Abstract
Background: TMPRSS2 plays an important role in the viral entry mechanisms of influenza viruses and coronaviruses. Therefore, TMPRSS2 seems to be a suitable antiviral drug target. To exclude possible side effects of TMPRSS2 truncation in an early stage of drug in-vitro testing, this study aims to analyze the impact of TMPRSS2 truncation via antisense peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO) targeting immune cells, using the example of regulatory T cells (Treg). Methods: TMPRSS2 was truncated in human Tregs using a splice-modulating PPMO. Effects on Treg function were analyzed by evaluation of surface marker and transcription factor expression, cytokine secretion, and effector cell suppression capability. Results: PPMO treatment led to a slight concentration-dependent toxicity in Tregs. Tregs with truncated TMPRSS2 behave similarly to untreated and control PPMO-treated cells in the analyzed assays. Conclusions: Treg function is not altered after TMPRSS2 truncation and therefore, no unwanted side effects in regard of Tregs are expected when using TMPRSS2-truncating PPMO as an anti-viral drug.
1. Introduction
Viral infections causing acute respiratory illnesses, like influenza or COVID-19, are responsible for billions of disease cases every year. Although most people recover without treatment, severe cases can lead to hospitalization and death, e.g., 3–5 million severe illnesses and 290,000 to 650,000 deaths annually in case of seasonal influenza (https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) accessed on 30 March 2024). Vaccinations are the best protection against serious illnesses, but do not prevent people from getting ill at all. Furthermore, vaccines, especially for influenza, have to be modified annually because viruses constantly undergo genetic changes, leading to antigen variation [1]. This evolution of the virus is also a problem for antiviral drugs since it can lead to drug resistance. Current antiviral drugs mainly use the approach of targeting viral proteins that play a key role in the viral life cycle, like neuraminidase inhibitors, M2 channel ion blockers or inhibitors of the viral RNA polymerase for influenza [1,2]. Another approach for antiviral agent development is to target indispensable host (human) factors, which may lead to broad-spectrum inhibitors that interfere with host cellular proteins used by multiple viruses. These drugs should have a low risk of drug resistance, but often cause more side effects in patients [3].
One of the potential host factor targets for drugs against influenza viruses and coronaviruses studied in recent years is transmembrane protease serine 2 (TMPRSS2). TMPRSS2 proteolytically activates envelope proteins of viruses, initializing viral entry via membrane fusion [4,5]. TMPRSS2 seems to be a good choice as a drug target, since (1) it is directly involved in virus host cell entry and (2) like many human proteases, it shows functional redundancy—its deficiency does not show a discernible phenotype (at least in mice [6]). Furthermore, it has been shown that a lack of TMPRSS2 expression prevents pathogenesis and virus replication of corona and influenza A viruses in mice [7,8,9,10,11,12]. This aroused initial hopes of rapidly finding a drug during the COVID-19 pandemic to meet the urgent need for treatment of severely ill patients, because with nafamostat and camostat mesylate, TMPRSS2 inhibitors were already approved for other diseases in Japan. Although both drugs show inhibition of TMPRSS2 and of SARS-CoV-2 in a cell culture of human lung cells [13,14], only minor and not statistically significant improvements could be detected in clinical studies (reviewed in [15]). Putative interference on the effect of TMPRSS2 inhibitors in humans might be attributed to the fact that not only the target cells—in case of influenza virus and coronavirus infection, mainly lung cells—but also interacting cells, like immune cells, are affected by the inhibitor. If an inhibition of TMPRSS2 in immune cells lead to altered function, this might counteract the effect in the target cells and lessen the overall positive outcome in patients.
Antisense peptide-conjugated phosphorodiamidate morpholino oligomers (PPMOs) offer huge hope in the fight against antimicrobial resistance, since resistance mechanisms observed for ‘standard antimicrobics’ seem to have no effects, as yet [16,17]. Morpholino oligomers have an uncharged backbone which reduce their deliverability via the traditional lipid-based delivery systems. However, the cellular uptake of morpholinos can be increased by conjugation with cell-penetrating peptides [18]. Furthermore, it has been shown that PPMO can help traditional antibiotics to better penetrate bacterial biofilms [19]. Antimicrobial resistance should be even more insignificant due to not targeting the virus directly, but rather the interacting host counterpart.
In the present study, we analyzed a functional knockdown of TMPRSS2 by PPMO. We used a PPMO against exon 5 of TMPRSS2 pre-mRNA (T-ex5 PPMO, described in [20]). PPMOs act by sterically blocking complementary sequences of mRNA. The T-ex5 PPMO specifically interferes with TMPRSS2 mRNA maturation, resulting in the production of TMPRSS2-mRNA lacking exon 5, and consequently expression of a truncated TMPRSS2 protein that is enzymatically inactive. The potential of the T-ex5 PPMO in inhibition of viral spread has been demonstrated by the lab of Prof. Dr. Böttcher-Friebertshäuser [4,5,20,21]. However, using a host protease as target could lead to significant side effects. This has to be examined and excluded before a drug can be used in patients.
Since the complexity of the immune system is difficult to recreate in vitro, we first examined which immune cells express TMPRSS2 mRNA before studying the influence of the T-ex5 PPMO. One interesting cell type is the regulatory T cell, which plays an essential role in modulating immune responses during viral infections. Tregs are crucial for maintaining immunological homeostasis and preventing excessive inflammation [22,23]. The use of T-ex5 PPMO may influence Treg populations and functionality. Although the impact of TMPRSS2 knockout on immune cells and especially regulatory T cells has yet to be investigated, it is essential to investigate how the T-ex5 PPMO interacts with the immune system before using it as an antiviral drug.
2. Results
2.1. Human Immune Cells Express TMPRSS2
Since TMPRSS2 expression in immune cells is not well-studied and the few existing publications report inconsistent results [24,25,26,27], we first examined the expression levels using RT- and qRT-PCR in different human immune cells. In Figure 1A, TMPRSS2 expression in human peripheral blood mononuclear cells (PBMCs) in comparison with the human lung cell line Calu-3 is depicted, showing that TMPRSS2 is expressed in immune cells. Using qRT-PCR, TMPRSS2 expression levels of the different immune cells, isolated from PBMCs, were analyzed (Figure 1B). Calu-3 cells were used as a positive control with good TMPRSS2 expression levels, and levels in tested immune cells were set in relation. Tregs, monocyte-derived dendritic cells (MdDC) and natural killer cells (NK) achieve ~80% of the expression level compared with the Calu-3 control.
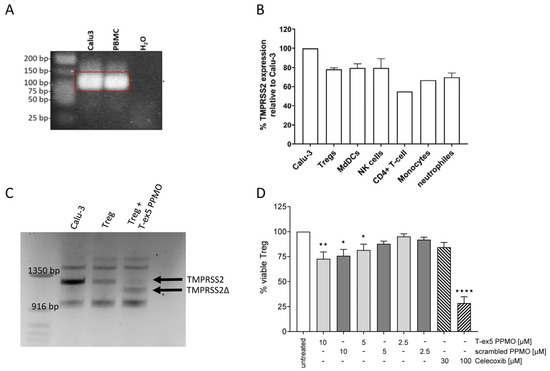
Figure 1.
TMPRSS2 expression and truncation and Treg viability. (A) Examples of agarose gels from a one-step RT-PCR, showing TMPRSS2 mRNA expression in PBMCs and Calu-3 as positive control. (B) TMPRSS2 mRNA expression in different immune cells relative to Calu-3 cells as positive control (n = 1–4). (C) Agarose gel with plotted one-step RT-PCR samples of Calu-3 (positive control), untreated Treg and T-ex5 PPMO treated Treg cells, showing a TMPRSS2 PCR fragment at around 1220 bp for Calu-3 and untreated Treg, and a shorter fragment (TMPRSS2Δ; approx. 120 bp smaller) for the T-ex5 PPMO treated Treg cells. (D) Viability assay using Orangu displaying viable Treg cells after treatment with different concentrations of T-ex5 PPMO, scrambled PPMO or Celecoxib (reference for cytotoxicity) in relation to untreated cells (n = 3–6). Statistic for D: ordinary one-way ANOVA with Dunnett’s multiple comparison test; * p ≤ 0.05; ** p ≤ 0.005; **** p < 0.0001.
2.2. T-ex5 PPMO Truncates TMPRSS2 mRNA in Treg Cells
The next step was to inactivate TMPRSS2. For that purpose, T-ex-5 PPMO was used which truncates the mRNA of TMPRSS2 and leads thereby to an inactive TMRPSS2. The TMPRSS2 mRNA was truncated in the immune cells with the highest TMPRSS2 levels. Successful truncation was analyzed by one-step RT-PCR using primers spanning a huge part of the TMPRSS2 mRNA, including exon 5 as the PPMO target site. In Treg cells treated with T-ex5 PPMO, a ~120 bp shorter PCR fragment was detected (Figure 1C), which corresponds to the size of exon 5 excluded from the TMPRSS2 mRNA. In monocyte-derived dendritic cells (MdDCs) and natural killer (NK) cells, no PCR fragment for TMPRSS2 could be generated using the one-Step RT-PCR, therefore only the Treg results are discussed here. Other immune cells will be investigated further.
2.3. PPMOs Have a Slight Cytotoxic Effect on Treg Cells
A first step in the analysis of potential drug candidates is to examine cell toxicity. Therefore, a viability assay using different PPMO concentrations was performed (Figure 1D). Celecoxib was used as a control for the toxic effect. A reduction of about 25% of viable cells was detected in test cultures treated with 10 µM PPMO, irrespective of whether the T-ex5 or the scrambled version was used. With decreasing PPMO concentration, the viability increased to around 100% at 2.5 µM PPMO. Since the assay only allowed for a low cell density, the ratio of PPMO to cells in the 10 µM treatment was very high, while in the other assays the ratio of PPMO to cells was more comparable to the 2.5 µM condition, although 10 µM in total volume was used. Therefore, a toxic effect on the cells in the other experimental settings should be negligible.
2.4. TMPRSS2 Truncation Has No Effect on Cytokine Release or Surface Marker Expression
As important Treg cytokines, the release of IL-10, IL-13 and CCL3 (MiP1α) were analyzed in the cell culture supernatant using a cytometric bead assay (Figure 2A–C). Treg cells were treated with T-ex5 PPMO, scrambled PPMO or vehicle for 24 h and subsequently stimulated with Phytohemagglutinin-L (PHA) or vehicle for 24 h. No significant differences in cytokine release due to the PPMO treatment after 24 h PPMO stimulation, further 24 h incubation (vehicle) or Treg cell activation (PHA), was observed. However, IL-13 concentration was downregulated in four of five donors in TMPRSS2-truncated Tregs in the 24 h, 24 h plus 24 h activation and the 24 h plus 24 h cultivation condition. The significant variability among donors hinders the achievement of significance. More IL-10, as well as IL-13, was released under Treg activation using PHA in all three conditions, while CCL3 release did not change at all. Furthermore, the expression of the surface markers CD69 and CD274 was analyzed by flow cytometry (Figure 2D,E). CD69 on Treg is an important sign of potent inhibitory activity and IL-10 production [28]. Therefore, similar to the IL-10 release, an increased cell surface expression of CD69 after PHA activation was observed, but no difference by PPMO treatment in the activated or non-activated state was detected. CD274 or PD-L1 expression on T cells is associated with a conversion towards iTregs and to a highly suppressive form [29]. TMPRSS2 truncation has no effect on PD-L1 surface expression. Even PHA stimulation only showed minor elevation. These results indicate that the truncation of TMPRSS2 did not influence the effector function of Treg cells.
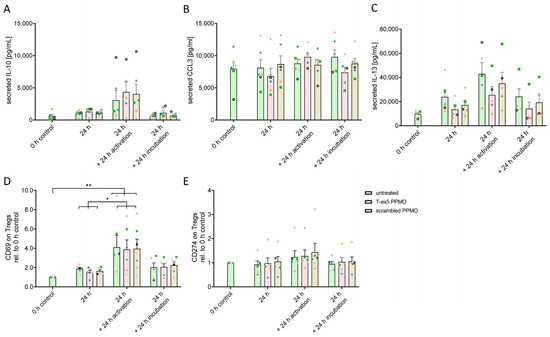
Figure 2.
Effects of TMPRSS2 truncation on cytokine release and surface marker expression. (A–C) Comparison of secreted IL-10, CCL3(MiP1α), and IL-13 levels using CBA in the supernatant of untreated, T-ex5 or scrambled PPMO-treated Treg cells after 24 h, 48 h (24 h + 24 h incubation), and 24 h + 24 h activation with PHA. (D,E) Expression levels of surface markers CD69 and PD-L1 (CD274) comparing TMPRSS2 truncation by T-ex5 PPMO with scrambled PPMO treatment and untreated Treg cells in activated Treg cells (PHA treatment) and non-activated Tregs after 24 h and 48 h. Analyzed by flow cytometry. Graphs display means with SEM in bars and the individual experiments (donors, V1–V6) indicated by different symbols (V1 = green square; V2 = orange triangle; V3 = blue rhombus; V4 = pink star; V5 = black wheel; V6 = grey circle). Statistics: Two-Way-ANOVA with Tukey (A) activation/time p < 0.0001, F(3,59) = 11.44; (B) no significance; (C) activation/time p < 0.0001, F(3,48) = 9.625; (D) activation/time p < 0.0001, F(3,60) = 14.57; (E) no significance; * p ≤ 0.05; ** p ≤ 0.005.
2.5. No Changes in the mRNA Expression Levels of Receptors, Transcription Factor or Released Cytokines by TMPRSS2 Truncation
Next, the mRNA expression levels of the receptors CCR7, CCR8, CCR10, CXCR3, CTLA-4 (CD152), and CD39, as well as the transcription factor FoxP3, the secreted serine protease granzyme B, and the anti-inflammatory cytokine IL-35 (EBI3) were analyzed by qRT-PCR (Figure 3 and Supplemental Figure S1). Truncation of TMPRSS2 by T-ex5 PPMO did not influence the expression levels of any of the analyzed mRNAs. Activation by PHA seems to induce the mRNA expression of CCR10, CTLA-4, and granzyme B. These data suggest that the deletion of TMPRSS2 does not affect the recruiting and activation of Treg cells.
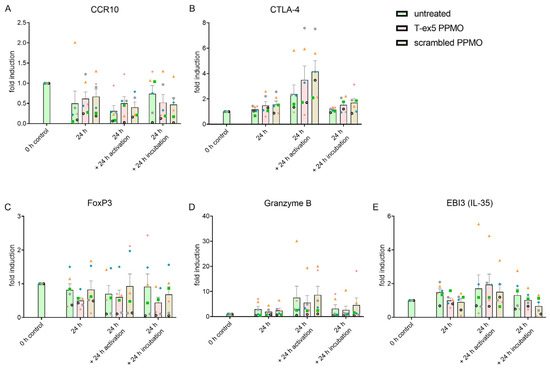
Figure 3.
Analysis of mRNA expression levels of receptors (A,B), transcription factor (C) or cytokines (E) after TMPRSS2 truncation. Graphs showing fold induction relative to the 0 h control of mRNA levels of receptors CCR10 (A) and CTLA-4 (B), Treg specific transcription factor FoxP3 (C), secreted serine protease granzyme B (D), and the cytokine IL-35 (E) in Treg cultures. The graphs compare Tregs after treatment with T-ex5 PPMO, and scrambled PPMO, as well as untreated Tregs after 24 h, 48 h (24 h + 24 h incubation) and under PHA activation (24 h + 24 h activation). Graphs display means with SEM in bars and the individual experiments (donors, V1–V6) indicated by different symbols (V1 = green square; V2 = orange triangle; V3 = blue rhombus; V4 = pink star; V5 = black wheel; V6 = grey circle). Significances were analyzed using Two-Way-ANOVA with Tukey CCR10: p = 0.0005, F(3,59) = 6.833; CTLA-4: activation/time p < 0.0001, F(3,60) = 13.94; FoxP3: no significance; Granzyme B: activation/time p = 0.0058, F(3,60) = 4.595; EBI3 (IL-35): activation/time p = 0.0478, F(3,60) = 2.796.
2.6. TMPRSS2 Truncation Does Not Alter Treg Suppressive Function on Responder T Cells
To analyze whether TMPRSS2 truncation influences Treg function, namely the suppression of effector cell proliferation, a co-culture of Treg and responder T cells was incubated for five days and the proliferation of the Tresp determined. As seen in Figure 4, in the control (only Tresp), 75% of the Tresp cells proliferated. As expected, a reduction of proliferating Tresp cells could be observed when Treg cells are co-cultured. In that case, a 25% reduction of proliferation was seen with Tresp to Treg ratios of 50:1 and 20:1. This reduction even increases with higher Treg ratios to a 50% reduction in a 1:1 proportion of Tregs and Tresp cells. However, no difference corresponding to TMPRSS2 truncation by T-ex5 PPMO treatment was observed. These data indicate that the truncation of TMPRSS2 did not affect the suppressive function of Tregs.
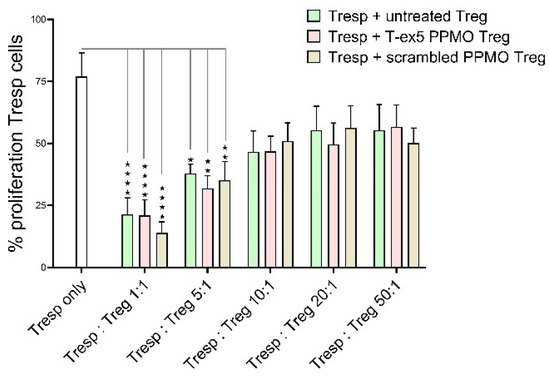
Figure 4.
Treg suppression assay. Graph displaying Treg suppressor function on Tresp proliferation rate with Tregs untreated or treated with T-ex5 PPMO (TMPRSS2 truncation) or scrambled PPMO (PPMO control) using different Tresp/Treg ratios. Statistics: Bars showing mean values of n = 4 experiments with SEM. Significances were analyzed using Two-Way-ANOVA with Tukey; overall significant difference in Treg:Tresp ratio p < 0.0001, F(5,54) = 18.76; * p ≤ 0.05; ** p ≤ 0.005; **** p < 0.0001.
3. Discussion
Soon after the beginning of the search for a treatment for severely ill COVID-19 patients, hopes were laid on TMPRSS2 inhibitors. Unfortunately, the drugs nafamostat and camostat mesylate have been shown not to improve disease outcome in clinical studies [15,30]. Since virus infection is inhibited by these two drugs in in vitro tests using lung cell lines and lung organoids, the question arises of why it does not work in the human in vivo setting. One explanation from the authors of the meta-analysis of the clinical trials was the inconsistency of the trials and co-medications used [15]. Another possibility is the influence of the TMPRSS2/serine protease inhibition on other cells in the body, which might mitigate the anti-viral effect on the lung cells. This hypothesis might be supported by the finding that inhaled aprotinin, a broad-spectrum protease inhibitor, resulted in a shorter treatment time and hospitalization compared with the placebo group in a multicenter, double-blind, randomized trial [31]. Since aprotinin was inhaled whereas nafamostat and camostat mesylate were given intravenously or orally, aprotinin was placed directly in contact with the lung epithelial cells and was not distributed throughout the body where it could interact with all other cell types. Important cells that might influence a drug’s outcome are immune cells, since they directly interconnect with the virus-target cells and are the cells responsible for infection clearance. Therefore, in this study, we examined the influence of TMPRSS2 inhibition by splice-modulating PPMO on immune cells.
The task of regulatory T cells is the maintenance of immunological self-tolerance and homeostasis. Tregs regulate the immune system by suppressing effector T cells and inhibiting proinflammatory cytokine release, which leads to reduced inflammation. An imbalance in immune regulation towards inflammation can lead to hyperinflammation and cytokine storm, which is often more harmful for the body than the underlying cause, e.g., viral infection, itself. Cytokine storm has been proposed as the main cause of death in SARS-CoV-2 and influenza virus infection [32,33]. The elevated level of multiple cytokines causes damage to the alveolar functions and can lead to multiple organ disfunction [34]. The specific role of Tregs in SARS-CoV-2 infection is currently unknown, but it has been shown that COVID-19 patients have significant fewer Tregs than the healthy population [32,35,36,37]. Moreover, the Middle East respiratory syndrome (MERS) coronavirus has been shown to efficiently infect T cells and induced substantial apoptosis in the infected cells [38]. Hence, Tregs seem to play an important role in coronavirus and influenza virus infections and therefore, special attention should be paid to Tregs for treatment of these respiratory diseases. Because of this, and due to our results showing that Tregs have one of the highest TMPRSS2 expression rates among immune cells, we analyzed the effects of TMPRSS2 truncation by T-ex5 PPMO in Tregs in detail.
Important cytokines secreted by Tregs are the immunosuppressive IL-10, the motility-inducing CCL-3 (MiP1α), and IL-13, which is known for its regenerative and reparative function. We could demonstrate that cytokine secretion is not significantly affected by TMPRSS2 truncation, although IL-13 tends to be slightly reduced when using PPMO and especially the T-ex5 PPMO. A downregulation of IL-13 could be a positive side effect of the TMPRSS2 truncation by T-ex5 PPMO, since elevated IL-13 levels have been associated with worse clinical outcomes in COVID-19 patients [39,40]. Furthermore, there seems to be a link between IL-13 and TMPRSS2, since IL-13 significantly increases TMPRSS2 expression in airway epithelial cells [41]. If—vice versa—TMPRSS2 expression also influences IL-13 secretion, this could be an interesting point for further studies.
Our further analysis of surface markers, receptors, cytokines, and the Treg-specific transcription factor FoxP3 showed no effects in response to TMPRSS2 truncation. Furthermore, in a functional assay testing the suppressive function of Tregs, no difference between Tregs with truncated TMPRSS2 and controls could be detected. Therefore, TMPRSS2 seems to play no role for Treg function. Moreover, TMPRSS2 truncation for the treatment of viral infection would not affect Treg main functions.
In our assays, we included Tregs in an activated state by PHA stimulation. However, we could detect an effect of the PHA activation only in some analytes. This might be due to the fact that the Tregs are already activated through IL-2 addition in the expansion and maintenance media. Alternatively, the PHA concentration used might have been too low for some analytes, and only the most activation-sensitive proteins/pathways reacted. In our case, these seem to be the activation marker CD69, the cytokines IL-10 and IL-13, and the inhibitory receptor CTLA-4.
In a viability assay, we observed a slight toxicity of PPMO at concentrations of 5 µM and 10 µM, both with the T-ex5 and the scrambled PPMO. Although we also used 10 µM in the other experiments, we did not detect loss of cells there (see Supplemental Figure S2). This discrepancy might be due to the number of cells used in the different assays. In the viability assay, only 1 × 106 Treg cells per ml were used, while for mRNA isolation and surface marker staining, 5 × 106 cells per ml were incubated with 10 µM PPMO. Consequently, the ratio of PPMO molecules per cell was five times higher in the viability assay. Furthermore, PPMOs have been reported to be toxic in a dose–dependent manner above a specific threshold [42]. Therefore, a stringent determination of toxic dose versus PPMO efficacy has to be specified for its use as an antiviral drug.
The study design does not allow us to distinguish if PBMC-isolated Tregs are natural, thymus-derived nTregs, or from conventional, naive CD4+ T cell-induced iTregs, or both. Furthermore, if a mixed culture is analyzed, we cannot ascertain whether both types express TMPRSS2 or not. In the latter case, when PPMO-mediated truncation only take place in part of the cells, effects might be masked by the untargeted cells and therefore no significant functional changes would be detected.
Moreover, the T-ex5 PPMO used resulted in a truncated version of TMPRSS2, rather than a complete loss of the full-length enzymatically active form. Previously, we demonstrated that a truncated, enzymatically inactive enzyme is expressed in T-ex5 PPMO-treated cells [20]. However, the levels of both full-length and truncated TMPRSS2 protein in Treg cells are too low for detection by Western blot analysis. Therefore, our conclusion that the truncation of TMPRSS2 in Treg cells does not affect their functions relies on the assumption that the truncated TMPRSS2 protein is expressed in T-ex5 PPMO-treated Treg cells, as indicated for the truncated mRNA shown in Figure 1C. In the T-ex5 PPMO-treated Tregs, a small full-length TMPRSS2 mRNA band was still detected, similar to what was observed in primary human airway epithelial cells [4,43]. Therefore, we cannot completely exclude the possibility that low levels of enzymatically active TMPRSS2 are still expressed in T-ex5-treated Tregs. However, in human airway cells, the low amount of enzymatically active TMPRSS2 is not sufficient to ensure efficient activation of viral envelope proteins and thus efficient virus replication [4,43]. We speculate that the low remaining amount of enzymatically active TMPRSS2 in both human airway cells and Tregs is also insufficient to fulfill the physiological function of the protease. It should be noted that the physiological role of TMPRSS2 is still unknown, and it is also not clear whether TMPRSS2 also fulfills functions that are independent of its catalytic activity. Thus, the truncated TMPRSS2 expressed in T-ex5 PPMO-treated Tregs may still perform a physiological function; inhibition of TMPRSS2 activity by T-ex5 PPMO, however, does not affect regulatory T cell function.
4. Materials and Methods
4.1. Cells and Reagents
Human regulatory T cells and responder T cells were cultured in X-VIVO 15 media (Lonza) supplemented with IL-2 (500 IU/mL and 236 IU/mL, respectively). Fresh Buffy coats from healthy donors were obtained from DRK-Blutspendedienst (Frankfurt/Main, Germany) on the day of cell isolation. Orangu assay was purchased from Cell guidance systems (Cambridge, UK). Human FcR Blocking Reagent, human CD4+CD25+ regulatory T Cell isolation kit, human Treg Expansion Kit, bovine serum albumin (BSA), and most antibodies for surface staining were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany) if not stated otherwise. Recombinant human IL-2, Carboxyfluorescein succinimidyl ester (CFSE) Cell Division Tracker Kit, and Zombie Violet Dye were purchased from BioLegend (Amsterdam, The Netherlands). Phytohemagglutinin-L (PHA; 500x = 1.25 mg/mL) was purchased from Invitrogen (Thermo Fisher scientific, Dreieich, Germany). P7-Tex5 and NC-705 scrambled PPMO were manufactured by AVI BioPharma Inc. (Bothell, WA, USA). Celecoxib was synthesized by WITEGA Laboratorien Berlin-Adlershof GmbH (Berlin, Germany). CellTrace VioletTM from Life Technologies was purchased from Thermo Fisher scientific (Life Technologies, Darmstadt, Germany), as well as the First Strand cDNA Synthesis kit. SCRIPTUM Standard One-step Reverse-Transcription-PCR kit was purchased from Bio&Sell (Feucht, Germany).
4.2. Isolation and Expansion of Human Treg Cells
PBMCs were isolated from six fresh buffy coats of healthy donors by density gradient. Within Sep-MateTM-50 Tubes (Stemcell Technologies, Cologne, Germany), 25 mL of blood was mixed 1:2 with Hanks’s balanced salt solution (HBSS, Thermo Fisher Scientific, Oberhausen, Germany), layered over with 15 mL of Biocoll (Bio&Sell, Feucht, Germany) and centrifuged for 10 min at 1200× g at RT. PBMCs found in the interface were carefully removed and washed four times with 2 mM EDTA/PBS. Using a MACSQuant® Analyzer 10 flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany), the cell count was determined. Afterwards, CD4+CD25+ Tregs cells were enriched using antibody-labeled magnetic beads from Miltenyi Biotec (Bergisch Gladbach, Germany) via magnetic cell separation (MACS) with LS columns. In detail, PBMCs were incubated for 5 min with CD4+ Tcell biotin antibody cocktail, before anti-biotin microbeads were added for another 10 min. Then, using an LD column, bead-labeled cells were isolated within the magnetic field of the MACS separator. After elution, CD4+ cells were incubated with CD25 microbeads and separated via an MS column. Unlabeled cells within the flow-through were collected as responder T cells (Tresp) cells for the suppression assay. Labeled cells (Tregs) were flushed out of the column without the magnetic field. Cells were counted and 1 × 105 cells were seeded in X-Vivo 15 media, supplemented with 500 U/mL rIL-2, in a 96-well round bottom plate and incubated with anti-Biotin MACSiBead Particles loaded with the biotinylated antibodies against human CD3 and CD28 for 14 days. Cells were fed several times with fresh media and split into bigger well formats. CD4+CD25−Tresp cells were cultivated at a density of 1 × 106 cells in X-Vivo 15 media + 10 ng/mL rIL-2 in a 24-well format for 14 days.
If not otherwise indicated, expanded Tregs were seeded as 2.5 × 106 cells in 500 µL in a 48-well plate.
4.3. Downregulation of TMPRSS2 and Activation of Treg Cells
TMPRSS2 downregulation was achieved by incubation of Tregs with T-ex5 PPMO. Phosphorodiamidate morpholino oligomers (PMO) were synthesized at AVI BioPharma Inc. (Corvallis, Oregon) as previously described [44]. The cell-penetrating peptide (CPP) (RXRRBR)2XB (R = arginine, X = 6 aminohexanoic acid, B = beta alanine), was covalently conjugated to the 5′ end of each PMO through a non-cleavable linker, to produce peptide-PMO (PPMO), by methods previously described [45]. The T-ex5 PPMO binds to TMPRSS2 pre-mRNA at the beginning of exon 5, which leads to an excision of exon 5 and a truncated, inactive version of the TMPRSS2 protein. In detail, after Treg cell expansion, cells were seeded in the required formats for the subsequent experiments and treated with 10 µM T-ex5 PPMO, 10 µM scrambled PPMO or left untreated for 24 h if not stated otherwise. Successful truncation of TMPRSS2 was controlled by one-step RT-PCR. For that, the Treg cells were harvested and total RNA was isolated using the RNeasy Mini Kit from Qiagen (Hilden, Germany). The RT-PCR was conducted after the manufacture’s manual of the one-step RT-PCR kit, using a forward primer (CTACGAGGTGCATCC) and a reverse primer (CCAGAGGCCCTCCAGCG TCACCCTGGCAA) to receive a 1228 bp cDNA fragment of TMPRSS2 and a 120 bp shorter fragment for the truncated mRNA. PCR products were analyzed using a 3% agarose gel.
For some experiments, 24 h PPMO-treated or untreated samples were further treated. Samples were either activated with PHA (0.5x = 1.25 µg/mL) or further incubated for another 24 h.
4.4. Cell Viability Assay
To test for PPMO toxicity, an Orangu assay was performed. Therefore, 0.1 × 106 Treg cells were seeded into a 96-well plate. After 24 h treatment with 2.5, 5 and 10 µM T-ex5 PPMO, scrambled PPMO or 30 µM and 100 µM Celecoxib (as reference for cytotoxicity), 10 µL Orangu solution was added to 100 µL cell suspension and incubated for 5 h at 3 °C, 5% CO2. Afterwards, absorbance at 450 nm was measured on a Spark plate reader (Tecan, Maennedorf, Switzerland) with 650 nm as a reference.
4.5. Flow Cytometry
Flow cytometry was used to analyze the purity of isolated cells and to assess the expression of surface markers. To test for Treg purity, PBMCs, cells after MACS isolation and after the 14 days of expansion were stained with Zombie Violet for viability (10 min, RT, dark), followed by 20 min incubation at 4 °C with CD4-VioGreen and CD25-APC-Vio770 antibodies. Cells were washed with DPBS + 0.5% BSA and then analyzed using the MACSQuant Analyzer 10. For surface marker staining, cells were blocked with FcR blocking reagent (15 min at 4 °C) after Zombie Violet staining and before incubation with the antibody mix including CD4 VioGreen, CD25 APC-Vio770 (both Miltenyi Biotec, Bergisch Gladbach, Germany), CD274-APC (BD Pharmingen, Heidelberg, Germany), CD152-PE, and CD69-PE-Vio770. FlowJo software V10 (Treestar, Ashland, TN, USA) was used to analyze the data. Treg cells were gated as CD4+/CD25+ cells and the geometric mean of the fluorescent intensity was calculated for the mentioned surface markers.
4.6. Cytometric Bead Array
To determine the concentrations of secreted cytokines in Treg cell culture supernatant, a cytometric bead array (CBA; BD Bioscience, Heidelberg, Germany) was performed according to the manufacturer’s information. We analyzed IL-10, MiP-1α, and IL-13 secretion. The samples were measured using a MACSQuant Analyzer 10. The resulting FC data were analyzed using BD FCAP Array Software (Version 3.0).
4.7. qRT-PCR
To analyze expression of cytokines, cytokine receptors and Treg transcription factor, qPCR was performed on mRNA samples. First, the mRNA was transcribed into cDNA using the random hexamer primer, according to the First Strand cDNA Synthesis kit manual. 10 ng cDNA were applied in the qPCR using the applied biosystems SYBR Select Master Mix. qPCR was run in a 396-well format on the QuantStudio 12K Flex (Applied Biosystems) with the following settings: UDG activation 50 °C, 2 min; polymerase activation 95 °C, 5 min; 45 cycles of 95 °C denaturation (15 s), 60 °C annealing (20 s) and 72 °C extension (30 s). Sequences of the used primers can be found in Supplementary Table S1. Results were analyzed using the 2−ΔΔCt method, with 18S and RPL37A as reference genes, and normalized to the untreated condition.
4.8. Suppression Assay
As a functional assay, the capacity of Treg cells to suppress effector cells was determined. To achieve this, PPMO-treated Tregs were co-cultivated with Tresp cells and cell proliferation was analyzed via CFSE dilution. The test setup was adapted from Miltenyi Biotec. and Collison et al. [46,47]. Briefly, the Tresp cells were stained with CFSE according to the manufacturer’s instructions. A quantity of 0.75 × 106 cells were seeded into a 96-well round bottom plate and incubated for 24 h with anti-Biotin MACSiBead particles loaded with the biotinylated antibodies against human CD3 and CD28. In parallel, Treg cells were incubated with 10 µM T-ex5 or scrambled PPMO or kept untreated in X-VIVO 15 + 500 IU/mL rIL-2. To separate Treg cells from Tresp cells during flow cytometry, Treg cells were stained with CellTrace Violet (CTV) after PPMO treatment. For co-culture, Treg cells were added to Tresp cells in the ratios 1:1, 1:5, 1:10, 1:20, and 1:50 and incubated for five days at 37 °C, 5% CO2. Afterwards, cells were measured using the MACSQuant® Analyzer 10 and data were analyzed using FlowJo software.
4.9. Statistics
For graphical presentation and statistical analysis, GraphPad Prism 9 (GraphPad Software Inc., Boston, MA, USA) was used. Untreated, T-ex5 PPMO and scrambled PPMO samples were tested for normality using Shapiro-Wilk test and QQ-plots, before ordinary one-way ANOVA with Dunnett’s multiple comparison test or two-way ANOVA with Tukey’s multiple comparisons was used to analyze the datasets.
5. Conclusions
In this study, the impact of the downregulation of TMPRSS2 enzymatic activity by PPMO treatment on immune cells was assessed, using the example of regulatory T-cells. No significant effect could be detected. Therefore, in the case of T-ex5 PPMO usage as an anti-viral drug, no unwanted side effects due to dysfunctional Tregs are expected.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ddc4020025/s1, Table S1. Listing Primer information; Figure S1. Showing further analyzed expressions of surface markers and other proteins; Figure S2. Showing percentage of viable cells during flow cytometry analysis; Figure S3 showing gating strategy for regulatory T cells. Refs. [48,49,50,51,52] have been cited in the Supplementary Materials.
Author Contributions
Conceptualization, E.B.-F. and S.S.; Data curation, S.G.; Formal analysis, S.G. and S.S.; Funding acquisition, E.B.-F. and S.S.; Investigation, S.G., F.K.S., L.K. and M.S.; Methodology, S.G., E.B.-F. and S.S.; Project administration, E.B.-F. and S.S.; Resources, H.M.M. and E.B.-F.; Supervision, S.G., E.B.-F. and S.S.; Validation, S.G. and M.S.; Writing—original draft, S.G.; Writing—review and editing, M.S., H.M.M., E.B.-F. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Landesoffensive zur Entwicklung wissenschaft-lich-ökonomischer Exzellenz (LOEWE) Research Centre for Novel Drug Targets against Poverty-Related and Neglected Tropical Infectious Diseases (DRUID), the LOEWE Centre for Translational Biodiversity Genomics (TBG), the Fraunhofer Cluster of Excellence Immune mediated diseases (CIMD) and the Leistungszentrum innovative Therapeutics (TheraNova). This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 853988. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and JDRF INTERNATIONAL.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BSA | Bovine serum albumin |
| CBA | cytometric bead array |
| CCR | C-C chemokine receptor |
| CD | Cluster of differentiation |
| cDNA | complementary deoxyribonucleic acid |
| CFSE | Carboxyfluorescein succinimidyl ester |
| COVID-19 | Coronavirus disease 2019 |
| CTLA-4 | Cytotoxic T-lymphocyte associated protein 4 |
| CTV | CellTrace Violet |
| FCAP | Flow Cytometric Analysis Program |
| FcR | Fc receptor |
| IL | Interleukin |
| MACS | magnetic cell separation |
| MdDC | Monocyte derived dendritic cell |
| NK | Natural killer cell |
| PBMCs | Peripheral blood mononuclear cells |
| PPMO | Antisense peptide-conjugated phosphorodiamidate morpholino oligomers |
| PHA | Phytohemagglutinin-L |
| qRT-PCR | Quantitative reverse transcribed polymerase chain reaction |
| RT-PCR | reverse transcribed polymerase chain reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TMPRSS2 | Transmembrane protease serine 2 |
| Treg | Regulatory T cell |
| Tresp | Responder T cells |
| UDG | Uracil-DNA glycosylases |
| WHO | World Health Organization |
References
- Yin, H.; Jiang, N.; Shi, W.; Chi, X.; Liu, S.; Chen, J.-L.; Wang, S. Development and Effects of Influenza Antiviral Drugs. Molecules 2021, 26, 810. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-L. Current and novel antiviral strategies for influenza infection. Curr. Opin. Virol. 2016, 18, 126–134. [Google Scholar] [CrossRef]
- Li, G.; de Clercq, E. Overview of Antiviral Drug Discovery and Development: Viral Versus Host Targets. In Antiviral Discovery for Highly Pathogenic Emerging Viruses; Muñoz-Fontela, C., Delgado, R., Eds.; The Royal Society of Chemistry: London, UK, 2021; pp. 1–27. ISBN 978-1-78801-564-6. [Google Scholar]
- Limburg, H.; Harbig, A.; Bestle, D.; Stein, D.A.; Moulton, H.M.; Jaeger, J.; Janga, H.; Hardes, K.; Koepke, J.; Schulte, L.; et al. TMPRSS2 Is the Major Activating Protease of Influenza A Virus in Primary Human Airway Cells and Influenza B Virus in Human Type II Pneumocytes. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; Rohde, C.; et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 2020, 3, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Heinlein, C.; Hackman, R.C.; Nelson, P.S. Phenotypic analysis of mice lacking the Tmprss2-encoded protease. Mol. Cell. Biol. 2006, 26, 965–975. [Google Scholar] [CrossRef]
- Hatesuer, B.; Bertram, S.; Mehnert, N.; Bahgat, M.M.; Nelson, P.S.; Pöhlmann, S.; Schughart, K. Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice. PLoS Pathog. 2013, 9, e1003774. [Google Scholar] [CrossRef]
- Sakai, K.; Ami, Y.; Tahara, M.; Kubota, T.; Anraku, M.; Abe, M.; Nakajima, N.; Sekizuka, T.; Shirato, K.; Suzaki, Y.; et al. The host protease TMPRSS2 plays a major role in in vivo replication of emerging H7N9 and seasonal influenza viruses. J. Virol. 2014, 88, 5608–5616. [Google Scholar] [CrossRef]
- Tarnow, C.; Engels, G.; Arendt, A.; Schwalm, F.; Sediri, H.; Preuss, A.; Nelson, P.S.; Garten, W.; Klenk, H.-D.; Gabriel, G.; et al. TMPRSS2 is a host factor that is essential for pneumotropism and pathogenicity of H7N9 influenza A virus in mice. J. Virol. 2014, 88, 4744–4751. [Google Scholar] [CrossRef] [PubMed]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J. Virol. 2019, 93, 6. [Google Scholar] [CrossRef]
- Lambertz, R.L.O.; Gerhauser, I.; Nehlmeier, I.; Gärtner, S.; Winkler, M.; Leist, S.R.; Kollmus, H.; Pöhlmann, S.; Schughart, K. H2 influenza A virus is not pathogenic in Tmprss2 knock-out mice. Virol. J. 2020, 17, 56. [Google Scholar] [CrossRef]
- Li, F.; Han, M.; Dai, P.; Xu, W.; He, J.; Tao, X.; Wu, Y.; Tong, X.; Xia, X.; Guo, W.; et al. Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide. Nat. Commun. 2021, 12, 866. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Hofmann-Winkler, H.; Smith, J.C.; Krüger, N.; Sørensen, L.K.; Søgaard, O.S.; Hasselstrøm, J.B.; Winkler, M.; Hempel, T.; Raich, L.; et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine 2020, 65, 103255. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Schroeder, S.; Kleine-Weber, H.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob. Agents Chemother. 2020, 64, 6. [Google Scholar] [CrossRef]
- Hernández-Mitre, M.P.; Morpeth, S.C.; Venkatesh, B.; Hills, T.E.; Davis, J.; Mahar, R.K.; McPhee, G.; Jones, M.; Totterdell, J.; Tong, S.Y.C.; et al. TMPRSS2 inhibitors for the treatment of COVID-19 in adults: A systematic review and meta-analysis of randomized clinical trials of nafamostat and camostat mesylate. Clin. Microbiol. Infect. 2024, 30, 743–754. [Google Scholar] [CrossRef]
- Sully, E.K.; Geller, B.L. Antisense antimicrobial therapeutics. Curr. Opin. Microbiol. 2016, 33, 47–55. [Google Scholar] [CrossRef]
- Bardouni, M.M.; Hashemi, A.; Lotfi, M.J.; Hamidi, S.; Khezri, F.; Karimi, M. Oligonucleotides: A therapeutic approach for tackling antimicrobial resistance. In Emerging Nanomaterials and Nano-Based Drug Delivery Approaches to Combat Antimicrobial Resistance; Elsevier: Amsterdam, The Netherlands, 2022; pp. 733–754. ISBN 9780323907927. [Google Scholar]
- Moulton, H.M. In vivo delivery of morpholino oligos by cell-penetrating peptides. Curr. Pharm. Des. 2013, 19, 2963–2969. [Google Scholar] [CrossRef]
- Moustafa, D.A.; Wu, A.W.; Zamora, D.; Daly, S.M.; Sturge, C.R.; Pybus, C.; Geller, B.L.; Goldberg, J.B.; Greenberg, D.E. Peptide-Conjugated Phosphorodiamidate Morpholino Oligomers Retain Activity against Multidrug-Resistant Pseudomonas aeruginosa In Vitro and In Vivo. mBio 2021, 12, 10-1128. [Google Scholar] [CrossRef]
- Böttcher-Friebertshäuser, E.; Stein, D.A.; Klenk, H.-D.; Garten, W. Inhibition of influenza virus infection in human airway cell cultures by an antisense peptide-conjugated morpholino oligomer targeting the hemagglutinin-activating protease TMPRSS2. J. Virol. 2011, 85, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Schwerdtner, M.; Schmacke, L.C.; Nave, J.; Limburg, H.; Steinmetzer, T.; Stein, D.A.; Moulton, H.M.; Böttcher-Friebertshäuser, E. Unveiling the Role of TMPRSS2 in the Proteolytic Activation of Pandemic and Zoonotic Influenza Viruses and Coronaviruses in Human Airway Cells. Viruses 2024, 16, 1798. [Google Scholar] [CrossRef]
- Singer, M.; Elsayed, A.M.; Husseiny, M.I. Regulatory T-cells: The Face-off of the Immune Balance. Front. Biosci. 2024, 29, 377. [Google Scholar] [CrossRef]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Devaprasad, A.; Pandit, A. Enrichment of SARS-CoV-2 Entry Factors and Interacting Intracellular Genes in Tissue and Circulating Immune Cells. Viruses 2021, 13, 1757. [Google Scholar] [CrossRef]
- Gunne, S.; Schwerdtner, M.; Henke, M.; Schneider, A.-K.; Keutmann, L.; Böttcher-Friebertshäuser, E.; Schiffmann, S. TMPRSS2 Impacts Cytokine Expression in Murine Dendritic Cells. Biomedicines 2023, 11, 419. [Google Scholar] [CrossRef]
- Zankharia, U.; Yadav, A.; Yi, Y.; Hahn, B.H.; Collman, R.G. Highly restricted SARS-CoV-2 receptor expression and resistance to infection by primary human monocytes and monocyte-derived macrophages. J. Leukoc. Biol. 2022, 112, 569–576. [Google Scholar] [CrossRef]
- Bao, R.; Hernandez, K.; Huang, L.; Luke, J.J. ACE2 and TMPRSS2 expression by clinical, HLA, immune, and microbial correlates across 34 human cancers and matched normal tissues: Implications for SARS-CoV-2 COVID-19. J. Immunother. Cancer 2020, 8, e001020. [Google Scholar] [CrossRef]
- Yu, L.; Yang, F.; Zhang, F.; Guo, D.; Li, L.; Wang, X.; Liang, T.; Wang, J.; Cai, Z.; Jin, H. CD69 enhances immunosuppressive function of regulatory T-cells and attenuates colitis by prompting IL-10 production. Cell Death Dis. 2018, 9, 905. [Google Scholar] [CrossRef]
- Fanelli, G.; Romano, M.; Nova-Lamperti, E.; Werner Sunderland, M.; Nerviani, A.; Scottà, C.; Bombardieri, M.; Quezada, S.A.; Sacks, S.H.; Noelle, R.J.; et al. PD-L1 signaling on human memory CD4+ T cells induces a regulatory phenotype. PLoS Biol. 2021, 19, e3001199. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Mubariz, M.; Khlidj, Y.; Nasir, M.M.; Ramadan, S.; Saeed, F.; Muhammad, A.; Abuelazm, M. Safety and Efficacy of Camostat Mesylate for COVID-19: A systematic review and Meta-analysis of Randomized controlled trials. BMC Infect. Dis. 2024, 24, 709. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Calvo, F.J.; Padín, J.F.; Muñoz-Rodríguez, J.R.; Serrano-Oviedo, L.; López-Juárez, P.; Porras Leal, M.L.; González Gasca, F.J.; Rodríguez Martínez, M.; Pérez Serrano, R.; Sánchez Cadena, A.; et al. Aprotinin treatment against SARS-CoV-2: A randomized phase III study to evaluate the safety and efficacy of a pan-protease inhibitor for moderate COVID-19. Eur. J. Clin. Investig. 2022, 52, e13776. [Google Scholar] [CrossRef]
- Dhawan, M.; Rabaan, A.A.; Alwarthan, S.; Alhajri, M.; Halwani, M.A.; Alshengeti, A.; Najim, M.A.; Alwashmi, A.S.S.; Alshehri, A.A.; Alshamrani, S.A.; et al. Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities with a Special Focus on Long COVID. Vaccines 2023, 11, 699. [Google Scholar] [CrossRef]
- Gu, Y.; Zuo, X.; Zhang, S.; Ouyang, Z.; Jiang, S.; Wang, F.; Wang, G. The Mechanism behind Influenza Virus Cytokine Storm. Viruses 2021, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.-Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Alahyari, S.; Rajaeinejad, M.; Jalaeikhoo, H.; Amani, D. Regulatory T Cells in Immunopathogenesis and Severity of COVID-19: A Systematic Review. Arch. Iran. Med. 2022, 25, 127–132. [Google Scholar] [CrossRef]
- Rahimzadeh, M.; Naderi, N. Toward an understanding of regulatory T cells in COVID-19: A systematic review. J. Med. Virol. 2021, 93, 4167–4181. [Google Scholar] [CrossRef]
- Alahdal, M.; Elkord, E. Exhaustion and over-activation of immune cells in COVID-19: Challenges and therapeutic opportunities. Clin. Immunol. 2022, 245, 109177. [Google Scholar] [CrossRef]
- Chu, H.; Zhou, J.; Wong, B.H.-Y.; Li, C.; Chan, J.F.-W.; Cheng, Z.-S.; Yang, D.; Wang, D.; Lee, A.C.-Y.; Li, C.; et al. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J. Infect. Dis. 2016, 213, 904–914. [Google Scholar] [CrossRef]
- Donlan, A.N.; Sutherland, T.E.; Marie, C.; Preissner, S.; Bradley, B.T.; Carpenter, R.M.; Sturek, J.M.; Ma, J.Z.; Moreau, G.B.; Donowitz, J.R.; et al. IL-13 is a driver of COVID-19 severity. J. Clin. Investig. Insight 2021, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Sasson, J.; Moreau, G.B.; Petri, W.A. The role of interleukin 13 and the type 2 immune pathway in COVID-19: A review. Ann. Allergy Asthma Immunol. 2023, 130, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Francisco, D.; Conway, M.; Martinez, F.D.; Vercelli, D.; Polverino, F.; Billheimer, D.; Kraft, M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J. Allergy Clin. Immunol. 2020, 146, 80–88.e8. [Google Scholar] [CrossRef]
- Moulton, H.M.; Moulton, J.D. Morpholinos and their peptide conjugates: Therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim. Biophys. Acta 2010, 1798, 2296–2303. [Google Scholar] [CrossRef]
- Bestle, D.; Limburg, H.; Kruhl, D.; Harbig, A.; Stein, D.A.; Moulton, H.; Matrosovich, M.; Abdelwhab, E.M.; Stech, J.; Böttcher-Friebertshäuser, E. Hemagglutinins of Avian Influenza Viruses Are Proteolytically Activated by TMPRSS2 in Human and Murine Airway Cells. J. Virol. 2021, 95, e0090621. [Google Scholar] [CrossRef] [PubMed]
- Summerton, J.; Weller, D. Morpholino antisense oligomers: Design, preparation, and properties. Antisense Nucleic Acid Drug 1997, 7, 187–195. [Google Scholar] [CrossRef]
- Wu, R.P.; Youngblood, D.S.; Hassinger, J.N.; Lovejoy, C.E.; Nelson, M.H.; Iversen, P.L.; Moulton, H.M. Cell-penetrating peptides as transporters for morpholino oligomers: Effects of amino acid composition on intracellular delivery and cytotoxicity. Nucleic Acids Res. 2007, 35, 5182–5191. [Google Scholar] [CrossRef] [PubMed]
- Miltenyi Biotec. In Vitro Human Regulatory T Cell Suppression Assay: Human CD4 + CD25 + Regulatory T Cell Isolation, In Vitro Suppression Assay and Analysis. Available online: https://www.miltenyibiotec.com/_Resources/Persistent/24ae7abc48492a7e8c0dea44b0dc5205997c11dc/Suppression_Assay_protocol_final.pdf (accessed on 16 January 2025).
- Collison, L.W.; Vignali, D.A.A. In vitro Treg suppression assays. Methods Mol. Biol. 2011, 707, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Barsheshet, Y.; Wildbaum, G.; Levy, E.; Vitenshtein, A.; Akinseye, C.; Griggs, J.; Lira, S.A.; Karin, N. CCR8+FOXp3+ Treg cells as master drivers of immune regulation. Proc. Natl. Acad. Sci. USA 2017, 114, 6086–6091. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, J.-X.; Chen, Y.; Cai, L.-L.; Wang, X.-Z.; Guo, W.-H.; Zheng, J.-F. The chemokine receptor CCR10 promotes inflammation-driven hepatocarcinogenesis via PI3K/Akt pathway activation. Cell Death Dis. 2018, 9, 232. [Google Scholar] [CrossRef]
- Roser, L.A.; Luckhardt, S.; Ziegler, N.; Thomas, D.; Wagner, P.V.; Damm, G.; Scheffschick, A.; Hewitt, P.; Parnham, M.J.; Schiffmann, S. Immuno-inflammatory in vitro hepatotoxicity models to assess side effects of biologicals exemplified by aldesleukin. Front. Immunol. 2023, 14, 1275368. [Google Scholar] [CrossRef]
- Shi, Y.; Dai, M.; Wu, G.; Zhou, P.; Fang, Y.; Yan, X. Levels of Interleukin-35 and Its Relationship with Regulatory T-Cells in Chronic Hepatitis B Patients. Viral Immunol. 2015, 28, 93–100. [Google Scholar] [CrossRef]
- Maess, M.B.; Sendelbach, S.; Lorkowski, S. Selection of reliable reference genes during THP-1 monocyte differentiation into macrophages. BMC Mol. Biol. 2010, 11, 90. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).