Nanoscale Self-Assemblies from Amphiphilic Block Copolymers as Proficient Templates in Drug Delivery
Abstract
1. Introduction
1.1. Structural Design
1.2. Updated Synthesis Route
2. Types of BCPs
2.1. Hydrophilic BCPs
2.2. Hydrophobic BCPs
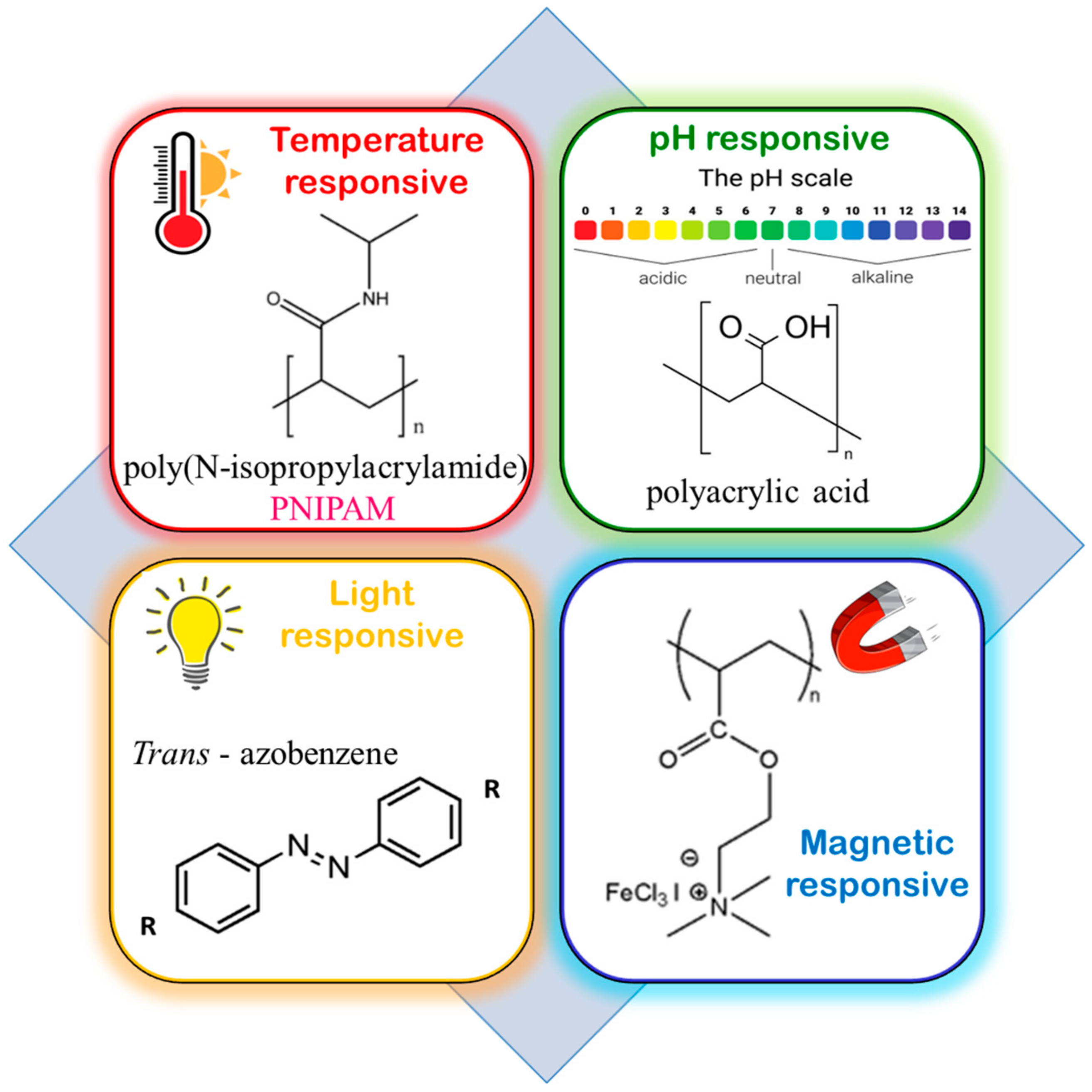
2.3. Stimuli-Responsive Block Copolymers (SRPs)
- (i)
- Temperature responsiveness
- (ii)
- pH responsiveness
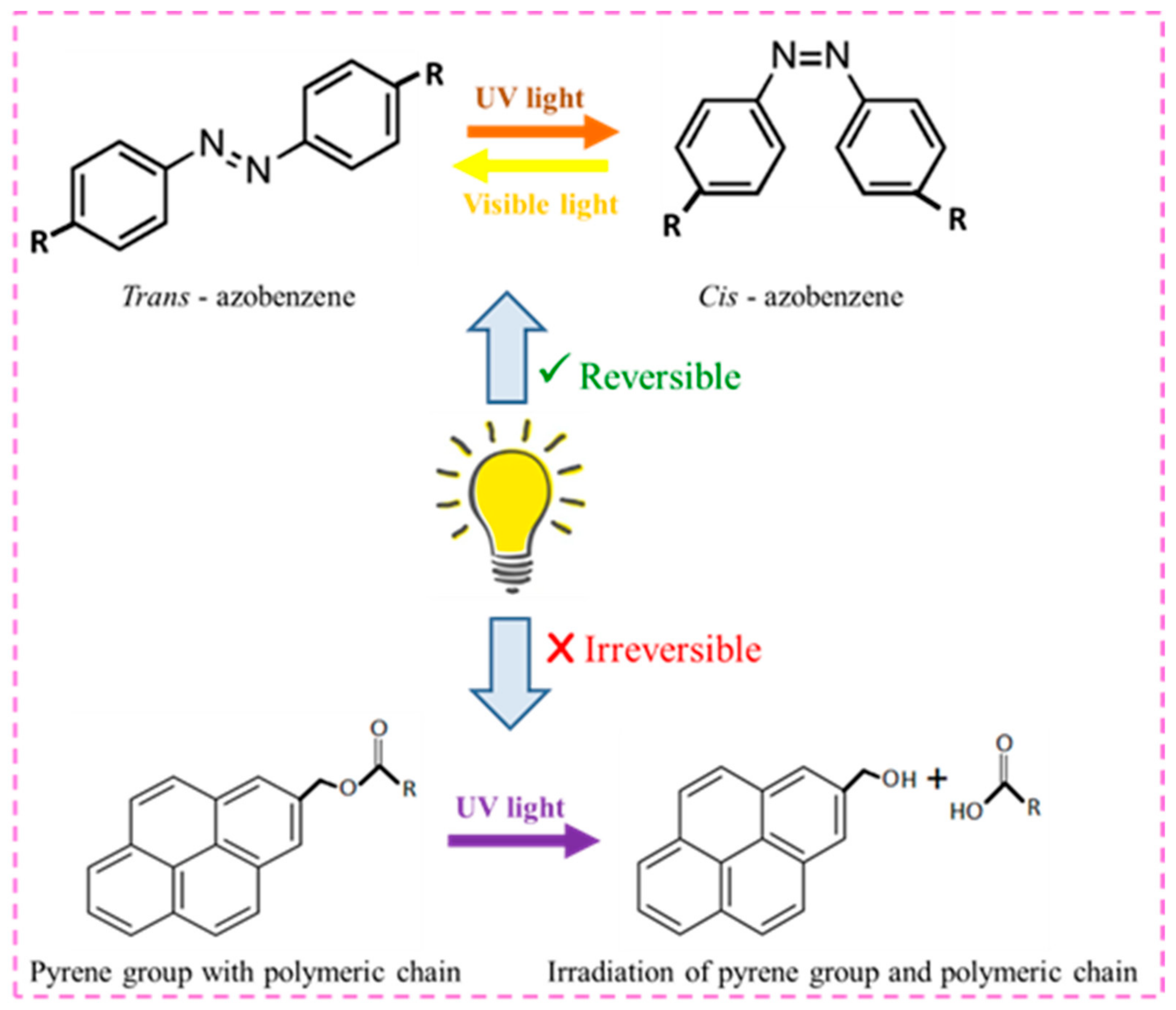
- (iii)
- Light responsiveness
- (iv)
- Magnetic responsiveness
- (v)
- Multi-responsiveness
3. Physicochemical Features of the Self-Assembly in BCPs
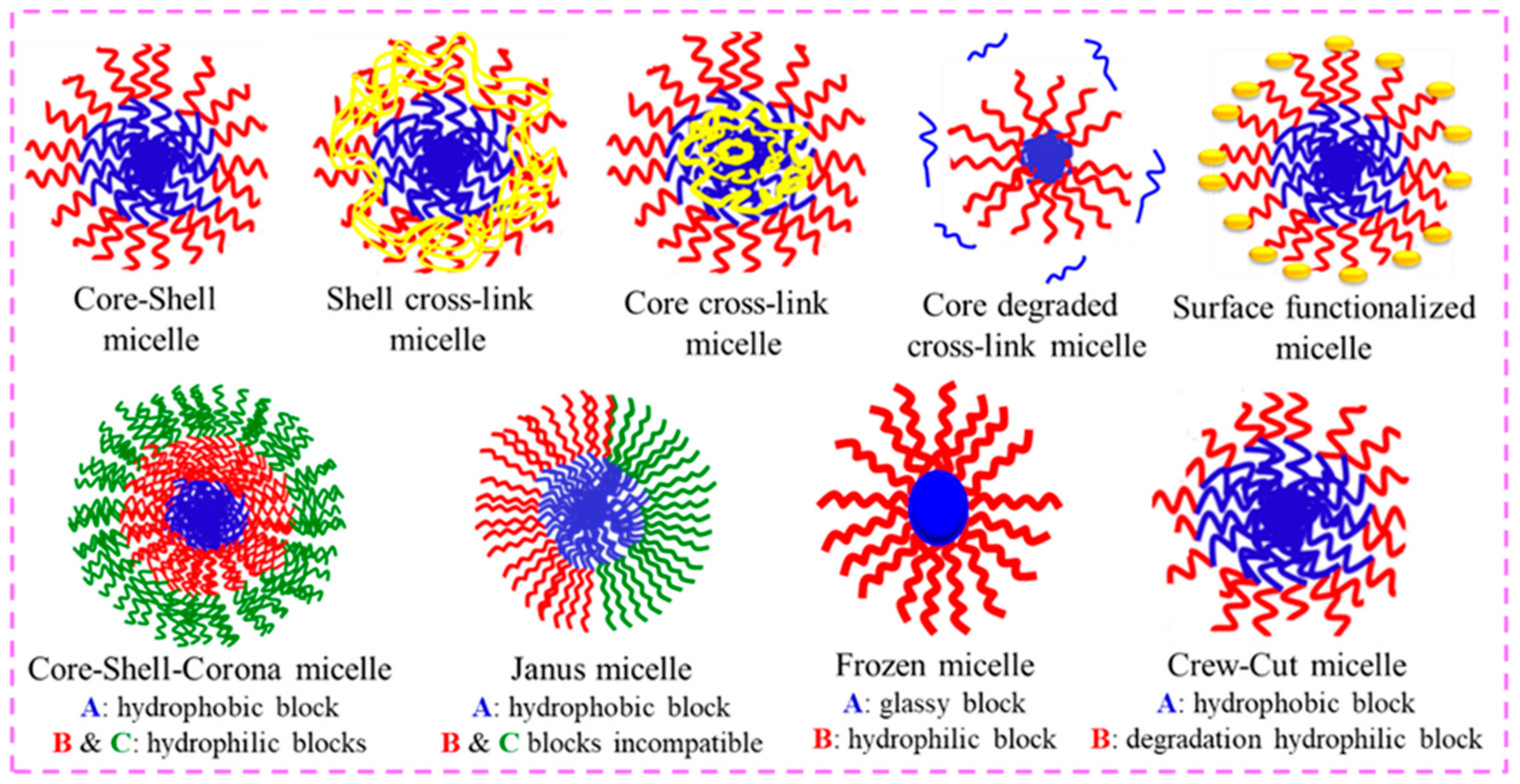
3.1. Polymeric Micelles (PMs)
3.2. Polymersomes
3.3. Ethylene Oxide (EO)-Propylene Oxide (PO)-Based BCP Micelles
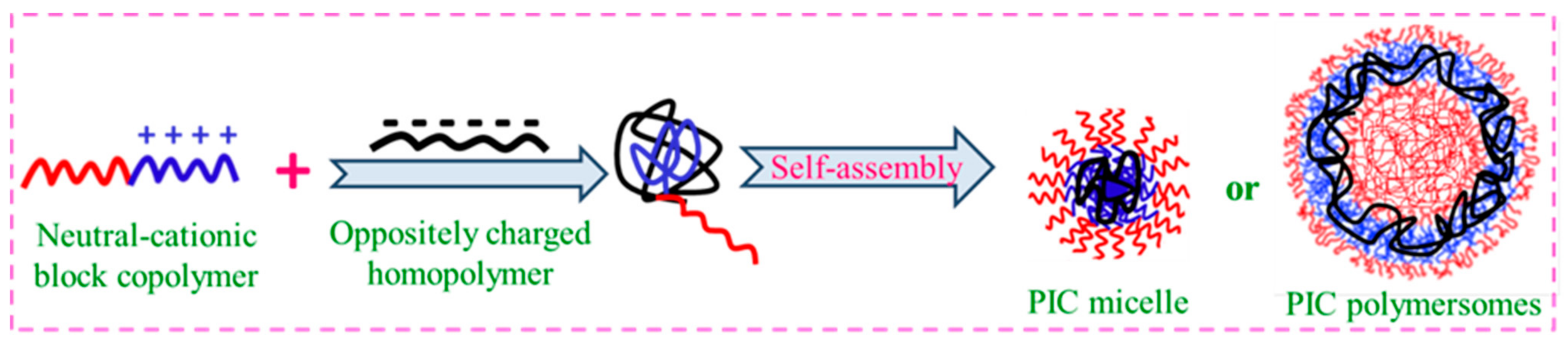
3.4. Polyion Complex Micelles (PICMs)
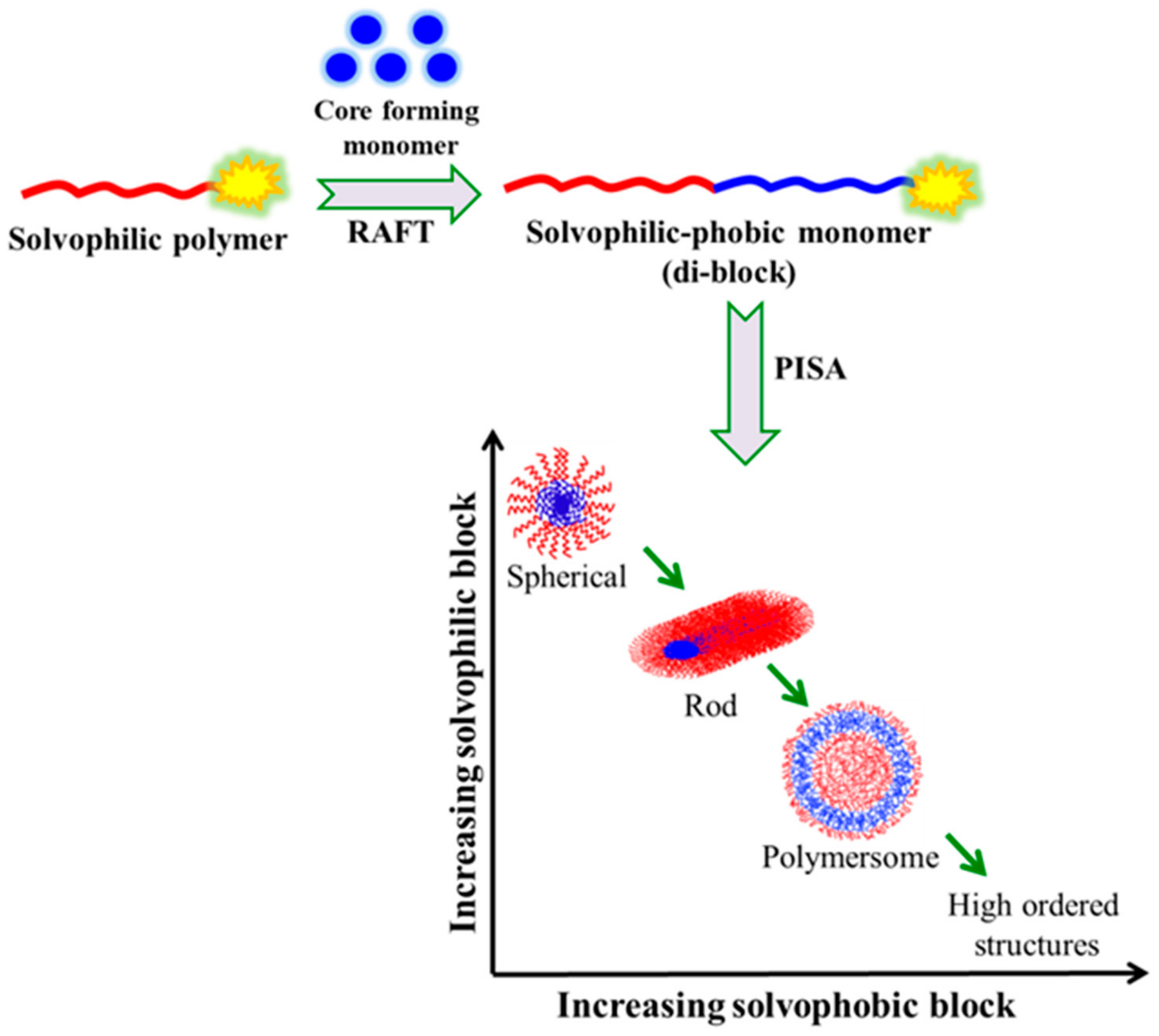
3.5. Polymerization-Induced Self-Assembly (PISA)
3.6. Crystallization-Driven Self-Assembly (CDSA)
4. Applications of BCPs
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riess, G.; Hurtrej, G.; Bahadur, P. Block Copolymers, Encyclopedia of Polymer Science Engineering. Wiley 1985, 2, 324–434. [Google Scholar]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Perin, F.; Motta, A.; Maniglio, D. Amphiphilic copolymers in biomedical applications: Synthesis routes and property control. Mater. Sci. Eng. C 2021, 123, 111952–111967. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Coenjarts, C.; Ober, C. Patternable block copolymers. Adv. Polym. Sci. 2005, 190, 183–226. [Google Scholar]
- Li, Z.; Lin, Z. Self-assembly of block copolymers for biological applications. Polym. Int. 2021, 71, 366–370. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pispas, S.; Floudas, G. Block Copolymers: Synthetic Strategies, Physical Properties, and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Lazzari, M.; Tornerio, M. A global view on block copolymers. Polymers 2020, 12, 869. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Lu, Y.; Lin, J.; Wang, L.; Zhang, L.; Cai, C. Self-Assembly of Copolymer Micelles: Higher-Level Assembly for Constructing Hierarchical Structure. Chem. Rev. 2020, 120, 4111–4140. [Google Scholar] [CrossRef]
- Hu, X.; Xiong, S. Fabrication of Nanodevices Through Block Copolymer Self-Assembly. Front. Nanotechnol. 2022, 4, 762996–763012. [Google Scholar] [CrossRef]
- Xi, S.; Zhu, Y.; Lu, J.; Chapmana, W. Block copolymer self-assembly: Melt and solution by molecular density functional theory. J. Chem. Phys. 2022, 156, 054902–054910. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4072. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Genevieve, G.; Marie-Helene, D.; Vinayak, P.; Sant, N.; Dusica, M.; Jean-Christophe, L. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar]
- Kazunori, K.; Atsushi, H.; Yukio, N. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar]
- Xiong, X.; Binkhathlan, Z.; Molavi, O.; Lavasanifar, A. Amphiphilic block co-polymers: Preparation and application in nanodrug and gene delivery. Acta Biomater. 2012, 8, 2017–2033. [Google Scholar] [CrossRef] [PubMed]
- Arotçaréna, M.; Heise, B.; Ishaya, S.; Laschewsky, A. Switching the inside and the outside of aggregates of water-soluble block copolymers with double thermoresponsivity. J. Am. Chem. Soc. 2002, 124, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, C.; Grubbs, R. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Braunecker, W.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J. Macromolecular architectures by living and controlled/living polymerizations. Prog. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Sumerlin, B.; Tsarevsky, N. Progress in Controlled Radical Polymerization: Mechanisms and Techniques. Am. Chem. Soc. 2012, 1100, 317–331. [Google Scholar]
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, 1706441–1706473. [Google Scholar] [CrossRef] [PubMed]
- Mertoglu, M.; Garnier, S.; Laschewsky, A.; Skrabania, K.; Storsberg, J. Stimuli responsive amphiphilic block copolymers for aqueous media synthesised via reversible addition fragmentation chain transfer polymerisation (RAFT). Polymer 2005, 46, 7726–7740. [Google Scholar] [CrossRef]
- Siegwart, D.; Oh, J.; Matyjaszewski, K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Szwarc, M. ‘Living’ polymers. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S. Radical addition-fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Nabiyan, A.; Max, J.B.; Schacher, F.H. Double hydrophilic copolymers-synthetic approaches, architectural variety, and current application fields. Chem. Soc. Rev. 2022, 51, 995–1044. [Google Scholar] [CrossRef] [PubMed]
- Nadal, C.; Gineste, S.; Coutelier, O.; Marty, J.D.; Destarac, M. A deeper insight into the dual temperature- and pH-responsiveness of poly(vinylamine)-b-poly(N-isopropylacrylamide) double hydrophilic block copolymers. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128502–128513. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J. Double Hydrophilic Block Copolymer Self-Assembly in Aqueous Solution. Macromol. Chem. Phys. 2018, 219, 1700494–1700508. [Google Scholar] [CrossRef]
- Butun, V.; Armes, S.; Billingham, N.; Tuzar, Z.; Rankin, A.; Eastoe, J.; Heenan, R. Synthesis and aqueous solution properties of a well-defined thermo-responsive schizophrenic diblock copolymer. Macromolecules 2001, 34, 1503. [Google Scholar]
- Liu, S.; Billingham, N.C.; Armes, S.P. A Schizophrenic Water-Soluble Diblock Copolymer. Angew. Chem. Int. Ed. 2001, 40, 2328–2332. [Google Scholar] [CrossRef]
- Liu, S.; Armes, S.P. Micelle Formation and Inversion Kinetics of a Schizophrenic Diblock Copolymer. Langmuir 2002, 19, 4432. [Google Scholar] [CrossRef]
- Papadakis, C.M.; Müller-Buschbaum, P.; Laschewsky, A. Switch it inside-out: “Schizophrenic” behavior of all thermoresponsive UCST-LCST diblock copolymers. Langmuir 2019, 35, 9660–9676. [Google Scholar] [CrossRef]
- Alam, M.; Keiko, H.; Yusa, S.; Nakashima, K. Schizophrenic micelle of a water-soluble diblock polymer and its application to a thermo-optical device. Colloid Polym. Sci. 2014, 292, 1611–1617. [Google Scholar] [CrossRef]
- Guragain, S.; Bastakoti, B.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-Stimuli-Responsive Polymeric Materials. Chem.-A Eur. J. 2021, 21, 13164–13174. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, T.; Urban, M.; Winnik, F.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Mizoue, Y.; Takahashi, R.; Sakurai, K.; Yusa, S. A Thermo-Responsive Polymer Micelle with a Liquid Crystalline Core. Polymers 2023, 15, 770. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Behera, B.; Sahu, S.; Ananthakrishnan, R.; Maiti, T.; Pramanik, P. Design of Dual Stimuli Responsive Polymer Modified Magnetic Nanoparticles for Targeted Anti-Cancer Drug Delivery and Enhanced MR Imaging. New J. Chem. 2016, 40, 545–557. [Google Scholar] [CrossRef]
- Appold, M.; Mari, C.; Lederle, C.; Elbert, J.; Schmidt, C.; Ott, I.; Stühn, B.; Gasser, G.; Gallei, M. Multi-stimuli responsive block copolymers as a smart release platform for a polypyridyl ruthenium complex. Polym. Chem. 2016, 8, 890–900. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug. Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- You, Y.; Oupicky, D. Synthesis of Temperature-Responsive Heterobifunctional Block Copolymers of Poly(ethylene glycol) and Poly(N-isopropylacrylamide). Biomacromolecules 2007, 8, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Mukerabigwi, J.; Yin, W.; Zha, Z.; Ke, W.; Wang, Y.; Chen, W.; Japir, A.; Wang, Y.; Ge, Z. Polymersome nanoreactors with tumor pH-triggered selective membrane permeability for prodrug delivery, activation, and combined oxidation-chemotherapy. J. Control. Release 2019, 303, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Huang, X.; Xu, F.; Pan, J.; Wang, Y.; Zhou, S. A pH-responsive polymersome depleting regulatory T cells and blocking A2A receptor for cancer immunotherapy. Nano Res. 2022, 15, 2324–2334. [Google Scholar] [CrossRef]
- Zhuang, J.; Gordon, M.; Ventura, J.; Li, L.; Thayumanavan, S. Multi-stimuli responsive macromolecules and their assemblies. Chem. Soc. Rev. 2013, 42, 7421–7436. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zhao, D.; Yuan, D.; Wang, H.; Tu, K.; Wang, L. Influence of indomethacin-loading on the micellization and drug release of thermosensitive dextran-graft-poly(N-isopropylacrylamide). Reactive Func. Polym. 2011, 71, 820–827. [Google Scholar] [CrossRef]
- Wei, H.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Thermo-sensitive polymeric micelles based on poly (N-isopropylacrylamide) as drug carriers. Progr. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- He, M.; Zhang, Z.; Jiao, Z.; Yan, M.; Miao, P.; Wei, Z.; Leng, X.; Li, Y.; Fan, J.; Sun, W.; et al. Redox-responsive phenyl-functionalized polylactide micelles for enhancing Ru complexes delivery and phototherapy. Chin. Chem. Lett. 2023, 34, 1075741–1075746. [Google Scholar] [CrossRef]
- Li, Y.; Tong, A.; Niu, P.; Guo, W.; Jin, Y.; Hu, Y.; Tao, P.; Miao, W. Light-Decomposable Polymeric Micelles with Hypoxia-Enhanced Phototherapeutic Efficacy for Combating Metastatic Breast Cancer. Pharmaceutics 2022, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Singh, K. Amphiphilic star block copolymer micelles in saline as effective vehicle for quercetin solubilization. J. Mol. Liq. 2021, 345, 118259–118265. [Google Scholar] [CrossRef]
- Kuperkar, K.; Tiwari, S.; Bahadur, P. Self-assembled block copolymer nanoaggregates for drug delivery applications. In Applications of Polymers in Drug Delivery, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 423–447. ISBN 978-0-12-819659-5. [Google Scholar]
- Nakashima, K.; Bahadur, P. Aggregation of water-soluble block copolymers in aqueous solutions: Recent trends. Adv. Colloid Interface Sci. 2006, 123, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S. Responsive Polymers for Detection and Sensing Applications: Current Status and Future Developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Stubenrauch, K.; Voets, I.; Fritz-Popovski, G.; Trimmel, G. pH and Ionic Strength Responsive Polyelectrolyte Block copolymer micelles prepared by ring opening metathesis polymerization. J. Polym. Sci. A 2008, 47, 1178–1191. [Google Scholar] [CrossRef]
- Qian, S.; Li, S.; Xiong, W.; Khan, H.; Huang, J.; Zhang, W. A new visible light and temperature responsive diblock copolymer. Polym. Chem. 2019, 10, 5001–5009. [Google Scholar] [CrossRef]
- Blanazs, A.; Armes, S.; Ryan, A. Self-assembled block copolymer aggregates: From micelles to vesicles and their biological applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Procházka, K.; Limpouchová, Z.; Štěpánek, M.; Šindelka, K.; Lísal, M. DPD Modelling of the Self-and Co-Assembly of Polymers and Polyelectrolytes in Aqueous Media: Impact on Polymer Science. Polymers 2022, 14, 404. [Google Scholar] [CrossRef]
- Tuzar, Z.; Kratochvil, P. Block and graft copolymer micelles in solution. Adv. Colloid Interface Sci. 1976, 6, 201–232. [Google Scholar] [CrossRef]
- Aqeel, R.; Srivastava, N.; Kushwaha, P. Micelles in Cancer Therapy: An Update on Preclinical and Clinical Status. Recent Pat. Nanotechnol. 2022, 16, 283–294. [Google Scholar]
- Wang, H.; Fliedel, C.; Manoury, E.; Poli, R. Core-crosslinked micelles with a poly-anionic poly(styrene sulfonate)-based outer shell made by RAFT polymerization. Polymer 2022, 243, 124640–124649. [Google Scholar] [CrossRef]
- Xiong, D.; Yao, N.; Gu, H.; Wang, J.; Zhang, L. Stimuli-responsive shell cross-linked micelles from amphiphilic four-arm star copolymers as potential nanocarriers for “pH/redox-triggered” anticancer drug release. Polymer 2017, 114, 161–172. [Google Scholar] [CrossRef]
- Bai, J.; Wang, J.; Feng, Y.; Yao, Y.; Zhao, X. Stability-tunable core-crosslinked polymeric micelles based on an imidazole-bearing block polymer for pH-responsive drug delivery. Colloids Surf. A 2022, 639, 128353–128361. [Google Scholar] [CrossRef]
- Kim, J.O.; Kabanov, A.V.; Bronich, T.K. Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J. Control. Release 2009, 138, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, W.; Xu, D. Stimuli-responsive nanoscale drug delivery systems for cancer therapy. J. Drug Target. 2018, 27, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, F.; Wang, Y.; Stenzel, M.; Chapman, R. Polyion Complex Micelles for Protein Delivery Benefit from Flexible Hydrophobic Spacers in the Binding Group. Macromol. Rapid Commun. 2022, 41, 2000208–2000214. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Xiao, Y.; Mao, A.; Lang, M. Phenylboronic acid conjugated mPEG-b-PCL micelles as DOX carriers for enhanced drug encapsulation and controlled drug release. Eur. Polym. J. 2022, 173, 111235. [Google Scholar] [CrossRef]
- Feng, R.; Li, Z.; Fangfang, T.; Min, W.; Shiyu, C.; Zhimei, S.; Hongmei, L. Phenylboronic acid-modified polymeric anhydride-F127 micelles for pH-activated targeting delivery of doxorubicin. Colloids Surf. B 2022, 216, 112559–112568. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tan, X.; Zhou, Q.; Geng, P.; Wang, J.; Zou, P.; Deng, A.; Hu, J. Co-delivery of doxorubicin and SIS3 by folate-targeted polymeric micelles for overcoming tumor multidrug resistance. Drug Deliv. Transl. Res. 2022, 12, 167–179. [Google Scholar] [CrossRef]
- Bastakoti, B.; Liao, S.; Inoue, M.; Yusa, S.; Imura, M.; Nakashima, K.; Wu, K.; Yamauchi, Y. pH-responsive polymeric micelles with core-shell-corona architectures as intracellular anti-cancer drug carriers. Sci. Technol. Adv. Mater. 2013, 14, 044402–044407. [Google Scholar] [CrossRef]
- Nicolai, T.; Colombani, O.; Chassenieux, C. Dynamic polymeric micelles versus frozen nanoparticles formed by block copolymers. Soft Matter. 2010, 6, 3111–3118. [Google Scholar] [CrossRef]
- Gohy, J.; Zhao, Y. Photo-responsive block copolymer micelles: Design and behavior. Chem. Soc. Rev. 2013, 42, 7117–7129. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Varshney, S.; Wong, S.; Eisenberg, A. Block Copolymer “Crew-Cut” Micelles in Water. Macromolecules 1994, 27, 7923–7927. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S. Ionic liquids: Versatile media for preparation of vesicles from polymerization-induced self-assembly. ACS Macro Lett. 2015, 4, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Becerra, E.; Quinchia, J.; Castro, C.; Orozco, J. Light-Triggered Polymersome-based anticancer therapeutics delivery. Nanomaterials 2022, 12, 836. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Meng, F.; Cheng, R.; Zhong, Z. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: A comparative study with micelles. J. Control. Release 2010, 142, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Robertson, J.; Yealland, G.; Avila-Olias, M.; Chierico, L.; Bandmann, O.; Renshaw, S.; Battaglia, G. pH-sensitive tubular polymersomes: Formation and applications in cellular delivery. ACS Nano 2014, 8, 4650–4661. [Google Scholar] [CrossRef]
- Pawar, P.; Gohil, S.; Jain, J.; Kumar, N. Functionalized polymersomes for biomedical applications. Polym. Chem. 2013, 4, 3160–3176. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ramezani, M.; Abnous, K.; Alibolandi, M. Biocompatible polymersomes-based cancer theranostics: Towards multifunctional nanomedicine. Int. J. Pharm. 2017, 519, 287–303. [Google Scholar] [CrossRef]
- Raffa, P.; Wever, D.; Picchioni, F.; Broekhuis, A. Polymeric Surfactants: Synthesis, Properties, and Links to Applications. Chem. Rev. 2015, 115, 8504–8563. [Google Scholar] [CrossRef] [PubMed]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Singla, P.; Garg, S.; McClements, J.; Jamieson, O.; Peeters, M.; Mahajan, R. Advances in the therapeutic delivery and applications of functionalized Pluronics: A critical review. Adv. Colloid Interface Sci. 2022, 299, 102563–102594. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Ezhov, A.A.; Ferberg, A.S.; Krylov, S.S.; Semenova, M.N.; Semenov, V.V.; Kudryashova, E.V. Polymeric Micelles Formulation of Combretastatin Derivatives with Enhanced Solubility, Cytostatic Activity and Selectivity against Cancer Cells. Pharmaceutics 2023, 15, 1613. [Google Scholar] [CrossRef] [PubMed]
- Jundi, A.E.; Buwalda, S.J.; Bakkour, Y.; Garric, X.; Nottelet, B. Double hydrophilic block copolymers self-assemblies in biomedical applications. Adv. Colloid Int. Sci. 2020, 283, 102213–102279. [Google Scholar] [CrossRef] [PubMed]
- Giaouzi, D.; Pispas, S. PNIPAM-b-PDMAEA double stimuli responsive copolymers: Effects of composition, end groups and chemical modification on solution self-assembly. Eur. Polym. J. 2020, 135, 109867. [Google Scholar] [CrossRef]
- Bhowmik, S.; Pham, T.; Takahashi, R.; Kim, D.; Matsuoka, H.; Ishihara, K.; Yusa, S. Preparation of Water-Soluble Polyion Complex (PIC) Micelles with Random Copolymers Containing Pendant Quaternary Ammonium and Sulfonate Groups. Langmuir 2023, 39, 8120–8129. [Google Scholar] [CrossRef]
- Pham, T.; Pham, T.; Yusa, S. Polyion complex (PIC) micelles formed from oppositely charged styrene-based polyelectrolytes via electrostatic, hydrophobic, and π–π interactions. Polym. J. 2022, 54, 1091–1101. [Google Scholar] [CrossRef]
- Song, Y.; Tian, Q.; Huang, Z.; Fan, D.; She, Z.; Liu, X.; Cheng, X.; Yu, B.; Deng, Y. Self-assembled micelles of novel amphiphilic copolymer cholesterol-coupled F68 containing cabazitaxel as a drug delivery system. Int. J. Nanomed. 2014, 9, 2307–2317. [Google Scholar]
- Kim, D.; Vitol, E.; Liu, J.; Balasubramanian, S.; Gosztola, D.; Cohen, E.; Novosad, V.; Rozhkova, E. Stimuli-Responsive Magnetic Nanomicelles as Multifunctional Heat and Cargo Delivery Vehicles. Langmuir 2013, 29, 7425–7432. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, S.; Santos, S.; Fessi, H.; Elaissari, A. Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm. 2011, 403, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; Pang, M.; Zhang, W. Synthesis and micellization of a multi-stimuli responsive block copolymer based on spiropyran. Polym. Chem. 2016, 7, 6880–6885. [Google Scholar] [CrossRef]
- Corten, C.; Kretschmer, K.; Kuckling, D. Novel multi-responsive P2VP-block-PNIPAAm block copolymers via nitroxide-mediated radical polymerization. Beilstein J. Org. Chem. 2010, 6, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Fuoss, R.; Sadek, H. Mutual interaction of polyelectrolytes. Science 1949, 110, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Nishiuchi, M.; Inoue, M.; Ishihara, K.; Sanada, Y.; Sakurai, K.; Yusa, S. Preparation and characterization of polyion complex micelles with phosphobetaine shells. Langmuir 2013, 29, 9651–9661. [Google Scholar] [CrossRef] [PubMed]
- Damsongsang, P.; Yusa, S.; Hoven, V. Zwitterionic nano-objects having functionalizable hydrophobic core: Formation via polymerization-induced self-assembly and their morphology. Eur. Polym. J. 2022, 179, 111536. [Google Scholar] [CrossRef]
- Ohno, S.; Ishihara, K.; Yusa, S. Formation of Polyion Complex (PIC) Micelles and Vesicles with Anionic pH-Responsive Unimer Micelles and Cationic Diblock Copolymers in Water. Langmuir 2016, 32, 3945–3953. [Google Scholar] [CrossRef]
- Yusa, S.; Yokoyama, Y.; Morishima, Y. Synthesis of oppositely charged block copolymers of polyethylene glycol via reversible addition-fragmentation chain transfer radical polymerization and characterization of their polyion complex micelles in water. Macromolecules 2009, 42, 376–383. [Google Scholar] [CrossRef]
- Chen, F.; Stenzel, M. Polyion Complex Micelles for Protein Delivery. Aust. J. Chem. 2018, 71, 768–780. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Polyion complex micelle formation from double-hydrophilic block copolymers composed of charged and non-charged segments in aqueous media. Polym. J. 2018, 50, 95–100. [Google Scholar] [CrossRef]
- Nakamura, N.; Mochida, Y.; Toh, K.; Anraku, Y.; Cabral, H. Effect of mixing ratio of oppositely charged block copolymers on polyion complex micelles for in vivo application. Polymers 2021, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Charleux, B.; Delaittre, G.; Rieger, J.; D’Agosto, F. Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step. Macromolecules 2012, 45, 6753–6765. [Google Scholar] [CrossRef]
- Derry, M.; Fielding, L.; Armes, S. Polymerization-induced self-assembly of block copolymer nanoparticles via RAFT non-aqueous dispersion polymerization. Prog. Polym. Sci. 2016, 52, 1–18. [Google Scholar] [CrossRef]
- Penfold, N.; Yeow, J.; Boyer, C.; Armes, S. Emerging Trends in Polymerization-Induced Self-Assembly. ACS Macro Lett. 2019, 8, 1029–1054. [Google Scholar] [CrossRef] [PubMed]
- Warren, N.; Armes, S. Polymerization-Induced Self-Assembly of Block Copolymer Nano-objects via RAFT Aqueous Dispersion Polymerization. J. Am. Chem. Soc. 2014, 136, 10174–10185. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Lowe, A. Polymerization-induced self-assembly: Ethanolic RAFT dispersion polymerization of 2-phenylethyl methacrylate. Polym. Chem. 2014, 5, 2342–2351. [Google Scholar] [CrossRef]
- Zhang, W.; Kadirkhanov, J.; Wang, C.; Ding, S.; Hong, C.; Wang, F.; You, Y. Polymerization-induced self-assembly for the fabrication of polymeric nano-objects with enhanced structural stability by cross-linking. Polym. Chem. 2020, 11, 3654–3672. [Google Scholar] [CrossRef]
- Cao, J.; Tan, Y.; Chen, Y.; Zhang, L.; Tan, J. Expanding the Scope of Polymerization-Induced Self-Assembly: Recent Advances and New Horizons. Macromol. Rapid Comm. 2021, 42, 2100498–2100510. [Google Scholar] [CrossRef]
- D’Agosto, F.; Rieger, J.; Lansalot, M. RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem. 2020, 59, 8368–8392. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hong, C.; Pan, C. Polymerization techniques in polymerization-induced self-assembly (PISA). Polym. Chem. 2020, 11, 3673–3689. [Google Scholar] [CrossRef]
- Gonzalez, J.; Schmarsow, R.; Rojo, U.; Puig, J.; Schroeder, W.; Zucchi, I. Block Copolymer Micelles Generated by Crystallization-Driven Self-Assembly in Polymer Matrices. Sci. Rev. 2020, 1, 47–64. [Google Scholar]
- Inam, M.; Cambridge, G.; Pitto-Barry, A.; Laker, Z.L.; Wilson, N.R.; Mathers, R.T.; Dove, A.P.; O’Reilly, R.K. 1D vs. 2D shape selectivity in the crystallization-driven self-assembly of polylactide block copolymers. Chem. Sci. 2017, 8, 4223–4230. [Google Scholar] [CrossRef] [PubMed]
- Gädt, T.; Ieong, N.; Cambridge, G.; Winnik, M.; Manners, I. Complex and hierarchical micelle architectures from diblock copolymers using living, crystallization-driven polymerizations. Nat. Mater. 2009, 8, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ganda, S.; Stenzel, M. Concepts, fabrication methods and applications of living crystallization-driven self-assembly of block copolymers. Prog. Polym. Sci. 2020, 101, 101195–101205. [Google Scholar] [CrossRef]
- He, W.; Xu, J. Crystallization assisted self-assembly of semicrystalline block copolymers. Prog. Polym. Sci. 2012, 37, 1350–1400. [Google Scholar] [CrossRef]
- Finnegan, J.; Pilkington, E.; Alt, K.; Rahim, A.; Kent, S.; Davis, T.; Kempe, K. Stealth Nanorods via the Aqueous Living Crystallisation-Driven Self-Assembly of Poly(2-oxazoline)s. Chem. Sci. 2021, 12, 7350–7360. [Google Scholar] [CrossRef]
- Gilroy, J.; Gädt, T.; Whittell, G.; Chabanne, L.; Mitchels, J.; Richardson, R.; Winnik, M.; Manners, I. Monodisperse cylindrical micelles by crystallization-driven living self-assembly. Nat. Chem. 2010, 2, 566–570. [Google Scholar] [CrossRef]
- MacFarlane, L.; Zhao, C.; Cai, J.; Qiu, H.; Manners, I. Emerging applications for living crystallization-driven self-assembly. Chem. Sci. 2021, 12, 4661–4682. [Google Scholar] [CrossRef]
- Sha, Y.; Rahman, A.; Zhu, T.; Cha, Y.; McAlister, C.; Tang, C. ROMPI-CDSA: Ring-opening metathesis polymerization-induced crystallization-driven self-assembly of metallo-block copolymers. Chem. Sci. 2019, 10, 9782–9787. [Google Scholar] [CrossRef] [PubMed]
- López-Lorente, A.; Mizaikoff, B. Recent advances on the characterization of nanoparticles using infrared spectroscopy. Trends Analyt. Chem. 2016, 84, 97–106. [Google Scholar] [CrossRef]
- Modena, M.; Rühle, B.; Burg, T.; Wuttke, S. Nanoparticle Characterization: What to Measure. Adv. Mater. 2019, 31, 1901556–1901580. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.; Thanh, N. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Z.; Wang, F.; Xie, D.; Yang, S.; Wang, T.; Feng, L.; Chu, C. Synthesis, characterization, and self-assembly of linear poly(ethyleneoxide)-block–poly(propylene oxide)-block–poly(e-caprolactone) (PEO–PPO–PCL) copolymers. J. Colloid Interf. Sci. 2013, 393, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, W.; Park, H.; Lee, D.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Ray, D.; Aswal, V.; Kuperkar, K.; Bahadur, P. Temperature stimulated self-association and micellar transition for star shaped normal and reverse EO-PO block copolymers and their mixed systems as potential use for anticancer drug solubilization. Soft Matter. 2022, 18, 4543–4553. [Google Scholar] [CrossRef]
- Patel, D.; Jana, R.; Lin, M.; Kuperkar, K.; Seth, D.; Chen, L.; Bahadur, P. Revisiting the salt-triggered self-assembly in very hydrophilic triblock copolymer Pluronic® F88 using multitechnique approach. Colloid Polym. Sci. 2021, 299, 229–239. [Google Scholar] [CrossRef]
- Lunagariya, J.; Sivakumar, N.; Asif, M.; Dhar, A.; Vekariya, R. Dependency of Anion and Chain Length of Imidazolium Based Ionic Liquid on Micellization of the Block Copolymer F127 in Aqueous Solution: An Experimental Deep Insight. Polymers 2017, 9, 285. [Google Scholar] [CrossRef]
- Patel, D.; Ray, D.; Kuperkar, K.; Pal, H.; Aswal, V.; Bahadur, P. Solubilization, micellar transition and biocidal assay of loaded multifunctional antioxidants in Tetronic® 1304 micelles. Polym. Int. 2020, 69, 1097–1104. [Google Scholar] [CrossRef]
- Patel, D.; Ray, D.; Kuperkar, K.; Aswal, V.; Bahadur, P. Parabens induced spherical micelle to polymersome transition in thermo-responsive amphiphilic linear and star-shaped EO-PO block copolymers. J. Mol. Liq. 2020, 316, 113897–113908. [Google Scholar] [CrossRef]
- Singla, P.; Singh, O.; Chabba, S.; Aswal, V.; Mahajan, R. Sodium deoxycholate mediated enhanced solubilization and stability of hydrophobic drug Clozapine in pluronic micelles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 15, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.; Hinsberg, W. Block copolymer-based nanostructures: Materials, processes, and applications to electronics. Chem. Rev. 2010, 110, 146–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J. Preparation and Properties of Poly(l-lactide)-block-poly(trimethylenecarbonate) as Biodegradable Thermoplastic Elastomer. Polym. J. 2002, 34, 203–208. [Google Scholar] [CrossRef]
- Chowdhry, B.; Snowdena, M.; Leharne, S. A scanning calorimetric investigation of phase transitions in a PPO-PEO-PPO block copolymer. Eur. Polym. J. 1999, 35, 273–278. [Google Scholar] [CrossRef]
- Sarolia, J.; Kumar, D.; Shah, S.; Bahadur, P.; Tiwari, S. Thermodynamics of pluronic 103 micellization in mannitol solution: Analyses based on isothermal titration calorimetry. Colloid Surf. A 2022, 648, 129240–129244. [Google Scholar] [CrossRef]
- Santiago, A.; Vargas, J.; Jorge, V.; Cruz-Morales, J.; Mikhail, T.; Gavino, R.; Malkanduev, Y.; Sivov, N. Synthesis of New Polymer Ionomers via Ring-Opening Metathesis Polymerization. Open J. Organic Polym. Mat. 2014, 4, 84–91. [Google Scholar] [CrossRef][Green Version]
- Patel, D.; Vaswani, P.; Sengupta, S.; Ray, D.; Bhatia, D.; Choudhury, S.D.; Aswal, V.K.; Kuperkar, K.; Bahadur, P. Thermoresponsive phase behavior and nanoscale self-assembly generation in normal and reverse Pluronics®. Colloid Polym. Sci. 2023, 301, 75–92. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Ribeiro, I.; Boyd, B.; Loh, W. Impact of preparation method and variables on the internal structure, morphology, and presence of liposomes in phytantriol-Pluronic® F127cubosomes. Colloids Surf. B 2016, 145, 845–853. [Google Scholar] [CrossRef]
- Rodrigues, E.; Morales, M.; Medeiros, S.; Suguihiro, M.; Baggio-Saitovitch, E. Pluronics coated sterically stabilized magnetite nanoparticles for hyperthermia applications. J. Magn. Magn. Mater. 2016, 416, 434–440. [Google Scholar] [CrossRef]
- Kanga, E.; Sharker, S.; Inc, I.; Park, S. Pluronic mimicking fluorescent carbon nanoparticles conjugated with doxorubicin via acid-cleavable linkage for tumor-targeted drug delivery and bioimaging. J. Ind. Eng. Chem. 2016, 43, 150–157. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Kumar, K.; Arora, A.; Katti, D. Fabrication and characterization of Pluronic modified poly(hydroxybutyrate) fibers for potential wound dressing applications. Mat. Sci. Eng. C 2016, 63, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Pellosi, D.; Calori, I.; Paula, L.; Hioka, N.; Quaglia, F.; Tedesco, A. Multifunctional theranostic Pluronic mixed micelles improve targeted photoactivity of Verteporfin in cancer cells. Mat. Sci. Eng. C 2017, 71, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yap, L.; Yang, M. Evaluation of hydrogel composing of Pluronic F127 and carboxymethyl hexanoyl chitosan as injectable scaffold for tissue engineering applications. Colloids Surf. B 2016, 146, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B. Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics 2023, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Barz, M.; Rijcken, C.; Kiessling, F.; Hennink, W.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Colfen, H. Double-Hydrophilic Block Copolymers: Synthesis and Application as Novel Surfactants and Crystal Growth Modifiers. Macromol. Rapid Commun. 2001, 4, 220–252. [Google Scholar]
- Zou, Y.; Zhou, X.; Ma, J.; Yang, X.; Deng, Y. Recent advances in amphiphilic block copolymer templated mesoporous metal-based materials: Assembly engineering and applications. Chem. Soc. Rev. 2020, 49, 1173–1208. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, K.; Liang, X.; Liu, H.; Li, Y. Small molecule-mediated self-assembly behaviors of Pluronic block copolymers in aqueous solution: Impact of hydrogen bonding on the morphological transition of Pluronic micelles. Soft Matter. 2020, 16, 142–152. [Google Scholar] [CrossRef]
- Dhapte, V.; Mehta, P. Advances in hydrotropic solutions: An updated review. Polytech. J. 2015, 1, 424–435. [Google Scholar] [CrossRef]
- Hodgdon, T.; Kaler, E. Hydrotropic solutions. Curr. Opin. Colloid Interface Sci. 2007, 12, 121–128. [Google Scholar] [CrossRef]
- Vemula, V.; Lagishetty, V.; Lingala, S. Solubility enhancement techniques. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 41–51. [Google Scholar]
- Klermund, L.; Castiglione, K. Polymersomes as nanoreactors for preparative biocatalytic applications: Current challenges and future perspectives. Bioprocess Biosyst. Eng. 2018, 41, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Ruttala, H.; Ramasamy, T.; Madeshwaran, T.; Hiep, T.; Kandasamy, U.; Oh, K.; Choi, H.; Yong, C.; Kim, J. Emerging potential of stimulus-responsive nanosized anticancer drug delivery systems for systemic applications. Arch. Pharm. Res. 2017, 41, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, V.; Nivetha, R.P.; Tamilanban, T.; Narayanan, J.; Vetriselvan, S.; Fuloria, N.K.; Chinni, S.V.; Sekar, M.; Fuloria, S.; Wong, L.S.; et al. Nanogels as novel drug nanocarriers for CNS drug delivery. Front. Mol. Biosci. 2023, 10, 1232109. [Google Scholar] [CrossRef] [PubMed]
- Hlavatovičová, E.; Fernandez-Alvarez, R.; Byś, K.; Kereïche, S.; Mandal, T.; Atanase, L.; Štěpánek, M.; Uchman, M. Stimuli-Responsive Triblock Terpolymer Conversion into Multi-Stimuli-Responsive Micelles with Dynamic Covalent Bonds for Drug Delivery through a Quick and Controllable Post-Polymerization Reaction. Pharmaceutics 2023, 15, 288. [Google Scholar] [CrossRef]
- Zlotnikov, I.; Streltsov, D.; Ezhov, A.; Kudryashova, E. Smart pH- and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. Pharmaceutics 2023, 15, 1135. [Google Scholar] [CrossRef]
- Szewczyk-Łagodzińska, M.; Plichta, A.; Dębowski, M.; Kowalczyk, S.; Iuliano, A.; Florjańczyk, Z. Recent Advances in the Application of ATRP in the Synthesis of Drug Delivery Systems. Polymers 2023, 15, 1234. [Google Scholar] [CrossRef]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Varela-Moreira, A.; Yang, S.; Marcel, H.F.; Twan, L.; Wim, E.H.; Raymond, M.S. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Favero, E.D.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release. 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Monica, G.; Niraj, K.J.; John, D.; Vandana, P.; Kamal, D.; Sachin, K.S. Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef] [PubMed]
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 4, 1860. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, D.; Kuperkar, K.; Yusa, S.-i.; Bahadur, P. Nanoscale Self-Assemblies from Amphiphilic Block Copolymers as Proficient Templates in Drug Delivery. Drugs Drug Candidates 2023, 2, 898-922. https://doi.org/10.3390/ddc2040045
Patel D, Kuperkar K, Yusa S-i, Bahadur P. Nanoscale Self-Assemblies from Amphiphilic Block Copolymers as Proficient Templates in Drug Delivery. Drugs and Drug Candidates. 2023; 2(4):898-922. https://doi.org/10.3390/ddc2040045
Chicago/Turabian StylePatel, Dhruvi, Ketan Kuperkar, Shin-ichi Yusa, and Pratap Bahadur. 2023. "Nanoscale Self-Assemblies from Amphiphilic Block Copolymers as Proficient Templates in Drug Delivery" Drugs and Drug Candidates 2, no. 4: 898-922. https://doi.org/10.3390/ddc2040045
APA StylePatel, D., Kuperkar, K., Yusa, S.-i., & Bahadur, P. (2023). Nanoscale Self-Assemblies from Amphiphilic Block Copolymers as Proficient Templates in Drug Delivery. Drugs and Drug Candidates, 2(4), 898-922. https://doi.org/10.3390/ddc2040045








