Abstract
Neuroendocrine tumors (NETs) represent a diverse group of neoplasms originating from neuroendocrine cells, presenting varied clinical behaviors and posing significant challenges in management. This review explores the emerging roles of receptor tyrosine kinases (RTKs) in the pathogenesis and progression of NETs, including vascular endothelial growth factor receptors (VEGFRs), insulin-like growth factor receptors (IGF-1R), RET, epidermal growth factor receptor (EGFR), and ALK. The dysregulation of RTK signaling pathways contributes to key cellular processes such as proliferation, survival, and invasion in NETs. We discuss the potential of targeting RTKs as therapeutic strategies in NETs, with a focus on recent developments in RET inhibitors and the therapeutic implications of RTK alterations.
1. Introduction
Neuroendocrine tumors (NETs) encompass a heterogeneous group of neoplasms originating from neuroendocrine cells scattered throughout the body, particularly in the digestive and respiratory systems [1].
Despite representing only 1% of newly diagnosed neoplasms, their incidence has markedly increased in recent decades [2]. These tumors exhibit varied clinical behaviors, ranging from indolent, slow-growing lesions to aggressive malignancies, often characterized by hormone secretion leading to diverse symptoms and complications. While diagnostic advancements have enhanced NET detection, managing these tumors remains challenging, particularly in cases where therapeutic options face limitations and failures [3].
Compared to other tumors, NETs present a relatively low mutation rate [4]. Genomic mutations in this group often occur in tumor suppressor genes associated with hereditary syndromes such as MEN1 (multiple endocrine neoplasia type 1), VHL (von Hippel-Lindau disease), and TSC1/2 (tuberous sclerosis) [5].
Numerous studies have sought to identify driver mutations and overexpression patterns in NETs, highlighting frequent mutations or overexpression of proteins involved in receptor tyrosine kinases (RTKs) and alterations in the PI3K/AKT or MAPK pathways [1,6,7].
RTKs play a significant role in the pathogenesis and progression of various cancers, including NETs [8]. Acting as cell surface receptors, RTKs initiate intracellular signaling cascades upon ligand activation, regulating critical cellular processes like proliferation, survival, and angiogenesis [9].
Currently, surgery remains the main treatment for the majority of NETs and can be curative in many cases. However, it may not be feasible for large, infiltrating, or metastatic disease [10]. In cases of advanced disease, medical therapy, primarily chemotherapy and radiation are often attempted to reduce tumor mass [11].
Given the heterogeneity of mutational status in NETs, only a few targeted therapies have been considered in vitro studies and clinical trials. Everolimus, an mTOR inhibitor, and sunitinib, a multi-target RTK inhibitor, have been widely investigated [12]. Notably, both agents target RTK activation and pathways, reflecting the crucial role of RTKs in NET onset and progression. In this review, we aim to summarize the main RTKs implicated in NETs and their potential as therapeutic targets.
2. EGFR
The epidermal growth factor receptor (EGFR) is a member of the ErbB family of receptor tyrosine kinases, and its dysregulation has been implicated in various cancers, including certain types of NETs [13,14] (Figure 4). The role of EGFR in NETs is complex, and its significance can vary among different subtypes of neuroendocrine tumors [15].
In some neuroendocrine tumors, particularly pulmonary carcinoids and a subset of gastrointestinal NETs, increased expression and activation of EGFR have been observed [16]. EGFR-targeting molecules demonstrate efficacy in vitro models of NETs, with erlotinib, a selective EGFR inhibitor, exhibiting activity in reducing cell viability in lung neuroendocrine cell lines and primary cultures [17]. Elevated EGFR expression was observed in highly glycosylated and aggressive pancreatic NETs, correlating with a poor prognosis [18]. Mutations of EGFR are infrequently detected in NETs. However, EGFR mutations have the potential to drive NET differentiation. Kogo et al. demonstrated this in a patient diagnosed with a pulmonary adenocarcinoma harboring an L858R mutation of EGFR. Following surgery, chemotherapy, and targeted therapy, the patient experienced relapse, developed resistance to EGFR inhibition, and saw the tumor differentiate into large cell neuroendocrine carcinoma [19]. But mutations are not the sole genetic alterations affecting EGFR behavior in NETs. Marinović et al. demonstrated an association between EGFR polymorphism +1562 AG and susceptibility to NET development [13]. In essence, genetic mutations or alterations affecting EGFR in NETs can significantly impact treatment strategies and tumor susceptibility. It is paramount to comprehend these genetic changes to tailor targeted therapies effectively and pinpoint individuals who may be predisposed to developing NETs.
3. IGF-1R
Insulin-like growth factor receptors (IGFRs) represent a crucial class of cell surface receptors involved in regulating fundamental cellular processes such as growth, survival, and differentiation. Emerging as key players in cancer biology, IGFRs have garnered significant attention for their role in neoplastic transformation and tumor progression [20]. IGFRs, particularly the type 1 receptor (IGF-1R), are recognized as key players in the intricate landscape of NETs, exerting a profound influence on their pathogenesis and progression [21]. These receptors play a crucial role in controlling cellular processes like growth, survival, and differentiation. Their dysregulation is linked to the development of various cancers in different types of tissues [22].
Despite the longstanding awareness of elevated expression levels of IGF-1R and its associated proteins in NET cells, dating back to seminal studies in the early 2000s [23,24,25,26] the precise mechanistic role of IGF-1R in the context of NETs remains tantalizingly elusive. While accumulating evidence underscores the involvement of IGF-1R in mediating responses to therapeutic interventions, including mTOR inhibition [17,27], the functional implications of its activity within NETs are far from straightforward. Compounding the complexity, IGF-1R’s potential to form heterodimers with insulin receptors introduces an additional layer of intricacy to its signaling dynamics, warranting careful consideration [28].
In the context of insulinomas, investigations have shed light on the activation of IGF-1R; yet, intriguingly, its pathway appears to undergo downregulation during the metastatic cascade, suggesting a nuanced and context-dependent role in the neoplastic transformation of NETs [29]. Furthermore, recent findings by Wang et al. have unveiled the paradoxical anticancer effects of IGF-1R. Through an exhaustive analysis of the secretome derived from a panel of 13 lung neuroendocrine cell lines, the study identified ASCL1 as a prominent overexpressed target. ASCL1, also known as achaete-scute homolog 1, stands as a master regulator of neuroendocrine differentiation, wielding profound influence over the phenotypic identity and functional attributes of neuroendocrine cells [30].
Remarkably, ASCL1 was found to exert its influence through the modulation of IGFBP5, an inhibitor of IGF-1R activity. Strikingly, the inhibition of ASCL1 led to the downregulation of IGFBP5 expression, consequently reactivating the IGF1R pathway and eliciting a profound reduction in cell proliferation [31].
Nevertheless, the enigmatic dual role of IGF-1R in NET biology remains a subject of intense scrutiny and warrants further in-depth investigation to unravel its intricacies and therapeutic implications (Figure 1). IGF-1R exhibits a dual role in NET biology, with its impact varying depending on the tumor stage. At different stages, IGF-1R can function both as a tumor promoter, facilitating growth, and as a tumor suppressor, exerting inhibitory effects. Understanding this dual role is essential for unravelling the complex dynamics of NETs and tailoring targeted therapeutic approaches to the specific stage of the disease.
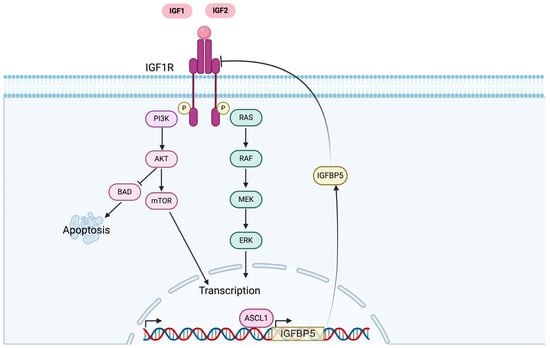
Figure 1.
IGF-1R activation and pathway in NET cells. The complex interactions and downstream effects initiated by IGF-1R activation, highlighting its central role in regulating key cellular processes such as proliferation, survival, and differentiation within NETs.
4. VEGFRs
Vascular endothelial growth factor receptors (VEGFRs) constitute a family of receptor tyrosine kinases pivotal in angiogenesis. This family comprises three isoforms: VEGFR1, VEGFR2, and VEGFR3. Correspondingly, their ligands, the VEGF family, consist of distinct isoforms—VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PIGF. VEGFR1 responds to VEGF-A, VEGF-B, and PIGF, while VEGFR2 interacts with VEGF-A, VEGF-C, and VEGF-D. VEGFR3, on the other hand, is activated by both VEGF-C and VEGF-D (Figure 2) [32].
In cancer cells, VEGFRs instigate the canonical RTK pathway, culminating in PI3K/AKT/mTOR activation [33]. Dysregulation of VEGFR signaling has been implicated in various cancers, including neuroendocrine tumors (NETs). Despite the demonstrated antiproliferative effects of VEGFR inhibitors in preclinical models [34], our understanding of the role of these receptors in NET biology remains limited.
Although research has revealed elevated levels of VEGFRs (and their ligands) in lung NETs compared to other bronchial neoplasia [35], the precise involvement of VEGFRs in neuroendocrine tumors is not fully elucidated. VEGFRs (and relative ligands) were found to be more highly expressed in lung NETs as compared to other bronchial neoplasia. Recently, Axitinib, a selective and potent VEGFR inhibitor, was found to inhibit lung NET growth in both in vitro and in vivo models by acting on cell cycle progression and the activation of apoptosis [36].
Chang et al. have elucidated the role of VEGFRs in gastroenteropancreatic NETs, highlighting the interplay between PTEN loss and DUSP19 inactivation. Their study revealed that the loss of PTEN in pancreatic NETs correlates with the inactivation of DUSP19, leading to heightened activation of VEGFR3 [37]. Despite being predominantly expressed in lymphatic vessels [38], this research illustrates that VEGFR3 activation in pancreatic NETs occurs independently of VEGF and drives the activation of the ERK pathway. This activation triggers epithelial–mesenchymal transition (EMT) and subsequently promotes cancer cell invasion [39]. The activation of VEGFRs in NET cells holds a central role in mediating epithelial–mesenchymal transition (EMT), promoting invasion, and ultimately contributing to metastasis. Understanding the intricate involvement of VEGFR in these processes is essential for deciphering the metastatic mechanisms in NETs and exploring potential therapeutic interventions.
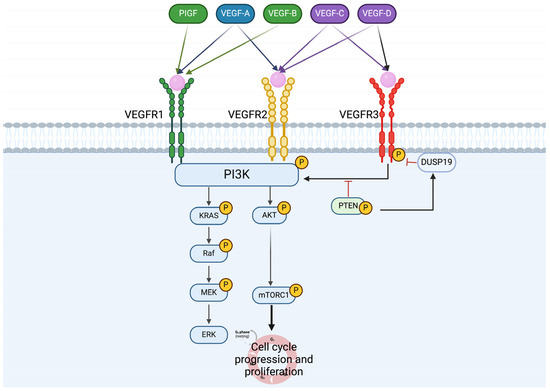
Figure 2.
VEGF ligands and receptors, activation pathway in NETs cells. The diagram outlines the activation pathways of vascular endothelial growth factor (VEGF) ligands and their receptors in neuroendocrine tumor (NET) cells. The figure delineates the interactions between VEGFR1, VEGFR2, and VEGFR3 receptors and their respective ligands, including VEGF-A, VEGF-B, VEGF-C, and VEGF-D. Through intricate signaling cascades, these ligands activate their corresponding receptors, leading to the modulation of angiogenesis and various cellular processes crucial for NET pathogenesis.
5. RET
The RET (rearranged during transfection) gene provides instructions for making a protein that plays a role in the development and maintenance of nerve cells and certain other cells [40]. In the context of oncogenesis, RET’s role as an oncogenic driver looms large, particularly in the realm of medullary thyroid cancer (MTC) [41]. Mutations in the RET gene, primarily germline mutations in hereditary cases or somatic mutations in sporadic cases, have been identified as the primary etiological factors underlying the pathogenesis of MTC [41]. These mutations result in constitutive activation of the RET kinase, unleashing a cascade of aberrant signaling events that drive uncontrolled cell proliferation, survival, and tumor progression within the thyroid gland [41] (Figure 3).
Activation of the RET receptor triggers a cascade of signaling events that impact multiple cellular pathways essential for cell growth, survival, and proliferation. Among these pathways, RET has been shown to activate the c-Jun N-terminal kinase (JNK), extracellular-signal-regulated kinase (ERK), and mammalian target of rapamycin (mTOR) pathways [42]. RET mutations have also been identified in other neuroendocrine tumors, including a subset of pancreatic neuroendocrine tumors (pNETs) [43].
While RET mutations are prominently associated with medullary thyroid cancer (MTC), accounting for the majority of hereditary cases and a subset of sporadic cases, their significance transcends the confines of thyroid malignancies. RET can undergo mutations or form fusion genes, leading to aberrant signaling pathways implicated in various cancers; RET alterations have been identified in a diverse array of cancer types, spanning lung cancer, colorectal cancer, breast cancer, and pheochromocytoma, among others [44].
While the role of RET in medullary thyroid cancer has been extensively explored in previous studies [41], our focus will be on recent findings that illuminate the significance of RET in other neuroendocrine tumors.
Researchers studied RET kinase as a potential target for treating certain types of metastatic castration-resistant prostate cancer (mCRPC). They found increased RET activity in AR-independent tumors, which are unresponsive to standard treatments. Inhibitors of RET, especially AD80, reduced RET activation and tumor growth in both cell and animal models, showing promise for treating mCRPC with high RET levels [45]. In a related study, Bae et al. utilizing a cancer dependency MAP, identified ZBTB7A, a transcription factor that plays a critical role in various cellular processes, including cell proliferation, differentiation, and survival, to be closely correlated with RET kinase activity within similar experimental settings. This finding provides additional insight into the regulatory network surrounding RET signaling [46].
In addition to its role in neuroendocrine prostate cancer, RET has also been implicated in lung neuroendocrine tumors. Notably, Kander et al. reported the efficacy of a RET selective inhibitor, Selpercatinib, in a patient harboring a CCDC6-RET fusion [47].
RET mutations and fusions are not only prevalent in medullary thyroid cancer (MTC) but also in other subtypes of neuroendocrine tumors (NETs). With the advancement in pharmacological development of RET inhibitors, this presents a significant opportunity for a subset of patients harboring RET mutations. Understanding the broader implications of RET alterations beyond MTC underscores the potential for targeted therapies to benefit a wider spectrum of NET patients.
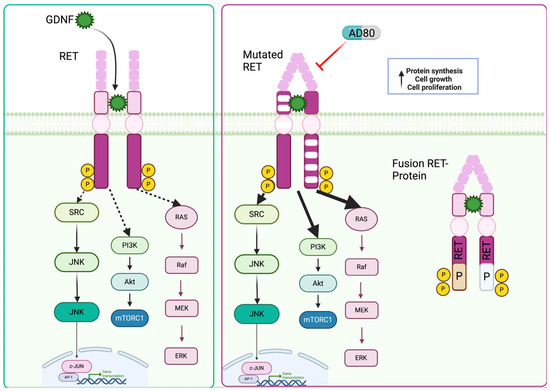
Figure 3.
RET and its mutation/fusion in NETs cells. RET wild-type, mutated, and fusion protein variants of the RET receptor tyrosine kinase in neuroendocrine tumors (NETs). The diagram delineates the structural differences between the wild-type RET protein and its mutated counterparts, highlighting specific amino acid alterations associated with mutations. Additionally, the figure illustrates fusion proteins resulting from genetic rearrangements involving RET, such as RET fusion with other genes.
6. ALK
The discovery of anaplastic lymphoma kinase (ALK) in 1994 marked a significant milestone in cancer research. Since its identification, ALK has garnered considerable attention as a promising therapeutic target due to its involvement in various malignancies, particularly in non-small-cell lung cancer (NSCLC) [48] and anaplastic large cell lymphoma (ALCL) [49]. ALK aberrations, including gene fusions and point mutations, lead to constitutive activation of the ALK kinase domain, driving oncogenic signaling pathways that promote cell proliferation, survival, and tumor growth (Figure 4) [50]. Multiple studies have underscored the clinical relevance of ALK alterations, highlighting their utility as actionable targets for precision medicine approaches. The development of ALK tyrosine kinase inhibitors (TKIs), such as crizotinib, ceritinib, and alectinib, has revolutionized the treatment landscape for ALK-positive cancers, leading to significant improvements in patient outcomes and survival rates [51]. Although ALK mutations or fusions have not been commonly reported in neuroendocrine tumors (NETs), some recent papers have reported on the alteration/fusion of ALK in a subset of NETs.
In a study by Leal et al., ALK staining was analyzed in 154 samples of pulmonary neuroendocrine tumors (NETs), revealing positive staining for ALK in 5.2% of the samples. Notably, one sample harbored an ALK-EML4 fusion [52]. Intriguingly, Nakajima et al. reported a case of successfully treated lung NETs in a patient with an ALK rearrangement, utilizing crizotinib, a selective ALK inhibitor [53]. Although ALK’s significance in neuroendocrine tumors (NETs) is underscored by recent reports, its therapeutic potential in a subset of NET cases warrants further validation. Continued research is crucial to fully elucidate ALK’s role in NETs and explore its implications for targeted therapies.
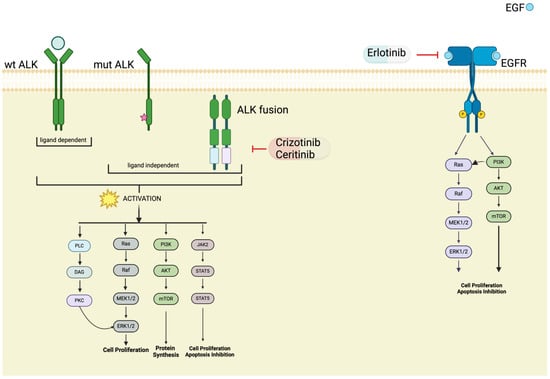
Figure 4.
ALK and EGFR pathway in NET. An overview of the various forms of the ALK (anaplastic lymphoma kinase) and EGFR (epidermal growth factor receptor) proteins observed in neuroendocrine tumors (NETs). The diagram illustrates wild-type (wt) ALK and EGFR proteins, as well as mutated and fusion variants resulting from genetic alterations.
7. Discussion
Neuroendocrine tumors (NETs) present a complex and heterogeneous group of neoplasms, posing significant challenges in their management and treatment [54]. Our review highlights the emerging roles of various receptor tyrosine kinases (RTKs), including vascular endothelial growth factor receptors (VEGFRs), insulin-like growth factor receptors (IGF-1R), RET, EGFR, and ALK, in the pathogenesis and progression of NETs.
The involvement of RTKs in NETs underscores their potential as therapeutic targets, offering new avenues for treatment strategies. For instance, the pivotal role of VEGFR activation in mediating epithelial–mesenchymal transition (EMT), invasion, and metastasis suggests that targeting VEGFR signaling pathways could be a promising therapeutic approach to impede tumor progression and metastatic spread in NETs [35].
Similarly, the dual role of IGF-1R in NETs biology, acting as both a tumor promoter and a tumor suppressor depending on the tumor stage, highlights the complexity of RTK signaling in driving NET pathogenesis. Understanding this dual role is crucial for designing tailored therapeutic interventions that take into account the stage-specific functions of IGF-1R in NETs [29].
Furthermore, the presence of RET mutations and fusions not only in medullary thyroid cancer (MTC) but also in other subtypes of NETs opens up new possibilities for targeted therapy. The recent development of RET inhibitors provides a promising opportunity for patients harboring RET alterations, emphasizing the importance of molecular profiling to identify actionable targets in NETs [40]. RTK pathway proteins, including TRK, are often found to be mutated or overexpressed in NETs, highlighting their significance in driving tumorigenesis and progression. This aberrant activation of RTK pathways contributes to the dysregulation of key cellular processes such as proliferation, survival, and angiogenesis, further underscoring their potential as therapeutic targets in NETs [55]. This aberrant activation of RTK pathways contributes to the dysregulation of key cellular processes such as proliferation, survival, and angiogenesis, further underscoring their potential as therapeutic targets in NETs. Currently, only Everolimus and sunitinib, both related to RTK inhibition, have been approved for targeted therapy in NETs [56]. These agents have shown efficacy in certain subsets of NETs, but their clinical benefits are often limited by adverse effects and acquired resistance. Thus, there remains a critical need for the development of novel RTK-targeted therapies with improved efficacy and safety profiles for the treatment of NETs.
While only a few recent reports have underscored the importance of ALK in NETs, further investigation is warranted to fully elucidate its role and therapeutic implications. More comprehensive studies are needed to validate the potential of ALK inhibitors as a treatment option for subsets of NET patients [53].
It is important to note that this review is not exhaustive. While we have focused on elucidating the roles of key RTKs such as VEGFRs, IGF-1R, RET, EGFR, ALK, and others such as TRK, it is acknowledged that there may be other receptors involved in NET pathogenesis, such as c-MET [57] and FGF [58]. However, for the purpose of this review, we have chosen to concentrate on the latest reports regarding RTKs to provide a comprehensive overview of the current understanding in this area.
8. Conclusions
In conclusion, the emerging understanding of RTKs in NETs sheds light on the molecular mechanisms driving tumor progression and metastasis. Targeting these pathways holds promise for developing novel therapeutic strategies and improving outcomes for patients with NETs. Continued research efforts are essential to further unravel the complexities of RTK signaling in NETs and translate these findings into clinical practice.
Author Contributions
L.T.: data curation, A.D. and L.T.: writing—original draft preparation, T.G.: writing—review and editing, T.G.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Departmental Strategic Plan (PSD) of the University of Udine—Interdepartmental Project on Healthy Ageing (2020-25).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, H.; Yu, Z.; Liu, Y.; Guo, L.; Teng, L.; Guo, L.; Liang, L.; Wang, J.; Gao, J.; Li, R.; et al. Genomic Characterization Reveals Distinct Mutation Landscapes and Therapeutic Implications in Neuroendocrine Carcinomas of the Gastrointestinal Tract. Cancer Commun. 2022, 42, 1367–1386. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.E.; Dowrey, T.W.; Martin, C.V.; Bi, K.; Titchen, B.; Johri, S.; DelloStritto, L.; Patel, M.; Mackichan, C.; Inga, S.; et al. Intertumoral Lineage Diversity and Immunosuppressive Transcriptional Programs in Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors. Sci. Adv. 2023, 9, eadd9668. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, A.; Wiedmer, T.; Marinoni, I.; Perren, A. Genetic and Epigenetic Drivers of Neuroendocrine Tumours (NET). Endocr. Relat. Cancer 2017, 24, R315–R334. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A. The Landscape of Molecular Alterations in Pancreatic and Small Intestinal Neuroendocrine Tumours. Ann. Endocrinol. 2019, 80, 153–158. [Google Scholar] [CrossRef]
- Sakane, T.; Sakamoto, Y.; Masaki, A.; Murase, T.; Okuda, K.; Nakanishi, R.; Inagaki, H. Mutation Profile of Thymic Carcinoma and Thymic Neuroendocrine Tumor by Targeted Next-Generation Sequencing. Clin. Lung Cancer 2021, 22, 92–99.e4. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, M.K.; Thomas, C.F.; Dong, J.; Schulte, S.C.; Khadka, P.; Sun, Z.; Kosari, F.; Jen, J.; Molina, J.; Vasmatzis, G.; et al. Pathways Impacted by Genomic Alterations in Pulmonary Carcinoid Tumors. Clin. Cancer Res. 2018, 24, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Umemura, S.; Ishii, G.; Tsuta, K.; Matsumoto, S.; Aokage, K.; Hishida, T.; Yoshida, J.; Ohe, Y.; Suzuki, H.; et al. Expression Profiling of Receptor Tyrosine Kinases in High-Grade Neuroendocrine Carcinoma of the Lung: A Comparative Analysis with Adenocarcinoma and Squamous Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2015, 141, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of Receptor Tyrosine Kinase Activation in Cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Uri, I.; Grozinsky-Glasberg, S. Current Treatment Strategies for Patients with Advanced Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs). Clin. Diabetes Endocrinol. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Albertelli, M.; Grillo, F.; Mohamed, A.; Saveanu, A.; Barlier, A.; Ferone, D.; Florio, T. Neuroendocrine Tumors: Insights into Innovative Therapeutic Options and Rational Development of Targeted Therapies. Drug Discov. Today 2014, 19, 458–468. [Google Scholar] [CrossRef] [PubMed]
- IEO ENETS Center of Excellence for GEP NETs; Martins, D.; Spada, F.; Lambrescu, I.; Rubino, M.; Cella, C.; Gibelli, B.; Grana, C.; Ribero, D.; Bertani, E.; et al. Predictive Markers of Response to Everolimus and Sunitinib in Neuroendocrine Tumors. Target. Oncol. 2017, 12, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Marinović, S.; Cigrovski Berković, M.; Zjačić-Rotkvić, V.; Kapitanović, S. Analysis of Polymorphisms in EGF, EGFR and HER2 Genes in Pancreatic Neuroendocrine Tumors (PNETs). Cancer Genet. 2022, 266–267, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Papouchado, B.; Erickson, L.A.; Rohlinger, A.L.; Hobday, T.J.; Erlichman, C.; Ames, M.M.; Lloyd, R.V. Epidermal Growth Factor Receptor and Activated Epidermal Growth Factor Receptor Expression in Gastrointestinal Carcinoids and Pancreatic Endocrine Carcinomas. Mod. Pathol. 2005, 18, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.M.; Ishizuka, J.; Thompson, J.C. Studies of Growth Regulation in a Neuroendocrine Cell Line. Acta Oncol. 1993, 32, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C. Molecular Targeted Therapy for Carcinoid and Islet-Cell Carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; Ditsiou, A.; Cilibrasi, C.; Vella, V.; Rea, F.; Schiavon, M.; Cavallesco, N.G.; Giamas, G.; Zatelli, M.C.; Gagliano, T. EGF and IGF1 Affect Sunitinib Activity in BP-NEN: New Putative Targets beyond VEGFR? Endocr. Connect. 2019, 8, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xu, H.; Strosberg, J.R.; Lu, R.; Zhu, X.; Deng, S.; Ding, L.; Ni, Q.; Warshaw, A.L.; Yu, X.; et al. EGFR Is a Potential Therapeutic Target for Highly Glycosylated and Aggressive Pancreatic Neuroendocrine Neoplasms. Int. J. Cancer 2023, 153, 164–172. [Google Scholar] [CrossRef]
- Kogo, M.; Shimizu, R.; Uehara, K.; Takahashi, Y.; Kokubo, M.; Imai, Y.; Tomii, K. Transformation to Large Cell Neuroendocrine Carcinoma as Acquired Resistance Mechanism of EGFR Tyrosine Kinase Inhibitor. Lung Cancer 2015, 90, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Benyoucef, S.; Surinya, K.H.; Hadaschik, D.; Siddle, K. Characterization of Insulin/IGF Hybrid Receptors: Contributions of the Insulin Receptor L2 and Fn1 Domains and the Alternatively Spliced Exon 11 Sequence to Ligand Binding and Receptor Activation. Biochem. J. 2007, 403, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Galal, M.A.; Alouch, S.S.; Alsultan, B.S.; Dahman, H.; Alyabis, N.A.; Alammar, S.A.; Aljada, A. Insulin Receptor Isoforms and Insulin Growth Factor-like Receptors: Implications in Cell Signaling, Carcinogenesis, and Chemoresistance. Int. J. Mol. Sci. 2023, 24, 15006. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, K.; Li, W.; Wang, C.; Liu, Y.; Zhang, H.; Pan, J.; Qi, S.; Peng, J. Expression of Insulin-Like Growth Factor Type 1 Receptor Is Linked to Inflammation in Adamantinomatous Craniopharyngioma. Neuroendocrinology 2022, 112, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Ludovini, V.; Bellezza, G.; Pistola, L.; Bianconi, F.; Di Carlo, L.; Sidoni, A.; Semeraro, A.; Del Sordo, R.; Tofanetti, F.R.; Mameli, M.G.; et al. High Coexpression of Both Insulin-like Growth Factor Receptor-1 (IGFR-1) and Epidermal Growth Factor Receptor (EGFR) Is Associated with Shorter Disease-Free Survival in Resected Non-Small-Cell Lung Cancer Patients. Ann. Oncol. 2009, 20, 842–849. [Google Scholar] [CrossRef]
- Wulbrand, U.; Remmert, G.; Zöfel, P.; Wied, M.; Arnold, R.; Fehmann, H.C. mRNA Expression Patterns of Insulin-like Growth Factor System Components in Human Neuroendocrine Tumours. Eur. J. Clin. Investig. 2000, 30, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Van Adrichem, R.C.S.; De Herder, W.W.; Kamp, K.; Brugts, M.P.; De Krijger, R.R.; Sprij-Mooij, D.M.; Lamberts, S.W.J.; Van Koetsveld, P.M.; Janssen, J.A.M.J.L.; Hofland, L.J. Effects of Somatostatin Analogs and Dopamine Agonists on Insulin-Like Growth Factor 2-Induced Insulin Receptor Isoform A Activation by Gastroenteropancreatic Neuroendocrine Tumor Cells. Neuroendocrinology 2016, 103, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Afargan, M. Novel Long-Acting Somatostatin Analog with Endocrine Selectivity: Potent Suppression of Growth Hormone But Not of Insulin. Endocrinology 2001, 142, 447–486. [Google Scholar] [CrossRef] [PubMed]
- Gentilin, E.; Di Pasquale, C.; Rossi, M.; Tagliati, F.; Gagliano, T.; Rossi, R.; Pelizzo, M.; Merante Boschin, I.; Degli Uberti, E.C.; Zatelli, M.C. IGF-I Influences Everolimus Activity in Medullary Thyroid Carcinoma. Front. Endocrinol. 2015, 6, 63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chitnis, M.M.; Yuen, J.S.P.; Protheroe, A.S.; Pollak, M.; Macaulay, V.M. The Type 1 Insulin-Like Growth Factor Receptor Pathway. Clin. Cancer Res. 2008, 14, 6364–6370. [Google Scholar] [CrossRef] [PubMed]
- Henfling, M.E.R.; Perren, A.A.; Schmitt, A.M.; Saddig, C.M.; Starke, A.A.; Riedl, R.G.; Versleijen-Jonkers, Y.M.H.; Sprij-Mooij, D.M.; Ramaekers, F.C.S.; Hofland, L.J.; et al. The IGF Pathway Is Activated in Insulinomas but Downregulated in Metastatic Disease. Endocr. Relat. Cancer 2018, 25, 1005–1018. [Google Scholar] [CrossRef]
- Borges, M.; Linnoila, R.I.; Van De Velde, H.J.K.; Chen, H.; Nelkin, B.D.; Mabry, M.; Baylin, S.B.; Ball, D.W. An Achaete-Scute Homologue Essential for Neuroendocrine Differentiation in the Lung. Nature 1997, 386, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Hu, R.; Ding, Q.; Savage, T.K.; Huffman, K.E.; Williams, N.; Cobb, M.H.; Minna, J.D.; Johnson, J.E.; Yu, Y. Subtype-Specific Secretomic Characterization of Pulmonary Neuroendocrine Tumor Cells. Nat. Commun. 2019, 10, 3201. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Gagliano, T.; Giamas, G. Direct Effects of Anti-Angiogenic Therapies on Tumor Cells: VEGF Signaling. Trends Mol. Med. 2017, 23, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; Hofland, L.J.; Dogan, F.; Giamas, G.; Gagliano, T.; Zatelli, M.C. Evaluation of Spheroid 3D Culture Methods to Study a Pancreatic Neuroendocrine Neoplasm Cell Line. Front. Endocrinol. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Mairinger, F.D.; Walter, R.F.H.; Werner, R.; Christoph, D.C.; Ting, S.; Vollbrecht, C.; Zarogoulidis, K.; Huang, H.; Li, Q.; Schmid, K.W.; et al. Activation of Angiogenesis Differs Strongly Between Pulmonary Carcinoids and Neuroendocrine Carinomas and Is Crucial for Carcinoid Tumourgenesis. J. Cancer 2014, 5, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Dicitore, A.; Gaudenzi, G.; Carra, S.; Cantone, M.C.; Oldani, M.; Saronni, D.; Borghi, M.O.; Grotteschi, J.; Persani, L.; Vitale, G. Antitumor Activity of Axitinib in Lung Carcinoids: A Preclinical Study. Cancers 2023, 15, 5375. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-M.; Chu, P.-Y.; Lin, H.-Y.; Huang, K.-W.; Hung, W.-C.; Shan, Y.-S.; Chen, L.-T.; Tsai, H.-J. PTEN Regulates Invasiveness in Pancreatic Neuroendocrine Tumors through DUSP19-Mediated VEGFR3 Dephosphorylation. J. Biomed. Sci. 2022, 29, 92. [Google Scholar] [CrossRef] [PubMed]
- Vaahtomeri, K.; Karaman, S.; Mäkinen, T.; Alitalo, K. Lymphangiogenesis Guidance by Paracrine and Pericellular Factors. Genes Dev. 2017, 31, 1615–1634. [Google Scholar] [CrossRef] [PubMed]
- Varney, M.L.; Singh, R.K. VEGF-C-VEGFR3/Flt4 Axis Regulates Mammary Tumor Growth and Metastasis in an Autocrine Manner. Am. J. Cancer Res. 2015, 5, 616–628. [Google Scholar] [PubMed]
- Rudin, C.M.; Drilon, A.; Poirier, J.T. RET Mutations in Neuroendocrine Tumors: Including Small-Cell Lung Cancer. J. Thorac. Oncol. 2014, 9, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Santoro, M.; Schlumberger, M. The Importance of the RET Gene in Thyroid Cancer and Therapeutic Implications. Nat. Rev. Endocrinol. 2021, 17, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, V.; Ting, S.; Herold, T.; Synoracki, S.; Latteyer, S.; Moeller, L.C.; Zwanziger, D.; Stuschke, M.; Fuehrer, D.; Schmid, K.W. NGS Based Identification of Mutational Hotspots for Targeted Therapy in Anaplastic Thyroid Carcinoma. Oncotarget 2017, 8, 42613–42620. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Anton-Pascual, B.; Modrego, A.; Del Carmen Riesco-Martinez, M.; Lens-Pardo, A.; Carretero-Puche, C.; Rubio-Cuesta, B.; Soldevilla, B. Advances in the Treatment of Gastroenteropancreatic Neuroendocrine Carcinomas: Are We Moving Forward? Endocr. Rev. 2023, 44, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Addeo, A.; Miranda-Morales, E.; Den Hollander, P.; Friedlaender, A.; Sintim, H.O.; Wu, J.; Mani, S.A.; Subbiah, V. RET Aberrant Cancers and RET Inhibitor Therapies: Current State-of-the-Art and Future Perspectives. Pharmacol. Ther. 2023, 242, 108344. [Google Scholar] [CrossRef] [PubMed]
- VanDeusen, H.R.; Ramroop, J.R.; Morel, K.L.; Bae, S.Y.; Sheahan, A.V.; Sychev, Z.; Lau, N.A.; Cheng, L.C.; Tan, V.M.; Li, Z.; et al. Targeting RET Kinase in Neuroendocrine Prostate Cancer. Mol. Cancer Res. 2020, 18, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.Y.; Bergom, H.E.; Day, A.; Greene, J.T.; Sychev, Z.E.; Larson, G.; Corey, E.; Plymate, S.R.; Freedman, T.S.; Hwang, J.H.; et al. ZBTB7A as a Novel Vulnerability in Neuroendocrine Prostate Cancer. Front. Endocrinol. 2023, 14, 1093332. [Google Scholar] [CrossRef] [PubMed]
- Kander, E.M.; Shah, M.H.; Zhou, Y.; Goyal, A.; Palmer, J.D.; Owen, D.H.; Shilo, K.; Patel, G.; Raval, R.R.; Gonzalez, J.; et al. Response to the Selective RET Inhibitor Selpercatinib (LOXO-292) in a Patient With RET Fusion-Positive Atypical Lung Carcinoid. Clin. Lung Cancer 2021, 22, e442–e445. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the Transforming EML4–ALK Fusion Gene in Non-Small-Cell Lung Cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a Kinase Gene, ALK, to a Nucleolar Protein Gene, NPM, in Non-Hodgkin’s Lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, J.; Xia, Q.; Liu, K.; Dong, Z. New Perspectives for Targeting Therapy in ALK-Positive Human Cancers. Oncogene 2023, 42, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Ou, S.H.; Bang, Y.J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.L.; Peters, G.; Szaumkessel, M.; Leong, T.; Asadi, K.; Rivalland, G.; Do, H.; Senko, C.; Mitchell, P.L.; Quing, C.Z.; et al. NTRK and ALK Rearrangements in Malignant Pleural Mesothelioma, Pulmonary Neuroendocrine Tumours and Non-Small Cell Lung Cancer. Lung Cancer 2020, 146, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Uchiyama, N.; Shigemasa, R.; Matsumura, T.; Matsuoka, R.; Nomura, A. Atypical Carcinoid Tumor with Anaplastic Lymphoma Kinase (ALK) Rearrangement Successfully Treated by an ALK Inhibitor. Intern. Med. 2016, 55, 3151–3153. [Google Scholar] [CrossRef] [PubMed]
- Ooki, A.; Osumi, H.; Fukuda, K.; Yamaguchi, K. Potent Molecular-Targeted Therapies for Gastro-Entero-Pancreatic Neuroendocrine Carcinoma. Cancer Metastasis Rev. 2023, 42, 1021–1054. [Google Scholar] [CrossRef] [PubMed]
- Aristizabal Prada, E.T.; Heinzle, V.; Knösel, T.; Nölting, S.; Spöttl, G.; Maurer, J.; Spitzweg, C.; Angele, M.; Schmidt, N.; Beuschlein, F.; et al. Tropomyosin Receptor Kinase: A Novel Target in Screened Neuroendocrine Tumors. Endocr. Relat. Cancer 2018, 25, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Passhak, M.; McNamara, M.G.; Hubner, R.A.; Ben-Aharon, I.; Valle, J.W. Choosing the Best Systemic Treatment Sequence for Control of Tumour Growth in Gastro-Enteropancreatic Neuroendocrine Tumours (GEP-NETs): What Is the Recent Evidence? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101836. [Google Scholar] [CrossRef] [PubMed]
- Corti, F.; Brizzi, M.P.; Amoroso, V.; Giuffrida, D.; Panzuto, F.; Campana, D.; Prinzi, N.; Milione, M.; Cascella, T.; Spreafico, C.; et al. Assessing the Safety and Activity of Cabozantinib Combined with Lanreotide in Gastroenteropancreatic and Thoracic Neuroendocrine Tumors: Rationale and Protocol of the Phase II LOLA Trial. BMC Cancer 2023, 23, 908. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Cozzolino, A.; Malandrino, P.; Minotta, R.; Puliani, G.; Saronni, D.; Faggiano, A.; Colao, A. Role of FGF System in Neuroendocrine Neoplasms: Potential Therapeutic Applications. Front. Endocrinol. 2021, 12, 665631. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).