancet 2023, 401, 789–794. [Google Scholar] [CrossRef]
- Liao, H.; Lyon, C.J.; Ying, B.; Hu, T. Climate Change, Its Impact on Emerging Infectious Diseases and New Technologies to Combat the Challenge. Emerg. Microbes Infect. 2024, 13, 2356143. [Google Scholar] [CrossRef] [PubMed]
- Maccaro, A.; Audia, C.; Stokes, K.; Masud, H.; Sekalala, S.; Pecchia, L.; Piaggio, D. Pandemic Preparedness: A Scoping Review of Best and Worst Practices from COVID-19. Healthcare 2023, 11, 2572. [Google Scholar] [CrossRef]
- Kellerborg, K.; Brouwer, W.; Van Baal, P. Costs and Benefits of Interventions Aimed at Major Infectious Disease Threats: Lessons from the Literature. Eur. J. Health Econ. 2020, 21, 1329–1350. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.S.; Ando, A.W.; Loch-Temzelides, T.; Vale, M.M.; Li, B.V.; Li, H.; Busch, J.; Chapman, C.A.; Kinnaird, M.; Nowak, K.; et al. The Costs and Benefits of Primary Prevention of Zoonotic Pandemics. Sci. Adv. 2022, 8, eabl4183. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, W.; Li, S.; Yang, H.; Zhang, X.; Lu, W.; Liu, J.; Wang, Y. Cost-Effectiveness of Interventions for the Prevention and Control of COVID-19: Systematic Review of 85 Modelling Studies. J. Glob. Health 2022, 12, 05022. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Paul, P.; Kumar, S.; Büsselberg, D.; Dwivedi, V.D.; Chaari, A. Promising Antiviral Activities of Natural Flavonoids against SARS-CoV-2 Targets: Systematic Review. Int. J. Mol. Sci. 2021, 22, 11069. [Google Scholar] [CrossRef]
- Murali, M.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Almehmadi, M.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Kalegowda, N.; et al. Repositioning Therapeutics for SARS-CoV-2: Virtual Screening of Plant-basedAnti-HIV Compounds as Possible Inhibitors against COVID-19 Viral RdRp. CPD 2022, 28, 969–980. [Google Scholar] [CrossRef]
- Basnet, S.; Marahatha, R.; Shrestha, A.; Bhattarai, S.; Katuwal, S.; Sharma, K.R.; Marasini, B.P.; Dahal, S.R.; Basnyat, R.C.; Patching, S.G.; et al. In Vitro and In Silico Studies for the Identification of Potent Metabolites of Some High-Altitude Medicinal Plants from Nepal Inhibiting SARS-CoV-2 Spike Protein. Molecules 2022, 27, 8957. [Google Scholar] [CrossRef] [PubMed]
- Lanrewaju, A.A.; Enitan-Folami, A.M.; Nyaga, M.M.; Sabiu, S.; Swalaha, F.M. Metabolites Profiling and Cheminformatics Bioprospection of Selected Medicinal Plants against the Main Protease and RNA-Dependent RNA Polymerase of SARS-CoV-2. J. Biomol. Struct. Dyn. 2023, 42, 6740–6760. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): A Global Pandemic and Treatment Strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Abiodun, O.A.; Ogboo, B.C.; Kola-Mustapha, A.T.; Attah, E.I.; Edemhanria, L.; Kumari, A.; Jaganathan, R.; Adelakun, N.S. Polypharmacology of Some Medicinal Plant Metabolites against SARS-CoV-2 and Host Targets: Molecular Dynamics Evaluation of NSP9 RNA Binding Protein. J. Biomol. Struct. Dyn. 2022, 40, 11467–11483. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Rizki, D.R.; Purnama, A.; Duta, T.F.; Harapan, H.; Idroes, R.; Ginting, B. Antiviral Molecular Targets of Essential Oils against SARS-CoV-2: A Systematic Review. Sci. Pharm. 2023, 91, 15. [Google Scholar] [CrossRef]
- Theodoridis, S.; Drakou, E.G.; Hickler, T.; Thines, M.; Nogues-Bravo, D. Evaluating Natural Medicinal Resources and Their Exposure to Global Change. Lancet Planet. Health 2023, 7, e155–e163. [Google Scholar] [CrossRef]
- Mohammadi, M.; Yahyapour, Y.; Nasrollahian, S.; Tayefeh-Arbab, M.H.; Javanian, M.; Rajabi Fadardi, M.; Mousavi Kani, S.N.; Honarvar Bakeshloo, P.; Saghebi, R.; Rajabnia, M.; et al. A Review on Herbal Secondary Metabolites Against COVID-19 Focusing on the Genetic Variants of SARS-CoV-2. Jundishapur J. Nat. Pharm. Prod. 2022, 17, e129618. [Google Scholar] [CrossRef]
- Aribisala, J.O.; Aruwa, C.E.; Uthman, T.O.; Nurain, I.O.; Idowu, K.; Sabiu, S. Cheminformatics Bioprospection of Broad Spectrum Plant Secondary Metabolites Targeting the Spike Proteins of Omicron Variant and Wild-Type SARS-CoV-2. Metabolites 2022, 12, 982. [Google Scholar] [CrossRef]
- Low, Z.; Lani, R.; Tiong, V.; Poh, C.; AbuBakar, S.; Hassandarvish, P. COVID-19 Therapeutic Potential of Natural Products. Int. J. Mol. Sci. 2023, 24, 9589. [Google Scholar] [CrossRef]
- Gomes, B.A.; Fernandes, D.A.; Mendonça, S.C.; Campos, M.F.; Da Fonseca, T.S.; Constant, L.E.C.; De Sousa, N.F.; Priscila Barros De Menezes, R.; De Oliveira, B.A.C.; Da Silva Costa, S.; et al. Predicting the Anti-SARS-CoV-2 Potential of Isoquinoline Alkaloids from Brazilian Siparunaceae Species Using Chemometric Tools. Int. J. Mol. Sci. 2025, 26, 633. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Facchiano, A.; Carbone, V. Food Plant Secondary Metabolites Antiviral Activity and Their Possible Roles in SARS-CoV-2 Treatment: An Overview. Molecules 2023, 28, 2470. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.A.; Gomes, B.A.; Da Silva, A.F.; De Carvalho, J.A.B.; Ricardo, N.S.; Leitão, S.G.; Leitão, G.G. Brazilian Medicinal Plants and Their Metabolites as Potential Antivirals Against SARS-CoV-2: A Systematic Review of Experimental Findings. Rev. Bras. Farmacogn. 2024, 34, 883–898. [Google Scholar] [CrossRef]
- Araújo, J.; De Sousa, L.; Sousa, A.; Bastos, R.; Santos, G.; Lage, M.; Stoyanov, S.; Passos, I.; De Azevedo, R.; Rocha, J. DFT, Molecular Docking, and ADME/Tox Screening Investigations of Market-Available Drugs against SARS-CoV-2. J. Braz. Chem. Soc. 2021, 32, 1628–1641. [Google Scholar] [CrossRef]
- Srivastava, V.; Yadav, A.; Sarkar, P. Molecular Docking and ADMET Study of Bioactive Compounds of Glycyrrhiza Glabra against Main Protease of SARS-CoV2. Mater. Today Proc. 2022, 49, 2999–3007. [Google Scholar] [CrossRef]
- Khaldan, A.; Bouamrane, S.; En-Nahli, F.; El-mernissi, R.; El Khatabi, K.; Hmamouchi, R.; Maghat, H.; Ajana, M.A.; Sbai, A.; Bouachrine, M.; et al. Prediction of Potential Inhibitors of SARS-CoV-2 Using 3D-QSAR, Molecular Docking Modeling and ADMET Properties. Heliyon 2021, 7, e06603. [Google Scholar] [CrossRef]
- Goyzueta-Mamani, L.D.; Barazorda-Ccahuana, H.L.; Mena-Ulecia, K.; Chávez-Fumagalli, M.A. Antiviral Activity of Metabolites from Peruvian Plants against SARS-CoV-2: An In Silico Approach. Molecules 2021, 26, 3882. [Google Scholar] [CrossRef]
- Amparo, T.R.; Seibert, J.B.; Almeida, T.C.; Costa, F.S.F.; Silveira, B.M.; Da Silva, G.N.; Dos Santos, O.D.H.; De Souza, G.H.B. In Silico Approach of Secondary Metabolites from Brazilian Herbal Medicines to Search for Potential Drugs against SARS-CoV-2. Phytother. Res. 2021, 35, 4297–4308. [Google Scholar] [CrossRef]
- Chan, W.K.B.; Olson, K.M.; Wotring, J.W.; Sexton, J.Z.; Carlson, H.A.; Traynor, J.R. In Silico Analysis of SARS-CoV-2 Proteins as Targets for Clinically Available Drugs. Sci. Rep. 2022, 12, 5320. [Google Scholar] [CrossRef]
- Das, S.; Singh, A.; Samanta, S.K.; Singha Roy, A. Naturally Occurring Anthraquinones as Potential Inhibitors of SARS-CoV-2 Main Protease: An Integrated Computational Study. Biologia 2022, 77, 1121–1134. [Google Scholar] [CrossRef]
- Rieder, A.S.; Deniz, B.F.; Netto, C.A.; Wyse, A.T.S. A Review of In Silico Research, SARS-CoV-2, and Neurodegeneration: Focus on Papain-Like Protease. Neurotox. Res. 2022, 40, 1553–1569. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 Genome, Structure, Evolution, Pathogenesis and Therapies: Structural Genomics Approach. Biochim. Biophys. Acta 2020, 1866, 165878. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.-Y.; Yang, J.-S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921.e10. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Jeong, G.U.; Song, H.; Yoon, G.Y.; Kim, D.; Kwon, Y.-C. Therapeutic Strategies Against COVID-19 and Structural Characterization of SARS-CoV-2: A Review. Front. Microbiol. 2020, 11, 1723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xu, M.; Chen, C.Z.; Guo, H.; Shen, M.; Hu, X.; Shinn, P.; Klumpp-Thomas, C.; Michael, S.G.; Zheng, W. Identification of SARS-CoV-2 3CL Protease Inhibitors by a Quantitative High-Throughput Screening. ACS Pharmacol. Transl. Sci. 2020, 3, 1008–1016. [Google Scholar] [CrossRef]
- Ziebuhr, J. The Coronavirus Replicase. In Coronavirus Replication and Reverse Genetics; Enjuanes, L., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 287, pp. 57–94. ISBN 978-3-540-21494-6. [Google Scholar]
- Bafna, K.; Cioffi, C.L.; Krug, R.M.; Montelione, G.T. Structural Similarities between SARS-CoV2 3CLpro and Other Viral Proteases Suggest Potential Lead Molecules for Developing Broad Spectrum Antivirals. Front. Chem. 2022, 10, 948553. [Google Scholar] [CrossRef]
- Vuong, W.; Khan, M.B.; Fischer, C.; Arutyunova, E.; Lamer, T.; Shields, J.; Saffran, H.A.; McKay, R.T.; Van Belkum, M.J.; Joyce, M.A.; et al. Feline Coronavirus Drug Inhibits the Main Protease of SARS-CoV-2 and Blocks Virus Replication. Nat. Commun. 2020, 11, 4282. [Google Scholar] [CrossRef]
- Klemm, T.; Ebert, G.; Calleja, D.J.; Allison, C.C.; Richardson, L.W.; Bernardini, J.P.; Lu, B.G.; Kuchel, N.W.; Grohmann, C.; Shibata, Y.; et al. Mechanism and Inhibition of the Papain-like Protease, PLpro, of SARS-CoV-2. EMBO J. 2020, 39, e106275. [Google Scholar] [CrossRef] [PubMed]
- Kuzikov, M.; Costanzi, E.; Reinshagen, J.; Esposito, F.; Vangeel, L.; Wolf, M.; Ellinger, B.; Claussen, C.; Geisslinger, G.; Corona, A.; et al. Identification of Inhibitors of SARS-CoV-2 3CL-Pro Enzymatic Activity Using a Small Molecule in Vitro Repurposing Screen. ACS Pharmacol. Transl. Sci. 2021, 4, 1096–1110. [Google Scholar] [CrossRef]
- Zhai, T.; Zhang, F.; Haider, S.; Kraut, D.; Huang, Z. An Integrated Computational and Experimental Approach to Identifying Inhibitors for SARS-CoV-2 3CL Protease. Front. Mol. Biosci. 2021, 8, 661424. [Google Scholar] [CrossRef] [PubMed]
- Glaab, E.; Manoharan, G.B.; Abankwa, D. Pharmacophore Model for SARS-CoV-2 3CLpro Small-Molecule Inhibitors and in Vitro Experimental Validation of Computationally Screened Inhibitors. J. Chem. Inf. Model. 2021, 61, 4082–4096. [Google Scholar] [CrossRef]
- Kuang, Y.; Ma, X.; Shen, W.; Rao, Q.; Yang, S. Discovery of 3CLpro Inhibitor of SARS-CoV-2 Main Protease. Future Sci. OA 2023, 9, FSO853. [Google Scholar] [CrossRef]
- De Souza, A.S.; De Souza, R.F.; Guzzo, C.R. Quantitative Structure-Activity Relationships, Molecular Docking and Molecular Dynamics Simulations Reveal Drug Repurposing Candidates as Potent SARS-CoV-2 Main Protease Inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 11339–11356. [Google Scholar] [CrossRef]
- Schake, P.; Dishnica, K.; Kaiser, F.; Leberecht, C.; Haupt, V.J.; Schroeder, M. An Interaction-Based Drug Discovery Screen Explains Known SARS-CoV-2 Inhibitors and Predicts New Compound Scaffolds. Sci. Rep. 2023, 13, 9204. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.; Ho, J.; Wills, S.; Mawri, A.; Lawson, L.; Ebert, M.C.C.J.C.; Fortin, G.M.; Rayalam, S.; Taval, S. Identification of 3-Chymotrypsin like Protease (3CLPro) Inhibitors as Potential Anti-SARS-CoV-2 Agents. Commun. Biol. 2021, 4, 93. [Google Scholar] [CrossRef]
- Luttens, A.; Gullberg, H.; Abdurakhmanov, E.; Vo, D.D.; Akaberi, D.; Talibov, V.O.; Nekhotiaeva, N.; Vangeel, L.; De Jonghe, S.; Jochmans, D.; et al. Ultralarge Virtual Screening Identifies SARS-CoV-2 Main Protease Inhibitors with Broad-Spectrum Activity against Coronaviruses. J. Am. Chem. Soc. 2022, 144, 2905–2920. [Google Scholar] [CrossRef]
- Pokharkar, O.; Zyryanov, G.V.; Tsurkan, M.V. Natural Products from Marine Actinomycete Genus Salinispora Might Inhibit 3CLpro and PLpro Proteins of SARS-CoV-2: An In Silico Evidence. Microbiol. Res. 2023, 14, 1907–1941. [Google Scholar] [CrossRef]
- Silva, R.C.; Freitas, H.F.; Campos, J.M.; Kimani, N.M.; Silva, C.H.T.P.; Borges, R.S.; Pita, S.S.R.; Santos, C.B.R. Natural Products-Based Drug Design against SARS-CoV-2 Mpro 3CLpro. Int. J. Mol. Sci. 2021, 22, 11739. [Google Scholar] [CrossRef] [PubMed]
- La Monica, G.; Bono, A.; Lauria, A.; Martorana, A. Targeting SARS-CoV-2 Main Protease for Treatment of COVID-19: Covalent Inhibitors Structure–Activity Relationship Insights and Evolution Perspectives. J. Med. Chem. 2022, 65, 12500–12534. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Amorim, V.M.; De Souza, R.F.; Guzzo, C.R.; De Souza, A.S. Molecular Dynamics Simulations Suggest SARS-CoV-2 3CLpro Mutations in Beta and Omicron Variants Do Not Alter Binding Affinities for Cleavage Sites of Non-Structural Proteins. COVID 2023, 3, 622–636. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Wasilewicz, A.; Kirchweger, B.; Bojkova, D.; Abi Saad, M.J.; Langeder, J.; Bütikofer, M.; Adelsberger, S.; Grienke, U.; Cinatl, J., Jr.; Petermann, O.; et al. Identification of Natural Products Inhibiting SARS-CoV-2 by Targeting Viral Proteases: A Combined in Silico and in Vitro Approach. J. Nat. Prod. 2023, 86, 264–275. [Google Scholar] [CrossRef]
- Anand, K.; Ziebuhr, J.; Wadhwani, P.; Mesters, J.R.; Hilgenfeld, R. Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs. Science 2003, 300, 1763–1767. [Google Scholar] [CrossRef]
- Li, C.; Teng, X.; Qi, Y.; Tang, B.; Shi, H.; Ma, X.; Lai, L. Conformational Flexibility of a Short Loop near the Active Site of the SARS-3CLpro Is Essential to Maintain Catalytic Activity. Sci. Rep. 2016, 6, 20918. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, C.; Xin, L.; Ren, X.; Tian, L.; Ju, X.; Li, H.; Wang, Y.; Zhao, Q.; Liu, H.; et al. The Development of Coronavirus 3C-Like Protease (3CLpro) Inhibitors from 2010 to 2020. Eur. J. Med. Chem. 2020, 206, 112711. [Google Scholar] [CrossRef]
- Banerjee, R.; Perera, L.; Tillekeratne, L.M.V. Potential SARS-CoV-2 Main Protease Inhibitors. Drug Discov. Today 2021, 26, 804–816. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Inhibition of the Main Protease of SARS-CoV-2 (Mpro) by Repurposing/Designing Drug-like Substances and Utilizing Nature’s Toolbox of Bioactive Compounds. Comput. Struct. Biotechnol. J. 2022, 20, 1306–1344. [Google Scholar] [CrossRef]
- McClain, C.B.; Vabret, N. SARS-CoV-2: The Many Pros of Targeting PLpro. Sig. Transduct. Target. Ther. 2020, 5, 223. [Google Scholar] [CrossRef] [PubMed]
- Rut, W.; Lv, Z.; Zmudzinski, M.; Patchett, S.; Nayak, D.; Snipas, S.J.; El Oualid, F.; Huang, T.T.; Bekes, M.; Drag, M.; et al. Activity Profiling and Crystal Structures of Inhibitor-Bound SARS-CoV-2 Papain-like Protease: A Framework for Anti–COVID-19 Drug Design. Sci. Adv. 2020, 6, eabd4596. [Google Scholar] [CrossRef]
- Drosten, C.; Günther, S.; Preiser, W.; Van Der Werf, S.; Brodt, H.-R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.M.; et al. Identification of a Novel Coronavirus in Patients with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Gao, X.; Qin, B.; Chen, P.; Zhu, K.; Hou, P.; Wojdyla, J.A.; Wang, M.; Cui, S. Crystal Structure of SARS-CoV-2 Papain-like Protease. Acta Pharm. Sin. B 2021, 11, 237–245. [Google Scholar] [CrossRef]
- Báez-Santos, Y.M.; St. John, S.E.; Mesecar, A.D. The SARS-Coronavirus Papain-like Protease: Structure, Function and Inhibition by Designed Antiviral Compounds. Antivir. Res. 2015, 115, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ye, G.; Si, Y.; Shen, Z.; Liu, Z.; Shi, Y.; Xiao, S.; Fu, Z.F.; Peng, G. Structure of the Multiple Functional Domains from Coronavirus Nonstructural Protein 3. Emerg. Microbes Infect. 2021, 10, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, B.H.; Jukneliene, D.; Kanjanahaluethai, A.; Bechill, J.; Severson, K.M.; Smith, C.M.; Rota, P.A.; Baker, S.C. Identification of Severe Acute Respiratory Syndrome Coronavirus Replicase Products and Characterization of Papain-Like Protease Activity. J. Virol. 2004, 78, 13600–13612. [Google Scholar] [CrossRef]
- Osipiuk, J.; Azizi, S.-A.; Dvorkin, S.; Endres, M.; Jedrzejczak, R.; Jones, K.A.; Kang, S.; Kathayat, R.S.; Kim, Y.; Lisnyak, V.G.; et al. Structure of Papain-like Protease from SARS-CoV-2 and Its Complexes with Non-Covalent Inhibitors. Nat. Commun. 2021, 12, 743. [Google Scholar] [CrossRef]
- Huynh, T.; Cornell, W.; Luan, B. In Silico Exploration of Inhibitors for SARS-CoV-2’s Papain-Like Protease. Front. Chem. 2021, 8, 624163. [Google Scholar] [CrossRef]
- Barretto, N.; Jukneliene, D.; Ratia, K.; Chen, Z.; Mesecar, A.D.; Baker, S.C. The Papain-Like Protease of Severe Acute Respiratory Syndrome Coronavirus Has Deubiquitinating Activity. J. Virol. 2005, 79, 15189–15198. [Google Scholar] [CrossRef]
- Békés, M.; van der Heden van Noort, G.J.; Ekkebus, R.; Ovaa, H.; Huang, T.T.; Lima, C.D. Recognition of Lys48-Linked Di-Ubiquitin and Deubiquitinating Activities of the SARS Coronavirus Papain-like Protease. Mol. Cell 2016, 62, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.G.; Wang, N.; Chen, Z.; Chen, Z.; Tseng, M.; Barretto, N.; Lin, R.; Peters, C.J.; Tseng, C.-T.K.; Baker, S.C.; et al. Regulation of IRF-3-Dependent Innate Immunity by the Papain-like Protease Domain of the Severe Acute Respiratory Syndrome Coronavirus. J. Biol. Chem. 2007, 282, 32208–32221. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, M.K.; Ploegh, H.L. Ubiquitination, Ubiquitin-like Modifiers, and Deubiquitination in Viral Infection. Cell Host Microbe 2009, 5, 559–570. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Zheng, Y.; Yang, Y.; Xing, Y.; Chen, Z. SARS Coronavirus Papain-like Protease Inhibits the Type I Interferon Signaling Pathway through Interaction with the STING-TRAF3-TBK1 Complex. Protein Cell 2014, 5, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Schäfer, A.; Pham, A.; Frieman, M. The SARS Coronavirus Papain like Protease Can Inhibit IRF3 at a Post Activation Step That Requires Deubiquitination Activity. Virol. J. 2014, 11, 209. [Google Scholar] [CrossRef]
- Heaton, S.M.; Borg, N.A.; Dixit, V.M. Ubiquitin in the Activation and Attenuation of Innate Antiviral Immunity. JEM 2016, 213, 1–13. [Google Scholar] [CrossRef]
- Li, S.-W.; Wang, C.-Y.; Jou, Y.-J.; Yang, T.-C.; Huang, S.-H.; Wan, L.; Lin, Y.-J.; Lin, C.-W. SARS Coronavirus Papain-like Protease Induces Egr-1-Dependent up-Regulation of TGF-Β1 via ROS/P38 MAPK/STAT3 Pathway. Sci. Rep. 2016, 6, 25754. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like Protease Regulates SARS-CoV-2 Viral Spread and Innate Immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef]
- Moustaqil, M.; Ollivier, E.; Chiu, H.-P.; Van Tol, S.; Rudolffi-Soto, P.; Stevens, C.; Bhumkar, A.; Hunter, D.J.B.; Freiberg, A.N.; Jacques, D.; et al. SARS-CoV-2 Proteases PLpro and 3CLpro Cleave IRF3 and Critical Modulators of Inflammatory Pathways (NLRP12 and TAB1): Implications for Disease Presentation across Species. Emerg. Microbes Infect. 2021, 10, 178–195. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J. Validation and Invalidation of SARS-CoV-2 Papain-like Protease Inhibitors. ACS Pharmacol. Transl. Sci. 2022, 5, 102–109. [Google Scholar] [CrossRef]
- Mahmoudvand, S.; Shokri, S. Interactions between SARS Coronavirus 2 Papain-like Protease and Immune System: A Potential Drug Target for the Treatment of COVID-19. Scand. J. Immunol. 2021, 94, e13044. [Google Scholar] [CrossRef] [PubMed]
- Ratia, K.; Pegan, S.; Takayama, J.; Sleeman, K.; Coughlin, M.; Baliji, S.; Chaudhuri, R.; Fu, W.; Prabhakar, B.S.; Johnson, M.E.; et al. A Noncovalent Class of Papain-like Protease/Deubiquitinase Inhibitors Blocks SARS Virus Replication. Proc. Natl. Acad. Sci. USA 2008, 105, 16119–16124. [Google Scholar] [CrossRef]
- Ma, C.; Sacco, M.D.; Xia, Z.; Lambrinidis, G.; Townsend, J.A.; Hu, Y.; Meng, X.; Szeto, T.; Ba, M.; Zhang, X.; et al. Discovery of SARS-CoV-2 Papain-like Protease Inhibitors through a Combination of High-Throughput Screening and a FlipGFP-Based Reporter Assay. ACS Cent. Sci. 2021, 7, 1245–1260. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, Y.; Lei, Y.; Wang, R.; Zhan, M.; Liu, J.; An, Y.; Zhou, Y.; Zhan, J.; Yin, F.; et al. Design and Evaluation of a Novel Peptide–Drug Conjugate Covalently Targeting SARS-CoV-2 Papain-like Protease. J. Med. Chem. 2022, 65, 876–884. [Google Scholar] [CrossRef]
- Zhou, T.; Tsybovsky, Y.; Gorman, J.; Rapp, M.; Cerutti, G.; Chuang, G.-Y.; Katsamba, P.S.; Sampson, J.M.; Schön, A.; Bimela, J.; et al. Cryo-EM Structures of SARS-CoV-2 Spike without and with ACE2 Reveal a pH-Dependent Switch to Mediate Endosomal Positioning of Receptor-Binding Domains. Cell Host Microbe 2020, 28, 867–879.e5. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.; Xu, W.; Liu, S. Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural Basis of Receptor Recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Goc, A.; Sumera, W.; Rath, M.; Niedzwiecki, A. Phenolic Compounds Disrupt Spike-Mediated Receptor-Binding and Entry of SARS-CoV-2 Pseudo-Virions. PLoS ONE 2021, 16, e0253489. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, H.; Guo, L.; Xie, D.; He, H.; Zhang, H.; Liu, Y.; Peng, L.; Zheng, L.; Lu, W.; et al. Potential Inhibitors for Blocking the Interaction of the Coronavirus SARS-CoV-2 Spike Protein and Its Host Cell Receptor ACE2. J. Transl. Med. 2022, 20, 314. [Google Scholar] [CrossRef]
- Katuwal, S.; Upadhyaya, S.R.; Marahatha, R.; Shrestha, A.; Regmi, B.P.; Khadayat, K.; Basnet, S.; Basnyat, R.C.; Parajuli, N. In Silico Study of Coumarins: Wedelolactone as a Potential Inhibitor of the Spike Protein of the SARS-CoV-2 Variants. J. Trop. Med. 2023, 2023, 4771745. [Google Scholar] [CrossRef]
- Delgado-Maldonado, T.; Gonzalez-Morales, L.D.; Juarez-Saldivar, A.; Lara-Ramírez, E.E.; Rojas-Verde, G.; Moreno-Rodriguez, A.; Bandyopadhyay, D.; Rivera, G. Structure-Based Virtual Screening from Natural Products as Inhibitors of SARS-CoV-2 Spike Protein and ACE2 Receptor Binding and Their Biological Evaluation In Vitro. Med. Chem. 2024, 20, 546–553. [Google Scholar] [CrossRef]
- Elfiky, A.A. SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp) Targeting: An in Silico Perspective. J. Biomol. Struct. Dyn. 2020, 39, 3204–3212. [Google Scholar] [CrossRef] [PubMed]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of Replicating SARS-CoV-2 Polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef]
- Lamb, Y.N. Remdesivir: First Approval. Drugs 2020, 80, 1355–1363. [Google Scholar] [CrossRef]
- Rubin, D.; Chan-Tack, K.; Farley, J.; Sherwat, A. FDA Approval of Remdesivir—A Step in the Right Direction. N. Engl. J. Med. 2020, 383, 2598–2600. [Google Scholar] [CrossRef]
- Mishra, A.; Rathore, A.S. RNA Dependent RNA Polymerase (RdRp) as a Drug Target for SARS-CoV2. J. Biomol. Struct. Dyn. 2022, 40, 6039–6051. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-Dependent RNA Polymerase from COVID-19 Virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Suručić, R.; Travar, M.; Petković, M.; Tubić, B.; Stojiljković, M.P.; Grabež, M.; Šavikin, K.; Zdunić, G.; Škrbić, R. Pomegranate Peel Extract Polyphenols Attenuate the SARS-CoV-2 S-Glycoprotein Binding Ability to ACE2 Receptor: In Silico and in Vitro Studies. Bioorg. Chem. 2021, 114, 105145. [Google Scholar] [CrossRef]
- Alves, J.; Engel, L.; De Vasconcelos Cabral, R.; Rodrigues, E.L.; De Jesus Ribeiro, L.; Higa, L.M.; Da Costa Ferreira Júnior, O.; Castiñeiras, T.M.P.P.; De Carvalho Leitão, I.; Tanuri, A.; et al. A Bioluminescent and Homogeneous SARS-CoV-2 Spike RBD and hACE2 Interaction Assay for Antiviral Screening and Monitoring Patient Neutralizing Antibody Levels. Sci. Rep. 2021, 11, 18428. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, I.; Cassel, J.; Register, E.T.; Zhou, X.Y.; Messick, T.E.; Keeney, F.; Lu, L.D.; Beattie, K.D.; Rali, T.; Tebas, P.; et al. The Natural Stilbenoid (–)-Hopeaphenol Inhibits Cellular Entry of SARS-CoV-2 USA-WA1/2020, B.1.1.7, and B.1.351 Variants. Antimicrob. Agents Chemother. 2021, 65, e00772-21. [Google Scholar] [CrossRef]
- Invernizzi, L.; Moyo, P.; Cassel, J.; Isaacs, F.J.; Salvino, J.M.; Montaner, L.J.; Tietjen, I.; Maharaj, V. Use of Hyphenated Analytical Techniques to Identify the Bioactive Constituents of Gunnera perpensa L., a South African Medicinal Plant, Which Potently Inhibit SARS-CoV-2 Spike Glycoprotein–Host ACE2 Binding. Anal. Bioanal. Chem. 2022, 414, 3971–3985. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Huang, V.; Chao, T.-C.; Hsiao, C.-D.; Lin, A.; Chang, M.-F.; Chow, L.-P. Screening of Drugs by FRET Analysis Identifies Inhibitors of SARS-CoV 3CL Protease. Biochem. Biophys. Res. Commun. 2005, 333, 194–199. [Google Scholar] [CrossRef]
- Cihlova, B.; Huskova, A.; Böserle, J.; Nencka, R.; Boura, E.; Silhan, J. High-Throughput Fluorescent Assay for Inhibitor Screening of Proteases from RNA Viruses. Molecules 2021, 26, 3792. [Google Scholar] [CrossRef]
- Sekar, R.B.; Periasamy, A. Fluorescence Resonance Energy Transfer (FRET) Microscopy Imaging of Live Cell Protein Localizations. J. Cell Sci. 2003, 160, 629–633. [Google Scholar] [CrossRef]
- Sabariegos, R.; Picazo, F.; Domingo, B.; Franco, S.; Martinez, M.-A.; Llopis, J. Fluorescence Resonance Energy Transfer-Based Assay for Characterization of Hepatitis C Virus NS3-4A Protease Activity in Live Cells. Antimicrob. Agents Chemother. 2009, 53, 728–734. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Antunes, M.S. Re-Engineering Plant Phenylpropanoid Metabolism with the Aid of Synthetic Biosensors. Front. Plant Sci. 2021, 12, 701385. [Google Scholar] [CrossRef]
- Puttaswamy, H.; Gowtham, H.G.; Ojha, M.D.; Yadav, A.; Choudhir, G.; Raguraman, V.; Kongkham, B.; Selvaraju, K.; Shareef, S.; Gehlot, P.; et al. In Silico Studies Evidenced the Role of Structurally Diverse Plant Secondary Metabolites in Reducing SARS-CoV-2 Pathogenesis. Sci. Rep. 2020, 10, 20584. [Google Scholar] [CrossRef]
- Wang, F.; Liu, D.; Gao, D.; Yuan, J.; Zhao, J.; Yuan, S.; Cen, Y.; Lin, G.-Q.; Zhao, J.; Tian, P. Discovery of Natural Catechol Derivatives as Covalent SARS-CoV-2 3CLpro Inhibitors. Int. J. Biol. Macromol. 2024, 264, 130377. [Google Scholar] [CrossRef] [PubMed]
- Shahhamzehei, N.; Abdelfatah, S.; Efferth, T. In Silico and In Vitro Identification of Pan-Coronaviral Main Protease Inhibitors from a Large Natural Product Library. Pharmaceuticals 2022, 15, 308. [Google Scholar] [CrossRef]
- Krüger, N.; Kronenberger, T.; Xie, H.; Rocha, C.; Pöhlmann, S.; Su, H.; Xu, Y.; Laufer, S.A.; Pillaiyar, T. Discovery of Polyphenolic Natural Products as SARS-CoV-2 Mpro Inhibitors for COVID-19. Pharmaceuticals 2023, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, N.; Mehroze, A.; Sarwar, W.; Arshad, U.; Parveen, S.; Rashid, M.; Farooq, A.; Rafiq, N.; Wondmie, G.F.; Bin Jardan, Y.A.; et al. Exploration of Phenolic Acid Derivatives as Inhibitors of SARS-CoV-2 Main Protease and Receptor Binding Domain: Potential Candidates for Anti-SARS-CoV-2 Therapy. Front. Chem. 2023, 11, 1251529. [Google Scholar] [CrossRef]
- Gancia, E.; Montana, J.G.; Manallack, D.T. Theoretical Hydrogen Bonding Parameters for Drug Design. J. Mol. Graph. Model. 2001, 19, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Ghiandoni, G.M.; Caldeweyher, E. Fast Calculation of Hydrogen-Bond Strengths and Free Energy of Hydration of Small Molecules. Sci. Rep. 2023, 13, 4143. [Google Scholar] [CrossRef]
- Wang, R.; Chen, X.; Li, H.; Chen, X.; Sun, D.; Yu, D.; Lu, J.; Xie, Y.; Zhang, Q.; Xu, J.; et al. Danshensu Inhibits SARS-CoV-2 by Targeting Its Main Protease as a Specific Covalent Inhibitor and Discovery of Bifunctional Compounds Eliciting Antiviral and Anti-Inflammatory Activity. Int. J. Biol. Macromol. 2024, 257, 128623. [Google Scholar] [CrossRef]
- Hicks, E.G.; Kandel, S.E.; Lampe, J.N. Identification of Aloe-Derived Natural Products as Prospective Lead Scaffolds for SARS-CoV-2 Main Protease (Mpro) Inhibitors. Bioorg Med. Chem. Lett. 2022, 66, 128732. [Google Scholar] [CrossRef]
- Chen, Z.; Cui, Q.; Cooper, L.; Zhang, P.; Lee, H.; Chen, Z.; Wang, Y.; Liu, X.; Rong, L.; Du, R. Ginkgolic Acid and Anacardic Acid Are Specific Covalent Inhibitors of SARS-CoV-2 Cysteine Proteases. Cell Biosci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Lee, H.; Mittal, A.; Patel, K.; Gatuz, J.L.; Truong, L.; Torres, J.; Mulhearn, D.C.; Johnson, M.E. Identification of Novel Drug Scaffolds for Inhibition of SARS-CoV 3-Chymotrypsin-like Protease Using Virtual and High-Throughput Screenings. Bioorg. Med. Chem. 2014, 22, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Soledade, M.; Pedras, C.; Zheng, Q. The Chemistry of Arabidopsis thaliana. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1297–1315. ISBN 978-0-08-045382-8. [Google Scholar]
- Abdallah, H.M.; El-Halawany, A.M.; Sirwi, A.; El-Araby, A.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Koshak, A.E.; Asfour, H.Z.; Awan, Z.A.; Elfaky, M.A. Repurposing of Some Natural Product Isolates as SARS-COV-2 Main Protease Inhibitors via In Vitro Cell Free and Cell-Based Antiviral Assessments and Molecular Modeling Approaches. Pharmaceuticals 2021, 14, 213. [Google Scholar] [CrossRef]
- Srinivasan, V.; Brognaro, H.; Prabhu, P.R.; De Souza, E.E.; Günther, S.; Reinke, P.Y.A.; Lane, T.J.; Ginn, H.; Han, H.; Ewert, W.; et al. Antiviral Activity of Natural Phenolic Compounds in Complex at an Allosteric Site of SARS-CoV-2 Papain-like Protease. Commun. Biol. 2022, 5, 805. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Al-Hashimi, I.; Al Moustafa, A.-E.; Khalil, A. A Comprehensive Review on the Antiviral Activities of Chalcones. J. Drug Target. 2021, 29, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef]
- Li, Z.; Xie, H.; Tang, C.; Feng, L.; Ke, C.; Xu, Y.; Su, H.; Yao, S.; Ye, Y. Flavonoids from the Roots and Rhizomes of Sophora Tonkinensis and Their in Vitro Anti-SARS-CoV-2 Activity. Chin. J. Integr. Med. 2023, 21, 65–80. [Google Scholar] [CrossRef]
- Herzog, A.-M.; Göbel, K.; Marongiu, L.; Ruetalo, N.; Alonso, M.C.; Leischner, C.; Busch, C.; Burkard, M.; Lauer, U.M.; Geurink, P.P.; et al. Compounds Derived from Humulus Lupulus Inhibit SARS-CoV-2 Papain-like Protease and Virus Replication. Phytomedicine 2024, 123, 155176. [Google Scholar] [CrossRef]
- Khamto, N.; Utama, K.; Tateing, S.; Sangthong, P.; Rithchumpon, P.; Cheechana, N.; Saiai, A.; Semakul, N.; Punyodom, W.; Meepowpan, P. Discovery of Natural Bisbenzylisoquinoline Analogs from the Library of Thai Traditional Plants as SARS-CoV-2 3CLPro Inhibitors: In Silico Molecular Docking, Molecular Dynamics, and In Vitro Enzymatic Activity. J. Chem. Inf. Model. 2023, 63, 2104–2121. [Google Scholar] [CrossRef] [PubMed]
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S.; Munyoo, B.; Tuchinda, P.; Jearawuttanakul, K.; Seemakhan, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Rangkasenee, N.; et al. High-Content Screening of Thai Medicinal Plants Reveals Boesenbergia Rotunda Extract and Its Component Panduratin A as Anti-SARS-CoV-2 Agents. Sci. Rep. 2020, 10, 19963. [Google Scholar] [CrossRef]
- Ma, M.; Luan, X.; Zheng, H.; Wang, X.; Wang, S.; Shen, T.; Ren, D. A Mulberry Diels-Alder-Type Adduct, Kuwanon M, Triggers Apoptosis and Paraptosis of Lung Cancer Cells through Inducing Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2023, 24, 1015. [Google Scholar] [CrossRef]
- Lin, Y.; Zang, R.; Ma, Y.; Wang, Z.; Li, L.; Ding, S.; Zhang, R.; Wei, Z.; Yang, J.; Wang, X. Xanthohumol Is a Potent Pan-Inhibitor of Coronaviruses Targeting Main Protease. Int. J. Mol. Sci. 2021, 22, 12134. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral Activities of Flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yao, S.; Zhao, W.; Li, M.; Liu, J.; Shang, W.; Xie, H.; Ke, C.; Hu, H.; Gao, M.; et al. Anti-SARS-CoV-2 Activities in Vitro of Shuanghuanglian Preparations and Bioactive Ingredients. Acta Pharmacol. Sin. 2020, 41, 1167–1177. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Hua, Y.; Zhang, W.; Zhou, X.; He, J.; Chen, D. Tannic Acid as a Natural Ferroptosis Inhibitor: Mechanisms and Beneficial Role of 3’-O-Galloylation. ChemistrySelect 2021, 6, 1562–1569. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, G.-H.; Wang, H.-N.; Hu, Q.; Chen, L.-L.; Guan, X.-Q.; Li, H.-L.; Chen, H.-Z.; Tang, H.; Ge, G.-B. Discovery of Naturally Occurring Inhibitors against SARS-CoV-2 3CLpro from Ginkgo biloba Leaves via Large-Scale Screening. Fitoterapia 2021, 152, 104909. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kwon, E.-B.; Kim, B.; Chung, H.-S.; Choi, G.; Kim, Y.-H.; Choi, J.-G. Mulberry Component Kuwanon C Exerts Potent Therapeutic Efficacy In Vitro against COVID-19 by Blocking the SARS-CoV-2 Spike S1 RBD:ACE2 Receptor Interaction. Int. J. Mol. Sci. 2022, 23, 12516. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, M.; Xue, H.; Yu, R.; Bao, Y.-O.; Kuang, Y.; Chai, Y.; Ma, W.; Wang, J.; Shi, X.; et al. Schaftoside Inhibits 3CLpro and PLpro of SARS-CoV-2 Virus and Regulates Immune Response and Inflammation of Host Cells for the Treatment of COVID-19. Acta Pharm. Sin. B 2022, 12, 4154–4164. [Google Scholar] [CrossRef]
- Zhu, D.; Su, H.; Ke, C.; Tang, C.; Witt, M.; Quinn, R.J.; Xu, Y.; Liu, J.; Ye, Y. Efficient Discovery of Potential Inhibitors for SARS-CoV-2 3C-like Protease from Herbal Extracts Using a Native MS-Based Affinity-Selection Method. J. Pharm. Biomed. Anal. 2022, 209, 114538. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, M.; Fu, B.; Li, M.; Yang, J.; Zhang, Z.; Li, C.; Zhang, H.; Wu, H.; Xue, W.; et al. Structure-Based Discovery of the SARS-CoV-2 Main Protease Noncovalent Inhibitors from Traditional Chinese Medicine. J. Chem. Inf. Model. 2024, 64, 1319–1330. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Hu, Y.; Ding, T.; Zafar, M.M.; Jia, X.; Zhang, L.; Ren, M.; Li, F.; Wang, W. Cotton Flower Metabolites Inhibit SARS-CoV-2 Main Protease. FEBS Open Bio 2022, 12, 1886–1895. [Google Scholar] [CrossRef]
- Su, H.; Yao, S.; Zhao, W.; Zhang, Y.; Liu, J.; Shao, Q.; Wang, Q.; Li, M.; Xie, H.; Shang, W.; et al. Identification of Pyrogallol as a Warhead in Design of Covalent Inhibitors for the SARS-CoV-2 3CL Protease. Nat. Commun. 2021, 12, 3623. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, D.-Y.; Scartelli, C.; Xie, H.; Merrill-Skoloff, G.; Yang, M.; Sun, L.; Saeed, M.; Flaumenhaft, R. Plant Flavonoid Inhibition of SARS-CoV-2 Main Protease and Viral Replication. iScience 2023, 26, 107602. [Google Scholar] [CrossRef]
- Li, H.; Sun, M.; Lei, F.; Liu, J.; Chen, X.; Li, Y.; Wang, Y.; Lu, J.; Yu, D.; Gao, Y.; et al. Methyl Rosmarinate Is an Allosteric Inhibitor of SARS-CoV-2 3 CL Protease as a Potential Candidate against SARS-Cov-2 Infection. Antiviral Res. 2024, 224, 105841. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.-G. Antiviral Effect of Methylated Flavonol Isorhamnetin against Influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; Hassan, H.M.; Youssif, K.A.; Gaber, Y.; Moatasim, Y.; Kutkat, O.; Mostafa, A.; Ali, M.A.; Rateb, M.E.; et al. Cnicin as an Anti-SARS-CoV-2: An Integrated In Silico and In Vitro Approach for the Rapid Identification of Potential COVID-19 Therapeutics. Antibiotics 2021, 10, 542. [Google Scholar] [CrossRef]
- Hu, X.; Wang, M.; Pan, Y.; Xie, Y.; Han, J.; Zhang, X.; Niayale, R.; He, H.; Li, Q.; Zhao, T.; et al. Anti-Inflammatory Effect of Astragalin and Chlorogenic Acid on Escherichia Coli-Induced Inflammation of Sheep Endometrial Epithelium Cells. Front. Vet. Sci. 2020, 7, 201. [Google Scholar] [CrossRef]
- Du, A.; Zheng, R.; Disoma, C.; Li, S.; Chen, Z.; Li, S.; Liu, P.; Zhou, Y.; Shen, Y.; Liu, S.; et al. Epigallocatechin-3-Gallate, an Active Ingredient of Traditional Chinese Medicines, Inhibits the 3CLpro Activity of SARS-CoV-2. Int. J. Biol. Macromol. 2021, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-H.; Rao, Y.K.; Tzeng, Y.-M. Inhibitory Effects of Flavonol Glycosides from Cinnamomum osmophloeum on Inflammatory Mediators in LPS/IFN-γ-Activated Murine Macrophages. Bioorg. Med. Chem. 2005, 13, 2381–2388. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kim, Y.S.; Kim, J.H.; Chung, H.-S. Antiviral Activity of Ethanol Extract of Geranii Herba and Its Components against Influenza Viruses via Neuraminidase Inhibition. Sci. Rep. 2019, 9, 12132. [Google Scholar] [CrossRef]
- Liao, Q.; Chen, Z.; Tao, Y.; Zhang, B.; Wu, X.; Yang, L.; Wang, Q.; Wang, Z. An Integrated Method for Optimized Identification of Effective Natural Inhibitors against SARS-CoV-2 3CLpro. Sci. Rep. 2021, 11, 22796. [Google Scholar] [CrossRef]
- Schwarz, S.; Sauter, D.; Wang, K.; Zhang, R.; Sun, B.; Karioti, A.; Bilia, A.; Efferth, T.; Schwarz, W. Kaempferol Derivatives as Antiviral Drugs against the 3a Channel Protein of Coronavirus. Planta Med. 2014, 80, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Iraci, N.; Denaro, M.; Mandalari, G.; Giofrè, S.V.; Trombetta, D. Synergistic Combination of Citrus Flavanones as Strong Antioxidant and COX-Inhibitor Agent. Antioxidants 2023, 12, 972. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a Flavanone with Antiviral and Anti-inflammatory Effects: A Promising Treatment Strategy against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Khamto, N.; Utama, K.; Boontawee, P.; Janthong, A.; Tatieng, S.; Arthan, S.; Choommongkol, V.; Sangthong, P.; Yenjai, C.; Suree, N.; et al. Inhibitory Activity of Flavonoid Scaffolds on SARS-CoV-2 3CLpro: Insights from the Computational and Experimental Investigations. J. Chem. Inf. Model. 2024, 64, 874–891. [Google Scholar] [CrossRef]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from Nature: A Phytochemistry and Biological Activity Review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Martínez, J.; Jiménez-Alesanco, A.; Ceballos-Laita, L.; Ortega-Alarcón, D.; Vega, S.; Calvo, C.; Benítez, C.; Abian, O.; Velázquez-Campoy, A.; Thomson, T.M.; et al. Discovery of Diverse Natural Products as Inhibitors of SARS-CoV-2 Mpro Protease through Virtual Screening. J. Chem. Inf. Model. 2021, 61, 6094–6106. [Google Scholar] [CrossRef]
- Tun, M.M.N.; Luvai, E.; Nwe, K.M.; Toume, K.; Mizukami, S.; Hirayama, K.; Komatsu, K.; Morita, K. Anti-SARS-CoV-2 Activity of Various PET-Bottled Japanese Green Teas and Tea Compounds in Vitro. Arch. Virol. 2022, 167, 1547–1557. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Wang, W.; Ke, J.-P.; Zhang, P.; Chu, G.-X.; Bao, G.-H. Discovery of Camellia Sinensis Catechins as SARS-CoV-2 3CL Protease Inhibitors through Molecular Docking, Intra and Extra Cellular Assays. Phytomedicine 2022, 96, 153853. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Bo, S.; Chang, S.K.; Chen, Y.; Sheng, Z.; Jiang, Y.; Yang, B. The Structure Characteristics, Biosynthesis and Health Benefits of Naturally Occurring Rare Flavonoids. Crit. Rev. Food Sci. Nutr. 2024, 64, 2490–2512. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Elissawy, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Efferth, T.; Singab, A.N.B. The Pharmacology of the Genus Sophora (Fabaceae): An Updated Review. Phytomedicine 2019, 64, 153070. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, H.I.; Kutkat, O.; Sweelam, H.M.; Eldehna, W.M.; Mostafa, M.A.; Ibrahim, M.T.; Moatasim, Y.; GabAllah, M.; Al-Karmalawy, A.A. Investigating the Potential Anti-SARS-CoV-2 and Anti-MERS-CoV Activities of Yellow Necklacepod among Three Selected Medicinal Plants: Extraction, Isolation, Identification, In Vitro, Modes of Action, and Molecular Docking Studies. Metabolites 2022, 12, 1109. [Google Scholar] [CrossRef]
- Püntener, A.G.; Schlesinger, U. Natural Dyes. In Colorants for Non-Textile Applications; Elsevier: Amsterdam, The Netherlands, 2000; pp. 382–455. ISBN 978-0-444-82888-0. [Google Scholar]
- Li, J.; Liao, W.; Huang, D.; Ou, M.; Chen, T.; Wang, X.; Zhao, R.; Zhang, L.; Mei, L.; Liu, J.; et al. Current Strategies of Detecting Aβ Species and Inhibiting Aβ Aggregation: Status and Prospects. Coord. Chem. Rev. 2023, 495, 215375. [Google Scholar] [CrossRef]
- Low, K.J.Y.; Venkatraman, A.; Mehta, J.S.; Pervushin, K. Molecular Mechanisms of Amyloid Disaggregation. J. Adv. Res. 2022, 36, 113–132. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Miao, P.; Xu, F.; Chen, J.; Jiang, X.; Hui, Z.; Wang, L.; Bai, R. Discovery of Anti-Inflammatory Natural Flavonoids: Diverse Scaffolds and Promising Leads for Drug Discovery. Eur. J. Med. Chem. 2023, 260, 115791. [Google Scholar] [CrossRef]
- Drouet, S.; Leclerc, E.A.; Garros, L.; Tungmunnithum, D.; Kabra, A.; Abbasi, B.H.; Lainé, É.; Hano, C. A Green Ultrasound-Assisted Extraction Optimization of the Natural Antioxidant and Anti-Aging Flavonolignans from Milk Thistle Silybum marianum (L.) Gaertn. Fruits for Cosmetic Applications. Antioxidants 2019, 8, 304. [Google Scholar] [CrossRef]
- Xiao, T.; Wei, Y.; Cui, M.; Li, X.; Ruan, H.; Zhang, L.; Bao, J.; Ren, S.; Gao, D.; Wang, M.; et al. Effect of Dihydromyricetin on SARS-CoV-2 Viral Replication and Pulmonary Inflammation and Fibrosis. Phytomedicine 2021, 91, 153704. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Leung, P.S. Pancreatic Cancer, Pancreatitis, and Oxidative Stress. In Gastrointestinal Tissue; Elsevier: Amsterdam, The Netherlands, 2017; pp. 173–186. ISBN 978-0-12-805377-5. [Google Scholar]
- Sun, Y.; Liu, S.; Yang, S.; Chen, C.; Yang, Y.; Lin, M.; Liu, C.; Wang, W.; Zhou, X.; Ai, Q.; et al. Mechanism of Dihydromyricetin on Inflammatory Diseases. Front. Pharmacol. 2022, 12, 794563. [Google Scholar] [CrossRef]
- Selim, S.; Albqmi, M.; Alanazi, A.; Alruwaili, Y.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; Al Jaouni, S.K.; Hussein, S.; Warrad, M.; et al. Antiviral Activities of Olive Oil Apigenin and Taxifolin against SARS-CoV-2 RNA-Dependent RNA Polymerase (RdRP): In Silico, Pharmacokinetic, ADMET, and in-Vitro Approaches. Cogent Food Agric. 2023, 9, 2236828. [Google Scholar] [CrossRef]
- Rudrapal, M.; Issahaku, A.R.; Agoni, C.; Bendale, A.R.; Nagar, A.; Soliman, M.E.S.; Lokwani, D. In Silico Screening of Phytopolyphenolics for the Identification of Bioactive Compounds as Novel Protease Inhibitors Effective against SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 10437–10453. [Google Scholar] [CrossRef]
- Hamdy, R.; Mostafa, A.; Abo Shama, N.M.; Soliman, S.S.M.; Fayed, B. Comparative Evaluation of Flavonoids Reveals the Superiority and Promising Inhibition Activity of Silibinin against SARS-CoV-2. Phytother. Res. 2022, 36, 2921–2939. [Google Scholar] [CrossRef]
- Pillai, J.U.; Cherian, L.; Taunk, K.; Iype, E.; Dutta, M. Identification of Antiviral Phytochemicals from Cranberry as Potential Inhibitors of SARS-CoV-2 Main Protease (Mpro). Int. J. Biol. Macromol. 2024, 261, 129655. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, N.; Faruk, O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health Effects, Sources, Utilization and Safety of Tannins: A Critical Review. Toxin Rev. 2019, 40, 432–444. [Google Scholar] [CrossRef]
- Alharbi, Y.T.M.; Abdel-Mageed, W.M.; Basudan, O.A.; Mothana, R.A.; Tabish Rehman, M.; ElGamal, A.A.; Alqahtani, A.S.; Fantoukh, O.I.; AlAjmi, M.F. Investigation of Phytochemicals Isolated from Selected Saudi Medicinal Plants as Natural Inhibitors of SARS CoV-2 Main Protease: In Vitro, Molecular Docking and Simulation Analysis. Saudi Pharm. J. 2024, 32, 102023. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Albohy, A.; Abdulrazik, B.S.; Bayoumi, S.A.L.; Malak, L.G.; Khallaf, I.S.A.; Bringmann, G.; Farag, S.F. Natural Coumarins as Potential Anti-SARS-CoV-2 Agents Supported by Docking Analysis. RSC Adv. 2021, 11, 16970–16979. [Google Scholar] [CrossRef]
- Barot, K.P.; Jain, S.V.; Kremer, L.; Singh, S.; Ghate, M.D. Recent Advances and Therapeutic Journey of Coumarins: Current Status and Perspectives. Med. Chem. Res. 2015, 24, 2771–2798. [Google Scholar] [CrossRef]
- Chidambaram, S.K.; Ali, D.; Alarifi, S.; Radhakrishnan, S.; Akbar, I. In Silico Molecular Docking: Evaluation of Coumarin Based Derivatives against SARS-CoV-2. J. Infect. Public Health 2020, 13, 1671–1677. [Google Scholar] [CrossRef]
- Salgado-Moran, G.; Cardona, V.W.; Gerli-Candia, L.; Mendoza-Huizar, L.H.; Abdizadeh, T. Identification of Novel Coumarin Based Compounds as Potential Inhibitors of the 3-Chymotrypsin-like Main Protease of SARS-CoV-2 Using DFT, Molecular Docking and Molecular Dynamics Simulation Studies. J. Chil. Chem. Soc. 2022, 67, 5521–5536. [Google Scholar] [CrossRef]
- Mir, S.A.; Meher, R.K.; Nayak, B. Molecular Modeling and Simulations of Some Antiviral Drugs, Benzylisoquinoline Alkaloid, and Coumarin Molecules to Investigate the Effects on Mpro Main Viral Protease Inhibition. Biochem. Biophys. Rep. 2023, 34, 101459. [Google Scholar] [CrossRef] [PubMed]
- Amen, Y.; Elsbaey, M.; Othman, A.; Sallam, M.; Shimizu, K. Naturally Occurring Chromone Glycosides: Sources, Bioactivities, and Spectroscopic Features. Molecules 2021, 26, 7646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, W.; Chen, Q.; Huang, W.; Yang, Z.; Li, X.; Wang, X. Inhibition Viral RNP and Anti-Inflammatory Activity of Coumarins against Influenza Virus. Biomed. Pharmacother. 2017, 87, 583–588. [Google Scholar] [CrossRef]
- Su, X.; Zhou, D.; Li, N. Bioactive Stilbenes from Plants. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 73, pp. 265–403. ISBN 978-0-323-91097-2. [Google Scholar]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source Plants, Chemistry, Biosynthesis, Pharmacology, Application and Problems Related to Their Clinical Application-A Comprehensive Review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.-C.; Wu, Y.-C.; Chen, Z.-W.; Yang, W.-C. Naturally Occurring Anthraquinones: Chemistry and Therapeutic Potential in Autoimmune Diabetes. Evid. Based Complement. Alternat Med. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Carvalho, N.K.G.; Wellisson Da Silva Mendes, J.; Martins Da Costa, J.G. Quinones: Biosynthesis, Characterization of 13C Spectroscopical Data and Pharmacological Activities. Chem. Biodivers. 2023, 20, e202301365. [Google Scholar] [CrossRef]
- Ntemafack, A.; Singh, R.V.; Ali, S.; Kuiate, J.-R.; Hassan, Q.P. Antiviral Potential of Anthraquinones from Polygonaceae, Rubiaceae and Asphodelaceae: Potent Candidates in the Treatment of SARS-COVID-19, A Comprehensive Review. S. Afr. J. Bot. 2022, 151, 146–155. [Google Scholar] [CrossRef]
- Abdullah; Hussain, Y. Anthraquinones and SARS-CoV-2. In Application of Natural Products in SARS-CoV-2; Elsevier: Amsterdam, The Netherlands, 2023; pp. 171–184. ISBN 978-0-323-95047-3. [Google Scholar]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Wang, D.-Y.; Li, Y.-P.; Deyrup, S.T.; Zhang, H.-J. Plant-Derived Lignans as Potential Antiviral Agents: A Systematic Review. Phytochem. Rev. 2022, 21, 239–289. [Google Scholar] [CrossRef]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and Their Derivatives from Plants as Antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef]
- Sureja, D.K.; Shah, A.P.; Gajjar, N.D.; Jadeja, S.B.; Bodiwala, K.B.; Dhameliya, T.M. In Silico Computational Investigations of AntiViral Lignan Derivatives as Potent Inhibitors of SARS-CoV-2. ChemistrySelect 2022, 7, e202202069. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Horváti, K.; Kraszni, M.; Ausbüttel, T.; Pályi, B.; Kis, Z.; Mucsi, Z.; Kovács, G.M.; Bősze, S.; Boldizsár, I. Arylnaphthalene Lignans with Anti-SARS-CoV-2 and Antiproliferative Activities from the Underground Organs of Linum austriacum and Linum perenne. J. Nat. Prod. 2023, 86, 672–682. [Google Scholar] [CrossRef]
- Li, B.; Qiao, L.; Xiao, Q.; Zhang, J.; Liu, J.; Zhang, B.; Liu, H. Effects of Diarylbutane Lignans from Schisandra Chinensis Fruit on SARS-CoV-2 3CLpro and PLpro and Their in Vitro Anti-Inflammatory Properties. Phytomedicine Plus 2023, 3, 100432. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qiao, L.; Zhang, J.; Xiao, Q.; Liu, J.; Zhang, B.; Liu, H. Natural 7,8-Secolignans from Schisandra sphenanthera Fruit Potently Inhibit SARS-CoV-2 3CLpro and Inflammation. J. Tradit. Complement. Med. 2024, 14, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Omar, F.; Tareq, A.M.; Alqahtani, A.M.; Dhama, K.; Sayeed, M.A.; Emran, T.B.; Simal-Gandara, J. Plant-Based Indole Alkaloids: A Comprehensive Overview from a Pharmacological Perspective. Molecules 2021, 26, 2297. [Google Scholar] [CrossRef]
- Sayed, A.M.; Ibrahim, A.H.; Tajuddeen, N.; Seibel, J.; Bodem, J.; Geiger, N.; Striffler, K.; Bringmann, G.; Abdelmohsen, U.R. Korupensamine A, but Not Its Atropisomer, Korupensamine B, Inhibits SARS-CoV-2 in Vitro by Targeting Its Main Protease (Mpro). Eur. J. Med. Chem. 2023, 251, 115226. [Google Scholar] [CrossRef]
- Ikram, N.K.B.K.; Zhan, X.; Pan, X.-W.; King, B.C.; Simonsen, H.T. Stable Heterologous Expression of Biologically Active Terpenoids in Green Plant Cells. Front. Plant Sci. 2015, 6, 129. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Z.; Gan, Y.; Xie, J.; Xia, Z.; Liu, T.; Chen, X.; Li, X.; Zhou, H.; Sun, P.; et al. From Ancient Remedy to Modern Medicine: Artemisia Argyi Sesquiterpenoids as a Promising Natural Treatment for COVID-19. Arab. J. Chem. 2023, 16, 105298. [Google Scholar] [CrossRef]
- Fan, Y.-W.; Ao, Z.-Y.; Zhang, W.-J.; Chen, J.-Y.; Lian, X.; Chen Pan, Y.; Chen, L.-P.; Wu, J.-W.; Yuan, J. The Sesquiterpenes with the COVID-19 Mpro Inhibitory Activity from the Carpesium abrotanoides L. Nat. Prod. Res. 2023, 38, 1909–1917. [Google Scholar] [CrossRef]
- Waqas, M.; Ullah, S.; Halim, S.A.; Rehman, N.U.; Ali, A.; Jan, A.; Muhsinah, A.B.; Khan, A.; Al-Harrasi, A. Targeting Papain-like Protease by Natural Products as Novel Therapeutic Potential SARS-CoV-2. Int. J. Biol. Macromol. 2024, 258, 128812. [Google Scholar] [CrossRef]
- Djouonzo, P.T.; Mukim, M.S.I.; Kemda, P.N.; Kowa, T.K.; Tchinda, A.T.; Agbor Agbor, G.; Pan, C.-H.; Song, D.-G. SARS-CoV-2 Main Protease Inhibitors from the Stem Barks of Discoglypremna caloneura (Pax) Prain (Euphorbiaceae) and Pterocarpus erinaceus Poir (Fabaceae) and Their Molecular Docking Investigation. Appl. Biol. Chem. 2023, 66, 76. [Google Scholar] [CrossRef]
- Zhao, L.; Qin, X.; Lin, T.; Xie, F.; Yao, L.; Li, Y.; Xiong, B.; Xu, Z.; Ye, Y.; Chen, H.; et al. Multi-Target Mechanisms against Coronaviruses of Constituents from Chinese Dagang Tea Revealed by Experimental and Docking Studies. J. Ethnopharmacol. 2022, 297, 115528. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, J.; Wan, X.; Li, B.; Guan, M.; Ning, X.; Hu, X.; Li, S.; Liu, S.; Song, G. Discovery and Structural Optimization of 3-O-β-Chacotriosyl Betulonic Acid Saponins as Potent Fusion Inhibitors of Omicron Virus Infections. Bioorg. Chem. 2023, 131, 106316. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Zhang, Y.; Zhang, Q.; Liu, Q.; Hu, Y.; Chen, X.; Wang, J.; Shi, Y.; Deng, C.; et al. Oridonin Inhibits SARS-CoV-2 Replication by Targeting Viral Proteinase and Polymerase. Virologica Sinica 2023, 38, 470–479. [Google Scholar] [CrossRef]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential Oil–Cyclodextrin Complexes: An Updated Review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
- Oleszek, M.; Oleszek, W. Saponins in Food. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2021; pp. 1501–1540. ISBN 978-981-15-4147-6. [Google Scholar]
- Dinda, B.; Debnath, S.; Mohanta, B.C.; Harigaya, Y. Naturally Occurring Triterpenoid Saponins. Chem. Biodivers. 2010, 7, 2327–2580. [Google Scholar] [CrossRef]
- Van de Sand, L.; Bormann, M.; Alt, M.; Schipper, L.; Heilingloh, C.S.; Steinmann, E.; Todt, D.; Dittmer, U.; Elsner, C.; Witzke, O.; et al. Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease. Viruses 2021, 13, 609. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Deng, W.; Ding, L.; Ye, X.; Yin, S.; Huang, W. Glycyrrhetinic Acid and Its Derivatives as Potential Alternative Medicine to Relieve Symptoms in Nonhospitalized COVID-19 Patients. J. Med. Virol. 2020, 92, 2200–2204. [Google Scholar] [CrossRef]
- Yi, Y.; Li, J.; Lai, X.; Zhang, M.; Kuang, Y.; Bao, Y.-O.; Yu, R.; Hong, W.; Muturi, E.; Xue, H.; et al. Natural Triterpenoids from Licorice Potently Inhibit SARS-CoV-2 Infection. J. Adv. Res. 2022, 36, 201–210. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Yekeen, N.; Malik, A.A.; Idris, A.K.; Reepei, N.I.; Ganie, K. Foaming Properties, Wettability Alteration and Interfacial Tension Reduction by Saponin Extracted from Soapnut (Sapindus mukorossi) at Room and Reservoir Conditions. J. Pet. Sci. Eng. 2020, 195, 107591. [Google Scholar] [CrossRef]
- Nicoliche, T.; Bartolomeo, C.S.; Lemes, R.M.R.; Pereira, G.C.; Nunes, T.A.; Oliveira, R.B.; Nicastro, A.L.M.; Soares, É.N.; Da Cunha Lima, B.F.; Rodrigues, B.M.; et al. Antiviral, Anti-Inflammatory and Antioxidant Effects of Curcumin and Curcuminoids in SH-SY5Y Cells Infected by SARS-CoV-2. Sci. Rep. 2024, 14, 10696. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, M.; Muller, F.L.; Van Remmen, H. An Objective Appraisal of the Free Radical Theory of Aging. In Handbook of the Biology of Aging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 177–202. ISBN 978-0-12-378638-8. [Google Scholar]
- Kao, Y.-W.; Hsu, S.-K.; Chen, J.Y.-F.; Lin, I.-L.; Chen, K.-J.; Lee, P.-Y.; Ng, H.-S.; Chiu, C.-C.; Cheng, K.-C. Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. Int. J. Mol. Sci. 2020, 22, 212. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. In Silico Studies to Identify Potential Natural Antiviral Agents to Treat and Control SARS-CoV-2 (COVID-19). J. Glob. Biosci. 2021, 10, 8720–8743. [Google Scholar]
- Dembitsky, V.M. Biological Activity and Structural Diversity of Steroids Containing Aromatic Rings, Phosphate Groups, or Halogen Atoms. Molecules 2023, 28, 5549. [Google Scholar] [CrossRef]
- Castilla, V.; Ramirez, J.; Coto, C.E. Plant and Animal Steroids a New Hope to Search for Antiviral Agents. CMC 2010, 17, 1858–1873. [Google Scholar] [CrossRef]
- Yerlikaya, P.O.; Arısan, E.; Mehdizadehtapeh, L.; Uysal Onganer, P.; Çoker Gürkan, A. The Use of Plant Steroids in Viral Disease Treatments: Current Status and Future Perspectives. Eur. J. Biol. 2023, 82, 86–94. [Google Scholar] [CrossRef]
- Carino, A.; Moraca, F.; Fiorillo, B.; Marchianò, S.; Sepe, V.; Biagioli, M.; Finamore, C.; Bozza, S.; Francisci, D.; Distrutti, E.; et al. Hijacking SARS-CoV-2/ACE2 Receptor Interaction by Natural and Semi-Synthetic Steroidal Agents Acting on Functional Pockets on the Receptor Binding Domain. Front. Chem. 2020, 8, 572885. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kolesarova, A. Plant Molecules and Their Influence on Health and Female Reproduction. In Environmental Contaminants and Medicinal Plants Action on Female Reproduction; Elsevier: Amsterdam, The Netherlands, 2022; pp. 245–399. ISBN 978-0-12-824292-6. [Google Scholar]
- Giacalone, M.; Forfori, F.; Giunta, F. Chili Pepper Compounds in the Management of Neuropathic Pain. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–195. ISBN 978-0-12-411462-3. [Google Scholar]
- Guldiken, B.; Catalkaya, G.; Ozkan, G.; Ceylan, F.D.; Capanoglu, E. Toxicological Effects of Commonly Used Herbs and Spices. In Toxicology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 201–213. ISBN 978-0-12-819092-0. [Google Scholar]
- Nag, A.; Paul, S.; Banerjee, R.; Kundu, R. In Silico Study of Some Selective Phytochemicals against a Hypothetical SARS-CoV-2 Spike RBD Using Molecular Docking Tools. Comput. Methods Programs Biomed. 2021, 137, 104818. [Google Scholar] [CrossRef]
- Rahmattullah, N.; Arumingtyas, E.; Widyananda, M.; Ahyar, A.; Tabroni, I. Bioinformatics Analysis of Bioactive Compounds of Four Capsicum Species against SARS-CoV-2 Infection. Int. J. Adv. Biol. Biomed. Res. 2021, 9, 298–319. [Google Scholar] [CrossRef]
- Pandey, A.K.; Verma, S. An in Silico Evaluation of Dietary Components for Structural Inhibition of SARS-Cov-2 Main Protease. J. Biomol. Struct. Dyn. 2022, 40, 136–142. [Google Scholar] [CrossRef]
- Badea, G.I.; Radu, G.L. Introductory Chapter: Carboxylic Acids-Key Role in Life Sciences. In Carboxylic Acid-Key Role in Life Sciences; Badea, G.I., Radu, G.L., Eds.; InTech: London, UK, 2018; ISBN 978-1-78923-278-3. [Google Scholar]
- Pedreira, A.; Taşkın, Y.; García, M.R. A Critical Review of Disinfection Processes to Control SARS-CoV-2 Transmission in the Food Industry. Foods 2021, 10, 283. [Google Scholar] [CrossRef]
- Shahab, S.; Kaviani, S.; Sheikhi, M.; Almodarresiyeh, H.A.; Al Saud, S. DFT Calculations and In Silico Study of Chlorogenic, Ellagic and Quisqualic Acids as Potential Inhibitors of SARS-CoV-2 Main Protease Mpro. Biointerface Res. Appl. Chem. 2021, 12, 61–73. [Google Scholar] [CrossRef]
- Opgrande, J.L.; Dobratz, C.J.; Brown, E.; Liang, J.; Conn, G.S.; Shelton, F.J.; With, J. Benzaldehyde. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley Online Library: Hoboken, NJ, USA, 2000; ISBN 978-0-471-48494-3. [Google Scholar]
- Khairan, K.; Idroes, R.; Tumilaar, S.G.; Tallei, T.E.; Idroes, G.M.; Rahmadhany, F.; Futri, M.U.; Dinura, N.M.; Mauliza, S.; Diana, M.; et al. Molecular Docking Study of Fatty Acids from Pliek U Oil in the Inhibition of SARS-CoV-2 Protein and Enzymes. IOP Conf. Ser: Mater. Sci. Eng. 2021, 1087, 012058. [Google Scholar] [CrossRef]
- Kang, N.; Heo, S.-Y.; Cha, S.-H.; Ahn, G.; Heo, S.-J. In Silico Virtual Screening of Marine Aldehyde Derivatives from Seaweeds against SARS-CoV-2. Mar. Drugs 2022, 20, 399. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-B.; Long, C.; Kennelly, E.J. Structural Diversity and Bioactivities of Natural Benzophenones. Nat. Prod. Rep. 2014, 31, 1158–1174. [Google Scholar] [CrossRef]
- Martiz, R.M.; Patil, S.M.; Ramu, R.; Jayanthi, M.K.; Ranganatha, L.V.; Khanum, S.A.; Silina, E.; Stupin, V.; Achar, R.R. Discovery of Novel Benzophenone Integrated Derivatives as Anti-Alzheimer’s Agents Targeting Presenilin-1 and Presenilin-2 Inhibition: A Computational Approach. PLoS ONE 2022, 17, e0265022. [Google Scholar] [CrossRef] [PubMed]
- Mustieles, V.; Balogh, R.K.; Axelstad, M.; Montazeri, P.; Márquez, S.; Vrijheid, M.; Draskau, M.K.; Taxvig, C.; Peinado, F.M.; Berman, T.; et al. Benzophenone-3: Comprehensive Review of the Toxicological and Human Evidence with Meta-Analysis of Human Biomonitoring Studies. Environ. Int. 2023, 173, 107739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q.; Liu, M.; Ruan, J.; Yu, H.; Li, J.; Wang, T. Effects of Benzophenones from Mango Leaves on Lipid Metabolism. Chem. Pharm. Bull. 2019, 67, 634–639. [Google Scholar] [CrossRef]
- Coelho, C.; Gallo, G.; Campos, C.B.; Hardy, L.; Würtele, M. Biochemical Screening for SARS-CoV-2 Main Protease Inhibitors. PLoS ONE 2020, 15, e0240079. [Google Scholar] [CrossRef]
- Malik, H.N.; Jabeen, A.; Ashraf, S.; Haq, Z.U.; Salar, U.; Arshia; Khan, K.M. Benzophenone and Coumarin Derivatives as 3-CLPro Inhibitors: Targeting Cytokine Storm through in Silico and in Vitro Approaches. J. Biomol. Struct. Dyn. 2022, 1265, 133478. [Google Scholar] [CrossRef]
- Costa, M.A.M.; De Sousa, N.F.; Mansur Pontes, C.L.; Scotti, M.T.; De Assis, F.F.; Braga, A.L.; Sandjo, L.P. Inhibitory Effects against SARSCoV-2 Main Protease (Mpro) of Biflavonoids and Benzophenones from the Fruit of Platonia insignis. Fitoterapia 2024, 173, 105784. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Yang, Y.; Li, Y.; He, C. Naturally Occurring Coumestans from Plants, Their Biological Activities and Therapeutic Effects on Human Diseases. Pharmacol. Res. 2021, 169, 105615. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.M.; Hop, N.Q.; Son, N.T. Wedelolactone: A Molecule of Interests. Fitoterapia 2023, 164, 105355. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Fu, L.; Xu, H. Natural Product-Based Screening for Lead Compounds Targeting SARS CoV-2 Mpro. Pharmaceuticals 2023, 16, 767. [Google Scholar] [CrossRef]
- Kapoor, N.; Ghorai, S.M.; Khuswaha, P.K.; Bandichhor, R.; Brogi, S. Butein as a Potential Binder of Human ACE2 Receptor for Interfering with SARS-CoV-2 Entry: A Computer-Aided Analysis. J. Mol. Model. 2022, 28, 270. [Google Scholar] [CrossRef]
- Mazziotti, I.; Petrarolo, G.; La Motta, C. Aurones: A Golden Resource for Active Compounds. Molecules 2022, 27, 2. [Google Scholar] [CrossRef]
- Caleffi, G.S.; Rosa, A.S.; De Souza, L.G.; Avelar, J.L.S.; Nascimento, S.M.R.; De Almeida, V.M.; Tucci, A.R.; Ferreira, V.N.; Da Silva, A.J.M.; Santos-Filho, O.A.; et al. Aurones: A Promising Scaffold to Inhibit SARS-CoV-2 Replication. J. Nat. Prod. 2023, 86, 1536–1549. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The Application of in Silico Drug-Likeness Predictions in Pharmaceutical Research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
- Ferreira, L.L.G.; Andricopulo, A.D. ADMET Modeling Approaches in Drug Discovery. Drug Discov. Today 2019, 24, 1157–1165. [Google Scholar] [CrossRef]
- Kar, S.; Leszczynski, J. Open Access in Silico Tools to Predict the ADMET Profiling of Drug Candidates. Expert. Opin. Drug Deliv. 2020, 15, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Żołek, T.; Maciejewska, D. Theoretical Evaluation of ADMET Properties for Coumarin Derivatives as Compounds with Therapeutic Potential. Eur. J. Pharm. Med. Res. 2017, 109, 486–502. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Saldívar-González, F.I. Cheminformatics to Characterize Pharmacologically Active Natural Products. Biomolecules 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Gupta, P.; Sharma, S.; Sharma, A.; Agarwal, S.M. ADMET Profiling of Geographically Diverse Phytochemical Using Chemoinformatic Tools. Future Med. Chem. 2019, 12, 69–87. [Google Scholar] [CrossRef]
- Falade, V.A.; Adelusi, T.I.; Adedotun, I.O.; Abdul-Hammed, M.; Lawal, T.A.; Agboluaje, S.A. In Silico Investigation of Saponins and Tannins as Potential Inhibitors of SARS-CoV-2 Main Protease (Mpro). In Silico Pharmacol. 2021, 9, 9. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Wimmerová, M.; Wimmer, Z. Saponins of Selected Triterpenoids as Potential Therapeutic Agents: A Review. Pharmaceuticals 2023, 16, 386. [Google Scholar] [CrossRef]
- Rajasekar, N.; Sivanantham, A.; Ravikumar, V.; Rajasekaran, S. An Overview on the Role of Plant-Derived Tannins for the Treatment of Lung Cancer. Phytochemistry 2021, 188, 112799. [Google Scholar] [CrossRef]
- Duran, N.; Polat, M.F.; Aktas, D.A.; Alagoz, M.A.; Ay, E.; Cimen, F.; Tek, E.; Anil, B.; Burmaoglu, S.; Algul, O. New Chalcone Derivatives as Effective against SARS-CoV-2 Agent. Int. J. Clin. Pract. 2021, 75, e14846. [Google Scholar] [CrossRef]
- Prabakaran, G.; Manivarman, S.; Bharanidharan, M. Catalytic Synthesis, ADMET, QSAR and Molecular Modeling Studies of Novel Chalcone Derivatives as Highly Potent Antioxidant Agents. Mater. Today Proc. 2022, 48, 400–408. [Google Scholar] [CrossRef]
- Norisham, M.; Husin, F. Elucidation of Stilbene-Derivatives as Potential Inhibitors of SARS-CoV-2 Mpro Binding Pocket: A Molecular Docking, and ADMET Prediction Studies. IJF 2024, 1, 56–68. [Google Scholar] [CrossRef]
- Mortuza, M.G.; Roni, M.A.H.; Kumer, A.; Biswas, S.; Saleh, M.A.; Islam, S.; Sadaf, S.; Akther, F. A Computational Study on Selected Alkaloids as SARS-CoV-2 Inhibitors: PASS Prediction, Molecular Docking, ADMET Analysis, DFT, and Molecular Dynamics Simulations. Biochem. Res. Int. 2023, 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wanat, K. Biological Barriers, and the Influence of Protein Binding on the Passage of Drugs across Them. Mol. Biol. Rep. 2020, 47, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mei, L.; Quach, T.; Porter, C.; Trevaskis, N. Lipophilic Conjugates of Drugs: A Tool to Improve Drug Pharmacokinetic and Therapeutic Profiles. Pharm. Res. 2021, 38, 1497–1518. [Google Scholar] [CrossRef]
- Pangal, A.; Ahmed, K. Synthesis and Biological Evaluation of Coumarin-Quinone Hybrids as Multifunctional Bioactive Agents. ADMET DMPK 2022, 11, 81–96. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E. Lipophilicity and ADMET Analysis of Quinoline-1,4-Quinone Hybrids. Pharmaceutics 2022, 15, 34. [Google Scholar] [CrossRef]
- Zuo, H.-L.; Huang, H.-Y.; Lin, Y.-C.-D.; Cai, X.-X.; Kong, X.-J.; Luo, D.-L.; Zhou, Y.-H.; Huang, H.-D. Enzyme Activity of Natural Products on Cytochrome P450. Molecules 2022, 27, 515. [Google Scholar] [CrossRef]
- Maia, M.D.S.; Raimundo E Silva, J.P.; De Lima Nunes, T.A.; Saraiva De Sousa, J.M.; Soares Rodrigues, G.C.; Messias Monteiro, A.F.; Fechine Tavares, J.; Da Franca Rodrigues, K.A.B.; Mendonça-Junior, F.J.; Scotti, L.; et al. Virtual Screening and the In Vitro Assessment of the Antileishmanial Activity of Lignans. Molecules 2020, 25, 2281. [Google Scholar] [CrossRef]
- Ye, L.; Fan, S.; Zhao, P.; Wu, C.; Liu, M.; Hu, S.; Wang, P.; Wang, H.; Bi, H. Potential Herb–Drug Interactions between Anti-COVID-19 Drugs and Traditional Chinese Medicine. Acta Pharm. Sin. B 2023, 13, 3598–3637. [Google Scholar] [CrossRef]
- Klomp, F.; Wenzel, C.; Drozdzik, M.; Oswald, S. Drug–Drug Interactions Involving Intestinal and Hepatic CYP1A Enzymes. Pharmaceutics 2020, 12, 1201. [Google Scholar] [CrossRef]
- Deodhar, M.; Al Rihani, S.B.; Arwood, M.J.; Darakjian, L.; Dow, P.; Turgeon, J.; Michaud, V. Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice. Pharmaceutics 2020, 12, 846. [Google Scholar] [CrossRef]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Relevance of Half-Life in Drug Design: Miniperspective. J. Med. Chem. 2018, 61, 4273–4282. [Google Scholar] [CrossRef] [PubMed]
- Dobrek, L. A Synopsis of Current Theories on Drug-Induced Nephrotoxicity. Life 2023, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Mercês, É.A.B.; Portela, F.S.; Malheiro, L.F.L.; Silva, H.B.L.; De Benedictis, L.M.; De Benedictis, J.M.; Silva, C.C.; d’Ávilla, E.; Santos, A.C.L.; et al. An Integrated View of Cisplatin-Induced Nephrotoxicity, Hepatotoxicity, and Cardiotoxicity: Characteristics, Common Molecular Mechanisms, and Current Clinical Management. Clin. Exp. Nephrol. 2024, 28, 711–727. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, C.; Hop, C.E.C.A.; Wright, M.R.; Hu, M.; Lai, Y.; Khojasteh, S.C.; Humphreys, W.G. Intestinal Excretion, Intestinal Recirculation, and Renal Tubule Reabsorption Are Underappreciated Mechanisms That Drive the Distribution and Pharmacokinetic Behavior of Small Molecule Drugs. J. Med. Chem. 2021, 64, 7045–7059. [Google Scholar] [CrossRef] [PubMed]
- Adibah, K.Z.M.; Azzreena, M.A. Plant Toxins: Alkaloids and Their Toxicities. GSC Biol. Pharm. Sci. 2018, 6, 21–29. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, R.; Bharti, R. Pharmacological Activities and Toxicities of Alkaloids on Human Health. Mater. Today Proc. 2022, 48, 1407–1415. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Zhang, B.-Y.; Qiu, L.-P.; Guan, R.-F.; Ye, Z.-H.; Yu, X.-P. Structure Properties and Mechanisms of Action of Naturally Originated Phenolic Acids and Their Derivatives against Human Viral Infections. CMC 2017, 24, 4279–4302. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. A Review of the Health Protective Effects of Phenolic Acids against a Range of Severe Pathologic Conditions (Including Coronavirus-Based Infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef]
- Gonçalves, C.P.; Marcucci, M.C.; Rocha, O.V.; Utuari, C.L.C.; Fortunato, C.R.M.; Souza, G.F.D.; Farias, A. Computational Studies of Caffeoylquinic Acids from Brazilian Green Propolis and Its Anti-Viral Potential against SARS-CoV-2 Mpro. RSD 2022, 11, e359111536917. [Google Scholar] [CrossRef]
- Gao, K.; Wang, R.; Chen, J.; Tepe, J.J.; Huang, F.; Wei, G.-W. Perspectives on SARS-CoV-2 Main Protease Inhibitors. J. Med. Chem. 2021, 64, 16922–16955. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanouil, C.; Chatziathanasiadou, M.V.; Chatzigiannis, C.; Chontzopoulou, E.; Mavromoustakos, T.; Grdadolnik, S.G.; Tzakos, A.G. Unveiling the Interaction Profile of Rosmarinic Acid and Its Bioactive Substructures with Serum Albumin. J. Enzym. Inhib. Med. Chem. 2020, 35, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhai, G.; Li, Y.; Wang, M.; Chen, X.; Wang, R.; Xie, H.; Zhang, W.; Ge, G.; Zhang, Q.; et al. Ginkgolic Acids Inhibit SARS-CoV-2 and Its Variants by Blocking the Spike Protein/ACE2 Interplay. Int. J. Biol. Macromol. 2023, 226, 780–792. [Google Scholar] [CrossRef]
- Geiger, N.; König, E.-M.; Oberwinkler, H.; Roll, V.; Diesendorf, V.; Fähr, S.; Obernolte, H.; Sewald, K.; Wronski, S.; Steinke, M.; et al. Acetylsalicylic Acid and Salicylic Acid Inhibit SARS-CoV-2 Replication in Precision-Cut Lung Slices. Vaccines 2022, 10, 1619. [Google Scholar] [CrossRef]
- Macario, A.; López, J.C.; Blanco, S. Molecular Structure of Salicylic Acid and Its Hydrates: A Rotational Spectroscopy Study. Int. J. Mol. Sci. 2024, 25, 4074. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Xu, X.; Yang, C.; Niu, X.; Yin, S.; Pan, X.; Xu, W.; Hu, G.; Wang, C.; et al. Danshensu Alleviates Pseudo-Typed SARS-CoV-2 Induced Mouse Acute Lung Inflammation. Acta Pharmacol. Sin. 2022, 43, 771–780. [Google Scholar] [CrossRef]
- Reis, B.; Martins, M.; Barreto, B.; Milhazes, N.; Garrido, E.M.; Silva, P.; Garrido, J.; Borges, F. Structure−Property−Activity Relationship of Phenolic Acids and Derivatives. Protocatechuic Acid Alkyl Esters. J. Agric. Food Chem. 2010, 58, 6986–6993. [Google Scholar] [CrossRef] [PubMed]
- Merkl, R.; Hrádková, I.; Filip, V.; Šmidrkal, J. Antimicrobial and Antioxidant Properties of Phenolic Acids Alkyl Esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological Effects of Protocatechuic Acid and Its Therapeutic Potential in Neurodegenerative Diseases: Review on the Basis of in Vitro and in Vivo Studies in Rodents and Humans. Nutr. Neurosci. 2017, 22, 72–82. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound Ingested in Daily Foods and Herbs Frequently and Heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, X.; Wu, J.; Li, H.; Yang, L.; Zhang, Y.; Wang, X.; Li, Z. Protocatechuic Acid Protects Mice from Influenza A Virus Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Chen, Y.; Zhuang, A.-Q.; Chen, S.-M. Natural Phenolic Acids and Their Derivatives against Human Viral Infections. In Infectious Diseases; Aditya Parikesit, A., Ed.; IntechOpen: London, UK, 2023; Volume 27, ISBN 978-1-80356-783-9. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).