Molecular Targets for Cannabinoids in Natural Killer Cells: Do They Modulate the Antitumor Activity?
Abstract
1. Introduction
2. Cannabinoids
3. Structural and Functional Diversity of the Cannabinoid Targets in NK Cells
3.1. Overview of the Cannabinoid Targets
3.2. Canonical CB1 and CB2 Expression in NK Cells
3.3. Non-Canonical Cannabinoid Receptors in NK Cells
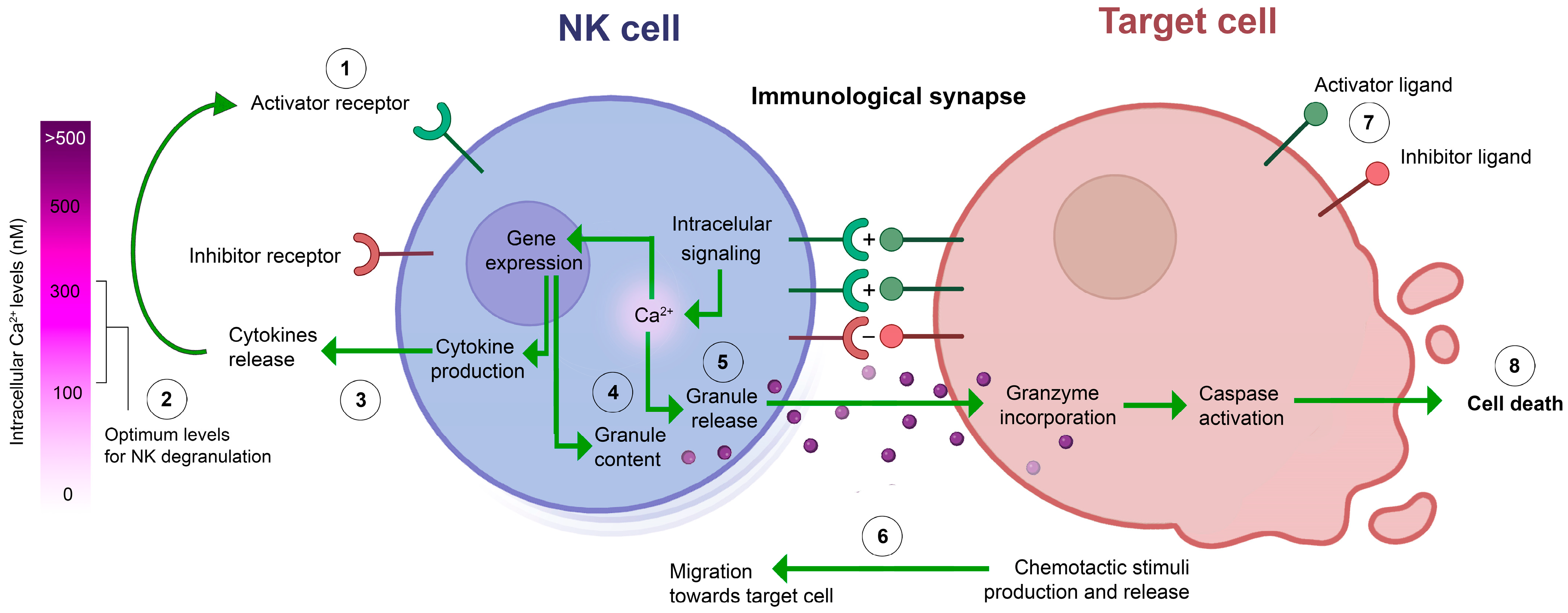
3.4. Ion Channels and Ca2+ Signaling
3.5. Enzymes
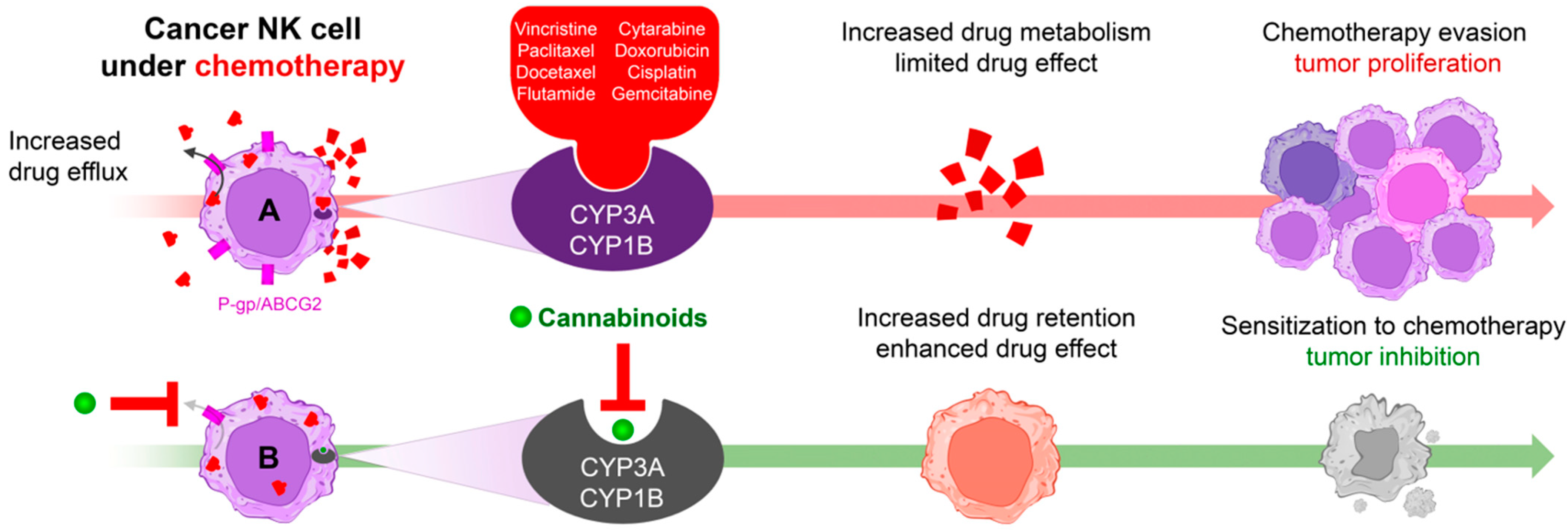
3.6. Transporters
4. Biological Effects of Cannabinoids on NK Cells in Animal Models and In Vitro Studies
4.1. Functional Role of CB2 in NK Cells: Evidence from Murine CB2-Knockout Models
4.2. Effects of Cannabinoids on NK Cell Viability and Proliferation
4.3. Effects of Cannabinoids on NK Cell Migration and Cytokine Production
4.4. Effect of Cannabinoids on Anticancer Activity of NK Cells
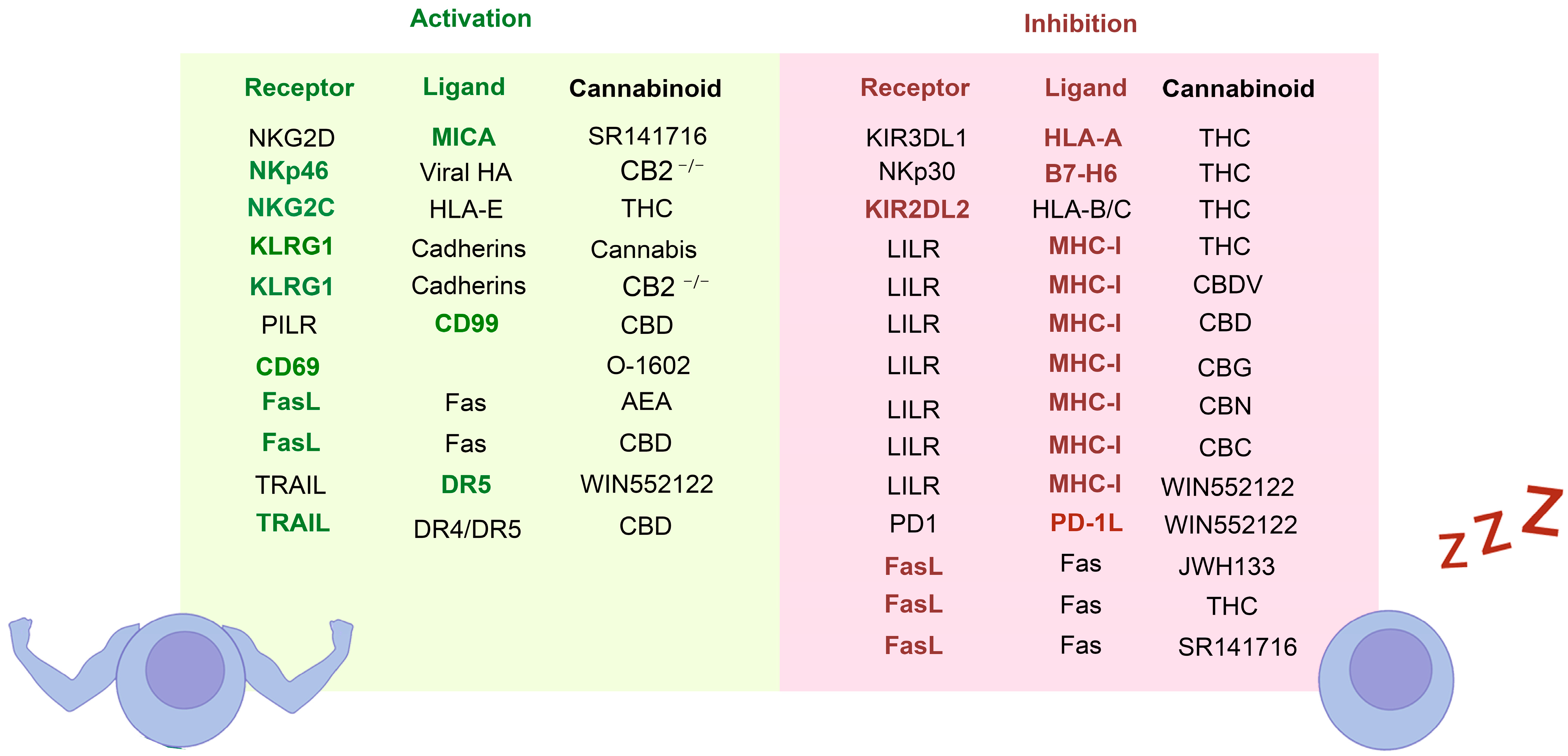
4.5. Effect of the Cannabinoids in the Interaction between NK and Target Cells
5. Cannabis Effect on NK-Related Branch of Immunity in Clinical Reports
6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mujal, A.M.; Delconte, R.B.; Sun, J.C. Natural Killer Cells: From Innate to Adaptive Features. Annu. Rev. Immunol. 2021, 39, 417–447. [Google Scholar] [CrossRef]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011, 89, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.G.M.; Keating, N.; Nicholson, S.E. Natural Killer Cells: Tumor Surveillance and Signaling. Cancers 2020, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.; Medlyn, M.; Billadeau, D.D. Locked and Loaded: Mechanisms Regulating Natural Killer Cell Lytic Granule Biogenesis and Release. Front. Immunol. 2022, 13, 871106–871124. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Cohen, I.; Bar-Sela, G. “The Two Sides of the Same Coin”—Medical Cannabis, Cannabinoids, and Immunity: Pros and Cons Explained. Pharmaceutics 2022, 14, 389. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef] [PubMed]
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the landscape of cancer. J. Cancer Res. Clin. 2021, 147, 2507–2534. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Villatoro-Gómez, K.; Perez-Tapia, S.M.; Pottosin, I.; Dobrovinskaya, O. Cannabidiol on the Path from the Lab to the Cancer Patient: Opportunities and Challenges. Pharmaceutics 2022, 15, 366. [Google Scholar] [CrossRef]
- Aziz, A.; Nguyen, L.C.; Oumeslakht, L.; Bensussan, A.; Mkaddem, S.B. Cannabinoids as Immune System Modulators: Cannabidiol Potential Therapeutic Approaches and Limitations. Cannabis Cannabinoid Res. 2023, 8, 254–269. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, 1373–1380. [Google Scholar] [CrossRef]
- Rahaman, O.; Ganguly, D. Endocannabinoids in immune regulation and immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The synthetic cannabinoids phenomenon: From structure to toxicological properties. A review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Hernández-Tiedra, S.; Dávila, D.; Lorente, M. The use of cannabinoids as anticancer agents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Andradas, C.; Truong, A.; Byrne, J.; Endersby, R. The Role of Cannabinoids as Anticancer Agents in Pediatric Oncology. Cancers 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Tanasescu, R.; Constantinescu, C.S. Cannabinoids and the immune system: An overview. Immunobiology 2010, 215, 588–597. [Google Scholar] [CrossRef]
- Parolaro, D.; Massi, P.; Rubino, T.; Monti, E. Endocannabinoids in the immune system and cancer. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2002, 66, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.S.; Angel, C.E.; Schwarcz, L.E.; Dunbar, P.R.; Glass, M. Detailed Characterisation of CB2 Receptor Protein Expression in Peripheral Blood Immune Cells from Healthy Human Volunteers Using Flow Cytometry. Int. J. Immunopathol. Pharmacol. 2009, 23, 25–34. [Google Scholar] [CrossRef]
- López, A.J.S.; Román-Vega, L.; Tojeiro, E.R.; Giuffrida, A.; García-Merino, A. Cannabinoids in treated multiple sclerosis. Clin. Exp. Immunol. 2015, 179, 119–127. [Google Scholar]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Blal, H.A.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Sarsembayeva, A.; Kienzl, M.; Gruden, E.; Ristic, D.; Maitz, K.; Valadez-Cosmes, P.; Santiso, A.; Hasenoehrl, C.; Brcic, L.; Lindenmann, J.; et al. Cannabinoid receptor 2 plays a pro-tumorigenic role in non-small cell lung cancer by limiting anti-tumor activity of CD8+ T and NK cells. Front. Immunol. 2023, 13, 997115. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Cunha, S.C.; Gonçalves, D.; Mendes, A.; Braga, J.; Correia-da-Silva, G.; Teixeira, N.A. Decidual NK cell-derived conditioned medium from miscarriages affects endometrial stromal cell decidualisation: Endocannabinoid anandamide and tumour necrosis factor-α crosstalk. Hum. Reprod. 2020, 35, 265–274. [Google Scholar] [CrossRef]
- Kishimoto, S.; Muramatsu, M.; Gokoh, M.; Oka, S.; Waku, K.; Sugiura, T. Endogenous Cannabinoid Receptor Ligand Induces the Migration of Human Natural Killer Cells. J. Biochem. 2005, 137, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Garofano, F.; Schmidt-Wolf, I.G.H. High Expression of Cannabinoid Receptor 2 on Cytokine-Induced Killer Cells and Multiple Myeloma Cells. Int. J. Mol. Sci. 2020, 21, 3800. [Google Scholar] [CrossRef]
- Garofano, F.; Sharma, A.; Abken, H.; Gonzalez-Carmona, M.A.; Schmidt-Wolf, I.G.H. A Low Dose of Pure Cannabidiol Is Sufficient to Stimulate the Cytotoxic Function of CIK Cells without Exerting the Downstream Mediators in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2022, 23, 3783. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Fu, B.; Jin, L.; Zheng, X.; Zhang, A.; Sun, R.; Tian, Z.; Wei, H. Programmed differentiated natural killer cells kill leukemia cells by engaging SLAM family receptors. Oncotarget 2017, 8, 57024–57038. [Google Scholar] [CrossRef][Green Version]
- Chiurchiù, V.; Lanuti, M.; Bardi, M.D.; Battistini, L.; Maccarrone, M. The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. Int. Immunol. 2015, 27, 153–160. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Y.; Liu, Z.; Cao, B.-B.; Peng, Y.-P.; Qiu, Y.-H. Dopamine Receptors Modulate Cytotoxicity of Natural Killer Cells via cAMP-PKA-CREB Signaling Pathway. PLoS ONE 2013, 8, e65860. [Google Scholar] [CrossRef]
- Hellstrand, K.; Hermodsson, S. Serotonergic 5-HT1a Receptors Regulate a Cell Contact-Mediated Interaction between Natural Killer Cells and Monocytes. Scand. J. Immunol. 1993, 37, 7–18. [Google Scholar] [CrossRef]
- Frank, M.G.; Johnson, D.R.; Hendricks, S.E.; Frank, J.L.W. Monocyte 5-HT1A receptors mediate pindobind suppression of natural killer cell activity: Modulation by catalase. Int. Immunopharmacol. 2001, 1, 247–253. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Maher, D.P.; Walia, D.; Heller, N.M. Suppression of Human Natural Killer Cells by Different Classes of Opioids. Anesthesia Analg. 2019, 128, 1013–1021. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Sengupta, A.; Zhang, C.; Boyadjieva, N.; Murugan, S. Opiate Antagonist Prevents μ- and δ-Opiate Receptor Dimerization to Facilitate Ability of Agonist to Control Ethanol-altered Natural Killer Cell Functions and Mammary Tumor Growth*. J. Biol. Chem. 2012, 287, 16734–16747. [Google Scholar] [CrossRef]
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion Channels in Innate and Adaptive Immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef] [PubMed]
- Maul-Pavicic, A.; Chiang, S.C.C.; Rensing-Ehl, A.; Jessen, B.; Fauriat, C.; Wood, S.M.; Sjöqvist, S.; Hufnagel, M.; Schulze, I.; Bass, T.; et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc. Natl. Acad. Sci. USA 2011, 108, 3324–3329. [Google Scholar] [CrossRef]
- Kaschek, L.; Zöphel, S.; Knörck, A.; Hoth, M. A calcium optimum for cytotoxic T lymphocyte and natural killer cell cytotoxicity. Semin. Cell Dev. Biol. 2021, 115, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, M.; Cruz-Aguilar, L.H.; Pottosin, I.; Dobrovinskaya, O. Reduction of Ca2+ Entry by a Specific Block of KCa3.1 Channels Optimizes Cytotoxic Activity of NK Cells against T-ALL Jurkat Cells. Cells 2023, 12, 2065. [Google Scholar] [CrossRef]
- Schwindling, C.; Quintana, A.; Krause, E.; Hoth, M. Mitochondria Positioning Controls Local Calcium Influx in T Cells. J. Immunol. 2010, 184, 184–190. [Google Scholar] [CrossRef]
- Rimmerman, N.; Ben-Hail, D.; Porat, Z.; Juknat, A.; Kozela, E.; Daniels, M.P.; Connelly, P.S.; Leishman, E.; Bradshaw, H.B.; Shoshan-Barmatz, V.; et al. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: A novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 2013, 4, e949. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Valle-Reyes, J.S.; Hernández-Cruz, A.; Pottosin, I.; Dobrovinskaya, O. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019, 10, 779–797. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Pottosin, I.; Dobrovinskaya, O. Phenolic Compounds Cannabidiol, Curcumin and Quercetin Cause Mitochondrial Dysfunction and Suppress Acute Lymphoblastic Leukemia Cells. Int. J. Mol. Sci. 2020, 22, 204. [Google Scholar] [CrossRef] [PubMed]
- Saul, S.; Stanisz, H.; Backes, C.S.; Schwarz, E.C.; Hoth, M. How ORAI and TRP channels interfere with each other: Interaction models and examples from the immune system and the skin. Eur. J. Pharmacol. 2014, 739, 49–59. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Petrocellis, L.D.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Marzo, V.D. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Storozhuk, M.V.; Zholos, A.V. TRP Channels as Novel Targets for Endogenous Ligands: Focus on Endocannabinoids and Nociceptive Signalling. Curr. Neuropharmacol. 2018, 16, 137–150. [Google Scholar] [CrossRef]
- Wang, W.; Cao, X.; Liu, C.; Liu, L. Cannabinoid WIN 55,212-2 inhibits TRPV1 in trigeminal ganglion neurons via PKA and PKC pathways. Neurol. Sci. 2012, 33, 79–85. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, H.-J.; Kim, G.E.; Cho, M.-H.; Yoon, S.-Y.; Davies, A.J.; Oh, S.B.; Lee, H.; Cho, Y.K.; Joo, C.H.; et al. Attenuation of natural killer cell functions by capsaicin through a direct and TRPV1-independent mechanism. Carcinogenesis 2014, 35, 1652–1660. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Samanta, A.; Liu, Y.; Hughes, T.E.; Zhao, S.; Yudin, Y.; Rohacs, T.; Han, S.; Moiseenkova-Bell, V.Y. Molecular mechanism of TRPV2 channel modulation by cannabidiol. eLife 2019, 8, e48792. [Google Scholar] [CrossRef]
- Zhang, L.; Simonsen, C.; Zimova, L.; Wang, K.; Moparthi, L.; Gaudet, R.; Ekoff, M.; Nilsson, G.; Hellmich, U.A.; Vlachova, V.; et al. Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function. Nat. Commun. 2022, 13, 7483–7500. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Farfariello, V.; Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Amantini, C. The role of transient receptor potential vanilloid type-2 ion channels in innate and adaptive immune responses. Front. Immunol. 2013, 4, 34–42. [Google Scholar] [CrossRef]
- Hassan, S.; Eldeeb, K.; Millns, P.J.; Bennett, A.J.; Alexander, S.P.H.; Kendall, D.A. Cannabidiol enhances microglial phagocytosis. Br. J. Pharmacol. 2014, 171, 2426–2439. [Google Scholar] [CrossRef]
- Zhang, D.; Spielman, A.; Wang, L.; Ding, G.; Huang, F.; Gu, Q.; Schwarz, W. Mast-Cell Degranulation Induced by Physical Stimuli Involves the Activation of Transient-Receptor-Potential Channel TRPV2. Physiol. Res. 2012, 61, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Rah, S.-Y.; Kwak, J.-Y.; Chung, Y.-J.; Kim, U.-H. ADP-ribose/TRPM2-mediated Ca2+ signaling is essential for cytolytic degranulation and antitumor activity of natural killer cells. Sci. Rep. 2015, 5, 9482. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, H.; Muraki, K.; Eaton, N.; Balinas, C.; Staines, D.; Marshall-Gradisnik, S. Loss of Transient Receptor Potential Melastatin 3 ion channel function in natural killer cells from Chronic Fatigue Syndrome/Myalgic Encephalomyelitis patients. Mol. Med. 2018, 24, 44. [Google Scholar] [CrossRef]
- Marshall-Gradisnik, S.; Huth, T.; Chacko, A.; Johnston, S.; Smith, P.; Staines, D. Natural killer cells and single nucleotide polymorphisms of specific ion channels and receptor genes in myalgic encephalomyelitis/chronic fatigue syndrome. Appl. Clin. Genet. 2016, 9, 39–47. [Google Scholar] [CrossRef]
- Froghi, S.; Grant, C.R.; Tandon, R.; Quaglia, A.; Davidson, B.; Fuller, B. New Insights on the Role of TRP Channels in Calcium Signalling and Immunomodulation: Review of Pathways and Implications for Clinical Practice. Clin. Rev. Allergy Immunol. 2021, 60, 271–292. [Google Scholar] [CrossRef]
- Scopelliti, F.; Dimartino, V.; Cattani, C.; Cavani, A. Functional TRPA1 Channels Regulate CD56dimCD16+ NK Cell Cytotoxicity against Tumor Cells. Int. J. Mol. Sci. 2023, 24, 14736. [Google Scholar] [CrossRef]
- Dobrovinskaya, O.; Valencia-Cruz, G.; Castro-Sánchez, L.; Bonales-Alatorre, E.O.; Liñan-Rico, L.; Pottosin, I. Cholinergic Machinery as Relevant Target in Acute Lymphoblastic T Leukemia. Front. Pharmacol. 2016, 7, 290. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, S.R.; Ziblat, A.; Torres, N.I.; Zwirner, N.W.; Bouzat, C. Expression and Functional Role of α7 Nicotinic Receptor in Human Cytokine-stimulated Natural Killer (NK) Cells*. J. Biol. Chem. 2016, 291, 16541–16552. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, D.; Han, R.; Zhang, C.; Jin, W.-N.; Wood, K.; Liu, Q.; Shi, F.-D.; Hao, J. Acetylcholine-producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/macrophages. Proc. Natl. Acad. Sci. USA 2017, 114, E6202–E6211. [Google Scholar] [CrossRef] [PubMed]
- Shelukhina, I.; Siniavin, A.; Kasheverov, I.; Ojomoko, L.; Tsetlin, V.; Utkin, Y. α7- and α9-Containing Nicotinic Acetylcholine Receptors in the Functioning of Immune System and in Pain. Int. J. Mol. Sci. 2023, 24, 6524. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, M.; Keun-Hang, S.Y.; Sydorenko, V.; Ashoor, A.; Kabbani, N.; Kury, L.A.; Sadek, B.; Howarth, C.F.; Isaev, D.; Galadari, S.; et al. Effects of cannabidiol on the function of α7-nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2013, 720, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Chrestia, J.F.; Esandi, M.d.C.; Bouzat, C. Cannabidiol as a modulator of α7 nicotinic receptors. Cell. Mol. Life Sci. 2022, 79, 564. [Google Scholar] [CrossRef]
- Bhandage, A.K.; Friedrich, L.M.; Kanatani, S.; Jakobsson-Björkén, S.; Escrig-Larena, J.I.; Wagner, A.K.; Chambers, B.J.; Barragan, A. GABAergic signaling in human and murine NK cells upon challenge with Toxoplasma gondii. J. Leukoc. Biol. 2021, 110, 617–628. [Google Scholar] [CrossRef]
- Cifelli, P.; Ruffolo, G.; Felice, E.D.; Alfano, V.; van Vliet, E.A.; Aronica, E.; Palma, E. Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? Int. J. Mol. Sci. 2020, 21, 723. [Google Scholar] [CrossRef]
- Bhandage, A.K.; Barragan, A. GABAergic signaling by cells of the immune system: More the rule than the exception. Cell. Mol. Life Sci. 2021, 78, 5667–5679. [Google Scholar] [CrossRef]
- den Eynden, J.V.; Ali, S.S.; Horwood, N.; Carmans, S.; Brône, B.; Hellings, N.; Steels, P.; Harvey, R.J.; Rigo, J.-M. Glycine and Glycine Receptor Signalling in Non-Neuronal Cells. Front. Mol. Neurosci. 2009, 2, 9. [Google Scholar] [CrossRef]
- Turk, S.; Baesmat, A.S.; Yılmaz, A.; Turk, C.; Malkan, U.Y.; Ucar, G.; Haznedaroğlu, I.C. NK-cell dysfunction of acute myeloid leukemia in relation to the renin–angiotensin system and neurotransmitter genes. Open Med. 2022, 17, 1495–1506. [Google Scholar] [CrossRef]
- Mandler, R.N.; Seamer, L.C.; Whitlinger, D.; Lennon, M.; Rosenberg, E.; Bankhurst, A.D. Human natural killer cells express Na+ channels. A pharmacologic flow cytometric study. J. Immunol. 1990, 144, 2365–2370. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Firmenich, L. Chapter Four-Novel immunotherapeutic approaches to cancer: Voltage-gated sodium channel expression in immune cells and tumors. In Cancer Immunology and Immunotherapy; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 83–109. ISBN 9780128233979. [Google Scholar]
- Wang, H.; Zhang, X.; Xue, L.; Xing, J.; Jouvin, M.-H.; Putney, J.W.; Anderson, M.P.; Trebak, M.; Kinet, J.-P. Low-Voltage-Activated CaV3.1 Calcium Channels Shape T Helper Cell Cytokine Profiles. Immunity 2016, 44, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Erdogmus, S.; Concepcion, A.R.; Yamashita, M.; Sidhu, I.; Tao, A.Y.; Li, W.; Rocha, P.P.; Huang, B.; Garippa, R.; Lee, B.; et al. Cavβ1 regulates T cell expansion and apoptosis independently of voltage-gated Ca2+ channel function. Nat. Commun. 2022, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S.; Budrow, J.; Debatis, M.; Hunter, S.A.; Subramanian, A. Phospholipase participation in cannabinoid-induced release of free arachidonic acid. Biochem. Pharmacol. 1994, 48, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Pick, H.; Vogel, H.; Held, W. NK Cells Respond to Haptens by the Activation of Calcium Permeable Plasma Membrane Channels. PLoS ONE 2016, 11, e0151031. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, X.; Tian, J.; Xiao, Y.; Tian, T.; Xu, F.; Hong, X.; Zhu, M.X. TRPC channels: Structure, function, regulation and recent advances in small molecular probes. Pharmacol. Ther. 2020, 209, 107497. [Google Scholar] [CrossRef] [PubMed]
- Trikha, P.; Moseman, J.E.; Thakkar, A.; Campbell, A.R.; Elmas, E.; Foltz, J.A.; Chakravarti, N.; Fitch, J.R.; Mardis, E.R.; Lee, D.A. Defining the AHR-regulated transcriptome in NK cells reveals gene expression programs relevant to development and function. Blood Adv. 2021, 5, 4605–4618. [Google Scholar] [CrossRef]
- Rodríguez-Antona, C.; Leskelä, S.; Zajac, M.; Cuadros, M.; Alvés, J.; Moneo, M.V.; Martín, C.; Cigudosa, J.C.; Carnero, A.; Robledo, M.; et al. Expression of CYP3A4 as a predictor of response to chemotherapy in peripheral T-cell lymphomas. Blood 2007, 110, 3345–3351. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Kushihara, M.; Yamamoto, I.; Watanabe, K. Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem. Pharmacol. 2010, 79, 1691–1698. [Google Scholar] [CrossRef]
- Doohan, P.T.; Oldfield, L.D.; Arnold, J.C.; Anderson, L.L. Cannabinoid Interactions with Cytochrome P450 Drug Metabolism: A Full-Spectrum Characterization. AAPS J. 2021, 23, 91. [Google Scholar] [CrossRef]
- Whalen, M.M.; Doshi, R.N.; Bader, B.W.; Bankhurst, A.D. Lysophosphatidylcholine and Arachidonic Acid Are Required in the Cytotoxic Response of Human Natural Killer Cells to Tumor Target Cells. Cell. Physiol. Biochem. 1999, 9, 297–309. [Google Scholar] [CrossRef]
- Hoffman, T.; Hirata, F.; Bougnoux, P.; Fraser, B.A.; Goldfarb, R.H.; Herberman, R.B.; Axelrod, J. Phospholipid methylation and phospholipase A2 activation in cytotoxicity by human natural killer cells. Proc. Natl. Acad. Sci. USA 1981, 78, 3839–3843. [Google Scholar] [CrossRef]
- Evans, A.T.; Formukong, E.; Evans, F.J. Activation of phospholipase A2 by cannabinoids. FEBS Lett. 1987, 211, 119–122. [Google Scholar] [CrossRef]
- Shim, S.J.; Yang, W.-I.; Shin, E.; Koom, W.S.; Kim, Y.B.; Cho, J.H.; Suh, C.O.; Kim, J.H.; Kim, G.E. Clinical significance of cyclooxygenase-2 expression in extranodal natural killer (NK)/T-cell lymphoma, nasal type. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 31–38. [Google Scholar] [CrossRef]
- Benish, M.; Bartal, I.; Goldfarb, Y.; Levi, B.; Avraham, R.; Raz, A.; Ben-Eliyahu, S. Perioperative Use of β-blockers and COX-2 Inhibitors May Improve Immune Competence and Reduce the Risk of Tumor Metastasis. Ann. Surg. Oncol. 2008, 15, 2042–2052. [Google Scholar] [CrossRef]
- Takeda, S.; Misawa, K.; Yamamoto, I.; Watanabe, K. Cannabidiolic Acid as a Selective Cyclooxygenase-2 Inhibitory Component in Cannabis. Drug Metab. Dispos. 2008, 36, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the Cyclooxygenase Inhibiting Effects of Six Major Cannabinoids Isolated from Cannabis sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Demarest, K.; Patricelli, M.P.; Bracey, M.H.; Giang, D.K.; Martin, B.R.; Lichtman, A.H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 2001, 98, 9371–9376. [Google Scholar] [CrossRef] [PubMed]
- Freigang, S.; Zadorozhny, V.; McKinney, M.K.; Krebs, P.; Herro, R.; Pawlak, J.; Kain, L.; Schrantz, N.; Masuda, K.; Liu, Y.; et al. Fatty acid amide hydrolase shapes NKT cell responses by influencing the serum transport of lipid antigen in mice. J. Clin. Investig. 2010, 120, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Shamran, H.; Singh, N.P.; Zumbrun, E.E.; Murphy, A.; Taub, D.D.; Mishra, M.K.; Price, R.L.; Chatterjee, S.; Nagarkatti, M.; Nagarkatti, P.S.; et al. Fatty acid amide hydrolase (FAAH) blockade ameliorates experimental colitis by altering microRNA expression and suppressing inflammation. Brain Behav. Immun. 2017, 59, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, P.; Pucci, M.; Siena, S.D.; Giacomo, D.D.; Pirazzi, V.; Geremia, R.; Maccarrone, M. The faah gene is the first direct target of estrogen in the testis: Role of histone demethylase LSD1. Cell. Mol. Life Sci. 2012, 69, 4177–4190. [Google Scholar] [CrossRef] [PubMed]
- Curran, E.M.; Berghaus, L.J.; Vernetti, N.J.; Saporita, A.J.; Lubahn, D.B.; Estes, D.M. Natural Killer Cells Express Estrogen Receptor-α and Estrogen Receptor-β and Can Respond to Estrogen Via a Non-Estrogen Receptor-α-Mediated Pathway. Cell. Immunol. 2001, 214, 12–20. [Google Scholar] [CrossRef]
- Surace, L.; Doisne, J.-M.; Escoll, P.; Marie, S.; Dardalhon, V.; Croft, C.; Thaller, A.; Topazio, D.; Sparaneo, A.; Cama, A.; et al. Polarized mitochondria as guardians of NK cell fitness. Blood Adv. 2021, 5, 26–38. [Google Scholar] [CrossRef]
- Keating, S.E.; Zaiatz-Bittencourt, V.; Loftus, R.M.; Keane, C.; Brennan, K.; Finlay, D.K.; Gardiner, C.M. Metabolic Reprogramming Supports IFN-γ Production by CD56bright NK Cells. J. Immunol. 2016, 196, 2552–2560. [Google Scholar] [CrossRef]
- Fišar, Z.; Singh, N.; Hroudová, J. Cannabinoid-induced changes in respiration of brain mitochondria. Toxicol. Lett. 2014, 231, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Klimecki, W.T.; Taylor, C.W.; Dalton, W.S. Inhibition of cell-mediated cytolysis and P-glycoprotein function in natural killer cells by verapamil isomers and cyclosporine a analogs. J. Clin. Immunol. 1995, 15, 152–158. [Google Scholar] [CrossRef]
- Takahashi, M.; Misawa, Y.; Watanabe, N.; Kawanishi, T.; Tanaka, H.; Shigenobu, K.; Kobayashi, Y. Role of P-glycoprotein in Human Natural Killer-Like Cell Line-Mediated Cytotoxicity. Exp. Cell Res. 1999, 253, 396–402. [Google Scholar] [CrossRef]
- Wilisch, A.; Noller, A.; Handgretinger, R.; Weger, S.; Nüssler, V.; Niethammer, D.; Probst, H.; Gekeler, V. Mdr1P-glycoprotein expression in natural killer (NK) cells enriched from peripheral or umbilical cord blood. Cancer Lett. 1993, 69, 139–148. [Google Scholar] [CrossRef]
- Perkovic, S.; Basic-Kinda, S.; Gasparovic, V.; Krznaric, Z.; Babel, J.; Ilic, I.; Aurer, I.; Batinic, D. Epstein-Barr virus-negative aggressive natural killer-cell leukaemia with high P-glycoprotein activity and phosphorylated extracellular signal-regulated protein kinases 1 and 2. Hematol. Rep. 2012, 4, e16. [Google Scholar] [CrossRef]
- Holland, M.L.; Lau, D.T.T.; Allen, J.D.; Arnold, J.C. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br. J. Pharmacol. 2007, 152, 815–824. [Google Scholar] [CrossRef]
- Zhu, H.-J.; Wang, J.-S.; Markowitz, J.S.; Donovan, J.L.; Gibson, B.B.; Gefroh, H.A.; DeVane, C.L. Characterization of P-glycoprotein Inhibition by Major Cannabinoids from Marijuana. J. Pharmacol. Exp. Ther. 2006, 317, 850–857. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Dong, M.; Yang, Z.; Zhang, M.; Chen, Q. sATP-binding cassette subfamily G member 2 enhances the multidrug resistance properties of human nasal natural killer/T cell lymphoma side population cells. Oncol. Rep. 2020, 44, 1467–1478. [Google Scholar] [CrossRef]
- Yang, S.; Kobayashi, S.; Sekino, K.; Kagawa, Y.; Miyazaki, H.; Shil, S.K.; Umaru, B.A.; Wannakul, T.; Owada, Y. Fatty acid-binding protein 5 controls lung tumor metastasis by regulating the maturation of natural killer cells in the lung. FEBS Lett. 2021, 595, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Haj-Dahmane, S.; Shen, R.-Y.; Elmes, M.W.; Studholme, K.; Kanjiya, M.P.; Bogdan, D.; Thanos, P.K.; Miyauchi, J.T.; Tsirka, S.E.; Deutsch, D.G.; et al. Fatty-acid–binding protein 5 controls retrograde endocannabinoid signaling at central glutamate synapses. Proc. Natl. Acad. Sci. USA 2018, 115, 3482–3487. [Google Scholar] [CrossRef] [PubMed]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; et al. Fatty Acid-binding Proteins (FABPs) Are Intracellular Carriers for Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD)*. J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef]
- Ferrini, M.E.; Hong, S.; Stierle, A.; Stierle, D.; Stella, N.; Roberts, K.; Jaffar, Z. CB2 receptors regulate natural killer cells that limit allergic airway inflammation in a murine model of asthma. Allergy 2017, 72, 937–947. [Google Scholar] [CrossRef]
- Barchi, M.; Innocenzi, E.; Giannattasio, T.; Dolci, S.; Rossi, P.; Grimaldi, P. Cannabinoid receptors signaling in the development, epigenetics, and tumours of male germ cells. Int. J. Mol. Sci. 2020, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.; Olsen, B.B. Cannabidiol induces cell death in human lung cancer cells and cancer stem cells. Pharmaceuticals 2021, 14, 1169. [Google Scholar] [CrossRef]
- Bogdanović, V.; Mrdjanović, J.; Borišev, I. A Review of the Therapeutic Antitumor Potential of Cannabinoids. J. Altern. Complement. Med. 2017, 23, 831–836. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Torres-Suárez, A.I. Phyto-, endo- and synthetic cannabinoids: Promising chemotherapeutic agents in the treatment of breast and prostate carcinomas. Expert Opin. Investig. Drugs 2016, 25, 1311–1323. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol—From Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef]
- Kawakami, Y.; Klein, T.W.; Newton, C.; Djeu, J.Y.; Dennert, G.; Specter, S.; Friedman, H. Suppression by Cannabinoids of a Cloned Cell Line with Natural Killer Cell Activity. Proc. Soc. Exp. Biol. Med. 1988, 187, 355–359. [Google Scholar] [CrossRef]
- Ignatowska-Jankowska, B.; Jankowski, M.; Glac, W.; Swiergel, A.H. Cannabidiol-induced lymphopenia does not involve NKT and NK cells. J Physiol. Pharmacol. 2008, 60 (Suppl. 3), 99–103. [Google Scholar]
- Singh, U.P.; Singh, N.P.; Singh, B.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10−/− mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol. Appl. Pharmacol. 2012, 258, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Fuzio, D.; Viganò, D.; Sacerdote, P.; Parolaro, D. Relative involvement of cannabinoid CB1 and CB2 receptors in the Δ9-tetrahydrocannabinol-induced inhibition of natural killer activity. Eur. J. Pharmacol. 2000, 387, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Borysenko, M.; Kumar, M.S.A.; Millard, W.J. Effects of Acute and Subchronic Δ9-tetrahydrocannabinol Administration on the Plasma Catecholamine, β-Endorphin, and Corticosterone Levels and Splenic Natural Killer Cell Activity in Rats. Proc. Soc. Exp. Biol. Med. 1985, 180, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W.; Newton, C.; Friedman, H. Inhibition of natural killer cell function by Marijuana components. J. Toxicol. Environ. Health 1987, 20, 321–332. [Google Scholar] [CrossRef]
- Specter, S.C.; Klein, T.W.; Newton, C.; Mondragon, M.; Widen, R.; Friedman, H. Marijuana effects on immunity: Suppression of human natural killer cell activity by delta-9-tetrahydrocannabinol. Int. J. Immunopharmacol. 1986, 8, 741–745. [Google Scholar] [CrossRef]
- Patrini, G.; Sacerdote, P.; Fuzio, D.; Manfredi, B.; Parolaro, D. Regulation of immune functions in rat splenocytes after acute and chronic in vivo treatment with CP-55,940, a synthetic cannabinoid compound. J. Neuroimmunol. 1997, 80, 143–148. [Google Scholar] [CrossRef]
- Massi, P.; Vaccani, A.; Romorini, S.; Parolaro, D. Comparative characterization in the rat of the interaction between cannabinoids and opiates for their immunosuppressive and analgesic effects. J. Neuroimmunol. 2001, 117, 116–124. [Google Scholar] [CrossRef]
- Takheaw, N.; Jindaphun, K.; Pata, S.; Laopajon, W.; Kasinrerk, W. Cannabinoid Receptor 1 Agonist ACEA and Cannabinoid Receptor 2 Agonist GW833972A Attenuates Cell-Mediated Immunity by Different Biological Mechanisms. Cells 2023, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Labrada, A.; Pesini, C.; Santiago, L.; Hidalgo, S.; Calvo-Pérez, A.; Oñate, C.; Andrés-Tovar, A.; Garzón-Tituaña, M.; Uranga-Murillo, I.; Arias, M.A.; et al. All About (NK Cell-Mediated) Death in Two Acts and an Unexpected Encore: Initiation, Execution and Activation of Adaptive Immunity. Front. Immunol. 2022, 13, 896228–896241. [Google Scholar] [CrossRef] [PubMed]
- EL–Gohary, M.; Eid, M.A. Effect of cannabinoid ingestion (in the form of bhang) on the immune system of high school and university students. Hum. Exp. Toxicol. 2004, 23, 149–156. [Google Scholar] [CrossRef]
- Pacifici, R.; Zuccaro, P.; Farré, M.; Poudevida, S.; Abanades, S.; Pichini, S.; Langohr, K.; Segura, J.; Torre, R.D.L. Combined immunomodulating properties of 3,4-methylenedioxymethamphetamine (MDMA) and cannabis in humans. Addiction 2007, 102, 931–936. [Google Scholar] [CrossRef]
- Evans, D.L.; Leserman, J.; Perkins, D.O.; Stern, R.A.; Murphy, C.; Tamul, K.; Liao, D.; van der Horst, C.M.; Hall, C.D.; Folds, J.D. Stress-associated reductions of cytotoxic T lymphocytes and natural killer cells in asymptomatic HIV infection. Am. J. Psychiatry 1995, 152, 543–550. [Google Scholar]
- Bredt, B.M.; Higuera-Alhino, D.; Shade, S.B.; Hebert, S.J.; McCune, J.M.; Abrams, D.I. Short-Term Effects of Cannabinoids on Immune Phenotype and Function in HIV-1-Infected Patients. J. Clin. Pharmacol. 2002, 42, 82S–89S. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.D.; Camarca, M.; Xu, J.; Durako, S.; Murphy, D.; Moscicki, B.; Wilson, C.M.; Network, R. for E. in A.C. and H.P., Adolescent Medicine HIV/AIDS Research the Relationships between Substance Abuse, Psychosocial Variables, and Natural Killer Cell Enumeration and Function in HIV-Infected and High-Risk Uninfected Adolescents. AIDS Res. Hum. Retroviruses 2003, 19, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kisiolek, J.N.; Flores, V.A.; Ramani, A.H.; Butler, B.; Haughian, J.M.; Stewart, L.K. Eight Weeks of Daily Cannabidiol Supplementation Improves Sleep Quality and Immune Cell Cytotoxicity. Nutrients 2023, 15, 4173. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, N.; Cirillo, A.; Siniscalco, D. Beneficial Effects of Palmitoylethanolamide on Expressive Language, Cognition, and Behaviors in Autism: A Report of Two Cases. Case Rep. Psychiatry 2015, 2015, 325061. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research, 1st ed.; The National Academies Press: Washington, DC, USA, 2017; pp. 199–216. [Google Scholar]
- Casey, T.M.; Meade, J.L.; Hewitt, E.W. Organelle Proteomics Identification of the Exocytic Machinery Associated with the Natural Killer Cell Secretory Lysosome*. Mol. Cell. Proteom. 2007, 6, 767–780. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Brito, C.X.L.; Teixeira, S.A.; Barboza, R.; Reis, A.S.d.; Azevedo-Santos, A.P.S.; Muscará, M.; Costa, S.K.P.; Marinho, C.R.F.; Brain, S.D.; et al. TRPV1 Antagonism by Capsazepine Modulates Innate Immune Response in Mice Infected with Plasmodium berghei ANKA. Mediat. Inflamm. 2014, 2014, 506450. [Google Scholar] [CrossRef] [PubMed]
- Su, A.I.; Wiltshire, T.; Batalov, S.; Lapp, H.; Ching, K.A.; Block, D.; Zhang, J.; Soden, R.; Hayakawa, M.; Kreiman, G.; et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 2004, 101, 6062–6067. [Google Scholar] [CrossRef] [PubMed]
- Deem, R.L.; Britvan, L.J.; Targan, S.R. Definition of a secondary target cell trigger during natural killer cell cytotoxicity: Possible role of phospholipase A2. Cell. Immunol. 1987, 110, 253–264. [Google Scholar] [CrossRef]
- Assmann, N.; O’Brien, K.L.; Donnelly, R.P.; Dyck, L.; Zaiatz-Bittencourt, V.; Loftus, R.M.; Heinrich, P.; Oefner, P.J.; Lynch, L.; Gardiner, C.M.; et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. 2017, 18, 1197–1206. [Google Scholar] [CrossRef]
- Ziegler, S.; Weiss, E.; Schmitt, A.-L.; Schlegel, J.; Burgert, A.; Terpitz, U.; Sauer, M.; Moretta, L.; Sivori, S.; Leonhardt, I.; et al. CD56 Is a Pathogen Recognition Receptor on Human Natural Killer Cells. Sci. Rep. 2017, 7, 6138. [Google Scholar] [CrossRef]
- Mah-Som, A.Y.; Keppel, M.P.; Tobin, J.M.; Kolicheski, A.; Saucier, N.; Sexl, V.; French, A.R.; Wagner, J.A.; Fehniger, T.A.; Cooper, M.A. Reliance on Cox10 and oxidative metabolism for antigen-specific NK cell expansion. Cell Rep. 2021, 35, 109209–109225. [Google Scholar] [CrossRef]
- Zhao, H.B.; Zhang, X.F.; Shi, F.; Zhang, M.Z.; Xue, W.L. Comparison of the expression of human equilibrative nucleotide transporter 1 (hENT1) and ribonucleotide reductase subunit M1 (RRM1) genes in seven non-Hodgkin lymphoma cell lines. Genet. Mol. Res. 2016, 15, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Kim, S.; Ahn, Y.-O.; Lee, S.-H.; Kim, D.-W.; Heo, D.S. Anti-cancer activity of gemcitabine against natural killer cell leukemia/lymphoma. Leuk. Lymphoma 2014, 55, 940–943. [Google Scholar] [CrossRef]
- Chen, C.; Chang, Z.; Tsai, F.; Chen, S. Cannabinoid receptor type 1 antagonist inhibits progression of obesity-associated nonalcoholic steatohepatitis in a mouse model by remodulating immune system disturbances. Immun. Inflamm. Dis. 2020, 8, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Rakib, A.; Moore, B.M.; Singh, U.P. Cannabinoid Receptor 2 (CB2) Inverse Agonist SMM-189 Induces Expression of Endogenous CB2 and Protein Kinase A That Differentially Modulates the Immune Response and Suppresses Experimental Colitis. Pharmaceutics 2022, 14, 936. [Google Scholar] [CrossRef]
- Ciaglia, E.; Torelli, G.; Pisanti, S.; Picardi, P.; D’Alessandro, A.; Laezza, C.; Malfitano, A.M.; Fiore, D.; Zottola, A.C.P.; Proto, M.C.; et al. Cannabinoid receptor CB1 regulates STAT3 activity and its expression dictates the responsiveness to SR141716 treatment in human glioma patients’ cells. Oncotarget 2015, 6, 15464–15481. [Google Scholar] [CrossRef]
- Ko, M.-W.; Breznik, B.; Senjor, E.; Jewett, A. Synthetic cannabinoid WIN 55,212–2 inhibits growth and induces cell death of oral and pancreatic stem-like/poorly differentiated tumor cells. Adv. Cancer Biol.-Metastasis 2022, 5, 100043–100052. [Google Scholar] [CrossRef]
- Hernández-Cervantes, R.; Pérez-Torres, A.; Prospéro-García, Ó.; Montor, J.M. Gestational exposure to the cannabinoid WIN 55,212-2 and its effect on the innate intestinal immune response. Sci. Rep. 2019, 9, 20340. [Google Scholar] [CrossRef]
- Hu, Y.; Ranganathan, M.; Shu, C.; Liang, X.; Ganesh, S.; Osafo-Addo, A.; Yan, C.; Zhang, X.; Aouizerat, B.E.; Krystal, J.H.; et al. Single-cell Transcriptome Mapping Identifies Common and Cell-type Specific Genes Affected by Acute Delta9-tetrahydrocannabinol in Humans. Sci. Rep. 2020, 10, 3450. [Google Scholar] [CrossRef]
- Karmaus, P.W.F.; Wagner, J.G.; Harkema, J.R.; Kaminski, N.E.; Kaplan, B.L.F. Cannabidiol (CBD) enhances lipopolysaccharide (LPS)-induced pulmonary inflammation in C57BL/6 mice. J. Immunotoxicol. 2013, 10, 321–328. [Google Scholar] [CrossRef]
- Dada, S.; Ellis, S.L.S.; Wood, C.; Nohara, L.L.; Dreier, C.; Garcia, N.H.; Saranchova, I.; Munro, L.; Pfeifer, C.G.; Eyford, B.A.; et al. Specific cannabinoids revive adaptive immunity by reversing immune evasion mechanisms in metastatic tumours. Front. Immunol. 2023, 13, 982082. [Google Scholar] [CrossRef]
- Hurrell, B.P.; Helou, D.G.; Shafiei-Jahani, P.; Howard, E.; Painter, J.D.; Quach, C.; Akbari, O. Cannabinoid receptor 2 engagement promotes group 2 innate lymphoid cell expansion and enhances airway hyperreactivity. J. Allergy Clin. Immunol. 2022, 149, 1628–1642.e10. [Google Scholar] [CrossRef]
- Falcinelli, S.D.; Cooper-Volkheimer, A.D.; Semenova, L.; Wu, E.; Richardson, A.; Ashokkumar, M.; Margolis, D.M.; Archin, N.M.; Rudin, C.D.; Murdoch, D.; et al. Impact of Cannabis Use on Immune Cell Populations and the Viral Reservoir in People with HIV on Suppressive Antiretroviral Therapy. J. Infect. Dis. 2023, 228, 1600–1609. [Google Scholar] [CrossRef]
- Libro, R.; Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells. Front. Physiol. 2016, 7, 559. [Google Scholar] [CrossRef]
- Huang, L.; Ramirez, J.C.; Frampton, G.A.; Golden, L.E.; Quinn, M.A.; Pae, H.Y.; Horvat, D.; Liang, L.; DeMorrow, S. Anandamide exerts its antiproliferative actions on cholangiocarcinoma by activation of the GPR55 receptor. Lab. Investig. 2011, 91, 1007–1017. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, L.; Wang, Y.; Deng, G.; Cao, X.; Ke, B.; Wu, X.; Gu, Y.; Cheng, H.; Xu, Q.; et al. Single-cell analyses reveal cannabidiol rewires tumor microenvironment via inhibiting alternative activation of macrophage and synergizes with anti-PD-1 in colon cancer. J. Pharm. Anal. 2023, 13, 726–744. [Google Scholar] [CrossRef]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Δ9-Tetrahydrocannabinol attenuates allogeneic host-versus-graft response and delays skin graft rejection through activation of cannabinoid receptor 1 and induction of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Greiner, B.; Sommerfeld, M.; Kintscher, U.; Unger, T.; Kappert, K.; Kaschina, E. Differential Regulation of MMPs, Apoptosis and Cell Proliferation by the Cannabinoid Receptors CB1 and CB2 in Vascular Smooth Muscle Cells and Cardiac Myocytes. Biomedicines 2022, 10, 3271. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, O.; Calvaruso, G.; Portanova, P.; Blasio, A.D.; Santulli, A.; Vento, R.; Tesoriere, G.; Giuliano, M. The Synthetic Cannabinoid WIN 55,212-2 Sensitizes Hepatocellular Carcinoma Cells to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis by Activating p8/CCAAT/Enhancer Binding Protein Homologous Protein (CHOP)/Death Receptor 5 (DR5) Axis. Mol. Pharmacol. 2010, 77, 854–863. [Google Scholar]
- Ivanov, V.N.; Wu, J.; Hei, T.K. Regulation of human glioblastoma cell death by combined treatment of cannabidiol, γ-radiation and small molecule inhibitors of cell signaling pathways. Oncotarget 2017, 8, 74068–74095. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivas-Aguirre, M.; Gutiérrez-Iñiguez, C.; Pottosin, I.; Dobrovinskaya, O. Molecular Targets for Cannabinoids in Natural Killer Cells: Do They Modulate the Antitumor Activity? Receptors 2024, 3, 122-144. https://doi.org/10.3390/receptors3020007
Olivas-Aguirre M, Gutiérrez-Iñiguez C, Pottosin I, Dobrovinskaya O. Molecular Targets for Cannabinoids in Natural Killer Cells: Do They Modulate the Antitumor Activity? Receptors. 2024; 3(2):122-144. https://doi.org/10.3390/receptors3020007
Chicago/Turabian StyleOlivas-Aguirre, Miguel, Cecilia Gutiérrez-Iñiguez, Igor Pottosin, and Oxana Dobrovinskaya. 2024. "Molecular Targets for Cannabinoids in Natural Killer Cells: Do They Modulate the Antitumor Activity?" Receptors 3, no. 2: 122-144. https://doi.org/10.3390/receptors3020007
APA StyleOlivas-Aguirre, M., Gutiérrez-Iñiguez, C., Pottosin, I., & Dobrovinskaya, O. (2024). Molecular Targets for Cannabinoids in Natural Killer Cells: Do They Modulate the Antitumor Activity? Receptors, 3(2), 122-144. https://doi.org/10.3390/receptors3020007







